Abstract
Although it is well-established that stress is linked to poor health, only more recently has psychoneuroimmunology research revealed the extensive and complex relationships between psychological stress and immunological processes in humans. The current chapter begins by introducing the relevant theories regarding stress and health. We address how chronic stressors are different from other forms of stress and why this distinction is important for health. Next, we review the basic anatomical and physiological nature of the immune system. Afterward, we present evidence of the links between chronic stressors and disease through their effects on the immune system, including responses to vaccination, systemic inflammation, and cellular aging, among other immune functions. We conclude by summarizing the extant findings and consider some of the next steps for this burgeoning area of research, including a focus on factors that may buffer against the effects of chronic stress on immunity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Psychoneuroimmunology
- Inflammation
- Immune system
- Transactional model of stress
- Appraisal
- Sympathetic-adrenal-medullary (SAM) system
- Hypothalamic-pituitary-adrenal (HPA) axis
- Sympathetic nervous system (SNS)
- Norepinephrine (NE)
- Epinephrine (EPI)
- Catecholamines
- Allostasis/allostatic load
- Trier Social Stress Test (TSST)
- Cytokine levels
- Vaccine response
- Latent viral infections
- Lymphocyte proliferation
- Natural killer (NK) cells
- Wound healing
- Cellular aging
Introduction to Stress and the Immune System
The fact that stress is linked to poor health is well-established. Individuals who report high levels of stress tend to also display a variety of poor health outcomes. For example, stress is associated with depression (Hammen, 2005), cardiovascular disease (Black & Garbutt, 2002), childhood asthma (Bloomberg & Chen, 2005), autoimmune diseases (Elenkov & Chrousos, 2002), HIV progression (Evans et al., 1997), and cancer (Godbout & Glaser, 2006). Moreover, particularly stressful events like the death of a spouse are associated with increased mortality (Bloom et al., 1978). However, not all stressors lead to disease and death. To the contrary, some forms of stress may enhance survival and promote positive outcomes. For example, acute stress can increase blood sugar levels, thus fueling the brain and body to deal with ongoing threats. To understand how stressors can have positive and negative effects on the body, we must acknowledge that not all stressors are created equal or rely on the same mechanisms to influence health.
The aim of this chapter is to review evidence linking long-term or chronic stressors to disease via their effects on the immune system. To understand the importance of focusing on chronic stressors and immune outcomes, a few questions must first be answered: (1) How are chronic stressors different from other forms of stress, and why is this distinction important? (2) Why is the immune system so important to health? To this end, we start by reviewing the concept of stress and pathways connecting stressors to health. Next, we provide an overview of the immune system and review prior work linking chronic stress to immune outcomes, including responses to vaccination, systemic inflammation, cellular aging, and other immune functions. We conclude with a summary of extant findings and discussion of future directions, including factors that may buffer against the effects of chronic stress on immunity.
The Concept of Stress
The term “stress” has been simultaneously used to refer to stressful stimuli, or stressors, as well as stress responses and stress-related appraisals, thus leading to some confusion. More formally, stress has been defined as “a process in which environmental demands tax or exceed the adaptive capacity of an organism, resulting in psychological and biological changes that may place persons at risk for disease” (Cohen et al., 1995, p. 3). According to the transactional model of stress (Lazarus & Folkman, 1984), situations are appraised as irrelevant, benign/positive, or potentially stressful. A potentially stressful encounter is further evaluated with respect to its potential costs and the resources that can be allocated toward managing this encounter. When perceived costs exceed resources, the event is perceived to be more threatening. From this perspective, the degree to which an event is perceived as stressful and threatening depends on appraisals of that situation and appraisals of resources available to manage it. Perceived stress , in turn, can elicit emotional and physiological responses, which may confer risk for illness. For example, perceived stress has been linked with increased susceptibility to the common cold (Cohen et al., 1993).
Nevertheless, it is possible to study stress and health without measuring stress appraisals. For example, some researchers have focused on identifying events hypothesized to be overwhelming for the majority of individuals (i.e., the epidemiological approach; for a review, see Cohen et al. (2016) and Cohen et al. (2007a)). This approach has led to the creation of life event scales which aim to measure stress by counting the number of stressful events (e.g., death of a close family member) which occurred during a predetermined time period (e.g., 12 months; Holmes & Rahe, 1967). Moreover, this line of research has been successful in linking groups of individuals thought to be under considerable pressure to a variety of health outcomes. For example, caring for a spouse with dementia has been linked to multiple forms of immune dysfunction that can place an individual at risk for disease (Gouin et al., 2008). In summary, both individuals’ perceptions of stressors and independently defined stressful events may lead to an increased risk for disease.
Pathways Linking Stress to Illness
Typically, stressors are thought to influence health via their effects on health behaviors and physiological stress response systems, including the immune system. For example, women who report a history of sexual assault also report greater substance use, increased frequency of risky sexual behaviors, and decreased exercise (Lang et al., 2003). Thus, to the extent that stressors can influence the frequency of health-related behaviors like diet, exercise, or drug use, they can influence health (see Park & Iacocca, 2014, for a more detailed discussion of this pathway).
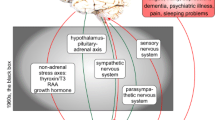
Moreover, stress can cause disease via its effects on biological stress responses. When threatened, the body undergoes a wide variety of functional changes. These changes are mediated by the interconnection of the nervous system, the endocrine system, and the immune systems (Glaser & Kiecolt-Glaser, 2005; Sarafino et al., 2008). For example, stressors have been shown to alter heart rate, blood pressure, cellular growth, and blood sugar levels (Sarafino et al., 2008). Among the biological systems involved in stress responding, the sympathetic-adrenal-medullary (SAM) and hypothalamic-pituitary-adrenocortical (HPA) axes are considered to be so intimately connected with the stress response that some have defined stressors as “a stimulus that activates the hypothalamic–pituitary–adrenal (HPA) axis and/or the sympathetic nervous system (SNS)” (Glaser & Kiecolt-Glaser, 2005. p. 243). This is because activation of the SAM and HPA axes results in the production of catecholamines (i.e., epinephrine and norepinephrine), adrenocorticotropic hormone, cortisol, growth hormone, and prolactin (Glaser & Kiecolt-Glaser, 2005). These hormonal stress mediators interact with an array of physiological systems, including the immune system (Black, 2003), and can promote survival when properly controlled or disease susceptibility when poorly regulated.
Prominent stress-health theories posit that repeated or excessive exposure to primary stress mediators of the SAM and HPA axis (e.g., epinephrine, norepinephrine, and cortisol) is the primary mechanism via which stress leads to disease (McEwen, 1998). More specifically, the allostatic load model states that adaptation in response to stressful circumstances involves activation of neural, neuroendocrine, and immune systems (e.g., the SAM and HPA axes) so as to allow the body to cope with challenges that it may not survive otherwise. For example, secretion of primary stress mediators leads to increased blood pressure, blood sugar levels, and analgesia, thereby promoting survival functions (e.g., fighting off a predator; Everly & Lating, 2013). However, under certain circumstances (e.g., repeated activation), the SAM and HPA axes may produce functional changes in organs regulated by hormones of the SAM and HPA axes (e.g., thickening of blood vessels) and in term contribute to disease. For example, primary stress mediators also influence the functioning of immune cells (e.g., cytotoxic T cells), and, when prolonged, this effect can increase the risk of developing infectious diseases (Glaser & Kiecolt-Glaser, 2005). Similarly, repeated cardiovascular activation is thought to cause hypertension via structural adaptation of blood vessels (i.e., vessel wall thickening; Johsson & Hansson, 1977). In sum, the cumulative effects of stressors on biological systems are thought to lead to disease susceptibility via excessive exposure to primary stress mediators.
