Abstract
Transcranial Doppler (TCD/TCCS) is a noninvasive technique that allows the study of cerebral vasculature and its disturbances in real-time, promoting timely therapeutic measures to control cerebral perfusion variables. In the setting of post-traumatic intracranial hypertension, one of the effective interventions for restoration of many intracranial properties (including compliance and elastance) is decompressive craniectomy (DC). Strict follow-up of outcome and complication results of this intervention is mandatory to determine additional therapeutic strategies to maintain brain homeostasis. A three-phase pattern of cerebral blood flow (CBF) after DC in traumatic brain injury (TBI) (hypoperfusion, vasospasm and hyperemia) measured by TCD/TCCS has been described. A fourth pattern is described, called the transition pattern, taking into account a wider spectrum of cerebral hemodynamic changes occurring in the early and intermediate period post-DC. The latter’s clinical implications are related to getting more physiologic-approach management of patients with TBI after DC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Decompressive craniectomy
- Transcranial Doppler ultrasonography
- Intracranial pressure
- Cerebral hemodynamics
- Traumatic brain injury
- Intracranial compartments
- Neurocritical care
-
1.
TCD/TCCS is a noninvasive monitoring technique in patients with acute neurological injury and/or neurosurgical pathology. TCD/TCCS is safe, which facilitates the study of cerebral blood flow velocities (CBFVs) and allows detection of possible complications.
-
2.
TCCS allows visualization of possible anatomical changes of the brain parenchyma and blood vessels, and achieves a greater accuracy measures of CBFVs compared with TCD method (blind technique), pre- and post-craniectomy.
-
3.
TCD/TCCS allows assessment of cerebral hemodynamic changes in real time during the intracranial hypertension management, as well as the hemodynamic changes due to decompressive craniectomy (DC).
-
4.
Decompressive craniectomy is considered a life-saving surgical technique to improve refractory intracranial hypertension, where ICP elevation is not controlled with medical management. TCD/TCCS provides useful information during clinical evolution and could contribute to determining the neurological prognosis of the critically ill patient.
1 Introduction
Transcranial Doppler/transcranial color-coded duplex sonography (TCD/TCCS) are noninvasive and reproducible monitoring tools that facilitate the study of the cerebral hemodynamics, allowing for the assessment in real time the CBFV variations and its association with clinical changes at bedside [1]. These noninvasive monitoring techniques are part of the diagnostic arsenal to provide timely and directed-therapy. Therefore, TCD/TCCS measurements can provide information to individualize interventions adjusted to the clinical needs of each patient [1].
TCCS, compared to other monitoring techniques, provides images of the brain parenchyma anatomical (B-mode) and cerebral blood vessels in real time, enabling a more accurate acoustic access [2, 3].
In patients with traumatic brain injury (TBI) , TCD/TCCS provides information on the cerebral hemodynamic status. Hyperemia and vasospasm are pathological conditions present during TBI. These clinical situations can be differentiated from each by calculating the Lindegaard ratio [6]. If the latter is elevated, then vasospasm is the most likely reason for the elevated CBFVs. The vasospasm and CBFV changes (spectral Doppler waveform analysis) may precede the clinical manifestations of neurological worsening (e.g., delayed cerebral ischemia). Therefore, TCD/TCCS is a useful technique to anticipate neurological worsening at bedside [4, 5]. It has been described that vasospasm findings evidenced by TCD/TCCS predict and are associated with documented vasospasm through invasive angiography (CTA, DSA) [6].
A three-phase CBF pattern has been described after TBI [6]. Initially, overall CBF is reduced leading to a hypoperfusion status followed by a hyperperfusion phenomenon in the following 24–72 hours [5, 6].
There are patterns for rapid and simple recognition of CBF disturbances (e.g., hyperflow, cerebral circulatory arrest), which can be identified in the interpretation of spectral Doppler waveform analysis and hemodynamic indexes (pulsatility indexes (PI), resistance index (RI)) derived from it [2], thus facilitating rapid real-time therapeutic decision (Fig. 55.1).
2 TCD/TCCS Technique
TCD technique uses a low-frequency transducer (2 MHz), and TCCS uses phased array transducer whose frequency oscillates between 1 and 5 MHz, being automatically configured at frequencies 1 and 2 MHz when the B-mode is selected [1, 2, 38].
CBFVs should be measured with an insonation angle less than 60 degrees. The following precaution is recommended [1]:
-
1.
