Abstract
Cancer immunotherapy has demonstrated great therapeutic responses in clinic by educating and awakening patients’ own immune system to against cancer cells. Along with development of immunotherapy in preclinic and clinic, the number of approved immunotherapeutics is increasing greatly. However, the key challenge of the extensive implementation of immunotherapy against cancer is the controlled modulation of immune systems, owing to serious adverse effects including nonspecific inflammation and autoimmunity. Fully understanding how to increase the immune responses of various immunotherapeutic strategies is important to improve their therapeutic efficacy and control the adverse effects. Phototherapy including photothermal therapy (PTT) and photodynamic therapy (PDT), as new emerging cancer therapeutic treatments, usually employs phototherapeutic agents to selectively kill tumor cells with certain light irradiation. Phototherapy could selectively kill the cancer cells under appropriate light irradiation. In addition to directly killing tumor cells, phototherapy also could induce strong anti-tumor immune responses. Such tumor-specific immunological responses induced after phototherapy, usually in combination with immune checkpoint blockade (ICB) therapy, could effectively improve the therapeutic efficacy and reduce toxic side effects, presenting remarkable potential in inhibiting tumor metastasis and recurrence. In this chapter, we would like to summarize the latest advances, as well as challenges and opportunities in utilizing nanoparticle-based phototherapy in combination with checkpoint blockade for enhancing anticancer immunity, and discuss the further prospects in this field together with how this strategy may possibly be transformed into clinic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phototherapy, an emerging cancer therapeutic strategy, usually utilizes phototherapeutic agents to selectively kill tumor cells under appropriate light irradiation. It generally falls into two categories, photothermal therapy (PTT) and photodynamic therapy (PDT) [1, 2]. PTT usually leverages optical absorbing agents to generate heat under appropriate light irradiation to burn cancer cells. Ideal photothermal agents should have high absorbance in the near-infrared (NIR) region (760–1000 nm), a transparency window for biological tissues, and should be able to efficiently convert the absorbed optical energy into heat. Various types of nanomaterials with high NIR absorbance have been exploited as PTT agents, exhibiting promising therapeutic efficacy in many preclinical animal studies. Examples include inorganic nanomaterials, such as gold nanostructures [3, 4], carbon-based nanomaterials [5], copper sulfide (CuS) nanoparticles [6, 7], palladium nanosheets [8], and other transition metal dichalcogenides [9, 10], and organic nanomaterials, such as NIR-light absorbing conjugated polymers [11, 12], porphysomes [13], and NIR dye-encapsulated polymeric nanoparticles [14], have been explored as effective PTT agents both in vitro and in vivo.
On the other hand, PDT relies on photosensitizers that can transfer the surrounding oxygen molecules to reactive oxygen species (ROS) or cytotoxic singlet oxygen (1O2) to kill the cancer cells under certain light irradiation [15]. PDT is an externally activatable treatment modality, which has been approved in clinic for cancer treatment. A wide range of photosensitizers, most of them containing porphyrin structures, have been applied for PDT. However, inefficient tumor accumulation and cell uptake of photosensitizers largely limit the application of PDT. Various nanomaterials, including liposomes [16], polymeric nanoparticles [17], quantum dots [18], magnetic nanoparticles [18], mesoporous silica nanoparticles [19], and carbon-based nanomaterials [20], have been developed to delivery photosensitizers, aiming to enhance their tumor accumulation and therapeutic efficacy. Moreover, most of the recently used photosensitizers are excited by visible light, which usually has limited the tissue penetration depth. To overcome this limitation, a unique type of nanomaterials, upconversion nanoparticles (UCNPs), which could emit visible light under NIR light excitation, has been explored to realize NIR-mediated PDT with improved tissue penetration [21].
