Abstract
Over the last few years, agents targeting immune checkpoints have shown potential to improve therapeutic outcomes in patients with lung cancer in multiple clinical settings. Inhibitors of PD-1/PD-L1 have been approved for the treatment of different types of lung cancer by the FDA either alone or in combination with chemotherapy or other immune checkpoint inhibitors, such as anti-CTLA-4 agents. The introduction of these agents in clinical practice has revolutionized the therapeutic approach to lung cancer, keeping the promises of long-term benefit in selected patient populations. The therapeutic indications of immunotherapy in lung cancer are rapidly growing, and multiple combinations entered clinical practice or are under active development. Furthermore, the quest for a reliable predictive biomarker is still ongoing to overcome the limits of currently approved tests for patients’ selection. In this review, we summarized the current status and progress of anti-PD-1/PD-L1 agents in lung cancer treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Clearly, we are seeing that lung cancer improves its survival year by year; NSCLC 2-year relative survival increased from 34% for persons diagnosed during 2009 through 2010 to 42% during 2015 through 2016, including absolute increases of 5% to 6% for every stage of diagnosis; survival for small cell lung cancer remained at 14% to 15%. This is due to the improvement in treatments, where immunotherapy plays a fundamental role [1].
Immunotherapy treatment is now a reality in clinical practice, and knowledge mechanism of action is key in understanding the benefit of the improved survival of the lung [2]. The development of immune checkpoint inhibitor (ICI) agents targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death protein ligand 1 (PD-L1) has garnered tremendous interests in the field of immuno-oncology because of the recent successful applications in multiple advanced cancers. Although CTLA-4 is the first immune checkpoint molecule identified, the PD-1/PD-L1 axis has been widely investigated due to the role in the exhaustion of CD8+ T cells. Physiologically, PD-1/PD-L1 has the task of limiting the activity of T cells in peripheral tissues at the time of an inflammatory response to infection, thereby limiting autoimmunity. Similar to CTLA-4, PD-1 is expressed on activated T cells and inhibits T-cell responses by interfering with T-cell receptor signaling. PD-1 has two ligands, PD-L1 (B7-H1) that is expressed on antigen-presenting cells (APCs), macrophages, fibroblasts, and T cells and PD-L2 (B7-DC) that is predominantly expressed on antigen-presenting cells (APCs). PD-L1 is also overexpressed in several solid tumors, while PD-L2 is expressed relatively rarely. The role of CTLA-4 and PD-1/PD-L1 in immune suppression and their expression in solid tumors provided the rationale for their therapeutic exploitation. Moreover, CTLA-4 and PD-1 exert their effects through separate pathways, and therefore simultaneous targeting of both pathways has also been evaluated to restore antitumor immunity [3, 4].
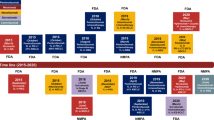
Since the first demonstration of activity of PD(L)-1 agents in lung cancer in early clinical trials in 2012, immune checkpoint blockade (ICB) has emerged as a novel effective therapeutic strategy in different clinical settings and determined a dramatic shift in the therapeutic landscape of both NSCLC and SCLC (Fig. 1) [5]. Several biological prognostic and predictive factors in blood and tissue samples have been identified, but unfortunately no single biomarker can perfectly discriminate between responders and nonresponders, and PD-L1 immunohistochemical expression still remains the only applicable marker in clinical practice to date [6].
Role of immune checkpoint inhibitors in lung cancer and immune-modulatory activities of conventional treatment strategies [3, 7,8,9].
Abbreviations: MHC major histocompatibility complex, TAA tumor-associated antigens, IFN interferon, TH1 T-helper 1, CTL cytotoxic T-cell lymphocyte, NK natural killer, Tregs regulatory T cells, MDSCs myeloid-derived suppressor cells, TCR T-cell receptor, DC dendritic cell (Credit: Created with BioRender.com)
To date, the primary biomarker used for lung cancer has been PD-L1 [10]. Different immunohistochemical assays have been developed, using different antibodies and scoring systems. However, multiple harmonization studies have consistently reported high concordance between most of these assays (22C3, SP263, 28-8, 73-10, and E1L3N) in terms of PD-L1 expression on tumor cells (TC) [11,12,13,14]. PD-L1 tumor proportion score (TPS) evaluated using the Dako 22C3 assay was developed and validated as the companion diagnostic for single-agent pembrolizumab in pretreated NSCLC in the randomized phase II/III KEYNOTE-010 trial [15]. Based on the positive results in pretreated patients, the use of ICIs was then moved to treatment-naïve patients, and PD-L1 expression represented the most extensively used biomarker for treatment selection of single-agent PD-1/PD-L1 inhibitors versus platinum-based chemotherapy [16]. PD-L1 TPS ≥50% identifies a subgroup of patients that accounts for approximately 30% of the patients with NSCLC that derives greater benefit from single-agent ICIs than platinum-based chemotherapy, and to date, three different agents (pembrolizumab, atezolizumab, and cemiplimab) [17,18,19] have been approved in this setting. Single-agent ICI in PD-L1 low expressors (TPS 1–49%) for first-line therapy is controversial, as the benefit seen in the KEYNOTE-042 trial with pembrolizumab [20] is likely driven by PD-L1 strong expressors [21].
Absence of PD-L1 expression does not conclusively identify patients who will not benefit from immunotherapy, leading to investigation of many other biomarkers [22,23,24].
In this review, we summarized the current status and progress of anti-PD-1/PD-L1 agents in lung cancer treatment.
2 Early-Stage NSCLC and Locally Advanced NSCLC
Approximately 40% of NSCLC patients are diagnosed with locoregional disease that is potentially resectable [25]. Adjuvant platinum-based chemotherapy has been shown to improve survival in patients with stages II–III disease and can be considered high-risk stage IB disease (4 cm, poorly differentiated carcinoma, post-wedge resection, lymphovascular invasion, visceral pleural involvement, unknown lymph node status) [26]. Meta-analyses of randomized phase III trials conducted in the 1990s and early 2000s reported an absolute survival benefit at 5 years of 5% from adjuvant/neoadjuvant approaches in stage IB–IIIA NSCLC compared with surgery alone [27, 28].
Since these trials, the therapeutic landscape of early-stage NSCLC has little improved over the last two decades, and only recently, a randomized phase III trial has reported a survival advantage in a selected patient population (activating EGFR mutations) using osimertinib after platinum-based chemotherapy [29]. ICIs might potentially revolutionize the adjuvant setting, given the well-known ability of immunotherapy of inducing long-term responses, and multiple clinical trials are ongoing (Table 1).
Recently, the preliminary results of a single-arm phase II study (NCT03053856) evaluated the postoperative role of pembrolizumab in stage IIIA-N2 NSCLC who has undergone neoadjuvant concurrent chemoradiotherapy (weekly carboplatin/paclitaxel and radiation therapy to 44 Gy in 22 fractions) with curative resection for up to 2 years or until disease recurrence. The primary endpoint is disease-free survival (DFS), with a statistical goal of more than 20 months. Thus far, of 37 patients treated in this trial, 14 patients have discontinued treatment owing to disease progression (n = 9), adverse events (n = 4), or consent withdrawal (n = 1). Adverse events have included grade 4 pneumonitis (n = 1) and grade 3 autoimmune hepatitis (n = 1), which have led to discontinuation, as well as grade 1 or 2 hypothyroidism (n = 6), pneumonitis (n = 5), and skin rash (n = 3) [30].
On March 2021, Roche announced that phase III IMpower010 trial met the primary endpoint, demonstrating a statistically significant improvement in terms of DFS with the use of the PD-L1 inhibitor atezolizumab as compared with best supportive care (BSC) in patients with PD-L1-positive, stages II–IIIA NSCLC who have undergone surgical resection and received up to four cycles of adjuvant cisplatin-based chemotherapy. The presentation of the full results of the study is eagerly awaited.
In addition to the adjuvant setting, immunotherapy might have a role also in the neoadjuvant setting either as monotherapy or in combination with platinum-based chemotherapy. Preliminary data of these studies are encouraging, especially when considering chemo-immunotherapy combinations (Table 2).
Collectively, chemo-immunotherapy seems associated with higher ORR and increased probability of major pathological response (MPR)/pathologic complete response (pCR). This therapeutic strategy seems more promising than single-agent ICB and moved quickly to phase III. Several randomized trials evaluating the addition of a PD-1/PD-L1 inhibitor to a platinum-based doublet as neoadjuvant therapy in resectable NSCLC (stages I–IIIA) are underway, including KEYNOTE-671 (pembrolizumab), AEGEAN (durvalumab), NCT04316364 (adebrelimab/SHR-1316), NCT04379635 (tislelizumab), JS001 028 III (toripalimab), CheckMate-77 T (nivolumab), and CheckMate-816 (nivolumab/chemotherapy vs. nivolumab-ipilimumab).
In October 2020, Bristol Myers Squibb announced that the CheckMate-816 met its primary endpoint of improved pCR in patients who received nivolumab plus chemotherapy before surgery. The presentation of the full results of the study is expected in the next few months.
In patients with inoperable stage III disease, the use of chemoradiotherapy has been shown to increase survival as compared with radiotherapy alone [44], and concurrent chemoradiation (cCRT) increases 5-year overall survival by 4.5% as compared with a sequential approach [45].
Several studies have shown promise for immunotherapy following cCRT in patients with unresectable stage III LA-NSCLC. The PACIFIC trial (A Global Study to Assess the Effects of MEDI4736 Following Concurrent Chemoradiation in Patients with Stage III Unresectable Non-Small Cell Lung Cancer) reported encouraging phase III data on the use of the anti-PD-L1 antibody durvalumab in this context. PACIFIC was the first study to demonstrate improved outcomes in patients with LA-NSCLC who received an immune checkpoint inhibitor. In this phase III trial, patients with stage III unresectable NSCLC were randomly assigned in a 2:1 ratio to receive either durvalumab (a PD-L1 inhibitor) or placebo as consolidation therapy every 2 weeks for as long as 1 year [46]. The study population consisted of 713 patients who had received cisplatin-based chemotherapy with concurrent radiation to 66 Gy and had no disease progression following treatment. Progression-free survival (PFS), the primary endpoint, was significantly longer in the durvalumab group than in the placebo group (median PFS, 16.8 vs. 5.6 months; P < 0.001). In addition, the co-primary OS remained consistent with that previously reported (stratified HR = 0.69 [95% CI: 0.55–0.86]); the median OS was not reached with durvalumab but was 29.1 months with placebo. The 12-, 24-, and 36-month OS rates with durvalumab and placebo were 83.1% versus 74.6%, 66.3% versus 55.3%, and 57.0% versus 43.5%, respectively [47]. Improved OS with durvalumab was broadly observed irrespective of PD-L1 expression, which is consistent with findings from prespecified and post hoc analyses carried out at the time of the primary OS analysis [46]. Remember that PD-L1 data were based on pre-cCRT samples, which may not reflect changes in expression potentially incurred by cCRT, and should also be taken into consideration when drawing definitive conclusions. PACIFIC was not designed to evaluate the efficacy of durvalumab based on PD-L1 status. Overall, the findings of this analysis underscore the long-term survival benefit with durvalumab after cCRT and further establish the PACIFIC regimen as the standard of care in patients with unresectable stage III NSCLC who do not progress while undergoing cCRT. An exploratory analysis showed that patients who started treatment with durvalumab <14 days from completion of radiation therapy had improved efficacy outcomes compared with those who started treatment ≥14 days from completion of radiation therapy [48].
Besides consolidation after cCRT, other therapeutic strategies under active investigation include the concomitant use of ICIs during chemoradiotherapy (PACIFIC-2, CheckMate73L, EA5181, DETERRED-PART II, NICOLAS, KEYNOTE-799) and the use after sequential chemoradiotherapy (PACIFIC-6) or radiotherapy alone (DUART). The results of these trials will provide additional insights on the role of PD-1/PD-L1 inhibitors in inoperable stage III NSCLC.
3 Pretreated NSCLC
After few years since early clinical sights of activity of PD-1/PD-L1 inhibitors in lung cancer [49, 50], three PD-1/PD-L1 therapies have been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) in the second-line setting (nivolumab, pembrolizumab, and atezolizumab), on the basis of phase III studies demonstrating improved overall survival (OS) in comparison with the former standard-of-care therapy docetaxel.
In two phase III trials (CheckMate-017 and CheckMate-057), nivolumab showed an improvement in OS and favorable safety versus docetaxel in patients with previously treated, advanced squamous, and non-squamous NSCLC [51, 52]. After follow-up of 64.2 and 64.5 months for CheckMate-017 and CheckMate-057 [53], respectively, 50 nivolumab-treated patients and 9 docetaxel-treated patients were alive. Five-year pooled OS rates were 13.4% versus 2.6%, respectively; 5-year PFS rates were 8.0% versus 0%, respectively. Nivolumab-treated patients without disease progression at 2 and 3 years had an 82.0% and 93.0% chance of survival, respectively, and a 59.6% and 78.3% chance of remaining progression-free at 5 years, respectively. Treatment-related adverse events (TRAEs) were reported in 8 of 31 (25.8%) nivolumab-treated patients between 3 and 5 years of follow-up, 7 of whom experienced new events; one (3.2%) TRAE was grade 3, and there were no grade 4 TRAEs. Clearly, nivolumab compared to docetaxel exhibited a fivefold increase in OS rate, with no new safety signals. Interestingly, PD-L1 expression as a predictive biomarker produced contrasting results between the two trials, despite similar study designs and the same assessment methods. The different mutational burden of squamous and non-squamous histology, as well as the frequency of oncogene-addicted tumors, might have contributed to this discrepancy. Moreover, a landmark analysis of the CheckMate-057 demonstrated that, excluding patients who had died in the first 3 months, nivolumab was superior to docetaxel in both PD-L1-positive and PD-L1-negative patients [54]. Atezolizumab was compared with docetaxel in pretreated NSCLC in phase II (POPLAR) and phase III randomized studies (OAK), showing improved OS across all PD-L1 expression levels with incremental efficacy results at the increase of PD-L1 IHC expression in tumor cells (TC) or tumor-infiltrating immune cells (IC) using the SP142 assay [55, 56]. A longer OS was observed in patients receiving atezolizumab vs. docetaxel in POPLAR (median OS, 12.6 months vs. 9.7 months; HR, 0.76 [95% CI: 0.58–1.00]) and OAK (median OS, 13.3 vs. 9.8 months; HR, 0.78 [95% CI: 0.68–0.89]. Four-year OS rates in POPLAR were 14.8% (8.7–20.8) and 8.1% (3.2–13.0) for atezolizumab and docetaxel, respectively, and 15.5% (12.4–18.7) and 8.7% (6.2–11.3) in OAK. Most 4-year survivors in the docetaxel arms received subsequent immunotherapy (POPLAR, 50%; OAK, 65%). Of 4-year survivors, most had ECOG PS 0 and non-squamous histology; approximately half were responders (POPLAR, atezolizumab, 7/15; docetaxel, 3/4; OAK, atezolizumab, 24/43; docetaxel, 11/26). Treatment-related grade 3/4 adverse events occurred in 27% and 16% of atezolizumab 4-year survivors in POPLAR and OAK, respectively [57].
The development of pembrolizumab in NSCLC started with the phase I multi-cohort study KEYNOTE-001, which evaluated the safety and activity of this compound and also validated the companion diagnostic 22C3 IHC assay for PD-L1 expression. Patients with squamous and non-squamous tumors were enrolled; however, PD-L1 expression had to be 1% or greater. All patients had progressed on first-line platinum-doublet therapy, and those with driver mutations had also progressed on appropriate TKI therapy [58]. The updated analysis with a 42.6 months follow-up [59] showed that the risk of death was reduced with pembrolizumab versus docetaxel in both the PD-L1 TPS ≥50% group (HR 0.53; P < 0.00001) and the TPS ≥1% group (HR 0.69; P < 0.00001). Median OS was 16.9 months (95% CI, 12.3 to 21.4 months) versus 8.2 months (95%CI, 6.4 to 9.8 months) in the TPS ≥50% group and 11.8 months (95% CI, 10.4 to 13.1 months) versus 8.4 months (95% CI, 7.6 to 9.5 months) in the TPS ≥1% group. Kaplan-Meier estimates of OS at 36 months were higher with pembrolizumab versus docetaxel in both TPS groups, with OS rates of 34.5% versus 12.7% in the TPS ≥50% group and 22.9% versus 11.0% in the TPS ≥1% group. The risk of disease progression or death (per RECIST v1.1 by BICR rather than per investigator) was reduced with pembrolizumab versus docetaxel in the PD-L1 TPS ≥50% (HR 0.57; P = 0.00001) and TPS ≥1% groups (HR, 0.83; P = 0.005). Kaplan-Meier estimates of PFS at 36 months were higher with pembrolizumab versus docetaxel in both TPS groups, with PFS rates of 21.9% versus 1.2% in the TPS ≥50% group and 12.7% versus 1.0% in the TPS ≥1% group.
