Abstract
Carbonic anhydrase IX (CAIX) is a transmembranous enzyme that is present in multiple carcinomas, including clear cell renal cell carcinoma (ccRCC). CAIX is a validated cell marker for hypoxia, an important asset in the prediction of radiotherapeutic efficacy. CAIX expression in ccRCC is high and homogenous, the consequence of mutations in the von Hippel Lindau gene, leading to a pseudohypoxic phenotype. CAIX is recognized, amongst others, by the monoclonal antibody G250. The high expression of CAIX in the most common malignant renal cancer in combination with very limited expression in normal tissue endorses CAIX as a promising candidate for multiple antibody-based applications in this disease. This chapter explores potential clinical applications, including the guidance of clinical decision making in case of diagnostic dilemmas concerning ccRCC suspicion, the use of real-time monitoring of surgical margins during renal surgery, the development of novel therapeutic options in patients with advanced ccRCC, and the use of CAIX imaging in hypoxic tumors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Carbonic anhydrases (CA) form a family of zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide, producing bicarbonate and a proton (Hilvo et al. 2008).
CAIX was initially named MN protein after being identified as a cell surface protein in a human cervical carcinoma cell line (HeLa) (Pastorek et al. 1994; Zavada et al. 1993; Pastorekova et al. 1992). When it became clear that this protein belonged to the family of Cas, it was adequately denominated as the ninth member of the CA family: CAIX. CAIX is a suitable pHi regulator in conditions of environmental acidosis (Innocenti et al. 2009). In normal tissue, CAIX expression is limited to the gastrointestinal tract, mainly the gastric mucosa and the bile ducts, where it plays a role in maintaining the acid–base balance.
Prominent expression of CAIX has been observed in multiple carcinomas, including lung, kidney, brain, colon, pancreas, liver, breast, endometrium, esophagus, ovary, and skin (Ivanov et al. 2001). The most homogeneous expression has been observed in clear cell Renal Cell Carcinoma (ccRCC). Elegant studies have demonstrated that regulation of CAIX expression is mainly dependent on transcription factor hypoxia inducible factor 1 (HIF-1α) (Wykoff et al. 2000). In normoxic conditions, HIF-1a is hydroxylated by prolyl hydroxylase domain proteins (PHDs) and bound by pVHL, inciting the polyubiquitylation of prolyl hydroxylated HIF-1a for subsequent degradation via the 26S proteasome (Aprelikova et al. 2004). Under hypoxic conditions, hydroxylation does not occur, leading to HIF accumulation since pVHL binding is inhibited and HIF degradation prevented (Mucaj et al. 2012). This leads to nuclear translocation, and after heterodimerization with HIF-1beta, the transcription factor binds to hypoxic responsive elements in gene promotor regions leading to expression of multiple genes, amongst others CAIX (Benej et al. 2014).
The main driver event in the development of ccRCC is a mutation of VHL. This also leads to expression of hypoxia responsive genes, as the mutated pVHL cannot bind HIF-1α, leading to HIF1α accumulation and nuclear translocation, a so-called pseudohypoxic response. The almost ubiquitous mutation of VHL in ccRCC also explains the homogeneous expression of CAIX in these tumors (Fig. 9.1).
CAIX is recognized by, amongst others, the monoclonal antibody (mAbG250). This antibody was discovered in 1986 (Oosterwijk et al. 1986). Initial studies with G250 indicated specific expression in renal cell carcinoma (RCC), while expression in normal renal tissue was absent. Other normal tissue sites with CAIX expression included bile-duct epithelium and mucous cells in the stomach. Interestingly, G250 antigen expression was also found in other tumor types, albeit less homogenous and at a lower rate. Molecular identification of the recognized antigen lasted until 2000, and it became clear that it was identical to the tumor-associated antigen MN/CAIX (Grabmaier et al. 2000).
This high expression of CAIX in the most common malignant renal cancer in combination with very limited expression in normal tissue endorsed CAIX as a promising candidate for diagnostic and therapeutic modalities in this disease. While current research has mostly focused on ccRCC, the potential of CAIX-targeting extends to other CAIX-expressing malignancies, albeit that the less homogeneous expression may hamper success.
9.2 Carbonic Anhydrase IX Imaging
9.2.1 CAIX Imaging in RCC
CAIX imaging in RCC has been extensively investigated over the last decades. Renal tumors are divergent and their behavior is highly dependent on the pathophysiological subtype. This ranges from benign (20%, including oncocytoma and angiomyolipoma) to indolent (papillary and chromophobe carcinoma) with limited metastatic potential to the more aggressive metastatic clear cell renal cell carcinoma (ccRCC) (Leibovich et al. 2010). Due to more frequent radiological evaluation of the abdomen, the incidence of RCC has increased (Capitanio et al. 2019). Identification of the tumor phenotype is paramount to improve clinical decision making and enable individualized treatment planning. Importantly, approximately 20–30% of kidney lesions are non-malignant and watchful waiting may be possible. Currently, the differential diagnosis is achieved through renal tumor biopsies. This is an invasive procedure with a poor predictive value. Conventional imaging methods (i.e., computed tomography (CT) and ultrasound) cannot reliably distinguish between the indolent and aggressive malignancies (Kutikov et al. 2006). Positron emission tomography (PET) and single photon emission computed tomography (SPECT) are nuclear imaging modalities that offer the ability to non-invasively visualize pathophysiological characteristics of tumors. For this purpose, noninvasive imaging of CAIX using radiolabeled G250 has been developed.
