Abstract
Infections have been the greatest challenge to the advancement of human race. With the advent of antibiotic therapy, successful treatment strategy was installed against infections. But, due to improper use and misuse of antibiotics along with the evolutionary advantage gained by microorganisms against antibiotics, resistant strains evolved that made the action of many antibiotics futile. Scientific world was after developing newer, more powerful antibiotics to counter the resistant strains, but later realised to change their strategy to adopt methods with the same set of antibiotic armamentaria used in a different way to overcome the resistance barrier created by the microbes. For implementing this strategy, nanoscience and technology came handy. This chapter describes the historical developments in the field of nanoscience briefly, the conventional management strategies for infections and the use of nanotechnology in the control of infections. A section on the use of nanotechnology in the control of other diseases is also added.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nanotechnology
- Nanomedicine
- Nanomaterials
- Infections
- Antibiotic resistance
- Antibiotic
- Non-antibiotic
- Neurodegeneration
- Cancer
- Diabetes
14.1 Introduction
Health has been the most important criteria to measure the development of a country. Health indices have always been dwindling throughout the world due to various factors that emerged time to time. It was infectious diseases that claimed the lives of millions during the initial stages of human settlement (Dobson and Carper 1996). With the discovery of antibiotics and the advancement of health care, we have succeeded in overcoming infections to a greater extent initially. Then came an era of non-communicable diseases with a steep rise in the death rate among different countries. The determinants of health have been redefined in the light of escalating rates of diseases like coronary artery disease, stroke, cancers etc. At the dawn of the twenty-first century we were facing a grave situation of highly prevalent non-communicable diseases with a soaring rate of infectious diseases contributed by newly emerging pandemics (Heinrich et al. 2020) of Ebola, SARS-CoV-2 etc. Making the matters more complicated is the resurgence of bacterial strains that are resistant to multiple antibiotics. The situation is warranting to shift the concentration from developing newer antibiotics to adopt newer strategies to make the available antibiotics more efficacious. For this, medical sciences embraced the newly emerged science of nanotechnology. The application of nanoscience in medical sciences have already developed different speciality streams like nanovaccines, nanodiagnostics, nanopharmaceutics so on and so forth (Islan et al. 2017; Saglam et al. 2021). In this chapter we discuss about the research and developments in the field of nanomedicine in controlling infections and other diseases.
14.2 Historical Development of Nanomaterials in Relevance to Biology and Medicine
Evolution of nanoscience can be traced back to oldest civilizations that existed on earth. In the fifth century B.C. philosophers debated on the continuity of matter and ascertained that any matter can be divided into infinitesimally smaller subunits culminating in indivisible and invisible matter (Bayda et al. 2020). This smallest subunit of matter is equivalent to the modern entity of ‘atom’, and can be considered as a recognition and acceptance of materials in micro- and nanoscales in those days. In the fourth century AD Romans created the Lycurgus cup , a dichroic glass cup which appears green on direct light and reddish purple in transmitted light, is considered as the oldest manmade nanomaterial. The dichroic property of Lycurgus cup was established in 1990 through transmission electron microscopy. It is due to the presence of silver-gold alloy nanoparticles in 7:3 proportion of 50–100 nm size containing dispersed copper of about 10% (Elsner 2013). The vast array of ceramic glazes used by the Islamic world and Europe contained silver and copper nanoparticles (Padovani et al. 2003; Barber and Freestone 1990). They were extensively used from the ninth to seventeenth centuries. Later Italians utilized similar nanoparticles during the Renaissance period for pottery making. They were influenced by the Ottoman techniques prevalent from the thirteenth to eighteenth centuries using ‘Damascus’ saber blades, cementite nanowires and carbon nanotubes which provided strength, resilience and keen edges respectively to produce ‘Damascus’ saber blades strength, made from cementite nanowires for resilience and carbon nanotubes for keen edges (Sciau 2012). Michael Faraday studied colloidal gold in suspension, its optical and electronic properties and demonstrated how the gold nanoparticles changed their colour in solutions under specific lighting conditions (Lin et al. 1986). The ancient Indian treatment systems like Ayurveda and Siddha also utilized metals and minerals in the nanoscale for the treatment of various ailments. Detailed procedures of calcining the metals and minerals into “Bhasma’ of nano-proportions were utilized in the treatment of various ailments at small doses. The science detailing these methods of purification and reduction along with their modes of use in the treatment branch termed ‘Rasasastra’ had enormous patronage from Buddhism (Ranade and Acharya 2015). The famous Indian Alchemy principles also lay hidden in these scriptures. Calcined gold, silver, copper, lead, tin, antimony, sulphur, arsenic etc. were utilized for the treatment of various ailments with the aid of appropriate vehicles. Even biological materials like amber, stag horn, conch shell and elephant tusk were also calcined and used as medicine in the form of nanoparticles. The qualities of certain metals like silver and gold were known to prevent infections from the tenth to thirteenth centuries. This knowledge was inculcated while making confectionaries prepared in India and its adjoining provinces by covering with finely thin silver or gold foils beaten up for several days to make them reach nano-proportions, which were called ‘Chandi ka warq’ and ‘Sone ka warq’ respectively. This practice is still in vogue in parts of Indian subcontinent.
The modern evolution of nanotechnology took place in 1959 when Richard Feynman, Nobel laureate and famous American physicist introduced it conceptually with the famous lecture “There’s Plenty of Room at the Bottom” given at California Institute of Technology (Richard 1960). The concept laid by Feynman helped in constructing machines of molecular proportions for performing humongous task of handling large volumes of data in a tiniest of space. The materialization of the concepts laid down by Feynman during the latter half of the twentieth century earned him the title ‘Father of modern nanotechnology’. The spark created by Feynman was carried forward by many other eminent personalities like Norio Taniguchi from Japan who coined the term “nanotechnology” in 1974 (Taniguchi 1974).
Towards the end of the twentieth century several more scientists were attracted to the opportunities hidden in the field of nanotechnology and came up with increased momentum of research activities. Several opportunities were suggested for the synthesis of nanomaterials and broadly they were classified as two categories: top-down and bottom-up approaches (Khan et al. 2011).
The breaking down of a material into nano-sized particles from a relatively large material by the use of precision engineering and lithography is termed top-down approach (Madou 2011). Majority of micro-electronics industry utilizes top-down approach with precision engineering while lithography utilizes patterning of surfaces through deposition of materials or through exposure ions, electrons or light (Biswas et al. 2012).Bottom-up approach on the other hand utilizes self-assembly of atoms or molecules by physical or chemical interactions to form materials in nanoscale range (1–100 nm) (Luby et al. 2015).
The first published book on nanotechnology was by K. Eric Drexler in the year 1986 entitled “Engines of Creation: The Coming Era of Nanotechnology”, which introduced the term ‘Molecular engineering’ (Bayda et al. 2020; Drexler 1981). He described the build-up of complex machines from self-assembly of individual atoms to form nanostructures. Later in 1991 he associated with Peterson and Pergamit to publish yet another milestone publication titled “Unbounding the Future: The Nanotechnology Revolution” in which they introduced terms like ‘nanobots’ or ‘assemblers’ for medical applications, and the term ‘nanomedicine’ was introduced henceforth (Drexler et al. 1991). The evolution of nanotechnology is subdivided into four distinct generations and as per Mihail Roco, we are presently in the fourth generation of nanotechnology era (Roco 2007) (Fig. 14.1). Nanomaterials and their uses in the field of medicine are presented in Table 14.1.
14.2.1 History of Nanobiology
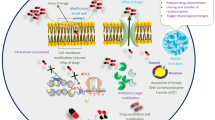
The history of use of nanotechnology in the fields of biology and medicine extends from diagnosis, drug delivery to molecular imaging. Biological systems including the human body are an assembly of nanoscaled structures undergoing self-assembly and self-organization to form higher-order structure of biological units and organisms ranging from micro-, meso- and macroscale. Life processes involving elementary biological units like cell-membrane, DNA, proteins or lipids are of nano-dimensions (Fig. 14.2). These biological units and their functions are better comprehended, guided and manipulated with the help of nanotechnology (Logothetidis 2006). The application of nanoscience in the field of biology is henceforth called bio-nanotechnology. Miniaturization is the essential feature of nanomedicine wherein the nanometer scale is similar in size to the macromolecules like enzymes, receptors, and carrier proteins. The nanoscale devices in the range of 20–50 nm can easily enter cells as well as exit the vascular circulation and enter tissues swiftly. The birth of a new stream of nanotechnology called “nano-pharmaceuticals” developed and marketed products using nanotechnology for drug delivery of regenerative medicine, nanoparticles with antimicrobial activities and nanochips, nanoelectrodes and nano-biosensors for the detection of biomarkers. In medicine, nanotechnology coupled with Biotechnology, Information technology and Cognitive science (NBIC developments) are thought to contribute immensely in the fields of in vitro detection, in vivo diagnosis, multimodal imaging, chemotherapy, phototherapy, gene therapy, immunotherapy, theranostics and their clinical translation (Logothetidis 2006; McGinn 2012).
Profound advancements are made in the field of nano-oncology to improve the efficacy of traditional chemotherapy by incorporating nanomaterials to target the tumour site (Kumar et al. 2016a, b; Palazzolo et al. 2019a, b). Researches are advancing in using nanomaterials to modulate essential biological process for cancer therapy like autophagy, quenching of oxidative stress and to exert cytotoxic activity against the cancer cells (Sharma et al. 2019). Other than cancers, diabetes mellitus, neurodegenerative diseases as well as detecting and curing bacteria, fungi and viruses associated with infections are also being investigated with the help of nanotechnology. Another instrumental advancement in the field of bio-nanotechnology is the development of “Scaffolded DNA origami” by Paul Rothemund in 2006 by self-assembling DNA nanostructures in a “one-pot” reaction (Rothemund 2006). This forms the first application of DNA nanotechnology, which was conceptualized by Nadrian Seeman way back in 1982 (Seeman 1982). DNA nanotechnology is the hot seat of interdisciplinary research presently with inputs from physics, chemistry, materials science, computer science and medicine. Nano-informatics incorporating the vast opportunities of computer science is another field which is not being used to its fullest potential in the field of medicine (Sharma et al. 2019). Predictive analysis of nanocarriers by employing powerful machine-learning algorithms predicts their cellular uptake, activity and cytotoxicity. Nano-informatics can utilize powerful tools like data mining, network analysis, quantitative structure-property relationship (QSPR), quantitative structure-activity relationship (QSAR) and ADMET (absorption, distribution, metabolism, excretion, and toxicity) for predictions. The opportunities and possibilities for nanoscience are many and they are being utilized elaborately during the twenty-first century and hence we aptly termed as the “next industrial revolution”.
The following properties of nanomaterials created tremendous attraction for them to be utilized as nanomedicine. (1) Biomolecules with nano-size dimensions are capable of regulating cellular biochemical pathways and in turn cellular homoeostasis. Properly engineered nanoparticles can interfere with the biomolecules at any stage in the molecular processes giving us the capability to hinder or promote any biochemical process in the system biology of humans. (2) Nanomaterials have fairly good solubility due to the advantage of their size, and this can be further enhanced by modifying their surface properties. (3) Owing to their higher surface-to-volume ratio and greater surface area, they are capable of carrying a higher therapeutic payload to their target site. (4) Due to their property of selective targeting, nanoparticles can deliver therapeutic dosages to their specific target, reducing the untoward effects on the nearby healthy tissues. (5) Nanoparticles can now be utilized for personalized diagnosis and therapy (Bisht and Rayamajhi 2016).
14.3 Infection and Their Treatment – Current Knowledge
Infectious diseases posed the greatest threat to the establishment of human race on earth. The human population of present day is to a greater extent due to the victory over infectious diseases that started with the discovery of antibiotics. From the discovery of penicillin in 1928 by Alexander Fleming, the number of antibiotics in treatment have increased at a sudden pace, now reaching to a sizeable population amounting to hundreds. Antibiotic resistance also emerged simultaneously leading to the development of ‘superbugs’ which pose a real challenge to researchers. Antibiotic resistance has provided an evolutionary advantage to the microbes to survive the newly emerging antibiotics. Methicillin-resistant Staphylococcus aureus , fluoroquinolone-resistant S. aureus , erythromycin-resistant Streptococcus pyogenes and S. pneumoniae and vancomycin-resistant enterococci are few of the resistant strains that gain medical attention on a public health purview recently (Kapoor et al. 2017).
Antibiotics evolved after 1928 are classified and dealt based on their mechanism of action (Fig. 14.3);
14.3.1 Antibiotics Targeting Cell Wall
Gram-positive bacteria are protected by an outer cell wall which is tough, rigid and mesh-like. Meanwhile, the Gram-negative bacteria is covered by a thin cell wall surrounded by a second outer membrane. The space enclosed between the cell wall and the outer membrane is termed periplasm. The outer membrane is an additional protective layer which protects the bacteria from the entry of external substances. However, they are provided with channels called porins. which allow the entry of molecules into the cell (Hauser 2015). The tough cell wall helps in maintaining their shape and protects them from the osmotic and mechanical stressors. The cytoplasmic membrane prevents the entry and exit of ions and maintains the cytoplasmic components.
Bacterial cell wall is made up of peptidoglycan, which is a polysaccharide layer cross-linked to glycan strands facilitated by the action of transglycosidases. The cross-linking extends from the sugars in the polymers to the peptides on the other strand. Precisely, the D-alanyl-alanine portion of peptide chain is cross-linked by glycine in the presence of penicillin binding proteins (PBPs) . This cross-linking makes the cell walls stronger, and this biochemical pathway is targeted by a group of antibiotics like β-lactams and glycopeptides (Kahne et al. 2005; Reynolds 1989; Strohl 1997; Benton et al. 2007; Leach et al. 2007).
