Abstract
Pancreaticojejunostomy is a longitudinal ductal drainage procedure often combined with a subtotal pancreatic head resection used for chronic pancreatitis. This chapter describes the available options for decompression of the pancreatic duct, technique, pitfalls and complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic pancreatitis
- Pancreaticojejunostomy
- Puestow Procedure
- Partington-Rochelle Procedure
- Beger Procedure
- Frey Procedure
- Hamburg Procedure
Indications
-
Disabling recurrent or continuous abdominal pain causing periodic sick leaves, frequent hospitalization and impairment
-
Persistent pain after conservative treatment with adequate analgesic medication
-
Failed endoscopic interventions
-
Local anatomic complications such as biliary, duodenal or venous obstruction due to an inflammatory mass in the head of the pancreas
-
Dilatation and hypertension of the main duct caused by fibrotic strictures, calculi or debris
-
Pseudocysts, fistulas or chronic calcification of the pancreas
Preoperative Preparation
A successful outcome depends on an adequate preoperative diagnostic work-up which includes imaging of the biliary and pancreatic duct system, preferably by magnetic resonance cholangiopancreaticography (MRCP). Patient comorbidities, especially hepatic function, portal hypertension and nutritional status need to be evaluated and if necessary treated prior to surgery.
Pitfalls and Danger Points
The primary pitfall is failure to diagnose pancreatic adenocarcinoma. A precise assessment of patients’ medical history can avoid mistreatment. A pancreatic mass without chronic pain, missing evidence of alcohol abuse or elevated CA 19-9 levels are indicators for a potential pancreatic carcinoma. In such cases, endoscopic ultrasound-guided biopsies may be performed but if a pancreatic carcinoma cannot be ruled out, biopsy proof is not required before proceeding with an oncological resection rather than a lesser procedure.
Operative Strategy
Chronic pancreatitis is characterized by distinctive recurrent or continuous abdominal pain. These disabling attacks cause periodic sick leaves, frequent hospitalization and impairment in the quality of life. The initial treatment of patients is conservative with adequate analgesic medication and endoscopic interventions as needed. Surgery has been shown to be superior to repeated endoscopic treatments in multiple randomized trials and is strongly recommended in cases of persistent pain or failed endoscopic interventions.
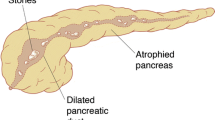
The exact operative strategy should be determined prior to surgery based on the preoperative findings. A variety of options for decompression of the pancreatic duct are available, such as the longitudinal incision of the pancreatic duct followed by a pancreaticojejunostomy (Partington-Rochelle Procedure, Fig. 100.1), the duodenum-preserving subtotal pancreatic head resection with complete transection of the pancreas above the portal vein (Beger Procedure), the duodenum-preserving local resection of the pancreatic head with a complete longitudinal incision of the pancreatic duct (Frey Procedure, Fig. 100.2) and the subtotal pancreatic head and uncinate resection with a V-shaped resection of the pancreatic duct (Hamburg Procedure, Fig. 100.3).
Each procedure has its assets and drawbacks. Whereas isolated incision of the pancreatic duct does preserve pancreatic parenchyma to the greatest extent, it does not include the resection of the pancreatic head, which is often the pacemaker of chronic pancreatitis. The term ‘pacemaker’ is used internationally for the function of the pancreatic head in chronic pancreatitis. Failure to address an inflammatory mass in the head of the pancreas will cause inadequate drainage of the organ, even if the main duct has been incised completely, which might lead to an ongoing pancreatitis and chronic pain. The Frey Procedure includes a limited resection of the pacemaker at little expense of pancreatic parenchyma but is not the optimal treatment for small-duct disease or extensive inflammation in the head of the pancreas. Such findings can be treated by an additional V-shape resection to drain second- and third-grade pancreatic branches (Hamburg Procedure).