Multiple preclinical studies in various mouse models using different radionuclides have shown excellent selective uptake of mAbG250 in CAIX-expressing xenografts (Brouwers et al. 2004a; Steffens et al. 1998,1999a; Dijk et al. 1991; Kranenborg et al. 1997). The very limited expression of CAIX in normal tissue combined with the homogenous CAIX expression in ccRCC led to the initiation of multiple clinical studies.
In 1993, the first clinical study was published, documenting the imaging and biodistribution characteristics of 131I-mAbG250 in patients suspected for RCC. The explicit visualization of primary and metastatic RCC lesions combined with remarkable uptake of antibody in CAIX-positive tumor tissue demonstrated its diagnostic potential. However, development of human-anti-mouse-antibodies (HAMA) prevented repeated administration (Oosterwijk et al. 1993).
The potential of CAIX imaging in RCC was supported after the chimeric version of the mAbG250 (cG250/girentuximab) was successfully used in a similar study. The characteristics and performance of cG250 were similar to the murine antibody in terms of optimal protein dose, tumor-specific uptake, and visual assessment (Steffens et al. 1997). More importantly, the chimerization greatly reduced the immunogenicity of the antibody, and development of human anti-chimeric antibody (HACA) was rare. Thus, multiple administrations became feasible.
At the same time, with a gradually increasing availability of various radionuclides, careful selection of the radionuclide based on physical half-life and other characteristics for specific indications became possible. Subsequently, the most promising radionuclides (131I (t1/2 = 8.0 days), 125I (t1/2 = 59.4 days), 124I (t1/2 = 4.2 days), 111In (t1/2 = 2.8 days), and 89Zr (t1/2 = 3.3 days)) were used in combination with cG250 in phase I/II studies.
Although the tumor uptake of the radiolabeled cG250 was high, intratumoral uptake was surprisingly heterogeneous and could not be explained by antigen expression alone. As intratumoral necrosis and tumor vasculature did not seem to consistently associate with the heterogenicity, the impact of multiple administrations on tumor uptake of the antibody was studied. Patients with primary kidney tumors received two presurgical administrations, separated by four days, of 125I- and 131I-cG250, respectively. Analysis of the surgically removed specimen showed an identical uptake pattern of both radiolabeled cG250, indicating that two consecutive administrations did not significantly alter the heterogeneous pattern (Steffens et al. 1999b).
In order to compare the CAIX targeting 131I-cG250 with the clinically available PET-tracer 18F-FDG, an intrapatient comparison study in patients with advanced RCC was initiated. Remarkably, the PET-tracer 18F-FDG proved to be superior in comparison to 131I-cG250 for detection of RCC metastases (Brouwers et al. 2002). Since 131I is physiologically excreted from the cell after intracellular proteolytic digestion of labeled antibody, the intracellular retainment of the radionuclide could play an important role in achieving improved tumor to background ratios. This intracellular retainment of a radioisotope after internalization is called residualization. Radiometals, such as 64Cu, 111In, and 89Zr are not excreted, and are also suited for diagnostic and therapeutic purposes when bound to (internalizing) antibodies.
In view of the former, an intrapatient comparison study was initiated using the residualizing 111In and the non-residualizing 131I as radiolabels. 111In-cG250 showed superior tumor to background ratios compared to 131I-cG250 (Brouwers et al. 2003a). Moreover, the diagnostic accuracy of 111In-cG250 in detecting ccRCC lesions in both primary as well as metastasized disease was excellent, providing evidence that antibody internalization does play a role for cG250 imaging (Muselaers et al. 2013).
Although 111In-cG250 showed promising diagnostic ability, the more favorable characteristics of PET compared to SPECT, such as higher spatial resolution and superior quantitative analysis of images, incited the development of immuno-PET tracers. To match the relatively slow pharmacokinetics of intravenously administrated mAb (~t1/2 = 70 h), the radioisotopes 89Zr and 124I were deemed good candidates for this modality.
The first clinical study using CAIX-directed immuno-PET was performed with 124I-cG250 in 26 patients with suspected renal lesions. The aim of the study was to unequivocally distinguish ccRCC from non-ccRCC. Sensitivity and specificity of 124I-cG250 PET/CT to detect ccRCC were 94% and 100%, respectively. The negative and predictive values were 90% and 100%, respectively (Divgi et al. 2007). In a large follow-up multicenter comparison study, 226 patients, who were scheduled for surgery of a renal mass were evaluated. 124I-cG250 PET imaging outperformed conventional imaging in the form of contrast-enhanced CT (CECT). In 195 patients, comparative data was available, and showed superior sensitivity and specificity of 124I-cG250 compared to CECT (86.2 versus. 75.5% and 85.9 versus 46.8%, respectively) (Divgi et al. 2013). However, the non-residualizing characteristics of 124I combined with a scarce availability of this radioisotope were hampering clinical implementation.
With the residualizing radioisotope 89Zr becoming more widely available, which was a limiting factor for clinical development, animal experiments with 89Zr-cG250 were performed to investigate its value as imaging agent. Comparison of 89Zr-cG250 with 124I-cG250 demonstrated superiority in terms of tumor to background ratio (Stillebroer et al. 2013a). This led to a phase I/II clinical trial to evaluate the value of 89Zr-cG250 in aiding clinicians when facing diagnostic dilemmas in RCC. A total of 30 patients were enrolled, in 16 patients immuno-PET imaging was used in the decision to perform either surgery or active surveillance. In 70% of patients, the clinical management was changed based on 89Zr-cG250 PET imaging. Nine patients showed no tumor uptake of 89Zr-cG250, suggesting non-malignancy progression of lesions during follow-up (mean 13.0 ± 4.9 mo) was absent. In seven patients, tumor uptake of 89Zr-cG250 was observed which led to surgical intervention in five cases: in all five cases, ccRCC was histologically proven after surgery. In 5/14 patients suspected of recurrent or metastatic ccRCC, use of 89Zr-cG250 PET/CT imaging led to a major change in clinical management. Additionally, in three patients, repeated biopsies were avoided. Therefore, the authors concluded that 89Zr-cG250 PET/CT is of diagnostic value, and it may guide clinical decision making when a diagnostic dilemma concerning ccRCC suspicion presents (Hekman et al. 2018).
