Abstract
Perioptic meningiomas, defined as those in contact or adjacent (within a 2 or 3 mm distance) to the anterior optic pathways (AOP, optic nerves, and chiasm), are challenging lesions to manage with radiation ablative therapies due to the vicinity to the radiation-sensible optic apparatus. Because of the perceived risk of damaging the AOP with single-session stereotactic radiosurgery, hypofractionated radiation delivery regimens have been introduced since the last two decades. Hypofractionated radiosurgery involves the delivery of an ablative radiation dose in 2–5 large fractions at 24- or 12-h intervals and allows the surgeon to combine the high conformality and targeting accuracy of SRS platforms with the radiobiological advantages of dose fractionation. Various studies have investigated the safety and efficacy of hypofractionated radiosurgery for the treatment of perioptic meningiomas using the Gamma Knife, the CyberKnife, and linear accelerators. Overall, those studies have confirmed that hypofractionated stereotactic radiosurgery is effective in controlling the growth of perioptic meningiomas and is safe to the anterior optic pathways, though follow-up assessment periods and number of patients included remain limited. The aim of this chapter is to review the literature about stereotactic hypofractionated radiosurgery for the treatment of perioptic meningiomas. These outcomes are compared with those of alternative therapies including conventionally fractionated radiation therapy and single-session radiosurgery. Finally, the radiation tolerance of the optic pathways when using the different radiation delivery regimens is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Perioptic meningiomas are defined as those in contact or adjacent (within a 2 or 3 mm distance) to the anterior optic pathways (AOP, optic nerves, and chiasm) [1,2,3,4,5,6,7,8,9,10,11]. The treatment of these tumors is comprised of microsurgical resection and ablative radiation therapies. The aim of microsurgical resection, which is usually proposed as the first-line treatment, is to immediately decompress the AOP, so as to restore visual function or prevent its decline. However, the complete surgical removal of these tumors is not always feasible due to the risk of damaging the AOP during surgical manipulation and due to the tumor’s infiltrative growth and invasion of the skull base dura and cavernous sinus [12, 13]. Radiation ablative therapies, which include conventionally fractionated stereotactic radiation therapy (FSRT) and stereotactic radiosurgery (SRS), are commonly used as salvage or adjuvant treatments for recurrent or residual meningiomas after surgical resection, respectively. Finally, they can be used as up-front treatments for small tumors or for patients who are not good surgical candidates due to advanced age and/or serious medical comorbidities. The main concern with SRS delivered in the usual single fraction is that a single large dose of radiation may damage the adjacent AOP and pituitary gland and stalk, which are exquisitely radiation sensitive [14, 15]. Therefore, in the last two decades, a handful of studies have investigated the effects of fractionating the radiosurgical dose in up to five larger fractions to control the growth of perioptic meningiomas while mitigating the risk of damaging the AOP [1, 2, 4,5,6,7,8,9,10,11, 16]. The rationale of this approach, namely hypofractionated radiosurgery, is to allow interfractional normal tissue repair of sublethal damage while delivering a biologically effective dose capable of controlling the tumor growth [17]. Those studies have demonstrated that hypofractionated SRS is effective in controlling the growth of perioptic meningiomas with little visual toxicity, though the follow-up assessment periods and number of patients are limited (Table 8.1) [1, 2, 4,5,6,7,8,9,10,11, 18]. More recently, advancements in neuroimaging and radiosurgical platforms have rekindled an interest in delivering single-session SRS for the management of perioptic meningiomas [19, 20]. As a matter of fact, some authors have demonstrated in their studies that single-session SRS for perioptic meningiomas is safe to the AOP as well as effective in controlling tumors’ growth, comparably with hypofractionated SRS or conventionally fractionated radiotherapy [15, 19,20,21]. However, the safe radiation dose to be delivered in a single session to perioptic meningiomas and the AOP is yet to be established. The aim of the following sections is to review the literature about stereotactic hypofractionated radiosurgery for the treatment of perioptic meningiomas. These are compared with the outcomes of alternative therapies including conventionally fractionated radiation therapy and single-session radiosurgery. Finally, the radiation tolerance of the optic pathways to the different radiation delivery regimens is discussed.