14.3.1.1 Beta-Lactam Antibiotics
They are the oldest and a broad class of antibiotics with a β-lactam ring in their molecular structure. They irreversibly inhibit the enzyme transpeptidase, an enzyme required for bacterial cell wall synthesis. The final step in transpeptidation in the synthesis of cell wall is facilitated by a transpeptidase called penicillin binding protein (PBP). PBP binds to D-alanyl-D-alanine attached at the end of the peptidoglycan precursors, muropeptides, to cross link the peptidoglycan. β-lactam antibiotics target the PBPs, as the β-lactam ring mimics the D-alanyl D-alanine portion of the peptide chain (Džidić et al. 2008). As the PBP links to the β-lactam ring, they will not be available to cross-link the peptidoglycan and the synthesis of the new cell wall abruptly ends. The disruption of peptidoglycan layer damages the permeability barrier of the bacterium. Penicillin derivatives, cephalosporins, monobactams and carbapenems belong to β-lactam antibiotics. In spite of escalating antimicrobial resistance, these antibiotics are still clinically important due to their short t1/2, low volume of distribution with significant kidney tubular secretion (MacDougall 2017).
14.3.1.2 Glycopeptides
Glycopeptides are cyclic or polycyclic glycosylated non-ribosomal peptides produced by filamentous actinomycetes belonging to various groups. The glycopeptides inhibit the cross-linking of the peptidoglycan by binding on to the D-alanyl D-alanine portion of peptide side chain. They in turn cross-link peptides within and between peptidoglycan on the surface of the cytoplasmic membrane. Vancomycin, a glycopeptide antibiotic prevents the binding of PBP with the D-alanyl subunit and binds noncovalently with the terminal carbohydrate, thus inhibiting the formation of cell wall (Grundmann et al. 2006).
14.3.2 Inhibition of Protein Biosynthesis
The genetic information in the bacterial DNA is first transcripted on to the m-RNA followed by translation of the triplet codons on the m-RNA by the ribosomes. The entire process of protein synthesis is catalysed by ribosomes and myriads of cytoplasmic factors. The bacterial 70S ribosomes are consisting of two sub-units, the 30S and 50S subunits which are integral to the functioning of the ribosomes (Yoneyama and Katsumata 2006; Vannuffel and Cocito 1996; Johnston et al. 2002). Antimicrobials target either the 30S or 50S sub-units of the bacterial ribosomes.
14.3.2.1 Inhibitors of 30S Subunits
14.3.2.1.1 Aminoglycosides
Aminoglycosides (AG) are positively charged molecules attaching on to the negatively charged outer membrane of the bacterial cell. This process leads to the formation of large pores, which allow the penetration of the antibiotic into the bacterial cell. The passage of the antibiotic through the cell membrane requires activation of energy-dependent transport channels which requires oxygen and an active proton motive force. Due to this peculiar requirement, AG are less active against anaerobic organisms, but they are synergistic to cell wall disrupting antibiotics like β-lactams and glycopeptides as they provide easier access to the AG into the cell. On their entry, AGs form hydrogen bonds with the conserved portions in 16S r-RNA of the 30S subunit near the A site, causing misreading and abrupt termination of translation of m-RNA. Tetracycline, chlortetracycline, doxycycline and minocycline exhibit antimicrobial effect following this mechanism (Wise 1999).
14.3.2.2 Inhibition of 50S Subunits
14.3.2.2.1 Chloramphenicol
Chloramphenicol is a broad-spectrumantibiotic isolated from Salmonella venezuelae. It is characterised by the presence of a nitrobenzene moiety derived from dichloroacetic acid. This antibiotic binds with the conserved sequences of the 23S r-RNA of the 50S subunit at the peptidyl transferase cavity and prevents the binding of t-RNA to the A site of the ribosome (Yoneyama and Katsumata 2006; Vannuffel and Cocito 1996).
14.3.2.2.2 Macrolides
Macrolides contain macrocyclic lactone ring and hence the name. Almost all members of this group are isolated from Streptomyces, and erythromycin is the best-known member of this group. Macrolides interferes with the early stage of protein synthesis, translocation, by targeting the conserved sequences of 23S r-RNA of 50S subunit and binds within the nascent peptide exit tunnel (NPET) adjacent to the peptidyl transferase centre and results in premature detachment of the newly formed incomplete peptide chains (Strohl 1997; Leach et al. 2007). Along with macrolides, linocosamides and streptogramins B also exhibit similar mechanism (Yoneyama and Katsumata 2006; Wise 1999).
14.3.2.2.3 Oxazolidinones
Oxazolidinones are compounds containing 2-oxazolidine, exhibiting good activity against Gram-positive bacteria. Oxazolidinones are a relatively newer group of antibiotics which are completely synthetic and act by interfering with protein synthesis at multiple stages like binding to 23S r-RNA of the 50S subunit and suppressing 70S inhibition and interact with peptidyl-t-RNA (Lambert 2005; Bozdogan and Appelbaum 2004; Strohl 1997; Leach et al. 2007).
14.3.3 Inhibitors of DNA Replication
14.3.3.1 Quinolones
Fluoroquinolones (FQ) inhibit DNA gyrase , the enzyme that nicks the double-stranded DNA, and introduces negative supercoils and reseals the nicked ends. They thus prevent the positive supercoiling essential for the transcription or replication of the strands. DNA gyrase enzyme consists of four chains – two A subunits and two B subunits. The A subunit is responsible for creating the nick in the DNA strand, while B subunit adds the negative supercoils and the other A subunit reseal the strands. FQs have high affinity to A subunit and perform strand cutting and resealing. In Gram-positive bacteria, topoisomerase IV is the enzyme responsible for nicking the DNA strands, and it shows high affinity towards FQs. Hence, FQs have more potency against Gram-positive bacteria. In mammals, the homologous enzyme for DNA gyrase is topoisomerase II, which has very low affinity for FQ (Yoneyama and Katsumata 2006; Wise 1999; Higgins et al. 2003; Strohl 1997).
14.3.4 Folic Acid Metabolism Inhibitors
14.3.4.1 Sulfonamides and Trimethoprim
These antibiotics interfere with the folic acid metabolism pathways. Sulpha drugs and trimethoprim act on the folic acid metabolic pathway at two different steps. Sulphonamides have high affinity towards dihydropteroate synthase and inhibit it competitively compared to its natural substrate p-amino benzoic acid. Trimethoprim on the other hand inhibits dihydrofolate reductase, which catalyses a step much farther to that of dihydropteroate synthase (Yoneyama and Katsumata 2006; Tenover 2006; Straus and Hancock 2006).
14.4 Mechanisms of Antimicrobial Resistance
14.4.1 Prevention of Accumulation of Antimicrobials
This is achieved by either decreasing the uptake of the drug or increasing the efflux from the cell by changing the permeability of the outer membrane. Bacterial cells uptake materials from the outside by diffusion through porins, diffusion through the plasma membrane or by self-uptake. Porins are located on the outer membrane of Gram-negative bacteria and admit small hydrophilic molecules to pass through it. As the number of porins on the outer membrane decreases, the organism develops resistance against antibiotics that gain entry into the cell through them. β -lactam antibiotics and FQ resistance to Gram-negative bacteria is due to this process (Fig. 14.4).
14.4.1.1 Efflux Pumps
Efflux pumps are the membrane proteins that export the antibiotics from the interior of the cell to outside. The resistance of the organism against an antibiotic is determined by the efficiency of the efflux pumps (Džidić et al. 2008). Some resistant strains have developed speedy efflux pumps, which pump out the antibiotics at the same rate as they enter the cells and prevent the drugs from reaching their targets (Nikaido 1994; Kumar and Schweizer 2005). These pumps are located on the cytoplasmic membrane as compared to porins which are present on the outer membrane. Efflux pumps can be specific to a particular antibiotic, but most of them are multidrug transporters capable of pumping out wide range of unrelated antibiotics (Nikaido and Zgurskaya 1999; Webber and Piddock 2003).
14.4.1.2 Modification of Target Molecule
The binding of the antibiotic molecule to its target site is governed by the symmetry and dynamics of the interaction. Any natural or acquired change in the target molecule could hamper the effective drug interaction. A bacterium can acquire the change in target molecule through mutations in bacterial genes. Alterations in the 30S and 50S subunits can lead to resistance to antibiotics that affect protein synthesis like macrolides, chloramphenicol etc. Modification of PBP is another mechanism for resistance favoured by Gram-positive bacteria. It creates decreased affinity of the β -lactam antibiotics to the PBP (Mobashery and Azucena 1999; Lambert 2005). β -lactamase production is the complementary mechanism adopted by Gram-negative microbes for resistance. E. faecium resistance to ampicillin, Streptococcus pneumoniae resistance to penicillin etc., are mediated through this mechanism. Cell wall precursors are changed by mutations by certain bacteria. Antibiotics that inhibit cell wall synthesis are performed by binding to the D-alanyl-D-alanine moieties of peptidoglycan. Changing the D-alanyl-D-alanine to D-alanyl-lactate prevents glycopeptides cross linking (Džidić et al. 2008; Grundmann et al. 2006). E.faecium and E. faecalis develop resistance through this mechanism. Mutation to DNA gyrase and topoisomerase IV genes produces defective enzymes that leads to replication failure and prevents antibiotics like FQ to bind. Ribosomal protection mechanisms and RNA polymerase mutations also impart resistance to bacterial strains (Kapoor et al. 2017).
14.4.1.3 Antibiotic Inactivation
Chiefly there are three enzymes that inactivate antibiotics – β-lactamases, aminoglycoside-modifying enzymes and chloramphenicol acetyltransferases (Alekshun and Levy 2007). β-lactamases hydrolyse β-lactam ring containing ester and amide bond like penicillin, cephalosporins, monobactams etc. Aminoglycoside modifying enzymes like phosphoryl-transferases, nucleotidyl-transferases and adenylyl-transferases reduce affinity to bind to 30S ribosomal subunit and generate resistance to multiple organisms (Maurice et al. 2008; Strateva and Yordanov 2009). Chloramphenicol-acetyl-transferases is another mechanism to build resistance to the antibiotic chloramphenicol (Tolmasky 2000). These enzymes present in some Gram-positive and some Gram-negative organisms along with few Haemophilus influenza strains acetylates hydroxyl groups of chloramphenicol making them unable to bind to the 50S subunits of ribosomes.
WHO has warned that several of the infectious diseases will become incurable due to resistance.For overcoming these several other treatment strategies have been scientifically developed. Here we list the major classes of alternatives for antibiotic treatment through non-antibiotic treatments to control infections.
14.5 Non-antibiotic Treatments for Infections
-
Phage therapy
-
Bacteriocins
-
Killing factors in microbes
-
Antibacterial activities of non-antibiotic drugs
-
Quorum quenching
14.5.1 Phage Therapy
Phages are a group of viruses that have the capability to infect and kill bacteria. The first mentioning on phages was done by Ernest Hankin in 1896 and later the name bacteriophage was coined by Felix d’Herelle. Among all the different methods of non-antibiotic treatment of infections above, phage therapy is the only treatment that entered clinical trials and was produced in large scale for the purpose of treatment during the 1940s. Humans are administered with phages orally, rectally, locally, parenterally as intravenous injections or through respiratory passages as aerosols or intrapleural injections (Sulakvelidze et al. 2001). Today, phage therapy is not considered as an armamentarium against bacterial infections in almost all parts of the world. One reason for its declined used is attributed to the early introduction of phage therapy before understanding its purpose. The advent of stronger and newer antibiotics pushed phage therapy to the verge of extinction (Kutter et al. 2010).
Bacteriophages replicate inside the bacterial cells following two major pathways:
-
1.
Lytic module
Lytic pathway involves the following steps:
-
(a)
Attachment of the phage on to the bacterial wall
-
(b)
Injection of its DNA inside the host cell
-
(c)
Termination of the synthesis of bacterial cell components
-
(d)
Phage DNA replication and formation of new phage capsids
-
(e)
Assembly and release of phage components by lysis of the host cell.
-
(a)
-
2.
Lysogenic module
In this pathway the initial steps of attachment and inoculation of the phage DNA into the bacterial host cell are similar to that of the lytic pathway. In the third step, the phage DNA will anneal on to the host chromosome and gets integrated (lysogenization), and the replication of the phage DNA occurs along with the replication of bacterial DNA for subsequent several multiplications (Prophage). The prophages after several cycles of multiplication free themselves from the bacterial genome and induce the bacterial genome to synthesize phage components, which are released after the lysis of the bacterial cell. Phages inhibit bacterial restriction enzyme through genome modification (Andriashvili et al. 1986). As this infection cycle can go on for several lifecycles, lysogenic phages are not ideally used for phage therapy (Lorch 1999).
Phage therapy had not been successful in combating bacterial infections as the scientists were more concerned with the application of the phages while the clinical data pertaining to their use were completely ignored. As the phages replicates inside the bacterium host, they exhibit the interesting phenomena of self-dosing. Phages are highly specific about their host and due to this reason, its usage was relatively safe as the friendly micro flora of the body is spared from the attack of the phages. But this also has a disadvantage that the causative agent of the disease needs to be identified properly before dosing. This specificity is lacking in the case of antibiotics and they became more popular than the phage therapy in the years that followed. The most feared complication from phage therapy is the release of endotoxins by the bacteria lysed by the phages. Even though phage therapy took up gradually crossing many geographical restrictions, none of its advocates recognized the role bacterial immunity. The bacterial immune responses are of two kinds – the innate and adaptive responses. The distinction between and the self and non-self-DNA through the restriction modification forms the innate response. This is achieved on the basis of the DNA methylation pattern and on the lack of phage replication machinery. The adaptive immunity in bacteria was discovered with the identification of CRISPR (Clustered regularly interspaced short palindromic repeats) sequences. CRISPR-mediated immune response involves complex processes like gene silencing mechanism (Brouns et al. 2008; Barrangou et al. 2007; Hale et al. 2009).
