Abstract
Nutritional approaches to the prevention or treatment of sarcopenia have previously focused on protein. However, several micronutrient vitamins and minerals, including the B group of vitamins, vitamins C, E and the carotenoids and minerals such as iron, magnesium, and selenium, may exert effects on sarcopenia or sarcopenic factors (skeletal muscle mass, function or strength). These may be indirect through effects on protein synthesis or direct through effects on mechanisms of aging for skeletal muscle such as inflammaging, counteracting reactive oxygen species and mitochondrial dysfunction. Other direct mechanisms also exist including the involvement of vitamin C in formation of collagen, a structural component of skeletal muscle, and of carnitine, required for muscle contraction. Better quality dietary intakes, as found with the Mediterranean Dietary pattern and other healthy eating patterns, may also be beneficial to prevention or treatment of sarcopenia though effects on skeletal muscle mass or function during aging. However, current research into the influence of micronutrient vitamins and minerals and optimal dietary patterns for the prevention or treatment of sarcopenia and loss of skeletal muscle mass and function with age is limited, and further research is required.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sarcopenia
- Skeletal muscle mass
- Skeletal muscle strength
- Nutrients
- Dietary patterns
- Vitamins
- Mineral trace elements
13.1 Introduction
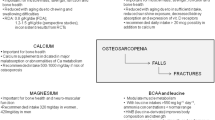
Sarcopenia is associated with the conditions of frailty, falls, osteoporosis and risk of fracture, as well as with malnutrition [1,2,3]. In addition, there are metabolic consequences of loss of skeletal muscle mass during aging which include reduced energy expenditure, which affects obesity as well as glucose dysregulation, effects of glycaemic control and the onset of type-2 diabetes [1, 4]. The loss of, and changes to, skeletal muscle mass with aging also contribute to age-associated reduction in utilisation of dietary protein and fat [1].
In this chapter, the term ‘sarcopenic factors’ refers to the loss of skeletal muscle strength or function or loss or changes in skeletal muscle mass or combinations of these. The term ‘fat free mass’ (FFM) is used, since FFM is often measured and used as a proxy for skeletal muscle mass (SMM). Since FFM increases both with increased body weight and size, measurements of FFM in human studies are scaled for body size by height (skeletal muscle mass index—SMI), percentage of total body weight or Body Mass Index (BMI) (FFM/BMI, FFM divided by BMI).
Nutrition is a modifiable lifestyle factor that can interact with the mechanisms of loss of skeletal muscle mass and function, as well as with the mechanisms of aging, and previous research has focused on a number of nutrients that are relevant for skeletal muscle physiology and metabolism. However, the major focus to date has been on protein intake. Lesser focus has been centred on the micronutrient vitamins and minerals, and on intake of fats and fatty acids. There have also been some recent studies examining the relevance of patterns of dietary intake. The relevance of dietary patterns may be due to the individual nutrients such as protein or the micronutrients which are associated with the optimal dietary patterns as well as to the synergistic effects of these nutrients and the foods within these dietary patterns.
This chapter covers the foods, nutrients and dietary patterns that have been linked to either prevention or treatment of sarcopenia and suggests areas for future research in relation to aspects of nutrition and prevention of sarcopenia.
13.2 Nitrogen Balance and Exogenous Antioxidants
Maintaining the balance between the continuous anabolism and catabolism of protein occurring in the body and thus ensuring nitrogen balance does not become negative are crucial for conserving skeletal muscle during aging [1, 5,6,7,8]. Thus, factors that interfere with this process such as inflammaging and insulin resistance have an adverse impact on nitrogen balance. There is also an increase in anabolic resistance, i.e. lower myofibrillar synthesis of protein, during aging.
A number of micronutrient vitamins or minerals are known to act as exogenous antioxidants or help counteract the increased circulating concentrations of inflammatory cytokines associated with aging, and thus may be beneficial to muscle health and prevention of sarcopenia. These include vitamins C, E, and D, the carotenoids and the minerals, magnesium, selenium and zinc, but there is also evidence for specific dietary patterns being relevant. Specific relationships between the mechanisms of aging with nutrients and patterns of dietary intake are described in the following sections.
13.2.1 Important Dietary Factors
13.2.1.1 Protein
Skeletal muscle is the main reserve of protein in the body, and protein is required to maintain this reserve of protein in the muscle through protein synthesis. It is thus logical that there has been extensive research into the relevance of protein intake to conservation and prevention of sarcopenia during aging [2, 3, 5, 6, 9]. As the relevance of protein to maintenance of sarcopenic factors and prevention of sarcopenia during aging has been studied extensively a summary of the importance of protein as well, as the most recent developments in this area of research are covered in this chapter. Readers may refer to the earlier work in the following publications [2, 3, 5, 6, 9].
A recent review of nutritional interventions to improve sarcopenic factors and sarcopenia found that the evidence was generally not of high quality and was insufficient to establish with any certainty the effects of supplementation with protein, essential amino acids or creatine, or ß-hydroxy-ß-methylbutyrate [10]. The authors determined that the level of evidence supporting most recommendations was low to moderate, but that the best evidence related to the amino acid leucine which has a significant effect on muscle mass in older people with sarcopenia. However, the review also recommended that increases in protein intake designed to increase muscle mass and strength should be accompanied by resistance exercise programmes [10]. By contrast, another systematic review found that protein does not augment the effects of resistance exercise on skeletal muscle mass and function in older people [11]. However, most recent research and clinical recommendations have noted that reducing the decline in sarcopenic factors during aging requires that the increase in intake of protein should accompanied by resistance exercise [9, 12,13,14,15]. This is because an increase in physical activity synergises with increased protein intake to affect regulation of protein synthesis.