Not all trials using PD-1 and PD-L1 checkpoint inhibitors for the second-line treatment of advanced NSCLC have yielded positive results. Avelumab, an anti-PD-L1 monoclonal antibody, was compared with docetaxel in the JAVELIN Lung 200 trial [60]. As a result, the OS was not significantly different between the avelumab and docetaxel groups, even in the subgroup with positive tumor PD-L1 expression. High post-study use of ICIs and the non-blinded design of the trial might have affected the results.
Currently, ICIs are now well established as the standard of care for second-line treatment of advanced NSCLC, but there is no data to suggest that one agent is superior to another in that setting. No head-to-head comparison has been conducted. Indeed, the meta-analyses of published studies with ICIs in pretreated NSCLC did not demonstrate significant evidence of survival differences between these agents [61, 62]. Therefore, in clinical practice, factors that could influence ICI selection might include drug access, dosing schedule, costs, and PD-L1 expression.
4 First-Line Metastatic NSCLC
The introduction of the anti-PD-1 nivolumab for metastatic lung cancer in second-line setting was just the beginning in the development of checkpoint inhibitors in different clinical scenarios [16], including first-line treatment either as monotherapy in selected patient populations or in combination with chemotherapy +/− antiangiogenic drugs or in combination with CTLA-4 inhibitors with or without chemotherapy.
Within the revolutionary first-line setting for metastatic lung cancer patients, two pivotal randomized phase III clinical trials have compared pembrolizumab vs. platinum-doublet regardless histology and without driver mutations (EGFR/ALK wild type) in patients with PD-L1 TPS ≥50% (KEYNOTE-024) and in those with PD-L1 TPS ≥1% (KEYNOTE-042).
KEYNOTE-024 met its primary endpoint, reaching its goal of demonstrating the superiority of pembrolizumab vs. chemotherapy for patients with strong PD-L1 expression (TPS ≥50%) regardless of tumor histology [17]. By reaching a median OS of 30 months [63], pembrolizumab is positioned as a less toxic and more effective treatment than platinum-doublet-based chemotherapy in this selected patient population, demonstrating for the first time a survival advantage over platinum-based chemotherapy as first-line treatment in non-oncogene addicted NSCLCs. Based on these results, PD-L1 ≥ 50% in absence of concomitant driver mutations identified a novel subgroup of patients that accounts for approximately 30% of all NSCLCs that benefits from a chemotherapy-free regimen in first line.
Similar results were more recently reported with two ICIs, atezolizumab and cemiplimab, in patients with strong PD-L1 expression without EGFR mutations and/or ALK rearrangements and have been recently approved by the US FDA as first-line options.
Atezolizumab was compared with platinum-based chemotherapy in the randomized phase III trial IMpower110 in PD-L1 selected NSCLCs (PD-L1 expression on at least 1% of tumor cells or at least 1% of tumor-infiltrating immune cells as assessed by the SP142 immunohistochemical assay) [18]. The study demonstrated a statistically significant improvement in OS in the intention-to-treat (ITT) population (patients whose tumors were wild-type with respect to EGFR mutations or ALK translocations) within the subgroup of patients with strong PD-L1 expression (20.2 months vs. 13.1 months; hazard ratio for death, 0.59; P = 0.01). Furthermore, OS and PFS favored atezolizumab in the subgroups with a high blood-based tumor mutational burden (bTMB), assessed through the plasma 394-gene NGS panel FoundationOne CDx Liquid, suggesting a potential utility of this biomarker for patient selection [18].
Cemiplimab was compared with platinum-based chemotherapy in the randomized phase III trial EMPOWER-Lung 1 as first-line treatment in advanced NSCLC with PD-L1 tumor expression ≥50% and no EGFR mutations, ALK translocations, or ROS1 fusions. Patients were ineligible if they had never smoked (defined as ≤100 cigarettes in a lifetime). This is the largest study in this setting (563 patients in the PD-L1 ≥ 50% population) and showed that cemiplimab was superior to chemotherapy in improving PFS (8.2 months vs. 5.7 months, HR 0.54; p < 0.0001) and OS (not reached vs. 14.2 months, HR 0.57; p = 0.0002) in PD-L1-strong positive NSCLC patients [19].
Recently, a multicenter retrospective study analyzed the impact of different PD-L1 expression levels on pembrolizumab outcome in the subgroup of patients with NSCLC PD-L1 TPS ≥50% without EGFR/ALK aberrations. Compared with patients with PD-L1 expression of 50%–89% (N = 107), patients with an expression level of 90%–100% (N = 80) had a significantly higher ORR (60.0% versus 32.7%), a significantly longer PFS (14.5 versus 4.1 months), and a significantly longer OS (not reached versus 15.9 months). These results suggest that in patients with NSCLC and PD-L1 expression ≥50% treated with first-line pembrolizumab, clinical outcomes are significantly improved in NSCLC with a very high PD-L1 expression of ≥90% [64].
Other studies have sought to expand the potential number of patients that might benefit from upfront PD-1/PD-L1 blockage as monotherapy, evaluating these agents in patients with PD-L1 expression ≥1%. The CheckMate-026 failed to demonstrate a survival benefit with nivolumab versus platinum-based chemotherapy. Nivolumab was not associated with significantly longer PFS than chemotherapy among patients with previously untreated stage IV or recurrent NSCLC with a PD-L1 expression level of 5% or more, the primary endpoint of the trial. Furthermore, no differences were observed in OS between groups, and no advantage was seen in the PD-L1 ≥ 50% subgroup (HR for PFS 1.07 and 0.90 for OS) [64]. However, an exploratory analysis evaluating the tumor mutation burden (TMB) with whole exome sequencing (WES), performed in a subgroup of patients (58% of the randomized patients), showed that nivolumab was associated with higher ORR (47% vs. 28%) and longer PFS (9.7 vs. 5.8 months; HR 0.62) in patients with high TMB (≥243 mutations). No correlation between TMB and PD-L1 expression level was observed. Interestingly, the subgroup of patients with both high TMB and strong PD-L1 expression identified the subgroup of patients with higher response rate (75%) than those with only one of these factors (32% among patients with a high TMB only and 34% among those with a PD-L1 ≥ 50% only) or neither factor (16%) [65].
In contrast, the KEYNOTE-042 met its primary endpoints, demonstrating a statistically significant OS benefit in patients with a TPS ≥50% (HR 0.69, p = 0.0003), ≥20% (HR 0.77; p = 0.0020), and ≥ 1% (HR 0.81; p = 0.0018) leading to the FDA approval of pembrolizumab in treatment-naïve EGFR/ALK wild-type NSCLC patients with a TPS ≥ 1% [20]. However, this decision raised some concerns as pembrolizumab monotherapy may not represent the best treatment strategy for patients with tumor PD-L1 expression of 1–49%, as survival curves cross approximately 7 months after treatment initiation, with chemotherapy performing better than pembrolizumab during the first 6 months from randomization. These results suggest that a substantial number of patients progress rapidly and die within the first 6 months of treatment without obtaining any meaningful benefit from immunotherapy, and therefore other therapeutic strategies might be preferable in this subgroup of patients [21], especially in light of the positive results of chemo-immunotherapy trials in the first line.
Multiple randomized phase III trials have investigated the efficacy and safety (Table 3) of different chemo-immunotherapy trials.
Phase III KEYNOTE-189 trial evaluated the use of pembrolizumab in association with platinum-pemetrexed chemotherapy in patients of non-squamous EGFR/ALK wild-type NSCLC, regardless of PD-L1 expression [66]. The trial met the two primary endpoints, demonstrating a statistically significant improvement in terms of both OS and PFS with the combination, as assessed by blinded, independent central radiologic review. First-line pembrolizumab plus chemotherapy demonstrate substantially improved OS and PFS in metastatic non-squamous NSCLC, regardless of PD-L1 expression or liver/brain metastases, with acceptable safety profile [66, 67]. Pembrolizumab plus platinum-pemetrexed was associated with a median OS of 22.0 months vs. 10.6 months with chemotherapy alone (HR 0.60) with a 3-year OS almost doubled (31.3% vs. 17.4%). Median PFS was longer for the experimental arm (9.0 vs. 4.9 months; HR 0.50), with a 3-year PFS rate of 11.8% vs.1.3% [68]. The PFS/OS benefit was seen across all the PD-L1 subgroups, with the strong PD-L1-positive subgroup benefitting more from the addition of pembrolizumab. Patients who completed the planned 35 cycles of treatment (2 years) were associated with durable responses and were most still alive at the 4-year follow-up (79.6% OS rate after 2 years from treatment completion) [68].
In a similar study design, pembrolizumab in combination with carboplatin plus paclitaxel/nab-paclitaxel demonstrated a PFS/OS as compared with chemotherapy in squamous NSCLC (KEYNOTE-407). The study met its two primary endpoints, demonstrating a statistically significant advantage for chemo-immunotherapy in terms of both OS (15.9 vs. 11.3 months, HR 0.64; P < 0.001) and PFS (6.4 vs. 4.8 months, HR 0.56; P < 0.001) [69]. Similar to the KEYNOTE-189 trial, the addition of pembrolizumab to chemotherapy was associated with survival benefit across all the PD-L1 subgroups, including among PD-L1-negative (TPS <1%) tumors [70]. At a 3-year follow-up, pembrolizumab plus carboplatin and paclitaxel/nab-paclitaxel continued to provide OS and PFS benefit vs. placebo plus chemotherapy (median OS 17.2 vs. 11.6 months with a 3-year OS rate of 29.7% vs. 18.2%; median PFS 8.0 vs. 5.1 months with a 3-year PFS rate of 16.1% vs. 6.5%). Among patients who completed the planned 35 cycles of treatment, durable responses were seen with a 1-year OS rate from completion of pembrolizumab of 96% [71].
In the IMpower150 was tested the addition of atezolizumab to bevacizumab plus chemotherapy as first-line treatment for metastatic non-squamous NSCLC, regardless of PD-L1 expression. In contrast with other chemo-immunotherapy trials, patients with known EGFR or ALK aberrations were included in the study but were excluded from the ITT population. Patients were randomly assigned, in a 1:1:1 ratio, to receive atezolizumab plus carboplatin plus paclitaxel (ACP group), atezolizumab plus bevacizumab plus carboplatin plus paclitaxel (ABCP group), or bevacizumab plus carboplatin plus paclitaxel (BCP group) [72].
The two primary endpoints were PFS both among patients in the ITT WT population and among patients in the WT population who had high expression of an effector T-cell (Teff) gene signature in the tumor (Teff-high WT population) and overall survival in the WT population. ABCP was associated with longer PFS (8.3 versus 6.8 months, HR 0.62; P < 0.001) and longer OS (19.2 versus 14.7 months, HR 0.78; P = 0.02) as compared with BCP in the ITT population [72]. Interestingly, an exploratory analysis of the study showed that ABCP was associated with improved OS compared with BCP in patients with sensitizing EGFR mutations (HR 0.31) and in those with baseline liver metastases (HR 0.52). In contrast, no OS benefit was seen with ACP versus BCP in patients with sensitizing EGFR mutations (HR 0.90), in the ITT population (HR 0.85), or in patients with baseline liver metastases (HR 0.87) [73]. These data should be interpreted with cautions, given the low number of patients included in this analysis, but suggest a potential synergistic effect between bevacizumab and atezolizumab.
Another therapeutic strategy explored in treatment-naïve advanced NSCLC is the dual immune checkpoint blockage with PD-1 plus CTLA-4 inhibitors. Checkmate-227 (Part 1) trial was a randomized phase III study evaluating the role of nivolumab plus ipilimumab in either PD-L1-positive (≥1%) versus chemotherapy or nivolumab (Part 1a) or PD-L1-negative (<1%) NSCLC patients versus chemotherapy +/− nivolumab (Part 1b). In Part 1a, nivolumab-ipilimumab was significantly associated with a longer median duration of OS as compared with chemotherapy alone (17.1 vs. 14.9 months; P = 0.007). The OS benefit was also observed in the Part 1b of the study (PD-L1 < 1%) with a median duration of 17.2 months with nivolumab plus ipilimumab and 12.2 months with chemotherapy. This combination was associated with similar serious adverse event (G3–4 AEs) rates compared with chemotherapy (32.8% with nivolumab plus ipilimumab and 36.0% with chemotherapy) [77]. At a 3-year follow-up, nivolumab-ipilimumab continues to provide a survival benefit as compared with chemotherapy in both PD-L1 ≥ 1% and PD-L1 < 1% with a similar 3-year OS rate (33% and 34%, respectively) [78]. This chemotherapy-free regimen was recently FDA-approved in PD-L1 ≥ 1%.
In contrast with nivolumab-ipilimumab, the dual blockage with durvalumab plus tremelimumab was not associated with significant survival benefit in the randomized phase III MYSTIC trial. The primary endpoints, assessed in patients with PD-L1 ≥ 25%, were OS for durvalumab vs. chemotherapy and OS and PFS for durvalumab plus tremelimumab vs. chemotherapy. The study did not meet its primary endpoints with no statistically significant improvement in terms of OS with durvalumab vs. chemotherapy (HR 0.76, p = 0.04) or OS/PFS with durvalumab plus tremelimumab vs. chemotherapy in patients PD-L1-positive tumors (HR 0.85 and 1.05, respectively) [79]. However, this combination was associated with OS improvement in patients with high blood TMB (≥20 mutations per megabase), assessed with the 500-gene plasma NGS platform GuardantOMNI [80].
Whether dual PD-1/CLTA-4 blockage is superior to PD-1 inhibition alone in patients with PD-L1 TPS ≥50% is still debated. The randomized, double-blind, phase III trial, KEYNOTE-598, addressed this issue and compared pembrolizumab plus ipilimumab vs. pembrolizumab alone. The primary endpoints were OS and PFS. The trial failed to demonstrate a survival benefit in terms of both OS (21.4 months for pembrolizumab-ipilimumab vs. 21.9 months for pembrolizumab-placebo; HR 1.08, p = 0.74) and PFS (8.2 months for pembrolizumab-ipilimumab vs. 8.4 months for pembrolizumab-placebo; HR1.06, p = 0.72). Differences in grades 3–5 AEs occurred in 62.4% vs. 50.2% and resulted in death in 13.1% versus 7.5%. Despite the study being early stopped due to futility by the external data and safety monitoring committee, it provides evidence that the addition of an anti-CTLA-4 inhibitor to pembrolizumab in PD-L1-strong positive NSCLC patients does not improve the efficacy but also worsens the toxicity profile [81].
To increase the disease control during the first few weeks of immunotherapy, another therapeutic strategy recently investigated is the addition of a limited course (two cycles) of a platinum-based chemotherapy to the dual checkpoint blockage. In the CheckMate-9LA, patients were randomly assigned (1:1) to nivolumab (360 mg intravenously every 3 weeks) plus ipilimumab (1 mg/kg intravenously every 6 weeks) combined with histology-based, platinum-doublet chemotherapy (intravenously every 3 weeks for two cycles, experimental group) or chemotherapy alone (every 3 weeks for four cycles, control group). Randomization was stratified by tumor histology, sex, and PD-L1 expression. The primary endpoint was OS in all randomly assigned patients [82]. The experimental group was associated with a significantly longer OS than control group (14.1 vs. 10.7 months, HR 0.69; p = 0.00065) at the preplanned interim analysis. In contrast with the CheckMate-227 study, the two OS curves early separated, suggesting that the addition of a short course of chemotherapy might overcome the limits of chemotherapy-free regimens that might be associated with a lower disease control in the first 3 months of treatment. No differences were observed across all PD-L1 TPS subgroup. This regimen was associated with an increased incidence of serious AEs as compared with chemotherapy alone (30% vs. 18%), although treatment-related deaths were similar in both groups (2%) [82]. Recently, the results of an exploratory analysis of the study, analyzing the role of tissue and blood TMB (tTMB and bTMB), were presented. Collectively, 64% and 73% of all randomized patients had tTMB (FoundationOne CDx assay) and bTMB (GuardantOMNI) evaluable samples, respectively. Similar to the CheckMate-227, the OS benefit with nivolumab-ipilimumab plus chemotherapy was observed regardless of TMB status with higher tTMB and bTMB associated with greater ORR and PFS benefit but similar OS outcomes. Collectively, these results support the use of nivolumab-ipilimumab plus two cycles of chemotherapy as first-line treatment option for patients with advanced NSCLC regardless of PD-L1 expression, TMB status, or their combination [83].
