Abstract
This chapter highlights the current evidence for use of neoadjuvant and adjuvant immunotherapy among patients with localized renal cell carcinoma. Here, we discuss clinical patient cases to assist clinicians in the management of patients with renal cell carcinoma and summarize pivotal clinical trials in this field. We begin by outlining completed trials of neoadjuvant therapy, including sunitinib, axitinib, pazopanib, and sorafenib. We then review ongoing trials of neoadjuvant nivolumab and durvalumab with or without tremelimumab. Next, we provide an overview of adjuvant therapies, including sorafenib/sunitinib, pazopanib, sunitinib, axitinib, and pembrolizumab. We then outline currently ongoing trials of adjuvant immunotherapy, including atezolizumab, nivolumab/ipilimumab and nivolumab, and durvalumab/tremelimumab and durvalumab. Though the tumors of some patients treated with neoadjuvant therapies have been successfully downstaged and are thereby amenable to surgical intervention, this has not yet become the standard of care. In the adjuvant setting, despite FDA approval for sunitinib as well as potentially beneficial outcomes in selected cohorts, the use of adjuvant vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) has not become the standard of care or even been commonly used in clinical practice. The recent finding regarding adjuvant pembrolizumab, as well as results of trials currently underway exploring the use of adjuvant or neoadjuvant immunotherapy, is an active area of research, and results are eagerly anticipated.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Survival and outcomes of patients diagnosed with renal cell carcinoma (RCC) vary greatly based on the stage of their disease. Approximately, 65% of patients with RCC are diagnosed with localized disease, 16% with locoregional disease, and 16% with metastatic disease [1]. Patients with localized disease have a significantly better 5-year survival rate of 93% compared to a dismal 12% among those with metastatic disease [1]. Though the frontline treatment for metastatic RCC is systemic therapy—largely based on vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR TKIs), immunotherapy (IO), and combination therapy with VEGFR TKIs and IO—localized and locally advanced RCC are primarily managed surgically with curative intent [2,3,4,5]. For these patients, radical nephrectomy and nephron-sparing surgery (partial nephrectomy) are accepted standard of care options. Surgical approach depends largely on the extent and position of the tumor along with other comorbid conditions (such as if the patient has a solitary kidney). However, many patients relapse either locally at the site of nephrectomy or at distant sites.
Recurrence risk is determined by pathological stage and Fuhrman nuclear grade as well as the patient’s Eastern Cooperative Oncology Group (ECOG) Performance Status (PS). It is very important to identify which patients are at a high risk through different prognostic models and evaluate the role of adjuvant therapy to treat microscopic disease. The goal of neoadjuvant therapy prior to surgery, as discussed below, has been to downstage tumors among patients with a high risk of recurrence to optimize potential surgical options and long-term outcomes. The goal of adjuvant therapy is to decrease recurrence risk following surgical removal of the tumor, and adjuvant therapy represents a potential of great therapeutic benefit, as 30–40% of patients with localized RCC develop metastatic disease after nephrectomy, which confers dismal survival outcomes [6].
Neoadjuvant Therapy
A 71-year-old male with a history of prior right radical nephrectomy for clear cell RCC was found to have a contralateral solid renal mass measuring up to 4.8 cm. The patient underwent a renal biopsy, which was again consistent with clear cell RCC. Workup showed that he did not have metastatic disease, though the patient had mild renal insufficiency, with a creatinine level of 1.4 mg/dL. Because of the size of the mass, there was concern that a partial nephrectomy would pose a high risk of insufficient renal reserve, whether because of the size of surgical margins necessary or the need for completion nephrectomy. As the mass appeared indolent, growing from 2 to 4.8 cm over 60 months, the decision was made to proceed with neoadjuvant treatment with axitinib 5 mg twice daily. On 3-month restaging scans, there was a noted partial response from 4.8 to 3.5 cm, and he received a partial nephrectomy 4 months after the initiation of treatment (Fig. 15.1). The final pathology report was consistent with grade 2 pT1aNxMx clear cell RCC, and his postoperative creatinine level was 1.6 mg/dL. The patient tolerated treatment without significant adverse effects (AEs). This illustrates the potential benefit of neoadjuvant therapy to optimize surgical outcomes and prevent future recurrence.
Localized RCC is primarily managed surgically with either partial or radical nephrectomy, depending on the extent of disease. The goal of neoadjuvant therapy is to optimize surgical resection and potentially downstage the disease to allow for a less extensive surgery, especially among patients with T4 disease. This may make a patient a candidate for nephron-sparing surgery and thereby preserve more of the patient’s renal function. It may also be used as a bridge to surgery among those patients for whom upfront surgical resection is not possible. For example, among patients with large disease burden or those who have RCC invading or extensively abutting adjacent organs, a response to neoadjuvant therapy can facilitate more complex surgical resections. Some also argue that upfront treatment with neoadjuvant therapy, when the burden of micrometastatic disease is at its lowest point, may portend better outcomes, decrease the likelihood of recurrence, and cure the patient [7].
Numerous trials have explored the role of neoadjuvant therapy in improving outcomes prior to surgical resection (Fig. 15.2). The bulk of evidence for neoadjuvant therapy comes from phase 2 trials examining different VEGF TKIs, though there is currently no standard of care. For patients treated with neoadjuvant therapy, it is important to monitor for AEs that may require dose reduction or discontinuation. It is also imperative to closely assess for any signs of disease progression that may change the disease stage or available treatment options. We now focus on examining the pivotal trials that have explored neoadjuvant therapy for patients with localized RCC.