14.5.2 Bacteriocins
Bacteriocins are peptides which are bactericidal, produced by bacteria belonging to several groups which is evolved for eliminating competition from other strains. E. coli strain V produces a dialyzable and heat-stable compound called colicin V that inhibits the growth of coliphages (E. coliφ) at very low concentration. The use of bacteriocins is not extensive in clinical infections but limited to food preservation (Cleveland et al. 2001; O’sullivan et al. 2002). Application of bacteriocins are seen in food industry, where the probiotic microorganisms used in packaged foods secretes bacteriocins and prevents the growth of harmful bacteria. Bacteriocins are of four major types; Class I (antibiotics) are <5 kDa small heat-resistant peptides; class II are <15 kDa small heat-stable, membrane-active and unmodified peptides; class III consists of heat-labile proteins with size >15 kDa and class IV bacteriocins are lipid or carbohydrate moieties bound to it (Sablon et al. 2000; Garneau et al. 2002; Klaenhammer 1993). Bacteriocins exert inhibitory action mainly on Gram positive bacteria than Gram-negative, as they possess an outer membrane made mainly out of lipopolysaccharides (LPS). This outer membrane provides an evolutionary advantage of preventing free diffusion of molecules heavier than 0.6 kDa. The smallest known bacteriocin is of about 3 kDa size. Bacteriocins act by binding to the cell membrane and alter their osmotic stability leading to cell death (Cotter et al. 2013). Even though Gram-negative bacteria evades the action of bacteriocins, some of them gain entry to the cell interior through specific receptors over the outer membrane like OmpF, FhuA or over the inner membrane like SbmA, YejABEF and TonB. Absence of the outer protective LPS layer makes Gram-negative bacteria more susceptible to bacteriocins leading to cell death (Stevens et al. 1991). The mode of mechanism of bacteriocin is doubtful yet it is postulated that they bind to the lipid-II, which is essential for the transport of peptidoglycan subunit from cytoplasm to cell wall, thus interrupting the synthesis of cell wall leading to cell death. Several other mechanisms have also been postulated regarding the mode of action of bacteriocins; they include pore formation in cell wall using lipid-II as a docking molecule, de-energizing membrane and dissipating proton motive force, preventing uptake of amino acids and triggering their release from the cell, excluding potassium ions, depolarizing cytoplasmic membrane, hydrolysis and partial efflux of cellular ATP (Cotter et al. 2005; Abee et al. 1994). The advantage of bacteriocin use is that they do not harm the beneficial microbiota of the body, but they are effective only against a small group of bacteria. Bacteriocins are short-acting than antibiotics, due to action of proteolytic enzymes on them which easily converts them to non-toxic amino acids. This can be overcome by the using dendrimers that prevent their degradation and deliver them at the site of infection (Tam et al. 2002; Bracci et al. 2003). Resistance development is another negative aspect of bacteriocin that limits its activity. Bacteriocin resistance is acquired through the immunity gene present in bacterial strain or through alteration in gene expression.
14.5.3 Killing Factors
Killing factors are the factors released by bacterial cells to kill sibling cells during starvation. This phenomenon was noticed in B. subtilis and is named as “cannibalism”. “Cannibalism” is exhibited through a set of genes that induce lysis of their sister cells in their milieu during nutrient scarcity (Nandy et al. 2007). The lysed cells provide the nutrients for the killer cells for their survival and spore formation. B. subtilis exhibits predation rather than cannibalism, that is, it lyses the bacterial cells of other species. The feature of cannibalism is due to two peptides – sporulation delaying protein and sporulation killing factor. Killer B. subtilis cells preferentially target non-B. subtilis cells. This shows the antibiotic action of killer peptides (Burbulys et al. 1991).
14.5.4 Antibacterial Activities of Non-antibiotic Drugs
The drugs that are developed to treat non-infectious diseases but having antimicrobial activities are called non-antibiotics (Williams 1995; Lind and Kristiansen et al. 1990). They are effective against some Gram-negative bacteria, Gram positive bacteria, viruses, fungi, protozoa etc. (Jones 1996). Barbiturates, diuretic drugs, beta-adrenergic receptor antagonists, antihistamines, mucolytic agents, non-steroid anti-inflammatory drugs, proton pump inhibitors and psychotherapeutic drugs are studied in this regard. Alteration of cell permeability is considered as their mode of action in this regard. Alternative mechanisms like affection of efflux pump of microbes, cross-membrane ion transport, cell energy transport and activity of membrane-bound enzymes are also studied (Cederlund and Mårdh 1993). The concentration at which these drugs produce their activity is much higher than the physiologically observed dose. The combination of nonantibiotic drug with antibiotics may make the resistant bacteria susceptible to the non-effective drug. The combination of beta-lactam with phenothiazines when administered to beta-lactam-resistant microorganisms like MRSA makes them sensitive (Amaral and Kristiansen 2000). The advantage of such an approach is that the physiological impacts of the drugs involved are well established.
14.5.5 Quorum Quenching
Quorum quenching is the process of communication between bacterial cells through messenger molecules. It is established that such communication plays a significant part in the beginning of virulence mechanism. Two mechanisms in this regard have been recognized and analyzed (Nigam et al. 2014). First type involves recognition of signal by means of cytosolic transcription factor while in the second type it is facilitatedvia an auto inducing recognized through a membrane receptor. Any microbe can own either type of quorum sensing system or both. The former form of quorum sensing is facilitated by derivatives of acyl homoserine lactone while the auto inducing system involves peptides. It is assumed that if this signalling is weakened, the virulence of microbes can be controlled without selection pressure. It is known that selection pressure leads to the development of new drug-resistant microbes. Therefore, this method that hinders the spread of microbes without imposing selection pressure may be crucial to prevent evolution of drug-resistant microbes. Quorum quenching is achieved either through mimicking of quorum sensing molecules that compete with analogous quorum sensing molecules or through inhibiting enzymes involved in synthesis of quorum sensing molecules. The report of Triclosan as an anti-bacterial agent inhibiting enoyl-ACP reductase which produces an important intermediate in AHL biosynthesissupport this mechanism (Hoang and Schweizer 1999). The important advantages of quorum sensing inhibitors are that it limits evolution of new drug-resistant forms as well as virulence and bio-film formation (Czajkowski and Jafra 2009).
14.6 Mechanisms of Antibacterial Activity of Nanoparticles
Mainly three mechanisms have been identified as the modes of action of nanoparticles in killing the microbes. They are as follows: (1) due to direct intake of nanoparticles, (2) reactive oxygen species (ROS) production and (3) disruption of cell wall by nanoparticles.
14.6.1 Direct Absorption of Nanoparticles
Studies have shown that nanoparticles of silver (AgNPs) leach out silver ions when it comes in contact with the body fluids due to variation in the pH. These silver ions have high affinity towards enzymes containing thiol group rich amino acids like Cysteine. Thus, they have high affinity towards respiratory (NADH dehydrogenase) and electron transport group of enzymes (Prasad and Swamy 2013; Swamy and Prasad 2012). This interaction results in the uncoupling of the ATP molecule from the respiratory chain. They create a phosphate deficient environment inside the bacterial cell by actively efflux phosphate ions and at the same time reduces their reuptake. They reduce proton motive force resulting from the loss of protons due to the binding of silver ions to the various transport proteins. They are also shown to increase the frequency of DNA mutations during the translation process and in leakage of intracellular contents from cytoplasm retrenchment and cell membrane degradation.
14.6.2 Reactive Oxygen Species (ROS) Production
ROS are formed as by-products of all metabolic pathways inside the cells of all respiring organisms. There are several inbuilt mechanisms in organisms to tackle this life-threatening escalation of ROS. Antioxidant defence mechanisms like glutathione, glutathione disulphide, reduced glutathione, glutathione peroxidases and peroxiredoxins play an important role in quenching the surge in oxidative stress. Excessive production of ROS brings in damages to the lipid bilayer of membranes, dysfunction of mitochondria and DNA damage. Metals in general and silver ions in particular catalyses ROS generation in the presence of oxygen dissolved in milieu. The generation of ROS is done either by inactivating the respiratory chain enzymes or the superoxide dismutase which scavenge the superoxide radicals or by a combined mechanism of both. Thiol group binding and inactivating metal ions thus provide significant antibacterial effect.
14.6.3 Cell Wall Damage
The low size of the metal ions and their electrostatic attraction to the negatively charged cell membrane allows them to easily adhere to them and penetrate to create pits by releasing lipopolysaccharides and proteins from them. This varies the permeability of the membrane by releasing muramic acid by binding to the N-acetylmuramic acid and N-acetylglucosamine of the peptidoglycan strands (Feng et al. 2000; Sondi and Salopek-Sondi 2004; Morones et al. 2005; Song et al. 2006).
14.7 Nanomaterials in Controlling Infections
Reducing the size of the particles to nanoscale has the advantage of easily surpassing the natural biological barriers inside living systems owing to their high surface-to-volume ratio. Nanosize of particles having high surface-to-volume ratio permits them to surpass the barriers to biological systems and molecules. Molecular interactions could be enhanced by manipulating the size, shape and chemical characteristics of the nanomaterials (Kim et al. 2010) and can be utilized as therapeutic and diagnostic agents in the form of vehicles. Nanotechnology could be used to overcome the bacterial resistance by creating new and improved antimicrobial agents. The use of nanomaterials in infectious diseases can be discussed under the following headings (Blecher et al. 2011);
-
Drug delivery systems
-
Drug infused nanoparticles
-
Immunomodulation
14.7.1 Nanotechnology-Based Drug Delivery Systems
14.7.1.1 Chitosan
Chitosan is a biopolymer made of natural polysaccharide exhibiting polycationic interactions with negatively charged microbial cell wall and other cytoplasmic membranes leading to the disruption of the cell membrane and subsequent leakage of intracellular elements from deranged osmotic stability.They also have the capability to enter the nucleus of the bacterial cell and other microorganisms like fungi and bind to the DNA and thus inhibits the mRNA and protein synthesis (Ma et al. 2008; Qi et al. 2004). Chitosan molecules have high affinity towards bacteria and fungi due to their relatively higher surface-to-charge ratio and surface charge density. Hence, they exhibit greater antimicrobial activity against Gram-positive as well as Gram-negative organisms. Thus, they exhibit antimicrobial activity against the most notorious pathogens like E. coli and Staphylococcus aureus. (Banerjee et al. 2010; Sanpui et al. 2008). Chitosan is derived from a natural substance, chitin, which is the structural ingredient of the exoskeleton of crustaceans. Studies have shown that the chitosan nanoparticles are more efficacious than chitosan alone or antibiotics like doxycycline. Its polycationic characteristic and high affinity towards metals are utilized in engaging it along with several other nanoparticles like metallic NPs (copper and silver), nitric oxide releasing nanoparticles and drug containing NPs used for targeted drug delivery and as carriers (Qi et al. 2004). Chitosan also escalates the antimicrobial property of these nanomaterials. In experimental models, silver nanoparticles-embedded membranes increased their zone of inhibition when incorporated with 70% of chitosan. Similarly, incorporation of chitosan in silver nanoparticles decreased the mean inhibitory concentration (MIC) against S. aureus (Ma et al. 2008).
14.7.1.2 Metallic Nanoparticles
14.7.1.2.1 Silver
Silver is traditionally used as an antimicrobial agent in treating conditions like burns and wounds. It is believed that silver ions on gaining entry into the bacterial cell wall and membranes target the DNA, respiratory enzymes and other proteins containing sulphur or thiol groups resulting in loss of capability to replicate and finally cell death results (Aziz et al. 2014, 2015, 2016, 2019). Silver nanoparticles (Ag-NP) are attributed with small size and large surface area, and this makes them capable of easily penetrating the bacterial cell wall and other biological membranes (Rai et al. 2009; Pal et al. 2007; Ruparelia et al. 2008; Prasad 2014). Thus, the nanoparticle size is proportionated with its antimicrobial activity; smaller the size, greater the effect. Shape of the nanoparticles also influences its activity. Small triangular and truncated nanoparticles are more efficacious than round or rod-shaped particles. Hence, small and triangular nanoparticles (<10 nm) exhibit more antimicrobial efficacy than large round or rod-shaped nanoparticles (Pal et al. 2007). Ag-NPs thus exhibit a very varied antimicrobial property against viruses, bacteria and fungi due to their surface interaction. Several bacterial species belonging to both Gram-negative and Gram-positive strains succumb to them. This wide range of activity is achieved at relatively lower concentration of Ag-NPs than the conventional silver preparations. This paves way to lower dosing and lesser toxicity from silver. These conclusions are relatively theoretical and studies pertaining to the toxicity of Ag-NPs are yet to be unravelled. Ag-NPs also exhibit synergism with other antibiotics. This is another area of interest in Ag-NP research. Activities of penicillin G, amoxicillin, erythromycin, clindamycin and vancomycin increased against organisms like S.aureus and E. coli. Among these agents, erythromycin showed the greatest inhibition. As Ag-NPs exhibit wide range of targets, microorganisms should develop multiple, simultaneous compensatory mutations to develop resistance. Hence, Ag-NPs can overcome bacterial resistance against antibiotics and at the same time enhance their efficacy (Sanpui et al. 2008). Owing to these qualities Ag-NPs find its application in medical devises that constantly come in contact with body fluids and poses a threat to infection by serving as acoatings on them to prevent microbial colonization, in wound dressings and in enhancing the potency of antibiotics.
