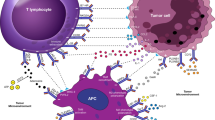
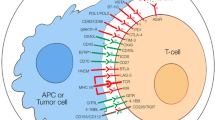
Abstract
Due to improved understanding of the tumor biology and immunology, cancer immunotherapy has rapidly developed as a promising strategy to treat various types of cancer and become the fourth most popular therapy after the conventional methods including surgery, radiotherapy, and chemotherapy. Immune checkpoint blockade (ICB) therapy played a vital role in cancer immunotherapy by interfering with various immunosuppressive mechanisms and utilizing the host’s immune system to kill tumor cells, including inhibitors of cytotoxic T lymphocyte-associated protein 4 (CTLA-4), programmed cell death 1 receptor (PD-1), and programmed cell death receptor ligand 1 (PD-L1). In addition, the emerging immune checkpoint blockers like anti-LAG-3 and anti-TIM-3 antibodies also perform promising therapeutic roles in the treatment of cancer. Therefore, this chapter will introduce a variety of immune checkpoints, elucidate the underlying mechanistic aspects of the immune checkpoint pathways in tumors, and further show the successful applications of nanotechnology in combination with immune checkpoint therapy to improve the antitumor efficacy.
Similar content being viewed by others
References
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, tim-3, and tigit: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44:989–1004
Boussiotis VA (2016) Molecular and biochemical aspects of the pd-1 checkpoint pathway. N Engl J Med 375:1767–1778
Chen DS, Mellman I (2017) Elements of cancer immunity and the cancer-immune set point. Nature 541:321–330
Chen C, Guo Q, Fu H, Yu J, Wang L, Sun Y et al (2021a) Asynchronous blockade of pd-l1 and cd155 by polymeric nanoparticles inhibits triple-negative breast cancer progression and metastasis. Biomaterials 275:120988
Chen P, Wang H, Zhao L, Guo H, Zhang L, Zhang W et al (2021b) Immune checkpoints ox40 and ox40l in small-cell lung cancer: predict prognosis and modulate immune microenvironment. Front Oncol 11:713853
Cremolini C, Vitale E, Rastaldo R, Giachino C (2021) Advanced nanotechnology for enhancing immune checkpoint blockade therapy. Nanomaterials (Basel) 11(3):661
Croft M (2010) Control of immunity by the tnfr-related molecule ox40 (cd134). Annu Rev Immunol 28:57–78
Curigliano G, Gelderblom H, Mach N, Doi T, Tai D, Forde PM et al (2021) Phase i/ib clinical trial of sabatolimab, an anti-tim-3 antibody, alone and in combination with spartalizumab, an anti-pd-1 antibody, in advanced solid tumors. Clin Cancer Res 27:3620–3629
Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K et al (2013) Ox40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 73:7189–7198
Deng H, Zhang Z (2018) The application of nanotechnology in immune checkpoint blockade for cancer treatment. J Control Release 290:28–45
Deng J, Zhao S, Zhang X, Jia K, Wang H, Zhou C, He Y (2019) Ox40 (cd134) and ox40 ligand, important immune checkpoints in cancer. Onco Targets Ther 12:7347–7353
Dixon KO, Tabaka M, Schramm MA, Xiao S, Tang R, Dionne D et al (2021) Tim-3 restrains anti-tumour immunity by regulating inflammasome activation. Nature 595:101–106
Gao S, Yang X, Xu J, Qiu N, Zhai G (2021) Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: the horizons in cancer treatment. ACS Nano 15(8):12567–12603
Golden EB, Apetoh L (2015) Radiotherapy and immunogenic cell death. Semin Radiat Oncol 25:11–17
Goleva E, Lyubchenko T, Kraehenbuehl L, Lacouture ME, Leung D, Kern JA (2021) Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann Allergy Asthma Immunol 126:630–638
Gu Z, Da SC, Van der Maaden K, Ossendorp F, Cruz LJ (2020) Liposome-based drug delivery systems in cancer immunotherapy. Pharmaceutics 12(11):1054
Halpert MM, Konduri V, Liang D, Chen Y, Wing JB, Paust S et al (2016) Dendritic cell-secreted cytotoxic t-lymphocyte-associated protein-4 regulates the t-cell response by downmodulating bystander surface b7. Stem Cells Dev 25:774–787