In addition to directly killing cancer cells, phototherapy is also useful to trigger and/or improve other therapeutic treatments, achieving synergistic therapeutic effects. Furthermore, it has now been well-established that the dying tumor cells after phototherapy could release tumor-associated antigens and self-antigens, which could attract a large number of antigen-presenting cells (APCs) [22]. APCs, particularly dendritic cells (DCs), are able to capture these antigens and present these antigens to adaptive immune cells, eliciting strong antigen-specific immune responses [23]. Various immunogenic factors, including the exposure of calreticulin (CAR) on the cell surface, release of pro-inflammatory cytokines and factors (IL-6, TNF-α, and IL-1β), and post-apoptotic exodus of high mobility groups, have been identified during the dying of tumor cells after phototherapy [24,25,26]. Thus, similar to cancer vaccines using cancer cell lysates, the tumor-associated antigens released after phototherapy in the presence of adjuvant nanoparticles could act as “tumor vaccines” [27]. To further promote the antitumor immune responses induced by phototherapy, immune checkpoint blockade could be selected to block the regulatory pathways that express on immune cells, enhancing immune stimulatory or subverting immune suppressive effects for the tumor infiltration, activation, and proliferation of T cells [28]. In this chapter, well-designed nanoparticle platforms used for phototherapy in combination with checkpoint blockade for cancer immunotherapy will be discussed.
2 Photothermal Therapy
In situ photoimmunotherapy (ISPI) which combines local PTT and immunological stimulation with immunoadjuvant was first raised in 1997 [29]. It utilized 805 nm diode laser to increase the temperature in the target tumor which was pretreated with the glycated chitosan gel containing indocyanine green (ICG), directly killing the tumor cells and releasing a large number of tumor-associated antigens, self-antigens, and heat shock proteins (HSPs). Concomitantly, the immunological defense system was stimulated to against residual and metastatic tumor cells, significantly inhibiting the tumor recurrence and metastasis. These findings indicated that PTT of the primary tumor indeed triggered the specific immunological response in tumor-bearing rats. To facilitate the antitumor immunological response induced by ISPI, additional immunological stimulations are required to activate the immune system, achieving the effective and continuable antitumor immune responses. In 2010, Chen and co-workers conducted a preliminary clinical study to evaluate the therapeutic efficacy of ISPI together with an immunological stimulator, imiquimod, a food and drug administration (FDA) approved Toll-like receptor 7 (TLR7) agonist, in late-stage melanoma patients [30]. Eleven patients with multiple cutaneous metastases received ISPI in one or multiple 6-week treatment cycles. During the ISPI treatment, three main components were applied directly in the cutaneous tumors: local injection of ICG and imiquimod, and local irradiation with 805 nm laser. Five patients were still alive at the time of their last follow-up and the 12-month overall survival was 70%. Thus, ISPI with imiquimod has clinical benefits for patients with advanced and late-stage melanoma.
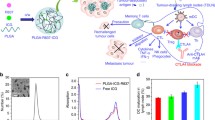
Various inorganic nano-agents such as gold nanostructures [31], carbon nanotubes [32], graphene oxide [33], CuS nanoparticles [34] and MoS2 nanosheets [35] with strong NIR absorbance have been explored for ISPI. Yata et al. modified gold nanoparticles with CpG oligodeoxynuleotides, and mixed them with hexapod-like structured DNA, obtaining the composite immunostimulatory gold–DNA hydrogel [31]. EG7-OVA tumors after injection of DNA hydrogel were irradiated with 780 nm laser, the local increased temperature could directly kill the cancer cells, consequently increasing the level of HSPs, improving the levels of tumor-associated antigen-specific IgG in the serum, and promoting the production of (IFN-γ) in the splenocytes. Thus, the DNA hydrogel-based PTT significantly inhibit the growth of tumor and prolong the survival of mice (Fig. 1a). Yata et al. reported the photothermally enhanced intracellular delivery of nanocarriers for CpG delivery [33]. In this work, they successfully used polyethylene glycol modified graphene oxide to load immune adjuvant CpG for photothermally enhanced immune response. The NIR optical absorbance of graphene oxide was further applied to promote the immunostimulatory activity of CpG, showing remarkably enhanced immune stimulation responses under laser irradiation, owing to the photothermally induced local heating that accelerated intracellular trafficking of CPG coated nanoparticles (Fig. 1b). In a following work, Lu and colleagues designed light-induced transformative CuS-based CpG systems for combined photothermal ablation and immunotherapy (Fig. 1c) [34]. After laser irradiation, the structures of nanoparticles break down, reassemble, and transform into polymer complexes that improve CpG tumor uptake and retention by plasmacytoid dendritic cells. Compared with immunotherapy or photothermal therapy alone, such CuS nanoparticles-mediated photothermal immunotherapy, exhibited more potent innate and adaptive immune responses, resulting in combined anticancer effects against primary treated and distant untreated tumors (Fig. 1d).