13.2.1.1.1 Variability in Response to Interventions with Dietary Protein
The limited effectiveness of interventions with protein in older people and the variability in response to interventions with dietary protein may be due to a number of reasons including the anabolic resistance that occurs during aging [5, 15]. Recent research studied the effect of protein supplementation on muscle disuse in young men and found substantial declines in muscle mass and myofibrillar protein synthesis rates during inactivity [16]. The authors also found that the high intake of protein used in their intervention, 1.6 g/kg body weight per day, did not attenuate the decreases in quadriceps muscle volume that occurred during inactivity when compared with the control interventions with low, or no, protein intake; the control groups received either 0.5 g or 0.15 g/kg/day, respectively [9, 16]. Therefore, increased protein intake did not counteract the reduced myofibrillar protein synthesis rates that occurred during inactivity, even in young men. Other recent work has also demonstrated that the incorporation of amino acids such as leucine into skeletal muscle, during muscle protein synthesis, occurs only for a period of 2–3 h in a rested state. This is known as the muscle full phenomenon which means that muscle becomes unresponsive to higher doses of protein intake after a short period following protein consumption [13]. This phenomenon may also explain the limited effectiveness of increases in protein intake that are designed to overcome the anabolic resistance to protein synthesis in muscle during older age.
Other factors such as sex and race potentially also influence the effectiveness of protein intake in interventions to improve sarcopenic factors during aging. Recent research found that associations between protein intake and sarcopenic factors differed according to sex and race, in a longitudinal study [17].
Some of the variability in response to interventions with protein may also be due to the type of protein used since protein from animal sources such as meat or dairy foods is more biologically available than protein from vegetable sources such as pulses (beans and lentils) and vegetables [18]. Animal sources of protein have a profile of amino acids that is higher in indispensable amino acids, including the branch chain amino acids that stimulate the production of mTOR that is required to increase the synthesis of protein [7]. However, a recent population study, The NU-AGE Study, found an interaction between plant and animal protein intakes on the risk of sarcopenia [19]. Though the risk of sarcopenia decreased with increased intakes of total protein, this decrease was greater when intakes of vegetable protein were also higher [19].
A number of the dietary interventions with protein-containing foods have found variability in sarcopenic outcomes which may be due to not only the protein composition of the foods that were administered but also to the associated nutrients in the foods. For instance, one intervention in an Australian population that was accompanied by resistance exercise training found positive effects of a red meat intervention on lean muscle mass in women when compared with a control group [20]. However, the increase in protein intake in the group provided with red meat was also accompanied by a significant increase in intake of zinc which may also have increased the effectiveness of the intervention. In a further study of similar design, which also included men, no significant effect of increased intake of meat was found. However, a similar increase in zinc intake occurred during the study that was also associated with the group with increased meat intake [21].
Finally, variation in the interaction between protein intake and the composition of the microbiome in the gut may occur. Thus, it has been hypothesised that the gut microbiome may modulate individual response to dietary protein and thus have effects on sarcopenia and sarcopenic factors [22, 23].
13.2.1.1.2 Intake of Dietary Amino Acids
To date, there is little data on intakes of amino acids in general populations of middle and older age who are at risk of sarcopenia. In recent unpublished research, we investigated the full range of amino acids in the diet and contributors to sarcopenia, skeletal muscle mass and function and found a number of differences in the associations between individual amino acids and sarcopenic factors in both younger and older women in the UK-Twin cohort.
13.2.1.1.3 Current Clinical Recommendations for Protein Intake
Current recommendations regarding protein intake during aging include increasing total protein intakes, between 1.0 g and 1.5 g protein per kg body weight per day, for individuals older than 65 years [5]. However, other reviews and dietary recommendations do not recommend intakes higher than 1.0 g protein/kg/day in older age groups [24]. It has also been suggested that ensuring protein intake balanced across meal occasions is important in older people, to ensure maximal utilisation of protein and muscle protein synthesis [5, 14].
In summary, adequate protein is undoubtably important for the maintenance of skeletal muscle function and structure during aging, but to date the evidence is mixed for the effectiveness of dietary interventions with protein to rectify or prevent sarcopenia. Accompanying increases in protein intake with sufficient micronutrient vitamins and minerals or as part of improvements in overall dietary intakes may be important in the prevention and treatment of sarcopenia, as described in the following sections.
13.2.1.2 The B Group Vitamins
The B vitamins are a diverse group of vitamins with important biological functions. Several of the B vitamins are highly relevant to muscle, acting as cofactors in processes involved in muscle synthesis and as neurotrophic agents that maintain neural integrity and function [25, 26]. In addition, deficiencies of a number of B vitamins result in neuromuscular problems (e.g. beri beri) and neurological symptoms (e.g. pellagra). So, maintenance of vitamin B status may be important for prevention of sarcopenia. However, very few studies have investigated relationships between dietary intake of B vitamins and circulating blood concentrations [25]. One study in the Netherlands found associations between dietary folate, vitamin B6, and B12 intakes and physical function in older adults in the Netherlands [27]. Another study, in adults older than 65 years, found lower intakes of vitamin B6 and folic acid in adults with sarcopenia compared with those without [28]. Two further studies found that lower intakes and concentrations of circulating vitamin B12 were associated with low SMI, sarcopenia or dynapenia [29, 30]. Lastly, in unpublished work from our group, using the Twins UK Study of adult women aged 18–79 years, significant positive associations were evident in multivariable models between dietary B vitamin intakes (niacin, folate, pantothenate, riboflavin, thiamine and B6) and measures of fat-free mass. Similarly, positive trends were observed across niacin, folate, pantothenate, riboflavin and thiamine dietary intakes and leg explosive power (measured using a Nottingham Power Rig). Therefore, although more research is required, the current evidence suggests that dietary intake of B vitamins is important for both skeletal muscle mass and function.
13.2.1.3 Vitamin C
Vitamin C has several mechanistic functions relevant to skeletal muscle metabolism and physiology, which could prevent age-related loss of skeletal muscle. Vitamin C in muscle is involved in synthesis of carnitine, an important factor involved in energy production, and collagen, an essential structural component of muscle [31, 32]. It also has a strong capability to act as an electron donor. Reactive oxygen species (ROS) are produced during normal oxidative metabolism in muscle, but capable of cellular damage if uncontrolled [33]. Under normal physiological conditions, the presence of ROS is controlled by antioxidant and enzymatic defence systems including superoxide dismutase and glutathione peroxidase as well as antioxidants from the diet [33, 34]. Age-related increases in ROS due to mitochondrial dysfunction, modification to enzymatic defences and changes to muscle fibres may lead to cellular damage in muscle, as does the age-related increase in circulating concentrations of inflammatory cytokines [33, 35]. If in sufficient supply, the antioxidant capacity of vitamin C may therefore help to reduce oxidative damage to muscle, as well as reducing potentially damaging concentrations of inflammatory cytokines in the circulation [35]. In previous observational studies, positive associations between dietary vitamin C and measures of skeletal muscle function were found in the Italian InCHIANTI Study, and for women only in the UK [36,37,38,39]. One study examined both FFM and muscle function with intake as well as circulating vitamin C, finding positive associations between measures of physical function but not FFM [38]. A further study found intake of vitamin C was associated with FFM after 2.6 years of follow up [36]. More recent observational evidence in women of all ages found that higher intakes of vitamin C were associated with significantly higher indices of FFM and leg explosive power [40]. The differences between the highest intakes in quintile 5 versus those in quintile 1 ranged between 2.0% and 12.8% (P < 0.01–0.02) [40]. Further observational study evidence shows positive associations of both dietary and circulating vitamin C with measures of skeletal muscle mass in middle- and older-aged men and women [41]. Overall, the evidence thus points towards potential protective effects of vitamin C on measures of skeletal muscle mass and function.