Importance of keeping the correct insonation angle-correction. It can increase 20–30% in CBFVs measurement (more details see Chap. 72).
One of the impediments for an adequate transcranial insonation of CBFVs is the absence of transtemporal and suboccipital acoustic windows (prevalent in elderly women). Therefore, in craniectomized patients [1, 38, 39], the evaluation of cerebral basal arteries is easier.
3 Descompressive Craniectomy (DC)
The utility of DC to manage intracranial hypertension syndrome has been well described. A space-occupying lesion (intracerebral hemorrhage, subdural hematoma, etc.) or diffuse lesions (brain edema) may generate midline shift and/or different classic herniation syndromes. According to the classical Monro-Kellie doctrine , intracranial components shift to compensate the ICP changes to maintain adequate brain oxygen delivery and cerebral perfusion pressure (CPP). The following have been described as compensatory mechanisms: cerebrospinal fluid (CSF) re-distributes from skull into the spinal canal (less production and increase reabsorption), venous systems compression (increase venous outflow), and arterial system compression (decrease blood entering the skull). These mechanisms are only temporarily effective. Hence, when these compensatory mechanisms are exhausted, neurological worsening ensues [4, 5, 17].
DC is a surgical procedure whereby a bone fragment with a diameter of at least 12–15 cm (supratentorially) is temporarily removed. This provides additional space for brain tissue expansion, thus restoring some balance of intracranial pressures [14, 15], to decrease ICP and CPP [3, 8]. After this procedure, the pressure gradient generated by the displacement of brain structures decreases according to the Monro-Kellie doctrine, therefore avoiding brain edema, cerebral hypoperfusion, and brain herniation syndromes [4, 16, 19, 20, 22, 23].
ICP control through DC leads to a theoretical improvement of cerebral hemodynamics and maintain adequate brain oxygen delivery due to optimization of CBF [10,11,12]. TCD/TCCS has a potential as a diagnostic and monitoring tool to indirectly record these hemodynamic changes, to obtaining real-time information and make crucial and individualized therapeutic decisions to optimize CPP, as well as its usefulness in the post-DC follow-up [3,4,5,6,7,8].
DC is employed when intracranial hypertension is refractory to medical treatment, such as in cases of severe TBI, acute ischemic stroke (AIS) , aneurysmal subarachnoid hemorrhage (aSAH) , CNS infections, and venous sinus thrombosis [3, 4], and can be classified in two ways: chronological and initial vs add-on therapy [26, 27] (Table 55.1).
In general, there are two types of surgical techniques for DC (Table 55.2).
4 Neurological Syndromes and Decompressive Craniectomy: The ¨Open Box¨ Concept
When a bone flap is removed, the skull or “closed box,” becomes an “open box” [3, 5, 29, 31, 32, 36]. Therefore, once swollen brain tissue herniates through the craniectomy defect, ICP and midline shift (mass effect) are immediately reduced, responding to the Monro-Kellie doctrine [29, 32]. After DC, the neurosurgical team and ICU team should pay attention to the new “invisible” variable, atmospheric pressure [37].
The “open box” concept has particular pathophysiological features. The lateral ventricle can migrate to the craniectomy defect. However, it is not clear whether this represents a localized effect of ex-vacuo hydrocephalus, altered CSF dynamics, or a combination of both phenomena [32, 37]. Hence, a disturbance of CSF dynamics may occur, including a “siphon effect” and subsequent reduction of CBF. This may be due to both the venous return disturbance and the subarachnoid space obliteration due to direct pressure on the brain parenchyma that compromise regional CPP [32].
Different neurological syndromes (some early and some late) associated with DC has been described [29, 32, 37], resulting from external and/or internal forces, such as pathophysiological consequences on intracranial compartments (Tables 55.3 and 55.4, Figs. 55.2 and 55.3).
Consequently, the usefulness of TCD/TCCS in this clinical scenario, is real-time early detection of hemodynamic changes (CBFVs, spectral Doppler waveform, PI, etc.) to improve cerebral perfusion through individualized therapeutic decisions [30, 34] (Fig. 55.3).
5 TCD/TCCS: Hemodynamic Changes Associated with Decompressive Craniectomy
TCD/TCCS provides information for the evaluation of the hemodynamic changes of the “exposed” brain after bone window removal [5, 6], which facilitates the measurements.