14.7.1.2.2 Copper
Copper has been less engaged as an antimicrobial agent when compared to silver. Yet its use as an antifungal agent is well documented in history as early as the nineteenth century (Cioffi et al. 2005). Copper oxide as a nanomaterial has recently gained importance due to its cost effectiveness and compatibility with other polymers. Yet copper oxide nanoparticles (CuO-NPs) are inferior to Ag-NP in their antimicrobial property against E. coli as well as methicillin-resistant S.aureus (MRSA) (Ruparelia et al. 2008; Ren et al. 2009). But they are found to be more efficacious against B. subtilis, which is attributed to the copper’s affinity to amine and carboxyl groups on the cell surface of these pathogens (Ruparelia et al. 2008; Yadav et al. 2017). Compared to Ag-NPs, CuO-NP exhibit a broader range of activity, especially against fungi. Copper loaded nanoparticle laden polymer thin films demonstrated significant antifungal activity against S. cervisiae yeast, moulds and bacteria including E. coli, S. aureus and Listeria monocytogenes. They significantly reduced the number of colony forming units (CFUs) especially in S. cervisiae, where no CFUs were noted (Cioffi et al. 2005). These results project out the biostatic property of CuO-NPs. On comparison with silver, CuO-NPs exhibit broader range of antimicrobial activity and weaker activity against most of the bacteria, they are strong antifungal agents and are capable of preventing surface microbial colonization especially on medical instruments.
14.7.1.2.3 Titanium
Titanium dioxide (TiO2) has gained importance as a nanomaterial recently due to its activation on exposure to ultraviolet light forming active oxygen species, a process termed as photocatalysis. The family of active oxygen species generated includes hydrogen peroxide and hydroxyl radicals. They are responsible for obliterating the bacterial cell membranes resulting in cell death (Kim et al. 2003). This property of TiO2 nanoparticles (TiO2-NP) has been engaged in water and air purification and their activity against pathogenic opportunistic microorganisms (Martinez-Gutierrez et al. 2010). TiO2-NP infused thin film composite membranes has been recently shown to have significant antimicrobial activity and prevents bacterial attachment to membrane surface and formation of E. coli biofilm on medical instruments by disrupting the bacterial cell membrane (Martinez-Gutierrez et al. 2010). Researches has been conducted by coupling TiO2-NP with Ag-NP for their antimicrobial property. Even though TiO2-AgNPs proved less efficacious than TiO2 alone or Ag-NP alone, against their activities against Gram-positive bacteria, Gram-negative bacteria and various fungi responsible for opportunistic infections and colonization of medical devices, they showed more pronounced activity against several strains of fungi of medical importance. These results highlight that it might be more beneficial to combine metal nanoparticles to augment the antimicrobial activity.
14.7.1.2.4 Magnesium
Several non-metals like chlorine, bromine and iodine has been traditionally used as antibacterial agents, but their toxicities limit their use especially in medical conditions. Their antimicrobial property is due to the formation of covalent metal-halogen complexes that interact with specific cellular enzymes or through oxidative stress leading to lipid peroxidation ending in the leakage of intracellular contents culminating in cell death (Lellouche et al. 2009). Magnesium- halogen nanoparticle also exhibit antimicrobial activity through the same pathway. Magnesium oxide has a unique place among them as they easily adsorb and retain halogens and at nanoparticle level, these activities increase fivefold . Other advantages of this compound are that, as they bind with halogens, the compound gets converted to powder form which helps in easy handling. Magnesium – halogen nanoparticles are found to be more effective against endospores of different bacterial strains like E. coli, B. megaterium and B. subtilis. Among them E. coli and B. megaterium are highly susceptible to MgO-halogen nanoparticle with complete destruction of entire endospores in less than 20 minutes. Several combinations of Mg-halogen combinations have been investigated for their antimicrobial activity. MgF2-NPs also showed similar antibacterial effect comparable to MgO-halogen nanoparticles . MgF2 – NPs exhibited dose-dependent inhibition of growth of E. coli and S. aureus and also inhibited biofilm formation on medical devices.
14.7.1.2.5 Zinc
Zinc oxide (ZnO) is another compound of interest in the field of antibacterial effect. It is the compound which is approved by FDA with respect to its antibacterial as well as safety profiles. Zinc oxide nanoparticles (ZnO-NPs) are demonstrated to be effective against major food-borne pathogens like E. coli O157:H7, Listeria monocytogenes and Salmonella spp. (Jin et al. 2009). ZnO-NPs are found to inhibit the growth of E. coli O157:H7 in a dose-dependent manner (Liu et al. 2009). Their antimicrobial action is attributed to membrane binding leading to lipid and protein destruction on membranes. This leads to altered membrane permeability and leakage of intracellular contents. These pathological changes are initiated by the generation of reactive oxygen species (Bhuyan et al. 2015).
14.7.1.3 Nitric Oxide – Releasing Nanoparticles
Nitric oxide (NO) has been identified as a molecule with varied physiological functions in the body. Several phagocytic cells like macrophages enhances the production and release of NO on stimulation through the transcription of inducible nitric oxide synthase (iNOS) (Englander and Friedman 2010). The NO thus released demonstrate antimicrobial activity by either of the several mechanisms identified like direct microbial DNA damage through generation of peroxynitrite, inactivation of zinc metalloproteins and interfering cellular respiration or by stimulating innate antimicrobial pathways that enhances host immune response. Also, there are research reports on the activity of NO as a wound healing agent. Considering these properties, a nanomaterial system has been created that donates or delivers NO to the site. NO – releasing nanoparticles (NO-NPs) entraps NO in a dry matrix and releases gaseous NO free radicals on exposure to moisture. This is utilized as a topical agent for promoting wound healing and preventing infections. NO–NPs can easily be applied on skin and ensures sustained delivery to the affected areas over prolonged period of time. These nanomaterials have been tested against S. aureus, Acinetobacter and MRSA – infected wounds and showed accelerated wound closure and decreased microbe burden (Martinez et al. 2009).
14.7.1.4 Drug – Infused Nanoparticles
Intracellular infections from organisms like Salmonella, Listeria and M. tuberculosis are difficult to eliminate due to the advantage they achieved from highly evolved evolutionary mechanisms like escaping phagosomes, phagosome–lysosome fusion inhibition and their ability to stay dormant (Pinto-Alphandary et al. 2000). These mechanisms downregulate several drug targets. Complicating the scenario is the fact that several antibiotics cannot penetrate intracellularly to act on these microorganisms. In such conditions, nanoparticles and liposomes are devised as potential drug carriers as they get endocytosed into phagocytic cells carrying intracellular pathogens. Several antibiotic-encapsulated liposomes are developed to deliver antibiotics like β-lactams like penicillin, ampicillin and cephalosporins, macrolides, aminoglycosides and fluoroquinolones for enhanced bacterial killing. Ampicillin-encapsulated liposomes against Salmonella, liposome-encapsulated tobramycin against P. aeruginosa and amphotericin B-encapsulated liposome against moulds and yeasts have been studied (Fattal et al. 1991). In spite of their success as a promising drug delivery system, there are limiting factors that impede their application in medicine . Some of the highly discussed limitations are their size, charge, purity, solubility of contents, stability, antigenicity, biocompatibility etc. There are two ways to deliver the drug to its target site by the nanocarriers – passive and active targeting. In passive targeting, nanocarriers are transported through the inter-endothelial cell spaces on the neovascularized vessels of the tumour mass or through the fenestrations on the vessels at the sites of inflammation. Passive transport is dependent on the plasma permeability and their time of retention. In the case of active targeting, nanocarriers are transported based on the receptor–ligand interaction, and this requires fenestrae or specific receptors. Change in the pH of the medium or a sudden oxidative burst can facilitate the delivery of the drug to its required target. Locally they can be transported with the aid of magnetic guidance and radio frequency-mediated delivery .
14.7.1.5 Immunomodulatory Effects
The immune system helps the body to fight against foreign substances including pathogens. It is broadly classified into innate and adaptive systems. The nonspecific immune response is termed innate immune response, which involves the recognition of the pathogen-specific molecular patterns (PAMPs) of the invading pathogens (Plummer and Manchester 2011). Antigen presenting cells (APCs) engulf the pathogen after the PAMPs have been recognized by the pattern recognition receptors (PRPs) present on cells. The cascade of events following the APCs engulfing and presenting the antigens to the cells of the adaptive immune system culminates in the activation of CD4 and CD8 lymphocytes subsequent to the induction of T and B cells (Plummer and Manchester 2011). The entire machinery works on an integrated and coordinated network of events mediated by cytokines which decides on the type of response to each pathogen. For example, Interferon gamma activation promotes T helper type 1 (Th1) response while Interleukin-4 (IL-4) and IL-5 activation results in a Th2 response (Sun et al. 2009). A synchronized interaction between APCs, T and B cells and inflammatory cytokines is essential for a functionally robust immune system.
Vaccines play an integral part of infectious disease control and require a balanced stimulation of both innate and acquired immune systems. Vaccines basically belong to either of the three categories viz., live attenuated, killed or fragmented (subunit) vaccines. With respect to the fairness of immunogenic response generated, live attenuated vaccines are the best, but they pose a threat of reactivation and attaining virulence inside the host. While killed and subunit vaccines are devoid of this threat, their immunogenicity is very poor, requiring repeated dosing and thereby increasing the cost. Since a trained personnel is essential to administer a vaccine in the form of injection, vaccine coverage is decreasing in many countries in spite of a global intervention. Mucosal application with the help of nanotechnology is one alternative to overcome this hurdle. There are several barriers to cross for the vaccine to reach the APC and induce a strong enough immune response. In intranasal or inhalational vaccines, the vaccine components should be smaller (< 5um) and not to be cleared by the exhalation (Chadwick et al. 2010). As the size specified is in accordance with that of a nanomaterial, nanoparticles are capable enough to modulate and influence the immune system at various levels of interaction. Nanoparticles can thus augment the efficacy of both oral and injected vaccines by enhancing the vaccine exposure time to the immune system as well as an increase in the uptake of antigens by APCs (Bal et al. 2010). Thus they improve the immune response to microbes like viruses and bacterial components and also lead to a more controlled and modified cytokine response (Huang et al. 2010).
14.7.2 Nanotechnology-Based Vaccines and Immunostimulatory Adjuvants
14.7.2.1 Synthetic Polymers
Polymeric nanoparticles are utilized as carriers for different vaccines like DNA vaccines and for proteins as well. Choice of nanomaterial is based on the requirements of the vaccine. Poly ε-caprolactone polymers are used to overcome the physicochemical differences in the digestive tract in the case of oral vaccines. Multicomponent particles are created encapsulating DNA in a PCL microparticle and this system is named nanoparticle-in-microsphere hybrid oral delivery system (NiMOS). The central core of NiMOS is susceptible to proteolytic degradation while the exterior is susceptible to lipase digestion. As the particles transit through the gut, the outer coat survives the proteolytic degradation and as they reach the intestines, got acted on by lipases and release the inner gelatinous core containing the vaccine which gets absorbed from the intestines and produce the antigenic effect. Many of the synthetic polymers used for vaccine delivery are found to be non-immunostimulatory. Poly(lactide-co-glycolide) (PLGA) particles does not lead to the rise in pro-inflammatory cytokine levels even after being taken up by the macrophages. But they produced stronger and sustained IgG response when encapsulating plasmid DNA owing to the stability of PLGA nanoparticles during their delivery into appropriate cells. This highlight the fact that nano polymers alone cannot be used as immunostimulants, but they can enhance immunization when they cross the mucosal barriers. A polymeric nanoparticle, Polymethyl methyl methacrylate (PMMA) is highly immunostimulant when administered as a vaccine adjuvant. It increased the antibody titre to 100-fold in HIV2 virus vaccine in animal models and also exhibited enhanced IgG and IgM antibody production against ovalbumin. Several other nanomaterials have also shown immunomodulatory activity for example; Carboxyfullerene nanoparticles increased immunologic activity by neutrophil activation resulting in microbial death in a S. pyogenes infection model in mouse. Some nanoparticles enhanced immune responsiveness in conjunction with Toll-like receptor agonists. PLGA NP modified with tetanus toxoid and MUCI lipopeptide (a TLR ligand) showed significant T cell activation and responsiveness in comparison to PLGA NP and tetanus toxoid or MUCI lipopeptide alone (Diwan et al. 2003).
Polymeric oligonucleotides of DNA or RNA termed Aptamers, which folds in 3-dimensional configuration with high affinity to target proteins, peptides or small molecules of drug or vitamin showed pronounced antimicrobial activity, inhibition of HIV reverse transcriptase and vaccinia virus replication and to overcome β-lactamase resistance in Gram-positive and Gram-negative bacteria. Aptamers are selected from random nucleic acid libraries and technologies are now available to prepare them with highest degree of purity, stability and selectivity.
14.7.2.2 Nanoemulsions
Nanoemulsions constitute lipophilic or hydrophilic substances dispersed as either water-in-oil or oil-in-water forms. Such carrier systems are engaged in vaccine delivery through mucosal barriers as they are easily endocytosed by the surface cells of the mucosa and easily presented to the APCs. Nanoemulions have also showed good immunostimulatory effect. The hepatitis B vaccine currently in use is given as an IM injection of recombinant hepatitis B surface antigen (HBsAg), containing aluminium salt (alum) as an adjuvant. Alum stimulates a Th2 immune response in the host with an ineffective CD8 response to the virus infected hepatocytes. Alum is also responsible for other complications like the formation of erythema and nodules over the injection site. On the contrary, recombinant HBsAg nanoemulsion (HBsAg – NE)-based intranasal vaccine produces an effective Th1 response in the host without any chance for local inflammation (Muttil et al. 2010). They are also capable of producing comparable levels of IgG antibody body level and significant levels of mucosal IgA antibodies as well. Nanoemulsion vaccines are also being tried for inactivated influenza and vaccinia virus infections.
14.7.2.3 Immune Stimulating Complexes
Immune-stimulating complexes (ISCOMs) are carriers and immunostimulatory adjuvants, which are nanosized spherical micelles containing saponin-derived components like Quil A, derived from the tree bark Quillaja (Helgeby et al. 2006). They are efficient carriers easily taken up by APCs and can activate and upregulate the expression of MHC I and II on APCs, even in the absence of an antigen. The overall result of this is the induction of pro-inflammatory cytokines like IL1, IL6, IL8 and IFNχ. Carriers containing saponin-derived components produce Th1 type response, but this may change depending on the adjuvant carried (Sun et al. 2009). ISCOMs carrying Leishmania antigen induce a Th2 response. Studies on various antigens incorporated on ISCOMs are available including influenza, hepatitis B, herpes virus, Helicobacter pylori and Corynebacterium (Sun et al. 2009).
