Abstract
Major depressive disorder (MDD) and other disorders on the depressive spectrum are more prevalent among HIV-infected individuals than in the general population. Depressive disorders have a significant impact on prevention, engagement, and adherence to HIV care, prognosis, illness-related disability, and quality of life. Diagnosis of depressive disorders may be delayed, and management may be inadequate, due to interfering factors such as overlapping symptoms, comorbid neurocognitive disorders, and risk of drug-drug interactions. Not only do HIV infection and disorders on the depressive spectrum act as mutually reciprocal risk factors, but their concomitance may provide an etiopathogenetic model; comorbid depressive disorders may contribute to understanding the complex role played by the neuro-endocrine-immune system in the development, maintenance, and chronicity of psychiatric symptoms. Many neurobiological pathways are involved, including the HPA axis and the gut microbiome. Various therapeutic options are available for MDD among HIV-infected patients, including antidepressant medications, other psychopharmacological interventions, and non-pharmacological strategies. Antidepressants are safe and appropriate for the care of persons with HIV and comorbid depressive disorders. The recognition and treatment of depressive symptoms and disorders among HIV-infected persons is a major clinical goal, considering the high prevalence of this comorbidity and its impact on prognosis and perceived quality of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Depressive disorders
- Depressive symptoms
- Major depressive disorder
- Diagnosis
- Inflammation
- Immune system
- Antidepressant medications
- Psychotherapy
- Mood
- Anxiety
Major depressive disorder (MDD), the most common neuropsychiatric complication in HIV-infected patients [1], is up to four times more prevalent among HIV-infected individuals than in the general population [2], particularly in certain subpopulations, such as women or socially disadvantaged persons [3]. Comorbidity of HIV/AIDS with MDD and other disorders in the depressive spectrum is described in all phases of HIV infection and impacts massively on various clinical aspects of both comorbid conditions [1, 4]. Unfortunately, MDD is often not recognized in persons living with HIV/AIDS (PLWHA), not only as a result of underestimation but also as a consequence of several confounding factors, such as HIV-associated neurocognitive disorders (HAND) and substance-related disorders. Moreover, even when properly diagnosed, MDD may be inadequately managed because of many reasons, including concerns related to side effects of psychotropics and potential drug-drug interactions [3, 5]. Since the introduction of effective antiretroviral therapy (ART), the impact and course of HIV infection have changed from a rapidly debilitating and deadly illness to a chronic, manageable illness with a long-term life expectancy, and the ability to provide an effective treatment of comorbid MDDs assumes even more relevance to the improvement of quality of life of patients [5].
The pathogenic basis for the association of MDD and HIV-infection is complex and remains to be fully clarified. Potential etiologies may include the disruption of immune balance, via HIV-promoted activation of inflammatory mediators, e.g., pro-inflammatory cytokines, chemokine receptors, extracellular matrix-degrading enzymes, and glutamate receptor-mediated excitotoxicity. The same pathogenetic pathways may be involved in determining effectiveness of antidepressant treatments [1, 6]. The identification of MDD biomarkers, such as pro-inflammatory cytokines, in PLWHA may improve diagnostic and therapeutic strategies for MDD, potentially increasing adherence to ART, improving quality of life, and decreasing morbidity and mortality [7].
In this chapter, we provide an update on the comorbidity of HIV/AIDS with MDD and other disorders on the depressive spectrum, synthesizing existing evidence from epidemiology, clinical features, explanatory models, and effective therapeutic options. Special attention is given to the role of inflammation and immune function as an explanatory model, as well as to the most frequent obstacles and pitfalls in clinical diagnosis and management.
Epidemiology
Prevalence and Risk Factors Associated with Depressive Disorders in Persons with HIV
The World Health Organization (WHO) estimated that, by the end of 2018, nearly 38 million people in the world were living with HIV, disproportionately affecting African regions where over two-thirds of the population living with HIV reside [8]. The US Centers for Disease Control and Prevention (CDC) estimates that there are just over one million PLWHA in the USA, with one in seven estimated to be undiagnosed [9]. Depressive disorders are even more prevalent, with 2017 data estimating 17.3 million adults in the USA having had at least one major depressive episode, representing over 7% of the entire population [10]. Given the significant morbidity associated with HIV infection, it is not surprising that depressive disorders are more common among this population than that of non-infected controls, as well as associated with an increased risk of HIV disease progression and mortality.