After performing a DC, mechanical, systemic, and atmospheric pressure changes involved in cerebral perfusion must be considered [8, 29, 32, 42, 44]. The impact of cerebral hemodynamic changes (CBFVs and hemodynamic indexes) can be recorded by TCD/TCCS during post-DC follow-up in ICU (Table 55.5), as they are progressively restored.
Understanding the different cerebral hemodynamic patterns (pre- and post-DC), help the implementation of timely therapeutic decisions individualized to optimize CPP for each patient. Therefore, keep in mind that generalized treatment goals [17] can lead to hypo- or hyperperfusion phenomena, being deleterious for the patient [11, 12, 39, 40] (Figs. 55.4a–c and 55.5).
(a) “Oligemic / hypoperfused” pattern in post-DC patient. This highlights an abnormally elevated PI and a very low amplitude of spectral Doppler wave, which in addition to low mean flow velocities (MFV, shown in the device screen as “TAMAX”) in MCA (<40 cm/s) suggest severe intracranial hypertension. ED End-diastolic volume, RIResistance index. (b) “Hyperemic/hyperperfused” pattern in the patient of Fig. A, this time post-DC (48 hr). Although literature [40] has defined “hyperemia” with MCA MFV >100 cm/s, there is an increase in MCA MFV >30% in comparison with pre-DC values. Turbulent flow, vascular congestion, increasing spectral Doppler waveform amplitude and a marked decrease in PI are also shown, which suggests a possible “exacerbated” reperfusion phenomena of the vascular territories secondary to intracranial pressure reduction after DC. (c) “Intermediate” pattern post-DC (6 hr). Although it has been described as “non-specific” by some authors [40], in this particular context of early post-DC, we propose a transition pattern between oligemia and hyperemia, with a tendency to PI improving (but still increased), a normal spectral-waveform morphology and stable values of MFV, reflecting the importance of “normoperfusion” as a neuroprotective measure and treatment goal in the neurocritical patient
6 Conclusion
During follow-up of cerebral hemodynamic changes in patients with acute brain injury (ABI), it is important to prevent and identify disturbances that extend their hospital stay and determine unfavorable long-term outcomes [38]. In the context of refractory intracranial hypertension, DC is a crucial intervention to restore adequate cerebral perfusion and brain oxygenation delivery. TCD/TCCS is a noninvasive monitoring tool that provides useful information for hemodynamic parameters analysis and estimate cerebral prognosis of the critically ill patients with ABI [6, 22].
TCD/TCCS is an useful monitoring method to assess indirectly CBF in neurocritical patients to demonstrate its optimization after therapeutic interventions such as DC or external ventricular drain (EVD), providing data to follow-up and control of CPP and ICP changes derived from medical and surgical therapeutic interventions [8, 9]. However, the measurement of the hemodynamic variables by TCD/TCCS (CBFVs, spectral Doppler waveform, PI, etc.) is an operator- and/or acoustic window-dependent technique [6, 10]. Hence, it is vitally important to have high-expertise staff to reduce as much as possible the error rate on clinical interpretation of data [10, 38]. Although there is increasing knowledge about the hemodynamic and vascular perfusion changes during ABI after a DC (Table 55.5), the heterogeneous therapeutic results in this clinical scenario cannot be overlooked. Therefore, a careful, detailed and specific analysis of each clinical situation is required to make individualize decisions [5, 6, 8, 9, 10,11,12, 39, 40].
DC is currently considered as a “definitive” management to control intracranial hypertension. This data assure that this procedure is effective for the management of cerebral oligemia without necessarily implying a post-DC cerebral hemodynamic equilibrium [41, 42, 46]. This surgical procedure may cause secondary harmful effects by hyperemia (paradoxical increase of ICP and/or risk of bleeding) or oligemia (hypoperfusion, ischemia). These cerebral hemodynamic events are possible causes of perpetuous secondary brain injury [18].
In consequence, the need for early and protocolized management of these probable neurological worsening variables, (1) arterial hypertension, (2) hypervolemia status, (3) hyperdynamic states (exacerbate hyperemia), (4) dehydration status and ICP increases (worsen the oligemia), which are considered commonly as late causes of clinical deterioration. Hence, the cerebral hemodynamic repercussion of these physiological/pathophysiological variables can be assessed indirectly by TCD/TCCS through pre-post DC cerebral flow patterns analysis [6,7,8, 38,39,40]. In the specific case of patients with TBI, the metabolic crisis is not only the result of cerebral ischemia and hypoperfusion [39, 40] but even can occur under apparently normal conditions of cerebral blood flow assessed by TCD/TCCS.