Sunitinib
Neoadjuvant therapy was first explored in a phase 2 trial examining sunitinib for unresectable advanced RCC [8]. Patients were eligible if they had RCC that was not suitable for nephrectomy, and patients with any histological subtype of RCC were eligible. Patients were treated with 50 mg of sunitinib daily for 4 weeks on a 6-week cycle and received a median of two cycles of sunitinib (range, 1–8 cycles). Of the 19 patients enrolled, none had a complete response (0%), three had a partial response (16%), seven had stable disease (37%), and nine had progressive disease (47%). Four patients (21%) underwent nephrectomy at a median 6-month follow-up. The most common AEs were fatigue (74%), dysgeusia (43%), hand-foot syndrome (32%), and diarrhea (31%). The dose of sunitinib was reduced for three patients to 37.5 mg daily because of toxicity and only one patient required discontinuation (5%) because of grade 3 hand-foot syndrome. Of note, this study included patients with both locally advanced and metastatic disease. Ultimately, the tumors of several patients with advanced RCC who received neoadjuvant sunitinib were downstaged, allowing the patient to undergo nephrectomy.
Axitinib
The next trial examining the role of neoadjuvant therapy among patients with locally advanced clear cell RCC was done several years later, in 2014 [9]. Unlike the sunitinib trial, this trial only enrolled patients with locally advanced disease and did not include patients who had metastases. In this phase 2 trial, which was conducted over 2 years from May 2011 to April 2013, patients with clear cell RCC received 12 weeks of 5 mg of axitinib twice daily, with the last dose 36 hours prior to nephrectomy. Of the 24 patients enrolled, 11 had a partial response, 13 had stable disease, and no patients had progressive disease. All patients were able to undergo surgical resection; 19 underwent radical nephrectomy and 5 underwent partial nephrectomy. The most common side effects were hypertension (79%), hoarseness (79%), fatigue (75%), mucositis (71%), hypothyroidism (71%), and hand-foot syndrome (63%). The most common grade 3 or higher AEs were hypertension (42%), transaminitis (8%), and abdominal pain (8%). This study showed that, among patients with advanced RCC, neoadjuvant axitinib was well tolerated and associated with a good response, making nephrectomy feasible.
Pazopanib
Rini and colleagues conducted a phase 2 trial examining the effects of neoadjuvant pazopanib among patients with localized RCC, with the goal of downsizing tumors and optimizing preservation of renal parenchyma [10]. Twenty-five patients with localized clear cell RCC were enrolled and were given 800 mg of pazopanib daily for 8 to 16 weeks prior to surgery. Of the 13 patients who were not able to undergo partial nephrectomy at baseline, 6 were able to undergo a partial nephrectomy after treatment with pazopanib. Neoadjuvant pazopanib helped to downsize some patients’ localized RCCs, allowing for partial nephrectomy among those who otherwise would have required radical nephrectomy.
Sorafenib
The use of neoadjuvant sorafenib among patients with high-risk RCC was explored by Zhang and colleagues in 2015 [11]. Patients were eligible if they had high-risk RCC, defined as (1) grade 2 or higher disease with inferior vena cava (IVC) thrombus, (2) tumor diameter >7 cm, (3) multiple tumors within a patient undergoing nephron-sparing surgery, (4) a tumor within a functional solitary kidney that was unsuitable to undergo nephron-sparing surgery, or (5) widespread metastatic RCC. This phase 2 trial took place over 6 years from April 2007 to October 2013. Of a total of 37 patients who received neoadjuvant sorafenib, 18 (48%) patients successfully received surgery and their characteristics were examined. The dose of sorafenib was 400 mg twice daily, and patients were treated for an average of 96 days (range, 30–278 days). Sorafenib was discontinued an average of 12 days prior to surgery (range, 7–30 days). The overall response rate (ORR) among patients who received neoadjuvant sorafenib was 94%; 4 patients had a partial response (22%) and 13 patients had stable disease (72%). The average tumor size decreased from 7.8 to 6.2 cm. Of note, there were also 5 patients with IVC tumor thrombus, and 4 of those patients had decreased tumor thrombus burden following neoadjuvant sorafenib. A total of 11 patients underwent radical nephrectomy, 5 patients underwent radical nephrectomy and IVC thromboembolectomy, and 2 patients underwent partial nephrectomy. This trial showed that neoadjuvant therapy can decrease primary tumor size, resulting in improved surgical outcomes. It may also reduce tumor thrombi and improve outcomes with thromboembolectomy.
Utility of Neoadjuvant Therapy
Phase 2 trials examining sunitinib, axitinib, pazopanib, and sorafenib among patients who were initially ineligible for nephrectomy have shown similar results regarding the reduction of tumor burden. This translates to expanded surgical options, including the potential of partial nephrectomy rather than radical nephrectomy. Despite these outcomes, however, the mainstay of treatment remains surgical resection, and neoadjuvant therapy with VEGFR TKIs has not been adopted as a standard practice. The role of IO in the neoadjuvant setting is currently being examined and remains under investigation. Compared to adjuvant IO trials, which are all phase 3, trials focusing on neoadjuvant IO are mainly in earlier phases of clinical investigation.
RCC has a predilection for vascular invasion, often seen as an IVC thrombus. This can be seen in 10–25% of RCC cases [12]. The effect of neoadjuvant VEGF TKIs on IVC thrombi has been mixed [13]. Neoadjuvant sunitinib has shown potential benefit [14, 15], whereas neoadjuvant sorafenib has shown mixed results [11, 16]. There has been case-level evidence of neoadjuvant nivolumab/ipilimumab causing a complete response for patients with IVC thrombus [17].