While more common in PLWHA, the exact prevalence of MDD and other disorders on the depressive spectrum among this group varies across the literature. There are a variety of factors contributing to this, largely due to the lack of a unified definition of “depression.” A systematic review by Sherr et al. found that studies rarely adopted the same cutoff points when using various scales to measure depressive symptoms. Studies using the Beck Depression Inventory (BDI), for example, had prevalence estimates that ranged from a cutoff of +/= 15 to a cutoff of +/= 20 among seven different studies, yielding prevalence rates which ranged from 12% to 71% [11]. This clearly contributes to the difficulty in determining the true prevalence of depressive disorders among PLWHA. Multiple studies suggest a prevalence of two- to fourfold the rate of depressive when compared to non-HIV-infected individuals in the general population [12]. The best recent estimate of the prevalence of depression in PLWHA comes from a 2019 systematic review and meta-analysis by Rezaei et al., the first study to analyze the global impact of comorbid depressive disorders, which found an overall prevalence of 31% across 118 studies including 51,143 subjects [3]. Of these PLWHA, 14,942 were diagnosed with moderate to severe depression [3]. Prevalence was noted to be highest in developing/underdeveloped countries, suggesting that governmental support, access to care, and increased awareness in developed countries positively impact on the rate of diagnosis and management of MDD among PLWHA.
Subgroup analyses of various studies can provide insight into prevalence rates differing from the standard PLWHA population, highlighting potential at-risk and vulnerable groups. Pregnant women with HIV, for example, have about one and a half times the odds of experiencing antenatal and postnatal depressive disorders when compared to non-HIV-infected controls [13]. Men who have sex with men and former blood/plasma donors were found to have significantly higher rates of depressive disorders in a Chinese systematic review and meta-analysis of over 20,000 PLWHA, with 43.9% and 85.6% experiencing depressive disorders, respectively [12]. Further analyses are warranted to identify other at-risk subgroups to guide targeted interventions.
Risk Factors
The relationship between HIV and depressive disorders is multifactorial and varies depending on geographic region. Depressive disorders can occur in all phases of HIV infection with more prevalence in advanced-stage HIV illness [1]. This suggests the importance of depressive symptom screening and clinical monitoring throughout treatment of HIV and AIDS. Some key risk factors include female sex, preexisting depressive disorder and family history of depressive disorders [5], as well as a combination of neurobiological changes and psychosocial and behavioral factors [1].
The neurobiological changes are related to lower CD4 counts, higher viral loads [1, 12], chronic neuroinflammation, reduction in trophic factors, and alterations in dopamine and other neurotransmitters [5]. Psychosocial factors such as early life trauma, violence exposure, limited healthcare access, financial instability, lower educational attainment, and underemployment can increase risk. Behavioral factors such as drug and alcohol abuse as well as limited physical activity are also implicated [5].
Unfortunately, depressive disorders continue to be underdiagnosed and undertreated [1] in the HIV-infected population. In addition to treatments focused on prevention and cure of HIV/AIDS, attention should be aimed at the quality of life in PLWHA [13], including the diagnosis and treatment of depressive disorders. Addressing and modifying the risk factors for depressive disorders may improve adherence to antiretrovirals and mitigate progression of HIV. This can help people cope with this illness and ultimately decrease mortality [13] (Table 6.1).
Diagnosis
Despite the importance of detecting and treating depressive disorders in HIV-infected patients, this syndrome frequently goes unrecognized [14, 15], and, even when clinically recognized, it often goes untreated. Moreover, even when treating depressive disorders concomitant with HIV, clinicians’ adherence to best-practices guidelines about dosing, duration, and monitoring of antidepressants is low, meaning that many patients fail to receive an adequate treatment course and therefore fail to benefit from treatment [14].
The ascertainment of depression in HIV-infected patients may be expected to be difficult or biased due to several factors:
-
1.
The etiology of depression in HIV is a complex, multidimensional phenomenon comprised of various components of psychiatric, psychological, neurological, systemic medical, and toxic factors which are summarized in Table 6.2. Stressful life events and other psychosocial factors (such as stigma) related to the fact of being infected by HIV may lead to an adjustment disorder with depressive symptoms (“psychological” depression). Further, HIV-related conditions and HIV-associated inflammation with an impact on the central nervous system could also present with depressive symptoms (“neuropsychiatric” depression). Moreover, some systemic medical conditions caused by HIV infection, such as hypothyroidism or hypogonadism, can produce depression-like symptoms (“medical” depression). Finally, some treatments given to PLWHA can produce psychiatric side effects (“toxic” depression). Once we have ruled out systemic medical or toxic conditions, it may be very difficult to differentiate between MDD (“psychiatric” depression), reactive depression (“psychological” depression), or depression due to HIV infection in the brain (“neuropsychiatric” depression). In many cases, in HIV patients, these three explanatory models of depressive disorders represent a continuum of depressive symptoms rather than three separate categorical diagnoses.
-
2.