Therefore, multimodal neuromonitoring (MMM) in ICU is crucial because microvascular alteration [41], cerebral excitotoxicity, electrical pathologic activity, and brain dysoxia [18, 39, 41] require the integration of different type of devices to arrive at a diagnosis, where information provided in real-time by TCD/TCCS at bedside is very useful to make-directed therapeutic decisions.
Finally, it is important to consider the skull multicompartmental theory at time to perform cerebral hemodynamic assess by TCD/TCCS [41, 46]. This theory refers to the fact that a patient with (or without) DC can develop hyperemia in one hemisphere and vasospasm in the contralateral hemisphere. These clinical conditions challenge classical management strategies, rendering them contradictory. However, bedside assessment by TCD/TCCS allows us to better understand these cerebral hemodynamic phenomena, motivating a more individualized treatment [37,38,39,40,41].
References
Fernández Domínguez J, et al. El Doppler Color Transcraneal en el estudio vascular cerebral. Elsevier Doyma. 2012;4(3):132–43.
Romero JA, Rivero OM. Chapter 19, Doppler transcraneal: Técnica y aplicaciones clínicas. In: Niño MC, Ferrer LE, editors. Neuroanestesia enfoque perioperatorio en el paciente neurológico, vol. 431–443. Editorial Distribuna; 2005.
Honeybul S, et al. Neurological susceptibility to a skull defect. Surg Neurol Int. 2014;5:83.
Lang S, et al. Monitoring and intraoperative management of elevated intracranial pressure and decompressive craniectomy. Elsevier; 2012. p. 289–310.
Song J, et al. Beneficial impact of early cranioplasty in patients with decompressive craniectomy: evidence from transcranial Doppler ultrasonography, vol. 156. Springer-Verlag Wien; 2013. p. 193–8.
Kalanuria A, Nyquist PA, Armonda RA, Razumovsky A, et al. Use of transcranial Doppler (TCD) ultrasound in the neurocritical care unit, vol. 24. Elsevier; 2013. p. 441–56.
Jaeger M, Soehle M, Meixensberger J, et al. Effects of decompressive craniectomy on brain tissue oxygen in patients with intracranial hypertension. J Neurol Neurosurg Psychiatry. 2003;74:513–5.
Daboussi A, et al. Cerebral hemodynamic changes in severe head injury patients undergoing decompressive craniectomy. J Neurosurg Anesthesiol. 2009;21:339–45.
Bor-Shen-Shu E, Jacobsen Teixeira M, Hirsch R, Ferreira de Andrade A, Marino R Jr, et al. Transcranial Doppler Sonography in tEwo patients who underwent Decompressive Craniectomy for Traumatic Brain Swelling. Arq Neuropsiquiatr. 2004;62(3-A)
Paredes I, et al. The effect of cranioplasty on cerebral hemodynamics as measured by perfusion computed tomography and Doppler ultrasonography. J Neurotrauma Mary Ann Liebert. 2006;33:1586–97.
Vicenzini E, et al. Transcranial Doppler for brain death after decompressive craniectomy: persistence of cerebral blood flow with flat EEG. 2010; Springer and ESICM. Intensive Care Med. 36:2163–4.
Lazaridis C, DeSantis SM, Vandergrift AW, Krishna V, et al. Cerebral blood flow velocity changes and the value of the pulsatility index post decompressive craniectomy. J Clin Neurosci. 2001;19:1052–4.
Rubiano AM, Villarreal W, Hakim EJ, Aristizabal J, Hakim F, Dìez JC, et al. Early decompressive craniectomy for neurotrauma: an institutional experience. Ulusal Travma ve Acil Cerrahi Dergisi = Turkish Journal of Trauma & Emergency Surgery: TJTES. 2009;15(1):28–38.
Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999;90:187–96.
Polin RS, Shaffrey ME, Bogaey CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 1997;41:84–94.
Stocchetti N, Zanaboni C, Colombo A, et al. Refractory intracranial hypertension and “second-tier” therapies in traumatic brain injury. Intensive Care Med. 2008;34:461–7. 6.
Stocchetti N, Colombo A, Ortolano F, et al. Time course of intracranial hypertension after traumatic brain injury. J Neurotrauma. 2007;24:1339–46.