14.7.2.4 Cytidine-Phosphate-Guanosine (CpG) Motifs
Bacterial oligodeoxynucleotides containing unmethylated CpG motifs are used as vaccine adjuvants with good immunostimulatory activity. They lead to enhanced secretion of IL12, surface expression of MHC, Th1 recruitment and activation and secretion of IgG antibodies as they are recognized by the APCs. They are recognized by the APCs through the activation of Toll-like-receptor 9 (TLR-9). CpG motifs incorporated into a nanoemulsion of water-oil-water has inactivated influenza virus and induced strong and sustained immune response when compared to conventional vaccines.
14.7.2.5 Chitosan
Chitosan improved vaccine delivery in the case of oral vaccines, as it proved to be an efficient carrier system to transport vaccines across mucous membrane. It also find its use as an immune adjuvant as it promotes antigen uptake and cytokine production. When incorporated with the glycoproteins present over the surface of influenza virus like HA and NA, a pronounced immunological response in the form of high titres of serum IgG and mucosal IgA antibodies were observed after intranasal administration (Brunner et al. 2000). Escalated IgA levels are attributed to the relatively strong interaction of chitosan with the sialic acid residues over the mucin. This interaction results in the opening up of the mucosal tight junctions enhancing the mucosal membrane transport and vaccine retention time (Florindo et al. 2009). Chitosan has gained importance as vaccine adjuvants with immunomodulatory stimulation capability. N-trimethyl chitosan (TMC) nanoparticles coupled with ovalbumin and diphtheria toxoid where swiftly internalized by the APC of the skin, Langerhans cells, and induced a profound expression of CD83, CD86 and MHC-II in murine models on intradermal administration. Once they are internalized, the antigens set free from the chitosan after lysosomal degradation, the immunological processes unfurl inside the body and the antigen will be presented to the T cells followed by a Th2-mediated antibody response. The antibody titre is higher than either of the antigen alone and this establishes its immunostimulatory effect as an adjuvant.
14.7.2.6 Metallic Nanoparticles
Every drug or gene transported in a nanoparticle should be well protected against the immune recognition by the host’s body because of the activation of the innate immune response and subsequent inactivation of the drug. To a great extend this property is well exhibited by metal nanoparticles (Massich et al. 2009). Polyvalent oligonucleotide coupled with gold nanoparticle produced only 25% less macrophage activation evidenced by IFNβ when compared to lipid-complexed DNA.
14.8 Role of Nanomaterials in Other Diseases
14.8.1 Neurodegeneration
Neurodegeneration is defined as the destruction in the structure and function of neurons leading to their loss. This basic event cascades to a plethora of incurable pathological anomalies to central nervous system collectively called as neurodegenerative disorders which include Alzheimer’s disease, Parkinson’s disease, Prion disease, amyotrophic lateral sclerosis and Huntington’s disease (Rubinsztein 2006). Neurodegenerative changes occur at different levels in the nervous system ranging from molecular level to systemic level. Distorted synaptic functions, a higher prevalence of intra-neuronal deposits resulting from misfolded proteins are some of the common events related with many neurodegenerative disorders, which helps in devising therapeutic modalities. Many more pathological events like genetic mutations, protein misfolding, protein aggregation, mitochondrial dysfunction, damage to the nucleic acid (DNA), structural and functional disruption of organelle membranes, neuronal and microglial apoptosis, autophagy and transglutaminase binding strongly influence the manifestation of neurodegenerative diseases. As neurodegenerative diseases occur during the later decades of life, the events leading to its manifestations ae attributed to mitochondrial DNA mutations and oxidative stress. Progression of neurodegenerative disease with advancing age is proportionated with the amount of neuronal loss and contributes a great deal of stress on the families emotionally, socially and financially. Considering the therapeutic challenges in neurodegenerative diseases, blood-brain-barrier (BBB) poses the greatest challenge. BBB represents a selectively semipermeable barrier between the nervous tissue and the blood compartments. It helps in the maintenance of neuronal cell functions, regulation of transport of nutrients and metabolites and the overall protection of the brain tissue. It avoids the entry of large therapeutic molecules as well a small molecule drugs from entering brain. Carrier-mediated transporters like large neutral amino-acid transporter, glucose transporter (GLUT1), cationic amino-acid transporter (CAT1), adenosine transporter (CNT2) and monocarboxylic acid transport small molecules to brain (Pardridge 2003). Certain circulating blood elements like leukocytes, erythrocytes, neutrophils, and other cells like exosomes can cross BBB at a faster rate. Drug delivery utilizing these living cells and exosomes paved way to the discovery of novel chemotherapeutic drug delivery to treat disorders of brain. Curcumin loaded exosomes experimentally showed improved cognitive function in mouse. Receptor-mediated transcytosis (RMT) is the common modality now used to deliver chemotherapeutic drugs to brain tissue across BBB. Endogenous macromolecular neuropeptides including transferrin, hormones, lipoproteins and insulin reaches brain tissue through RMT from blood through specific receptors. RMT are expressed on the luminal side of the endothelial cells and they favour endocytosis and transcytosis of molecules across the BBB (Abdul Razzak 2019). In RMT, nanoparticles are utilized after surface modification to bind to the transmembrane receptor associated with its transport. Macromolecular drug delivery to brainrelated diseases can also be increased tremendously by delivering drugs directly into the brain tissue with the help of electrostatic interactions between nanoparticle, which bears a positive charge, and the negatively charged BBB membrane through a process termed adsorptive-mediated transcytosis (AMT). AMT does not interfere with the normal cellular physiology as other drug delivery methods do. Nanocarriers used to cross BBB are;
14.8.1.1 Metal Nanoparticles
Transition metals like Zn, Cu and Fe are present in a sufficiently large amount in the brain and they play a crucial role in their cellular physiology including the metallo-enzyme function. They act as a therapeutic agent as well as an agent that carries diagnostic agent due to its specific physicochemical properties. These metal NPs are also used for the detection of biomarkers. They are used in various shapes and sizes, surface charge and by coupling with various surface ligands for targeting for effective drug delivery for NDs. Functionalized super-paramagnetic iron oxide (SPION) is presently used for theranostic applications in imaging techniques like MRI and targeted therapy in NDs (Luo et al. 2020).
14.8.1.2 Lipid-Based Nanoparticles
Lipid-based NPs are developed as theranostic agents in the treatment of various NDs, and they are unique in the aspect of negligible side effects when compared to other technologies. This is achieved through modifying their properties by enabling drugs and ligands to bind on to their surface (Niu et al. 2019). The well-known phytoconstituents in NDs is curcumin, which has many limitations due to its poor solubility, low bioavailability and instability. Soft lipid-based nanocurcumin has provided a new hope in inhibiting neuronal loss in Parkinson’s, Alzheimer’s, ALS and Huntington’s diseases (Rakotoarisoa and Angelova 2018).
14.8.1.3 Hydrogels
Hydrogels are 3D polymeric mesh-works capable of holding water. They find extensive use as a neuroprotective agent. Hydrogels are capable of systemic delivery of drugs directly into the brain tissue for targeted action in NDs. Activin B-loaded hydrogels have been developed, capable of slowly releasing activin-B over a period of several weeks for the treatment of Parkinson’s disease (Albani et al. 2013).
14.8.1.4 Dendrimers
Dendrimers are credited with the position of one of the smallest nano-formulations used in the treatment of NDs. Dendrimers are manipulated by changing their size, core-shell or the surface functional groups to be used as nanocarriers for drug or gene delivery to the brain tissue. Moreover, they have strong anti-amyloidogenic activity that makes them ideal for the treatment of PD, Prion diseases and Alzheimer’s. They also find their application in other areas like sterilization of medical instruments by phosphorus-containing dendrimers specially to prevent transmission of Prion disease (Šebestík et al. 2012).
14.8.1.5 Polymeric Nanoparticles
Polymer nanoparticles have the advantage of very low toxicity due to their fast excretion rate. They are block co-polymeric molecules that are biocompatible and biodegradable, and made up of lactic-co-glycolic acid (PLGA), polylactic acid (PLA), PLGA-PEG etc. (Calzoni et al. 2019; Prasad et al. 2017). Promising results have been shown by curcumin-loaded PLGA nanoparticles in the treatment of Alzheimer’s disease, showing an enhanced drug delivery and reduced oxidative stress and inflammation (Barbara et al. 2017; Mukherjee et al. 2020).
14.8.2 Cancer Therapy
Cancer therapy protocols of current era are restricted to surgery, radiation and chemotherapy. These treatment regimens, though successful to some extent, will not provide complete eradication of disease and on the contrary damage healthy tissues also. The limited success rate of the conventionally used chemotherapy is due to the lack of water-soluble chemotherapeutic agents, lack of drug sensitivity of the cancer cells and resistance to multiple chemotherapeutic drugs developed due to their repetitive administration. The modern-day cancer therapeutics aims at boosting the natural capacity of the body to identify and destroy abnormal cells. Cancer cells have also adapted to evade the body’s immune response by downregulating tumour surface antigen expression, extrusion of certain proteins to deactivate the immune cells or altering the cells in the surrounding microenvironment in order to suppress immune response. Immunotherapy acts by either stimulating the activities of components of immune system or by inhibiting the signals produced by the cancer cells that suppresses the immune responses.
14.8.2.1 Treatment Modalities in cancer
14.8.2.1.1 Immune Checkpoint Modulators
Natural proteins secreted by the cancer cells to prevent the damage of the normal and tumour cells by triggering the immunological responses are called immune checkpoint modulators. Suppressing the stimulation of immune response proteins will result in the activation of immune responses and their capability to destroy cancer cells. Activated cytotoxic T lymphocytes expressing CTLA4 on their surface is an FDA approved immune checkpoint inhibitor.
14.8.2.1.2 Adoptive Cell Transfer
Adoptive cell transfer is a promising treatment modality in cancer therapy where infiltrated T cells are isolated from the tumour samples of the patient and they are segregated based on their highest response shown to recognize patient’s tumour cells. These cells are isolated in a laboratory, cloned and cultured to generate a large population, which are later activated by immune signalling proteins, cytokines, and are reinfused into the patient’s body (Perica et al. 2015).
14.8.2.1.3 Therapeutic Antibodies
Therapeutic antibodies are lab-designed therapeutic molecules like antibody-drug conjugates (ADCs) that selectively destroy the cancer cells. In ADCs, a cytotoxic substance like bacterial toxin or cytotoxic small molecule drug or a radioactive compound is coated with antibodies or fragment antibodies by chemically linking them on to their surface. The target molecule expressed over the surface of the tumour cells will bind with the antibodies over the ADCs. The ADCs will be subsequently internalized by the tumour cells and the cytotoxic substance will destroy the cell (Scott et al. 2012).
14.8.2.1.4 Cancer Treatment Vaccines
Cancer vaccines are prepared from patients’ own tumour cells or substances produced by the tumour cells. They are designed to treat already developed cancers by strengthening the body’s natural defences against the cancer (Guo et al. 2013). Several new approaches have been developed in this novel treatment strategy. Contrary to the vaccines developed for other diseases, which prevent the occurrence of the disease, cancer vaccines are effectively utilized for their curative aspects. Vaccines targeting the tumour antigens form a major group of vaccines. As the tumour antigens vary drastically with the types of tumour as well as with individuals, two categories of cancer vaccines are identified; vaccines against tumour antigens that are specific to a particular tumour (Molecular vaccines) and those that are non-specific (Cellular vaccines) (Cheever et al. 2009). The efficacy of a vaccine relies on effectiveness of the adjuvant. Different adjuvants like incomplete Freund’s adjuvant in emulsified form, particulates and saponins are extensively researched. Many cancer vaccines act by modulating T cell immunity. Generation of CD8+ T effector and memory cells from CD4+ T cell help response is crucial in the effectiveness of cancer vaccines. Several other strategies including patient-derived immune cell vaccines, expression of tumour antigens with the help of recombinant viral vaccines, peptide vaccines, DNA vaccines and whole cell vaccines have been developed against cancers (Hearnden et al. 2013).
14.8.2.1.5 Chimeric Antigen Receptor T-Cell Therapy (CAR-T Cell Therapy)
CAR-T cell therapy which is an advanced Adoptive cell transfer technology involving harvesting of T cells which are then genetically engineered to express specific chimeric antigen receptors (CARs) on their surface with which the tumour cells are identified. The CAR-T cells are then proliferated to a population of billions or more in laboratory and are then introduced to the bloodstream of the patient where they proliferate and identify the tumour cells with the help of the genetically engineered receptor and destroys them. Though they are being used clinically, they have some untoward effects like toxicity related to uncontrolled T cell activity, cytokine release syndrome, shared expression of tumour-specific antigens by healthy cells and “on-target, off-tumour” toxicity (Zhang and Xu 2017).
14.8.2.1.6 Stem Cell Transplant
Stem cell transplant is another area of interest that provided positive results. Autologous, allogenic and syngeneic transplantation of marrow cells collected from healthy subjects are transfused through a vein of the patient which gets engrafted in the bone marrow produces a new lineage of blood cells. Autologous transfusion represents self stem cell transplantation before chemotherapy, while allogenic transplantation is the stem cell transplantation from a suitable donor; whereas syngeneic transplantation is done using stem cells from an identical sibling. In all the three types of transplantations, the marrow stem cells are harvested in aseptic conditions and are stored in a specific nutrient medium at particular freezing temperature after filtration.