13.2.1.4 Vitamin D
Vitamin D may be protective for development of sarcopenia through a number of direct or indirect mechanisms [7]. Receptors for vitamin D are found in skeletal muscle, but discussion is still ongoing as to the importance of this and the relevance to aging, such as whether levels or expression of these receptors decline during aging and whether they are important for the morphological changes that affect both skeletal muscle mass and function during aging [42]. Known roles of vitamin D are participation in myogenesis, cell proliferation, differentiation and regulation of cell signalling cascades, as well as signalling for potential genomic targets [43]. The functional effects of vitamin D in muscle may be through calcium and phosphate handling and signalling, particularly in relation to muscle strength and contraction [42]. Deficiency of vitamin D leads to muscle weakness which is one of the symptoms found in rickets in children, as well as in adults, where it is accompanied by muscle pain, in the condition of osteomalacia [42].
A recent systematic review summarised the evidence for supplementation of vitamin D in community-dwelling older adults aged 65 years and older in relation effects on muscle strength and function [44]. Studies included were those testing supplementation with vitamin D alone, or alongside calcium supplementation. Of the 15 studies included in the review, the majority found no improvement in muscle strength and mobility after administration of vitamin D with or without calcium supplements. In the meta-analyses performed, non-significant changes in hand-grip strength were found in the seven studies analysed and a small, but significant, increase in the timed-up and-go test of 0.3 s (95% CI = 0.1–0.5 s) in five studies. However, there was a high degree of heterogeneity between the studies. The overall conclusion was supplementation with vitamin D or with calcium did not result in improvements in skeletal muscle function [44]. A more recent intervention study with vitamin D in men aged 60 years and over, with low concentrations of circulating vitamin D, found no effect on lower extremity power, strength or lean mass over a period of 12 months of supplementation [45]. Further recent intervention studies, over shorter periods of 3–6 months, found either effects on appendicular skeletal muscle mass, but not grip strength [46], or improvements in skeletal muscle mass, but not strength.
There is also limited evidence that co-administration of vitamin D alongside the amino acid leucine is more likely to improve the efficacy of leucine supplementation on skeletal muscle mass [47]. It is clear from the existing evidence that vitamin D plays a role in maintenance of skeletal muscle mass strength or function, but in older populations the effects of supplementation on sarcopenic outcomes are mixed. Differences in the findings of intervention trials with vitamin D may be due to initial concentrations of circulating vitamin D, as well as to dosages used in the interventions, the age of the intervention groups and duration of the studies.
13.2.1.5 Vitamin E
Vitamin E, like vitamin C, has the potential to act as an antioxidant, preventing build-up of free radicals in cell membranes and in plasma lipoproteins. Vitamin E consists of two classes of molecules, tocopherols and tocotrienols, which are categorised according to the saturation of their phytyl tail groups. Observational data of individuals 65 years or over has shown a positive association between plasma alpha-tocopherol concentration and knee extension strength and physical performance, and between gamma-tocopherol and physical performance [48, 49]. In addition, low circulating vitamin E concentrations have been identified in frail individuals, compared to non-frail individuals, suggesting a lack of vitamin E may be linked to the transition from non-frail to frail [50]. Other cross-sectional analyses, of data from women aged 18–79 years in the Twins UK cohort, found that higher intake of vitamin E was associated with higher indices of skeletal muscle mass, but not function [40]. Further work in the EPIC-Norfolk study has found potentially protective associations in fat-free mass with higher intakes of dietary vitamin E or circulating concentrations of α-tocopherol [51].
The observational data are supported by mechanistic evidence from a number of animal studies, which demonstrate the role of vitamin E as an antioxidant and anti-inflammatory agent. This includes evidence that: in a rat model, vitamin E prevents increased nuclear translocation of NF-kB, increased expression of chemokines and the resultant leukocyte infiltration associated with H2O2-induced oxidative stress [52]; and in a mouse model, vitamin E reduces lipopolysaccharide (LPS)-induced inflammation by modulating the LPS-induced, and NF-kB mediated, upregulation of IL-6 gene and protein expression [53].
13.2.1.6 Carotenoids
The carotenoid family of phytochemical vitamins is found in yellow, orange and green leafy fruits and vegetables and includes β-carotene, β-cryptoxanthin, lycopene, lutein and zeaxthanin. These carotenoids function as exogenous antioxidants and anti-inflammatory agents thus interacting with the mechanisms of muscle aging.
Relatively few studies have investigated the relevance of total carotene, carotenoid intakes, or circulating concentrations, in relation to sarcopenia and sarcopenic factors. Two studies from a UK cohort found positive associations between higher intakes of carotene on grip strength or physical activity, with the latter finding of associations only in women [37, 39]. Several studies also found protective associations with higher circulating concentrations of β-carotene and indices of knee strength or function, or rate of decline in walking speed [49, 51,52,53,54,55,56,57].