Operative Technique
Exposure
Separate the greater omentum from the transverse colon between the hepatic and splenic flexures to expose the lesser sac and pancreas. Divide remaining peritoneal attachments between the pancreas and the posterior wall of the stomach. Ligate and divide the right gastroepiploic artery and vein to expose the head and neck of the pancreas to the full extent (Fig. 100.4). If the exploration or resection of the pancreatic head is intended, an extended Kocher maneuver and the exposure of the superior mesenteric vein is necessary. The Kocher maneuver allows a manual dorsal and ventral palpation and assessment of the pancreatic head and control of potential bleeding during the resection (Fig. 100.5a, b). The exposure of the superior mesenteric vein adjacent to the pancreas is necessary for subtotal resection of the head and uncinate with preservation of an adequate rim of pancreatic tissue for the anastomosis.
Incising the Pancreatic Duct
A dilated main pancreatic duct is usually palpable to the left of the superior mesenteric artery and vein. Localize a small, non-palpable duct confidently by puncturing the duct with a 22-gauge needle (Fig. 100.6a, b). After successful aspiration of pancreatic juice, incise the pancreas along the duct with electrocautery, using the needle as guidance. Next, extend the incision of the anterior wall using a right-angle clamp to define the direction of the duct towards the head and tail of the pancreas (Fig. 100.7a–c). Remove any calculi or debris in the main duct and side branches. It is crucial to open the entire duct from the head to the tail of the pancreas; in the end, the clamp should pass through the ampulla into the duodenum against a finger placed at the duodenum. The last part of the incision to within the 0.5 to 1 cm of the ampulla is necessary to achieve complete drainage of the duct. If the operative strategy is limited to the Partington-Rochelle procedure, the next step would be the construction of the Roux-en-Y pancreaticojejunostomy. In the presence of an inflammatory mass in the head of the pancreas, many surgeons prefer to continue with the duodenum preserving resection of the pancreatic head (e.g. Beger, Frey, Hamburg Procedure) to treat or to avoid local anatomic complications in the future.
(a) Local resection of the pancreatic head: careful excision of disc-shaped slices of parenchyma, beginning anteriorly and working posteriorly toward the retroperitoneum. (b) Completed local resection of the pancreatic head. In this case, a pancreatic as well as a bile duct stent was placed before surgery. During the decompression, the bile duct could not be preserved. In such cases, a biliodigestive anastomosis should be given preference instead of reinsertion of the bile duct into the pancreatic parenchyma
Pancreatic Head Resection
Local resection of the pancreatic head/uncinate is achieved by careful excision of disc-shaped slices of parenchyma, beginning anteriorly and working posteriorly towards the retroperitoneum, using a scalpel or electrocautery (Fig. 100.8a, b). Hemostasis during resection using fine suture ligatures is crucial to avoid postoperative anastomotic leakage and bleeding. Avoid excision beyond the posterior wall of the main duct to preserve the posterior capsule of the pancreas. A small rim of pancreatic tissue (5–10 mm) at the duodenal curve and to the right of the superior mesenteric artery and vein needs to be spared for preservation of duodenal blood supply and to facilitate the pancreaticojejunostomy. Preoperative findings of common bile duct obstruction indicate the need for identification and decompression of the intrapancreatic segment of the common bile duct.
V-Shape Resection of the Body and Tail
Coring out the pancreatic head and uncinate might be insufficient in the presence of small duct disease or extensive calcification of the pancreatic tissue. In such cases, the anterior V-shaped excision of the main duct and second- and third-grade pancreatic branches achieves sufficient drainage and prevents relapse (Hamburg-Procedure) (Fig. 100.9a, b).
Roux-en-Y Reconstruction
The reconstruction phase begins with the localization of the first jejunal loop and transection 10–15 cm beyond the ligament of Treitz using a linear stapler. The division of the mesentery is done with careful preservation of the vascular arc using clamps and ligatures. The alimentary limb is now passed through an avascular area of the transverse mesocolon and placed side-to-side against the pancreas. This should be possible without any tension, if necessary the division of the jejunal mesentery can be extended for laxity. Now, incise the antimesenteric aspect of the jejunum over a length approximately equal to the incision in the pancreatic duct using electrocautery.