14.8.2.2 Nanotechnology Used in cancer Treatment
14.8.2.2.1 Nanocarriers
Chemotherapy utilizes the drugs that kill cancer cells, but many of these agents also destroys healthy cells also leading to adverse events. Nanoparticles are utilized as the vehicles to deliver chemotherapeutic medications directly to the tumour site sparing the healthy tissue. Nanomaterials provide an effective size of the particles between 10 and 100 nm which enables them to reach the cells more efficiently. Secondly, nanoparticles can easily adsorb onto the cell matrix and thus becomes easily integrated into the cell and thus increases the effectiveness of the drug. This is partly contributed by the high dissolution rate of the nanoparticles owing to its larger surface-to-volume ratio. Thirdly, they exhibit drug delivery to specific target site and thus overcomes the hurdle of poor solubility and drug uptake. Fourthly, they can prevent dose related adverse events effectively by controlled drug release. Various pharmaceutical techniques like nanosphere encapsulation has provided efficient methods to prolong exposure to drug by slow and controlled drug release which is the most desirable property for a chemotherapeutic agent. Thus, utilization of nanotechnology in pharmaceutical industry improves therapeutic index, solves problems in drug delivery, protects drug from degradation prior to their binding to the target site, enhances bioavailability, facilitates drug absorption by the tumours cells and prevents interaction with normal cells.
14.8.2.2.2 Passive Targeting
Nanoparticles can easily pervade to tissues from the blood stream due to their nanoscale size and surface properties. This property is utilized in cancer therapy as many tumours contain blood vessels with leaky walls which help the drug to concentrate in the tumour tissue and exert its cytotoxicity only to the tumour cells, sparing the healthy normal cells. This property is termed as enhanced permeability and retention (Jasim 2017; Greish 2010).
14.8.2.2.3 Active Targeting
Active targeting refers to the properties of a nanoparticle to target cancer cells selectively based on the chemical affinity to the molecules expressed on their surface. Molecules attached to a nanoparticle can target and interact with the receptor expressed over the cancer cells. Thus, the drug can be delivered into the cancerous cells letting free the normal cells (Kumar 2012).
14.8.2.2.4 Destruction from inside the Cell (Photothermal Targeting)
Photothermal targeting is utilizing the capability of nanomaterials to convert specific wavelengths of light to thermal energy which can be used for the purpose of treatment in conditions like cancers. Photothermal therapy (PTT) are highly efficacious yet with minimal side effects when compared to traditional therapies (O'Neal et al. 2004. They precisely exterminated the tumour tissue without damaging any of the normal tissues. PTT is attributed with the advantage of producing minimal trauma, less toxicity, precise targeting, wide applicability, repeatability in targeting, usefulness in palliative treatment, improvising disease curability in association with surgical procedures, elimination of occult cancers, preservation of aesthetic appeal of the patients and protection of organ functions. They utilize the near-infrared region of the electromagnetic spectrum (700–1000 nm), which has the highest penetrating power in biological tissue. Nanomaterials with photothermal properties are injected into the patient which then accumulates in the cancer cells due to its enhanced permeability and retention (EPR) effect (Yavuz et al. 2009). On reaching the target site, they absorb wavelengths in the near-infrared region and generate heat energy from them sufficient to kill the tumour cells. It is explained that the light energy is absorbed by the nanomaterial in the form of photons, a part of which is then transmitted as photons and part of it is converted into heat energy which raises the temperature of the nanomaterial. The local rise in temperature around the tumour cells causes dilatation of vessels around the tumour mass leading to accelerated blood flow into the tumour tissue dissipating the enhanced temperature into the cancerous cells. As tumour mass is attributed with poor blood flow rate (less than 5% of normal tissues), the hyperpyrexia induced by the photothermal agent will not be dissipated out of the tumour mass leading to a swift rise in temperature of the tumour of the order of 42 degrees, which is sufficient to damage and kill the tumour (Shah et al. 2008). The nanosized photothermal agent injected into the body are excreted through two major routes – through kidneys (when their diameter is around 5 nm) or through liver (when their diameter is around 20–25 nm). Based on the nature of the nanomaterial used, there are four broad categories of photothermal agents: (1) Noble metal photothermal agents: gold, silver, platinum etc. (2) Photothermal agents made of carbon materials: graphene oxide, carbon nanotubes etc. (3) Photothermal agents from transition metal dichalcogenides: copper sulphide, zinc sulphide, bismuth sulphide, tungsten sulphide, bismuth selenide etc. (4) Organic- and dye-based photothermal agents: prussian blue, indocyanine green, polypyrrole, polyaniline, dopamine, melanin, thiophene etc. Noble metals like gold, silver and platinum exhibit a phenomenon called surface plasmon resonance (SPR) effect due to their highly ordered electromagnetic field and a greater electromagnetic absorption owing to their electronic structure in the conductive band. (Zhang et al. 2018). Gold is considered as an ideal material for photothermal effect due to its strong surface plasmon resonance effect. Gold nanoparticles of varied shapes like nanorods, nanoshells, nanostars, nanocages and nanobranches are used as photothermal agents owing to its extended stability, biocompatibility and non-toxic nature (Cobley et al. 2010). Silver nanoparticles, being one of the most widely used and cheapest of all noble metal in nanoscale, is possessing a unique property of surface enhanced Raman scattering (SERS) effect which is considered as an abnormal surface optical phenomenon due to which it has an immense absorptive capability at near-infrared wavelengths. Silver also exhibits strong localized surface plasmon resonance quality and as the near-infrared wavelengths fall on them, causes surface free electron resonance leading to the formation of an exothermic electron gas which transfers its energy to the surrounding environment through the medium of the nanoparticle resulting in rise of temperature. Platinum is also sharing properties similar to gold and silver yet will lower toxicity and high biocompatibility. They are being used in cancer therapy to scavenge oxygen free radicals generated in cancer cells by enzyme mimetics. Carbon materials like Graphene has greater absorption coefficient in near-infrared wavelengths with additional electrical, mechanical, optical and thermal properties making it more advantageous in PTT. They have added advantages of having large functional groups containing oxygen, low toxicity and production cost and highly biocompatible. It is being considered as the most promising and reputed carbon nanomaterial of the twenty-first century. Nanotubes made of carbon also show tremendous photothermal properties and can be utilized for thermal destruction of tumour cells. They can also be used as a drug carrier and can load chemotherapeutic agents on their surface and helps in combining phototherapy with chemotherapy synergistically. Transition metal chalcogenides attracted tremendous research interest recently due to its excellent thermal effect. They are being used as layered structures like tungsten disulphide and molybdenum disulphide or bismuth selenide, bismuth sulphide, silver sulphide and copper sulphide exhibiting high extinction coefficient and enhanced absorption in the near-infrared region and visible light.
14.8.2.2.5 Simultaneous Delivery of Two Drugs
Multidrug delivery using nanoparticles is utilized in resistant cancers with the tendency to relapse frequently. In the most resistant form of cancers like the triple-negative breast cancer treatment, they have been successfully implemented. Simultaneous delivery of multiple interventions like small interfering RNA (siRNA) along with chemotherapeutic agent doxorubicin in the treatment of breast cancer has been carried out successfully (Jiang et al. 2014; Sebastian 2017). These researches promise a new ray of hope even to the most resistant type of cancers.
14.8.3 Nanotechnology in Diabetes Mellitus
With the rising prevalence of obesity and aberrant lifestyle habits, the diabetes disease burden is rising globally. The global prevalence of diabetes is expected to touch 600 million mark by the turn of 2035 (Forouhi and Wareham 2010). Diabetes mellitus (DM) is a metabolic disorder caused by insufficient or absent secretion of insulin, which is secreted by a group cells in the pancreas termed islets of Langerhans. DM is classified mainly into two – Type 1 and Type 2 DM. Type 1 DM also called insulin-dependent DM is caused by the extensive destruction of the beta cells of islets of Langerhans by an autoimmune response involving the T-cells. Type 2 DM on the other hand is caused due to insulin resistance or sensitivity associated with decreased production (Subramani et al. 2012).
14.8.3.1 Nanoparticles for Insulin Delivery
Some of the common drug delivery systems utilizing nanotechnology are listed below; (Yih and Al-Fandi 2006; Attivi et al. 2005)
-
Polymeric biodegradable nanoparticles
-
Ceramic nanoparticles
-
Polymeric nanoparticles
-
Dendrimer
-
Liposomes
Polymeric nanoparticles are manufactured as colloidal solid particles (with dimensions ranging from 10 to 1000 nm) and based on the difference in their preparation they are of two types – Nanospheres and Nano-capsules. Nanospheres are polymeric nanoparticles having the drugs uniformly dispersed. Nano-capsules on the other hand have drugs dispersed in polymer membrane bound cavities that form vesicular systems (Tsapis et al. 2002; Si et al. 2003). These nanomaterials degrade on hydrolysis and delivers the medication to the targeted tissue.Polymeric nanoparticles are utilized for insulin transport utilizing a nonporous membrane bound polymer-insulin matrix containing glucose oxidase grafted on to their surface. An escalation in blood glucose level results in release of insulin after the biodegradation of the membrane of these nanoparticles (Morishita et al. 1992). Successful trials for oral insulin regimens with casein combined polymeric calcium phosphate-polyethylene glycol containing insulin have been undertaken (Sarmento et al. 2007). Casein prevents inactivation of insulin by gastric enzymes and the nanoparticles remain in small intestine for a longer duration favouring prolonged slower absorption and bioavailability due to its muco-adhesive property. Ceramic nanoparticles have high biocompatibility, low size (50 nm) and dimensional stability and are made up of materials such as calcium phosphate, alumina, titanium or silica (Rai et al. 2016). Porous hydroxyapatite nanoparticles have been used for delivering insulin into the intestines. Similarly, insulin-loaded poly nanoparticles are developed as nasally administered insulin. Bio Micro Electro Mechanical Systems (BioMEMS) are developed to implant them for controlled and prolonged release of insulin for long-term blood glucose control. Apart from these systems, gold nanoparticles along with chitosan as a reducing agent are being developed to carry insulin (Rai et al. 2016). Gold nanoparticles served a dual purpose in this case as a reducing agent as well as enhancing the penetration and uptake of insulin.
DM is also associated with lot of systemic complications like retinopathy, coronary artery disease (CAD) , nephropathy, inflammatory gum diseases, delayed wound healing, inflammatory skin diseases and neuropathy. Polyacrylic acid, polylactide and chitosan have been used as carriers for ophthalmic drug delivery in DM.
Though nanomaterials find their application in almost all types of diseases, this section has been devoted to the most ‘notorious’ and most prevalent diseases of public health importance. Hence, we discussed the use of nanomaterials in cancers and neurodegenerative diseases. This does not in any way cut down the importance of research and use of nanomaterials in other diseases like cardiovascular, respiratory, reproductive and endocrine disorders. It is also worthwhile to discuss here the rising concern regarding the use of nanomaterials in medicine as diagnostics and therapeutics, that is, their role as an endocrine disruptor is gaining momentum in many spheres of health. Though they appear as a ‘sole curse to a billion boons’, yet researches in this area must go on and, in its run, should also solve this ‘blotch on its face’ permanently.
14.9 Future Perspective of Nanomedicine and Biology
The possibilities of nanotechnologies are immense and beyond every known boundary. As we have been discussing the positive aspects of nanotechnology so far, it is time to see some of vices that this technology brings to humans. When it comes to its application in the field of medicine and biology, the dose and toxicity are of major concern. Even with the first medically used nanoparticle, nanosilver, itself toxicities have been noticed in the form of permanent pigmentation over the skin at higher doses which has been termed ‘Argyrosis’ (McShan et al. 2014; Korani et al. 2011). Similarly, titanium dioxide nanoparticles in inhalation and ingestion have produced pathological lesions on lung, liver, kidneys, spleen and brain in animal models. The first and foremost concern in the future perspective is to bring down the toxicity. As we are facing the global transmission from SARS -CoV-2, the world is still lurking in the dark for a permanent cure in the form of a vaccine (Liu et al. 2020; Singh 2020). The percentage of susceptible population is very high and the infection can take a serious turn at any point in the form of cytokine storm, septic shock, metabolic acidosis, coagulation dysfunction, acute respiratory distress syndrome (ARDS) finally leading to death. An effective treatment is still lacking due to drug resistance and non-specific targeting. This highlights the need for a vaccine immediately to put a stop to this scourge. Nanotechnology is the only solution to design new strategies to design vaccines against this virus and the preparedness to oversee the emerging outbreaks yet to come in the future. Cancer treatment is yet another field requiring improvisation. We are still not in a position to get rid of the cytotoxic drugs out of our cancer armamentarium. Targeted therapies are to be refined to spare the healthy tissue from the toxicity of the chemotherapeutic agents. As we are moving back to the era of high mortality from infectious diseases, it is high time to move to the phytoremedies coupled with nanotechnologies in the cure of infectious diseases. In-depth researches are needed to ascertain effective drug delivery systems in this regard. Nanotechnology still stands the forerunner to advance all technologies to the next millennium.
14.10 Conclusion
Nanotechnology has been in existence from the time humans started to live in communities in this planet. Every aspect of life has been touched by nanoscience. Application of nanotechnology in biology and medicine revolutionized health care. Nanoscience gained entry in prevention, diagnosis and treatment of diseases. The spectrum of diseases ranging from the non-communicable diseases to communicable diseases equally shared the benefits of nanoscience. Though the world has been benefited from nanotechnology and its applications, the inherent toxicity is masquerading its benefits. The future is for the technologies that address the toxicities arising from the nanomaterials used for medical purposes.