More recently, a phase I safety and dosimetry study using 89Zr-cG250 was performed [NCT03556046]. The primary aim of this study was safety assessment, while secondary outcomes included radiation dosimetry, tumor dosimetry, and diagnostic efficacy (Merkx et al. 2021).
Furthermore, a prospective, multi-center phase III study to evaluate sensitivity and specificity of 89Zr-cG250 PET/CT-imaging to detect ccRCC in patients with suspected primary renal mass for which surgery is scheduled is currently recruiting [NCT03849118].
As described, the tumor-targeting properties of the intact antibodies cG250 and mG250 have been extensively studied. However, these macromolecular radiotracers are known for their slow blood-clearing (~70 h). To optimize imaging properties, radioisotopes with matching half-life are required. As a result, the radiation burden to the patient are relatively high. Use of CAIX-targeting, rapid clearing small-molecules could decrease this radiation burden. However, the development of these fast-clearing tracers has been limited to preclinical work so far. One of these newly developing small molecules is a acetazolamide derivative, a carbonic anhydrase ligand with high affinity for CAIX that is able to transport cytotoxic drugs. The 99mTc-labeled acetazolamide conjugate showed high tumor uptake and decent tumor-to-organ ratios at 3 h p.i. in a mice model using SK-RC-52 xenografts (Krall et al. 2016). Similarly, a range of affibodies radiolabeled with 99mTc and 125I showed specific targeting of CAIX-expressing xenografts at 4 h p.i. Even though the development of these rapid-clearing CAIX-targeting tracers looks promising, clinical studies are required to fully assess their potential (Honarvar et al. 2015; Garousi et al. 2016).
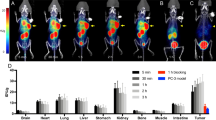
In conclusion, clinical trials have convincingly shown that CAIX-directed antibody imaging is capable of detecting ccRCC lesion in primary as well as metastatic settings. Furthermore, the ongoing development leads to improved tumor to background ratios, thus, enhancing diagnostic accuracy (Fig. 9.2).
Visualization of clear cell renal cell cancer metastases in different regions using 131I-cG250, 111In-cG250, and 89Zr-cG250. A) An intrapatient comparison in which the 131I-cG250 image shows faint accumulation in the chest region, whereas the 111In-cG250 image convincingly demonstrates lung and pleural metastases. B) PET image of 89Zr-cG250 accumulation in a tumor in the right kidney
However, implementation in regular clinical practice still has to be achieved. A currently recruited phase III trial might lead to clinical implementation of CAIX-targeting antibody imaging.
9.2.2 CAIX Imaging in Non-RCC
While current clinical studies with CAIX-targeting antibodies have been limited to RCC, another area of interest for CAIX imaging is the hypoxic tumor. Tumor hypoxia is associated with a poor prognosis, related to an increased resistance to conventional treatment modalities such as radiotherapy and chemotherapy (Bussink et al. 2003; Hockel and Vaupel 2001). As mentioned earlier, hypoxia can increase the levels of HIF-1α and as a result lead to CAIX expression. In non-RCC tumors, CAIX expression is mostly detected in perinecrotic areas and regions that are distanced from perfused vasculature. As a result, you see a much more variable and heterogenous expression in non-RCC tumors compared to ccRCC. Despite the heterogenous CAIX expression in hypoxic tumors, it has been validated as an intrinsic hypoxia-related cell marker and is particularly attractive for in vivo assessment of hypoxia (Wykoff et al. 2000; Pastorekova et al. 2006). However, the half-life of HIF-1α (5–8 min) is significantly lower than that of CAIX (38 h) (Rafajova et al. 2004; Moroz et al. 2009). In the context of transient and fluctuating hypoxia, the evaluation of CAIX expression could lead to an overestimation of actual hypoxic regions. This potential discrepancy requires awareness when considering CAIX quantification as an indicator of hypoxia.
The leaky vasculature of solid tumors is known to increase permeability of macromolecules. As the diffusion of an antibody within a tumor is dependent on the size, it was initially thought that intact IgG might not be suitable for imaging purposes in these tumors. Antibody fragments (F(ab′)2) are smaller than intact IgG (~100 and ~150 kDa, respectively) and have shown higher tumor penetration (Schmidt and Wittrup 2009). Additionally, the F(ab′)2 fragments are more rapidly cleared in comparison to intact IgG. A fast-clearing tracer allows for imaging at early timepoints, this is of particular interest in the setting of the intratumoral hypoxic region, which are susceptible to fluctuation. To evaluate the potential of these antibody fragments in detecting and quantifying hypoxic areas, several preclinical studies have been performed (Hoeben et al. 2010; Huizing et al. 2017, 2019).