However, the cumulative effect of stressors on health as outlined by the allostatic load model does not apply uniformly to all stressors or all individuals. Consistent with Lazarus and Folkman’s approach to understanding stress, individual appraisals can influence this relationship. For example, perceptions of high status inhibit responses to stress (Adler et al., 2000; Akinola & Mendes, 2014) and, thus, limit the rate of stress-related disease in high-status populations (Adler et al., 1994; Sapolsky, 2005). In addition, stressor characteristics can influence biological response patterns (e.g., Dickerson and Kemeny, 2004, and Segerstrom and Miller, 2004). For example, pain-inducing physical stressors (e.g., submerging ones that had in ice water) reliably lead to the secretion of epinephrine and norepinephrine (Biondi & Picardi, 1999), whereas psychological stressors characterized by social evaluative threat, or events that challenge self-esteem and social status (e.g., a hostile job interview), reliably lead to increased cortisol secretion (Dickerson & Kemeny, 2004; Dickerson et al., 2008). In sum, some stressors are more potent activators of certain physiological stress responses than others and, thus, may contribute more significantly to physical “wear and tear” and disease.
Why Is Chronic Stress Toxic?
Yet, a single short-lived stressor is unlikely to lead to disease. Instead, excessive exposure to primary stress mediators is a lengthy process, whereby recurrent or ongoing stressors (or mental representation of stressors; Brosschot et al., 2006) repeatedly activate the SAM and HPA axes. Consistent with this view, mounting evidence suggests that the duration of a stressor is an important determinant of its eventual health effects (Dhabhar, 2014; Juster et al., 2010; Mcewen, 2004; Segerstrom & Miller, 2004; Taylor et al., 2008). Acute stressors are a relative short-term, non-recurring, or low-frequency events (e.g., a single argument with a spouse) and typically do not continue to be appraised as overwhelming long after they have ended (Cohen et al., 2007a, b). In contrast, chronic stressors include persistent events (e.g., caring for a sick spouse), frequent or recurring forms of acute stressors (e.g., daily arguments with a spouse), and non-recurring events that continue to be experienced as overwhelming long after they have ended (e.g., sexual assault).
The effect of acute stressors on health is commonly studied in humans using laboratory tasks like the Trier Social Stress Test (Kirschbaum et al., 1993), which combines public speaking with mental arithmetic or short naturalistic stressors like academic examinations. In contrast, the study of chronic stress is restricted to naturalistic studies among humans. Some work on chronic stress has focused on measuring self-reported major life events and daily hassles over a specific period of time (Dimsdale et al., 1994; Miller et al., 2004). Additional work has focused on traumatic stressors like natural disasters (Solomon et al., 1997). Most notably, chronic stress has been measured by examining populations facing ongoing challenges like individuals caring for a spouse suffering from dementia (Kiecolt-Glaser et al., 1987), mothers of pre-term very-low-birthweight infants (Gennaro et al., 1997b), professional soldiers (Lauc et al., 1998), prisoners of war (Dekaris, 1993), unemployed adults (Ockenfels et al., 1995), bereaved individuals (Kemeny et al., 1994), and victims of child abuse (Felitti et al., 1998). Among these chronically stressed populations, spousal dementia caregivers are among the most extensively studied. Caring for a spouse with dementia is thought to be particularly challenging because dementia caregivers find themselves providing care for over 10 h per day and may do so for over 5 years (Donelan et al., 2002; Wimo et al., 2002). Moreover, dementia caregivers face significant distress as they see their spouse slowly lose their personality and intellect (Kiecolt-Glaser et al., 1991).
The distinction between acute and chronic stresses is particularly important in the context of immune function. Among the work examining the effect of acute and chronic stressors on immunity, some of the most compelling evidence comes from rodent studies that manipulated both acute and chronic stresses. In a study by Dhabhar and McEwen (1997), rats were restrained, shook, or both restrained and shook (a stressor nicknamed the “New York City Subway Stress”) for 2 h to manipulate varying intensities of acute stress. Chronic stress was manipulated by applying a random sequence of restraint, shaking, or restraint and shaking each hour, 6 h per day, for 3 weeks. Rats which were acutely stressed showed improvement in some aspects of their immune response, whereas chronically stressed rats showed widespread immunodeficiency. This work is consistent with reviews of human studies suggesting that chronic and acute stresses produce different immune effects (Segerstrom & Miller, 2004).
Why Focus on the Immune System?
Maintaining a functional immune system is essential to survival. The immune system serves to protect organisms against external threats like virus and bacteria as well as internal threats like cancerous cells. When one or more components of an organism’s immune system is no longer active, that organism is said to be immunodeficient and is subject to increased frequency or complications of common infections (Chinen & Shearer, 2010). In extreme cases, immunodeficiency can lead to death. For example, the majority individuals infected by human immunodeficiency virus (HIV) are expected to die within 2 years of the onset of acquired immunodeficiency syndrome (AIDS) if they remain untreated (Poorolajal et al., 2016). It is also possible for some components of the immune system to become too active and damage cells of the body (i.e., autoimmune disease, chronic inflammation). For example, in rheumatoid arthritis, the immune system is overactive and wrongfully attacks the body by degrading cartilage and bone tissue in articulations. Like AIDS, some diseases resulting from immune overactivity can lead to death (e.g., multiple sclerosis; Brønnum-Hansen et al., 2004). Thus, a balance in immune activity is necessary to maintain health. Indeed, dysregulated immune function implicates the development of many major and potentially fatal diseases, including cardiovascular disease (Hansson & Hermansson, 2011), cancer (de Visser et al., 2006), and diabetes (Pickup & Crook, 1998). In summary, to the extent that chronic stress can influence the immune system, it yields a powerful influence on health and well-being.
The Immune System
Overview
Understanding the effects of stressors on immune functioning relies to some degree on understanding how the immune system functions under normal conditions. The present chapter only briefly reviews major components of the immune system to provide a general frame of reference for studies linking chronic stress to immunity. However, the human immune system is remarkably complex, and those wishing to fully appreciate the complexity of the immune system may benefit from reviewing textbooks which more closely focus on the subject (e.g., Abbas et al., 2014, and Daruna, 2012).
The immune system is a complex array of cells, proteins, and physical barriers aimed at protecting the body against pathogens or foreign proteins, viruses, bacteria, parasites, and fungi that can cause illness and result in death. Over many generations, the human body has acquired numerous mechanisms to keep pathogens out of the body or prevent them from causing harm. The skin, gastrointestinal tract, respiratory tract, nasopharynx, cilia, eyelashes, and body hair all serve to physically prevent pathogens from entering or infecting the body. In addition, the body secretes a variety of substances to hinder pathogen entry. For example, the stomach tends to produce an acidic chemical environment which is hostile to foreign bacteria. If a foreign pathogen manages to penetrate physical barriers, cells and proteins of the innate immune system promptly mobilize to neutralize it. Finally, pathogens are also targeted by the adaptive immune system which mobilizes cell-mediated and antibody-mediated responses to neutralize the pathogen on initial exposure and improve the body’s defense against future exposure to the same pathogen. The immune system can therefore be divided in two parts: the innate and adaptive immune system. These branches of the immune system rely on distinct strategies to protect the body against pathogens and are considered complementary.