The clinical criteria commonly used to diagnose depressive disorder in the general population have some limitations when they were applied to PLWHA, due to the confounding effect of physiological changes associated with HIV illness progression or multimorbid other medical systemic illnesses (such as cancer, cardiovascular illness, or diabetes mellitus), the physical symptoms of infection, and/or side effects of HIV medications. For example, both symptoms of depression and physiological changes associated with HIV illness share appetite changes, lack of motivation, fatigue, sleep disturbances, anergia, loss of libido, impaired sexual functioning, and cognitive impairment. Thus, prevalence rates vary greatly across studies [16]. Relying excessively on somatic symptoms for depressive disorder diagnoses may lead clinicians to mistakes in diagnosis, either over- or underestimation of depression risk, since these somatic symptoms are less specific in patients with systemic medical comorbidities. Cognitive-affective criteria, similar to those proposed by Endicott for diagnosing depression in cancer patients, could be more helpful in HIV patients and make diagnosis more accurate [17]. Symptoms and signs such as depressed appearance, self-pity/pessimism, hopelessness, and social withdrawal or lack of reactivity, instead of poor appetite, fatigue, insomnia, or diminished ability to think clearly could be more useful to diagnose psychiatric/psychological depression in PLWHA (see Table 6.2).
-
3.
The clinical setting of care may complicate the diagnosis of depressive disorders in PLWHA. Depressed patients may only tend to report somatic symptoms to their HIV physician, and they may not refer to their mood state if not assessed specifically. Further, physicians who are not psychiatrists may feel less confident asking about depressive symptoms. In some cases, the brief duration of medical encounters may prevent clinicians from including psychosocial issues or evening asking the simple question “Are you depressed?”. Finally, there may be a misconception that depression is “normal” or even “acceptable” in PLWHA, leading to diagnostic and therapeutic nihilism.
Tools for Assessment
Clinicians need effective tools to diagnose depressive disorders in PLWHA. The utilization of self-report scales can also improve clinicians’ ability to screen for depression in patients with HIV and AIDS. Most of the instruments commonly validated and used to screen or measure depressive symptoms were created to be used in the general population. Table 6.3 describes some of the scales used to screen and measure depressive symptoms. Most of the scales include somatic symptoms of depression, which, as mentioned before, may be confounded with somatic symptoms due to HIV infection or other multimorbid illness. The Hospital Anxiety and Depression Scale (HADS) [21] is a self-report scale specially designed to assess anxiety and depression in people with systemic medical illness. The depression subscale mainly assesses anhedonia and does not include any somatic item that can be confused with symptoms of systemic medical illness. The HADS has a major advantage over other existing depression measures. Accumulating data suggest that the HADS provides a valid and reliable assessment of depression and anxiety for a wide variety of populations.
Case Vignette 6.1
J was a 68-year-old man known to be infected by HIV for 25 years, when he was using substances intravenously. He was on ART with undetectable viral load. Past medical history included type 2 diabetes mellitus on insulin for 2 years, diabetic nephropathy, and benign prostate hypertrophy and chronic obstructive pulmonary diseases because of smoking. The patient was on beta-adrenergic-blocking drugs for hypertension and on allopurinol for hyperuricemia. He had surgery for facial lipo-injection and abdominal and cervical liposuction in 2007.
He had had AD treatment with paroxetine in the past, due to depression and anxiety. He had a very good job as chief operating officer in a company, but had retired. He had a stable partner relationship for years. With the time, he reduced the amount of social relations. He preferred to avoid interpersonal relationships because he did not want to disclose his HIV condition. He used to be a meticulous person with obsessive traits of personality. After a long time, he went back to the psychiatrist who had given him treatment in his previous depressive and anxiety episode, because he started to have depressive symptoms, with feelings of hopelessness and suicidal thoughts. As concurrent events he related that he had started a long-distance relationship and was considering not continuing with his current partner. However, he was afraid of abandonment, if he broke his long-time relationship. He also referred difficulties in assuming the deterioration due to getting older and due to his medical illnesses. He admitted having difficulties in being adherent to the ART treatment. He often forgot to take the medication. He was also afraid of starting to have cognitive impairment. Neuropsychological examination was recommended, but he refused.
What care strategies would you consider for the case?
What clinical problems would you prioritize?
Case Vignette 6.2
D was a 30-year-old gay man, known to be infected by HIV and HCV through unprotected sex for 10 years. He started ART when his CD4 lymphocyte count was 212 cell/ml and his viral load was 182,000 copies. After starting ART, these values improved to almost 600 CD4 cells/ml and undetectable viral load. He went to his regular visit to his HIV physician, and after a short time of interview, he reported that he had feelings of low energy and some memory difficulties for the previous 8 months. He started to increase the intake of cocaine to get more energy, but this was useful only during a short time. He was living alone after he broke off his relationship with his partner, 1 year before. Laboratory tests showed no relevant deviations of the normal ranges, except from low testosterone levels. Scores on the HADS were 6 on the anxiety subscale and 17 on the depression subscale. The HIV care provider started testosterone treatment and sent D to the psychiatrist. After 8 weeks of testosterone replacement, D still referred depressive symptoms such as anhedonia, hopelessness, suicidal thoughts, increased social isolation, poor appetite, and sleep disturbances. Difficulties with memory and concentration also persisted. The depressive subscale of the HADS still scored above 10. D was given, antidepressant medications and almost all depressive symptoms improved after 4 weeks of treatment. Cognitive impairment did not improve. Neuropsychological testing showed Frascati criteria for mild neurocognitive disorder.