Neoadjuvant Immunotherapy
A 60-year-old male presented with a large right renal mass, IVC thrombus, and a large retroperitoneal lymph node with anterior displacement of the IVC. The patient underwent confirmatory biopsy of the retroperitoneal node, which was consistent with clear cell RCC with focal rhabdoid features. Given the large renal mass, lymphadenopathy, and IVC thrombus, the decision was made to proceed with nivolumab (3 mg/kg) plus ipilimumab (1 mg/kg) every 3 weeks for 4 doses, followed by maintenance nivolumab of 240 mg every 2 weeks or 480 mg every 4 weeks preoperatively. Restaging scans after 4 cycles of combined nivolumab/ipilimumab demonstrated a 3 mm decrease in the primary renal mass and a significant decrease in the retroperitoneal lymph node from 9.5 to 6.7 cm (Fig. 15.3). The decision was then made to proceed with right radical nephrectomy, IVC thrombectomy, right adrenalectomy, and retroperitoneal lymph node dissection. The final pathology report showed grade 4 clear cell RCC with focal rhabdoid features and extensive necrosis. The retroperitoneal node and thrombus were consistent with the primary tumor. This illustrates the potential benefit of IO to optimize surgical outcomes and prevent future recurrence.
Most active trials exploring neoadjuvant IO involve nivolumab (Table 15.1 and Fig. 15.2). The first phase 1 trial opened in February 2016 and is examining the effects of neoadjuvant nivolumab on nonmetastatic high-risk clear cell RCC. Patients were eligible if they had nonmetastatic high-risk clear cell RCC (T2a-T4N[any]M0 or T[any]N1M0), ECOG PS of 0 or 1, and adequate organ and marrow function and planned to have either a partial or radical nephrectomy. Seventeen patients were enrolled and received 3 mg/kg of nivolumab every 2 weeks for a total of 3 cycles prior to nephrectomy. The primary outcome was safety, with secondary outcomes including ORR, quality of life, metastasis-free survival, and overall survival (OS). This study finished enrollment in June 2020 and results are eagerly anticipated (NCT02575222).
A subsequent phase 1 study, which began enrolling patients in May 2016, is examining preoperative nivolumab among high-risk patients with RCC and includes patients with metastatic disease undergoing planned cytoreductive nephrectomy or metastasectomy. Patients are eligible if they have confirmed clear cell RCC; ECOG PS of 0 or 1; and adequate hematological, kidney, and liver function. This study is still actively recruiting (29 patients thus far), with an estimated accrual completion date of April 2021. Patients will receive nivolumab a total of 4 times on an every-other-week cycle at 8 weeks, 6 weeks, 4 weeks, and 2 weeks prior to surgery. The primary outcome is to determine if patients can receive at least 3 doses of nivolumab and undergo surgical resection without delay. Secondary outcomes include toxicity, ORR, and recurrence-free survival (NCT02595918).
The PROSPER trial (Perioperative Nivolumab vs. Observation in Patients with Renal Cell Carcinoma Undergoing Nephrectomy) is a phase 3 study comparing perioperative nivolumab to observation. The study began enrolling patients in February 2017 and is currently still recruiting, with an estimated enrollment goal of 805 patients. Patients randomized to the treatment arm will receive preoperative nivolumab every 2 weeks for 2 cycles, followed by partial or radical nephrectomy within 1–4 weeks. They then receive nivolumab every 2 weeks for 6 cycles, then monthly for 6 cycles or until toxicity or disease progression. The primary outcome in this trial is event-free survival. OS and toxicity are secondary outcomes. The estimated completion is November 2023 (NCT03055013).
Durvalumab with or without tremelimumab is also currently being examined as neoadjuvant therapy for locally advanced RCC. A phase 1 trial opened in December 2016 and has finished accruing. Twenty-nine patients have been randomized to multiple cohorts, including durvalumab monotherapy, durvalumab + tremelimumab combination therapy as a neoadjuvant approach, and durvalumab + tremelimumab combination therapy as an adjuvant therapy within 4–6 weeks postnephrectomy. The primary outcome is toxicity, with secondary outcomes exploring postsurgical complications and ORR. This trial is estimated to be completed in November 2020 (NCT02762006).
Adjuvant Therapy
A 57-year-old male was diagnosed with a 10-cm left renal mass with renal vein involvement and associated para-aortic adenopathy during a workup for abdominal pain. He underwent upfront surgical management of his disease via an open left radical nephrectomy and adrenalectomy. His final pathology report was consistent with clear cell pT3NxMx RCC. Because of his high-risk pathology, he was then evaluated by the medical oncology team for further treatment, and after discussion, the decision was made to proceed with adjuvant sunitinib therapy. He received sunitinib for a total of 1 year and experienced grade 2 neutropenia and grade 1 hand-foot syndrome. He has since been followed up with and demonstrates no evidence of disease 1 year following treatment, with no long-term treatment-related adverse effects (TRAEs). This illustrates the potential benefit of adjuvant therapy to reduce the risk of recurrence among otherwise high-risk patients receiving upfront surgical management.
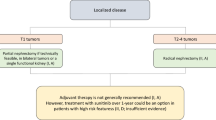
The role of adjuvant therapy has been the subject of numerous published and ongoing trials (Fig. 15.4) and remains controversial. Among patients with stage I disease, nephrectomy—partial or radical—is the mainstay of treatment, with active surveillance a consideration for select patients. For patients with stage II disease, nephrectomy followed by surveillance is preferred. However, for patients with stage III disease, though nephrectomy remains the standard of care, multiple clinical trials have explored the role of adjuvant therapy compared to standard surveillance. Current challenges in this field include heterogenous inclusion criteria in clinical trials as well as a lack of clinically apparent radiographic disease to assess response outcomes.