The Innate Immune System
The innate immune system is often called non-specific because the proteins and cells that comprise the innate immune system respond to a broad class of foreign pathogens but do not typically attempt to recognize or adapt their response to a specific type of pathogen. This is in contrast to the adaptive immune response which develops a form of cellular memory aimed at protecting the body against future exposure to a unique pathogen. Albeit non-specific, the innate immune system is a powerful first line of defense. Within hours of infection, cells and proteins of the innate immune system will migrate to the site of infection, ingest pathogens, and coordinate a wide array of cell-to-cell signaling.
Cells of the innate immune system include macrophages, mast cells, neutrophils, eosinophils, basophils, natural killer cells, and dendritic cells. Accumulation of these cells at the site of infection along with increased vascular dilation and permeability is part of inflammation (Abbas et al., 2014). Many of these cells (i.e., neutrophils, monocytes, and dendritic cells) travel to the site of infection to ingest and kill pathogens (via a process known as phagocytosis). For example, neutrophils are mass produced in the bone marrow following infection and promptly move to the site of infection where they ingest microbes for intracellular killing. In addition, damaged cells and some cells of the innate immune system (e.g., macrophages) produce soluble proteins known as cytokines. Cytokines like interleukin-1 and tumor necrosis factor alpha (TNF-α) circulate in the blood and attract other macrophages to the site of infection, stimulating inflammation. In addition, mast cells produce other signaling molecules known as anaphylatoxins (e.g., histamine, serotonin, and prostaglandins) which increase vascular dilation and permeability to ease the movement of immune cells to the site of infection. Thus, the innate immune response could be summarized as a form of cellular redeployment or migration to the site of infection aided by a variety of chemical alarm signals.
The innate immune response is not exclusively carried out by cells. Another notable component of the innate immune system is the complement cascade. The complement cascade is an array of proteins which are activated following infection and circulate in the blood to supplement other parts of the immune response. The complement cascade is able to mark pathogens and infected cells for ingestion, recruit neutrophils to the site of infection, break down the membrane of some pathogens (e.g., bacteria), and cluster pathogens together to ease the action of other immune cells.
Finally, the innate immune system also plays an important role in initiating the adaptive immune response via a process known as antigen presentation. After ingesting and breaking down the ingested pathogen into small protein fragments, cells of the innate immune system (e.g., dendritic cells and macrophages) will present these fragments on the surface of their membrane. The protein fragment then becomes visible to some cells of the adaptive immune system, including T cells. Numerous T cells are then able to bind to the protein fragments and check if they produce a matching protein or the one that physically fits the pathogen fragment. If they do, they will proliferate, thus initiating the cell-mediated component of the adaptive immune response.
The Adaptive Immune System
The hallmark of adaptive immunity is the ability to identify and neutralize specific pathogens. Humoral, or antibody-mediated, responses rely on the ability of B cells to identify specific pathogens using antibodies, or Y-shaped proteins, which bind to part of a pathogen (e.g., a foreign surface protein). The part of a pathogen which binds to an antibody is called an antigen. Much like a lock and a key, a given antibody can only bind to a relatively unique protein structure and thus is considered specific to an antigen. The variable part of antibodies can take on many shapes (1010 possible combinations) because the portion of DNA which codes for the variable portion of antibodies is shuffled during the development of B cells. As a result, the body produces a diverse population of B cells which each can only react with a unique protein structure. B-cell activation therefore relies on a potentially lengthy trial-and-error process whereby various antibody-producing B cells try to bind with an antigen until a match is produced. Once a naïve B cell has found an antigen matching the membrane-bound antibody it produces, that B cell will activate (with the help of other immune cells) and rapidly multiply in the form of plasma cells and memory B cells. Plasma cells secrete large quantities of antibodies which travel through blood and lymph to neutralize target antigens. Memory B cells remain dormant until the pathogen presents itself again. Memory B cells can therefore accelerate the antibody-mediated immune response as soon as the second exposure to the same pathogen. The cellular memory of B cells is the principle mechanisms underlying the use of vaccine where a weakened or inactive virus is injected in an organism to activate its “matching” B cell and protect the organism against future exposure to the active virus. Circulating antibodies protect against infections by blocking parts of a pathogen needed for it to function (neutralization), clustering pathogens together (thus facilitating the ingestion of pathogens by other immune cells through the process called phagocytosis), and activating the complement cascade.
Another important function of B cells (along with dendritic cells and macrophages) is antigen presentation. The cell-mediated adaptive immune response relies on antigen-presenting cells to interact with the pathogen. More specifically, helper T cells and cytotoxic T cells produce T-cell receptor which acts much like antibodies in that they express a highly variable structure and thus can bind to a specific antigen. However, the receptors of helper T cells and cytotoxic T cells cannot bind to free (i.e., non-ingested) pathogens; they can only bind to pathogen fragments processed by professional antigen-presenting cells (e.g., B cells, dendritic cells, or macrophages). Once again, through a process of trial and error, numerous naïve T cells will attempt to bind to the antigen displayed by antigen-presenting cells, and once a match is found, these T cells will activate. Activated T cells will proliferate in the form of effector T cells and memory T cells for current and future action.
Summary and Implications
In summary, the innate immune system protects the body from a broad range of pathogens via inflammation, whereas the adaptive immune system relies on humoral and cell-mediated responses to neutralize a unique pathogen on initial exposure and improve immune resistance to future encounters with the same pathogen. This process relies on the interaction of numerous cells and highlights the degree of interconnectivity present in the human immune system. However, this interconnectivity is not limited to the immune system. The central nervous system and neuroendocrine system also participate in the chemical cross talk carried out by the immune system. Psychoneuroimmunology is the study of this cross talk or the interdisciplinary research field that addresses the interactions of the central nervous system, the neuroendocrine system, and the immune system (Glaser & Kiecolt-Glaser, 2005). A major focus of psychoneuroimmunology research is on the topic of chronic stress and immune function.
Chronic Stress and Immune Function
Chronic stress may influence immune function by increasing the production of primary stress mediators (e.g., epinephrine, norepinephrine, and cortisol) over long periods of time. The complexity of the immune system provides researchers with numerous opportunities to assess the effect of chronic stress on immune function. For example, measuring the quantity of antibodies secreted in response to a vaccine can serve as a valuable indicator of the antibody-mediated response. Similarly, antibody secretion to latent infections (e.g., herpesvirus), immune cell proliferation (e.g., NK cells), and cytokine production (e.g., TNF-α) have all been used to infer the activity of distinct components of the immune system (e.g., Kiecolt-Glaser et al., 1987; Nakano et al., 1998; and Vedhara et al., 1999). Although animal researchers are able to manipulate stressors’ intensity and duration to examine the effects of chronic stress on immune function (e.g., Dhabhar and Mcewen, 1997), researchers examining the association between chronic stress and immunity in humans have had to rely on different methodology. For example, prior work has examined the association between self-reported stress and immune function (e.g., Miller et al., 2004) or compared groups of people facing chronic stressors to groups of individuals facing no chronic stress (e.g., Kiecolt-Glaser et al., 1991). Fewer have followed chronically stressed individuals and longitudinally examined immune outcomes (e.g., comparing immune function of unemployed adults before and after they obtained a new job; Cohen et al., 2007a, b). As a result, the current state of psychoneuroimmunology literature suggests a fairly reliable association between chronic stress and immune function in humans (rather than causation per se). To illustrate the association between chronic stress and immune function, we review evidence linking chronic stress to vaccine response, immune control over latent infections, lymphocyte proliferation, natural killer cell activity, systemic inflammation, wound healing, and cellular aging of immune cells.