Stocchetti N, Maas AIR. Traumatic intracranial hypertension. N Engl J Med. 2014;370:2121–30.
Delashaw JB, Broaddus WC, Kassell NF, et al. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke. 1990;21:874–81.
Weiner GM, Lacey MR, Mackenzie L, et al. Decompressive craniectomy for elevated intracranial pressure and its effect on the cumulative ischemic burden and therapeutic intensity levels after severe traumatic brain injury. Neurosurgery. 2010;66:1111–8.
Kjellberg RN, Prieto A Jr. Bifrontal decompressive craniotomy for massive cerebral edema. J Neurosurg. 1971;34:488–93.
Larach DR, Larach DB, Larach MG. A life worth living: seven years after craniectomy. Neurocrit Care. 2009;11:106–11.
Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–502.
Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22.
Michel P, Arnold M, Hungerbuhler HJ, et al. Decompressive craniectomy for space occupying hemispheric and cerebellar ischemic strokes: Swiss recommendations. Int J Stroke. 2009;4:218–23.
AI-Jishi, Saluja RS, AI-Jehani H, Lamoureux J, Maleki M, Marcoux J. Primary or secondary decompressive craniectomy: different indication and outcome. Can J Neurol Sci. 2011;38(4):612–20.
Wang R, Li M, Gao WW, Guo Y, Chen J, Tian HL. Outcomes of early decompressive craniectomy versus conventional medical management after severe traumatic brain injury: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(43):e1733.
Van der Meer C, Van Lindert E, Petru R. Late decompressive craniectomy as rescue treatment for refractory high intracranial pressure in children and adults. Acta Neurochir Suppl. 2012;114:305–10.
Fodstad H, Love JA, Ekstedt J, Fridén H, Liliequist B. Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir. 1984;70:21–30.
Granthan E, Landis H. Cranioplasty and the post traumatic syndrome. J Neurosurg. 1947;5:19–22.
Grant FC, Norcross NC. Repair of cranial defects by cranioplasty. Ann Surg. 1939;110:488–512.
Stiver SI, Wintermark M, Manley GT. Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg. 2008;109:245–54.
Yamaura A, Makino H. Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol Med Chir (Tokyo). 1977;17:43–53.
Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47:231–7.
Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34:729–31.
Gordon WA, Zafonte R, Cicerone K, Cantor J, Brown M, Lombard L, et al. Traumatic brain injury rehabilitation: state of science. Am J Phys Med Rehabil. 2006;85:343–82.
Akins P, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Cara. 2008;9:269–76.
Blanco P, Abdo-Cuza A. Transcranial Doppler ultrasound in neurocritical care. J Ultrasound. 2018;21:1–16.
Chang T, et al. Transcranial Doppler ultrasonography for the management of severe traumatic brain injury after decompressive craniectomy. World Neurosurg. 2019;126:e116–24.
Bor-Seng-Shu E, de-Lima-Oliveira M, Nogueira RC, Almeida KJ, EHA P, Paschoal FM Jr. Decompressive craniectomy for traumatic brain injury: postoperative TCD cerebral hemodynamic evaluation. Front Neurol. 2019;10:354.
Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
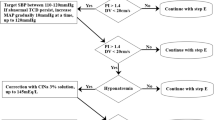
Algorithm
Algorithm

DC-POP Decompressive craniectomy postoperative, ABCD Airway-breathing-circulation-disability, MAP Mean arterial pressure, IMV Invasive mechanical ventilation, NIMV Non-invasive mechanical ventilation, GCS Glasgow coma scale, Fx Fracture, IV Intravenous, CO Cardiac output, CPP Cerebral perfusión pressure, DCI Delayed cerebral ischemia, e/ Every, Pre-OP Pre-Operative
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vásquez, S., Mantilla, J.M., Suárez, M.N., Bustamante, L.A., Guzman, J., Rubiano, A.M. (2022). Decompressive Craniectomy in the ICU: Usefulness of Transcranial Doppler (TCD/TCCS) in the Monitoring of Hemodynamic Changes. In: Rodríguez, C.N., et al. Neurosonology in Critical Care . Springer, Cham. https://doi.org/10.1007/978-3-030-81419-9_55
Download citation
DOI: https://doi.org/10.1007/978-3-030-81419-9_55
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81418-2
Online ISBN: 978-3-030-81419-9
eBook Packages: MedicineMedicine (R0)