The feasibility of noninvasive hypoxia imaging with 89Zr-cG250-F(ab′)2 in head and neck carcinomas has been studied, and demonstrated that the maximum tumor uptake of 89Zr-cG250-F(ab′)2 was reached at day 1 post injection (p.i.), while specific accumulation was seen at 4 h p.i. In comparison, the maximum tumor uptake and earliest specific accumulation for 89Zr-cG250 were 3 days and 1 day, respectively. A significant spatial correlation between the binding of the radiolabeled fragment and CAIX at the microscopic level was established, indicating that sufficient tumor penetration is achieved and accurate microscopic hypoxia localization is possible (Hoeben et al. 2010). Additionally, it was shown that CAIX expression was quantifiable in two different head and neck carcinoma xenograft models (Huizing et al. 2019). Similar results were found in a preclinical study that was designed to compare molecular targeting properties of 111In- Fab-cG250, 111In-F(ab′)2-cG250, and 111In-cG250 in a colorectal xenograft model (Carlin et al. 2010). It was demonstrated that the antibody fragments are capable of targeting CAIX in hypoxic regions at shorter time periods p.i, compared to intact IgG. However, this comes at the cost of reduced absolute uptake and severely reduced tumor-to-muscle ratio. Therefore, the authors concluded that noninvasive imaging with the intact IgG was favored.
Besides the intact cG250 and fragments thereof (Fab), numerous CAIX-targeted imaging agents have been studied in preclinical setting. While none of these surpass the radiolabeled cG250 in terms of tumor uptake, they might prove to be of added values in specific niches, such as visualizing intratumoral hypoxia.
A quantitative biodistribution study of the radiolabeled CAIX ligand VM4-037 in an HT-29 model (human colorectal tumor model) showed no tumor-specific uptake (Peeters et al. 2015). Comparable results were observed with 99mTc-labeled derivatives of phenylsulfonamide in mice bearing HT-29 xenografts (Akurathi et al. 2014). More success was achieved with 68Ga-labeled sulfonamide inhibitors. In a preclinical study using HT-29 xenografted mice, the biodistribution of three tracers (68Ga-DOTA-AEBSA, 68Ga-DOTA-(AEBSA)2, and 68Ga-DOTA-(AEBSA)3) was assessed. While tumor-to-muscle ratios were decent (3.18–9.55), the absolute tumor uptake remained low (Lau et al. 2016).
While CAIX imaging of hypoxic regions has been validated as a prognostic marker in several tumor types, a more interesting application might be predicting therapy resistance and expected efficacy. The visualization and quantification of intratumor hypoxic regions could be used to individualize treatment modalities (i.e., radiotherapeutic “dose painting”). Whether an intact antibody, antibody fragment(s), or other small molecules are best fit for this purpose is yet to be determined.
9.3 Intraoperative Imaging
Intraoperative (fluorescence) imaging is a valuable asset in cancer surgery. It improves tumor visualization in challenging situations such as multifocal disease or organ-sparing surgery by enabling differentiation of tumor and normal tissue. In fluorescence imaging, a fluorophore is excited by light of a distinct wavelength, resulting in emission of a photon. These emitted photons are detected by a fluorescence camera and converted into an image. In a first-in-man trial, fluorescent CAIX imaging was combined with radiodetection to intraoperatively detect ccRCC with cG250. The aim of the dual-label modality technique is to enable real-time monitoring of the surgical margins, ensuring radical surgery. This combination has the advantage that the emitted gamma radiation with its high penetration depth can be used to guide the surgeon to the place of interest, whereas the fluorescent label with the limited penetration depth can be used for live visualization, for instance, to detect positive surgical margins. Complete intraoperative assessment of CAIX-expressing tumors was achieved (Hekman et al. 2016), showing that this can aid the surgeon in achieving complete tumor resection, while sparing normal tissue (Hekman et al. 2018). Further research is needed to advance the use of cG250 for this detection method to show whether this novel technique reduces positive surgical margins and recurrence of tumor. Particularly, the technical hardware needs to be improved.
9.4 CAIX-Directed Radioimmunotherapy
Over the last decades, the armamentarium of therapies against cancer has rapidly expanded with antibody therapies becoming more prominent. One of the most promising application is combining the monoclonal antibody with radiation therapy (radioimmunotherapy). Initial studies were mainly limited to hematologic malignancies, which are perceived as the most radiosensitive tumors. For radioimmunotherapy (RIT) to be successful, several variables are imperative. Most obviously, the best cell surface target antigen and corresponding targeting antibody need to be selected and developed. To elaborate, the ideal target antigen should preferably be highly and homogenously expressed on the surface of all tumor cells with minimal expression on normal tissues. Additionally, the antibody should be internalized after binding, be able to rapidly achieve tumor penetration, and effectively bind the antigen without interacting with non-malignant tissues. Lastly, the antibody should be rapidly cleared from the body after reaching maximum tumor binding. The antibody–antigen pair that meet all these criteria has yet to be discovered. Nonetheless, cG250 in combination with CAIX seems to be a promising pair. Since the high and homogenous expression of CAIX has been observed in ccRCC, the potential of RIT in this disease has been extensively studied.
The choice of therapeutic radionuclide has been a point of discussion since the RIT was studied. In theory, several radionuclides are more favorable than others, but historically it has been shown that availability plays an important role in the development of RIT.