Vaccine Response
Vaccines serve to protect individuals/organisms by eliciting humoral and cell-mediated immune responses to a weakened or inactive virus, which generate memory cells that allow for faster and more robust immune responses to future infections. As such, measuring the degree to which individuals mount an immune response to vaccination can serve as a valuable indicator of immune functioning, whereby larger responses indicate stronger immunity. Multiple studies have linked chronic stress to immune responses to vaccination. For example, a study of 32 individuals caring for a spouse with dementia showed that dementia caregivers had diminished responses to an influenza vaccine relative to 32 control (non-caregiving) participants of similar age, sex, and socioeconomic status (Kiecolt-Glaser et al., 1996). More specifically, participants in this study were administered an influenza vaccine and provided periodic blood draws for up to 6 months post-vaccination to assess immune responses. Results showed that dementia caregivers showed significantly lower increases in interleukin-1β and interleukin-2 relative to the control (non-caregiving) group, suggesting that chronically stressed older adults may suffer from diminished natural and specific immune responses to vaccines . Similarly, some work indicates that spousal dementia caregivers have a reduced antibody response to an influenza vaccine relative to non-caregiving controls (Vedhara et al., 1999). Additional work implies that dementia caregivers do not continue to produce viral antibodies following vaccination for as long as non-chronically stressed (control) participants (Glaser et al., 2000), suggesting that the protective effect of vaccination may subside more rapidly in chronically stressed older adults compared to their non-stressed counterparts. Chronic stress also influences vaccine responses in young adults (Miller et al., 2004). In one such study, healthy young adults (N = 83) were followed for a 13-day period, during which they were vaccinated for influenza (on day 3) and reported the degree to which they felt stressed or overwhelmed daily. Young adults who reported being more stressed across the entire 13-day observation period were found to produce fewer antibodies for the influenza virus. In summary, chronic stress is associated with reduced immune responses to vaccination.
Control over Latent Viral Infections
Herpesvirus , including the Epstein-Barr virus, the herpes simplex virus type I, and varicella-zoster virus, are common (Glaser & Kiecolt-Glaser, 1994; Nahmias & Roizman, 1973) and are able to permanently infect a host by copying their viral sequence into the host’s DNA. However, latent viruses are not always active; the immune system generally keeps such viruses in a dormant state by inhibiting viral replication. When immunity is compromised, the latent virus can reactivate; in turn, the body increases its production of antibodies to neutralize the active virus. As such, monitoring the degree to which individuals produce antibodies specific to latent viruses can serve as a valuable indicator of immune function, whereby greater antibody production to latent viruses implies compromised immunity for the host.
Chronic stress predicts antibody production to latent herpesvirus (i.e., compromised immunity) in multiple studies (Kiecolt-Glaser et al., 1991; Kiecolt-Glaser et al., 1987; McKinnon et al., 1989). In one cross-sectional study, individuals caregiving for a family member with dementia displayed elevated Epstein-Barr virus antibody production relative to sociodemographically matched non-caregiving controls (Kiecolt-Glaser et al., 1987). Similarly, a longitudinal study assessing Epstein-Barr virus antibody production over two measurements (13 months apart on average) found that dementia caregivers displayed elevated viral antibody production during the second measurement relative to non-caregiving control participants who were matched on the basis of age, education, and income (Kiecolt-Glaser et al., 1991). Finally, residents living within 5 miles of the damaged Three Mile Island nuclear power plant (a population thought to be chronically stressed due to their proximity with a damaged nuclear plant; Baum et al. , 1983) exhibited increased antibody production to herpes simplex virus type I relative to control participants living more than 80 miles away (McKinnon et al., 1989). Taken together, these studies show that the immune systems of chronically stressed individuals may be impaired in their ability to maintain herpesviruses in a dormant state.
Lymphocyte Proliferation
In response to immune challenge , such as exposure to bacterial toxins, numerous cells of the innate and adaptive immune system must undergo rapid cell division (i.e., cell proliferation). As a result, researchers have measured the rate of immune cell proliferation to broadly infer immune function among individuals, with greater lymphocyte proliferation indicating better immune functioning. Among this line of work, some have observed lower lymphocyte (i.e., T cells, B cells, and NK cells) proliferation in chronically stressed populations. For example, one study examined lymphocyte proliferation among Japanese taxi drivers under conditions of low economic strain as well as under conditions of high economic strain and healthy control participants (Nakano et al., 1998). Results implied that lymphocyte proliferation was comparable for control participants and taxi drivers under low economic strain. However, taxi drivers operating under high economic strain displayed reduced lymphocyte proliferation relative to controls. Similarly, multiple other studies have found that dementia caregivers tend to display lower lymphocyte proliferation than non-caregiving controls (Bauer et al., 2000; Cacioppo et al., 1998; Fonareva et al., 2011; Kiecolt-Glaser et al., 1991). Finally, lower lymphocyte proliferation is also observed among chronically stressed populations including mothers of pre-term low-birthweight infants (Gennaro et al., 1997a) and women who were forced out of their home in a war-ravaged region (Sabioncello et al., 2000). In summary, chronically stressed individuals may be less able to mass produce immune cells through lymphocyte proliferation during acute infection.
Natural Killer Cell Activity
Natural killer (NK) cells owe their name to their ability to bind to cancerous and virus-infected cells without antigen stimulation to induce cell death. Nearly all cells of the body present fragments of the proteins they produce on their surface such that most cells of the immune system can identify them as foreign or non-foreign (self) cells. For example, when an infected cell produces viral protein fragments, these fragments are presented on the cell’s surface and allow cytotoxic T cells to recognize that the cell is infected. Related NK cells rely on a similar mechanism to identify infected cells but can trigger cell death even when cells stop presenting protein fragments on their surface. This ability allows NK cells to identify and kill some cancerous cells. NK cells also secrete antimicrobial molecules which kill bacteria by disrupting their cell wall (Iannello et al., 2008). NK cells are therefore an important part of the innate immune system because they can protect the body immediately and with no prior exposure to pathogen against a wide variety of foreign or infected cells as well as cancerous cells.
As such, the ability of NK cells to destroy foreign cells (i.e., NK cell activity or NK cell cytotoxicity) serves as a valuable indicator to the innate immune response. In the context of chronic stress, NK cell activity has been examined in a variety of chronically stressed populations (Dekaris, 1993; Lutgendorf et al., 1999; Vitaliano et al., 1998). For example, war prisoners suffering from prolonged stress and malnutrition were found to show decreased NK cell cytotoxicity and decreased phagocytic functions of ingestion and digestion (Dekaris, 1993), suggesting that their NK cells were less able to damage, ingest, and digest foreign/cancerous cells. Similar links between chronic stress and decreased NK cell activity have been documented in the context of older adults undergoing voluntarily housing relocation (Lutgendorf et al., 1999) and among dementia caregivers with a history of cancer (Vitaliano et al., 1998). Finally, a study that compared mothers of pre-term low-birthweight infants to mothers of healthy full-term infants showed that pre-term mothers exhibited decreased NK cell activity relative to mothers of healthy infants after delivery (Gennaro et al., 1997a). Women who give birth pre-term to low-birthweight infant may experience ongoing stress during the perinatal period, and this study suggests that this type of chronic stress may reduce NK cell activity.