ECOG-ACRIN E2805 ASSURE Trial
The first trial to examine the role of adjuvant therapy for localized RCC was the ECOG-ACRIN E2805 ASSURE (adjuvant sunitinib or sorafenib for high-risk nonmetastatic renal cell carcinoma) trial [18]. Investigators examined whether VEGFR TKIs sorafenib or sunitinib conferred a survival advantage when used in the adjuvant setting for locoregional disease. This study was initiated based on prior data showing improved progression-free survival (PFS) among patients with metastatic RCC who were treated with sorafenib or sunitinib.
This study was conducted over a 4-year period from April 2006 to September 2010, and 1943 patients were enrolled from 226 centers in the USA and Canada. Eligibility criteria included high-risk clear cell or non–clear cell RCC with complete surgical resection within 12 weeks of trial enrollment. High risk was defined as having pT1b G3-4 N0 M0 to T(any) G(any) N+ (fully resected) M0 disease. Patients also needed to be treatment-naive; have good cardiac function (defined as left ventricular ejection fraction >50%); have an ECOG PS of 0–1; and have intact liver, kidney (CrCl > 30 mL/min), and hematological function. Exclusion criteria included uncontrolled hypertension, thyroid disease, or HIV infection.
The 1943 patients were randomized in a double-blind fashion to 1 of 3 groups: (a) 50 mg of sunitinib daily for 4 weeks of a 6-week cycle, (b) 400 mg of sorafenib twice daily, or (c) placebo. Because of toxicity, 3 years into the study (May 2009), the sunitinib starting dose was decreased to 37.5 mg daily for 4 weeks of a 6-week treatment cycle, and, if the medication was well tolerated after the first or second cycle, it was increased to the 50 mg dose. Patients were treated for a total of 54 weeks.
The primary endpoint of the study was disease-free survival (DFS), with secondary endpoints including OS and toxic effects. Median DFS was 70 months (5.8 years) for sunitinib, 73.4 months (6.1 years) for sorafenib, and 79.6 months (6.6 years) for placebo. Statistically, the DFS did not differ significantly between these groups. When sunitinib was compared to placebo, the hazard ratio (HR) was 1.02 (95% CI, 0.85–1.23; P = 0.804), and when sorafenib was compared to placebo, the HR was 0.97 (95% CI, 0.80–1.17; P = 0.718). The 5-year OS also did not differ significantly between groups, as it was 77.9% in the sunitinib group (95% CI, 74.1–81.9), 80.5% in the sorafenib group (95% CI, 76.8–84.2), and 80.3% in the placebo group (95% CI, 76.7–84.0).
Patients experienced many side effects in this trial, with a large cohort of patients withdrawing from the study because of treatment toxicity. The most common grade 3 or higher side effects in the sunitinib group were hypertension (17%), fatigue (17%), hand-foot syndrome (15%), and diarrhea (10%). For the sorafenib group, the most common grade 3 or higher side effects were hand-foot syndrome (33%), hypertension (16%), rash (15%), and diarrhea (9%). Prior to the dose decrease because of toxicity in May 2009, 3 years into the trial, 44% of patients on sunitinib, 45% of patients on sorafenib, and 11% of patients in the placebo arm withdrew from the study because of TRAEs. After the dose reduction, fewer patients withdrew due to TRAEs—34% of the sunitinib cohort, 30% of the sorafenib cohort, and 10% of the placebo arm. The number of patients who discontinued the trial because of TRAEs was significantly lower after dose reduction in the sunitinib (P = 0.014) and sorafenib groups (P = 0.0001) but not in the placebo group (P = 0.696).
The findings of the ECOG-ACRIN E2805 ASSURE trial suggest that there is no benefit (DFS or OS) from adjuvant sorafenib or sunitinib compared to placebo and that treatment with an adjuvant TKI can cause significant toxicity without additional benefit.
S-TRAC Trial
The next trial to examine the role of adjuvant therapy for localized RCC was the S-TRAC (Sunitinib as Adjuvant Treatment for Patients at High Risk of Recurrence of Renal Cell Carcinoma Following Nephrectomy) trial [19]. The aim of the trial was to determine if sunitinib conferred a survival advantage in the adjuvant setting after nephrectomy.
The trial was conducted over a 3.5-year period from September 2007 to April 2011, overlapping with the aforementioned ASSURE trial. A total of 615 patients at 99 centers across 21 countries were enrolled. Eligible patients were required to have locoregional RCC, defined as stage III disease or higher or regional lymph node metastases; successfully undergone nephrectomy with the absence of residual disease; and enrolled on the trial between 3 and 12 weeks after surgery. Patients also needed to have clear cell RCC, compared to ASSURE, in which both clear cell and non–clear cell subtypes were allowed. Other notable inclusion criteria included an ECOG PS of 0, 1, or 2, compared to ASSURE, which enrolled only patients with an ECOG PS of 0 or 1. Exclusion criteria included metastatic disease, histologically undifferentiated tumors, a second malignancy diagnosed within 5 years, cardiovascular disease/major event in the past 6 months, and uncontrolled hypertension (defined as blood pressure >150/100 mmHg).
The 615 patients were randomized in a 1:1 ratio on the basis of ECOG score and country of residence into either a group receiving sunitinib 50 mg daily or placebo daily for 4 weeks of a 6-week cycle. Patients were treated for a total of 1 year. The primary endpoint of the study was DFS. Secondary endpoints included OS and safety assessments. The median DFS in the sunitinib group was 6.8 years (95% CI, 5.8-not reached) compared to 5.6 years in the placebo group (95% CI, 3.8–6.6). The HR was 0.76 (95% CI, 0.59–0.98; P = 0.03). Of note, there was an improved DFS with sunitinib compared to placebo according to both the independent central review group (6.8 years vs 5.6 years) and the investigators’ review (6.5 years vs 4.5 years), but this improvement was only statistically significant according to the independent central review group, not the investigators’ review group (HR, 0.81 [95% CI, 0.64–1.02]; P = 0.08). OS data was not mature at the time of data cutoff, with an HR of 1.01 (95% CI, 0.72–1.44; P = 0.94).