In summary, we have an arsenal of options when choosing first-line treatment for patients with metastatic lung cancer. The choice of the scheme will depend on a series of factors to take into account, considering that patients in everyday practice often do not always resemble the group selected and suitable for clinical trials.
5 ICIs and SCLC
Small cell lung cancer (SCLC) accounts for ~15% of all lung cancers and ~ 30,000 deaths in the USA annually, owing to the elusive pathophysiology of the disease, the poor prognosis of patients, and the minimal improvement in the effectiveness of therapies over the past decades. By the time that small cell lung cancer (SCLC) is diagnosed, nearly two-thirds of patients already have extensive stage disease (ES-SCLC) [84, 85].
ES-SCLC has a poor prognosis and a 5-year survival rate of <7% [84, 86]. For more than 20 years, the standard of care for ES-SCLC was platinum chemotherapy, which is associated with high initial response rates but a median survival of only 10 months. These findings highlight an unmet need for first-line (1 L) treatment of ES-SCLC [84, 86, 87]. In recent years, PD-L1/PD-1 inhibitors have demonstrated improved outcomes in patients with ES-SCLC.
Recent studies have shown that the efficacy of immunotherapy is related to a high tumor mutation burden (TMB), high genomic instability, and high immunogenicity in tumor cells. Some studies have shown that SCLC may have some advantages in immunotherapy.
PD-L1 expression in >1% of tumor cells is present in only a minority (~20%) of SCLC specimens [87, 88]. High counts of tumor-infiltrating lymphocytes (TILs) have been associated with better prognosis in SCLC in the pre-immunotherapy era [89]. Indeed, the presence of suppressive FOXP3+ regulatory T cells has been associated with a better prognosis in patients with LS-SCLC (HR 0.37; P = 0.013), and the presence of CD45RO+ memory T cells in brain metastases from ED-SCLC has been correlated with prolonged OS (11 vs. 5 months; P = 0.007) [90, 91]. However, data from studies designed to investigate the presence or absence of alternative, potentially clinically important immune checkpoints in SCLC, such as LAG3, TIM3, TIGIT, OX40, and ICOS, are currently unavailable. A better understanding of the immune microenvironment is an important area of unmet need in the immunobiology of SCLC. To better understand the ES-SCLC treatment, it can be divided into first line, maintenance, and second or more lines.
6 First-Line Therapy
The first immune checkpoint inhibitor evaluated in SCLC was the CTLA-4 inhibitor ipilimumab, following the promising results of a randomized phase II study [92]. In a phase III, placebo-controlled randomized trial, ipilimumab was evaluated in combination with platinum-etoposide with a phased schedule (two cycles of chemotherapy followed by two cycles of ipilimumab plus chemotherapy and then two additional cycles of ipilimumab) vs. chemotherapy alone in patients with ES-SCLC. Addition of ipilimumab to chemotherapy did not prolong OS versus chemotherapy alone in patients with newly diagnosed ES-SCLC (13.4 vs. 12.4 months, HR 0.91; p = 0.25) and was associated with higher serious AEs and discontinuation rates due to treatment-related AEs [93].
In the IMpower 133 trial, the efficacy of atezolizumab in combination with carboplatin-etoposide was assessed in patients with ES-SCLC. Patients were randomized to receive four 21-day cycles of carboplatin-etoposide plus atezolizumab or placebo and then maintenance atezolizumab or placebo until unacceptable toxicity, disease progression, or loss of clinical benefit. The study met its two primary endpoints (investigator-assessed PFS and OS) [94]. The addition of atezolizumab was associated with a significantly longer median OS (12.3 vs. 10.3 months, HR 0.76; p = 0.154) compared with chemotherapy alone with an 18-month OS of 34% vs. 21%. The survival benefit was seen regardless of PD-L1 expression or bTMB status [95]. Atezolizumab was the first ICI approved in first-line ES-SCLC, and this trial was the first randomized phase III study reporting a survival benefit in this setting as compared with platinum/etoposide after three decades of inconsistent results.
A second PD-L1 inhibitor that demonstrated a survival benefit in first-line ES-SCLC was durvalumab in combination with cisplatin/carboplatin plus etoposide. The randomized phase III trial CASPIAN randomized 805 ES-SCLC patients to receive durvalumab/tremelimumab plus platinum/etoposide or durvalumab plus platinum/etoposide or platinum/etoposide alone. Primary endpoint was OS [96]. Durvalumab/tremelimumab plus platinum/etoposide failed to demonstrate a significant improvement in OS versus platinum/etoposide (10.4 vs. 10.5 months, HR 0.82; p = 0.045). In contrast, durvalumab plus platinum/etoposide showed a sustained improvement in OS versus platinum-etoposide (12.9 vs. 10.5 months, HR 0.75, p = 0.0032). The survival benefit observed with durvalumab plus platinum/etoposide versus platinum/etoposide consistently favored the combination across all prespecified patient subgroups, as well as post hoc subgroups defined by liver metastases at baseline [97]. The overall survival benefit observed with durvalumab plus platinum-etoposide in CASPIAN aligns with findings from the IMpower133 trial, adding a novel therapeutic option in the therapeutic armamentarium of ES-SCLC.
In contrast with the positive results of IMpower133 and CASPIAN, the randomized phase III trial KEYNOTE-604 failed to demonstrate a statistically significant OS benefit with the addition of pembrolizumab to platinum/etoposide. The study randomized 453 ES-SCLC patients to receive pembrolizumab plus platinum/etoposide for 4 cycles followed by pembrolizumab for up to 35 cycles vs. platinum/etoposide for 4 cycles. Primary endpoints were PFS (by blinded central review) and OS with prespecified efficacy boundaries where one-sided P = 0.0048 for PFS and P = 0.0128 for OS. The addition of pembrolizumab significantly improved PFS (HR 0.75; P = 0.0023) and was associated with durable responses (12-month PFS rates: 13.6% vs. 3.1%). Albeit median OS was longer in the experimental arm, the significance threshold was not met (HR, 0.80; P = 0.0164). Twenty-four-month OS estimates were 22.5% and 11.2%, respectively. The PFS and OS HRs were similar between PD-L1-positive and PD-L1-negative tumors and regardless of the choice of platinum [98]. Albeit formally negative, the results of this trial along with those of IMpower133 and CASPIAN consolidate the use of platinum/etoposide plus an ICI as the novel standard of care for first-line ES-SCLC, which is associated with long-term clinical benefit in a small subgroup of patients. Inherited differences in the three trials and the enrollment of a poorer prognosis population in the KEYNOTE-604 trial might account for the survival differences seen in these studies. The identification of predictive biomarkers and the correlation with SCLC molecular subtypes might provide novel insights on patients benefitting most from this strategy.
7 Second-Line or Later Monotherapy
ICI monotherapy with nivolumab or pembrolizumab is FDA-approved for patients with ES-SCLC, independent of PD-L1 status, as a third or subsequent line of therapy.
The approval of nivolumab was based on the preliminary results of the phase II study CheckMate-032. Nivolumab monotherapy provided durable responses (median duration of response 17.9 months with 12-month and 18-month OS rates of 28.3% and 20.0%, respectively) and was well tolerated as a third- or later-line treatment for recurrent SCLC [99]. The randomized study compares nivolumab plus ipilimumab with nivolumab alone in pretreated ES-SCLC with ORR by blinded independent central review as primary endpoint. Although nivolumab plus ipilimumab was associated with higher ORR compared with nivolumab monotherapy (21.9% vs. 11.6%; p = 0.03), addition of ipilimumab did not prolong OS (median OS 4.7 vs. 5.7 months; 24-month OS rates 16.9% vs. 17.9%, respectively), at cost of higher-grade 3/4 treatment-related AEs (37.5% vs. 12.9%) [100].
The randomized phase III trial CheckMate-331 evaluated the efficacy of nivolumab in second line versus an active comparator (topotecan or amrubicin). The primary endpoint was OS. The trial failed to demonstrate a significant OS benefit with nivolumab compared with chemotherapy (7.5 vs. 8.4 months, HR 0.86; P = 0.11). No differences were noted between PD-L1-positive and PD-L1-negative tumors. Patients with baseline lactate dehydrogenase (LDH) under the upper limit of normal and those without baseline liver metastases seemed to benefit from nivolumab. A delayed separation in the survival curves at 12 months was observed, suggesting long-term benefit with nivolumab [101].
The FDA approval of pembrolizumab as third or subsequent line of therapy for ES-SCLC was based on the results of KEYNOTE-028 and KEYNOTE-158 trials. In the phase Ib KEYNOTE-028 trial, pembrolizumab was evaluated in PD-L1 selected patients with a tumor cell, immune infiltrate, and stromal summative PD-L1 combined positive score (CPS) ≥1%. The study included 24 patients (31.7% of all samples evaluated for PD-L1) with relapsed SCLC (12.5% receiving pembrolizumab as second line and 50% as third line). Pembrolizumab showed encouraging signals of activity in this setting with an ORR of 33%, a median PFS of 1.9 months (1-year PFS 23.8%), and a median OS of 9.7 months (1-year OS 37.7%) [102]. The KEYNOTE-158 was a phase II basket trial that enrolled 107 patients with relapsed SCLC (79% received pembrolizumab in the second-line or third-line setting), regardless of PD-L1 status (47% of patients had PD-L1-negative tumors). This study confirmed that promising antitumor activity (ORR 18.7%, median PFS 2.0 months, and OS 9.1 months) and durable responses (77% of the patients had a duration of response ≥9 months) were seen with pembrolizumab in pretreated SCLC, especially in patients with PD-L1-positive tumors (ORR 35.7% vs. 6.0% for PD-L1-positive and PD-L1-negative subgroups, respectively) [103]. A pooled analysis of these two trials, including 83 patients with recurrent SCLC, confirmed these findings. Pembrolizumab was associated with an ORR of 19.3% (2 complete responses and 14 partial responses) and a median duration of response not reached (61% of responders had responses lasting ≥18 months) [104].
In a phase II randomized clinical trial, the efficacy of atezolizumab monotherapy was compared with that of chemotherapy (with either topotecan or platinum rechallenge) in second-line SCLC, independent of PD-L1 expression. The study included 73 patients (49 in the atezolizumab arm and 24 in the chemotherapy arm), and 64% had platinum-sensitive disease (defined as disease progression ≥90 days after completion of induction chemotherapy). No significant differences were observed in median OS (9.5 vs. 8.7 months, HR 0.84; P = 0.60), and the median PFS was statistically inferior in patients who received atezolizumab (1.4 vs. 4.3 months; P = 0.004). ORRs were low in both groups (2.3% in the atezolizumab arm and 10% the chemotherapy arm) [105].
In summary, both nivolumab and atezolizumab have failed to improve OS compared with standard chemotherapy in RCTs involving patients with relapsed SCLC requiring second-line therapy. FDA approval of ICI monotherapy, with either nivolumab or pembrolizumab, has been granted only in the third-line or later setting based on ORRs of 10–30% in single-arm studies.
8 Activity of ICIs in Special Populations
Poor Performance Status (PS)
Patients with disease burden-determined poor performance status (PS) have generally poor prognosis. Evidence on first-line ICIs in PS ≥2 NSCLC with PD-L1 ≥ 50% expression is relatively scant, as this population is usually excluded from clinical trials. A recent retrospective multicenter study in a real-world setting addressed this issue. Among 153 patients included, the median PFS and OS were 2.4 (95% CI, 1.6–2.5) and 3.0 months (95% CI: 2.4–3.5), respectively. The 6-month PFS rate was 27% (95% CI, 21–35%). Patients with a PS 2 determined by comorbidities had significantly better results compared with PS 2 induced by disease burden (6-month PFS rate, 49% vs. 19%; median OS 11.8 vs. 2.8 months, respectively) [106]. Additional data are required to determine the best therapeutic approach for this poor prognosis subgroup of patients.
HIV/AIDS
Anti-PD-1/PD-L1 checkpoint inhibitors have been approved for a variety of cancers that occur with higher incidence in people with HIV, including lung cancer. However, HIV-infected patients were excluded from all registrative trials with ICIs in solid tumors, and therefore the evidence on safety and activity of these agents in this population are relatively poor and mostly derived from small case series or case reports [107].
A recent prospective study explored the safety of pembrolizumab immunotherapy in solid tumor patients with HIV infection (CD4 count greater than or equal to 100 cells/μL, antiretroviral therapy for 4 or more weeks, and an HIV viral load of less than 200 copies/mL were eligible) and showed that pembrolizumab has a similar irAE profile for people with HIV and advanced cancer who have suppressed antiretroviral treatment for HIV as seen in HIV-negative participants in published studies [108]. The proportion of serious events was similar to that previously described in patients receiving anti-PD-1 therapy for FDA-approved indications. Hypothyroidism was the most frequent immune-mediated event in 20% of the participants and was adequately controlled with standard treatment [108].
Evidence available to date in HIV-infected NSCLC suggests that single-agent PD-1/PD-L1 inhibitors can be used safely in this subgroup of patients with similar efficacy results observed in the overall NSCLC population. The results of ongoing clinical trials evaluating ICIs in HIV-infected patients with NSCLC (CHIVA-2/NCT03304093) and/or different solid tumors (NCT03094286, NCT02408861) will provide definitive conclusions in this setting [109].
Preexisting Autoimmune Disorders
The vast majority of clinical trials have excluded patients with significant preexisting autoimmune disorders (AID). However, AIDs are relatively common in clinical practice. Safety and efficacy of ICIs in patients with preexisting AIDs are largely unknown, and evidence available to date are mostly based on retrospective analyses.
In a large retrospective study including 751 patients, of whom 65.5% had an advanced NSCLC, 11.3% had preexisting AID, including both clinically active (17.6%) and inactive (82.4%) diseases. Patients with preexisting AID experienced higher incidence of immune-related adverse events (irAEs) of any grade compared with patients without AIDs (65.9% vs. 39.9%). However, no significant differences were observed regarding grade 3/4 irAEs. Interestingly, preexisting AIDs were not significantly associated with ICI efficacy [110]. Similarly, another retrospective multicenter study evaluating the safety of PD-1/PD-L1 inhibitors in NSCLC patients with preexisting AID showed that exacerbation of AID occurred in a minority of patients (23%). Thirty-eight percent of the patients experienced an irAE (74% G1/2, 26% G3/4), and 14% discontinued treatment because of irAEs [111].
Given the paucity of data, treatment with ICIs in this patient population should be evaluated with caution and after an accurate evaluation of the risk-benefit ratio within a multidisciplinary team [112].
Solid Organ Transplant
A scenario of the daily clinic surrounds the aspect that carries safety problems, solid organ transplant recipients (SOTR) who are routinely excluded from immunotherapy trials; therefore, there is limited data for these agents in this population. A first approximation to the information in relation to cancer patients and solid organ transplants was published in 2018 evaluating 26 solid organ recipients treated with ICIs. 3/7 had graft rejection with ipilimumab, 6/15 with PD-1 inhibitor, and 2/4 patients treated with sequential ipilimumab and PD-1 inhibitors. Graft rejection was observed in 7/10 patients who received prednisolone with or without cyclosporine and 4/16 patients treated with different immunosuppressive regimens (tacrolimus, everolimus, sirolimus, or mycophenolate mofetil), suggesting that solid organ transplant recipients considered for ICIs might need more intensive immunosuppressive therapy than prednisolone monotherapy. Tumor response was reported in 27% of patients treated with ipilimumab and in 32% in those treated with PD-1 inhibitors. Of the nine patients who obtained a CR/PR to ICIs, four patients were immunosuppressed with tacrolimus or sirolimus, while the other five were treated with prednisolone. This could suggest that immunosuppressive regimens containing tacrolimus or sirolimus can be continued when ICIs are administered to organ transplant recipients [113].