8.2 Results of Hypofractionated Radiosurgery for Perioptic Meningiomas
According to a consensus of the Congress of Neurological Surgeons (CNS) and the American Association of Neurological Surgeons (AANS), “SRS typically is performed in a single session, using a rigidly attached stereotactic guiding device, other immobilization technology and/or a stereotactic image-guidance system, but can be performed in a limited number of sessions, up to a maximum of five” [22].
SRS delivered in a number of large fractions between two and five is referred to as hypofractionated SRS. Since the last two decades, hypofractionated stereotactic radiosurgery has been introduced to treat some large neoplasms or intracranial tumors abutting critical and radiosensible structures, due to the perceived risks with single-session radiosurgery. In the case of perioptic tumors, the radiation gradient falloff typical of commonly used radiosurgery delivery platforms, including the Gamma Knife, was thought to be not steep enough to effectively control tumor’s growth while at the same time not injuring the AOP with a single-session treatment [7]. Contrastingly, hypofractionated SRS which integrates the benefits of focused high-dose radiation and conformity typically associated with SRS platforms with the radiobiological advantages of fractionation was considered safer to the AOP [23]. As such, a handful of studies have investigated the effectiveness of hypofractionated SRS for the treatment of perioptic tumors (Table 8.1) [1, 3,4,5,6,7,8,9, 11, 16, 24,25,26]. Initially, the Stanford’s group reported preliminary positive results in the treatment of perioptic tumors with hypofractionated SRS using the CyberKnife (Accuray, Inc., Sunnyvale, CA). No patients developed RION in that study; however the follow-up assessment time was short [8]. In the most recent clinical report of that group, Adler et al. reported on 49 patients treated with multisession CyberKnife-mediated SRS and observed that radiation-induced optic neuropathy (RION) developed in a single patient after a mean follow-up period of 46 months. Notably, that patient’s tumor had received multiple radiation treatments before SRS. For all patients, the reported tumor control rate was 94% at final evaluations [1]. Following the Stanford’s experience, other groups confirmed the safety and efficacy of hypofractionated SRS for the management of meningiomas and a range of slow-growing benign tumors adjacent to the visual pathways, including pituitary adenoma and craniopharyngioma [3,4,5,6,7, 9,10,11, 16]. In those studies, various radiosurgical devices were used to deliver hypofractionated/multisession SRS. The CyberKnife was the most popular [1, 5,6,7, 9,10,11, 16, 24], whereas Gamma Knife radiosurgery (GKRS) was used in a limited number of studies [3, 4]. Kim and colleagues treated 22 patients with perioptic benign tumors using GKRS with a tumor control rate of 96% and no visual compromise at a mean follow-up of 29 months [4]. That series was extended to include 38 patients and found comparable results after a mean period of 38.2 months following radiosurgery. A single patient developed RION at the last clinical assessment [3]. Finally, in an attempt to reduce the discomfort associated with prolonged stereotactic frame application, some authors investigated the use of a relocatable stereotactic frame compatible with the Gamma Knife Perfexion system (Extend system, Elekta AB instruments, Stockholm, Sweden). Nguyen reported on 15 patients with perioptic tumors (including 12 meningiomas) who were treated with hypofractionated GKRS using the relocatable Extend system and found that tumor’s growth was controlled in all patients, with no case of visual deterioration after a median follow-up of 13.8 months following radiosurgery. Similar results were achieved in the study of Devriendt et al. All the 11 patients with perioptic meningiomas who were included in that study did not develop RION or had tumor progression following five-session GKRS using the relocatable Extend system (mean follow-up time of 19 months) [2, 7, 9, 16].
Overall, hypofractionated SRS delivered with frameless or frame-based devices was demonstrated to be safe in terms of visual function preservation and effective in controlling perioptic meningiomas’ growth. In most series, visual function deterioration was caused by tumor progression rather than radiation damage. Most recently, the Italian Gamma Knife Research Study Group (IGKRS) has collected clinical and radiosurgical data of 167 patients treated with three-session hypofractionated GKRS for meningiomas in contact with the AOP (unpublished data). After a mean follow-up period of 51.8 months, longer than in most published studies, four patients developed RION, thus confirming the safety of three-session radiosurgery. The investigators observed that tumor control rate was lower in those patients treated with hypofractionated GKRS as a salvage or adjuvant treatment than in those treated with up-front GKRS. Since radiosurgery is an image-guided surgery, an unsuccessful prior resection can make defining the radiosurgical target as well as delineating the critical structures more difficult [19]. Therefore, some parts of recurrent or residual tumors might have received a lower non-ablative dose. This finding is concordant with some previous reports [27,28,29].