Han X, Li H, Zhou D, Chen Z, Gu Z (2020) Local and targeted delivery of immune checkpoint blockade therapeutics. Acc Chem Res 53:2521–2533
Hargadon KM, Johnson CE, Williams CJ (2018) Immune checkpoint blockade therapy for cancer: an overview of fda-approved immune checkpoint inhibitors. Int Immunopharmacol 62:29–39
Harris-Bookman S, Mathios D, Martin AM, Xia Y, Kim E, Xu H et al (2018) Expression of lag-3 and efficacy of combination treatment with anti-lag-3 and anti-pd-1 monoclonal antibodies in glioblastoma. Int J Cancer 143:3201–3208
Hodi FS, O’Day SJ, Mcdermott DF, Weber RW, Sosman JA, Haanen JB et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Hu M, Zhang J, Kong L, Yu Y, Hu Q, Yang T et al (2021) Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. ACS Nano 15:3123–3138
Huang TY, Huang GL, Zhang CY, Zhuang BW, Liu BX, Su LY et al (2020) Supramolecular photothermal nanomedicine mediated distant tumor inhibition via pd-1 and tim-3 blockage. Front Chem 8:1
Kandel S, Adhikary P, Li G, Cheng K (2021) The tim3/gal9 signaling pathway: an emerging target for cancer immunotherapy. Cancer Lett 510:67–78
Keir ME, Butte MJ, Freeman GJ, Sharpe AH (2008) Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol 26:677–704
Kiaie SH, Sanaei MJ, Heshmati M, Asadzadeh Z, Azimi I, Hadidi S et al (2021) Immune checkpoints in targeted-immunotherapy of pancreatic cancer: new hope for clinical development. Acta Pharm Sin B 11:1083–1097
Kim J, Hong J, Lee J, Fakhraei LS, Kim YH (2021) Recent advances in tumor microenvironment-targeted nanomedicine delivery approaches to overcome limitations of immune checkpoint blockade-based immunotherapy. J Control Release 332:109–126
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG et al (2016) Adaptive resistance to therapeutic pd-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 7:10501
Laurent S, Carrega P, Saverino D, Piccioli P, Camoriano M, Morabito A et al (2010) Ctla-4 is expressed by human monocyte-derived dendritic cells and regulates their functions. Hum Immunol 71:934–941
Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by ctla-4 blockade. Science 271:1734–1736
Lecocq Q, Keyaerts M, Devoogdt N, Breckpot K (2020) The next-generation immune checkpoint lag-3 and its therapeutic potential in oncology: third time’s a charm. Int J Mol Sci 22(1):75
Lee HT, Lee SH, Heo YS (2019) Molecular interactions of antibody drugs targeting pd-1, pd-l1, and ctla-4 in immuno-oncology. Molecules 24(6):1190
Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B et al (2008) The pd-1/pd-l1 complex resembles the antigen-binding fv domains of antibodies and t cell receptors. Proc Natl Acad Sci U S A 105:3011–3016
Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H et al (2018a) Blockade of tim3 relieves immunosuppression through reducing regulatory t cells in head and neck cancer. J Exp Clin Cancer Res 37:44
Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L (2018b) Combination immunotherapy of muc1 mrna nano-vaccine and ctla-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther 26:45–55
Liu J, Zhang R, Xu ZP (2019a) Nanoparticle-based nanomedicines to promote cancer immunotherapy: recent advances and future directions. Small 15:e1900262
Liu Y, Chen XG, Yang PP, Qiao ZY, Wang H (2019b) Tumor microenvironmental ph and enzyme dual responsive polymer-liposomes for synergistic treatment of cancer immuno-chemotherapy. Biomacromolecules 20:882–892
Liu Q, Duo Y, Fu J, Qiu M, Sun Z, Adah D et al (2021) Nano-immunotherapy: unique mechanisms of nanomaterials in synergizing cancer immunotherapy. Nano Today 36:101023
Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, Chen H (2018) The promising immune checkpoint lag-3: from tumor microenvironment to cancer immunotherapy. Genes Cancer 9:176–189