NK cells work in concert with other immune cells to increase their activity during infection. For example, inflammatory cytokines can render NK cells more active as well as promote NK cell proliferation. As such, NK cells that are less responsive to cytokines may indicate a less coordinated or impoverished innate immune response. Therefore, examining NK cell responses to cytokine stimulation is another useful functional immune measure to consider in the context of stress. In one study, current and former dementia caregivers were found to have NK cells which were less responsive to interferon gamma (IFN-γ) and interleukin-2 stimulation compared to non-caregiving controls who were matched on the basis of age, sex, education, and race (Esterling et al., 1994). This finding suggests that NK cells of chronically stressed older adults are less responsive to chemical signaling and that such deficits may endure even after the chronic stressor has ended. However, it should be noted that former caregivers may still experience some chronic stress post-caregiving in the form of bereavement. Additional work shows that unemployed adults displayed reduced NK cell cytotoxicity relative to employed adults, but that NK cell cytotoxicity also substantially increased for unemployed adults who eventually obtained a job (Cohen et al., 2007a, b). Taken together, these results indicate that NK cells of chronically stressed individuals may be less active and less well coordinated with other immune responses, but these stress-related effects may recover post-stressor.
Systemic Inflammation
Much like other stress responses (e.g., activation of the HPA and SAM axes), inflammation is an adaptive process in the short term. Inflammation is driven by a redistribution of immune cells at the site of infection aided by cytokine and anaphylatoxins release. However, when inflammation endures for too long, it can also damage the body. For example, cytokines also influence metabolism, and chronic elevation of cytokine levels is thought to increase risk for cardiovascular disease by promoting the formation of fatty plaques in arteries (Sattar et al., 2003). Given that cells of the innate immune system rely on chemical messages to move to the site of infection, measuring the amount of pro-inflammatory cytokines and other inflammatory markers (e.g., C-reactive protein or CRP) can serve as a valuable indicator of inflammation . Related, chronically stressed individuals tend to display elevated levels of inflammatory markers like CRP and TNF-α. For example, in a 3-year longitudinal study, dementia caregivers exhibited greater levels of TNF-α than matched controls, and longer duration of caregiving was associated with elevated CRP levels (von Känel et al., 2012). Furthermore, Känel et al. (2012) found that circulating CRP levels decreased in caregivers 3 months after the death of the spouse with Alzheimer’s disease, suggesting that CRP production was temporally tied to caregiving duties. Consistent with this finding, dementia caregivers tend to display greater levels of CRP than non-caregiving controls (Fonareva et al., 2011; Gouin et al., 2012), and lymphocytes of dementia caregivers tend to produce more TNF-α than non-caregiving controls (Damjanovic et al., 2007). In summary, chronic stress may promote varied and impactful disease states (e.g., cardiovascular disease) by inducing systemic inflammation.
Wound Healing
Mounting research suggests that stressors can influence wound healing . In humans, numerous studies show that stressors of varied duration influence the healing of wounds, which are commonly inflicted experimentally by researchers in a standardized way (see Gouin & Kiecolt-Glaser, 2011, for a review). For example, women caring for a relative suffering from dementia showed slower wound healing than controls matched for age and income (Kiecolt-Glaser et al., 1995). In this study, a small wound was applied to the forearm of all participants, and wound size was monitored every 2–8 days thereafter. On average, dementia caregivers needed 9 more days to heal fully. In addition, dementia caregivers produced less IL-1β (a cytokine implicated in wound healing via its effect on tissue remodeling; Barbul, 1990). Similar effects of stressors on wound healing have been documented in dental students facing examinations (Marucha et al., 1998), as well as couples discussing marital disagreements (Kiecolt-Glaser et al., 2005). In summary, prior work suggests that chronic as well as acute forms of stress slow wound healing across varied populations.
Cellular Aging
The innate and adaptive immune responses often rely on rapid cell division to protect the body against infections. For example, neutrophils tend to be mass produced in the bone marrow following infection. Yet, cell division is finite because DNA polymerase (the protein complex responsible for copying DNA during cell division) cannot produce full copies of a chromosomal DNA strand. Instead DNA polymerase always produces a slightly shorter copy with each replication. As a result, the protective ends of chromosomes (i.e., telomeres) progressively shorten and can serve as a useful indicator of cellular aging . Moreover, if stress is able to activate the immune system, then chronic stress may accelerate the rate of cellular aging in immune cells (which in turn may impair immune function). To investigate this claim, researchers collected blood samples from mothers of chronically ill children to assess the rate of cellular aging of white blood cells (Epel et al., 2004). Mothers of chronically ill children who had been caregiving for longer (i.e., were more chronically stressed) showed shortened white blood cell telomere length, suggesting accelerated cellular aging . Consistent with this finding, others have found that the peripheral blood mononuclear cells (i.e., T cells, B cells, NK cells, macrophages, and dendritic cells) of dementia caregivers also display shorter telomeres than non-caregiving controls (Damjanovic et al., 2007). Since telomere shortening is expected to continue at a relatively steady rate with normal aging , this marker allows one to compare telomere shortening expected under normal conditions to stress-induced telomere shortening. In this fashion, Epel et al. (2004) estimated that white blood cells of women who reported high levels of stress could be considered to be approximately 10 years older than those of women with low stress. In summary, chronic stressors may accelerate the rate of cellular aging of immune cells and thus impair immunity.
Concluding Remarks
Summary
Chronic stressors are thought to over activate physiological stress responses and thus may promote disease via their prolonged effect on the nervous, neuroendocrine, and immune systems. The present review provides support for the links between chronic stressors and diverse components of the immune system among humans. Relative to healthy controls, chronically stressed individuals tend to exhibit impoverished immune responses to vaccines, reduced control over latent infections, diminished lymphocyte proliferation, reduced NK cell activity and cytotoxicity, chronically elevated cytokine levels (i.e., systemic inflammation), slowed wound healing, and accelerated cellular aging of immune cells. Altogether, this review implies that chronic stressors may lead to widespread dysregulation of the immune system and thus yield a strong influence on health and well-being.
Additional Considerations
It is important to note that the present review of evidence linking chronic stress to immune outcomes is far from exhaustive. For example, other work has examined the association between stressor chronicity and oxidative stress (e.g., Aschbacher et al., 2013), platelet activity (e.g., Aschbacher et al., 2009), mucosal immunity (e.g., Bristow et al., 2008), β2-adrenergic receptor sensitivity (e.g., Mausbach et al., 2007), markers of blood coagulation (e.g., Aschbacher et al., 2006), and other markers of inflammation (e.g., von Känel et al., 2006). The literature linking chronic stress to immune functioning therefore includes numerous outcomes beyond those reviewed in this chapter. It is also worth mentioning that some work has linked chronic appraisals of threat to immune function. For example, HIV-infected men who report greater rejection sensitivity also display increase CD4+ T-cell decline, earlier AIDS onset, and mortality (over 9 years) than less sensitive individuals (Cole et al., 1997), thus suggesting that chronic stressor appraisals could also contribute to poor immune function. In addition, the reader should be aware that the effects of chronic stressors on immune functioning are not unequivocally supported in the literature. For example, prior work reports divergent findings with regard to the association between chronic stress and some immune parameters, including CRP (Vitaliano et al., 2007) and NK cell activity (Irwin et al., 1991, 1997). As such, it will be important for future work to continue to investigate the factors that may contribute to such mixed findings.