What do you think may be the “take-home” message in this case?
Would you have acted differently in a similar situation?
Etiology
The pathogenic causal pathways for the increased risk of MDD in HIV-infected subjects remain to be fully understood but are based on the complex interaction among biopsychosocial factors. The biological mechanisms include neurobiological changes caused by the persistent viral presence in the CNS and systemic and intra-CNS immune-inflammatory and neuroendocrine phenomena induced by HIV. The HIV-related immune imbalance could contribute to the development of depression via activation of inflammatory mediators, cytokines, chemokine receptors, extracellular matrix-degrading enzymes, and glutamate receptor-mediated excitotoxicity. All of these mediators have been proposed to play a role in mediating depressive symptoms and response to depression treatment in general (not only in HIV-related depression), but such a role could be even more important or apparent in persons with HIV. Additionally, HIV may act via direct infection of astrocytes, oligodendrocytes, and neuronal progenitor cells, and HIV proteins may activate brain glial cells with a further contribution to inflammation. Evidence favoring the role of pro-inflammatory cytokines in the pathogenesis of MDD has been confirmed in recent years and includes different molecules and mechanisms, as described in Table 6.4.
Among the cytokines likely to be involved in MDD and its treatment, there are pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, IL-12, IL-23, tumor necrosis factor (TNF)-α and IFN-α, and chemokines, such as monocyte chemoattractant protein (MCP)-1 [25]. The same pro-inflammatory cytokines may play a role in the development of MDD in HIV-infected persons, as significant changes in the levels of expression of pro-inflammatory cytokines (including IL-18 and INF-γ and TNF-α) in peripheral blood mononuclear cells of a sample of HIV-infected subjects were confirmed [26]. The neurotrophin brain-derived neurotrophic factor (BDNF) is also known to be involved in the pathogenesis and treatment of MDD: significantly lower levels of BDNF mRNA were found than in healthy subjects compared to the expression of BDNF in peripheral blood mononuclear cells of HIV-infected patients [26]. Pro-inflammatory cytokines may affect BDNF expression and release in a human glioblastoma-astrocytoma cell line, suggesting that cytokines may contribute to the development of depression by reducing the neurotrophic support in the brain.
Systemic immune activation during HIV infection is also a consequence of the effects of HIV on the structure and functioning of the gut mucosa; HIV negatively impacts on the functioning of the enteric immune system, reducing its effectiveness in surveillance to the gut barrier. As a consequence, translocation across the gut mucosa of bacterial products, such as LPS, may be easier, leading to engagement of a massive inflammatory response mediated by further production of pro-inflammatory cytokines. Further events include interference with the tryptophan metabolism, activation of inflammasomes, and direct stimulation of the CNS immune system. Neuroinflammation is also enhanced by the occurrence of co-infection by opportunistic pathogens or by abuse of drugs and alcohol.
Pro-inflammatory cytokines are known to also impact on the effectiveness of the treatment of MDD, since increased inflammatory markers in depressed patients have been associated with non-response to treatment with AD, and anti-inflammatory therapy is beneficial in depression, including observations of the adjuvant role of NSAIDs such as ASA toward serotoninergic AD [27]. A circular etiopathogenetic interrelationship among effects of AD medications and biomarkers of depression may be hypothesized, with biomarkers possibly associated with or predicting clinical response to AD [6]. The rate and severity of depression also seem to correlate to prescription and effectiveness of ART, a further demonstration that de-activation of the immune system and consequent reduction in cytokine levels also impact on psycho-behavioral phenomenology.
In HIV-infected individuals, identification of specific biomarkers for MDD may support diagnostic and therapeutic strategies by informing clinicians about the onset of comorbid depression or supporting recommendations for AD therapy. The final positive results of identifying biomarkers may be better adherence to ART, improvement of quality of life, and decreased morbidity and mortality.
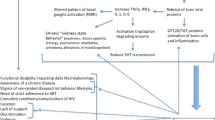
Psychosocial mechanisms are also relevant, such as the burden of having a life-threatening and chronic disabling disease and factors more specific to HIV such as isolation, stigma, lack of support, violence, and drug abuse; such issues become even more significant when other psychosocial risk factors are present, e.g., migrant status and poor coping. HIV-related psychosocial mechanisms may lead to depressive disorders via activation of some of the neurobiological pathways as previously described, e.g., altered activity of the hypothalamic-pituitary-adrenal axis as a consequence of chronic exposition to stress [1]. Figure 6.1 sums up mechanisms that could be implied in determining depression among HIV-infected subjects.