Few studies have investigated associations with more detailed dietary intakes of the individual carotenoids and sarcopenic factors. In the UK Twin cohort, we found that higher intakes of total and individual carotenoids were significantly associated with indices of FFM and leg explosive power with differences across quintiles of between 1.0 and 7.5% [41]. The strongest associations for indices of FFM were found with α-carotene intake (Q5-Q1 0.24 kg/m 2 ± 0.1 P-trend = 0.03), a 1.6% difference across quintiles. Significant associations were also found with FFM% for ß-cryptoxthanin and with FFM% and FFMBMI for lutein and zeaxthanin, with interquintle differences ranging from 1.1 to 7.2% [40]. LEP was associated significantly with carotenoid intakes, with the exception of α-carotene, with differences in LEP ranging from 6.3 to 7.5% when comparing extreme quintiles of carotenoid intakes [40]. A previous study found that dietary carotenoid intake as total carotene, ß-cryptoxthanin, and combined lutein and zeaxanthin was positively associated with FFM, expressed as percentage body weight, in both men and women, with lycopene associated only in women [58]. The greatest association was found for combined lutein and zeaxanthin in women with an interquintile difference of 2.5%. A more recent longitudinal analysis from the Framingham cohort study also found protective effects with higher intakes of total carotenoids, lycopene and combined lutein and zeathanin with annualised change in grip strength or faster gait speed, over a period of follow up which ranged from 4.5 to 15.4 years [59]. However, replication of the analyses in the Cardiovascular Heart Study found no associations between total carotenoid intake and either grip strength or gait speed [59]. The research findings, though limited, indicate that future intervention studies with carotenoid containing foods are warranted.
13.2.1.7 Minerals: Magnesium
Skeletal muscle acts as a major store of magnesium where it is important for energy metabolism, protein synthesis and turnover, transmembrane transport and muscle contraction and relaxation [60, 61]. Magnesium is also integral to function of the mitochondria thus influencing muscle performance through energy metabolism (ATP generation).
A number of observational studies have shown dietary magnesium intake, serum magnesium or muscle magnesium concentrations are positively associated with measures of skeletal muscle mass [62, 63] and function [64]. Magnesium supplementation has also been shown to increase the muscle strength in young adults gained through exercise [65] and improve physical performance in older individuals [66]. Lower magnesium intake has also been associated with sarcopenia [28, 29]. However, the mechanisms by which magnesium may be acting in muscle are not fully understood. Cell culture and animal studies have demonstrated that magnesium depletion can cause structural damage to muscle cells due to oxidative stress and disrupted calcium homeostasis [67]. It has also been suggested that magnesium protects against inflammaging, a known risk factor for sarcopenia [7]. Indeed, circulating concentrations of inflammatory cytokines, including C-reactive protein (CRP), IL-6 and TNF-α, have been negatively associated with skeletal muscle measures of both mass and function in a number of studies [64, 68,69,70]. Systematic review evidence also indicates that dietary magnesium intake is inversely associated with serum CRP concentration [71]. Furthermore, it is relevant that age-related physiological decline in function of the gastrointestinal and renal systems may lead to an increased susceptibility of older individuals to develop low magnesium status [72].
13.2.1.8 Minerals and Trace Elements: Calcium, Iron, Potassium, Phosphorus, Selenium and Zinc
Although a number of minerals and trace elements, such as selenium, zinc, potassium, iron and phosphorus, play roles in muscle metabolism and function, comparatively little research has focused on this area [7, 73]. The minerals calcium, potassium, and sodium are necessary for healthy muscle and nerve activity. Calcium is the main regulatory signalling molecule for skeletal muscle fibres. Also, low iron blood serum concentrations may be associated with poor physical performance. Phosphorus can lead to muscle weakness, and selenium deficiency is associated with several muscular diseases that also include symptoms of weakness. Both selenium and zinc potentially play a role in protecting skeletal muscle from oxidative damage, and zinc is also integral to protein synthesis, and in animal studies, zinc deficiency has been shown to impaired protein synthesis in skeletal muscle [74].
One observational study in older people found that higher iron, phosphorus and zinc intakes were associated with conservation of lean mass over a period of 2.6 years, indicating a potential role for minerals in sarcopenic factors or prevention of sarcopenia [36]. A more recent systematic review identified only six studies investigating the role of minerals on prevention or treatment of sarcopenia in individuals, aged 65 years or over [73]. Evidence was provided mainly from observational studies, finding that serum selenium and calcium intakes were significantly associated with muscle mass, and selenium, iron, and zinc intakes were significantly and positively associated with physical performance in older adults [73]. Also, selenium, calcium and phosphorus intakes were associated with the prevalence of sarcopenia [74]. Although the majority of studies in this review reported on dietary intakes only, a study of community-dwelling older individuals that measured selenium in the serum of participants found that those in the lowest tertile of circulating selenium concentrations were at an increased risk of low skeletal muscle mass [75]. Also, an earlier study in men and women, which was not included in the review, found those individuals in the lowest quartile of circulating selenium concentration had lower measures of grip, knee and hip strength [76].
As comparatively little research has involved the relevance of minerals and trace elements to skeletal muscle health and sarcopenia, further research is needed to improve our knowledge and understanding in this area.
13.2.1.9 Fatty Acids
Dietary sources of fat exist as a combination of different classes of fatty acid. Thus, dietary fat intake may vary significantly between individuals with regard to both total consumption and the ratios of different fatty acids including saturated (SFA), monounsaturated (MUFA), polyunsaturated (PUFA) and trans (TFA) fatty acids [77, 78].
There are several aspects to the rationale for dietary fat being important to muscle health. During aerobic exercise, fatty acids provide energy by acting as a critical substrate for production of ATP [79]. Phospholipid fatty acids also act as key structural components of muscle cell membranes (sarcolemma), and incorporation of different types of fatty acids may influence cellular signalling and function [80]. Fatty acids may also affect inflammatory pathways, which could have consequences on muscle. Indeed, in general terms, it is though that higher SFA and total fat intakes are associated with higher risk of inflammation, while other fatty acids, including n-3 PUFA, are associated with anti-inflammatory properties and protein synthesis [81].
A number of observational studies have suggested a role for fatty acids and their dietary profiles and measures of skeletal muscle mass or sarcopenia. In an analysis of Twins UK data, positive associations were evident between the PUFA to SFA ratio and indices of FFM, and negative associations were evident with the proportion of energy from fat in the diet, and SFA, MUFA and TFA, individually as a percentage of total dietary energy [82]. There is also some suggestion that a higher omega-3: omega-6 ratio is desirable as omega-3 fatty acids may provide protective effects for muscle, while omega-6 has pro-inflammatory effects which result in adverse effects. However, a recent systematic review showed no significant effects of total PUFA or specific omega-3 or omega-6 fatty acids on indices of skeletal muscle mass [83]. In conclusion therefore, there is rationale for the importance of different profiles of fatty acid in the diet, with ratios of different fatty acids relevant to measures of muscle health and sarcopenic risk factors. However, further investigation is required before definitive conclusions can be made, and recommendations given to optimise fatty acid intakes for muscle health.