In addition, few studies have examined the ability of the human immune system to recover after a chronic stressor has ended. This may be in part because much of the previous research on chronic stress has focused spousal caregivers. For this population, the end of caregiving duties is typically followed by another stressor: bereavement. We reviewed one exception by Cohen et al. (2007a, b), who followed unemployed adults pre- and post-employment and found some evidence for immune recovery. To move forward in examining immunological recovery from chronic stress, it will be necessary to use novel methodology and not rely solely on spousal caregivers. In addition to examining unemployment, researchers might consider chronic interpersonal or environmental stressors such as relationship difficulties, workplace stress, or discrimination—all of which may end with positive and/or negative outcomes. Further complicating the issue of examining post-stressor recovery is the need to examine pre-stressor functioning. In other words, future study designs may need to follow participants before chronic stress begins and after it has ended to thoroughly assess the extent to which individuals return to pre-stressor levels of immune functioning. In summary, the logistics to following individuals before and after they experience a chronic stressor remains a major barrier to the study of immune recovery in humans.
Future Directions
A promising venue for future research lies in identifying potential mechanisms and moderators of the relationship between chronic stress and immunity and leveraging the influence of such factors to promote health. Indeed, a variety of past studies support the notion that improving psychological outcomes can contribute to improved immune status. For example, a review of the effects of psychological interventions on neuroendocrine and immune functioning among HIV-infected individuals indicates that interventions which improve psychological outcomes (e.g., cognitive behavioral stress management) tend to have salutary effects on immunity (Carrico & Antoni, 2008). Similarly, stressed individuals who reported more active coping strategies also tended to display greater leukocyte (i.e., white blood cells) proliferation than stressed individuals who report more passive coping strategies (Stowell et al. 2001). In closing, although chronic stressors are often overwhelming, the experience of a chronic stressor is unique to each individual, and future research has the potential to leverage individual variability in coping and stress appraisals to minimize the effect of chronic stressors on health and well-being.
References
Abbas, A. K., Lichtman, A. H., & Pillai, S. (2014). Basic immunology: Functions and disorders of the immune system. Elsevier.
Adler, N. E., Boyce, T., Chesney, M. A., Cohen, S., Folkman, S., Kahn, R. L., & Leonard, S. (1994). Socioeconomic status and health: The challenge of the gradient. American Psychologist, 49(1), 15–24.
Adler, N. E., Epel, E. S., Castellazzo, G., & Ickovics, J. R. (2000). Relationship of subjective and objective social status with psychological and physiological functioning: Preliminary data in healthy, white women. Health Psychology, 19(6), 586–592.
Akinola, M., & Mendes, W. B. (2014). It’s good to be the king: Neurobiological benefits of higher social standing. Social Psychological and Personality Science, 5(1), 43–51.
Aschbacher, K., O’Donovan, A., Wolkowitz, O. M., Dhabhar, F. S., Su, Y., & Epel, E. (2013). Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology, 38(9), 1698–1708.
Aschbacher, K., Roepke, S. K., von Känel, R., Mills, P. J., Mausbach, B. T., Patterson, T. L., … Grant, I. (2009). Persistent versus transient depressive symptoms in relation to platelet hyperactivation: A longitudinal analysis of dementia caregivers. Journal of Affective Disorders, 116(1–2), 80–87.
Aschbacher, K., von Känel, R., Dimsdale, J. E., Patterson, T. L., Mills, P. J., Mausbach, B. T., … Grant, I. (2006). Dementia severity of the care receiver predicts procoagulant response in Alzheimer caregivers. The American Journal of Geriatric Psychiatry, 14(8), 694–703.
Barbul, A. (1990). Immune aspects of wound repair. Clinics in Plastic Surgery, 17(3), 433–442.
Bauer, M. E., Vedhara, K., Perks, P., Wilcock, G. K., Lightman, S. L., & Shanks, N. (2000). Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. Journal of Neuroimmunology, 103(1), 84–92.
Baum, A., Gatchel, R. J., & Schaeffer, M. A. (1983). Emotional, behavioral, and physiological effects of chronic stress at Three Mile Island. Journal of Consulting and Clinical Psychology, 51(4), 565–572.
Biondi, M., & Picardi, A. (1999). Psychological stress and neuroendocrine function in humans: The last two decades of research. Psychotherapy and Psychosomatics, 68(3), 114–150.
Black, P. H. (2003). The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behavior, and Immunity, 17(5), 350–364.
Black, P. H., & Garbutt, L. D. (2002). Stress, inflammation and cardiovascular disease. Journal of Psychosomatic Research, 52(1), 1–23.
Bloom, B. L., Asher, S. J., & White, S. W. (1978). Marital disruption as a stressor: A review and analysis. Psychological Bulletin, 85(4), 867–894.
Bloomberg, G. R., & Chen, E. (2005). The relationship of psychologic stress with childhood asthma. Immunology and Allergy Clinics, 25(1), 83–105.
Bristow, M., Cook, R., Erzinclioglu, S., & Hodges, J. (2008). Stress, distress and mucosal immunity in carers of a partner with fronto-temporal dementia. Aging & Mental Health, 12(5), 595–604.
Brønnum-Hansen, H., Koch-Henriksen, N., & Stenager, E. (2004). Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain, 127(4), 844–850.
Brosschot, J. F., Gerin, W., & Thayer, J. F. (2006). The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–124.
Cacioppo, J. T., Poehlmann, K. M., Kiecolt-Glaser, J. K., Malarkey, W. B., & Burleson, M. H. (1998). Cellular immune responses to acute stress in female caregivers of dementia patients and matched controls. Health Psychology, 17(2), 182–189.
Carrico, A. W., & Antoni, M. H. (2008). Effects of psychological interventions on neuroendocrine hormone regulation and immune status in HIV-positive persons: A review of randomized controlled trials. Psychosomatic Medicine, 70(5), 575–584.
Chinen, J., & Shearer, W. T. (2010). Secondary immunodeficiencies, including HIV infection. Journal of Allergy and Clinical Immunology, 125(2), S195–S203.
Cohen, F., Kemeny, M. E., Zegans, L. S., Johnson, P., Kearney, K. A., & Stites, D. P. (2007b). Immune function declines with unemployment and recovers after stressor termination. Psychosomatic Medicine, 69(3), 225–234.
Cohen, S., Tyrrell, D. A., & Smith, A. P. (1993). Negative life events, perceived stress, negative affect, and susceptibility to the common cold. Journal of Personality and Social Psychology, 64(1), 131–140.
Cohen, S., Gianaros, P. J., & Manuck, S. B. (2016). A stage model of stress and disease. Perspectives on Psychological Science, 11(4), 456–463.
Cohen, S., Janicki-Deverts, D., & Miller, G. E. (2007a). Psychological stress and disease. JAMA, 298(14), 1685–1687.
Cohen, S., Kessler, R., & Gordon, L. (1995). Strategies for measuring stress in studies of psychiatric and physical disorders. In Measuring stress: A guide for health and social scientists (pp. 3–26).