Inorganic nano-agents based PTT mediated immunotherapy. (a) DNA hydrogel consisting of a hexapod-like structured DNA with CpG sequences and gold nanoparticles for photothermal immunotherapy. (b) Graphene oxide loaded immunostimulator CpG for photothermally enhanced cancer immunotherapy. (c, d) Chitosan-coated hollow copper sulfide nanoparticles for combined photothermal and immune cancer therapy, inhibiting the growth of primary treated and distant untreated tumors. Copyright from Elsevier, 2017 [31], Elsevier, 2014 [33], ACS, 2014 [34]
Besides the use of inorganic nano-agents, some small molecule dyes with high NIR absorbance have also been co-encapsulated with immunological stimulations into polymeric nanoparticles or liposomes for effective combination of PTT and immunotherapy. Li et al. developed an endogenous vaccine based on fluorophore-loaded liposomes (IR-7-lipo) coated with a multivalent immunoadjuvant (HA-CpG). Upon irradiation with 808 nm laser, IR-7-lipo induced cancer cell necrosis and released tumor-associated antigens, while the immunoadjuvant improved the expression of co-stimulatory molecules on DCs and promoted antigen presentation. The combination therapy of PTT and immunotherapy regulated the tumor micro-environment, enhanced antitumor immune response, eradicated tumors in mice and inhibited cancer metastasis [36]. Kumar et al. developed biodegradable and biocompatible poloxamer/glycated chitosan/polycaprolactone based nanoparticles encapsulated with IR 820 for fluorescence imaging guided photo-immunotherapy [37]. Excitingly, glycol chitosan used here could work as the immunostimulatory agent and the IR 820 as the photothermal agent for PPT, both of them working together to kill drug and TNF-α dual-resistant breast cancer cells.
As an effective stimulator of the immune system, PTT also exhibits synergistic effects with immune checkpoint blockade to enhance immune stimulatory or reverse immune suppressive effects for T cell activation and proliferation. Liu and co-workers demonstrated the combination of anti-CTLA-4 with PTT using PEGylated single-wall carbon nanotubes (SWNTs) [38]. They found that polymer-coated SWNTs not only was used for photothermal tumor destruction, but also act as an immunological adjuvant to greatly promote maturation of DCs and production of anti-tumor cytokines. Moreover, CTLA-4 blockade applied after SWNT-based PTT of primary tumors would promote the infiltration of effective T cells and greatly abrogate regulatory T cells at distant tumors. Thus, such combined SWNT-based PTT and anti–CTLA-4 treatment was able to inhibit the growth of remaining cancer cells in both subcutaneous tumor model and lung metastasis model, promising for the treatment of cancer metastasis, the major cause of cancer death (Fig. 2). In a following work [39], they used three FDA-approved agents approved agents, PLGA as the encapsulating polymer, R837 as the immune adjuvant, and indocyanine green (ICG) as the near-infrared dye to enable PTT (Fig. 3). Upon photothermal ablation of primary tumors injected with PLGA-ICG-R837, the released tumor-associated antigens together with adjuvant (R837), could act as cancer vaccine, activing strong immunological responses. With the help of anti-CTLA4, which was able to inhibit the activities of Tregs, this strategy appeared to be particularly effective in inhibiting tumor metastasis. More importantly, such strategy exhibited strong immune-memory effect to protect mice from cancer recurrence.