A recent meta-analysis of published data reported that 37% of the patients experienced organ rejection and 14% died as a result of graft rejection. Nivolumab was associated with the highest rejection rate (52.2%), followed by pembrolizumab (26.7%) and ipilimumab (25%). When analyzing transplant rejection by organ, the highest rejection rate was observed in patients with kidney transplants (40.1%), followed by liver (35%) and heart (20%) transplants, and 64% presented disease progression. In terms of efficacy, the response rate was highest for pembrolizumab (40%), followed by nivolumab (30%) and ipilimumab (25%) [114].
Integrating Special Populations
An example of an inclusive clinical trial seeking to shed light on a daily problem in healthcare practice was given by a prospective cohort investigation with ICIs in special populations with stage IV or recurrent NSCLC, and no known sensitizing EGFR or ALK alterations, regardless of PD-L1 expression. CheckMate-817 was a phase IIIb/IV trial initiated due to limited available data on safety and efficacy of immunotherapy in patients with advanced NSCLC with poor performance status (ECOG PS 2) or other comorbidities, such as kidney and renal disease, and HIV-infected. First-line flat-dose nivolumab plus weight-based ipilimumab showed a consistent safety profile in special populations with advanced NSCLC, including those with ECOG performance score 2. Patients with either high TMB or higher PD-L1 1 expression exhibited improved outcomes. The safety profile was similar between the special population and a reference cohort. The mean time to the appearance of adverse events was similar between the cohorts [115]. Similarly, the TAIL study evaluated the safety and activity of atezolizumab in pretreated NSCLC with ECOG PS 2, renal failure, or preexisting autoimmune disease [116]. 615 patients received atezolizumab. Serious AEs occurred in 7.8% of patients and irAEs in 8.3%. The median OS was 11.1 months (95% CI: 8.9, 12.9), the ORR was 11.1% (95% CI: 8.7, 13.8), and the median of DOR was 14.6 months (95% CI: 8.4, 15.4) [116]. Medium- and long-term safety profile data are awaited for this population.
Clinical trials in lung cancer with anti-PD-1/PD-L1 treatment have generally excluded patients with ECOG PS ≥ 2, organ transplantation, AIDS, chronic viral infection, or organ dysfunction. This group of patients does not have scientific support that the use of immunotherapy in these special populations is scarce and is derived mainly from case series or real-world experience. Therefore, cautions should be used in clinical practice when considering these agents in special patient populations, as the evidence available to date are low. The results of ongoing clinical trials in these peculiar clinical scenarios will provide definitive conclusions on the safety and efficacy of ICIs in these subgroups of patients.
9 Impact of Molecular Characterization in the era of Immunotherapy and Future Directions
In the era of personalized medicine, the increasing use of next-generation sequencing (NGS) in both tissue and plasma is rapidly expanding our knowledge on the molecular characteristics of lung tumors. The multitude of information that can be obtained with these techniques has considerably improved the therapeutic landscape of advanced NSCLC trough the identification of oncogene drivers exploitable with targeted therapies [117]. In the context of non-oncogene addicted tumors, the molecular characterization of the tumor might provide useful prognostic and predictive information, overcoming the limits of PD-L1 tumor expression.
There are reports regarding the increase in the acquisition of somatic mutations during tumorigenesis which is associated with the formation of neoantigens and the subsequent development of immunogenicity; therefore, it has been postulated that tumors with a higher number of somatic mutations could be more sensitive to blocking immune checkpoints. According to international consensus, tumor mutational burden (TMB) is defined as the total number of non-synonymous mutations per coding area of a tumor genome and is calculated as mutations per DNA megabase (Mb). This emerging biomarker has been variably associated with ICI efficacy, although its clinical utility in clinical practice is unclear. On the one hand, TMB on either tissue (tTMB) or plasma (bTMB) has been clearly associated with improved efficacy with single-agent PD-1/PD-L1 inhibitors in exploratory analyses of large randomized studies in advanced NSCLC [65; 118, 119] and is a tumor-agnostic FDA-approved biomarker for pembrolizumab [120]. On the other hand, the predictive role of tTMB and bTMB is questioned when using chemo-immunotherapy combinations [121, 122].
Recently, the presence of concomitant mutations has been associated with inferior outcomes in NSCLC patients treated with single-agent ICIs.
Retrospective studies have shown that the presence of TP53 mutations without co-occurring STK11 or EGFR alterations (TP53-mut/STK11-EGFR-WT), independent of KRAS mutations, identifies a group of tumors with the highest CD8 T-cell density and PD-L1 expression that is associated with a prolonged PFS with single-agent ICIs. In contrast, STK11/LKB1 alterations are the most prevalent genomic driver of primary resistance to PD-1 axis inhibitors in KRAS-mutant lung adenocarcinoma [123, 124]. One of the possible explanations for these findings is that STK11, EGFR, or SMARC4 mutations are usually enriched among PD-L1-negative tumors, whereas TP53 mutations are more often seen in PD-L1-strong positive patients, as recently reported [125]. Collectively, these data suggest that concomitant mutations might influence ICI activity due to the presence of a tumor microenvironment less immunogenic (“cold tumors”), despite a higher TMB than wild-type tumors [126]. However, the debate on the prognostic/predictive role of these mutations is still open, and recent studies have shown that these mutations represent a poor prognostic factor rather a predictive factor that is independent of treatment received. Interestingly, the blood biomarker analysis of the MYSTIC trial showed that OS was shorter for patients with KEAP1-mutated or STK11-mutated NSCLC compared to wild-type patients irrespective of treatment received (durvalumab, durvalumab-tremelimumab, or chemotherapy) [127], suggesting that these mutations are likely a poor prognostic factor rather than a predictive factor to ICIs. Similar conclusions were recently reported in a large pan-cancer analysis evaluating the prognostic and predictive role of STK11 mutations. Across multiple solid tumors, STK11 alterations correlated with a poor prognosis regardless of therapy and were not associated with inferior immunotherapy outcome in the pan-cancer setting or in NSCLC. Furthermore, pan-cancer patients with co-altered STK11/KRAS did worse, regardless of treatment type [126]. In addition, the impact of these concomitant mutations among patients treated with chemo-immunotherapy combinations is still unclear, as initial retrospective studies reported that STK11 and KEAP1 genomic alterations are associated with shorter PFS with both platinum-pemetrexed-pembrolizumab chemo-immunotherapy and platinum-pemetrexed chemotherapy in non-squamous NSCLC and therefore represent adverse prognostic biomarkers, but the addition of pembrolizumab to platinum-pemetrexed does not result in prolonged PFS in PD-L1-positive STK11 and/or KEAP1-mutant non-squamous NSCLC [127], suggesting a potential negative predictive role [128, 129]. However, an exploratory analysis of the KEYNOTE-189 study did not confirm these findings, as pembrolizumab plus platinum/pemetrexed is associated with better outcomes than chemotherapy regardless of STK11 or KEAP1 mutational status [130].
The scenario of permanent evolution in the search for the best therapeutic strategy for a first line entails the need for robust predictive biomarkers to advanced NSCLC [131], which can potentially allow counseling of these patients who do not benefit from the use of ICI alone or in combination with chemotherapy or a combination of different checkpoint inhibitors.
The cornerstone of treatment for advanced NSCLC is focused on the search for biomarkers capable of predicting the response with an adequate safety profile. The biomarkers reported so far showed limitations in the capacity to effectively predict therapeutic efficacy of ICIs either alone or in different combinations. The use of ICIs is rapidly revolutionizing the therapeutic landscape of lung tumors, providing a significant improvement in the overall survival of these patients in multiple clinical settings. Long-term follow-up of registrative trials of these agents is constantly demonstrating long-term survivals in an unprecedented percentage of the patients, transforming an incurable disease into a chronic disease. The next step will be to extend the survival benefit to a higher percentage of patients, through the identification of novel predicting biomarkers and the introduction of more effective therapeutic strategies in tumors with less immunogenic microenvironment.
References
Siegel, R. L., Miller, K. D., Fuchs, H. E., & Jemal, A. (2021). Cancer statistics, 2021. CA: a Cancer Journal for Clinicians, 71(1), 7–33. https://doi.org/10.3322/caac.21654
Thomas, A., & Giaccone, G. (2015). Why has active immunotherapy not worked in lung cancer? Annals of Oncology, 26(11), 2213–2220. https://doi.org/10.1093/annonc/mdv323
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews. Cancer, 12(4), 252–264. https://doi.org/10.1038/nrc3239
Wei, S. C., Duffy, C. R., & Allison, J. P. (2018). Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discovery, 8(9), 1069–1086. https://doi.org/10.1158/2159-8290.CD-18-0367
Russo, A., McCusker, M. G., Scilla, K. A., Arensmeyer, K. E., Mehra, R., Adamo, V., & Rolfo, C. (2020). Immunotherapy in lung Cancer: From a minor god to the Olympus. Advances in Experimental Medicine and Biology, 1244, 69–92. https://doi.org/10.1007/978-3-030-41008-7_4
Rossi, G., Russo, A., Tagliamento, M., Tuzi, A., Nigro, O., Vallome, G., Sini, C., Grassi, M., Dal Bello, M. G., Coco, S., Longo, L., Zullo, L., Tanda, E. T., Dellepiane, C., Pronzato, P., & Genova, C. (2020). Precision medicine for NSCLC in the era of immunotherapy: New biomarkers to select the Most suitable treatment or the Most suitable patient. Cancers (Basel), 12(5). https://doi.org/10.3390/cancers12051125
Weichselbaum, R. R., Liang, H., Deng, L., & Fu, Y.-X. (2017). Radiotherapy and immunotherapy: A beneficial liaison? Nature Reviews. Clinical Oncology, 14(6), 365–379. https://doi.org/10.1038/nrclinonc.2016.211
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L., & Kroemer, G. (2020). Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nature Reviews. Clinical Oncology, 17(12), 725–741. https://doi.org/10.1038/s41571-020-0413-z
Ribas, A. (2012). Tumor immunotherapy directed at PD-1. The New England Journal of Medicine, 366(26), 2517–2519. https://doi.org/10.1056/NEJMe1205943
Doroshow, D. B., Bhalla, S., Beasley, M. B., Sholl, L. M., Kerr, K. M., Gnjatic, S., Wistuba, I. I., Rimm, D. L., Tsao, M. S., & Hirsch, F. R. (2021). PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nature Reviews. Clinical Oncology. https://doi.org/10.1038/s41571-021-00473-5
Rimm, D. L., Han, G., Taube, J. M., Yi, E. S., Bridge, J. A., Flieder, D. B., Homer, R., West, W. W., Wu, H., Roden, A. C., Fujimoto, J., Yu, H., Anders, R., Kowalewski, A., Rivard, C., Rehman, J., Batenchuk, C., Burns, V., Hirsch, F. R., & Wistuba, I. I. (2017). A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung Cancer. JAMA Oncology, 3(8), 1051–1058. https://doi.org/10.1001/jamaoncol.2017.0013
Marchetti, A., Barberis, M., Franco, R., De Luca, G., Pace, M. V., Staibano, S., Volante, M., Buttitta, F., Guerini-Rocco, E., Righi, L., D’antuono, T., Scagliotti, G. V., Pinto, C., De Rosa, G., & Papotti, M. (2017). Multicenter comparison of 22C3 pharm dx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. Journal of Thoracic Oncology, 12(11), 1654–1663. https://doi.org/10.1016/j.jtho.2017.07.031
Hirsch, F. R., McElhinny, A., Stanforth, D., Ranger-Moore, J., Jansson, M., Kulangara, K., Richardson, W., Towne, P., Hanks, D., Vennapusa, B., Mistry, A., Kalamegham, R., Averbuch, S., Novotny, J., Rubin, E., Emancipator, K., McCaffery, I., Williams, J. A., Walker, J., Longshore, J., Tsao, M. S., & Kerr, K. M. (2017). PD-L1 immunohistochemistry assays for lung Cancer: Results from phase 1 of the blueprint PD-L1 IHC assay comparison project. Journal of Thoracic Oncology, 12(2), 208–222. https://doi.org/10.1016/j.jtho.2016.11.2228
Tsao, M. S., Kerr, K. M., Kockx, M., Beasley, M.-B., Borczuk, A. C., Botling, J., Bubendorf, L., Chirieac, L., Chen, G., Chou, T.-Y., Chung, J.-H., Dacic, S., Lantuejoul, S., Mino-Kenudson, M., Moreira, A. L., Nicholson, A. G., Noguchi, M., Pelosi, G., Poleri, C., Russell, P. A., Sauter, J., Thunnissen, E., Wistuba, I., Yu, H., Wynes, M. W., Pintilie, M., Yatabe, Y., & Hirsch, F. R. (2018). PD-L1 immunohistochemistry comparability study in real-life clinical samples: Results of blueprint phase 2 project. Journal of Thoracic Oncology, 13(9), 1302–1311. https://doi.org/10.1016/j.jtho.2018.05.013
Herbst, R. S., Baas, P., Kim, D.-W., Felip, E., Pérez-Gracia, J. L., Han, J.-Y., Molina, J., Kim, J.-H., Arvis, C. D., Ahn, M.-J., Majem, M., Fidler, M. J., de Castro, G. J., Garrido, M., Lubiniecki, G. M., Shentu, Y., Im, E., Dolled-Filhart, M., & Garon, E. B. (2016). Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet, 387(10027), 1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Russo, A., Franchina, T., Ricciardi, G. R. R., Toscano, G., Schifano, S., Lo Certo, G., Battaglia, A., Pantò, E., Scaffidi Fonti, M., & Adamo, V. (2018). The changing scenario of 1(st) line therapy in non-oncogene addicted NSCLCs in the era of immunotherapy. Critical Reviews in Oncology/Hematology, 130, 1–12. https://doi.org/10.1016/j.critrevonc.2018.06.007
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., Gottfried, M., Peled, N., Tafreshi, A., Cuffe, S., O’Brien, M., Rao, S., Hotta, K., Leiby, M. A., Lubiniecki, G. M., Shentu, Y., Rangwala, R., & Brahmer, J. R. (2016). Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung Cancer. The New England Journal of Medicine, 375(19), 1823–1833. https://doi.org/10.1056/NEJMoa1606774
Herbst, R. S., Giaccone, G., de Marinis, F., Reinmuth, N., Vergnenegre, A., Barrios, C. H., Morise, M., Felip, E., Andric, Z., Geater, S., Özgüroğlu, M., Zou, W., Sandler, A., Enquist, I., Komatsubara, K., Deng, Y., Kuriki, H., Wen, X., McCleland, M., Mocci, S., Jassem, J., & Spigel, D. R. (2020). Atezolizumab for first-Line treatment of PD-L1-selected patients with NSCLC. The New England Journal of Medicine, 383(14), 1328–1339. https://doi.org/10.1056/NEJMoa1917346
Sezer, A., Kilickap, S., Gümüş, M., Bondarenko, I., Özgüroğlu, M., Gogishvili, M., Turk, H. M., Cicin, I., Bentsion, D., Gladkov, O., Clingan, P., Sriuranpong, V., Rizvi, N., Gao, B., Li, S., Lee, S., McGuire, K., Chen, C.-I., Makharadze, T., Paydas, S., Nechaeva, M., Seebach, F., Weinreich, D. M., Yancopoulos, G. D., Gullo, G., Lowy, I., & Rietschel, P. (2021). Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet, 397(10274), 592–604. https://doi.org/10.1016/S0140-6736(21)00228-2
Mok, T. S. K., Wu, Y.-L., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., Castro, G. J., Srimuninnimit, V., Laktionov, K. K., Bondarenko, I., Kubota, K., Lubiniecki, G. M., Zhang, J., Kush, D., & Lopes, G. (2019). Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet, 393(10183), 1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Mountzios, G., Remon, J., Novello, S., Blais, N., Califano, R., Cufer, T., Dingemans, A. M., Liu, S. V., Peled, N., Pennell, N. A., Reck, M., Rolfo, C., Tan, D., Vansteenkiste, J., West, H., & Besse, B. (2019). Position of an international panel of lung cancer experts on the decision for expansion of approval for pembrolizumab in advanced non-small-cell lung cancer with a PD-L1 expression level of ≥1% by the USA Food and Drug Administration. Annals of Oncology, 30(11), 1686–1688. https://doi.org/10.1093/annonc/mdz295
Russo, A., De Miguel, P. D., Gunasekaran, M., Scilla, K., Lapidus, R., Cooper, B., Mehra, R., Adamo, V., Malapelle, U., & Rolfo, C. (2019). Liquid biopsy tracking of lung tumor evolutions over time. Expert Review of Molecular Diagnostics, 19(12), 1099–1108. https://doi.org/10.1080/14737159.2020.1680287
Huang, Q., Zhang, H., Hai, J., Socinski, M. A., Lim, E., Chen, H., & Stebbing, J. (2018). Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology, 7(12), e1396403. https://doi.org/10.1080/2162402X.2017.1396403
Russo, A., Russano, M., Franchina, T., Migliorino, M. R., Aprile, G., Mansueto, G., Berruti, A., Falcone, A., Aieta, M., Gelibter, A., Russo, A., Barni, S., Maio, M., Martelli, O., Pantano, F., Iacono, D., Calvetti, L., Quadrini, S., Roca, E., Vasile, E., Imperatori, M., Occhipinti, M., Galvano, A., Petrelli, F., Calabrò, L., Pasquini, G., Intagliata, S., Ricciardi, G. R. R., Tonini, G., Santini, D., & Adamo, V. (2020). Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Outcomes with Nivolumab in Pretreated Non-Small Cell Lung Cancer (NSCLC): A large retrospective multicenter study. Advances in Therapy, 37(3), 1145–1155. https://doi.org/10.1007/s12325-020-01229-w
Howlader, N, Noone, A., Krapcho, M., Miller, D., Bishop, K., Kosary, C.L., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D.R., Chen, H.S., Feuer, E.J., Cronin, K.A. (Eds.), SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2019 SEER data submission, posted to the SEER web site, April 2019.