Possible causative mechanisms of depression in HIV subjects. (Reprinted from Nanni et al. [1]. With permission from Springer Nature)
Clinical Interventions
Pharmacotherapy
AD pharmacotherapy remains a mainstay of treatment for PLWHA and concomitant MDD. The indications to begin pharmacotherapy are the same regardless of HIV status, but with HIV-positive patients, there are additional considerations. Challenges related to antidepressant treatment of persons with HIV and depressive symptoms include an increased risk of nonadherence as well as drug-drug interactions with the patient’s antiretroviral medications [28]. Additionally, adequate treatment in the HIV population is critical, as HIV-positive individuals with depressive symptoms are shown to have increased HIV illness progression and higher mortality rates compared with HIV-positive individuals without symptoms of depressive disorders [29]. However, treatment of depressive symptoms has also been shown to increase adherence to ART, thus emphasizing that treatment of depression may also lead to better treatment of the HIV infection [1].
A recent meta-analysis of over 700 patients showed that treatment with AD pharmacotherapy was superior to placebo when assessing depressive symptoms with the Hamilton Depression Rating Scale (HAM-D) score [30]. This study supports that the pharmacotherapies we use to treat the general population will also be effective for PLWHA. Many classes of ADs have been studied in this patient population with similar results; thus, choosing therapy is based on tolerability and side effects. In general, SSRIs/SNRIs are first-line agents that tend to be tolerated well with few side effects, with sexual dysfunction being a common complaint. See also Chap. 17 for a detailed discussion on combining antiretrovirals and psychotropics. In addition, bupropion seems to be well tolerated and effective with more favorable side effect profiles [31]. Bupropion’s activating characteristics may also help with the fatigue that may be experienced by individuals living with HIV and depression and has been shown to be effective regardless of HIV clinical staging [32].
In a survey performed by 62 HIV mental health clinicians, almost all of whom were psychiatrists, citalopram/escitalopram emerged as the preferred first-line treatment option for MDD. In the case of no response to treatment for 8 weeks, a switch to a serotonin/norepinephrine reuptake inhibitor (SNRI) AD was considered a first-line treatment by consensus, followed by bupropion or another SSRI as other appropriate first-line options. Respondents considered a switch to mirtazapine as a second-line option. Fluvoxamine, amitriptyline, and monoamine oxidase inhibitors (MAOIs) were clearly considered third-line treatments.
In the case of augmenting a partial response to the initial treatment, clinicians chose to add a second (non-SSRI) AD as a first-line strategy. Use of lithium, psychostimulants, second-generation antipsychotics, and thyroid hormone replacement were viewed by a majority as second-line options. The addition of buspirone was relegated to third-line status [33].
In addition to starting AD therapy, it is also important to assure close follow-up of patients for re-assessment and dose adjustments, since only about half of patients fully respond to initial treatment [34].
To be noted, there is also a theoretical risk of CYP modulation that could lead to altered drug levels as well as lead to dangerous adverse events such as serotonin syndrome: this is specific to persons with HIV since they are likely to be taking other medications such as antiretrovirals that may alter drug metabolism [31, 35]. The use of ADs in patients who are on ART should follow similar principles as in the case of older patients or patients with other systemic medical illnesses: start with lower doses and increase progressively to standard full-recommended dose, and use the least complicated possible dosing schedules [36].
In addition to managing the pharmacotherapy for the treatment of the patient’s depressive symptoms, it is important to consider that many of the ART commonly used to treat HIV infection have their own neuropsychiatric side effects. The US CDC recommends screening for depression and suicidality for people with HIV who are taking a regimen that includes efavirenz. The same CDC guidelines include concern about an increased risk of suicidal ideation in patients taking dolutegravir. Moreover, integrase strand transfer inhibitors report depression as an adverse event [37]. In general, clinicians should discuss in advance the potential onset of depressive symptoms with their patients taking ART, to plan adequate management accordingly, if necessary (Table 6.5).
Psychotherapy
Alternatively, many patients and clinicians desire psychotherapy as a first-line treatment of MDD. In the American College of Physicians’ 2016 clinical guidelines for the treatment of MDD, they found that most comparative studies showed no significant difference in outcomes between pharmacologic and non-pharmacologic therapy for mild/moderate cases, although this was studied in the general population [38]. More specifically for PLWHA, large meta-analyses have shown that cognitive behavioral therapy has improved depressive symptoms in PLWHA patients with both depression and anxiety. Additionally, a review of group psychotherapy has shown that it may be beneficial as a treatment for depression in PLWHA [39]. As seen in Table 6.6, there have been many studies demonstrating the effectiveness of multiple different psychotherapies. While cognitive behavioral therapy has been the most studied [40], the specific approach to psychotherapy may be left to the patient and clinician to choose.