AEs occurred among 99.7% of patients in the sunitinib group and 88.5% of patients in the placebo group. The most common grade 3 or higher AEs in the sunitinib group were hand-foot syndrome (16%), neutropenia (8.5%), hypertension (7.8%), thrombocytopenia (6.2%), fatigue (4.9%), mucositis (4.6%), and diarrhea (3.9%).
The findings of the S-TRAC trial showed a DFS benefit for adjuvant sunitinib following nephrectomy compared to placebo. This was not seen in the ASSURE trial, which compared adjuvant sunitinib, sorafenib, and placebo. The authors of the S-TRAC trial posited numerous reasons for this discrepancy in outcomes, including different trial methods and doses of sunitinib. In S-TRAC, all patients got sunitinib at a 50 mg dose, compared to ASSURE, in which patients in the sunitinib arm initially received the 50 mg dose and then TRAEs caused a dose reduction to an initial dose of 37.5 mg among patients who enrolled after the third year of the study. The ASSURE trial also enrolled patients who had non–clear cell histology (21% of patients), compared to the S-TRAC trial, which only enrolled patients with clear cell RCC.
Based on the results of the S-TRAC trial, the FDA approved sunitinib in the adjuvant setting in November 2017 [20]. It is important to note that, in clinical practice, sunitinib is not commonly used for multiple reasons, which are expanded upon later in the chapter “Impact of VEGFR TKIs as adjuvant therapy.”
PROTECT Trial
The PROTECT (Pazopanib as Adjuvant Therapy in Localized/Locally Advanced RCC After Nephrectomy) trial, conducted after the ASSURE and S-TRAC trials, assessed the role of pazopanib in the adjuvant setting, given its efficacy as a first-line treatment [21]. The trial was conducted over a nearly 3-year span from December 2010 to September 2013. It enrolled 1538 patients at 263 centers across 26 countries. Patients were eligible if they had nonmetastatic clear cell or predominantly clear cell RCC histology that had been resected, along with Karnofsky PS > 80 and “adequate organ function.”
Patients were originally randomized to 800 mg of pazopanib daily or placebo, but because of TRAEs and patients withdrawing from the study, the starting pazopanib dose was decreased to 600 mg daily. If patients tolerated the 600 mg dose, then it could be escalated to 800 mg after 8–12 weeks. A total of 403 patients were enrolled and randomized when the starting dose was 800 mg (198 patients were randomized to pazopanib and 205 to placebo), and 1135 patients were enrolled and randomized on the subsequent lower starting dose of 600 mg (571 patients were randomized to pazopanib and 564 to placebo). Patients received treatment for a total of 1 year.
The primary endpoint of the study was DFS. Initially, the primary outcome was DFS for the cohort receiving 800 mg of pazopanib, but after the dose reduction, the primary outcome was changed to the DFS of the 600 mg cohort. The secondary endpoints were OS, DFS for the 800 mg cohort, DFS at yearly timepoints, safety, and patient-reported outcomes/quality of life. With respect to the primary endpoint (DFS for the 600 mg cohort), the HR was 0.86 (95% CI, 0.70–1.06; P = 0.16) but was not statistically significant. The secondary outcome of DFS for the 800 mg cohort showed a benefit from pazopanib (HR 0.69 [95% CI, 0.51–0.94]; P = 0.02). When both subgroups (600 mg and 800 mg) were combined, the HR was 0.80 (95% CI, 0.68–0.95; P = 0.01). There was not an OS benefit in any of the subgroups, as the OS in the 600 mg group had an HR of 0.79 (95% CI, 0.57–1.09; P = 0.16), the OS in the 800 mg group had an HR of 0.89 (95% CI, 0.54–1.46; P = 0.65), and the OS of the combined groups had an HR of 0.82 (95% CI, 0.62–1.07; P = 0.15).
A total of 98% of patients in the pazopanib group and 90% of patients in the placebo group experienced at least one AE. In terms of the different pazopanib dosage groups, 51% of patients in the 600 mg cohort and 60% of patients in the 800 mg cohort had dose reductions during treatment. Thirty-five percent of patients in the 600 mg cohort discontinued the drug because of TRAEs, whereas 39% of patients in the 800 mg cohort discontinued pazopanib because of TRAEs. Of note, 21% of patients in the 600 mg of pazopanib cohort had a dose escalation by week 12 because of tolerability. The most common AEs were diarrhea (64%), hypertension (52%), hair color changes (41%), nausea (40%), and fatigue (39%). The most common grade 3 and above AEs were hypertension (25%), increased alanine aminotransferase (16%), diarrhea (7%), and increased aspartate aminotransferase (6%).
The PROTECT trial showed that there was not an increased DFS in the cohort of patients who received 600 mg of pazopanib; however, its secondary endpoint of improved DFS for the 800 mg cohort was met. It is important to consider that the cohort that received the 800 mg dose was roughly one-third the size of the 600 mg cohort, largely because of toxicity and TRAEs.
ATLAS Trial
The most recent phase 3, randomized trial to explore the effect of adjuvant TKI therapy after nephrectomy was the ATLAS (Adjuvant Axitinib Therapy of Renal Cell Cancer in High Risk Patients) trial. This study evaluated the effect of axitinib among patients with locoregional RCC with high risk of recurrence after nephrectomy [22]. Like the previous trials (ASSURE, S-TRAC, and PROTECT), investigators of this study questioned whether the benefit of VEGFR TKIs in the metastatic setting could be extended into the adjuvant setting following nephrectomy.