Immunological responses triggered by carbon nanotubes-based photothermal therapy in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Copyright from Wiley-VCH, 2014 [38]
Photothermal therapy with immune-adjuvant nanoparticles in combination with checkpoint blockade for effective cancer immunotherapy. (a) Scheme showing the mechanism of antitumor immune responses induced by PLGA-ICG-R837-based PTT together with checkpoint-blockade. (b) Tumor growth curves of mice after different treatment on subcutaneous 4T1 tumor model. (c) Morbidity-free survival of different groups of mice-bearing orthotopic 4T1 tumors with spontaneous metastases after various treatments. Copyright from Springer Nature, 2017 [39]
3 Photodynamic Therapy
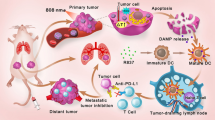
PDT is a clinically approved, minimally invasive therapeutic modality, depending on 1O2 or ROS generated by photosensitizers under the appropriate light irradiation. Apart from destroying local tumor tissue and increasing the expression of several stress proteins, PDT could increase the tumor immunogenicity by increasing the expression of CAR and releasing tumor-associated antigens [40]. Moreover, PDT is able to cause acute local inflammation, which could attract different immune cells especially activated neutrophils into the tumor, activating both innate and adaptive immune systems (Fig. 4). Korbelik group investigated the impact of PDT on the systemic and local kinetics of neutrophil trafficking and activity in different tumor models in mice. This study demonstrated that treatment of solid tumors by PDT indeed induced strong and protracted increase in systemic neutrophil numbers mediated by complement activation especially in the tumor [41]. In another wok, Gollnick et al. also investigated the immunogenicity induced by PDT [42]. They found that the PDT-generated tumor cell lysates were potent vaccines, which was more effective than other modes of creating whole tumor vaccines, such as UV or ionizing irradiation. PDT-generated lysates could activate DCs to express IL-12, which is critical to the development of cellular immune responses.
Scheme showing the inflammation and immune responses induced by photodynamic therapy. The damage of endothelial cells after photodynamic therapy activates a case of events that lead to local inflammation, releasing different cytokines including interleukin 1β (IL1β), IL6 and IL8, the production of tumor-necrosis factor-α (TNFα), and infiltration of immune cells in the treated tumor. Necrotic and apoptotic tumor cells express heat-shock proteins (HSPs) and release tumor-associated antigens to DCs that migrate to lymph nodes to active immune system. Copyright from Springer Nature, 2006 [40]
Despite the immune responses induced by PDT alone, combination of PDT with immunological stimulators can further promote the immune responses. Posakony and colleagues characterized and elucidated the nature of the interaction of optimized protocols of adjuvant Bacillus Calmette–Gue’rin (BCG) immunotherapy with PDT [43]. They found that BCG does not improve the efficiency of PDT during the early phase of tumor ablation, but significantly enhance the efficiency in preventing tumor recurrence by activating the T cells. Marrach et al. encapsulated ZnPc which is a long-wavelength absorbing PS within poly(D,L-lactic-co-glycolic acid)-b-poly(ethylene glycol) (PLGA-b-PEG). Then, coated the outside of the polymeric core with gold NPs (AuNPs) and modified with CpG-ODN. The hybrid nanoparticles containing both ZnPc and CpG-ODN after irradiation with a 660 nm light showed obvious photocytotoxicity to 4T1 metastatic mouse breast carcinoma cells. It was found that the combination of PDT with a synergistic immunostimulant in a single NP system resulted in significant immune response, which could be used for the treatment of metastatic cancer [44].
Considering the immunosuppression, immune checkpoint blockade also can be used enhance the immune responses induced by PDT. Lin and colleagues reported a treatment strategy that combined PDT based on a new chlorin-based nanoscale metal−organic framework (nMOF), TBC-Hf, and a small-molecule immunotherapy agent that inhibited IDO, encapsulated in the nMOF channels to induce systemic antitumor immunity. They detected increased T cell infiltration in the tumor microenvironment after activation of the immune system with the combination of IDO inhibition by the small-molecule immunotherapy agent and immunogenic cell death induced by PDT (Fig. 5a) [45]. Thus, the in situ vaccination induced by PDT working together with IDO inhibitors effectively trigger strong antitumor immune responses. In their a following work, they developed Zn-pyrophosphate (ZnP) nanoparticles loaded with photosensitizer pyrolipid (ZnP@pyro), which could cause the apoptosis and necrosis of tumor cells under the light irradiation, thus improving the tumor immunogenicity (Fig. 5b). Moreover, the immunogenic ZnP@pyro-based PDT, particularly sensitizing tumor to anti-PD-L1 blockade, not only significantly eradicated the primary tumor but also prevented the tumor metastasis [46]. In another work, Wang et al. demonstrated that PDT-mediated cancer immunotherapy can be augmented by PD-L1 knockdown in cancer cells. They designed a versatile micelleplex by integrating an acid-activatable cationic micelle, photosensitizer (PS), and small interfering RNA (siRNA). Compared with PDT alone, the combination of PDT and PD-L1 KD showed significantly enhanced efficacy to inhibit tumor growth and metastasis in a B16-F10 melanoma tumor model [47].