8.3 Radiation Tolerance of the Optic Apparatus During Radiosurgery
Visual impairment from RION is uncommon but disabling. It usually presents with painless rapid visual loss. Vascular injury has been suggested as a significant contributor to RION, although other factors may play a role in its development. The interval between radiation therapy and development of visual symptoms is generally ≤3 years (mode, 1–1.5; median, 2.5) [21]. To mitigate the risk of RION when targeting a perioptic meningioma with stereotactic radiation therapies, the knowledge of the radiation dose-response characteristics of the AOP is essential. At the present time, the safe radiation dose for such delicate nerve structures delivered with single or fractionated SRS treatments is controversial [30]. The seminal study investigating the AOP’s tolerance to single-session SRS was published by Tishler and colleagues in 1993 [31]. In that retrospective study, 17 patients with perioptic meningiomas were treated with a radiation dose to the AOP exceeding 8 Gy using either a linear accelerator or a GKRS. The authors found that radiation-induced optic nerve injury occurred in four of these patients after a median period of 19 months following SRS. Contrastingly, none of the 35 patients who were treated with a dose below 8 Gy developed RION. According to their results, Duma and colleagues found no visual complications when the dose of the radiation delivered to the AOP was below 9 Gy [32]. Based on those initial investigations, the safe dose to the AOP during single-session radiosurgery was kept below 8 Gy by many radiosurgeons. However, those initial studies were conducted early in the overall radiosurgical experience and had major limitations in the determination of the dose delivered to the AOP. First, the majority of patients included underwent computed tomography (CT) as the imaging modality used for dose planning. CT is limited in clearly identifying some intracranial structures, especially those near the skull base, such as the AOP. Assigning an exact dose to the AOP may have therefore either overestimated or underestimated the actual dose delivered. With modern treatment delivery platforms, the AOP is identified and contoured on high-resolution MRI exams that allow for more accurate volumes’ definition and dose estimates. Second, in the initial studies the maximum dose received by the AOP was based on computer-generated isodose curves being laid over the actual images, which is a fairly inaccurate method for estimating the dose delivered to a single structure, especially when using a treatment delivery technology with a rapid dose gradient falloff such as the Gamma Knife. Data analyzed to derive the 8 Gy threshold were thus relatively imprecise compared with those analyzed with more current and precise dose planning software that provide point dose statistics and dose-volume histograms for any chosen structures [33]. As a result, in 1998, Leber and colleagues investigated the neuro-ophthalmological outcomes of SRS using the Gamma Knife in 66 eyes of 45 patients treated with single-session SRS for benign skull base tumors involving the cavernous sinus. After a mean follow-up period of 40 months, the actuarial risk of developing RION was zero for patients receiving a maximum dose below 10 Gy, 26.7% for patients receiving 10–15 Gy, and 77.8% for those receiving more than 15 Gy. The authors concluded that the visual pathways appear to tolerate doses up to 10 Gy with acceptable risk [30]. Subsequently, similar studies examined the radiation tolerance of the optic pathways and suggested that the 8 Gy threshold is likely a conservative estimate for the single-fraction tolerance of the optic apparatus, and concluded that up to 10 Gy can be justified on a theoretical basis [34, 35]. That is, Morita et al. at the Mayo Clinic reviewed their experience with radiosurgery for skull base meningiomas, and observed that in 35 patients that were treated with a dose superior to 8 Gy to the optic apparatus (the median dose to the optic apparatus was 10 Gy, range 1–16 Gy), none developed RION after a median period of 35 months following SRS [35]. Stafford and colleagues later extended this series to include 215 patients and observed that the risk of developing a clinically significant RION was 1.9% (4 patients) for patients receiving 12 Gy or less to the AOP after a median period of 31 months following SRS. Three out of the four patients who developed RION in that study had been previously treated with radiotherapy, which, in accord with previous studies [30, 36], is a known risk factor for RION. Thereafter, several other studies analyzed the dose-volume tolerance of the AOP to single-fraction SRS delivered with contemporary radiation delivery techniques. Overall, those investigations confirmed that patients with parasellar benign lesions who have not had prior irradiation can receive doses up to 12 Gy to the AOP with a low risk of RION (see Fig. 8.1) [25]. For example, Hasegawa et al. [37] reported on 100 patients with craniopharyngiomas treated with single-fraction SRS using GKRS. Of the three patients who developed RION, one had undergone external body radiotherapy prior to SRS, whereas the other two patients received a maximum radiation dose to the AOP of 15 Gy and 18 Gy, respectively. They concluded that radiation doses up to 14 Gy to small portions of the AOP are safe with a low risk of RION. Leavitt et al. [20] reviewed 222 patients who underwent GKRS for perioptic tumors and who had not undergone previous irradiation. One patient who received a maximum radiation dose of 12.8 Gy to the AOP developed unilateral blindness 18 months after GKRS, and the overall risk of RION in patients receiving a dose greater than 8 Gy to the AOP was 1%. In 2014, Pollock et al. [25] reported on their series of patients with parasellar tumors treated with single-session GKRS and without prior irradiation. Overall, no patient developed RION after a median follow-up of 32 months. Taken as a whole, all these studies suggest that a dose below 12 Gy can safely be delivered in a single fraction to the AOP with little risk of causing RION [21].