References
Abdul Razzak R, Florence GJ, Gunn-Moore FJ (2019) Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int J Mol Sci 20(12):3108
Abee T, Rombouts FM, Hugenholtz J, Guihard G, Letellier L (1994) Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol 60(6):1962–1968
Albani D, Gloria A, Giordano C, Rodilossi S, Russo T, D’Amora U, Tunesi M, Cigada A, Ambrosio L, Forloni G (2013) Hydrogel-based nanocomposites and mesenchymal stem cells: a promising synergistic strategy for neurodegenerative disorders therapy. Sci World J. https://doi.org/10.1155/2013/270260
Alekshun MN, Levy SB (2007) Molecular mechanisms of antibacterial multidrug resistance. Cell 128(6):1037–1050
Amaral L, Kristiansen JE (2000) Phenothiazines: an alternative to conventional therapy for the initial management of suspected multidrug resistant tuberculosis. A call for studies. Int J Antimicrob Agents 14(3):173–176
Andriashvili IA, Kvachadze LI, Bashakidze RP, Adamiia R, Chanishvili TG (1986) Molecular mechanism of phage DNA protection from the restriction endonucleases of Staphylococcus aureus cells. Mol Gen Mikrobiol Virusol (8):43
Attivi D, Wehrle P, Ubrich N, Damge C, Hoffman M, Maincent P (2005) Formulation of insulin-loaded polymeric nanoparticles using response surface methodology. Drug Dev Ind Pharm 31(2):179–189
Aziz N, Fatma T, Varma A, Prasad R (2014) Biogenic synthesis of silver nanoparticles using Scenedesmus abundans and evaluation of their antibacterial activity. Journal of Nanoparticles, Article ID 689419. http://dx.doi.org/10.1155/2014/689419
Aziz N, Faraz M, Pandey R, Sakir M, Fatma T, Varma A, Barman I, Prasad R (2015) Facile algae-derived route to biogenic silver nanoparticles: Synthesis, antibacterial and photocatalytic properties. Langmuir 31:11605−11612. https://doi.org/10.1021/acs.langmuir.5b03081
Aziz N, Pandey R, Barman I, Prasad R (2016) Leveraging the attributes of Mucor hiemalis-derived silver nanoparticles for a synergistic broad-spectrum antimicrobial platform. Front Microbiol 7:1984. https://doi.org/10.3389/fmicb.2016.01984
Aziz N, Faraz M, Sherwani MA, Fatma T, Prasad R (2019) Illuminating the anticancerous efficacy of a new fungal chassis for silver nanoparticle synthesis. Front Chem 7:65. https://doi.org/10.3389/fchem.2019.00065
Bal SM, Slütter B, van Riet E, Kruithof AC, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA (2010) Efficient induction of immune responses through intradermal vaccination with N-trimethyl chitosan containing antigen formulations. J Control Release 142(3):374–383
Banerjee M, Mallick S, Paul A, Chattopadhyay A, Ghosh SS (2010) Heightened reactive oxygen species generation in the antimicrobial activity of a three component iodinated chitosan− silver nanoparticle composite. Langmuir 26(8):5901–5908
Barbara R, Belletti D, Pederzoli F, Masoni M, Keller J, Ballestrazzi A, Vandelli MA, Tosi G, Grabrucker AM (2017) Novel Curcumin loaded nanoparticles engineered for Blood-Brain barrier crossing and able to disrupt Abeta aggregates. Int J Pharm 526(1-2):413–424
Barber DJ, Freestone IC (1990) An investigation of the origin of the colour of the Lycurgus cup by analytical transmission electron microscopy. Archaeometry 32(1):33–45
Barrangou R, Fremaux C, Deveau H, Richards M, Boyaval P, Moineau S, Romero DA, Horvath P (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315(5819):1709–1712
Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F (2020) The history of nanoscience and nanotechnology: from chemical–physical applications to nanomedicine. Molecules 25(1):112
Benton B, Breukink E, Visscher I, Debabov D, Lunde C, Janc J, Mammen M, Humphrey P (2007) Telavancin inhibits peptidoglycan biosynthesis through preferential targeting of transglycosylation: evidence for a multivalent interaction between telavancin and lipid II. Int J Antimicrob Agents 29:S51–S52
Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A (2015) Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process 32:55–61
Bisht G, Rayamajhi S (2016) ZnO nanoparticles: a promising anticancer agent. Nano 3(Godište 2016):3–9
Biswas A, Bayer IS, Biris AS, Wang T, Dervishi E, Faupel F (2012) Advances in top–down and bottom–up surface nanofabrication: techniques, applications & future prospects. Adv Colloid Interf Sci 170(1-2):2–7
Blecher K, Nasir A, Friedman A (2011) The growing role of nanotechnology in combating infectious disease. Virulence 2(5):395–401
Bozdogan B, Appelbaum PC (2004) Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 23(2):113–119
Bracci L, Falciani C, Lelli B, Lozzi L, Runci Y, Pini A, De Montis MG, Tagliamonte A, Neri P (2003) Synthetic peptides in the form of dendrimers become resistant to protease activity. J Biol Chem 278(47):46590–46595
Brouns SJ, Jore MM, Lundgren M, Westra ER, Slijkhuis RJ, Snijders AP, Dickman MJ, Makarova KS, Koonin EV, Van Der Oost J (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321(5891):960–964
Brunner C, Seiderer J, Schlamp A, Bidlingmaier M, Eigler A, Haimerl W, Lehr HA, Krieg AM, Hartmann G, Endres S (2000) Enhanced dendritic cell maturation by TNF-α or cytidine-phosphate-guanosine DNA drives T cell activation in vitro and therapeutic anti-tumor immune responses in vivo. J Immunol 165(11):6278–6286
Burbulys D, Trach KA, Hoch JA (1991) Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell 64(3):545–552
Calzoni E, Cesaretti A, Polchi A, Di Michele A, Tancini B, Emiliani C (2019) Biocompatible polymer nanoparticles for drug delivery applications in cancer and neurodegenerative disorder therapies. J Funct Biomater 10(1):4
Cederlund H, Mårdh PA (1993) Antibacterial activities of non-antibiotic drugs. J Antimicrob Chemother 32(3):355–365
Chadwick S, Kriegel C, Amiji M (2010) Nanotechnology solutions for mucosal immunization. Adv Drug Deliv Rev 62(4-5):394–407
Cheever MA, Allison JP, Ferris AS et al. (2009). The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clinical cancer research 15(17):5323–5337.
Cioffi N, Torsi L, Ditaranto N, Tantillo G, Ghibelli L, Sabbatini L, Bleve-Zacheo T, D'Alessio M, Zambonin PG, Traversa E (2005) Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem Mater 17(21):5255–5262
Cleveland J, Montville TJ, Nes IF, Chikindas ML (2001) Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol 71(1):1–20
Cobley CM, Au L, Chen J, Xia Y (2010) Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Expert Opin Drug Deliv 7(5):577–587
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nature Reviews Microbiology 3(10):777–788.
Cotter PD, Ross RP, Hill C (2013) Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol 11(2):95–105
Czajkowski R, Jafra S (2009) Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochim Pol 56(1)
Diwan M, Elamanchili P, Lane H, Gainer A, Samuel J (2003) Biodegradable nanoparticle mediated antigen delivery to human cord blood derived dendritic cells for induction of primary T cell responses. J Drug Target 11(8–10):495–507
Dobson AP, Carper ER (1996) Infectious diseases and human population history. Bioscience 46(2):115–126
Drexler EK (1981) Molecular engineering: an approach to the development of general capabilities for molecular manipulation. Proc Natl Acad Sci U S A 78:5275–5278
Drexler EK, Peterson C, Pergamit G (1991) Unbounding the future: the nanotechnology revolution. William Morrow and Company, Inc., New York, NY
Džidić S, Šušković J, Kos B (2008) Antibiotic resistance mechanisms in bacteria: biochemical and genetic aspects. Food Technol Biotechnol 46(1)
Elsner J (2013) The Lycurgus cup. New light on old glass: recent research on byzantine mosaics and glass. The British Museum, London, UK, pp 103–111
Englander L, Friedman A (2010) Nitric oxide nanoparticle technology: a novel antimicrobial agent in the context of current treatment of skin and soft tissue infection. J Clin Aesthet Dermatol 3(6):45
Fattal E, Rojas J, Youssef M, Couvreur P, Andremont A (1991) Liposome-entrapped ampicillin in the treatment of experimental murine listeriosis and salmonellosis. Antimicrob Agents Chemother 35(4):770–772
Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52(4):662–668
Florindo HF, Pandit S, Lacerda L, Gonçalves LM, Alpar HO, Almeida AJ (2009) The enhancement of the immune response against S. equi antigens through the intranasal administration of poly-ɛ-caprolactone-based nanoparticles. Biomaterials 30(5):879–891
Forouhi NG, Wareham NJ (2010) Epidemiology of diabetes. Medicine 38(11):602–606
Garneau S, Martin NI, Vederas JC (2002) Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84(5–6):577–592
Greish K (2010) Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. In: Cancer Nanotechnol. Humana Press, pp 25–37
Grundmann H, Aires-de-Sousa M, Boyce J, Tiemersma E (2006) Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 368(9538):874–885
Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY (2013) Therapeutic cancer vaccines: past, present, and future. In: Advances in cancer research, vol 119. Academic Press, pp 421–475
Hale CR, Zhao P, Olson S, Duff MO, Graveley BR, Wells L, Terns RM, Terns MP (2009) RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139(5):945–956
Hauser AR (2015) Cell envelope. In: Antibiotic basic for clinicians, 2nd edn. Wolters Kluwer (India) Pvt. Ltd., New Delhi, pp 3–5
Heinrich MA, Martina B, Prakash J (2020) Nanomedicine strategies to target coronavirus. Nano Today:100961
Hearnden C, Lavelle EC (2013) Adjuvant strategies for vaccines: the use of adjuvants within the cancer vaccine setting. In: Prendergast GC,Jaffee EM (eds) Cancer immunotherapy: immune suppression and tumor growth, 2nd edn. Elsevier, New York, 655
Helgeby A, Robson NC, Donachie AM, Beackock-Sharp H, Lövgren K, Schön K, Mowat A, Lycke NY (2006) The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B cells. J Immunol 176(6):3697–3706
Higgins PG, Fluit AC, Schmitz FJ (2003) Fluoroquinolones: structure and target sites. Curr Drug Targets 4(2):181–190
Hoang TT, Schweizer HP (1999) Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J Bacteriol 181(17):5489–5497
Huang MH, Lin SC, Hsiao CH, Chao HJ, Yang HR, Liao CC, Chuang PW, Wu HP, Huang CY, Leng CH, Liu SJ (2010) Emulsified nanoparticles containing inactivated influenza virus and CpG oligodeoxynucleotides critically influences the host immune responses in mice. PLoS One 5(8):e12279
Islan GA, Durán M, Cacicedo ML, Nakazato G, Kobayashi RK, Martinez DS, Castro GR, Durán N (2017) Nanopharmaceuticals as a solution to neglected diseases: is it possible? Acta Trop 170:16–42
Jasim A, Abdelghany S, Greish K (2017) Current update on the role of enhanced permeability and retention effect in cancer nanomedicine. In Nanotechnology-based approaches for targeting and delivery of drugs and genes. Academic Press, 62–109.
Jiang T, Mo R, Bellotti A, Zhou J, Gu Z (2014) Gel–liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv Funct Mater 24(16):2295–2304
Jin T, Sun D, Su JY, Zhang H, Sue HJ (2009) Antimicrobial efficacy of zinc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J Food Sci 74(1):M46–M52
Johnston NJ, Mukhtar TA, Wright GD (2002) Streptogramin antibiotics: mode of action and resistance. Curr Drug Targets 3(4):335–344
Jones GR (1996) Successful cancer therapy with promethazine: the rationale. Med Hypotheses 46(1):25–29
Kahne D, Leimkuhler C, Lu W, Walsh C (2005) Glycopeptide and lipoglycopeptide antibiotics. Chem Rev 105(2):425–448
Kapoor G, Saigal S, Elongavan A (2017) Action and resistance mechanisms of antibiotics: a guide for clinicians. J Anaesthesiol Clin Pharmacol 33(3):300
Khan F, Khattak NS, Khan US, Rahman A (2011) Historical development of magnetite nanoparticles synthesis. J Chem Soc Pak 33(6):793
Kim BY, Rutka JT, Chan WC (2010) Nanomedicine. N Engl J Med 363(25):2434–2443
Kim SH, Kwak SY, Sohn BH, Park TH (2003) Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J Membr Sci 211(1):157–165
Klaenhammer TR (1993) Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev 12(1–3):39–85
Korani M, Rezayat SM, Gilani K, Bidgoli SA, Adeli S (2011) Acute and subchronic dermal toxicity of nanosilver in guinea pig. Int J Nanomedicine 6:855
Kumar A, Schweizer HP (2005) Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev 57(10):1486–1513
Kumar Khanna V (2012) Targeted delivery of nanomedicines. ISRN Pharmacol 2012
Kumar V, Bayda S, Hadla M, Caligiuri I, Russo Spena C, Palazzolo S, Kempter S, Corona G, Tooli G, Rizzolio F (2016a) Enhanced chemotherapeutic behavior of open-caged DNA@doxorubicin nanostructures for cancer cells. J Cell Physiol 231:106–110
Kumar V, Palazzolo S, Bayda S, Corona G, Tooli G, Rizzolio F (2016b) DNA nanotechnology for cancer therapy. Theranostics 6:710–725
Kutter E, De Vos D, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST (2010) Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11(1):69–86
Lambert PA (2005) Bacterial resistance to antibiotics: modified target sites. Adv Drug Deliv Rev 57(10):1471–1485
Leach KL, Swaney SM, Colca JR, McDonald WG, Blinn JR, Thomasco LM, Gadwood RC, Shinabarger D, Xiong L, Mankin AS (2007) The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol Cell 26(3):393–402
Lellouche J, Kahana E, Elias S, Gedanken A, Banin E (2009) Antibiofilm activity of nanosized magnesium fluoride. Biomaterials 30(30):5969–5978
Lin ST, Franklin MT, Klabunde KJ (1986) Nonaqueous colloidal gold. Clustering of metal atoms in organic media. 12. Langmuir 2(2):259–260
Lind K, Kristiansen JE (2000) Effect of some psychotropic drugs and a barbiturate on mycoplasmas. Int J Antimicrob Agents 14(3):235–238
Liu L, Liu Z, Chen H, Liu H, Gao Q, Cong F, Gao G, Chen Y (2020) Subunit nanovaccine with potent cellular and mucosal immunity for COVID-19. ACS Appl Biol Mater
Liu YJ, He LL, Mustapha A, Li H, Hu ZQ, Lin MS (2009) Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157: H7. J Appl Microbiol 107(4):1193–1201
Lorch A (1999) Bacteriophages: an alternative to antibiotics. Biotechnol Dev Monit 39:14–17
Logothetidis S (2006) Nanotechnology in medicine: The medicine of tomorrow and nanomedicine. Hippokratia 10:7–21
Luby Š, Lubyová M, Šiffalovič P, Jergel M, Majková E (2015) A brief history of nanoscience and foresight in nanotechnology. In: Nanomaterials and nanoarchitectures. Springer, Dordrecht, pp 63–86
Luo S, Ma C, Zhu MQ, Ju WN, Yang Y, Wang X (2020) Application of Iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer’s disease. Front Cell Neurosci 14
Ma Y, Zhou T, Zhao C (2008) Preparation of chitosan–nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr Res 343(2):230–237
MacDougall C (2017) Penicillins, cephalosporins, and other β-lactam antibiotics. In: Brunton LL, Hilal-Dandan R, Knollmann BC (eds) Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th edn. McGraw-Hill Education, New York, 1023–1038.