Cole, S. W., Kemeny, M. E., & Taylor, S. E. (1997). Social identity and physical health: Accelerated HIV progression in rejection-sensitive gay men. Journal of Personality and Social Psychology, 72(2), 320–335.
Damjanovic, A. K., Yang, Y., Glaser, R., Kiecolt-Glaser, J. K., Nguyen, H., Laskowski, B., … Weng, N. -p. (2007). Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. The Journal of Immunology, 179(6), 4249–4254.
Daruna, J. H. (2012). Introduction to psychoneuroimmunology. Academic Press.
de Visser, K. E., Eichten, A., & Coussens, L. M. (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer, 6(1), 24–37.
Dekaris, D. (1993). Multiple changes of immunologic parameters in prisoners of war: Assessments after release from a camp in Manjača, Bosnia. JAMA, 270(5), 595.
Dhabhar, F. S. (2014). Effects of stress on immune function: The good, the bad, and the beautiful. Immunologic Research, 58(2–3), 193–210.
Dhabhar, F. S., & Mcewen, B. S. (1997). Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain, Behavior, and Immunity, 11(4), 286–306.
Dickerson, S. S., & Kemeny, M. E. (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130(3), 355–391.
Dickerson, S. S., Mycek, P. J., & Zaldivar, F. (2008). Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychology, 27(1), 116–121.
Dimsdale, J. E., Mills, P., Patterson, T., Ziegler, M., & Dillon, E. (1994). Effects of chronic stress on beta-adrenergic receptors in the homeless. Psychosomatic Medicine, 56(4), 290–295.
Donelan, K., Hill, C. A., Hoffman, C., Scoles, K., Feldman, P. H., Levine, C., & Gould, D. (2002). Challenged to care: Informal caregivers in a changing health system. Health Affairs, 21(4), 222–231.
Elenkov, I. J., & Chrousos, G. P. (2002). Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences, 966(1), 290–303.
Epel, E. S., Blackburn, E. H., Lin, J., Dhabhar, F. S., Adler, N. E., Morrow, J. D., & Cawthon, R. M. (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences, 101(49), 17312–17315.
Esterling, B. A., Kiecolt-Glaser, J. K., Bodnar, J. C., & Glaser, R. (1994). Chronic stress, social support, and persistent alterations in the natural killer cell response to cytokines in older adults. Health Psychology, 13(4), 291–298.
Evans, D. L., Leserman, J., Perkins, D. O., Stern, R. A., Murphy, C., Zheng, B., … Petitto, J. M. (1997). Severe life stress as a predictor of early disease progression in HIV infection. The American Journal of Psychiatry, 154(5), 630–634.
Everly, G. S., & Lating, J. M. (2013). The anatomy and physiology of the human stress response. In G. S. Everly & J. M. Lating (Eds.), A clinical guide to the treatment of the human stress response (pp. 17–51). Springer.
Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., … Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. American Journal of Preventive Medicine, 14(4), 245–258.
Fonareva, I., Amen, A. M., Zajdel, D. P., Ellingson, R. M., & Oken, B. S. (2011). Assessing sleep architecture in dementia caregivers at home using an ambulatory polysomnographic system. Journal of Geriatric Psychiatry and Neurology, 24(1), 50–59.
Gennaro, S., Fehder, W., Nuamah, I. F., Campbell, D. E., & Douglas, S. D. (1997b). Caregiving to very low birthweight infants: A model of stress and immune response. Brain, Behavior, and Immunity, 11(3), 201–215.
Gennaro, S., Fehder, W. P., Cnaan, A., York, R., Campbell, D. E., Gallagher, P. R., & Douglas, S. D. (1997a). Immune responses in mothers of term and preterm very-low-birth-weight infants. Clinical and Diagnostic Laboratory Immunology, 4, 7.
Glaser, R., & Kiecolt-Glaser, J. K. (1994). Stress-associated immune modulation and its implications for reactivation of latent herpesviruses. In Human Herpesvirus infections (pp. 245–270).
Glaser, R., & Kiecolt-Glaser, J. K. (2005). Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology, 5(3), 243.
Glaser, R., Sheridan, J., Malarkey, W. B., MacCallum, R. C., & Kiecolt-Glaser, J. K. (2000). Chronic stress modulates the immune response to a pneumococcal pneumonia vaccine. Psychosomatic Medicine, 62(6), 804–807.
Godbout, J. P., & Glaser, R. (2006). Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. Journal of Neuroimmune Pharmacology, 1(4), 421–427.
Gouin, J.-P., Glaser, R., Malarkey, W. B., Beversdorf, D., & Kiecolt-Glaser, J. (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology, 31(2), 264–268.
Gouin, J.-P., Hantsoo, L., & Kiecolt-Glaser, J. K. (2008). Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation, 15(4–6), 251–259.
Gouin, J.-P., & Kiecolt-Glaser, J. K. (2011). The impact of psychological stress on wound healing: Methods and mechanisms. Immunology and Allergy Clinics of North America, 31(1), 81–93.
Hammen, C. (2005). Stress and depression. Annual Review of Clinical Psychology, 1(1), 293–319.
Hansson, G. K., & Hermansson, A. (2011). The immune system in atherosclerosis. Nature Immunology, 12(3), 204–212.
Holmes, T. H., & Rahe, R. H. (1967). The social readjustment rating scale. Journal of Psychosomatic Research, 11(2), 213–218.
Iannello, A., Debbeche, O., Samarani, S., & Ahmad, A. (2008). Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. Journal of Leukocyte Biology, 84(1), 1–26.
Irwin, M., Brown, M., Patterson, T., Hauger, R., Mascovich, A., & Grant, I. (1991). Neuropeptide Y and natural killer cell activity: Findings in depression and Alzheimer caregiver stress. The FASEB Journal, 5(15), 3100–3107.
Irwin, M., Hauger, R., Patterson, T. L., Semple, S., Ziegler, M., & Grant, I. (1997). Alzheimer caregiver stress: Basal natural killer cell activity, pituitary-adrenal cortical function, and sympathetic tone. Annals of Behavioral Medicine, 19(2), 83–90.
Johsson, A., & Hansson, L. (1977). Prolonged exposure to a stressful stimulus (noise) as a cause of raised blood-pressure in man. Lancet, 1(8002), 86–87.
Juster, R.-P., McEwen, B. S., & Lupien, S. J. (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience & Biobehavioral Reviews, 35(1), 2–16.
Kemeny, M. E., Weiner, H., Taylor, S. E., Schneider, S., Visscher, B., & Fahey, J. L. (1994). Repeated bereavement, depressed mood, and immune parameters in HIV seropositive and seronegative gay men. Health Psychology, 13(1), 14–24.
Kiecolt-Glaser, J. K., Dura, J. R., Speicher, C. E., Trask, O. J., & Glaser, R. (1991). Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosomatic Medicine, 53(4), 345–362.
Kiecolt-Glaser, J. K., Glaser, R., Gravenstein, S., Malarkey, W. B., & Sheridan, J. (1996). Chronic stress alters the immune response to influenza virus vaccine in older adults. Proceedings of the National Academy of Sciences, 93(7), 3043–3047.
Kiecolt-Glaser, J. K., Loving, T. J., Stowell, J. R., Malarkey, W. B., Lemeshow, S., Dickinson, S. L., & Glaser, R. (2005). Hostile marital interactions, proinflammatory cytokine production, and wound healing. Archives of General Psychiatry, 62(12), 1377–1384.