Axial (a), coronal (b), and sagittal (c) post-contrast T1-weighted magnetic resonance images showing a perioptic meningioma treated with Gamma Knife radiosurgery. At the 2-year imaging follow-up assessment, the tumor’s volume significantly decreased, as showed in the postoperative axial (d), coronal (e), and sagittal (f) scans
The primary factors associated with the development of RION after single-fraction SRS include previous radiation treatment to the perioptic area and maximum radiation dose to the optic apparatus. A recent literature review suggested a crude approximately tenfold increased risk of RION in patients previously treated with radiation therapy to the perioptic area [38]. The other factors that are assumed to contribute to radiation-related vision loss include comorbid conditions (i.e., vasculopathies, hypertension, diabetes), tumor volume, extent of optic apparatus involvement and high-irradiated volume of the AOP, prior surgery, and optic pathway compression [25, 39]. Whereas abundant data about the radiation tolerance of the optic apparatus using single-session SRS have been published, minimal data exists relatively to patients receiving hypofractionated schedules [21], although it is recognized that hypofractionation may reduce the risk of normal tissue toxicity [18]. Therefore, appropriate dose constraints to the AOP for hypofractionated radiosurgery remain poorly defined [18]. Timmermann et al. proposed the unvalidated maximum AOP dose constraints of 19.5 Gy in three fractions and 25 Gy in five fractions [18, 40]. Subsequently, the detailed analysis of Hiniker and coworkers regarding the tolerance of the visual pathway to radiosurgery estimated that delivering to the AOP a cumulative maximum radiation dose up to 24 Gy in three fractions and 30 Gy in five fractions is associated with a limited risk of RION (1.9% in both cases). Therefore, the previously unvalidated estimates of Timmerman et al. may underestimate the tolerance of the AOP, particularly in patients without prior radiation [18].
8.4 Alternatives to Hypofractionated Radiosurgery for Perioptic Meningiomas
Given the results of the abovementioned recent studies, single-session SRS can be considered as a valid treatment option for perioptic meningiomas, alternative to hypofractionation radiosurgery (see Fig. 8.2). However, prospective studies investigating the radiation tolerance of the AOP with single-session SRS and comparing the outcomes of that treatment delivery modality with hypofractionated SRS are lacking in the literature. Ultimately, a definitive dose-volume relationship cannot be established as of yet. The devices that have been used to deliver single-session SRS for the management of perioptic meningiomas have included the Gamma Knife [18, 20] and linear accelerators (LINACs) [41, 42], which both achieved high rates of tumor growth control with little risk of RION. That is, Spiegelmann and colleagues reported on a series of 117 patients treated with frame-based LINAC radiosurgery for meningiomas involving the cavernous sinus. 10 Gy was their maximal exposure limit dose to the AOP. With that limit respected, after a mean follow-up period of 67 months, visual function deteriorated in one case, although the authors did not specify if such visual decline was due to tumor progression or RION. Also, when the authors considered the whole cohort of patients with lesions in the perisellar area that had been treated with single-session LINAC radiosurgery at their institution, they found out that the incidence of optic neuropathy was below 1% (2 cases in 234 patients at risk). These authors conclude therefore that LINAC SRS in a single fraction can be delivered safely and effectively to control the growth of benign tumors adjacent to the AOP. Such low risk of RION may be related to the frame-based head fixation, which eliminates head positioning inaccuracies inherent to frameless fixation devices (e.g., molded face masks) [31]. FSRT is also an established treatment option for perioptic meningiomas. This irradiation technique combines the accuracy of stereotactic positioning and targeting with the radiobiological advantage of fractionation and leads to a reduction in the volume of normal brain irradiated at high doses in comparison to conventional external beam radiotherapy [43]. The frequency and severity of radiation-induced complications using these techniques [44], including induced carcinogenesis, neurocognitive