13.2.2 Dietary Patterns
Most previous research has studied associations between individual components of the diet and musculoskeletal health, but it is likely that the balance of dietary components is also important. Indeed, we consume nutrients in combinations in food, and thus there may be synergistic and cumulative effects of different dietary components including protein and micronutrients on health and disease which might not be seen by examining the effects of nutrients or foods individually.
The Mediterranean diet (MD) pattern is characterised by high intakes of fruits and vegetables, legumes, nuts, cereals and olive oil with low intakes of saturated fat, moderately high intakes of fish, low to moderate intakes of dairy products, low intake of meat and regular but moderate intake of alcohol [84]. This micronutrient rich diet is associated with a number of favourable health outcomes, including overall mortality and protective effects on cardiovascular disease, hypertension and cancer [85, 86]. Comparatively few studies have explored the relationship between the MD and sarcopenia or sarcopenic factors [87]. In terms of muscle health and relevance to sarcopenia, observational studies, including data from the Twins UK study of adult women, have demonstrated that higher adherence to the MD is associated with higher measures of fat-free mass, and leg explosive power [88]. Likewise in the EPIC-Norfolk cohort, higher adherence to a Mediterranean diet was associated with significantly higher indices of FFM [89].
Potential Renal Acid Load (PRAL) is a means to quantify acid-base load of the diet as well as the effect of diet on systemic acid-base balance. A more alkalinogenic load, low PRAL, is considered protective. Fruits and vegetables have a low PRAL and tend to promote systemic alkalinity due to the bicarbonate present, while hepatic oxidation of the sulphur-containing amino acids, cysteine and methionine found in meats, grains and cheeses generates hydrogen ions and thus has the opposite effect [90].
Metabolic acidosis may be detrimental to skeletal muscle by decreasing protein synthesis and increasing proteolysis and oxidation of amino acids, through actions of the ubiquitin proteasome pathway and insulin-like growth factor-1 signalling [91]. It has been associated with muscle wasting in patients with chronic renal failure [92], and in acidotic obese individuals undergoing very low calorie diets for weight loss [93, 94]. While this process is a useful adaptive response to acidosis resulting in release of amino acids in the blood as a substrate for synthesis of glutamine and in turn ammonia, which helps mop up excess hydrogen ions for excretion as ammonium ions and thus reduce the acidosis [95], it does nevertheless occur to the detriment of muscle. A number of population studies have therefore investigated PRAL in relation to muscle health in young and old individuals [96, 97]. For example, evidence from the Twins UK study in women [97] aged 18–79 years showed a positive association between a more alkaline diet and muscle mass indexes, and this association was also evident in the middle- to older aged men and women in the EPIC-Norfolk cohort [98].
13.3 Summary, Recommendations, and Guidelines
To date, published observational studies, both cross-sectional and longitudinal in design, have demonstrated significant relationships between specific dietary factors and dietary patterns with muscle measures of mass and function, and thus sarcopenic risk factors. They have also highlighted a number of differences in these relationships according to sex. The major evidence for micronutrients that may be relevant during muscle aging involves vitamin C and the mineral magnesium, with much less evidence available for the carotenoids, vitamin E, and other minerals and trace elements including iron, selenium, calcium and phosphorus. Many of these findings have not been translated into intervention study designs, but evidence from observational studies is nevertheless somewhat convincing. There is also a small but growing body of evidence to suggest that adherence to specific dietary patterns, including the Mediterranean diet, and diets with a more alkalinogenic, low PRAL, also has improved muscle measures and thus reduced sarcopenic risk factors. Within the observational studies for both micronutrients and dietary patterns, there are differences between extremes of population intakes of a magnitude that could be clinically relevant. These suggest that more optimal dietary intakes may have beneficial effects on sarcopenic factors during aging.
Until recently, the impact of nutrition on muscle health and sarcopenia has been largely underestimated. Indeed, efforts to promote or retain muscle mass and strength and thus reduce the risk of sarcopenia have mainly been focused on a combination of increased protein intake alongside resistance exercise. However, as summarised here, increasing evidence is emerging to show that overall diet quality and intake of a range of nutrients including vitamins and minerals, not only protein, may play an important role in muscle health in older people.
Dietary recommendations for older adults in relation to skeletal muscle health are limited. The current recommendations are summarised in Table 13.1. In making recommendations for intakes of specific nutrients for muscle health, it is important to consider that current dietary recommendations and clinical deficiency or sufficiency criteria have not been generated using muscle health outcomes. The current published recommended nutrient intakes or the body composition criteria utilised to derive dietary recommendations may therefore not be directly relevant to maintaining or improving muscle health. Indeed, it may be necessary to achieve higher intakes for optimal muscle health than might be predicted using other health outcomes, and further investigation will be required to determine this in the future. With this caveat, it is nevertheless reasonable to deduce from the current evidence that individuals should, in conjunction with an active lifestyle, aim to consume sufficient fruits and vegetables, and protein, and to limit their saturated fat intake, for muscle health in later life.
References
Welch AA, Hayhoe RPG, Cameron D. The relationships between sarcopenic skeletal muscle loss during ageing and macronutrient metabolism, obesity and onset of diabetes. Proc Nutr Soc. 2019:1–12.
Landi F, Camprubi-Robles M, Bear DE, Cederholm T, Malafarina V, Welch AA, et al. Muscle loss: the new malnutrition challenge in clinical practice. Clin Nutr. 2019;38(5):2113–20.
Deutz NEP, Ashurst I, Ballesteros MD, Bear DE, Cruz-Jentoft AJ, Genton L, et al. The underappreciated role of low muscle mass in the management of malnutrition. J Am Med Dir Assoc. 2019;20(1):22–7.
Scott D, de Courten B, Ebeling PR. Sarcopenia: a potential cause and consequence of type 2 diabetes in Australia’s ageing population? Med J Aust. 2017;207(2):89.