Pignon, J.-P., Tribodet, H., Scagliotti, G. V., Douillard, J.-Y., Shepherd, F. A., Stephens, R. J., Dunant, A., Torri, V., Rosell, R., Seymour, L., Spiro, S. G., Rolland, E., Fossati, R., Aubert, D., Ding, K., Waller, D., & Le Chevalier, T. (2008). Lung adjuvant cisplatin evaluation: A pooled analysis by the LACE collaborative group. Journal of Clinical Oncology, 26(21), 3552–3559. https://doi.org/10.1200/JCO.2007.13.9030
NSCLC collaborative group. (2014). Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet, 383(9928), 1561–1571. https://doi.org/10.1016/S0140-6736(13)62159-5
Burdett, S., Pignon, J. P., Tierney, J., Tribodet, H., Stewart, L., Le Pechoux, C., Aupérin, A., Le Chevalier, T., Stephens, R. J., Arriagada, R., Higgins, J. P. T., Johnson, D. H., Van Meerbeeck, J., Parmar, M. K. B., Souhami, R. L., Bergman, B., Douillard, J.-Y., Dunant, A., Endo, C., Girling, D., Kato, H., Keller, S. M., Kimura, H., Knuuttila, A., Kodama, K., Komaki, R., Kris, M. G., Lad, T., Mineo, T., Piantadosi, S., Rosell, R., Scagliotti, G., Seymour, L. K., Shepherd, F. A., Sylvester, R., Tada, H., Tanaka, F., Torri, V., Waller, D., & Liang, Y. (2015). Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database of Systematic Reviews, 3, CD011430. https://doi.org/10.1002/14651858.CD011430
Wu, Y.-L., Tsuboi, M., He, J., John, T., Grohe, C., Majem, M., Goldman, J. W., Laktionov, K., Kim, S.-W., Kato, T., Vu, H.-V., Lu, S., Lee, K.-Y., Akewanlop, C., Yu, C.-J., de Marinis, F., Bonanno, L., Domine, M., Shepherd, F. A., Zeng, L., Hodge, R., Atasoy, A., Rukazenkov, Y., & Herbst, R. S. (2020). Osimertinib in resected EGFR-mutated non-small-cell lung Cancer. The New England Journal of Medicine, 383(18), 1711–1723. https://doi.org/10.1056/NEJMoa2027071
Ahn, M.-J., Park, S., Jung, H. A., Cho, J. H., Sun, J.-M., Lee, S.-H., Choi, Y. S., Ahn, J. S., Kim, J., Park, K., Zo, J. I., Shim, Y. M., Kim, K. H., Shin, E.-C., & Kim, H. K. (2019). Phase II, prospective single-arm study of adjuvant pembrolizumab in N2 positive non-small cell lung cancer (NSCLC) treated with neoadjuvant concurrent chemoradiotherapy followed by curative resection: Preliminary results. JCO, 37(15_suppl), 8520–8520. https://doi.org/10.1200/JCO.2019.37.15_suppl.8520
Forde, P. M., Chaft, J. E., Smith, K. N., Anagnostou, V., Cottrell, T. R., Hellmann, M. D., Zahurak, M., Yang, S. C., Jones, D. R., Broderick, S., Battafarano, R. J., Velez, M. J., Rekhtman, N., Olah, Z., Naidoo, J., Marrone, K. A., Verde, F., Guo, H., Zhang, J., Caushi, J. X., Chan, H. Y., Sidhom, J.-W., Scharpf, R. B., White, J., Gabrielson, E., Wang, H., Rosner, G. L., Rusch, V., Wolchok, J. D., Merghoub, T., Taube, J. M., Velculescu, V. E., Topalian, S. L., Brahmer, J. R., & Pardoll, D. M. (2018). Neoadjuvant PD-1 blockade in Resectable lung Cancer. The New England Journal of Medicine, 378(21), 1976–1986. https://doi.org/10.1056/NEJMoa1716078
Lee, J., Chaft, J., Nicholas, A., Patterson, A., Waqar, S., Toloza, E., Haura, E., Raz, D., Reckamp, K., Merritt, R., Owen, D., Finley, D., Mcnamee, C., Blasberg, J., Garon, E., Mitchell, J., Doebele, R., Baciewicz, F., Nagasaka, M., Pass, H., Schulze, K., Phan, S., Johnson, A., Bunn, P., Johnson, B., Kris, M., Kwiatkowski, D., Wistuba, I., Carbone, D., & Rusch, V. (2021). PS01.05 surgical and clinical outcomes with neoadjuvant Atezolizumab in Resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. Journal of Thoracic Oncology, 16(3), S59–S61. https://doi.org/10.1016/j.jtho.2021.01.320
Cascone, T., William, W. N. J., Weissferdt, A., Leung, C. H., Lin, H. Y., Pataer, A., Godoy, M. C. B., Carter, B. W., Federico, L., Reuben, A., Khan, M. A. W., Dejima, H., Francisco-Cruz, A., Parra, E. R., Solis, L. M., Fujimoto, J., Tran, H. T., Kalhor, N., Fossella, F. V., Mott, F. E., Tsao, A. S., Blumenschein, G. J., Le, X., Zhang, J., Skoulidis, F., Kurie, J. M., Altan, M., Lu, C., Glisson, B. S., Byers, L. A., Elamin, Y. Y., Mehran, R. J., Rice, D. C., Walsh, G. L., Hofstetter, W. L., Roth, J. A., Antonoff, M. B., Kadara, H., Haymaker, C., Bernatchez, C., Ajami, N. J., Jenq, R. R., Sharma, P., Allison, J. P., Futreal, A., Wargo, J. A., Wistuba, I. I., Swisher, S. G., Lee, J. J., Gibbons, D. L., Vaporciyan, A. A., Heymach, J. V., & Sepesi, B. (2021). Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: The phase 2 randomized NEOSTAR trial. Nature Medicine, 27(3), 504–514. https://doi.org/10.1038/s41591-020-01224-2
Provencio, M., Nadal, E., Insa, A., García-Campelo, M. R., Casal-Rubio, J., Dómine, M., Majem, M., Rodríguez-Abreu, D., Martínez-Martí, A., De Castro, C. J., Cobo, M., López Vivanco, G., Del Barco, E., Bernabé Caro, R., Viñolas, N., Barneto Aranda, I., Viteri, S., Pereira, E., Royuela, A., Casarrubios, M., Salas Antón, C., Parra, E. R., Wistuba, I., Calvo, V., Laza-Briviesca, R., Romero, A., Massuti, B., & Cruz-Bermúdez, A. (2020). Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology, 21(11), 1413–1422. https://doi.org/10.1016/S1470-2045(20)30453-8
Shu, C. A., Gainor, J. F., Awad, M. M., Chiuzan, C., Grigg, C. M., Pabani, A., Garofano, R. F., Stoopler, M. B., Cheng, S. K., White, A., Lanuti, M., D’Ovidio, F., Bacchetta, M., Sonett, J. R., Saqi, A., & Rizvi, N. A. (2020). Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. The Lancet Oncology, 21(6), 786–795. https://doi.org/10.1016/S1470-2045(20)30140-6
Ready, N., Tong, B., Clarke, J., Gu, L., Wigle, D., Dragnev, K., Sporn, T., Stinchcombe, T., & D’Amico, T. (2019). P2.04-89 neoadjuvant Pembrolizumab in early stage Non-Small Cell Lung Cancer (NSCLC): Toxicity, efficacy, and surgical outcomes. Journal of Thoracic Oncology, 14(10), S745. https://doi.org/10.1016/j.jtho.2019.08.1594
Bar, J., Urban, D., Ofek, E., Ackerstein, A., Redinsky, I., Golan, N., Kamer, I., Simansky, D., Onn, A., Raskin, S., Shulimzon, T., Peled, M., Zeitlin, N., Halparin, S., Jurkowicz, M., Abukhalil, R., Perelman, M., & Ben-Nun, A. (2019). Neoadjuvant pembrolizumab (Pembro) for early stage non-small cell lung cancer (NSCLC): Updated report of a phase I study, MK3475-223. JCO, 37(15_suppl), 8534–8534. https://doi.org/10.1200/JCO.2019.37.15_suppl.8534
Gao, S., Li, N., Gao, S., Xue, Q., Ying, J., Wang, S., Tao, X., Zhao, J., Mao, Y., Wang, B., Shao, K., Lei, W., Wang, D., Lv, F., Zhao, L., Zhang, F., Zhao, Z., Su, K., Tan, F., Gao, Y., Sun, N., Wu, D., Yu, Y., Ling, Y., Wang, Z., Duan, C., Tang, W., Zhang, L., He, S., Wu, N., Wang, J., & He, J. (2020). Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. Journal of Thoracic Oncology, 15(5), 816–826. https://doi.org/10.1016/j.jtho.2020.01.017
Wislez, M., Mazieres, J., Lavole, A., Zalcman, G., Carre, O., Egenod, T., Caliandro, R., Gervais, R., Jeannin, G., Molinier, O., Massiani, M. A., Langlais, A., Morin, F., Le Pimpec, B. F., Brouchet, L., Assouad, J., Milleron, B., Damotte, D., Antoine, M., & Westeel, V. (2020). 1214O neoadjuvant durvalumab in resectable non-small cell lung cancer (NSCLC): Preliminary results from a multicenter study (IFCT-1601 IONESCO). Annals of Oncology, 31, S794. https://doi.org/10.1016/j.annonc.2020.08.1416
Besse, B., Adam, J., Cozic, N., Chaput-Gras, N., Planchard, D., Mezquita, L., Masip, J. R., Lavaud, P., Naltet, C., Gazzah, A., Thomas de Montpreville, V., Ghigna, M.-R., Mussot, S., Fadel, E., Mabille, L., Duchemann, B., Barlesi, F., Soria, J.-C., Caramella, C., & Mercier, O. (2020). 1215O – SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): Results from the phase II PRINCEPS trial. Annals of Oncology, 31, S794–S795. https://doi.org/10.1016/j.annonc.2020.08.1417
Zinner, R., Axelrod, R., Solomides, C. C., Cowan, S., Leiby, B., Bhatia, A. K., Sundermeyer, M. L., Hooper, D. C., Harshyne, L., Lu-Yao, G. L., Quereda-Bernabeu, B. C., Whang, S. C., OHara, S. C., Vernau, D. C., Werner-Wasik, M., Lu, B., Johnson, J. M., Scott, W. C., Argiris, A., & Evans, N. R. (2020). Neoadjuvant nivolumab (N) plus cisplatin (C)/pemetrexed (P) or cisplatin/gemcitabine (G) in resectable NSCLC. JCO, 38(15_suppl), 9051–9051. https://doi.org/10.1200/JCO.2020.38.15_suppl.9051
Yang, C.-F. J., McSherry, F., Mayne, N. R., Wang, X., Berry, M. F., Tong, B., Harpole, D. H. J., D’Amico, T. A., Christensen, J. D., Ready, N. E., & Klapper, J. A. (2018). Surgical outcomes after neoadjuvant chemotherapy and Ipilimumab for non-small cell lung Cancer. The Annals of Thoracic Surgery, 105(3), 924–929. https://doi.org/10.1016/j.athoracsur.2017.09.030
Rothschild, S., Zippelius, A., Eboulet, E. I., Savic, S., Betticher, D. C., Bettini, A., Frueh, M., Joerger, M., Britschgi, C., Peters, S., Mark, M. T., Ochsenbein, A., Janthur, W. D., Waibel, C., Mach, N., Gonzalez, M., Froesch, P., Godar, G., Rusterholz, C., & Pless, M. (2020). SAKK 16/14: Anti-PD-L1 antibody durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small cell lung cancer (NSCLC)—A multicenter single-arm phase II trial. JCO, 38(15_suppl), 9016–9016. https://doi.org/10.1200/JCO.2020.38.15_suppl.9016
Aupérin, A., Le Péchoux, C., Pignon, J. P., Koning, C., Jeremic, B., Clamon, G., Einhorn, L., Ball, D., Trovo, M. G., Groen, H. J. M., Bonner, J. A., Le Chevalier, T., & Arriagada, R. (2006). Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Annals of Oncology, 17(3), 473–483. https://doi.org/10.1093/annonc/mdj117
Aupérin, A., Le Péchoux, C., Rolland, E., Curran, W. J., Furuse, K., Fournel, P., Belderbos, J., Clamon, G., Ulutin, H. C., Paulus, R., Yamanaka, T., Bozonnat, M.-C., Uitterhoeve, A., Wang, X., Stewart, L., Arriagada, R., Burdett, S., & Pignon, J.-P. (2010). Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of Clinical Oncology, 28(13), 2181–2190. https://doi.org/10.1200/JCO.2009.26.2543
Antonia, S. J., Villegas, A., Daniel, D., Vicente, D., Murakami, S., Hui, R., Kurata, T., Chiappori, A., Lee, K. H., de Wit, M., Cho, B. C., Bourhaba, M., Quantin, X., Tokito, T., Mekhail, T., Planchard, D., Kim, Y.-C., Karapetis, C. S., Hiret, S., Ostoros, G., Kubota, K., Gray, J. E., Paz-Ares, L., de Castro, C. J., Faivre-Finn, C., Reck, M., Vansteenkiste, J., Spigel, D. R., Wadsworth, C., Melillo, G., Taboada, M., Dennis, P. A., & Özgüroğlu, M. (2018). Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. The New England Journal of Medicine, 379(24), 2342–2350. https://doi.org/10.1056/NEJMoa1809697
Gray, J. E., Villegas, A., Daniel, D., Vicente, D., Murakami, S., Hui, R., Kurata, T., Chiappori, A., Lee, K. H., Cho, B. C., Planchard, D., Paz-Ares, L., Faivre-Finn, C., Vansteenkiste, J. F., Spigel, D. R., Wadsworth, C., Taboada, M., Dennis, P. A., Özgüroğlu, M., & Antonia, S. J. (2020). Three-year overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC-update from PACIFIC. Journal of Thoracic Oncology, 15(2), 288–293. https://doi.org/10.1016/j.jtho.2019.10.002
Faivre-Finn, C., Spigel, D. R., Senan, S., Langer, C., Perez, B. A., Özgüroğlu, M., Daniel, D., Villegas, A., Vicente, D., Hui, R., Murakami, S., Paz-Ares, L., Broadhurst, H., Wadsworth, C., Dennis, P. A., & Antonia, S. J. (2021). Impact of prior chemoradiotherapy-related variables on outcomes with durvalumab in unresectable stage III NSCLC (PACIFIC). Lung Cancer, 151, 30–38. https://doi.org/10.1016/j.lungcan.2020.11.024
Brahmer, J. R., Tykodi, S. S., Chow, L. Q. M., Hwu, W.-J., Topalian, S. L., Hwu, P., Drake, C. G., Camacho, L. H., Kauh, J., Odunsi, K., Pitot, H. C., Hamid, O., Bhatia, S., Martins, R., Eaton, K., Chen, S., Salay, T. M., Alaparthy, S., Grosso, J. F., Korman, A. J., Parker, S. M., Agrawal, S., Goldberg, S. M., Pardoll, D. M., Gupta, A., & Wigginton, J. M. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England Journal of Medicine, 366(26), 2455–2465. https://doi.org/10.1056/NEJMoa1200694
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., Powderly, J. D., Carvajal, R. D., Sosman, J. A., Atkins, M. B., Leming, P. D., Spigel, D. R., Antonia, S. J., Horn, L., Drake, C. G., Pardoll, D. M., Chen, L., Sharfman, W. H., Anders, R. A., Taube, J. M., McMiller, T. L., Xu, H., Korman, A. J., Jure-Kunkel, M., Agrawal, S., McDonald, D., Kollia, G. D., Gupta, A., Wigginton, J. M., & Sznol, M. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine, 366(26), 2443–2454. https://doi.org/10.1056/NEJMoa1200690
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E. E., Poddubskaya, E., Antonia, S., Pluzanski, A., Vokes, E. E., Holgado, E., Waterhouse, D., Ready, N., Gainor, J., Arén Frontera, O., Havel, L., Steins, M., Garassino, M. C., Aerts, J. G., Domine, M., Paz-Ares, L., Reck, M., Baudelet, C., Harbison, C. T., Lestini, B., & Spigel, D. R. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung Cancer. The New England Journal of Medicine, 373(2), 123–135. https://doi.org/10.1056/NEJMoa1504627