decline, delayed pituitary failure, and cranial neuropathies, are extremely low, due to the improvement in radiotherapy techniques and the advent of modern devices with mini–multileaf collimators [45, 46]. Several studies investigating FSRT for skull base meningiomas have reported good outcomes both in terms of tumor growth control and visual function preservation, with a low 0–6% incidence of visual loss for meningiomas around the anterior visual pathways [46,47,48,49,50,51,52,53,54]. However, despite these positive results, the outcomes of one of the largest studies investigating FSRT for perioptic meningiomas that was conducted by Astradsson et al. compared unfavorably with earlier series, as 10% of patients with perioptic meningiomas developed RION [55]. In line with these data, Stiebel-Kalish et al. found an overall 12% incidence of worsening visual function in their reported series of patients [56]. Although studies are controversial about visual function preservation, FSRT results as an especially valuable technique for large meningiomas in close proximity to the visual pathways, or those severely distorting or encasing the AOP for which single-session or hypofractionated SRS may not be suitable due to excessive radiation-induced toxicity [55]. As a matter of fact, in their landmark study comparing the outcomes of single-session SRS and FSRT in the treatment of cavernous sinus meningiomas, Metellus and colleagues pointed out that FSRT and SRS are aimed at two different types of tumors. Single-session SRS is reserved for small tumors, or residual and recurrent meningiomas after microsurgical resection, which do not severely compress or encase the AOP. Contrastingly, FSRT is deemed to be reserved for inoperable patients with voluminous, extensive tumors showing close relationship with the optic apparatus and skull base dural spreading [44]. A main difference between the two techniques is that the larger dose per session that characterizes radiosurgery results in a higher biological equivalent dose and subsequently correlates with greater tumor shrinkage on follow-up imaging, although tumor control rates are overlapping [1, 44, 57]. Ultimately, both SRS and FSRT are effective treatment options for benign skull base meningiomas and the choice of stereotactic technique should be based on the characteristics of the tumors [43]. In most centers single- or hypofractionated SRS is usually reserved for tumors less than 3 cm of maximum diameter not encasing or compressing the AOP, whereas FSRT is employed for larger meningiomas.
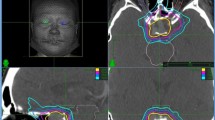
Axial (a), coronal (b), and sagittal (c) post-contrast magnetic resonance images showing the radiosurgical plan for the single-session radiosurgical treatment of a meningioma adjacent to the anterior optic pathways. The treatment is planned so that the 12 Gy isodose line is contacting with the optic apparatus. At the same time, the meningioma is completely covered by the 14 Gy isodose line
8.5 Conclusions
According to the present literature, hypofractionated SRS seems to be an effective technique for the control of perioptic meningiomas’ growth with little risk of causing RION. Notably, different radiosurgical platforms employing a frameless or frame-based head fixation system can be used, with little difference in terms of outcomes and safety. Alternative radiotherapy modalities are available and effective. These include single-session SRS and FSRT. Single-session SRS, owing to recent studies, which have defined the radiation tolerance of the AOP during single-session SRS, can be safely used for perioptic tumors. FSRT is mostly reserved to the treatment of large tumors or those which cannot be treated with single-session or hypofractionated SRS, due to an increased risk of normal tissue toxicity. Ultimately, the decision whether to use one technique over the other should be made in a case-by-case basis and should take into account various factors such as the volume of the target tumor, the treating center’s experience with each technique, and patient’s preference to undergo a single- or multiple-session treatment schedule. Available literature regarding the long-term efficacy and safety of hypofractionated radiosurgery for perioptic meningiomas is scarce. Further studies including large group of patients who have been followed up for long periods are needed to detect the actual recurrence rate with various fractionation protocols (Fig. 8.3).