Immunological responses triggered by nanoparticles-based photodynamic therapy in combination with immune checkpoint blockade. (a) The core–shell ZnP@pyro-based PDT treatment sensitizes tumors to anti-PD-L1 antibody, not only eradicating the primary 4 T1 breast tumor but also significantly preventing metastasis to the lung. (b) Chlorin-based nanoscale metal−organic framework (nMOF) loaded with a small-molecule IDO inhibitor for synergistic photodynamic and immunotherapy. Copyright from ACS, 2016 [46], ACS, 2016 [45]
The conventional photosensitizers for PDT are usually excited by visible light, which usually hampers the wide application of PDT owning to the limited tissue penetration depth. Many groups explored PDT using UCNPs, which are able to emit visible light under NIR light irradiation, showing much deeper tissue penetration compared to traditional visible light mediated PDT [21, 48]. Xu et al. utilized UCNPs to load chlorin e6(Ce6), a photosensitizer, and R837, a Toll-like-receptor-7 agonist (UCNP-Ce6-R837) simultaneously for NIR-laser triggered PDT (Fig. 6). Similarly, UCNP-Ce6-R837 based PDT would enable effective photodynamic destruction of tumors to generate tumor-associated antigens, which in the presence of R837 were able to promote strong antitumor immune responses. More importantly, in combination with CTLA-4 checkpoint blockade, this strategy not only showed excellent efficacy in eliminating the primary tumors with treatment, but also resulted in strong antitumor immunities to inhibit the growth of distant tumors without treatment. Furthermore, such strategy also could provide a strong immune memory function to prevent cancer recurrence [49].
Scheme showing the mechanism of combining NIR-mediated PDT with CTLA-4 checkpoint blockade for cancer immunotherapy. UCNP-Ce6-R837 nanoparticles under NIR light could enable effective photodynamic destruction of tumors and release tumor-associated antigens, which working together with adjuvant nanoparticles, inducing strong antitumor immune responses. With the help of a CTLA-4 checkpoint blockade, this strategy could effectively inhibit the tumor metastasis and recurrence. Copyright from ACS, 2017 [49]
In addition to the limited penetration depth of the irradiation light, hypoxia (low oxygenation) in the tumor sites is another important barrier of PDT, as oxygen is required to generate ROS to kill cancer cells [50]. Moreover, the hypoxic tumor microenvironment usually limits the effective functions of T cells and promotes immunosuppression by multiple immunosuppressive cells including Tregs, M2 tumor-associated macrophages (TAM), and myeloid-derived suppressor cells (MDSC) [51]. To overcome the hypoxia in the solid tumor, Yang et al. fabricated hollow mesoporous MnO2 nanoshells with PEG coating and photodynamic agent Ce6 and a chemotherapy drug doxorubicin (DOX) loading (H-MnO2-PEG/C&D), as a multifunctional theranostic platform that was responsive to tumor microenvironment and was able to modulate TME, for enhanced cancer combination chemo-PDT therapy [52]. The relieved tumor hypoxia by MnO2-triggered decomposition of endogenous H2O2 provided remarkable benefits not only for improving the efficacy of chemo-PDT, but also for reversing the immunosuppressive TME such as the polarization of tumor-associated macrophage to M1-phenotype to favor anti-tumor immunities post treatment. Further combination of with PD-L1 checkpoint blockade, such strategy offered an abscopal effect to inhibit the growth of not only primary tumors but also distant tumors without treatment owning to cytotoxic T cells (Fig. 7). Using a similar strategy, Lan et al. synthesized a nanoscale metal–organic framework (nMOF), consisting of Fe3O clusters and photosensitizer 5,10,15,20-tetra(p-benzoato)porphyrin (TBP) ligands. Under the light irradiation in hypoxic tumors, Fe3O clusters could induce the decomposition of endogenous H2O2 to produce O2 via the Fenton-like reaction. nMOF-based PDT induced anti-tumor immune responses, achieving synergistic therapeutic effect with PD-L1 checkpoint blockade [53].