The first RIT studies in ccRCC were performed in 1998 and used the murine version of the G250 antibody radiolabeled with 131I, a β-emitter. 131I had shown its therapeutic efficacy in thyroid-related diseases and was readily available. Additionally, radiolabeling of the antibody with 131I was technically straightforward. Initial results with a single injection of 131I-mG250 showed stabilization of disease in half of the patients. Despite the lack of major responses, the overall survival of patients treated with 131I-mG250 when compared to historic control patients suggested clinical benefit (Divgi et al. 1998). As mentioned earlier, the development of human anti-mouse monoclonal antibody (HAMA), prevented retreatment. For this purpose, cG250/girentuximab was developed. In the subsequent study, patients with RCC metastases received a single therapeutic-dose 131I-cG250 injection preceded by an imaging dose of the tracer to ensure the presence of CAIX-positive metastases. Due to extended serum half-life of the chimeric version, maximum tolerable dose (MTD) of 131I-cG250 was significantly lower than 131I-mG250, and hematological toxicity remained the dose limiting factor (Steffens et al. 1999c). Development of human-anti-chimeric-antibodies (HACA) was limited to a single patient, who received multiple cG250 injection in a previous study. Unfortunately, the therapeutic efficacy of a single dose 131I-cG250 was lacking. In an attempt to increase the RIT efficacy, a study in which patients received fractionated doses of 131I-cG250 was designed. The fractionated approach is associated with lower toxicity profile, possibly due to hematopoietic recovery in between the administrations. Moreover, multiple administration potentially increases the therapeutic efficacy by achieving higher absorbed tumor dose. However, this could not be confirmed in the study as fractionated radionuclide therapy with 131I-cG250 in metastatic ccRCC did not show major clinical responses in two separate studies (Divgi et al. 2004; Brouwers et al. 2005). The hematological toxicity remained an issue, and due to the limited efficacy of 131I-cG250, the search for more suitable radionuclides was initiated.
As more radionuclides became available for use in pre- and clinical studies, new studies were initiated. In a preclinical study, the therapeutic efficacy of lutetium-177 (177Lu)-, yttrium-90 (90Y)-and rhenium-186 (186Re) labeled to girentuximab were compared to 131-cG250. The tumor growth in mice with subcutaneous xenografts was delayed most effectively by 177Lu-cG250 (Brouwers et al. 2004b; Muselaers et al. 2014). This led to the initiation of a clinical study to investigate the therapeutic efficacy and maximum tolerable dose (MTD) of 177Lu-cG250 in patients with advanced RCC. 177Lu-cG250 RIT was well tolerated and resulted in disease stabilization in the majority of patients (Stillebroer et al. 2013b). The ensuing study used the observed MTD of 2405 MBq/m2 in a similar patient population. In absence of persistent toxicity and progressive disease after initial treatment, patients were eligible for retreatment of 177Lu-cG250 after 3 months with 75% of the initial activity dose. Similar to the phase I study, 177Lu-cG250 RIT led to stabilized disease in the majority of patients. After the first cycle of 177Lu-cG250 RIT, grade 3–4 thrombocytopenia and grade 3–4 leukocytopenia were observed in all but one patient (Muselaers et al. 2016). This is caused by the relatively long circulation combined with the range of the beta-emitting radionuclide. No clinical studies with cG250 RIT have been performed since then. To prevent irreversible myelotoxicity, treatment regimen need to be adjusted properly. Preclinical studies suggest that this might be achieved by combining RIT in a lower dose with VEGFR-TKIs or immune-checkpoint inhibitors (Stewart et al. 2014). Alternatively, modifying monoclonal antibodies to decrease the circulation time may provide a solution.
A promising alternative might be the use of cG250 labeled with α-emitting radionuclides such as 225Ac and 227Th. Αlpha-emitting radionuclides provide a higher linear energy transfer (LET) and have a lower range, avoiding damage to unrelated nearby tissues. The higher LET causes irreversible double-strand DNA breaks, resulting in a higher relative biological effectiveness (RBE) compared to beta radiation. Targeted α therapy (TAT) has shown impressive anti-tumor effects in multiple clinical studies (Kratochwil et al. 2016). However, TAT with monoclonal antibodies is challenging: whenever an α-emitting radionuclide decays, the daughter nuclide is released from the chelater. This so-called recoil effect leads to excretion of unbound radionuclides in the bloodstream. If these accumulate, other organs will be at risk. Moreover, the availability of α-emitting radionuclides is limited, inhibiting their use in clinical studies. Nevertheless, based on the results with cG250 in RIT, the potential of TAT in CAIX-targeted therapy needs to be further explored. Considering that the hypoxic regions within a tumor are resistant to conventional radiotherapy, CAIX-targeted RIT might be an effective alternative.
9.5 Carbonic Anhydrase IX Immunotherapy
Monoclonal antibodies have been approved for cancer treatment in multiple oncological entities. The therapeutic effect of antibodies may depend on the capacity to lyse cells by complement activation or by antibody-dependent cellular toxicity (ADCC). In vitro studies showed that cG250 initiates cell lysis through ADCC of CAIX-positive cells. Additionally, preclinical mouse studies showed significant tumor growth reduction after treatment with unmodified G250. These findings led to the first clinical study using unmodified cG250 in RCC patients in which optimal dosing and safety were assessed. Weekly infusions with unmodified cG250 were deemed safe and well tolerated (Davis et al. 2007a). The subsequent study in 36 patients with advanced RCC suggested clinical benefit after one treatment cycle of 50mg cG250 in 28% of progressive patients. During the follow-up, one minor partial response (<50% decrease) and one complete response (CR) were observed (Bleumer et al. 2004). The complete response was observed in patient who underwent a nephrectomy before starting treatment, therefore, it was unclear whether the CR was part of the natural disease course or an antibody-induced response. At the same time, the Adjuvant Rencarex® Immunotherapy (ARISER) study was initiated to study if cG250 was able to reduce the recurrence of disease in high risk patients who underwent nephrectomy for non-metastasized RCC. There was no improvement in median disease free survival (DFS) for patients that received adjuvant cG250 compared to placebo which was the primary objective of this study, and therefore, the study was pre-emptively terminated (Chamie et al. 2017). A retrospective subanalysis showed that adjuvant cG250 therapy did increase the DFS in patients with high expressing CAIX tumors, emphasizing that high CAIX expression is a prerequisite to achieve this benefit.