Abbreviations
- AANS:
-
American Association of Neurological Surgeons
- AOP:
-
Anterior optic pathways
- CNS:
-
Congress of Neurological Surgeons
- FSRT:
-
Fractionated stereotactic radiotherapy
- GKRS:
-
Gamma Knife radiosurgery
- LINAC:
-
Linear accelerator
- RION:
-
Radiation-induced optic neuropathy
- SRS:
-
Stereotactic radiosurgery
References
Adler JR Jr, Gibbs IC, Puataweepong P, Chang SD. Visual field preservation after multisession cyberknife radiosurgery for perioptic lesions. Neurosurgery. 2006;59(2):244–54. discussion-54
Devriendt D, De Smedt F, Glineur R, Massager N. Five-fraction Gamma Knife radiosurgery using the extend relocatable system for benign neoplasms close to optic pathways. Pract Radiat Oncol. 2015;5(3):e119–25.
Jee TK, Seol HJ, Im YS, Kong DS, Nam DH, Park K, et al. Fractionated gamma knife radiosurgery for benign perioptic tumors: outcomes of 38 patients in a single institute. Brain Tumor Res Treat. 2014;2(2):56–61.
Kim JW, Im YS, Nam DH, Park K, Kim JH, Lee JI. Preliminary report of multisession gamma knife radiosurgery for benign perioptic lesions: visual outcome in 22 patients. J Korean Neurosurg Soc. 2008;44(2):67–71.
Marchetti M, Bianchi S, Pinzi V, Tramacere I, Fumagalli ML, Milanesi IM, et al. Multisession radiosurgery for sellar and parasellar benign meningiomas: long-term tumor growth control and visual outcome. Neurosurgery. 2016;78(5):638–46.
Marchetti M, Conti A, Beltramo G, Pinzi V, Pontoriero A, Tramacere I, et al. Multisession radiosurgery for perioptic meningiomas: medium-to-long term results from a CyberKnife cooperative study. J Neuro-Oncol. 2019;143(3):597–604.
McTyre E, Helis CA, Farris M, Wilkins L, Sloan D, Hinson WH, et al. Emerging indications for fractionated gamma knife radiosurgery. Neurosurgery. 2017;80(2):210–6.
Mehta VK, Lee QT, Chang SD, Cherney S, Adler JR Jr. Image guided stereotactic radiosurgery for lesions in proximity to the anterior visual pathways: a preliminary report. Technol Cancer Res Treat. 2002;1(3):173–80.
Nguyen JH, Chen CJ, Lee CC, Yen CP, Xu Z, Schlesinger D, et al. Multisession gamma knife radiosurgery: a preliminary experience with a noninvasive, relocatable frame. World Neurosurg. 2014;82(6):1256–63.
Pham CJ, Chang SD, Gibbs IC, Jones P, Heilbrun MP, Adler JR Jr. Preliminary visual field preservation after staged CyberKnife radiosurgery for perioptic lesions. Neurosurgery. 2004;54(4):799–810. discussion -2
Puataweepong P, Dhanachai M, Hansasuta A, Dangprasert S, Sitathanee C, Ruangkanchanasetr R, et al. Clinical outcomes of perioptic tumors treated with hypofractionated stereotactic radiotherapy using CyberKnife(R) stereotactic radiosurgery. J Neuro-Oncol. 2018;139(3):679–88.
DeMonte F, Smith HK. al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81(2):245–51.
O'Sullivan MG, van Loveren HR, Tew JM Jr. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40(2):238–44. discussion 45-7
Shrieve DC, Hazard L, Boucher K, Jensen RL. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerance. J Neurosurg. 2004;101(Suppl. 3):390–5.
Goldsmith BJ, Rosenthal SA, Wara WM, Larson DA. Optic neuropathy after irradiation of meningioma. Radiology. 1992;185(1):71–6.
Sayer FT, Sherman JH, Yen CP, Schlesinger DJ, Kersh R, Sheehan JP. Initial experience with the eXtend System: a relocatable frame system for multiple-session gamma knife radiosurgery. World Neurosurg. 2011;75(5–6):665–72.
Barber SM, Teh BS, Baskin DS. Fractionated stereotactic radiotherapy for pituitary adenomas: single-center experience in 75 consecutive patients. Neurosurgery. 2016;79(3):406–17.