Kiecolt-Glaser, J. K., Shuttleworth, E. C., Dyer, C. S., Ogrocki, P., & Speicher, C. E. (1987). Chronic stress and immunity in family caregivers of Alzheimer’s disease victims. Psychosomatic Medicine, 13.
Kiecolt-Glaser, J. K., Marucha, P. T., Mercado, A. M., Malarkey, W. B., & Glaser, R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346(8984), 1194–1196.
Kirschbaum, C., Pirke, K.-M., & Hellhammer, D. H. (1993). The “Trier social stress test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81.
Lang, A. J., Rodgers, C. S., Laffaye, C., Satz, L. E., Dresselhaus, T. R., & Stein, M. B. (2003). Sexual trauma, posttraumatic stress disorder, and health behavior. Behavioral Medicine, 28(4), 150–158.
Lauc, G., Dabelic, S., Dumic, J., & Flogel, M. (1998). Stressin and natural killer cell activity in professional soldiers. Annals of the New York Academy of Sciences, 851(1), 526–530.
Lazarus, R., & Folkman, S. (1984). Stress, appraisal, and coping. .
Lutgendorf, S. K., Vitaliano, P. P., Tripp-Reimer, T., Harvey, J. H., & Lubaroff, D. M. (1999). Sense of coherence moderates the relationship between life stress and natural killer cell activity in healthy older adults. Psychology and Aging, 14(4), 12.
Marucha, P. T., Kiecolt-Glaser, J. K., & Favagehi, M. (1998). Mucosal wound healing is impaired by examination stress. Psychosomatic Medicine, 60(3), 362–365.
Mausbach, B. T., Mills, P. J., Patterson, T. L., Aschbacher, K., Dimsdale, J. E., Ancoli-Israel, S., … Grant, I. (2007). Stress-related reduction in personal mastery is associated with reduced immune cell β2-adrenergic receptor sensitivity. International Psychogeriatrics, 19(05), 935.
McEwen, B. S. (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840(1), 33–44.
Mcewen, B. S. (2004). Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences, 1032(1), 1–7.
McKinnon, W., Weisse, C. S., Reynolds, C. P., Bowles, C. A., & Baum, A. (1989). Chronic stress, leukocyte subpopulations, and humoral response to latent viruses. Health Psychology, 8(4), 389–402.
Miller, G. E., Cohen, S., Pressman, S., Barkin, A., Rabin, B. S., & Treanor, J. J. (2004). Psychological stress and antibody response to influenza vaccination: When is the critical period for stress, and how does it get inside the body? Psychosomatic Medicine, 66(2), 215–223.
Nahmias, A. J., & Roizman, B. (1973). Infection with herpes-simplex viruses 1 and 2. The New England Journal of Medicine, 289(13), 667–674.
Nakano, Y., Nakamura, S., Hirata, M., Harada, K., Ando, K., Tabuchi, T., … Oda, H. (1998). Immune function and lifestyle of taxi drivers in Japan. Industrial Health, 36(1), 32–39.
Ockenfels, M. C., Porter, L., Smyth, J., Kirschbaum, C., Hellhammer, D. H., & Stone, A. A. (1995). Effect of chronic stress associated with unemployment on salivary cortisol: Overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine, 57(5), 460.
Park, C. L., & Iacocca, M. O. (2014). A stress and coping perspective on health behaviors: Theoretical and methodological considerations. Anxiety, Stress, & Coping, 27(2), 123–137.
Pickup, J. C., & Crook, M. A. (1998). Is type II diabetes mellitus a disease of the innate immune system? Diabetologia, 41(10), 1241–1248.
Poorolajal, J., Hooshmand, E., Mahjub, H., Esmailnasab, N., & Jenabi, E. (2016). Survival rate of AIDS disease and mortality in HIV-infected patients: A meta-analysis. Public Health, 139, 3–12.
Sabioncello, A., Kocijan-Hercigonja, D., Rabatić, S., Tomašić, J., Jeren, T., Matijević, L., … Dekaris, D. (2000). Immune, endocrine, and psychological responses in civilians displaced by war. Psychosomatic Medicine, 62(4), 502–508.
Sapolsky, R. M. (2005). The influence of social hierarchy on primate health. Science, 308(5722), 648–652.
Sarafino, E. P., Caltabiano, M. L., & Byrne, D. (2008). Health psychology: Biopsychosocial interactions [second Australasian edition]. Wiley. Retrieved from http://au.wiley.com/WileyCDA/WileyTitle/productCd-EHEP002216.html
Sattar, N., McCarey, D. W., Capell, H., & McInnes, I. B. (2003). Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation, 108(24), 2957–2963.
Segerstrom, S. C., & Miller, G. E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630.
Solomon, G. F., Segerstrom, S. C., Grohr, P., Kemeny, M., & Fahey, J. (1997). Shaking up immunity: Psychological and immunologic changes after a natural disaster. Psychosomatic Medicine, 59(2), 114–127.
Stowell, J. R., Kiecolt-Glaser, J. K., & Glaser, R. (2001). Perceived stress and cellular immunity: When coping counts. Journal of Behavioral Medicine, 24(4), 323–339.
Taylor, D. H., Ezell, M., Kuchibhatla, M., Østbye, T., & Clipp, E. C. (2008). Identifying trajectories of depressive symptoms for women caring for their husbands with dementia. Journal of the American Geriatrics Society, 56(2), 322–327.
Vedhara, K., Cox, N. K., Wilcock, G. K., Perks, P., Hunt, M., Anderson, S., … Shanks, N. M. (1999). Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination. The Lancet, 353(9153), 627–631.
Vitaliano, P., Echeverria, D., Shelkey, M., Zhang, J., & Scanlan, J. (2007). A cognitive psychophysiological model to predict functional decline in chronically stressed older adults. Journal of Clinical Psychology in Medical Settings, 14(3), 177–190.
Vitaliano, P., Scanlan, J. M., Ochs, H. D., Syrjala, K., Siegler, I. C., & Snyder, E. A. (1998). Psychosocial stress moderates the relationship of cancer history with natural killer cell activity. Annals of Behavioral Medicine, 20(3), 199–208.
von Känel, R., Dimsdale, J. E., Mills, P. J., Ancoli-Israel, S., Patterson, T. L., Mausbach, B. T., & Grant, I. (2006). Effect of Alzheimer caregiving stress and age on frailty markers interleukin-6, C-reactive protein, and D-dimer. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(9), 963–969.
von Känel, R., Mills, P. J., Mausbach, B. T., Dimsdale, J. E., Patterson, T. L., Ziegler, M. G., … Grant, I. (2012). Effect of Alzheimer caregiving on circulating levels of C-reactive protein and other biomarkers relevant to cardiovascular disease risk: A longitudinal study. Gerontology, 58(4), 354–365.
Wimo, A., von Strauss, E., Nordberg, G., Sassi, F., & Johansson, L. (2002). Time spent on informal and formal care giving for persons with dementia in Sweden. Health Policy, 61(3), 255–268.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Manigault, A.W., Zoccola, P.M. (2021). Psychoneuroimmunology: How Chronic Stress Makes Us Sick. In: Hazlett-Stevens, H. (eds) Biopsychosocial Factors of Stress, and Mindfulness for Stress Reduction. Springer, Cham. https://doi.org/10.1007/978-3-030-81245-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-81245-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81244-7
Online ISBN: 978-3-030-81245-4
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)