The trial enrolled 724 patients across 137 multinational centers in 8 countries. Patients were eligible if they had RCC following nephrectomy (with greater than a 50% clear cell component) without metastatic disease, had not received any prior systemic treatment, and had an ECOG PS of 0–1. Patients were randomized 1:1 to receive axitinib 5 mg twice daily or placebo and were treated for a minimum of 1 year and up to 3 years based on individualized decision-making by the patient and the site investigator. Thirty-one percent of patients on the axitinib arm were treated for less than 1 year, 27% of patients were treated for 1–2 years, 23% of patients were treated for 2–3 years, and 20% of patients completed 3 years of treatment.
The primary endpoint was DFS and secondary endpoints were OS and safety. The trial was stopped early because of futility at a preplanned interim analysis because 203 DFS events were reached. The HR was 0.87 (95% CI, 0.66–1.147; P = 0.321). Prespecified subgroup analyses of high-risk and low-risk recurrence showed a potential difference in HR depending on analyses from an independent review committee (IRC) vs investigators assessment. In the high-risk patient subgroup, IRC review showed an HR of 0.735 (95% CI, 0.525–1.028; P = 0.07) and investigator review showed an HR of 0.641 (95% CI, 0.468–0.879; P = 0.005). In the low-risk patient subgroup, there was no statistically significant difference between axitinib and placebo, as the IRC review showed an HR of 1.016 (95% CI, 0.62–1.666; P = 0.948) and the investigator review showed an HR of 1.048 (95% CI, 0.654–1.681; P = 0.845).
The most common side effects with axitinib were hypertension (64%), diarrhea (47%), dysphonia (42%), and hand-foot syndrome (32%). The most common TRAEs with axitinib were hypertension (60%) and dysphonia (38%). Due to AEs, the dose of axitinib was reduced for 56% of patients, interrupted for 51% of patients, and discontinued for 23% of patients. The most common AEs requiring discontinuation of axitinib were hypertension (4%), proteinuria (3%), and hand-foot syndrome (2%).
ATLAS was stopped early for futility at a preset interim analysis and did not meet its primary endpoint for improved DFS with axitinib. However, prespecified subset analyses showed potential improvement in DFS in the high-risk patient cohort, although this effect is questionable as it was statistically significant per the investigators’ review but not according to IRC review.
Impact of VEGFR TKIs as Adjuvant Therapy
To date, 4 trials have explored the impact of VEGFR TKIs as adjuvant therapies following nephrectomy for localized/locoregional RCC. The ASSURE trial found no benefit for either adjuvant sunitinib or adjuvant sorafenib. The S-TRAC trial found a DFS benefit for adjuvant sunitinib following nephrectomy. The PROTECT trial did not meet its primary endpoint of improved DFS for patients treated with 600 mg of adjuvant pazopanib but did show improvement in DFS at the 800 mg dose. Lastly, the ATLAS trial was stopped early because of futility and did not meet its primary endpoint of improved DFS with axitinib, but preplanned subset analyses showed a potential benefit in the high-risk patient cohort.
There are several limitations to comparing these trials. ASSURE, S-TRAC, PROTECT, and ATLAS varied greatly in their inclusion criteria as they all had slightly different definitions of high-risk disease and guidelines for what stages of RCC were included (local vs locoregional). Baseline histology also differed between studies—S-TRAC included pure clear cell RCC, PROTECT included predominantly clear cell RCC, ATLAS included RCCs with over 50% clear cell component, and ASSURE allowed all RCC histologies (21% of patients had non–clear cell histology). Patients enrolled in ASSURE and S-TRAC were required to start adjuvant therapy within 12 weeks of surgery, whereas the PROTECT and ATLAS trials did not mandate this. Length of treatment also differed between trials—1 year of sunitinib in S-TRAC, 1 year of pazopanib in PROTECT, 54 weeks of either sunitinib or sorafenib in ASSURE, and up to 3 years of axitinib in ATLAS.
Currently, only sunitinib is FDA approved as adjuvant therapy following nephrectomy for patients with stage III RCC [20]. However, its use is controversial, and it is a category 3 recommendation in the NCCN guidelines [5]. The use of sorafenib, axitinib, or pazopanib is not standard practice, despite some potential marginal benefit seen in certain subgroups. A meta-analysis by Sun and colleagues examining the ASSURE, S-TRAC, and PROTECT trials (but not ATLAS) found that their pooled analyses did not show statistically significant benefit in DFS (HR, 0.92 [95% CI, 0.82–1.03]; P = 0.16) or OS (HR 0.98 [95% CI, 0.84–1.15]; P = 0.84) from adjuvant VEGFR TKIs but did show higher risk of grade 3 and grade 4 AEs (OR, 5.89 [95% CI, 4.85–7.15]; P < 0.001). They found in exploratory analyses that patients who were initiated on full-dose VEGFR TKIs had improved DFS with adjuvant therapy (HR, 0.83 [95% CI, 0.73–0.95]; P = 0.005). Based on the results of the 4 trials, the questionable benefit of adjuvant therapy in the setting of numerous side effects means that many oncologists do not view this as a good treatment option and it is not routinely used in standard practice.
KEYNOTE-564
KEYNOTE-564 was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial which explored the use of adjuvant pembrolizumab following nephrectomy [23]. It was the first published investigation exploring the use of adjuvant immunotherapy following nephrectomy (unlike the ASSURE, S-TRAC, PROTECT, and ATLAS trials, which all examined the effect of adjuvant tyrosine kinase inhibitors).