Hiniker SM, Modlin LA, Choi CY, Atalar B, Seiger K, Binkley MS, et al. Dose-response modeling of the visual pathway tolerance to single-fraction and hypofractionated stereotactic radiosurgery. Semin Radiat Oncol. 2016;26(2):97–104.
Sheehan JP, Starke RM, Kano H, Kaufmann AM, Mathieu D, Zeiler FA, et al. Gamma Knife radiosurgery for sellar and parasellar meningiomas: a multicenter study. J Neurosurg. 2014;120(6):1268–77.
Leavitt JA, Stafford SL, Link MJ, Pollock BE. Long-term evaluation of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(3):524–7.
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28–35.
Barnett GH, Linskey ME, Adler JR, Cozzens JW, Friedman WA, Heilbrun MP, et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106(1):1–5.
Cohen-Inbar O, Lee CC, Sheehan JP. The contemporary role of stereotactic radiosurgery in the treatment of meningiomas. Neurosurg Clin N Am. 2016;27(2):215–28.
Conti A, Pontoriero A, Midili F, Iati G, Siragusa C, Tomasello C, et al. CyberKnife multisession stereotactic radiosurgery and hypofractionated stereotactic radiotherapy for perioptic meningiomas: intermediate-term results and radiobiological considerations. Springerplus. 2015;4:37.
Pollock BE, Link MJ, Leavitt JA, Stafford SL. Dose-volume analysis of radiation-induced optic neuropathy after single-fraction stereotactic radiosurgery. Neurosurgery. 2014;75(4):456–60. discussion 60
Yamamoto M, Aiyama H, Koiso T, Watanabe S, Kawabe T, Sato Y, et al. Postsurgical salvage radiosurgery for nonfunctioning pituitary adenomas touching/compressing the optic chiasm: median 13-year postirradiation imaging follow-up results. Neurosurgery. 2019;85(4):476–85.
Kano H, Park KJ, Kondziolka D, Iyer A, Liu X, Tonetti D, et al. Does prior microsurgery improve or worsen the outcomes of stereotactic radiosurgery for cavernous sinus meningiomas? Neurosurgery. 2013;73(3):401–10.
Kaprealian T, Raleigh DR, Sneed PK, Nabavizadeh N, Nakamura JL, McDermott MW. Parameters influencing local control of meningiomas treated with radiosurgery. J Neuro-Oncol. 2016;128(2):357–64.
Han J, Girvigian MR, Chen JC, Miller MJ, Lodin K, Rahimian J, et al. A comparative study of stereotactic radiosurgery, hypofractionated, and fractionated stereotactic radiotherapy in the treatment of skull base meningioma. Am J Clin Oncol. 2014;37(3):255–60.
Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43–50.
Tishler RB, Loeffler JS, Lunsford LD, Duma C, Alexander E 3rd, Kooy HM, et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27(2):215–21.
Duma CM, Lunsford LD, Kondziolka D, Bissonette DJ, Somaza S, Flickinger JC. Radiosurgery for vascular malformations of the brain stem. Acta Neurochir Suppl (Wien). 1993;58:92–7.
Stafford SL, Pollock BE, Leavitt JA, Foote RL, Brown PD, Link MJ, et al. A study on the radiation tolerance of the optic nerves and chiasm after stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2003;55(5):1177–81.
Ove R, Kelman S, Amin PP, Chin LS. Preservation of visual fields after peri-sellar gamma-knife radiosurgery. Int J Cancer. 2000;90(6):343–50.
Morita A, Coffey RJ, Foote RL, Schiff D, Gorman D. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg. 1999;90(1):42–9.
Cifarelli CP, Schlesinger DJ, Sheehan JP. Cranial nerve dysfunction following Gamma Knife surgery for pituitary adenomas: long-term incidence and risk factors. J Neurosurg. 2012;116(6):1304–10.
Hasegawa T, Kobayashi T, Kida Y. Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: evaluation in 100 patients with craniopharyngioma. Neurosurgery. 2010;66(4):688–94. discussion 94-5
Milano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tome WA, et al. Single- and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. 2021;110(1):87–99.
Deng X, Yang Z, Liu R, Yi M, Lei D, Wang Z, et al. The maximum tolerated dose of gamma radiation to the optic nerve during gamma knife radiosurgery in an animal study. Stereotact Funct Neurosurg. 2013;91(2):79–91.
Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215–22.