To enhance the therapeutic potential of cG250 mAb in RCC therapy, combination therapy with immune agents such as interleukin-2 and interferon-α have been used (Davis et al. 2007b; Bleumer et al. 2006). Interleukin-2 (IL-2) has the ability to enhance ADCC of monoclonal antibodies including cG250 (Brouwers et al. 2003b). Admission of cG250 combined with IL-2 was well tolerated with little toxicity, but the value of adding IL-2 in this therapeutic setting was unclear. Currently, the role of CAIX targeting with cG250 in the immunotherapy is not well defined, particularly because the patient numbers in these phase I trials were small, and randomization and comparison with control groups were not performed. Larger controlled trials are needed to define the role of cG250 (or derivatives thereof) as a therapy modality.
9.6 Conclusion and Future Perspectives
The landscape of cancer treatment is constantly changing, and CAIX-targeted therapy has the potential to become an important asset in the growing armamentarium against cancer. CAIX represents an ideal target for treatment of clear cell renal cell carcinoma and hypoxic tumors. The rare expression in normal tissues enables the exploration of tumor-specific therapies such as RIT. RIT is considered to have a high anti-tumor effect, but a balance between therapeutic efficacy and toxicity is challenging. The search for the most suitable radionuclide is ongoing, and the increased availability of α-emitters has opened up a new path.
With immunotherapy finding its way into clinical practice, combination of RIT and immunotherapy are of future interest.
Beyond the clear therapeutic potential, CAIX has shown to be an excellent target for accurate diagnosis of clear cell renal cell carcinoma. This aids clinical decision making and enables individualized treatment plans. Other modalities that are being explored are intraoperative use of CAIX-targeting in patients planned for partial or total nephrectomy for RCC.
References
Akurathi V, Dubois L, Celen S, Lieuwes NG, Chitneni SK, Cleynhens BJ et al (2014) Development and biological evaluation of (9)(9)mTc-sulfonamide derivatives for in vivo visualization of CA IX as surrogate tumor hypoxia markers. Eur J Med Chem 71:374–384
Aprelikova O, Chandramouli GV, Wood M, Vasselli JR, Riss J, Maranchie JK et al (2004) Regulation of HIF prolyl hydroxylases by hypoxia-inducible factors. J Cell Biochem 92(3):491–501
Benej M, Pastorekova S, Pastorek J (2014) Carbonic anhydrase IX: regulation and role in cancer. Subcell Biochem 75:199–219
Bleumer I, Knuth A, Oosterwijk E, Hofmann R, Varga Z, Lamers C et al (2004) A phase II trial of chimeric monoclonal antibody G250 for advanced renal cell carcinoma patients. Br J Cancer 90(5):985–990
Bleumer I, Oosterwijk E, Oosterwijk-Wakka JC, Voller MC, Melchior S, Warnaar SO et al (2006) A clinical trial with chimeric monoclonal antibody WX-G250 and low dose interleukin-2 pulsing scheme for advanced renal cell carcinoma. J Urol 175(1):57–62
Brouwers AH, Buijs WC, Oosterwijk E, Boerman OC, Mala C, De Mulder PH et al (2003a) Targeting of metastatic renal cell carcinoma with the chimeric monoclonal antibody G250 labeled with (131)I or (111)In: an intrapatient comparison. Clin. Cancer Res. 9(10 Pt 2):3953s-s3960
Brouwers AH, Dorr U, Lang O, Boerman OC, Oyen WJ, Steffens MG et al (2002) 131 I-cG250 monoclonal antibody immunoscintigraphy versus [18 F]FDG-PET imaging in patients with metastatic renal cell carcinoma: a comparative study. Nucl Med Commun 23(3):229–236
Brouwers AH, Frielink C, Oosterwijk E, Oyen WJ, Corstens FH, Boerman OC (2003b) Interferons can upregulate the expression of the tumor associated antigen G250-MN/CA IX, a potential target for (radio)immunotherapy of renal cell carcinoma. Cancer Biother Radiopharm 18(4):539–547
Brouwers A, Verel I, Van Eerd J, Visser G, Steffens M, Oosterwijk E et al (2004a) PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother Radiopharm 19(2):155–163
Brouwers AH, van Eerd JE, Frielink C, Oosterwijk E, Oyen WJ, Corstens FH et al (2004b) Optimization of radioimmunotherapy of renal cell carcinoma: labeling of monoclonal antibody cG250 with 131I, 90Y, 177Lu, or 186Re. J Nucl Med Offi Publ Soc Nucl Med 45(2):327–337
Brouwers AH, Mulders PF, de Mulder PH, van den Broek WJ, Buijs WC, Mala C et al (2005) Lack of efficacy of two consecutive treatments of radioimmunotherapy with 131I-cG250 in patients with metastasized clear cell renal cell carcinoma. J Clin Oncol Offi J Am Soc Clin Oncol 23(27):6540–6548
Bussink J, Kaanders JH, van der Kogel AJ (2003) Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol J Eur Soc Ther Radiol Oncol 67(1):3–15
Capitanio U, Bensalah K, Bex A, Boorjian SA, Bray F, Coleman J et al (2019) Epidemiology of renal cell carcinoma. Eur Urol 75(1):74–84
Carlin S, Khan N, Ku T, Longo VA, Larson SM, Smith-Jones PM (2010) Molecular targeting of carbonic anhydrase IX in mice with hypoxic HT29 colorectal tumor xenografts. PLoS One 5(5):e10857
Chamie K, Donin NM, Klopfer P, Bevan P, Fall B, Wilhelm O et al (2017) Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: the ARISER randomized clinical trial. JAMA Oncol 3(7):913–920