Notably, HIV often affects individuals in lower socioeconomic areas with poor access to healthcare. Typically, this may be a barrier to psychotherapy treatment, as the patient may be unable to travel to obtain ongoing psychotherapy from their mental health clinician or may have economic limitations to access. Although traditionally a significant barrier to time-intensive psychotherapy, recent data suggest that brief telehealth/teletherapy appointments may be able to bridge this gap and have meaningful reductions in patients’ depressive symptoms [41]. As telehealth capabilities continue to improve, psychotherapy becomes an increasingly viable treatment option also for PLWHA. This increase in accessibility of care should encourage greater use of psychotherapy for the treatment of depression in PLWHA regardless of location. Please see Chap. 16 for further discussion on psychotherapy and other psychotherapeutic modalities.
Case Vignette 6.3
A 22-year-old man with known HIV-positive status presented to the clinic with complaints of fatigue for the previous 6 months. He stated that he just felt overly exhausted by the end of the day despite having no increase in activity level. He also found it exceedingly difficult to get out of bed each morning, which had a significant impact on his schoolwork as a college student. In addition to this, he was having trouble at work as he cannot concentrate as well as before. Despite these problems, he stated he “knows [he] should care about these problems” but feels “almost too tired to care.” On further questioning, he also noted that he had feelings of guilt regarding his HIV-positive status and was anxious about how this would affect him long term for both his health and relationships. He also had noticed a decreased appetite that started about 6 months previously, but he was unsure if he lost any weight. His HIV diagnosis was made over a year previously, and the patient had not had any AIDS-defining illnesses. The patient was taking a combination pill of an integrase strand transfer inhibitor, a nucleoside reverse transcriptase inhibitor (NRTI), and a prodrug of another NRTI. He stated that he had been taking the medication as instructed without any side effects. In addition to his HIV medications, he also took a daily multivitamin and drank whey protein shakes following workouts, but had not been to the gym recently due to his symptoms. Recently, he saw his clinician managing his HIV and stated his CD4+ counts were “good” and the rest of his laboratory work came back “normal.” He had no additional past medical or surgical history. His family history was pertinent for a mother with anxiety disorder and a father with type 2 diabetes mellitus. He denied any suicidal ideation or homicidal ideation.
What is the patient’s most likely diagnosis?
How would you initially manage this patient if you saw him in clinic?
Other Therapeutic Options
Pharmacotherapy and psychotherapy remain the most researched and supported therapies for MDD in HIV-positive individuals; however, additional therapeutic options are being studied. These therapies include a wide array of approaches and levels of invasiveness. A more invasive technique is the use of vagal nerve stimulators to modulate CNS function. While the traditional surgical approach to implant the stimulators is invasive, newer noninvasive transcutaneous VNS may be a future intervention for refractory depression in HIV patients, but future studies and official approval are still needed [42].
Electroconvulsive therapy (ECT) has been used to treat depressive disorders that are refractory to traditional therapies; however, response to ECT for PLWHA has not been widely studied. There are a series of case reports that show profound improvement of symptoms from a MADRS with a mean score of 36 before treatment to 6 following ECT [43]. Given the other medical risks for these immunocompromised patients, it is important to exclude other central nervous system pathology with neuroimaging and laboratory studies prior to considering ECT.
Another neuromodulation therapy being studied for its potential benefit in depressive disorders is transcranial magnetic stimulation (TMS). As a potentially effective therapy for treatment-resistant depressive disorder with fewer cognitive side effects than ECT, it is a promising new technology [44, 45]. There have not been major studies investigating TMS specifically in depressive disorder in PLWHA specifically, but it is an emerging area of interest. Additionally, there may be future uses of TMS for the treatment of neuropathic pain in PLWHA [46].
Ketamine, a dissociative anesthetic, has been demonstrated to be effective in the treatment of MDD and clinically useful in treatment-resistant MDD, including ECT-resistant MDD. While IV ketamine is studied in most trials, oral and intranasal ketamine have demonstrated significant antidepressant effects with good overall tolerability, making it a preferred treatment option , given its lower cost and ability to be administered outside the hospital setting [47]. Unfortunately, data on the efficacy of ketamine in PLWHA with MDD are not yet available in the literature. Future randomized-controlled trials, especially among treatment-resistant MDD in PLWHA, are warranted in this population.
Finally, there have been studies showing that improvements in lifestyle factors may be effective on depression scores as an adjunct to traditional treatment. Aerobic exercise, acupuncture, meditation/mindfulness, and massage therapy all have multiple small sample size studies that both support and refute their ability to augment depression treatment in PLWHA [48]. These interventions have little or no side effects and thus are encouraged in addition to traditional pharmacotherapy or psychotherapy. Please see Chap. 16 for further discussion of psychotherapy and other psychotherapeutic modalities.
Conclusions
A timely and precise diagnostic framing of depressive disorders in HIV-positive patients is essential to provide appropriate management of the two comorbid conditions. Both psychiatrists and specialists in infectious diseases should be aware of the high rates of depression in their HIV-positive patients as well as of the many factors interfering with appropriate management. The study of MDD in HIV provides a specific model of the roles of inflammation and immune system activation in the etiology of depressive disorders in general and not only facilitates improvements in basic science knowledge on the topic but also may identify powerful and reliable tools for the clinician to be used in everyday practice. The evolution of HIV from a life-threatening, acute condition to a manageable, chronic illness presents an imperative for clinicians and persons with HIV to diagnose and treat depressive disorders.