Wolfe RR, Miller SL, Miller KB. Optimal protein intake in the elderly. Clin Nutr. 2008;27(5):675–84.
Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84(3):475–82.
Welch AA. Nutritional influences on age-related skeletal muscle loss. Proc Nutr Soc. 2014;73(1):16–33.
Millward DJ. Limiting deconditioned muscle atrophy and strength loss with appropriate nutrition: can it be done? Am J Clin Nutr. 2020;112(3):499–500.
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN expert group. Clin Nutr. 2014;33(6):929–36.
Gielen E, Beckwee D, Delaere A, De Breucker S, Vandewoude M, Bautmans I, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. 2021;79(2):121–47.
Thomas DK, Quinn MA, Saunders DH, Greig CA. Protein supplementation does not significantly augment the effects of resistance exercise training in older adults: a systematic review. J Am Med Dir Assoc. 2016;17(10):959 e1–9.
Cruz-Jentoft AJ, Dawson Hughes B, Scott D, Sanders KM, Rizzoli R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: a narrative review. Maturitas. 2020;132:57–64.
Deane CS, Ely IA, Wilkinson DJ, Smith K, Phillips BE, Atherton PJ. Dietary protein, exercise, ageing and physical inactivity: interactive influences on skeletal muscle proteostasis. Proc Nutr Soc. 2020:1–12.
Theodorakopoulos C, Jones J, Bannerman E, Greig CA. Effectiveness of nutritional and exercise interventions to improve body composition and muscle strength or function in sarcopenic obese older adults: a systematic review. Nutr Res. 2017;43:3–15.
Nowson C, O'Connell S. Protein requirements and recommendations for older people: a review. Nutrients. 2015;7(8):6874–99.
Kilroe SP, Fulford J, Jackman S, Holwerda A, Gijsen A, van Loon L, et al. Dietary protein intake does not modulate daily myofibrillar protein synthesis rates or loss of muscle mass and function during short-term immobilization in young men: a randomized controlled trial. Am J Clin Nutr. 2020;113(3):548–61.
Elstgeest LEM, Schaap LA, Heymans MW, Hengeveld LM, Naumann E, Houston DK, et al. Sex-and race-specific associations of protein intake with change in muscle mass and physical function in older adults: the health, aging, and body composition (health ABC) study. Am J Clin Nutr. 2020;112(1):84–95.
Aubertin-Leheudre M, Adlercreutz H. Relationship between animal protein intake and muscle mass index in healthy women. Br J Nutr. 2009;102(12):1803–10.
Montiel-Rojas D, Nilsson A, Santoro A, Bazzocchi A, de Groot L, Feskens EJM, et al. Fighting sarcopenia in ageing european adults: the importance of the amount and source of dietary proteins. Nutrients. 2020;12(12):3601.
Daly RM, O’Connell SL, Mundell NL, Grimes CA, Dunstan DW, Nowson CA. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99(4):899–910.
Formica MB, Gianoudis J, Nowson CA, O’Connell SL, Milte C, Ellis KA, et al. Effect of lean red meat combined with a multicomponent exercise program on muscle and cognitive function in older adults: a 6-month randomized controlled trial. Am J Clin Nutr. 2020;112(1):113–28.
Bowyer RCE, Jackson MA, Pallister T, Skinner J, Spector TD, Welch AA, et al. Use of dietary indices to control for diet in human gut microbiota studies. Microbiome. 2018;6(1):77.
Ni Lochlainn M, Bowyer RCE, Steves CJ. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10(7):929.
Committee on Medical Aspects of Food Policy. Panel on Dietary Reference V, Great Britain. Dept. of H. Dietary reference values for food energy and nutrients for the United Kingdom: Report of the Panel on Dietary Reference Values of the Committee on Medical Aspects of Food Policy. London: HMSO; 1991.
Aytekin N, Mileva KN, Cunliffe AD. Selected B vitamins and their possible link to the aetiology of age-related sarcopenia: relevance of UK dietary recommendations. Nutr Res Rev. 2018;31(2):204–24.
Hwang SY, Sung B, Kim ND. Roles of folate in skeletal muscle cell development and functions. Arch Pharm Res. 2019;42(4):319–25.
Behrouzi P, Grootswagers P, Keizer PLC, Smeets E, Feskens EJM, de Groot L, et al. Dietary intakes of vegetable protein, folate, and vitamins B-6 and B-12 are partially correlated with physical functioning of Dutch older adults using copula graphical models. J Nutr. 2020;150(3):634–43.
Ter Borg S, de Groot LC, Mijnarends DM, de Vries JH, Verlaan S, Meijboom S, et al. Differences in nutrient intake and biochemical nutrient status between Sarcopenic and Nonsarcopenic older adults-results from the Maastricht sarcopenia study. J Am Med Dir Assoc. 2016;17(5):393–401.
Verlaan S, Aspray TJ, Bauer JM, Cederholm T, Hemsworth J, Hill TR, et al. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr. 2017;36(1):267–74.
Ates Bulut E, Soysal P, Aydin AE, Dokuzlar O, Kocyigit SE, Isik AT. Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp Gerontol. 2017;95:136–40.
Rebouche CJ. Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr. 1991;54(6 Suppl):1147S–52S.
Franceschi RT. The role of ascorbic acid in mesenchymal differentiation. Nutr Rev. 1992;50(3):65–70.
Gomes MJ, Martinez PF, Pagan LU, Damatto RL, Cezar MDM, Lima ARR, et al. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428–40.
Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle. 2015;6(3):197–207.
Cerullo F, Gambassi G, Cesari M. Rationale for antioxidant supplementation in sarcopenia. J Aging Res. 2012;2012:316943.
Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community-dwelling older adults: the Tasmanian older adult cohort study. J Am Geriatr Soc. 2010;58(11):2129–34.
Martin H, Aihie Sayer A, Jameson K, Syddall H, Dennison EM, Cooper C, et al. Does diet influence physical performance in community-dwelling older people? Findings from the Hertfordshire cohort study. Age Ageing. 2011;40(2):181–6.
Saito K, Yokoyama T, Yoshida H, Kim H, Shimada H, Yoshida Y, et al. A significant relationship between plasma vitamin C concentration and physical performance among Japanese elderly women. J Gerontol A Biol Sci Med Sci. 2012;67(3):295–301.