Davis ID, Wiseman GA, Lee FT, Gansen DN, Hopkins W, Papenfuss AT et al (2007a) A phase I multiple dose, dose escalation study of cG250 monoclonal antibody in patients with advanced renal cell carcinoma. Cancer Immun 7:13
Davis ID, Liu Z, Saunders W, Lee FT, Spirkoska V, Hopkins W et al (2007b) A pilot study of monoclonal antibody cG250 and low dose subcutaneous IL-2 in patients with advanced renal cell carcinoma. Cancer Immun 7:14
Divgi CR, Bander NH, Scott AM, O’Donoghue JA, Sgouros G, Welt S et al (1998) Phase I/II radioimmunotherapy trial with iodine-131-labeled monoclonal antibody G250 in metastatic renal cell carcinoma. Clin Cancer Res Offi J Am Assoc Cancer Res 4(11):2729–2739
Divgi CR, O’Donoghue JA, Welt S, O’Neel J, Finn R, Motzer RJ et al (2004) Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med Offi Publ Soc Nucl Med 45(8):1412–1421
Divgi CR, Pandit-Taskar N, Jungbluth AA, Reuter VE, Gonen M, Ruan S et al (2007) Preoperative characterisation of clear-cell renal carcinoma using iodine-124-labelled antibody chimeric G250 (124I-cG250) and PET in patients with renal masses: a phase I trial. Lancet Oncol 8(4):304–310
Divgi CR, Uzzo RG, Gatsonis C, Bartz R, Treutner S, Yu JQ et al (2013) Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol 31(2):187–194
Garousi J, Honarvar H, Andersson KG, Mitran B, Orlova A, Buijs J et al (2016) Comparative evaluation of affibody molecules for radionuclide imaging of in vivo expression of carbonic anhydrase IX. Mol Pharm 13(11):3676–3687
Grabmaier K, Vissers JL, De Weijert MC, Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH et al (2000) Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer 85(6):865–870
Hekman MC, Boerman OC, de Weijert M, Bos DL, Oosterwijk E, Langenhuijsen JF et al (2016) Targeted dual-modality imaging in renal cell carcinoma: an ex vivo kidney perfusion study. Clin Cancer Res Offi J Am Assoc Cancer Res 22(18):4634–4642
Hekman MCH, Rijpkema M, Aarntzen EH, Mulder SF, Langenhuijsen JF, Oosterwijk E et al (2018) Positron emission tomography/computed tomography with (89)Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur Urol 74(3):257–260
Hekman MC, Rijpkema M, Muselaers CH, Oosterwijk E, Hulsbergen-Van de Kaa CA, Boerman OC, et al (2018) Tumor-targeted dual-modality imaging to improve intraoperative visualization of clear cell renal cell carcinoma: a first in man study. Theranostics 8(8):2161–70.
Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al (2008) Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 283(41):27799–27809
Hockel M, Vaupel P (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 93(4):266–276
Hoeben BA, Kaanders JH, Franssen GM, Troost EG, Rijken PF, Oosterwijk E et al (2010) PET of hypoxia with 89Zr-labeled cG250-F(ab’)2 in head and neck tumors. J Nucl Med Off Publ Soc Nucl Med 51(7):1076–1083
Honarvar H, Garousi J, Gunneriusson E, Hoiden-Guthenberg I, Altai M, Widstrom C et al (2015) Imaging of CAIX-expressing xenografts in vivo using 99mTc-HEHEHE-ZCAIX:1 affibody molecule. Int J Oncol 46(2):513–520
Huizing FJ, Hoeben BAW, Franssen G, Lok J, Heskamp S, Oosterwijk E et al (2017) Preclinical validation of (111)In-girentuximab-F(ab’)2 as a tracer to image hypoxia related marker CAIX expression in head and neck cancer xenografts. Radiother Oncol J Eur Soc Ther Radiol Oncol 124(3):521–525
Huizing FJ, Hoeben BAW, Franssen GM, Boerman OC, Heskamp S, Bussink J (2019) Quantitative imaging of the hypoxia-related marker CAIX in head and neck squamous cell carcinoma xenograft models. Mol Pharm 16(2):701–708
Innocenti A, Pastorekova S, Pastorek J, Scozzafava A, De Simone G, Supuran CT (2009) The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg Med Chem Lett 19(20):5825–5828
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G et al (2001) Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158(3):905–919
Krall N, Pretto F, Mattarella M, Muller C, Neri D (2016) A 99mTc-labeled ligand of carbonic anhydrase IX selectively targets renal cell carcinoma in vivo. J Nucl Med 57(6):943–949
Kranenborg MH, Boerman OC, de Weijert MC, Oosterwijk-Wakka JC, Corstens FH, Oosterwijk E (1997) The effect of antibody protein dose of anti-renal cell carcinoma monoclonal antibodies in nude mice with renal cell carcinoma xenografts. Cancer 80(12 Suppl):2390–2397
Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F et al (2016) 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med Offi Publ Soc Nucl Med 57(12):1941–1944
Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP et al (2006) Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68(4):737–740
Lau J, Zhang Z, Jenni S, Kuo HT, Liu Z, Vullo D et al (2016) PET imaging of carbonic anhydrase IX expression of HT-29 tumor xenograft mice with (68)Ga-Labeled Benzenesulfonamides. Mol Pharm 13(3):1137–1146
Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML et al (2010) Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol 183(4):1309–1315
Merkx et al (2021) https://pubmed.ncbi.nlm.nih.gov/33651116/
Moroz E, Carlin S, Dyomina K, Burke S, Thaler HT, Blasberg R, et al (2009) Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One 4(4):e5077
Mucaj V, Shay JES, Simon MC (2012) Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol 95(5):464–470