Spiegelmann R, Cohen ZR, Nissim O, Alezra D, Pfeffer R. Cavernous sinus meningiomas: a large LINAC radiosurgery series. J Neuro-Oncol. 2010;98(2):195–202.
Spiegelmann R, Nissim O, Menhel J, Alezra D, Pfeffer MR. Linear accelerator radiosurgery for meningiomas in and around the cavernous sinus. Neurosurgery. 2002;51(6):1373–9. discussion 9-80
Minniti G, Amichetti M, Enrici RM. Radiotherapy and radiosurgery for benign skull base meningiomas. Radiat Oncol. 2009;4:42.
Metellus P, Regis J, Muracciole X, Fuentes S, Dufour H, Nanni I, et al. Evaluation of fractionated radiotherapy and gamma knife radiosurgery in cavernous sinus meningiomas: treatment strategy. Neurosurgery. 2005;57(5):873–86. discussion -86
al-Mefty O, Kersh JE, Routh A, Smith RR. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73(4):502–12.
Brell M, Villa S, Teixidor P, Lucas A, Ferran E, Marin S, et al. Fractionated stereotactic radiotherapy in the treatment of exclusive cavernous sinus meningioma: functional outcome, local control, and tolerance. Surg Neurol. 2006;65(1):28–33. discussion -4
Jalali R, Loughrey C, Baumert B, Perks J, Warrington AP, Traish D, et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominantly in the skull base location. Clin Oncol (R Coll Radiol). 2002;14(2):103–9.
Kocher M, Treuer H, Hoevels M, Semrau R, Sturm V, Mueller RP. Endocrine and visual function after fractionated stereotactic radiotherapy of perioptic tumors. Strahlenther Onkol. 2013;189(2):137–41.
Litre CF, Colin P, Noudel R, Peruzzi P, Bazin A, Sherpereel B, et al. Fractionated stereotactic radiotherapy treatment of cavernous sinus meningiomas: a study of 100 cases. Int J Radiat Oncol Biol Phys. 2009;74(4):1012–7.
Metellus P, Batra S, Karkar S, Kapoor S, Weiss S, Kleinberg L, et al. Fractionated conformal radiotherapy in the management of cavernous sinus meningiomas: long-term functional outcome and tumor control at a single institution. Int J Radiat Oncol Biol Phys. 2010;78(3):836–43.
Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809–16.
Milker-Zabel S, Zabel-du Bois A, Huber P, Schlegel W, Debus J. Fractionated stereotactic radiation therapy in the management of benign cavernous sinus meningiomas: long-term experience and review of the literature. Strahlenther Onkol. 2006;182(11):635–40.
Milker-Zabel S, Zabel-du Bois A, Huber P, Schlegel W, Debus J. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68(3):858–63.
Pirzkall A, Debus J, Haering P, Rhein B, Grosser KH, Hoss A, et al. Intensity modulated radiotherapy (IMRT) for recurrent, residual, or untreated skull-base meningiomas: preliminary clinical experience. Int J Radiat Oncol Biol Phys. 2003;55(2):362–72.
Astradsson A, Wiencke AK, Munck AF, Rosenschold P, Engelholm SA, Ohlhues L, Roed H, et al. Visual outcome after fractionated stereotactic radiation therapy of benign anterior skull base tumors. J Neuro-Oncol. 2014;118(1):101–8.
Stiebel-Kalish H, Reich E, Gal L, Rappaport ZH, Nissim O, Pfeffer R, et al. Visual outcome in meningiomas around anterior visual pathways treated with linear accelerator fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(2):779–88.
Leroy HA, Tuleasca C, Reyns N, Levivier M. Radiosurgery and fractionated radiotherapy for cavernous sinus meningioma: a systematic review and meta-analysis. Acta Neurochir. 2018;160(12):2367–78.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Franzini, A., Attuati, L., Zaed, I., Picozzi, P. (2021). Hypofractionated Radiosurgery for Perioptic Meningiomas: Current Practice, Principles, and Treatment Quandary. In: Longhi, M., Motti, E.D.F., Nicolato, A., Picozzi, P. (eds) Stereotactic Radiosurgery for the Treatment of Central Nervous System Meningiomas. Springer, Cham. https://doi.org/10.1007/978-3-030-79419-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-79419-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79418-7
Online ISBN: 978-3-030-79419-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)