Ma Y, Li J, Wang H, Chiu Y, Kingsley CV, Fry D et al (2020) Combination of pd-1 inhibitor and ox40 agonist induces tumor rejection and immune memory in mouse models of pancreatic cancer. Gastroenterology 159:306–319
Marhelava K, Pilch Z, Bajor M, Graczyk-Jarzynka A, Zagozdzon R (2019) Targeting negative and positive immune checkpoints with monoclonal antibodies in therapy of cancer. Cancers (Basel) 11(11):1756
Meng X, Wang J, Zhou J, Tian Q, Qie B, Zhou G et al (2021) Tumor cell membrane-based peptide delivery system targeting the tumor microenvironment for cancer immunotherapy and diagnosis. Acta Biomater 127:266–275
Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T et al (2002) Th1-specific cell surface protein tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536–541
Mu W, Chu Q, Liu Y, Zhang N (2020) A review on nano-based drug delivery system for cancer chemoimmunotherapy. Nanomicro Lett 12:142
Mu X, Zhang M, Wei A, Yin F, Wang Y, Hu K, Jiang J (2021) Doxorubicin and pd-l1 sirna co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of pca bone metastases. Nanoscale 13:8998–9008
Nikpoor AR, Tavakkol-Afshari J, Sadri K, Jalali SA, Jaafari MR (2017) Improved tumor accumulation and therapeutic efficacy of ctla-4-blocking antibody using liposome-encapsulated antibody: in vitro and in vivo studies. Nanomedicine-Uk 13:2671–2682
Nuebling T, Schumacher CE, Hofmann M, Hagelstein I, Schmiedel BJ, Maurer S et al (2018) The immune checkpoint modulator ox40 and its ligand ox40l in nk-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol Res 6:209–221
Okazaki T, Honjo T (2007) Pd-1 and pd-1 ligands: from discovery to clinical application. Int Immunol 19:813–824
Palmieri DJ, Carlino MS (2018) Immune checkpoint inhibitor toxicity. Curr Oncol Rep 20:72
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Patsoukis N, Wang Q, Strauss L, Boussiotis VA (2020) Revisiting the pd-1 pathway. Sci Adv 6(38):eabd2712
Perez-Ruiz E, Melero I, Kopecka J, Sarmento-Ribeiro AB, Garcia-Aranda M, De Las RJ (2020) Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug Resist Updat 53:100718
Pham LM, Poudel K, Ou W, Phung CD, Nguyen HT, Nguyen BL et al (2021) Combination chemotherapeutic and immune-therapeutic anticancer approach via anti-pd-l1 antibody conjugated albumin nanoparticles. Int J Pharm 605:120816
Qin S, Xu L, Yi M, Yu S, Wu K, Luo S (2019) Novel immune checkpoint targets: moving beyond pd-1 and ctla-4. Mol Cancer 18:155
Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM et al (2011) Trans-endocytosis of cd80 and cd86: a molecular basis for the cell-extrinsic function of ctla-4. Science 332:600–603
Redmond WL, Linch SN, Kasiewicz MJ (2014) Combined targeting of costimulatory (ox40) and coinhibitory (ctla-4) pathways elicits potent effector t cells capable of driving robust antitumor immunity. Cancer Immunol Res 2:142–153
Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M et al (2013) Phase iii randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 31:616–622
Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526
Sanaei MJ, Pourbagheri-Sigaroodi A, Kaveh V, Sheikholeslami SA, Salari S, Bashash D (2021) The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: recent advances and opportunities. Crit Rev Oncol Hematol 157:103160
Shang Q, Zhou S, Jiang Y, Wang D, Wang J, Song A, Luan Y (2020) Rational design of a robust antibody-like small-molecule inhibitor nanoplatform for enhanced photoimmunotherapy. ACS Appl Mater Interfaces 12:40085–40093
Sugamura K, Ishii N, Weinberg AD (2004) Therapeutic targeting of the effector t-cell co-stimulatory molecule ox40. Nat Rev Immunol 4:420–431
Tang X, Rao J, Yin S, Wei J, Xia C, Li M et al (2019) Pd-l1 knockdown via hybrid micelle promotes paclitaxel induced cancer-immunity cycle for melanoma treatment. Eur J Pharm Sci 127:161–174