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., Chow, L. Q., Vokes, E. E., Felip, E., Holgado, E., Barlesi, F., Kohlhäufl, M., Arrieta, O., Burgio, M. A., Fayette, J., Lena, H., Poddubskaya, E., Gerber, D. E., Gettinger, S. N., Rudin, C. M., Rizvi, N., Crinò, L., Blumenschein, G. R. J., Antonia, S. J., Dorange, C., Harbison, C. T., Graf Finckenstein, F., & Brahmer, J. R. (2015). Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung Cancer. The New England Journal of Medicine, 373(17), 1627–1639. https://doi.org/10.1056/NEJMoa1507643
Borghaei, H., Gettinger, S., Vokes, E. E., Chow, L. Q. M., Burgio, M. A., de Castro, C. J., Pluzanski, A., Arrieta, O., Frontera, O. A., Chiari, R., Butts, C., Wójcik-Tomaszewska, J., Coudert, B., Garassino, M. C., Ready, N., Felip, E., García, M. A., Waterhouse, D., Domine, M., Barlesi, F., Antonia, S., Wohlleber, M., Gerber, D. E., Czyzewicz, G., Spigel, D. R., Crino, L., Eberhardt, W. E. E., Li, A., Marimuthu, S., & Brahmer, J. (2021). Five-year outcomes from the randomized, phase III trials CheckMate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung Cancer. Journal of Clinical Oncology, 39(7), 723–733. https://doi.org/10.1200/JCO.20.01605
Peters, S., Cappuzzo, F., Horn, L., Paz-Ares, L., Borghaei, H., Barlesi, F., Steins, M., Felip, E., Spigel, D., Dorange, C., Lu, H., Healey, D., Kong Sanchez, T., Bhagavatheeswaran, P., Novotny, J., Jr., Lestini, B., & Brahmer, J. (2017). OA03.05 analysis of early survival in patients with advanced non-squamous NSCLC treated with Nivolumab vs docetaxel in CheckMate 057. Journal of Thoracic Oncology, 12(1), S253. https://doi.org/10.1016/j.jtho.2016.11.241
Fehrenbacher, L., Spira, A., Ballinger, M., Kowanetz, M., Vansteenkiste, J., Mazieres, J., Park, K., Smith, D., Artal-Cortes, A., Lewanski, C., Braiteh, F., Waterkamp, D., He, P., Zou, W., Chen, D. S., Yi, J., Sandler, A., & Rittmeyer, A. (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet, 387(10030), 1837–1846. https://doi.org/10.1016/S0140-6736(16)00587-0
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., Gadgeel, S. M., Hida, T., Kowalski, D. M., Dols, M. C., Cortinovis, D. L., Leach, J., Polikoff, J., Barrios, C., Kabbinavar, F., Frontera, O. A., De Marinis, F., Turna, H., Lee, J.-S., Ballinger, M., Kowanetz, M., He, P., Chen, D. S., Sandler, A., & Gandara, D. R. (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet, 389(10066), 255–265. https://doi.org/10.1016/S0140-6736(16)32517-X
Mazieres, J., Rittmeyer, A., Gadgeel, S., Hida, T., Gandara, D. R., Cortinovis, D. L., Barlesi, F., Yu, W., Matheny, C., Ballinger, M., & Park, K. (2021). Atezolizumab versus docetaxel in pretreated patients with NSCLC: Final results from the randomized phase 2 POPLAR and phase 3 OAK clinical trials. Journal of Thoracic Oncology, 16(1), 140–150. https://doi.org/10.1016/j.jtho.2020.09.022
Garon, E. B., Rizvi, N. A., Hui, R., Leighl, N., Balmanoukian, A. S., Eder, J. P., Patnaik, A., Aggarwal, C., Gubens, M., Horn, L., Carcereny, E., Ahn, M.-J., Felip, E., Lee, J.-S., Hellmann, M. D., Hamid, O., Goldman, J. W., Soria, J.-C., Dolled-Filhart, M., Rutledge, R. Z., Zhang, J., Lunceford, J. K., Rangwala, R., Lubiniecki, G. M., Roach, C., Emancipator, K., & Gandhi, L. (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine, 372(21), 2018–2028. https://doi.org/10.1056/NEJMoa1501824
Herbst, R. S., Garon, E. B., Kim, D.-W., Cho, B. C., Perez-Gracia, J. L., Han, J.-Y., Arvis, C. D., Majem, M., Forster, M. D., Monnet, I., Novello, S., Szalai, Z., Gubens, M. A., Su, W.-C., Ceresoli, G. L., Samkari, A., Jensen, E. H., Lubiniecki, G. M., & Baas, P. (2020). Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1–positive, advanced non–small-cell lung Cancer in the KEYNOTE-010 study. Journal of Clinical Oncology, 38(14), 1580–1590. https://doi.org/10.1200/JCO.19.02446
Barlesi, F., Vansteenkiste, J., Spigel, D., Ishii, H., Garassino, M., de Marinis, F., Özgüroğlu, M., Szczesna, A., Polychronis, A., Uslu, R., Krzakowski, M., Lee, J.-S., Calabrò, L., Arén Frontera, O., Ellers-Lenz, B., Bajars, M., Ruisi, M., & Park, K. (2018). Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN lung 200): An open-label, randomised, phase 3 study. The Lancet Oncology, 19(11), 1468–1479. https://doi.org/10.1016/S1470-2045(18)30673-9
Tan, P. S., Aguiar, P. J., Haaland, B., & Lopes, G. (2018). Comparative effectiveness of immune-checkpoint inhibitors for previously treated advanced non-small cell lung cancer – a systematic review and network meta-analysis of 3024 participants. Lung Cancer, 115, 84–88. https://doi.org/10.1016/j.lungcan.2017.11.017
Lee, C. K., Man, J., Lord, S., Cooper, W., Links, M., Gebski, V., Herbst, R. S., Gralla, R. J., Mok, T., & Yang, J. C.-H. (2018). Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: A systematic review and meta-analysis. JAMA Oncology, 4(2), 210–216. https://doi.org/10.1001/jamaoncol.2017.4427
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., Gottfried, M., Peled, N., Tafreshi, A., Cuffe, S., O’Brien, M., Rao, S., Hotta, K., Vandormael, K., Riccio, A., Yang, J., Pietanza, M. C., & Brahmer, J. R. (2019). Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung Cancer with PD-L1 tumor proportion score of 50% or greater. Journal of Clinical Oncology, 37(7), 537–546. https://doi.org/10.1200/JCO.18.00149
Aguilar, E. J., Ricciuti, B., Gainor, J. F., Kehl, K. L., Kravets, S., Dahlberg, S., Nishino, M., Sholl, L. M., Adeni, A., Subegdjo, S., Khosrowjerdi, S., Peterson, R. M., Digumarthy, S., Liu, C., Sauter, J., Rizvi, H., Arbour, K. C., Carter, B. W., Heymach, J. V., Altan, M., Hellmann, M. D., & Awad, M. M. (2019). Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Annals of Oncology, 30(10), 1653–1659. https://doi.org/10.1093/annonc/mdz288
Carbone, D. P., Reck, M., Paz-Ares, L., Creelan, B., Horn, L., Steins, M., Felip, E., van den Heuvel, M. M., Ciuleanu, T.-E., Badin, F., Ready, N., Hiltermann, T. J. N., Nair, S., Juergens, R., Peters, S., Minenza, E., Wrangle, J. M., Rodriguez-Abreu, D., Borghaei, H., Blumenschein, G. R. J., Villaruz, L. C., Havel, L., Krejci, J., Corral Jaime, J., Chang, H., Geese, W. J., Bhagavatheeswaran, P., Chen, A. C., & Socinski, M. A. (2017). First-Line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. The New England Journal of Medicine, 376(25), 2415–2426. https://doi.org/10.1056/NEJMoa1613493
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., Domine, M., Clingan, P., Hochmair, M. J., Powell, S. F., Cheng, S. Y.-S., Bischoff, H. G., Peled, N., Grossi, F., Jennens, R. R., Reck, M., Hui, R., Garon, E. B., Boyer, M., Rubio-Viqueira, B., Novello, S., Kurata, T., Gray, J. E., Vida, J., Wei, Z., Yang, J., Raftopoulos, H., Pietanza, M. C., & Garassino, M. C. (2018). Pembrolizumab plus chemotherapy in metastatic non-small-cell lung Cancer. The New England Journal of Medicine, 378(22), 2078–2092. https://doi.org/10.1056/NEJMoa1801005
Gadgeel, S., Rodríguez-Abreu, D., Speranza, G., Esteban, E., Felip, E., Dómine, M., Hui, R., Hochmair, M. J., Clingan, P., Powell, S. F., Cheng, S. Y.-S., Bischoff, H. G., Peled, N., Grossi, F., Jennens, R. R., Reck, M., Garon, E. B., Novello, S., Rubio-Viqueira, B., Boyer, M., Kurata, T., Gray, J. E., Yang, J., Bas, T., Pietanza, M. C., & Garassino, M. C. (2020). Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus Pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung Cancer. Journal of Clinical Oncology, 38(14), 1505–1517. https://doi.org/10.1200/JCO.19.03136
Gray, J., Rodríguez-Abreu, D., Powell, S. F., Hochmair, M. J., Gadgeel, S., Esteban, E., Felip, E., Speranza, G., De Angelis, F., Dómine, M., Cheng, S. Y., Bischoff, H. G., Peled, N., Reck, M., Hui, R., Garon, E. B., Boyer, M., Kurata, T., Yang, J., Jensen, E., Souza, F., & Garassino, M. C. (2021). FP13.02 Pembrolizumab + Pemetrexed-platinum vs Pemetrexed-platinum for metastatic NSCLC: 4-year follow-up from KEYNOTE-189. Journal of Thoracic Oncology, 16(3), S224. https://doi.org/10.1016/j.jtho.2021.01.141
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gümüş, M., Mazières, J., Hermes, B., Çay Şenler, F., Csőszi, T., Fülöp, A., Rodríguez-Cid, J., Wilson, J., Sugawara, S., Kato, T., Lee, K. H., Cheng, Y., Novello, S., Halmos, B., Li, X., Lubiniecki, G. M., Piperdi, B., & Kowalski, D. M. (2018). Pembrolizumab plus chemotherapy for squamous non-small-cell lung Cancer. The New England Journal of Medicine, 379(21), 2040–2051. https://doi.org/10.1056/NEJMoa1810865
Borghaei, H., Langer, C. J., Paz-Ares, L., Rodríguez-Abreu, D., Halmos, B., Garassino, M. C., Houghton, B., Kurata, T., Cheng, Y., Lin, J., Pietanza, M. C., Piperdi, B., & Gadgeel, S. M. (2020). Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non-small cell lung cancer without tumor PD-L1 expression: A pooled analysis of 3 randomized controlled trials. Cancer, 126(22), 4867–4877. https://doi.org/10.1002/cncr.33142
Robinson, A. G., Vicente, D., Tafreshi, A., Parra, H. S., Mazieres, J., Cicin, I., Medgyasszay, B., Rodríguez-Cid, J., Okamoto, I., Lee, S., Ramlau, R., Vladimirov, V., Cheng, Y., Halmos, B., Liu, C.-C., Schwarzenberger, P., Piperdi, B., & Paz-Ares, L. (2021). 97O first-line pembrolizumab plus chemotherapy for patients with advanced squamous NSCLC: 3-year follow-up from KEYNOTE-407. Journal of Thoracic Oncology, 16(4), S748–S749. https://doi.org/10.1016/S1556-0864(21)01939-0
Socinski, M. A., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., Rodríguez-Abreu, D., Moro-Sibilot, D., Thomas, C. A., Barlesi, F., Finley, G., Kelsch, C., Lee, A., Coleman, S., Deng, Y., Shen, Y., Kowanetz, M., Lopez-Chavez, A., Sandler, A., & Reck, M. (2018). Atezolizumab for first-Line treatment of metastatic nonsquamous NSCLC. The New England Journal of Medicine, 378(24), 2288–2301. https://doi.org/10.1056/NEJMoa1716948
Reck, M., Mok, T. S. K., Nishio, M., Jotte, R. M., Cappuzzo, F., Orlandi, F., Stroyakovskiy, D., Nogami, N., Rodríguez-Abreu, D., Moro-Sibilot, D., Thomas, C. A., Barlesi, F., Finley, G., Lee, A., Coleman, S., Deng, Y., Kowanetz, M., Shankar, G., Lin, W., & Socinski, M. A. (2019). Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. The Lancet Respiratory Medicine, 7(5), 387–401. https://doi.org/10.1016/S2213-2600(19)30084-0
West, H., McCleod, M., Hussein, M., Morabito, A., Rittmeyer, A., Conter, H. J., Kopp, H.-G., Daniel, D., McCune, S., Mekhail, T., Zer, A., Reinmuth, N., Sadiq, A., Sandler, A., Lin, W., Ochi Lohmann, T., Archer, V., Wang, L., Kowanetz, M., & Cappuzzo, F. (2019). Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology, 20(7), 924–937. https://doi.org/10.1016/S1470-2045(19)30167-6
Jotte, R., Cappuzzo, F., Vynnychenko, I., Stroyakovskiy, D., Rodríguez-Abreu, D., Hussein, M., Soo, R., Conter, H. J., Kozuki, T., Huang, K.-C., Graupner, V., Sun, S. W., Hoang, T., Jessop, H., McCleland, M., Ballinger, M., Sandler, A., & Socinski, M. A. (2020). Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. Journal of Thoracic Oncology, 15(8), 1351–1360. https://doi.org/10.1016/j.jtho.2020.03.028
Nishio, M., Barlesi, F., West, H., Ball, S., Bordoni, R., Cobo, M., Longeras, P. D., Goldschmidt, J., Jr., Novello, S., Orlandi, F., Sanborn, R. E., Szalai, Z., Ursol, G., Mendus, D., Wang, L., Wen, X., McCleland, M., Hoang, T., Phan, S., & Socinski, M. A. (2021). Atezolizumab plus chemotherapy for first-Line treatment of nonsquamous NSCLC: Results from the randomized phase 3 IMpower132 trial. Journal of Thoracic Oncology, 16(4), 653–664. https://doi.org/10.1016/j.jtho.2020.11.025
Hellmann, M. D., Paz-Ares, L., Bernabe Caro, R., Zurawski, B., Kim, S.-W., Carcereny Costa, E., Park, K., Alexandru, A., Lupinacci, L., de la Mora, J. E., Sakai, H., Albert, I., Vergnenegre, A., Peters, S., Syrigos, K., Barlesi, F., Reck, M., Borghaei, H., Brahmer, J. R., O’Byrne, K. J., Geese, W. J., Bhagavatheeswaran, P., Rabindran, S. K., Kasinathan, R. S., Nathan, F. E., & Ramalingam, S. S. (2019). Nivolumab plus Ipilimumab in advanced non-small-cell lung Cancer. The New England Journal of Medicine, 381(21), 2020–2031. https://doi.org/10.1056/NEJMoa1910231
Ramalingam, S. S., Ciuleanu, T. E., Pluzanski, A., Lee, J.-S., Schenker, M., Bernabe Caro, R., Lee, K. H., Zurawski, B., Audigier-Valette, C., Provencio, M., Linardou, H., Kim, S.-W., Borghaei, H., Hellmann, M. D., O’Byrne, K. J., Paz-Ares, L. G., Reck, M., Nathan, F. E., & Brahmer, J. R. (2020). Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. JCO, 38(15_suppl), 9500–9500. https://doi.org/10.1200/JCO.2020.38.15_suppl.9500