Madou MJ (2011) Manufacturing techniques for microfabrication and nanotechnology. CRC press
Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM (2009) Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Investig Dermatol 129(10):2463–2469
Martinez-Gutierrez F, Olive PL, Banuelos A, Orrantia E, Nino N, Sanchez EM, Ruiz F, Bach H, Av-Gay Y (2010) Synthesis, characterization, and evaluation of antimicrobial and cytotoxic effect of silver and titanium nanoparticles. Nanomedicine 6(5):681–688
Massich MD, Giljohann DA, Seferos DS, Ludlow LE, Horvath CM, Mirkin CA (2009) Regulating immune response using polyvalent nucleic acid− gold nanoparticle conjugates. Mol Pharm 6(6):1934–1940
Maurice F, Broutin I, Podglajen I, Benas P, Collatz E, Dardel F (2008) Enzyme structural plasticity and the emergence of broad-spectrum antibiotic resistance. EMBO Rep 9(4):344–349
McGinn RE (2012) What’s Different, Ethically, About Nanotechnology? Foundational Questions and Answers. In A. S. Khan (Ed.), Nanotechnology: ethical and Social Implications. CRC Press, Boca Raton, Florida, pp. 67–89
McShan D, Ray PC, Yu H (2014) Molecular toxicity mechanism of nanosilver. J Food Drug Anal 22(1):116–127
Morishita M, Morishita I, Takayama K, Machida Y, Nagai T (1992) Novel oral microspheres of insulin with protease inhibitor protecting from enzymatic degradation. Int J Pharm 78(1–3):1–7
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346
Mukherjee S, Madamsetty VS, Bhattacharya D, Roy Chowdhury S, Paul MK, Mukherjee A (2020) Recent advancements of nanomedicine in neurodegenerative disorders theranostics. Adv Funct Mater 2003054
Muttil P, Prego C, Garcia-Contreras L, Pulliam B, Fallon JK, Wang C, Hickey AJ, Edwards D (2010) Immunization of guinea pigs with novel hepatitis B antigen as nanoparticle aggregate powders administered by the pulmonary route. AAPS J 12(3):330–337
Nandy SK, Bapat PM, Venkatesh KV (2007) Sporulating bacteria prefers predation to cannibalism in mixed cultures. FEBS Lett 581(1):151–156
Nigam A, Gupta D, Sharma A (2014) Treatment of infectious disease: beyond antibiotics. Microbiol Res 169(9-10):643–651
Nikaido H, Zgurskaya HI (1999) Antibiotic efflux mechanisms. Curr Opin Infect Dis 12(6):529–536
Nikaido H (1994) Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science 264(5157):382–388
Niu X, Chen J, Gao J (2019) Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci 14(5):480–496
O’sullivan L, Ross RP, Hill C (2002) Potential of bacteriocin-producing lactic acid bacteria for improvements in food safety and quality. Biochimie 84(5–6):593–604
O'Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL (2004) Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 209(2):171–176
Padovani S, Sada C, Mazzoldi P, Brunetti B, Borgia I, Sgamellotti A, Giulivi A, d’Acapito F, Battaglin G (2003) Copper in glazes of renaissance luster pottery: nanoparticles, ions, and local environment. J Appl Phys 93(12):10058–10063
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73(6):1712–1720
Palazzolo S, Hadla M, Spena CR, Bayda S, Kumar V, Lo Re F, Adeel M, Caligiuri I, Romano F, Corona G et al (2019a) Proof-of-concept multistage biomimetic liposomal DNA origami nanosystem for the remote loading of doxorubicin. ACS Med Chem Lett 10:517–521
Palazzolo S, Hadla M, Russo Spena C, Caligiuri I, Rotondo R, Adeel M, Kumar V, Corona G, Canzonieri V, Tooli G et al (2019b) An effective multi-stage liposomal DNA origami nanosystem for in vivo cancer therapy. Cancers 11:1997
Pardridge WM (2003) Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv 3(2):90
Perica K, Varela JC, Oelke M, Schneck J (2015) Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med J 6(1):e0004
Pinto-Alphandary H, Andremont A, Couvreur P (2000) Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents 13(3):155–168
Plummer EM, Manchester M (2011) Viral nanoparticles and virus-like particles: platforms for contemporary vaccine design. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2:174–196
Prasad R (2014) Synthesis of silver nanoparticles in photosynthetic plants. Journal of Nanoparticles, Article ID 963961, 2014, http://dx.doi.org/10.1155/2014/963961
Prasad R, Pandey R, Varma A, Barman I (2017) Polymer based nanoparticles for drug delivery systems and cancer therapeutics. In: Natural Polymers for Drug Delivery (eds. Kharkwal H and Janaswamy S), CAB International, UK 53–70
Prasad R, Swamy VS (2013) Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. Journal of Nanoparticles 2013, http://dx.doi.org/10.1155/2013/431218
Qi L, Xu Z, Jiang X, Hu C, Zou X (2004) Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 339(16):2693–2700
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27(1):76–83
Rai VK, Mishra N, Agrawal AK, Jain S, Yadav NP (2016) Novel drug delivery system: an immense hope for diabetics. Drug Deliv 23(7):2371–2390
Rakotoarisoa M, Angelova A (2018) Amphiphilic nanocarrier systems for curcumin delivery in neurodegenerative disorders. Medicines 5(4):126
Ranade AV, Acharya R (2015) Arka and its pharmaceutical attributes in Indian alchemy (Rasashastra): a comprehensive review. Int J Ayurvedic Med 6(4):280–288
Ren G, Hu D, Cheng EW, Vargas-Reus MA, Reip P, Allaker RP (2009) Characterisation of copper oxide nanoparticles for antimicrobial applications. Int J Antimicrob Agents 33(6):587–590
Reynolds PE (1989) Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 8(11):943–950
Richard F (1960) There’s plenty of space at the bottom. Caltech Eng Sci 23(5):22–36
Rothemund PWK (2006) Folding DNA to create nanoscale shapes and patterns. Nature 440:297–302
Roco MC (2007). National nanotechnology initiative-past, present, future. In Goddard III WA, Brenner D, Lyshevski SE and Iafrate GJ (eds) Handbook on nanoscience, engineering and technology, 2nd ed, CRC Press, Boca Raton, Florida, pp. 3.1–3.26.
Rubinsztein DC (2006) The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443(7113):780–786
Ruparelia JP, Chatterjee AK, Duttagupta SP, Mukherji S (2008) Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater 4(3):707–716
Mobashery S, Azucena EF (1999) Bacterial antibiotic resistance. In: Encyclopedia of life sciences. Nature Publishing Group, London, UK
Sablon E, Contreras B, Vandamme E (2000) Antimicrobial peptides of lactic acid bacteria: mode of action, genetics and biosynthesis. In: New products and new areas of bioprocess engineering. Springer, Berlin, Heidelberg, pp 21–60
Saglam N, Korkusuz, F, Prasad R (2021) Nanotechnology Applications in Health and Environmental Sciences. Springer International Publishing (ISBN: 978-3-030-64410-9) https://www.springer.com/gp/book/9783030644093
Sanpui P, Murugadoss A, Prasad PD, Ghosh SS, Chattopadhyay A (2008) The antibacterial properties of a novel chitosan–Ag-nanoparticle composite. Int J Food Microbiol 124(2):142–146
Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D (2007) Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 24(12):2198–2206
Sciau P (2012) Nanoparticles in ancient materials: the metallic lustre decorations of medieval ceramics. INTECH Open Access Publisher
Scott AM, Allison JP, Wolchok JD (2012) Monoclonal antibodies in cancer therapy. Cancer Immun Arch 12(1)
Sebastian R (2017) Nanomedicine-the future of cancer treatment: a review. J Cancer Prev Curr Res 8(1):00–265
Šebestík J, Reiniš M, Ježek J (2012) Dendrimers in Neurodegenerative Diseases. In: Biomedical applications of peptide-, glyco-and glycopeptide dendrimers, and analogous dendrimeric structures. Springer, Vienna, pp 209–221
Seeman NC (1982) Nucleic acid junctions and lattices. J Theor Biol 99:237–247
Shah J, Park S, Aglyamov SR, Larson T, Ma L, Sokolov KV, Johnston KP, Milner TE, Emelianov SY (2008) Photoacoustic imaging and temperature measurement for photothermal cancer therapy. J Biomed Opt 13(3):034024
Sharma N, Sharma M, Sajid Jamal QM, Kamal MA, Akhtar S (2019) Nanoinformatics and biomolecular nanomodeling: a novel move en route for e_ective cancer treatment. Environ Sci Pollut Res Int:1–15
Si PZ, Zhang ZD, Geng DY, You CY, Zhao XG, Zhang WS (2003) Synthesis and characteristics of carbon-coated iron and nickel nanocapsules produced by arc discharge in ethanol vapor. Carbon 41(2):247–251
Singh B (2020) Biomimetic nanovaccines for COVID-19. Appl Sci Technol Ann 1(1):176–182
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275(1):177–182
Song HY, Ko KK, Oh LH, Lee BT (2006) Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cells Mater 11(Suppl 1):58
Stevens KA, Sheldon BW, Klapes NA, Klaenhammer TR (1991) Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol 57(12):3613–3615
Strateva T, Yordanov D (2009) Pseudomonas aeruginosa–a phenomenon of bacterial resistance. J Med Microbiol 58(9):1133–1148
Straus SK, Hancock RE (2006) Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta (BBA) 1758(9):1215–1223
Subramani K, Pathak S, Hosseinkhani H (2012) Recent trends in diabetes treatment using nanotechnology. Dig J Nanomater Bios 7(1)
Sulakvelidze A, Alavidze Z, Morris JG (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45(3):649–659
Sun HX, Xie Y, Ye YP (2009) ISCOMs and ISCOMATRIX™. Vaccine 27(33):4388–4401
Swamy VS, Prasad R (2012) Green synthesis of silver nanoparticles from the leaf extract of Santalum album and its antimicrobial activity. J Optoelectronic and Biomedical Materials 4(3):53–59
Tam JP, Lu YA, Yang JL (2002) Antimicrobial dendrimeric peptides. Eur J Biochem 269(3):923–932
Taniguchi N (1974) On the basic concept of “nano-technology”. In: Proceedings of international conference on production engineering. Part II, Japan Society of Precision Engineering, Tokyo
Tenover FC (2006) Mechanisms of antimicrobial resistance in bacteria. Am J Med 119(6):S3–10
Tolmasky ME (2000) Bacterial resistance to aminoglycosides and beta-lactams: the Tn1331 transposon paradigm. RNA 5(10):11
Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards DA (2002) Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci 99(19):12001–12005
Vannuffel P, Cocito C (1996) Mechanism of action of streptogramins and macrolides. Drugs 51(1):20–30
Strohl WR (1997) Biotechnology of antibiotics. Marcel Dekker Inc., New York, NY
Webber MA, Piddock LJ (2003) The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 51(1):9–11
Williams JD (1995) Selective toxicity and concordant pharmacodynamics of antibiotics and other drugs. J Antimicrob Chemother 35(6):721–737
Wise R (1999) A review of the mechanisms of action and resistance of antimicrobial agents. Can Respir J 6:20A-A
Yadav L, Tripathi RM, Prasad R, Pudake RN, Mittal J (2017) Antibacterial activity of Cu nanoparticles against E. coli, Staphylococcus aureus and Pseudomonas aeruginosa. Nano Biomed Eng 9(1):9–14. https://doi.org/10.5101/nbe.v9i1.p9-14
Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG, Wang LV (2009) Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat Mater 8(12):935–939
Yih TC, Al-Fandi M (2006) Engineered nanoparticles as precise drug delivery systems. J Cell Biochem 97(6):1184–1190
Yoneyama H, Katsumata R (2006) Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem 70(5):1060–1075
Zhang E, Xu H (2017) A new insight in chimeric antigen receptor-engineered T cells for cancer immunotherapy. J Hematol Oncol 10(1):1–1
Zhang H, Chen G, Yu B, Cong H (2018) Emerging advanced nanomaterials for cancer photothermal therapy. Rev Adv Mater Sci 53(2):131–146
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bharathan, B.P., Rajagopal, R., Alfarhan, A., Arasu, M.V., Al-Dhabi, N.A. (2022). Advancement in Nanomaterial Synthesis and its Biomedical Applications. In: Krishnan, A., Ravindran, B., Balasubramanian, B., Swart, H.C., Panchu, S.J., Prasad, R. (eds) Emerging Nanomaterials for Advanced Technologies. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-80371-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-80371-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80370-4
Online ISBN: 978-3-030-80371-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)