Robinson SM, Jameson KA, Batelaan SF, Martin HJ, Syddall HE, Dennison EM, et al. Diet and its relationship with grip strength in community-dwelling older men and women: the Hertfordshire cohort study. J Am Geriatr Soc. 2008;56(1):84–90.
Welch AA, Jennings A, Kelaiditi E, Skinner J, Steves CJ. Cross-sectional associations between dietary antioxidant vitamins C, E and carotenoid intakes and Sarcopenic indices in women aged 18–79 years. Calcif Tissue Int. 2020;106(4):331–42.
Lewis LN, Hayhoe RPG, Mulligan AA, Luben RN, Khaw KT, Welch AA. Lower dietary and circulating vitamin C in middle- and older-aged men and women are associated with lower estimated skeletal muscle mass. J Nutr. 2020;150(10):2789–98.
Girgis CM. Vitamin D and skeletal muscle: emerging roles in development, anabolism and repair. Calcif Tissue Int. 2020;106(1):47–57.
Montenegro KR, Cruzat V, Carlessi R, Newsholme P. Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev. 2019;32(2):192–204.
Rosendahl-Riise H, Spielau U, Ranhoff AH, Gudbrandsen OA, Dierkes J. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J Hum Nutr Diet. 2017;30(1):3–15.
Shea MK, Fielding RA, Dawson-Hughes B. The effect of vitamin D supplementation on lower-extremity power and function in older adults: a randomized controlled trial. Am J Clin Nutr. 2019;109(2):369–79.
Suebthawinkul C, Panyakhamlerd K, Yotnuengnit P, Suwan A, Chaiyasit N, Taechakraichana N. The effect of vitamin D2 supplementation on muscle strength in early postmenopausal women: a randomized, double-blind, placebo-controlled trial. Climacteric. 2018;21(5):491–7.
El Hajj C, Fares S, Chardigny JM, Boirie Y, Walrand S. Vitamin D supplementation and muscle strength in pre-sarcopenic elderly Lebanese people: a randomized controlled trial. Arch Osteoporos. 2018;14(1):4.
Martinez-Arnau FM, Fonfria-Vivas R, Cauli O. Beneficial effects of leucine supplementation on criteria for sarcopenia: a systematic review. Nutrients. 2019;11(10):2504.
Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79(2):289–94.
Ble A, Cherubini A, Volpato S, Bartali B, Walston JD, Windham BG, et al. Lower plasma vitamin E levels are associated with the frailty syndrome: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61(3):278–83.
Mulligan AA, Hayhoe R, Luben R, Welch A. Positive associations of dietary intake and plasma concentrations of vitamin E with skeletal muscle mass, heel bone ultrasound attenuation and fracture risk in the EPIC-Norfolk cohort. Antioxidants (Basel). 2021. Accessed 17 Jan 2021.
Aoi W, Naito Y, Takanami Y, Kawai Y, Sakuma K, Ichikawa H, et al. Oxidative stress and delayed-onset muscle damage after exercise. Free Radic Biol Med. 2004;37(4):480–7.
Huey KA, Fiscus G, Richwine AF, Johnson RW, Meador BM. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1beta responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp Physiol. 2008;93(12):1263–72.
Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Giacomini V, Corsi AM, et al. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63(4):376–83.
Semba RD, Blaum C, Guralnik JM, Moncrief DT, Ricks MO, Fried LP. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin Exp Res. 2003;15(6):482–7.
Semba RD, Varadhan R, Bartali B, Ferrucci L, Ricks MO, Blaum C, et al. Low serum carotenoids and development of severe walking disability among older women living in the community: the women’s health and aging study I. Age Ageing. 2007;36(1):62–7.
Alipanah N, Varadhan R, Sun K, Ferrucci L, Fried LP, Semba RD. Low serum carotenoids are associated with a decline in walking speed in older women. J Nutr Health Aging. 2009;13(3):170–5.
Hayhoe RPG, Lentjes MAH, Luben RN, Khaw KT, Welch AA. Dietary carotenoid intake of individuals in the EPIC-Norfolk cohort is positively associated with percentage fat-free mass. Proc Nutr Soc. 2016;75(75):E216.
Sahni S, Dufour AB, Fielding RA, Newman AB, Kiel DP, Hannan MT, et al. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: the Framingham offspring study. Am J Clin Nutr. 2020;113(2):437–45.
Jahnen-Dechent W, Ketteler M. Magnesium basics. Clin Kidney J. 2012;5(Suppl 1):i3–i14.
de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95(1):1–46.
Hayhoe RPG, Lentjes MAH, Mulligan AA, Luben RN, Khaw KT, Welch AA. Cross-sectional associations of dietary and circulating magnesium with skeletal muscle mass in the EPIC-Norfolk cohort. Clin Nutr. 2019;38(1):317–23.
Welch AA, Skinner J, Hickson M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK Biobank Cohort. Nutrients. 2017;9(11):1189.
Welch AA, Kelaiditi E, Jennings A, Steves CJ, Spector TD, MacGregor A. Dietary magnesium is positively associated with skeletal muscle power and indices of muscle mass and may attenuate the association between circulating C-reactive protein and muscle mass in women. J Bone Miner Res. 2016;31(2):317–25.
Brilla LR, Haley TF. Effect of magnesium supplementation on strength training in humans. J Am Coll Nutr. 1992;11(3):326–9.
Veronese N, Berton L, Carraro S, Bolzetta F, De Rui M, Perissinotto E, et al. Effect of oral magnesium supplementation on physical performance in healthy elderly women involved in a weekly exercise program: a randomized controlled trial. Am J Clin Nutr. 2014;100(3):974–81.
Rock E, Astier C, Lab C, Vignon X, Gueux E, Motta C, et al. Dietary magnesium deficiency in rats enhances free radical production in skeletal muscle. J Nutr. 1995;125(5):1205–10.
Aleman H, Esparza J, Ramirez FA, Astiazaran H, Payette H. Longitudinal evidence on the association between interleukin-6 and C-reactive protein with the loss of total appendicular skeletal muscle in free-living older men and women. Age Ageing. 2011;40(4):469–75.
Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64(11):1183–9.
Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–8.
Dibaba DT, Xun P, He K. Dietary magnesium intake is inversely associated with serum C-reactive protein levels: meta-analysis and systematic review. Eur J Clin Nutr. 2014;68(4):510–6.
Veronese N, Zanforlini BM, Manzato E, Sergi G. Magnesium and healthy aging. Magnes Res. 2015;28(3):112–5.
van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and sarcopenia; the role of calcium, Iron, magnesium, phosphorus, potassium, selenium, sodium, and zinc on muscle mass, muscle strength, and physical performance in older adults: a systematic review. J Am Med Dir Assoc. 2018;19(1):6–11 e3.
Giugliano R, Millward DJ. The effects of severe zinc deficiency on protein turnover in muscle and thymus. Br J Nutr. 1987;57(1):139–55.
Chen YL, Yang KC, Chang HH, Lee LT, Lu CW, Huang KC. Low serum selenium level is associated with low muscle mass in the community-dwelling elderly. J Am Med Dir Assoc. 2014;15(11):807–11.
Lauretani F, Semba RD, Bandinelli S, Ray AL, Guralnik JM, Ferrucci L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: the InCHIANTI study. Am J Clin Nutr. 2007;86(2):347–52.
Welch AA, Hayhoe RG. The relationship between dietary fat and sarcopenia, skeletal muscle loss, osteoporosis and risk of fractures in aging. In: Weaver CM, Bischoff-Ferrari H, Daly RM, Wong M-S, editors. Nutritional influences on bone health: 10th International Symposium. Cham: Springer; 2019. p. 211–27.
Welch A, Hayhoe R. The relationship between dietary fat and sarcopenia, skeletal muscle loss, osteoporosis and risk of fractures in aging. In: Weaver CM, Bischoff-Ferrari H, Daly RM, Wong M-S, editors. Nutritional influences on bone health. Cham: Springer; 2019. p. 211–25.
Melzer K. Carbohydrate and fat utilization during rest and physical activity. E-SPEN. 2011;6(2):e45–52.
Gerling CJ, Mukai K, Chabowski A, Heigenhauser GJF, Holloway GP, Spriet LL, et al. Incorporation of Omega-3 fatty acids into human skeletal muscle Sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front Physiol. 2019;10:348.
Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55(1–3):123–39.
Welch AA, Macgregor AJ, Minihane AM, Skinner J, Valdes AA, Spector TD, et al. Dietary fat and fatty acid profile are associated with indices of skeletal muscle mass in women aged 18–79 years. J Nutr. 2014;144(3):327–34.
Abdelhamid A, Hooper L, Sivakaran R, Hayhoe RPG, Welch A, Group P. The relationship between Omega-3, Omega-6 and Total polyunsaturated Fat and musculoskeletal health and functional status in adults: a systematic review and meta-analysis of RCTs. Calcif Tissue Int. 2019;105(4):353–72.
Milaneschi Y, Bandinelli S, Corsi AM, Lauretani F, Paolisso G, Dominguez LJ, et al. Mediterranean diet and mobility decline in older persons. Exp Gerontol. 2011;46(4):303–8.
Gotsis E, Anagnostis P, Mariolis A, Vlachou A, Katsiki N, Karagiannis A. Health benefits of the Mediterranean diet: an update of research over the last 5 years. Angiology. 2015;66(4):304–18.
Ostan R, Lanzarini C, Pini E, Scurti M, Vianello D, Bertarelli C, et al. Inflammaging and cancer: a challenge for the Mediterranean diet. Nutrients. 2015;7(4):2589–621.
Craig JV, Bunn DK, Hayhoe RP, Appleyard WO, Lenaghan EA, Welch AA. Relationship between the Mediterranean dietary pattern and musculoskeletal health in children, adolescents, and adults: systematic review and evidence map. Nutr Rev. 2017;75(10):830–57.
Kelaiditi E, Jennings A, Steves CJ, Skinner J, Cassidy A, MacGregor AJ, et al. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos Int. 2016;27(11):3251–60.
Jennings A, Mulligan AA, Khaw KT, Luben RN, Welch AA. A Mediterranean diet is positively associated with bone and muscle health in a non-Mediterranean region in 25,450 men and women from EPIC-Norfolk. Nutrients. 2020;12(4):1154.
Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc. 1995;95(7):791–7.
Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91(4):1128S–32S.
Garibotto G, Russo R, Sofia A, Sala MR, Sabatino C, Moscatelli P, et al. Muscle protein turnover in chronic renal failure patients with metabolic acidosis or normal acid-base balance. Miner Electrolyte Metab. 1996;22(1–3):58–61.
Vazquez JA, Adibi SA. Protein sparing during treatment of obesity: ketogenic versus nonketogenic very low calorie diet. Metabolism. 1992;41(4):406–14.
Bell JD, Margen S, Calloway DH. Ketosis, weight loss, uric acid, and nitrogen balance in obese women fed single nutrients at low caloric levels. Metabolism. 1969;18(3):193–208.
Dawson-Hughes B, Harris SS, Ceglia L. Alkaline diets favor lean tissue mass in older adults. Am J Clin Nutr. 2008;87(3):662–5.
Faure AM, Fischer K, Dawson-Hughes B, Egli A, Bischoff-Ferrari HA. Gender-specific association between dietary acid load and total lean body mass and its dependency on protein intake in seniors. Osteoporos Int. 2017;28(12):3451–62.
Welch AA, MacGregor AJ, Skinner J, Spector TD, Moayyeri A, Cassidy A. A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos Int. 2013;24(6):1899–908.
Hayhoe RPG, Abdelhamid A, Luben RN, Khaw KT, Welch AA. Dietary acid-base load and its association with risk of osteoporotic fractures and low estimated skeletal muscle mass. Eur J Clin Nutr. 2020;74(Suppl 1):33–42.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Welch, A.A., Hayhoe, R.P.G. (2021). Nutritional Approaches for Sarcopenia. In: Veronese, N., Beaudart, C., Sabico, S. (eds) Sarcopenia. Practical Issues in Geriatrics. Springer, Cham. https://doi.org/10.1007/978-3-030-80038-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-80038-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-80037-6
Online ISBN: 978-3-030-80038-3
eBook Packages: MedicineMedicine (R0)