Rizvi, N. A., Cho, B. C., Reinmuth, N., Lee, K. H., Luft, A., Ahn, M.-J., van den Heuvel, M. M., Cobo, M., Vicente, D., Smolin, A., Moiseyenko, V., Antonia, S. J., Le Moulec, S., Robinet, G., Natale, R., Schneider, J., Shepherd, F. A., Geater, S. L., Garon, E. B., Kim, E. S., Goldberg, S. B., Nakagawa, K., Raja, R., Higgs, B. W., Boothman, A.-M., Zhao, L., Scheuring, U., Stockman, P. K., Chand, V. K., & Peters, S. (2020). Durvalumab with or without Tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung Cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncology, 6(5), 661–674. https://doi.org/10.1001/jamaoncol.2020.0237
Si, H., Kuziora, M., Quinn, K. J., Helman, E., Ye, J., Liu, F., Scheuring, U., Peters, S., Rizvi, N. A., Brohawn, P. Z., Ranade, K., Higgs, B. W., Banks, K. C., Chand, V. K., & Raja, R. (2021). A blood-based assay for assessment of tumor mutational burden in first-line metastatic NSCLC treatment: Results from the MYSTIC study. Clinical Cancer Research, 27(6), 1631–1640. https://doi.org/10.1158/1078-0432.CCR-20-3771
Boyer, M., Şendur, M. A. N., Rodríguez-Abreu, D., Park, K., Lee, D. H., Çiçin, I., Yumuk, P. F., Orlandi, F. J., Leal, T. A., Molinier, O., Soparattanapaisarn, N., Langleben, A., Califano, R., Medgyasszay, B., Hsia, T.-C., Otterson, G. A., Xu, L., Piperdi, B., Samkari, A., & Reck, M. (2021). Pembrolizumab plus Ipilimumab or placebo for metastatic non-small-cell lung Cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. Journal of Clinical Oncology, JCO2003579. https://doi.org/10.1200/JCO.20.03579
Paz-Ares, L., Ciuleanu, T.-E., Cobo, M., Schenker, M., Zurawski, B., Menezes, J., Richardet, E., Bennouna, J., Felip, E., Juan-Vidal, O., Alexandru, A., Sakai, H., Lingua, A., Salman, P., Souquet, P.-J., De Marchi, P., Martin, C., Pérol, M., Scherpereel, A., Lu, S., John, T., Carbone, D. P., Meadows-Shropshire, S., Agrawal, S., Oukessou, A., Yan, J., & Reck, M. (2021). First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. The Lancet Oncology, 22(2), 198–211. https://doi.org/10.1016/S1470-2045(20)30641-0
Paz-Ares, L., Ciuleanu, T.-E., Cobo, M., Schenker, M., Zurawski, B., Menezes, J., Richardet, E., Bennouna, J., Felip, E., Juan-Vidal, O., Alexandru, A., Sakai, H., Scherpereel, A., Reck, M., Lu, S., John, T., Meadows-Shropshire, S., Balli, D., Agrawal, S., & Carbone, D. P. (2021). 98O first-line nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles chemotherapy (chemo) vs 4 cycles chemo in advanced non-small cell lung cancer (aNSCLC): Association of blood and tissue tumor mutational burden (TMB) with efficacy in CheckMate 9LA. Journal of Thoracic Oncology, 16(4), S750–S751. https://doi.org/10.1016/S1556-0864(21)01940-7
Bernhardt, E. B., & Jalal, S. I. (2016). Small Cell Lung Cancer. Cancer Treatment and Research, 170, 301–322. https://doi.org/10.1007/978-3-319-40389-2_14
Denninghoff, V., Russo, A., de Miguel-Pérez, D., Malapelle, U., Benyounes, A., Gittens, A., Cardona, A. F., & Rolfo, C. (2021). Small cell lung Cancer: State of the art of the molecular and genetic landscape and novel perspective. Cancers, 13(7). https://doi.org/10.3390/cancers13071723
Rudin, C. M., Brambilla, E., Faivre-Finn, C., & Sage, J. (2021). Small-cell lung cancer. Nature Reviews. Disease Primers, 7(1), 3. https://doi.org/10.1038/s41572-020-00235-0
Armstrong, S. A., & Liu, S. V. (2020). Dashing decades of defeat: Long anticipated advances in the first-line treatment of extensive-stage small cell lung Cancer. Current Oncology Reports, 22(2), 20. https://doi.org/10.1007/s11912-020-0887-y
Schultheis, A. M., Scheel, A. H., Ozretić, L., George, J., Thomas, R. K., Hagemann, T., Zander, T., Wolf, J., & Buettner, R. (2015). PD-L1 expression in small cell neuroendocrine carcinomas. European Journal of Cancer, 51(3), 421–426. https://doi.org/10.1016/j.ejca.2014.12.006
Iams, W. T., Shiuan, E., Meador, C. B., Roth, M., Bordeaux, J., Vaupel, C., Boyd, K. L., Summitt, I. B., Wang, L. L., Schneider, J. T., Warner, J. L., Zhao, Z., & Lovly, C. M. (2019). Improved prognosis and increased tumor-infiltrating lymphocytes in patients who have SCLC with neurologic paraneoplastic syndromes. Journal of Thoracic Oncology, 14(11), 1970–1981. https://doi.org/10.1016/j.jtho.2019.05.042
Bonanno, L., Pavan, A., Dieci, M. V., Di Liso, E., Schiavon, M., Comacchio, G., Attili, I., Pasello, G., Calabrese, F., Rea, F., Favaretto, A., Rugge, M., Guarneri, V., Fassan, M., & Conte, P. F. (2018). The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. European Journal of Cancer, 101, 191–200. https://doi.org/10.1016/j.ejca.2018.06.023
Berghoff, A. S., Ricken, G., Wilhelm, D., Rajky, O., Widhalm, G., Dieckmann, K., Birner, P., Bartsch, R., & Preusser, M. (2016). Tumor infiltrating lymphocytes and PD-L1 expression in brain metastases of small cell lung cancer (SCLC). Journal of Neuro-Oncology, 130(1), 19–29. https://doi.org/10.1007/s11060-016-2216-8
Reck, M., Bondarenko, I., Luft, A., Serwatowski, P., Barlesi, F., Chacko, R., Sebastian, M., Lu, H., Cuillerot, J.-M., & Lynch, T. J. (2013). Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: Results from a randomized, double-blind, multicenter phase 2 trial. Annals of Oncology, 24(1), 75–83. https://doi.org/10.1093/annonc/mds213
Reck, M., Luft, A., Szczesna, A., Havel, L., Kim, S.-W., Akerley, W., Pietanza, M. C., Wu, Y.-L., Zielinski, C., Thomas, M., Felip, E., Gold, K., Horn, L., Aerts, J., Nakagawa, K., Lorigan, P., Pieters, A., Kong Sanchez, T., Fairchild, J., & Spigel, D. (2016). Phase III randomized trial of Ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung Cancer. Journal of Clinical Oncology, 34(31), 3740–3748. https://doi.org/10.1200/JCO.2016.67.6601
Horn, L., Mansfield, A. S., Szczęsna, A., Havel, L., Krzakowski, M., Hochmair, M. J., Huemer, F., Losonczy, G., Johnson, M. L., Nishio, M., Reck, M., Mok, T., Lam, S., Shames, D. S., Liu, J., Ding, B., Lopez-Chavez, A., Kabbinavar, F., Lin, W., Sandler, A., & Liu, S. V. (2018). First-Line Atezolizumab plus chemotherapy in extensive-stage small-cell lung Cancer. The New England Journal of Medicine, 379(23), 2220–2229. https://doi.org/10.1056/NEJMoa1809064
Liu, S. V., Reck, M., Mansfield, A. S., Mok, T., Scherpereel, A., Reinmuth, N., Garassino, M. C., De Castro, C. J., Califano, R., Nishio, M., Orlandi, F., Alatorre-Alexander, J., Leal, T., Cheng, Y., Lee, J.-S., Lam, S., McCleland, M., Deng, Y., Phan, S., & Horn, L. (2021). Updated overall survival and PD-L1 subgroup analysis of patients with extensive-stage small-cell lung Cancer treated with Atezolizumab, carboplatin, and etoposide (IMpower133). Journal of Clinical Oncology, 39(6), 619–630. https://doi.org/10.1200/JCO.20.01055
Paz-Ares, L., Dvorkin, M., Chen, Y., Reinmuth, N., Hotta, K., Trukhin, D., Statsenko, G., Hochmair, M. J., Özgüroğlu, M., Ji, J. H., Voitko, O., Poltoratskiy, A., Ponce, S., Verderame, F., Havel, L., Bondarenko, I., Kazarnowicz, A., Losonczy, G., Conev, N. V., Armstrong, J., Byrne, N., Shire, N., Jiang, H., & Goldman, J. W. (2019). Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet, 394(10212), 1929–1939. https://doi.org/10.1016/S0140-6736(19)32222-6
Goldman, J. W., Dvorkin, M., Chen, Y., Reinmuth, N., Hotta, K., Trukhin, D., Statsenko, G., Hochmair, M. J., Özgüroğlu, M., Ji, J. H., Garassino, M. C., Voitko, O., Poltoratskiy, A., Ponce, S., Verderame, F., Havel, L., Bondarenko, I., Każarnowicz, A., Losonczy, G., Conev, N. V., Armstrong, J., Byrne, N., Thiyagarajah, P., Jiang, H., & Paz-Ares, L. (2021). Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. The Lancet Oncology, 22(1), 51–65. https://doi.org/10.1016/S1470-2045(20)30539-8
Rudin, C. M., Awad, M. M., Navarro, A., Gottfried, M., Peters, S., Csőszi, T., Cheema, P. K., Rodriguez-Abreu, D., Wollner, M., Yang, J. C.-H., Mazieres, J., Orlandi, F. J., Luft, A., Gümüş, M., Kato, T., Kalemkerian, G. P., Luo, Y., Ebiana, V., Pietanza, M. C., & Kim, H. R. (2020). Pembrolizumab or placebo plus etoposide and platinum as first-Line therapy for extensive-stage small-cell lung Cancer: Randomized, double-blind, phase III KEYNOTE-604 study. Journal of Clinical Oncology, 38(21), 2369–2379. https://doi.org/10.1200/JCO.20.00793
Ready, N., Farago, A. F., de Braud, F., Atmaca, A., Hellmann, M. D., Schneider, J. G., Spigel, D. R., Moreno, V., Chau, I., Hann, C. L., Eder, J. P., Steele, N. L., Pieters, A., Fairchild, J., & Antonia, S. J. (2019). Third-Line Nivolumab monotherapy in recurrent SCLC: CheckMate 032. Journal of Thoracic Oncology, 14(2), 237–244. https://doi.org/10.1016/j.jtho.2018.10.003
Ready, N. E., Ott, P. A., Hellmann, M. D., Zugazagoitia, J., Hann, C. L., de Braud, F., Antonia, S. J., Ascierto, P. A., Moreno, V., Atmaca, A., Salvagni, S., Taylor, M., Amin, A., Camidge, D. R., Horn, L., Calvo, E., Li, A., Lin, W. H., Callahan, M. K., & Spigel, D. R. (2020). Nivolumab monotherapy and Nivolumab plus Ipilimumab in recurrent small cell lung Cancer: Results from the CheckMate 032 randomized cohort. Journal of Thoracic Oncology, 15(3), 426–435. https://doi.org/10.1016/j.jtho.2019.10.004
Spigel, D. R., Vicente, D., Ciuleanu, T. E., Gettinger, S., Peters, S., Horn, L., Audigier-Valette, C., Pardo Aranda, N., Juan-Vidal, O., Cheng, Y., Zhang, H., Shi, M., Luft, A., Wolf, J., Antonia, S., Nakagawa, K., Fairchild, J., Baudelet, C., Pandya, D., Doshi, P., Chang, H., & Reck, M. (2021). Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331(☆). Annals of Oncology. https://doi.org/10.1016/j.annonc.2021.01.071
Ott, P. A., Elez, E., Hiret, S., Kim, D.-W., Morosky, A., Saraf, S., Piperdi, B., & Mehnert, J. M. (2017). Pembrolizumab in patients with extensive-stage small-cell lung Cancer: Results from the phase Ib KEYNOTE-028 study. Journal of Clinical Oncology, 35(34), 3823–3829. https://doi.org/10.1200/JCO.2017.72.5069
Chung, H. C., Lopez-Martin, J. A., Kao, S. C.-H., Miller, W. H., Ros, W., Gao, B., Marabelle, A., Gottfried, M., Zer, A., Delord, J.-P., Penel, N., Jalal, S. I., Xu, L., Zeigenfuss, S., Pruitt, S. K., & Piha-Paul, S. A. (2018). Phase 2 study of pembrolizumab in advanced small-cell lung cancer (SCLC): KEYNOTE-158. JCO, 36(15_suppl), 8506–8506. https://doi.org/10.1200/JCO.2018.36.15_suppl.8506
Chung, H. C., Piha-Paul, S. A., Lopez-Martin, J., Schellens, J. H. M., Kao, S., Miller, W. H. J., Delord, J.-P., Gao, B., Planchard, D., Gottfried, M., Zer, A., Jalal, S. I., Penel, N., Mehnert, J. M., Matos, I., Bennouna, J., Kim, D.-W., Xu, L., Krishnan, S., Norwood, K., & Ott, P. A. (2020). Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: Results from the KEYNOTE-028 and KEYNOTE-158 studies. Journal of Thoracic Oncology, 15(4), 618–627. https://doi.org/10.1016/j.jtho.2019.12.109
Pujol, J.-L., Greillier, L., Audigier-Valette, C., Moro-Sibilot, D., Uwer, L., Hureaux, J., Guisier, F., Carmier, D., Madelaine, J., Otto, J., Gounant, V., Merle, P., Mourlanette, P., Molinier, O., Renault, A., Rabeau, A., Antoine, M., Denis, M. G., Bommart, S., Langlais, A., Morin, F., & Souquet, P.-J. (2019). A randomized non-comparative phase II study of anti-programmed cell death-ligand 1 Atezolizumab or chemotherapy as second-Line therapy in patients with small cell lung Cancer: Results from the IFCT-1603 trial. Journal of Thoracic Oncology, 14(5), 903–913. https://doi.org/10.1016/j.jtho.2019.01.008
Facchinetti, F., Mazzaschi, G., Barbieri, F., Passiglia, F., Mazzoni, F., Berardi, R., Proto, C., Cecere, F. L., Pilotto, S., Scotti, V., Rossi, S., Del Conte, A., Vita, E., Bennati, C., Ardizzoni, A., Cerea, G., Migliorino, M. R., Sala, E., Camerini, A., Bearz, A., De Carlo, E., Zanelli, F., Guaitoli, G., Garassino, M. C., Ciccone, L. P., Sartori, G., Toschi, L., Dall’Olio, F. G., Landi, L., Pizzutilo, E. G., Bartoli, G., Baldessari, C., Novello, S., Bria, E., Cortinovis, D. L., Rossi, G., Rossi, A., Banna, G. L., Camisa, R., Di Maio, M., & Tiseo, M. (2020). First-line pembrolizumab in advanced non-small cell lung cancer patients with poor performance status. European Journal of Cancer, 130, 155–167. https://doi.org/10.1016/j.ejca.2020.02.023
Hajjar, J. (2019). Cancer immunotherapy for the immunosuppressed: Dissecting the conundrum of safety and efficacy. Journal of Immunotherapy and Precision Oncology, 2(3), 53–54. https://doi.org/10.4103/JIPO.JIPO_15_19
Uldrick, T. S., Gonçalves, P. H., Abdul-Hay, M., Claeys, A. J., Emu, B., Ernstoff, M. S., Fling, S. P., Fong, L., Kaiser, J. C., Lacroix, A. M., Lee, S. Y., Lundgren, L. M., Lurain, K., Parsons, C. H., Peeramsetti, S., Ramaswami, R., Sharon, E., Sznol, M., Wang, C.-C. J., Yarchoan, R., & Cheever, M. A. (2019). Assessment of the safety of Pembrolizumab in patients with HIV and advanced Cancer-a phase 1 study. JAMA Oncology, 5(9), 1332–1339. https://doi.org/10.1001/jamaoncol.2019.2244
Scilla, K. A., Russo, A., & Rolfo, C. (2019). Immunotherapy use in patients with HIV and non-small-cell lung Cancer: Current data. Journal of Immunotherapy and Precision Oncology, 2(3), 55–58. https://doi.org/10.4103/JIPO.JIPO_13_19