References
Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17:530.
Dubé B, Benton T, Cruess DG, Evans DL. Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci. 2005;30(4):237–46. Erratum in: J Psychiatry Neurosci. 2005;30(5):365.
Rezaei S, Ahmadi S, Rahmati J, Hosseinifard H, Dehnad A, Aryankhesal A, et al. Global prevalence of depression in HIV/AIDS: a systematic review and meta-analysis. BMJ Support Palliat Care. 2019;9(4):401–12.
Owe-Larsson B, Säll L, Salamon E, Allgulander C. HIV infection and psychiatric illness. Afr J Psychiatry. 2009;12(2):115–28.
Rubin LH, Maki PM. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep. 2019;16(1):82–95.
Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167(11):1305–20.
Carrico AW, Riley ED, Johnson MO, Charlebois ED, Neilands TB, Remien RH, et al. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56:146–50.
Global Health Observatory (GHO) data: HIV/AIDS. World Health Organization 2018. https://www.who.int/gho/hiv/en/.
CDC. HIV: statistics overview. https://www.cdc.gov/hiv/statistics/overview/index.html.
Major depression: definitions. National institute of mental HEALTH. https://www.nimh.nih.gov/health/statistics/majordepression.shtml.
Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression-a systematic review of interventions. Psychol Health Med. 2011;16(5):493–527.
Wang T, Fu H, Kaminga AC, Li Z, Guo G, Chen L, Li Q, et al. Prevalence of depression or depressive symptoms among people living with HIV/AIDS in China: a systematic review and meta-analysis. BMC Psychiatry. 2018;18(1):160.
Zhu QY, Huang DS, Lv JD, Guan P, Bai XH. Prevalence of perinatal depression among HIV-positive women: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):330.
Pence BW, O’Donnell JK, Gaynes BN. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS. 2012;26(5):656–8.
Rodkjaer L, Laursen T, Balle N, Sodemann M. Depression in patients with HIV is under-diagnosed: a cross-sectional study in Denmark. HIV Med. 2010;11(1):46–53.
Relf MV, Eisbach S, Okine KN, Ward T. Evidence-based clinical practice guidelines for managing depression in persons living with HIV. JANAC. 2013;24:S15–28.
Endicott J. Measurement of depression in patients with cancer. Cancer. 1984;53(S10):2243–8.
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–14.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15(17):5534–40.
Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:9.
Nasi M, Alboni S, Pinti M, Tascedda F, Benatti C, Benatti S, et al. Successful treatment of HIV-1 infection increases the expression of a novel, short transcript for IL-18 receptor alpha-chain. J Acquir Immune Defic Syndr. 2014;67:254–7.
Brunello N, Alboni S, Capone G, Benatti C, Blom JM, Tascedda F, et al. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21(4):219–25.
van Servellen G, Chang B, Garcia L, Lombardi E. Individual and system level factors associated with treatment nonadherence in human immunodeficiency virus-infected men and women. AIDS Patient Care STDs. 2002;16(6):269–81.
Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA. 2001;285(11):1466–74.
Eshun-Wilson I, Siegfried N, Akena DH, Stein DJ, Obuku EA, Joska JA. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev. 2018;1(1):CD008525.
Yanofski J, Croarkin P. Choosing antidepressants for HIV and AIDS patients: insights on safety and side effects. Psychiatry (Edgmont). 2008;5(5):61–6.
Currier MB, Molina G, Kato M. A prospective trial of sustained-release bupropion for depression in HIV-seropositive and AIDS patients. Psychosomatics. 2003;44(2):120–5.
Freudenreich O, Goforth HW, Cozza KL, Mimiaga MJ, Safren SA, Bachmann G, et al. Psychiatric treatment of persons with HIV/AIDS: an HIV-psychiatry consensus survey of current practices. Psychosomatics. 2010;51(6):480–8.
Cholera R, Pence BW, Bengtson AM, Crane HM, Christopoulos K, Cole SR, et al. Mind the gap: gaps in antidepressant treatment, treatment adjustments, and outcomes among patients in routine HIV care in a multisite U.S. clinical cohort. PLoS One. 2017;12(1):e0166435.
DeSilva KE, Le Flore DB, Marston BJ, Rimland D. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS. 2001;15(10):1281–5.
American Psychiatric Association. Practice guideline for the treatment of patients with HIV/AIDS. Washington DC: American Psychiatric Association; 2000.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. https://aidsinfo-nihgov.lrc1.usuhs.edu/contentfiles/lvguidelines/adultandadolescentgl.pdf (January 28, 2016 version).
Qaseem A, Barry MJ, Kansagara D. for the Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;164:350–9.