The trial enrolled 994 patients between June 2017 and September 2019, and at the prespecified interim analysis in December 2020, the median follow-up was 24.1 months (range 14.9–41.5 months). Patients were eligible if they had biopsy-proven clear cell RCC (did allow for a sarcomatoid component), intermediate/high risk disease (or M1 metastatic disease with no evidence of disease following nephrectomy and metastasectomy of soft tissue metastases), had not received prior systemic therapy, were less than 12 weeks from nephrectomy, and had ECOG PS 0–1.
Patients were randomized to pembrolizumab 200 mg intravenously every 3 weeks for up to 17 cycles or placebo. The primary outcome was DFS (assessed by the investigator), and secondary outcomes included OS, safety, and tolerability. DFS was not reached in either arm (HR 0.68, 95% CI 0.53–0.87), p = 0.0010, with an estimated 24-month DFS of 77.3% with pembrolizumab compared to 68.1% with placebo. The median OS was also not reached in either arm (HR 0.54, 95% CI 0.30–0.96), p = 0.0164, with an estimated 24-month OS of 96.6% with pembrolizumab vs 93.5% with placebo.
Treatment-related adverse effects were reported in 79.1% of the pembrolizumab population and 53.4% of those treated with placebo. Grade 3–5 AEs were seen in 18.9% of patients treated with pembrolizumab but only 1.2% of those treated with placebo. The most common TRAEs were fatigue (20.3% pembrolizumab, 14.3% placebo), pruritus (18.6% pembrolizumab, 11.5% placebo), diarrhea (15.8% pembrolizumab, 10.3% placebo), and rash (15.0% pembrolizumab, 7.3% placebo). The most common immune-mediated AEs were hypothyroidism (21.1% pembrolizumab, 3.6% placebo), hyperthyroidism (11.9% pembrolizumab, 0.2% placebo), and pneumonitis (2.3% pembrolizumab, 1.0% placebo).
The findings of KEYNOTE-564 are important as they represent a potential new therapeutic avenue in treating high-risk localized RCC following nephrectomy using immunotherapy, an area that is currently lacking beneficial options. While the data is still in the process of maturing, statistically significant benefits in DFS and OS are promising, and it will be of utmost importance to continue to follow future updates from this trial. To determine the effect of pembrolizumab on this patient population, this data, as well as data from other clinical trials exploring adjuvant immunotherapy following nephrectomy (see below), will be of utmost importance to determine if there is a potential role for immunotherapy in this setting, and potentially improve patient outcomes.
Trials Exploring Adjuvant Immunotherapy Following Nephrectomy
There are now a variety of ongoing clinical trials exploring the benefit of adjuvant IO after nephrectomy among patients with localized or locoregional RCC (Table 15.2 and Fig. 15.4). IMmotion010 is a phase 3, multicenter, randomized, placebo-controlled, double-blind study using atezolizumab. Patients randomized to the treatment arm receive 1200 mg of atezolizumab intravenously every 3 weeks for 16 cycles or 1 year, whichever occurs first. The primary outcome is DFS, which will be assessed by an IRC. Enrollment began in January 2017, and 778 patients have been accrued. It is no longer recruiting and is estimated to be completed in February 2024 (NCT03024996).
CheckMate914 is a phase 3, randomized, double-blind study comparing nivolumab monotherapy, nivolumab and ipilimumab combination therapy, and placebo among high-risk patients with localized RCC after nephrectomy. The primary outcome is DFS, which will be assessed by an independent central review. It began enrolling in July 2017 and has an estimated enrollment of 1600 patients. It is currently recruiting and is estimated to finish in July 2024 (NCT03138512).
RAMPART (Renal Adjuvant Multiple Arm Randomized Trial) is a phase 3, randomized, open-label trial examining adjuvant durvalumab, durvalumab and tremelimumab combination, and placebo among patients with RCC following primary resection. Durvalumab is given intravenously in 1500 mg doses every 4 weeks for a maximum of 13 cycles (1 year), and tremelimumab is given intravenously in 75 mg doses every 4 weeks for 2 total cycles. The primary outcomes are DFS and OS. It began enrolling in July 2018 and has an estimated enrollment of 1750 patients. It is currently recruiting and is estimated to finish in December 2034 (NCT03288532).
It is important to note the different inclusion criteria with respect to histological subtypes of RCC. The first two trials discussed, NCT03024996 examining atezolizumab, and NCT03138512 examining nivolumab and nivolumab with ipilimumab require patients to have clear cell RCC but allow for a sarcomatoid component. NCT03288532, on the other hand, which is examining durvalumab and durvalumab with tremelimumab, allows for variant histologies of all RCC cell types, with the exception of collecting duct, pure oncocytoma, medullary, and transitional cell cancer.
Conclusion
The current treatment paradigms for locally advanced RCC are nearly entirely based on surgical management followed by surveillance with serial imaging. More focus should be placed on identifying patients who are at the highest risk of disease recurrence and identifying molecular and radiomic markers of disease response to therapies. Numerous clinical trials have explored the role of neoadjuvant and adjuvant therapies among this patient population, but they have not been practice-changing or incorporated into the standard of care (with the possible exception of adjuvant pembrolizumab). Neoadjuvant trials exploring the roles of axitinib, pazopanib, sorafenib, and sunitinib showed that neoadjuvant therapy with these TKIs can lead to downstaging, allowing patients to undergo nephrectomy when they would not have been able to without neoadjuvant therapy or even allowing some patients to undergo partial nephrectomy rather than a total nephrectomy. These studies, however, have numerous limitations, including small sample sizes, different study endpoints, and a lack of data with single-agent IO in the neoadjuvant setting at the current time. Adjuvant trials, on the other hand, examining the same 4 TKIs—sunitinib, sorafenib, pazopanib, and axitinib—have shown mixed results, with none yet impacting the standard of care of postnephrectomy surveillance thus far. The S-TRAC trial showed a DFS benefit from adjuvant sunitinib, but this was not shown in the ASSURE trial. The PROTECT trial showed a potential benefit of adjuvant pazopanib as a secondary outcome at a higher dose. Lastly, the ATLAS trial showed a potential benefit of adjuvant axitinib in a high-risk patient cohort. Optimal selection of high-risk patients who would benefit from adjuvant therapy, including those who can tolerate higher doses, should be a future direction of examination.