Taraban VY, Rowley TF, O’Brien L, Chan HT, Haswell LE, Green MH et al (2002) Expression and costimulatory effects of the tnf receptor superfamily members cd134 (ox40) and cd137 (4-1bb), and their role in the generation of anti-tumor immune responses. Eur J Immunol 32:3617–3627
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27:450–461
Trapani JA, Darcy PK (2017) Immunotherapy of cancer. Aust Fam Physician 46:194–199
Tu L, Guan R, Yang H, Zhou Y, Hong W, Ma L et al (2020) Assessment of the expression of the immune checkpoint molecules pd-1, ctla4, tim-3 and lag-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int J Cancer 147:423–439
Tu K, Yu Y, Wang Y, Yang T, Hu Q, Qin X et al (2021) Combination of chidamide-mediated epigenetic modulation with immunotherapy: boosting tumor immunogenicity and response to pd-1/pd-l1 blockade. ACS Appl Mater Interfaces 13:39003–39017
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L et al (2014) Pd-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515:568–571
Venkatraman S, Meller J, Hongeng S, Tohtong R, Chutipongtanate S (2020) Transcriptional regulation of cancer immune checkpoints: emerging strategies for immunotherapy. Vaccines (Basel) 8(4):735
Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM et al (1994) Ctla-4 can function as a negative regulator of t cell activation. Immunity 1:405–413
Wan Z, Zheng R, Moharil P, Liu Y, Chen J, Sun R et al (2021) Polymeric micelles in cancer immunotherapy. Molecules 26(5):1220
Wang H, Tang Y, Fang Y, Zhang M, Wang H, He Z et al (2019) Reprogramming tumor immune microenvironment (time) and metabolism via biomimetic targeting codelivery of shikonin/jq1. Nano Lett 19:2935–2944
Wei G, Zhang H, Zhao H, Wang J, Wu N, Li L et al (2021) Emerging immune checkpoints in the tumor microenvironment: implications for cancer immunotherapy. Cancer Lett 511:68–76
Wolf Y, Anderson AC, Kuchroo VK (2020) Tim3 comes of age as an inhibitory receptor. Nat Rev Immunol 20:173–185
Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ et al (2012) Immune inhibitory molecules lag-3 and pd-1 synergistically regulate t-cell function to promote tumoral immune escape. Cancer Res 72:917–927
Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, He W (2021) Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B 11:941–960
Yang Q, Shi G, Chen X, Lin Y, Cheng L, Jiang Q et al (2020) Nanomicelle protects the immune activation effects of paclitaxel and sensitizes tumors to anti-pd-1 immunotherapy. Theranostics 10:8382–8399
Yu X, Huang X, Chen X, Liu J, Wu C, Pu Q et al (2019) Characterization of a novel anti-human lymphocyte activation gene 3 (lag-3) antibody for cancer immunotherapy. Mabs-Austin 11:1139–1148
Zhang D, Jiang F, Zaynagetdinov R, Huang H, Sood VD, Wang H et al (2020) Identification and characterization of m6903, an antagonistic anti-tim-3 monoclonal antibody. Onco Targets Ther 9:1744921
Zhang Y, Hughes KR, Raghani RM, Ma J, Orbach S, Jeruss JS, Shea LD (2021) Cargo-free immunomodulatory nanoparticles combined with anti-pd-1 antibody for treating metastatic breast cancer. Biomaterials 269:120666
Zhou L, Zhang P, Wang H, Wang D, Li Y (2020) Smart nanosized drug delivery systems inducing immunogenic cell death for combination with cancer immunotherapy. Acc Chem Res 53:1761–1772
Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ et al (2005) The tim-3 ligand galectin-9 negatively regulates t helper type 1 immunity. Nat Immunol 6:1245–1252
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry
Zhang, Z., Huang, W. (2023). Immune Checkpoint Therapy: A New Opportunity for Cancer Treatment. In: Rezaei, N. (eds) Handbook of Cancer and Immunology. Springer, Cham. https://doi.org/10.1007/978-3-030-80962-1_162-1
Download citation
DOI: https://doi.org/10.1007/978-3-030-80962-1_162-1
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80962-1
Online ISBN: 978-3-030-80962-1
eBook Packages: Springer Reference Biomedicine and Life SciencesReference Module Biomedical and Life Sciences