The posterior layer of the pancreaticojejunostomy should begin at the pancreatic tail using a double-armed 3-0 monofilament suture. Insert the needle through both the mucosal and seromuscular portions of the jejunal wall. Then pass the needle through the fibrotic parenchyma of pancreas. The suture is tied with the knot inside the lumen of the anastomosis. After stitching out one arm of the suture, the remaining intraluminal arm is used for a running suture attaching the full thickness of the jejunum to the cut surface of the pancreas along the capsule. Depending on the length of the anastomosis, a second monofilament suture can be used for continuing along the cored-out head of pancreas and towards the anterior layer of the anastomosis (Fig. 100.10). The anterior layer is performed using running seromuscular stitches, at the pancreatic tail using the remaining arm of the double-armed suture. The tying of the anterior and posterior suture at the pancreatic head completes the pancreaticojejunostomy. At a point at least 30 cm distal to the pancreaticojejunostomy, construct an end-to-side jejunojejunostomy to complete the Roux-en-Y anastomosis. If desired, a silicone drain may be left in the region of the pancreaticojejunal anastomosis. Close the abdomen in routine fashion.
Postoperative Care
The procedure itself does not necessitate postoperative intensive care monitoring, but this might be indicated due to the frequently existing comorbidities. The nasogastric tube should be removed as soon as possible along with the immediate enteral nutrition and mobilization whenever possible (fast-track surgery). Close monitoring of vital signs, fluid balance and serum glucose concentrations is advised.
Complications
The most common complications are pancreatic fistulas and the formation of intra-abdominal abscesses. Computed tomography (CT)-guided percutaneous drainage might be necessary and avoids reoperation in most cases. Intra-abdominal or intraluminal bleeding, especially late onset bleeding, is a rare but serious adverse event which needs prompt intervention. These patients should undergo early diagnostic angiography followed by embolization. If this does not control the bleeding, an emergency laparotomy should be performed.
Further Reading
Attasarany S, Abdel Aziz AM, Lehman GA. Endoscopic management of acute and chronic pancreatitis. Surg Clin North Am. 2007;87:1379.
Bachmann K, et al. Surgical treatment in chronic pancreatitis timing and type of procedure. Best Pract Res Clin Gastroenterol. 2010;24(3):299–310. https://doi.org/10.1016/j.bpg.2010.03.003. Review.
Beger HG, et al. Experiences with duodenum-sparing pancreas head resection in chronic pancreatitis. Der Chirurg. 1980;51(5):303–7.
Frey CF, et al. Description and rationale of a new operation for chronic pancreatitis. Pancreas. 1987;2:701–7.
Izbicki JR, et al. Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis: prospective evaluation of a new surgical procedure. Ann Surg. 1998;227:213–9.
Kutup A, et al. For which type of chronic pancreatitis is the Hamburg procedure indicated? J Hepatobiliary Pancreat Sci. 2010;17:758–62.
Negi S, Singh A, Chaudhary A. Pain relief after Frey’s procedure for chronic pancreatitis. Br J Surg. 2010;97:1087.
Schneider C, et al. Longitudinal V-shaped excision of the ventral pancreas for small duct disease in severe chronic pancreatitis: prospective evaluation of a new surgical procedure. Der Chirurg. 2009;80(1):28–33.
Yekebas EF, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Uzunoglu, F.G., Izbicki, J.R. (2022). Pancreaticojejunostomy for Chronic Pancreatitis. In: Scott-Conner, C.E.H., Kaiser, A.M., Nguyen, N.T., Sarpel, U., Sugg, S.L. (eds) Chassin's Operative Strategy in General Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-81415-1_100
Download citation
DOI: https://doi.org/10.1007/978-3-030-81415-1_100
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81414-4
Online ISBN: 978-3-030-81415-1
eBook Packages: MedicineMedicine (R0)