Overall, though neither neoadjuvant therapy nor adjuvant therapy is currently the standard of care, there is evidence that neoadjuvant therapy can lead to preoperative downstaging and improved surgical outcomes, and adjuvant therapy for a select patient subset may also improve outcomes. It is important to note that all evidence to date has been with VEGFR TKIs (with the exception of KEYNOTE-564 exploring adjuvant pembrolizumab), and the multitude of open and currently accruing clinical trials are exploring the utility of neoadjuvant or adjuvant IOs. Pending the results of these ongoing clinical trials, the incorporation of IOs may prove to be useful for this patient population (as in the metastatic setting), and future clinical trials incorporating both IO and a VEGF TKI as a combination therapy may be a potential avenue to explore as well. It will be important to monitor the treatment landscape of these trials, as, if patients treated with combination therapies including IOs in the neoadjuvant setting have recurrence of disease, this will affect potential subsequent treatment options as well as monitoring for and exploring patterns of resistance.
References
Surveillance, Epidemiology, and End Results (SEER) program populations (1969–2018) (www.seer.cancer.gov/), National Cancer Institute, DCCPS, Surveillance Research Program. Cancer Stat Facts: kidney and renal pelvis cancer.
Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370–85.
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–15.
Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–27.
National Comprehensive Cancer Network. Kidney cancer. Version 1.2021. https://www.nccn.org/professionals/physician_gls/pdf/kidney_blocks.pdf.
Abara E, Chivulescu I, Clerk N, Cano P, Goth A. Recurrent renal cell cancer: 10 years or more after nephrectomy. Can Urol Assoc J. 2010;4(2):E45–9.
Grivas NK. Neoadjuvant targeted therapy for advanced renal cell carcinoma: where do we stand. Urol Ann. 2019;11(1):115–6.
Thomas AA, Rini BI, Lane BR, Garcia J, Dreicer R, Klein EA, et al. Response of the primary tumor to neoadjuvant sunitinib in patients with advanced renal cell carcinoma. J Urol. 2009;181(2):518–23; discussion 523.
Karam JA, Devine CE, Urbauer DL, Lozano M, Maity T, Ahrar K, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol. 2014;66(5):874–80.
Rini BI, Plimack ER, Takagi T, Elson P, Wood LS, Dreicer R, et al. A phase II study of pazopanib in patients with localized renal cell carcinoma to optimize preservation of renal parenchyma. J Urol. 2015;194(2):297–303.
Zhang Y, Li Y, Deng J, Ji Z, Yu H, Li H. Sorafenib neoadjuvant therapy in the treatment of high risk renal cell carcinoma. PLoS One. 2015;10(2):e0115896.
Psutka SP, Leibovich BC. Management of inferior vena cava tumor thrombus in locally advanced renal cell carcinoma. Ther Adv Urol. 2015;7(4):216–29.
Berquist SW, Yim K, Ryan ST, Patel SH, Eldefrawy A, Cotta BH, et al. Systemic therapy in the management of localized and locally advanced renal cell carcinoma: current state and future perspectives. Int J Urol. 2019;26(5):532–42.
Cost NG, Delacroix SE, Sleeper JP, Smith PJ, Youssef RF, Chapin BF, et al. The impact of targeted molecular therapies on the level of renal cell carcinoma vena caval tumor thrombus. Eur Urol. 2011;59(6):912–8.
Field CA, Cotta BH, Jimenez J, Lane BR, Yim K, Lee HJ, et al. Neoadjuvant sunitinib decreases inferior vena caval thrombus size and is associated with improved oncologic outcomes: a multicenter comparative analysis. Clin Genitourin Cancer. 2019;17(3):e505–12.
Bigot P, Fardoun T, Bernhard JC, Xylinas E, Berger J, Rouprêt M, et al. Neoadjuvant targeted molecular therapies in patients undergoing nephrectomy and inferior vena cava thrombectomy: is it useful. World J Urol. 2014;32(1):109–14.
Labbate C, Hatogai K, Werntz R, Stadler WM, Steinberg GD, Eggener S, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer. 2019;7(1):66.
Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–16.
Ravaud A, Motzer RJ, Pandha HS, George DJ, Pantuck AJ, Patel A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–54.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. FDA expands approval of Sutent to reduce the risk of kidney cancer returning. 2017 11. https://www.fda.gov/news-events/press-announcements/fda-expands-approval-sutent-reduce-risk-kidney-cancer-returning.
Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol. 2017;35(35):3916–23.
Gross-Goupil M, Kwon TG, Eto M, Ye D, Miyake H, Seo SI, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol. 2018;29(12):2371–8.
Choueiri TK, Tomczak P, Park SH, Venguopal B, Ferguson T, Chang Y, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385(8):683–94.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Skelton, W.P., Dahmen, A., Chatwal, M., Jain, R.K., Chahoud, J., Spiess, P.E. (2022). Clinical Cases Debate: Neoadjuvant Versus Adjuvant Immunotherapy in Localized Renal Cell Carcinoma (RCC). In: Necchi, A., Spiess, P.E. (eds) Neoadjuvant Immunotherapy Treatment of Localized Genitourinary Cancers. Springer, Cham. https://doi.org/10.1007/978-3-030-80546-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-80546-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80545-6
Online ISBN: 978-3-030-80546-3
eBook Packages: MedicineMedicine (R0)