Honagodu AR, Krishna M, Sundarachar R, Lepping P. Group psychotherapies for depression in persons with HIV: a systematic review. Indian J Psychiatry. 2013;55(4):323–30.
Safren SA, Bedoya CA, O’Cleirigh C, Biello KB, Pinkston MM, Stein MD, et al. Cognitive behavioural therapy for adherence and depression in patients with HIV: a three-arm randomised controlled trial. Lancet HIV. 2016;3(11):e529–38.
Heckman TG, Heckman BD, Anderson T, Lovejoy TI, Markowitz JC, Shen Y, et al. Tele-interpersonal psychotherapy acutely reduces depressive symptoms in depressed HIV-infected rural persons: a randomized clinical trial. Behav Med. 2017;43(4):285–95.
Nicholson WC, Kempf MC, Moneyham L, Vance DE. The potential role of vagus-nerve stimulation in the treatment of HIV-associated depression: a review of literature. Neuropsychiatr Dis Treat. 2017;13:1677–89.
Schaerf FW, Miller RR, Lipsey JR, McPherson RW. ECT for major depression in four patients infected with human immunodeficiency virus. Am J Psychiatry. 1989;146:782–4.
Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549–60.
Magnezi R, Aminov E, Shmuel D, Dreifuss M, Dannon P. Comparison between neurostimulation techniques repetitive transcranial magnetic stimulation vs electroconvulsive therapy for the treatment of resistant depression: patient preference and cost-effectiveness. Patient Prefer Adherence. 2016;10:1481–7.
Treister R, Lang M, Klein MM, Oaklander AL. Non-invasive transcranial magnetic stimulation (TMS) of the motor cortex for neuropathic pain-at the tipping point? Rambam Maimonides Med J. 2013;4(4):e0023.
Rosenblat JD, Carvalho AF, Li M, Lee Y, Subramanieapillai M, McIntyre RS. Oral ketamine for depression: a systematic review. J Clin Psychiatry. 2019;80(3):18r12475.
Fulk LJ, Kane BE, Phillips KD, Bopp CM, Hand GA. Depression in HIV-infected patients: allopathic, complementary, and alternative treatments. J Psychosom Res. 2004;57:339–51.
Disclosure
The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Multiple-Choice Questions
Multiple-Choice Questions
-
1.
Which ART medications have the CDC recommended for a careful assessment of suicidality?
-
(a)
Efavirenz, zidovudine, and ritonavir
-
(b)
Efavirenz and dolutegravir (correct)
-
(c)
Ritonavir and dolutegravir
-
(d)
Efavirenz and ritonavir
-
(e)
All integrase inhibitors
-
(a)
-
2.
Among the following, which psychometric tool to screen persons with HIV for anxiety and depression may be considered to be more suitable?
-
(a)
Hospital Anxiety and Depression Scale (correct)
-
(b)
Beck Depression Inventory
-
(c)
Hamilton Depression Rating Scale
-
(d)
PHQ-9
-
(e)
Montgomery-Asberg Depression Rating Scale
-
(a)
-
3.
Which of the following are relevant risk factors for depressive disorders among HIV patients?
-
(a)
Male gender, practicing regular physical activity, high CD4 count
-
(b)
Low viral load, male gender, migrant status
-
(c)
Female gender, high viral load, family history (correct)
-
(d)
Heterosexuality, high socioeconomic status, female gender
-
(e)
Neuroticism, low CD4 count, long-term ongoing ART
-
(a)
-
4.
Which of the following mechanisms are considered to be relevant in the pathogenesis of MDD in HIV-positive subjects?
-
(a)
De-sensitization of CD4 and CD8, stimulation of anti-inflammatory cytokines, altered thyroid metabolism
-
(b)
Reduced effectiveness of the gut barrier, activation of inflammatory mediators, direct stimulation of the CNS immune system by HIV (correct)
-
(c)
Hypo-activation of the HPA axis, increased permeability of the gut barrier, release of histamine
-
(d)
Increased effectiveness of the gut barrier, release of anti-inflammatory cytokines, high levels of BDNF mRNA
-
(e)
Low levels of BDNF mRNA, hypo-activation of the HPA axis, altered thyroid metabolism, poor response to antidepressant medications
-
(a)
-
5.
According to the recent meta-analysis by Rezaei and colleagues (2019), what is the prevalence of MDD and other depressive disorders among PLWHA?
-
(a)
55%
-
(b)
20%
-
(c)
15%
-
(d)
30% (correct)
-
(e)
75%
-
(a)
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ferrari, S. et al. (2022). Depressive Disorders. In: Bourgeois, J.A., Cohen, M.A.A., Makurumidze, G. (eds) HIV Psychiatry. Springer, Cham. https://doi.org/10.1007/978-3-030-80665-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-80665-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80664-4
Online ISBN: 978-3-030-80665-1
eBook Packages: MedicineMedicine (R0)