Cortellini, A., Buti, S., Santini, D., Perrone, F., Giusti, R., Tiseo, M., Bersanelli, M., Michiara, M., Grassadonia, A., Brocco, D., Tinari, N., De Tursi, M., Zoratto, F., Veltri, E., Marconcini, R., Malorgio, F., Garufi, C., Russano, M., Anesi, C., Zeppola, T., Filetti, M., Marchetti, P., Botticelli, A., Antonini Cappellini, G. C., De Galitiis, F., Vitale, M. G., Sabbatini, R., Bracarda, S., Berardi, R., Rinaldi, S., Tudini, M., Silva, R. R., Pireddu, A., Atzori, F., Chiari, R., Ricciuti, B., Iacono, D., Migliorino, M. R., Rossi, A., Porzio, G., Cannita, K., Ciciarelli, V., Fargnoli, M. C., Ascierto, P. A., & Ficorella, C. (2019). Clinical outcomes of patients with advanced Cancer and pre-existing autoimmune diseases treated with anti-programmed Death-1 immunotherapy: A real-world transverse study. The Oncologist, 24(6), e327–e337. https://doi.org/10.1634/theoncologist.2018-0618
Leonardi, G. C., Gainor, J. F., Altan, M., Kravets, S., Dahlberg, S. E., Gedmintas, L., Azimi, R., Rizvi, H., Riess, J. W., Hellmann, M. D., & Awad, M. M. (2018). Safety of programmed Death-1 pathway inhibitors among patients with non-small-cell lung Cancer and preexisting autoimmune disorders. Journal of Clinical Oncology, 36(19), 1905–1912. https://doi.org/10.1200/JCO.2017.77.0305
Naing, A., Hajjar, J., Gulley, J. L., Atkins, M. B., Ciliberto, G., Meric-Bernstam, F., & Hwu, P. (2020). Strategies for improving the management of immune-related adverse events. Journal for Immunotherapy of Cancer, 8(2). https://doi.org/10.1136/jitc-2020-001754
Smedman, T. M., Line, P.-D., Guren, T. K., & Dueland, S. (2018). Graft rejection after immune checkpoint inhibitor therapy in solid organ transplant recipients. Acta Oncologica, 57(10), 1414–1418. https://doi.org/10.1080/0284186X.2018.1479069
Fisher, J., Zeitouni, N., Fan, W., & Samie, F. H. (2020). Immune checkpoint inhibitor therapy in solid organ transplant recipients: A patient-centered systematic review. Journal of the American Academy of Dermatology, 82(6), 1490–1500. https://doi.org/10.1016/j.jaad.2019.07.005
Barlesi, F., Audigier-Valette, C., Felip, E., Ciuleanu, T.-E., Jao, K., Rijavec, E., Urban, L., Aucoin, J.-S., Zannori, C., Vermaelen, K., Frontera, O. A., Ready, N., Curioni, A., Linardou, H., Poddubskaya, E., Fischer, J. R., Pillai, R., Li, S., Acevedo, A., & Paz-Ares, L. (2019). Nivolumab plus low-dose IPILIMUMAB as first-Line treatment of advanced NSCLC: Overall survival analysis of Checkmate 817. Annals of Oncology, 30, xi33–xi34. https://doi.org/10.1093/annonc/mdz451.001
Ardizzoni, A., Azevedo, S., Rubio Viquiera, B., Rodriguez Abreu, D., Alatorre-Alexander, J., Smit, H. J., Yu, J., Syrigos, K., Patel, H., Tolson, J., Cardona, A., Perez Moreno, P., & Newsom-Davis, T. (2019). LBA84 – primary results from TAIL, a global single-arm safety study of atezolizumab (atezo) monotherapy in a diverse population of patients with previously treated advanced non-small cell lung cancer (NSCLC). Annals of Oncology, 30, v920–v921. https://doi.org/10.1093/annonc/mdz394.082
Russo, A., Lopes, A. R., McCusker, M. G., Garrigues, S. G., Ricciardi, G. R., Arensmeyer, K. E., Scilla, K. A., Mehra, R., & Rolfo, C. (2020). New targets in lung Cancer (excluding EGFR, ALK, ROS1). Current Oncology Reports, 22(5), 48. https://doi.org/10.1007/s11912-020-00909-8
Gandara, D. R., Paul, S. M., Kowanetz, M., Schleifman, E., Zou, W., Li, Y., Rittmeyer, A., Fehrenbacher, L., Otto, G., Malboeuf, C., Lieber, D. S., Lipson, D., Silterra, J., Amler, L., Riehl, T., Cummings, C. A., Hegde, P. S., Sandler, A., Ballinger, M., Fabrizio, D., Mok, T., & Shames, D. S. (2018). Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nature Medicine, 24(9), 1441–1448. https://doi.org/10.1038/s41591-018-0134-3
Herbst, R. S., Lopes, G., Kowalski, D. M., Nishio, M., Wu, Y.-L., de Castro, J. G., Baas, P., Kim, D.-W., Gubens, M. A., Cristescu, R., Aurora-Garg, D., Albright, A., Ayers, M., Loboda, A., Lunceford, J., Kobie, J., Lubiniecki, G. M., Pietanza, M. C., Piperdi, B., & Mok, T. S. K. (2019). Association between tissue TMB (tTMB) and clinical outcomes with pembrolizumab monotherapy (pembro) in PD-L1-positive advanced NSCLC in the KEYNOTE-010 and -042 trials. Annals of Oncology, 30, v916–v917. https://doi.org/10.1093/annonc/mdz394.077
Marabelle, A., Fakih, M., Lopez, J., Shah, M., Shapira-Frommer, R., Nakagawa, K., Chung, H. C., Kindler, H. L., Lopez-Martin, J. A., Miller, W. H. J., Italiano, A., Kao, S., Piha-Paul, S. A., Delord, J.-P., McWilliams, R. R., Fabrizio, D. A., Aurora-Garg, D., Xu, L., Jin, F., Norwood, K., & Bang, Y.-J. (2020). Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. The Lancet Oncology, 21(10), 1353–1365. https://doi.org/10.1016/S1470-2045(20)30445-9
Paz-Ares, L., Langer, C. J., Novello, S., Halmos, B., Cheng, Y., Gadgeel, S. M., Hui, R., Sugawara, S., Borghaei, H., Cristescu, R., Aurora-Garg, D., Albright, A., Loboda, A., Kobie, J., Lunceford, J., Ayers, M., Lubiniecki, G. M., Pietanza, M. C., Piperdi, B., & Garassino, M. C. (2019). LBA80 – Pembrolizumab (pembro) plus platinum-based chemotherapy (chemo) for metastatic NSCLC: Tissue TMB (tTMB) and outcomes in KEYNOTE-021, 189, and 407. Annals of Oncology, 30, v917–v918. https://doi.org/10.1093/annonc/mdz394.078
Garassino, M. C., Gadgeel, S. M., Rodriguez-Abreu, D., Felip, E., Esteban, E., Speranza, G., Hochmair, M., Powell, S. F., Garon, E. B., Hui, R., Nogami, N., Cristescu, R., Morrissey, M., Loboda, A., Kobie, J., Ayers, M., Piperdi, B., Pietanza, M. C., Snyder, A., & Reck, M. (2020). Evaluation of blood TMB (bTMB) in KEYNOTE-189: Pembrolizumab (pembro) plus chemotherapy (chemo) with pemetrexed and platinum versus placebo plus chemo as first-line therapy for metastatic nonsquamous NSCLC. JCO, 38(15_suppl), 9521–9521. https://doi.org/10.1200/JCO.2020.38.15_suppl.9521
Biton, J., Mansuet-Lupo, A., Pécuchet, N., Alifano, M., Ouakrim, H., Arrondeau, J., Boudou-Rouquette, P., Goldwasser, F., Leroy, K., Goc, J., Wislez, M., Germain, C., Laurent-Puig, P., Dieu-Nosjean, M.-C., Cremer, I., Herbst, R., Blons, H., & Damotte, D. (2018). TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clinical Cancer Research, 24(22), 5710–5723. https://doi.org/10.1158/1078-0432.CCR-18-0163
Skoulidis, F., Goldberg, M. E., Greenawalt, D. M., Hellmann, M. D., Awad, M. M., Gainor, J. F., Schrock, A. B., Hartmaier, R. J., Trabucco, S. E., Gay, L., Ali, S. M., Elvin, J. A., Singal, G., Ross, J. S., Fabrizio, D., Szabo, P. M., Chang, H., Sasson, A., Srinivasan, S., Kirov, S., Szustakowski, J., Vitazka, P., Edwards, R., Bufill, J. A., Sharma, N., Ou, S.-H. I., Peled, N., Spigel, D. R., Rizvi, H., Aguilar, E. J., Carter, B. W., Erasmus, J., Halpenny, D. F., Plodkowski, A. J., Long, N. M., Nishino, M., Denning, W. L., Galan-Cobo, A., Hamdi, H., Hirz, T., Tong, P., Wang, J., Rodriguez-Canales, J., Villalobos, P. A., Parra, E. R., Kalhor, N., Sholl, L. M., Sauter, J. L., Jungbluth, A. A., Mino-Kenudson, M., Azimi, R., Elamin, Y. Y., Zhang, J., Leonardi, G. C., Jiang, F., Wong, K.-K., Lee, J. J., Papadimitrakopoulou, V. A., Wistuba, I. I., Miller, V. A., Frampton, G. M., Wolchok, J. D., Shaw, A. T., Jänne, P. A., Stephens, P. J., Rudin, C. M., Geese, W. J., Albacker, L. A., & Heymach, J. V. (2018). STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discovery, 8(7), 822–835. https://doi.org/10.1158/2159-8290.CD-18-0099
Lamberti, G., Spurr, L. F., Li, Y., Ricciuti, B., Recondo, G., Umeton, R., Nishino, M., Sholl, L. M., Meyerson, M. L., Cherniack, A. D., & Awad, M. M. (2020). Clinicopathological and genomic correlates of programmed cell death ligand 1 (PD-L1) expression in nonsquamous non-small-cell lung cancer. Annals of Oncology, 31(6), 807–814. https://doi.org/10.1016/j.annonc.2020.02.017
Marinelli, D., Mazzotta, M., Scalera, S., Terrenato, I., Sperati, F., D’Ambrosio, L., Pallocca, M., Corleone, G., Krasniqi, E., Pizzuti, L., Barba, M., Carpano, S., Vici, P., Filetti, M., Giusti, R., Vecchione, A., Occhipinti, M., Gelibter, A., Botticelli, A., De Nicola, F., Ciuffreda, L., Goeman, F., Gallo, E., Visca, P., Pescarmona, E., Fanciulli, M., De Maria, R., Marchetti, P., Ciliberto, G., & Maugeri-Saccà, M. (2020). KEAP1-driven co-mutations in lung adenocarcinoma unresponsive to immunotherapy despite high tumor mutational burden. Annals of Oncology, 31(12), 1746–1754. https://doi.org/10.1016/j.annonc.2020.08.2105
Rizvi, N., Cho, B. C., Reinmuth, N., Lee, K. H., Luft, A., Ahn, M., Papadimitrakopoulou, V., Heymach, J., Scheuring, U., Higgs, B., Ye, J., Kuziora, M., Wu, S., Liu, F., Si, H., & Peters, S. (2019). OA04.07 mutations associated with sensitivity or resistance to immunotherapy in mNSCLC: Analysis from the MYSTIC trial. Journal of Thoracic Oncology, 14(10), S217. https://doi.org/10.1016/j.jtho.2019.08.428
Krishnamurthy, N., Goodman, A. M., Barkauskas, D. A., & Kurzrock, R. (2021). STK11 alterations in the pan-cancer setting: Prognostic and therapeutic implications. European Journal of Cancer, 148, 215–229. https://doi.org/10.1016/j.ejca.2021.01.050
Skoulidis, F., Arbour, K., Hellmann, M., Patil, P., Marmarelis, M., Owen, D., Awad, M., Murray, J., Levy, B., Hellyer, J., Gainor, J., Stewart, T., Goldberg, S., Dimou, A., Bestvina, C., Cummings, A., Elamin, Y., Lam, V., Zhang, J., Shu, C., Riess, J., Blakely, C., Pecot, C., Mezquita, L., Tabbò, F., Sacher, A., Scheffler, M., Ricciuti, B., Venkatraman, D., Rizvi, H., Liu, C., Johnston, R., Ni, Y., Azok, J., Kier, M., Katz, S., Davies, K., Segal, J., Ritterhouse, L., Shaish, H., Lacroix, L., Memmott, R., Madrigal, J., Goldman, J., Lau, S., Killam, J., Walther, Z., Carter, B., Woodcock, M., Roth, J., Swisher, S., Leighl, N., Digumarthy, S., Mooradian, M., Rotow, J., Wolf, J., Scagliotti, G., Planchard, D., Besse, B., Bivona, T., Gandara, D., Garon, E., Rizvi, N., Camidge, D. R., Schalper, K., Herbst, R., Shaw, A., Neal, J., Wakelee, H., Brahmer, J., Jänne, P., Carbone, D., Aggarwal, C., Pennell, N., Rudin, C., Papadimitrakopoulou, V., & Heymach, J. (2019). MA11.11 STK11/LKB1 genomic alterations are associated with inferior clinical outcomes with chemo-immunotherapy in non-squamous NSCLC. Journal of Thoracic Oncology, 14(10), S294–S295. https://doi.org/10.1016/j.jtho.2019.08.591
Gadgeel, S. M., Rodriguez-Abreu, D., Felip, E., Esteban, E., Speranza, G., Reck, M., Hui, R., Boyer, M., Garon, E. B., Horinouchi, H., Cristescu, R., Aurora-Garg, D., Loboda, A., Lunceford, J., Kobie, J., Ayers, M., Piperdi, B., Pietanza, M. C., & Garassino, M. C. (2020). Abstract LB-397: Pembrolizumab plus pemetrexed and platinum vs placebo plus pemetrexed and platinum as first-line therapy for metastatic nonsquamous NSCLC: Analysis of KEYNOTE-189 by STK11 and KEAP1 status. Cancer Research, 80(16 Supplement), LB-397. https://doi.org/10.1158/1538-7445.AM2020-LB-397
Fujii, T., Naing, A., Rolfo, C., & Hajjar, J. (2018). Biomarkers of response to immune checkpoint blockade in cancer treatment. Critical Reviews in Oncology/Hematology, 130, 108–120. https://doi.org/10.1016/j.critrevonc.2018.07.010
Acknowledgement
Dr. Kaen reports clinical trial activities for Roche, Lilly Oncology, Clovis, MSD, AbbVie, Takeda, Novartis, Pfizer, Array BioPharma Inc., Servier, Nektar Therapeutics, Merck Healthcare KGaA, and GlaxoSmithKline and consultancy for Roche, Boehringer Ingelheim, Pfizer, MSD, BMS, Novartis, AstraZeneca, Raffo Tecnofarma, Varifarma, and Bayer. Dr. Minatta reports speaker bureau for Pfizer, Roche, and Takeda and advisory board role for LATAM MSD. Dr. Russo reports consultancy for AstraZeneca and MSD outside the submitted work. Dr. Malapelle has received personal fees (as consultant and/or speaker bureau) from Boehringer Ingelheim, Roche, MSD, Amgen, Thermo Fisher Scientific, Eli Lilly, Diaceutics, GSK, Merck, and AstraZeneca, unrelated to the current work. Dr. Rolfo reports grants from MSD, AstraZeneca, Archer, Inivata, Merck Serono, and Mylan, nonfinancial support from Oncompass, grants from Lung Cancer Research Foundation-Pfizer, and nonfinancial support from Guardant Health and Biomark Inc. outside the submitted work. The other authors have no conflict of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaen, D.L., Minatta, N., Russo, A., Malapelle, U., de Miguel-Pérez, D., Rolfo, C. (2021). Immunotherapy in Lung Cancer: Are the Promises of Long-Term Benefit Finally Met?. In: Naing, A., Hajjar, J. (eds) Immunotherapy. Advances in Experimental Medicine and Biology, vol 1342. Springer, Cham. https://doi.org/10.1007/978-3-030-79308-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-79308-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-79307-4
Online ISBN: 978-3-030-79308-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)