Muselaers CH, Boerman OC, Oosterwijk E, Langenhuijsen JF, Oyen WJ, Mulders PF (2013) Indium-111-labeled girentuximab immunoSPECT as a diagnostic tool in clear cell renal cell carcinoma. Eur Urol 63(6):1101–1106
Muselaers CH, Oosterwijk E, Bos DL, Oyen WJ, Mulders PF, Boerman OC (2014) Optimizing lutetium 177-anti-carbonic anhydrase IX radioimmunotherapy in an intraperitoneal clear cell renal cell carcinoma xenograft model. Mol Imaging 13:1–7
Muselaers CH, Boers-Sonderen MJ, van Oostenbrugge TJ, Boerman OC, Desar IM, Stillebroer AB et al (2016) Phase 2 study of lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 69(5):767–770
Oosterwijk E, Ruiter DJ, Hoedemaeker PJ, Pauwels EK, Jonas U, Zwartendijk J et al (1986) Monoclonal antibody G 250 recognizes a determinant present in renal-cell carcinoma and absent from normal kidney. Int J Cancer 38(4):489–494
Oosterwijk E, Bander NH, Divgi CR, Welt S, Wakka JC, Finn RD et al (1993) Antibody localization in human renal cell carcinoma: a phase I study of monoclonal antibody G250. J Clin Oncol 11(4):738–750
Pastorekova S, Zavadova Z, Kostal M, Babusikova O, Zavada J (1992) A novel quasi-viral agent, MaTu, is a two-component system. Virology 187(2):620–626
Pastorekova S, Parkkila S, Zavada J (2006) Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem 42:167–216
Pastorek J, Pastorekova S, Callebaut I, Mornon JP, Zelnik V, Opavsky R et al (1994) Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene 9(10):2877–2888
Peeters SG, Dubois L, Lieuwes NG, Laan D, Mooijer M, Schuit RC et al (2015) [(18)F]VM4-037 MicroPET imaging and biodistribution of two in vivo CAIX-expressing tumor models. Mol Imaging Biol MIB Offi Publ Acad Mol Imaging 17(5):615–619
Rafajova M, Zatovicova M, Kettmann R, Pastorek J, Pastorekova S (2004) Induction by hypoxia combined with low glucose or low bicarbonate and high posttranslational stability upon reoxygenation contribute to carbonic anhydrase IX expression in cancer cells. Int J Oncol 24(4):995–1004
Schmidt MM, Wittrup KD (2009) A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 8(10):2861–2871
Steffens MG, Boerman OC, Oosterwijk-Wakka JC, Oosterhof GO, Witjes JA, Koenders EB et al (1997) Targeting of renal cell carcinoma with iodine-131-labeled chimeric monoclonal antibody G250. J Clin Oncol 15(4):1529–1537
Steffens MG, Kranenborg MH, Boerman OC, Zegwaart-Hagemeier NE, Debruyne FM, Corstens FH et al (1998) Tumor retention of 186Re-MAG3, 111In-DTPA and 125I labeled monoclonal antibody G250 in nude mice with renal cell carcinoma xenografts. Cancer Biother Radiopharm 13(2):133–139
Steffens MG, Oosterwijk E, Kranenborg MH, Manders JM, Debruyne FM, Corstens FH et al (1999a) In vivo and in vitro characterizations of three 99mTc-labeled monoclonal antibody G250 preparations. J Nuclear Med 40(5):829–836
Steffens MG, Boerman OC, Oyen WJ, Kniest PH, Witjes JA, Oosterhof GO et al (1999b) Intratumoral distribution of two consecutive injections of chimeric antibody G250 in primary renal cell carcinoma: implications for fractionated dose radioimmunotherapy. Can Res 59(7):1615–1619
Steffens MG, Boerman OC, de Mulder PH, Oyen WJ, Buijs WC, Witjes JA et al (1999c) Phase I radioimmunotherapy of metastatic renal cell carcinoma with 131I-labeled chimeric monoclonal antibody G250. Clin Cancer Res Offi J Am Assoc Cancer Res 5(10 Suppl):3268s-s3274
Stewart GD, O’Mahony FC, Laird A, Rashid S, Martin SA, Eory L et al (2014) Carbonic anhydrase 9 expression increases with vascular endothelial growth factor-targeted therapy and is predictive of outcome in metastatic clear cell renal cancer. Eur Urol 66(5):956–963
Stillebroer AB, Franssen GM, Mulders PF, Oyen WJ, van Dongen GA, Laverman P et al (2013a) ImmunoPET imaging of renal cell carcinoma with (124)I- and (89)Zr-labeled anti-CAIX monoclonal antibody cG250 in mice. Cancer Biother Radiopharm 28(7):510–515
Stillebroer AB, Boerman OC, Desar IM, Boers-Sonderen MJ, van Herpen CM, Langenhuijsen JF et al (2013b) Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 64(3):478–485
van Dijk J, Zegveld ST, Fleuren GJ, Warnaar SO (1991) Localization of monoclonal antibody G250 and bispecific monoclonal antibody CD3/G250 in human renal-cell carcinoma xenografts: relative effects of size and affinity. Int J Cancer 48(5):738–743
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A et al (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Can Res 60(24):7075–7083
Zavada J, Zavadova Z, Pastorekova S, Ciampor F, Pastorek J, Zelnik V (1993) Expression of MaTu-MN protein in human tumor cultures and in clinical specimens. Int J Cancer 54(2):268–274
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Merkx, R.I.J., Mulders, P.F.A., Oosterwijk, E. (2021). Carbonic Anhydrase IX: Current and Emerging Therapies. In: Chegwidden, W.R., Carter, N.D. (eds) The Carbonic Anhydrases: Current and Emerging Therapeutic Targets. Progress in Drug Research, vol 75. Springer, Cham. https://doi.org/10.1007/978-3-030-79511-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-79511-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79510-8
Online ISBN: 978-3-030-79511-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)