Hollow MnO2 as a tumor-microenvironment responsive nano-platform for combination therapy favoring antitumor immune responses. (a) scheme showing the mechanism of anti-tumor immune responses induced by H-MnO2 complex in combination with anti-PD-L1 therapy. (b) The percentage of cytotoxic T lymphocytes (CTL) infiltrated in distant tumors. (c) The concentration of TNF-α in sera of mice after different treatments. Copyright from Springer Nature, 2017 [52]
4 Conclusion and Future Perspectives
Photo-immunotherapy has exhibited promising pre-clinical responses on different tumor models due to its unique superiorities including specific antitumor immunity and long-term immunological memory responses. As shown in this chapter, novel nanoparticles-enabled phototherapy could induce apoptotic and necrotic tumor cell death, which is different from most traditional cytotoxic agents that usually induce apoptotic tumor cell death. In case of necrosis, also called immunogenic cell death, cytoplasmic components spill over into extracellular space via the damaged plasma membrane and induce strong inflammatory responses, and the debris of tumor cells after phototherapy could act as tumor-associated antigens. The acute inflammation caused by phototherapy could further potentiate immune responses by attracting various immune cells and promoting the tumor-associated antigen presentation to the active cellular immune system. The immune responses induced by phototherapy can work synergistically with immune checkpoint blockade to improve the therapeutic outcomes with limited side effects.
Despite the progress achieved, more work is still needed to investigate the dynamic immune response and understand how phototherapy impacts the specific cellular aspects of antitumor immunity, with the aim of providing basic principles or guidance for combination of phototherapy and immunotherapy for an individual patient. Specifically, one point to be considered is to optimize phototherapy for inducing local tumor cures and producing inflammation to stimulate the immune system. Furthermore, it is also important to design suitable nanoparticle mediated phototherapy that is suitable to in combination with immune adjuvant or immune checkpoint blockade. The timing and dosing frequency of immune checkpoint blockade also are very important to the therapeutic efficacy and should be explored in more details.
References
Cheng, L., Wang, C., Feng, L., Yang, K., Liu, Z.: Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114, 10869–10939 (2014)
Bown, S.: Phototherapy of tumors. World J. Surg. 7, 700–709 (1983)
Xia, Y., et al.: Gold nanocages: from synthesis to theranostic applications. Acc. Chem. Res. 44, 914–924 (2011)
Nikoobakht, B., El-Sayed, M.A.: Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003)
Yang, K., Feng, L., Shi, X., Liu, Z.: Nano-graphene in biomedicine: theranostic applications. Chem. Soc. Rev. 42, 530–547 (2013)
Tian, Q., et al.: Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 23, 3542–3547 (2011)
Zhou, M., et al.: A chelator-free multifunctional [64Cu] CuS nanoparticle platform for simultaneous micro-PET/CT imaging and photothermal ablation therapy. J. Am. Chem. Soc. 132, 15351–15358 (2010)
Huang, X., et al.: Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 6, 28 (2011)
Cheng, L., et al.: PEGylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 26, 1886–1893 (2014)
Liu, T., et al.: Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mater. 26, 3433–3440 (2014)
Cheng, L., Yang, K., Chen, Q., Liu, Z.: Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano. 6, 5605–5613 (2012)
Yang, K., et al.: In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 24, 5586–5592 (2012)
Lovell, J.F., et al.: Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10, 324 (2011)
Cheng, L., et al.: PEGylated micelle nanoparticles encapsulating a non-fluorescent near-infrared organic dye as a safe and highly-effective photothermal agent for in vivo cancer therapy. Adv. Funct. Mater. 23, 5893–5902 (2013)
Nie, S.: Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 5, 523–528 (2010)
Jiang, F., et al.: Photodynamic therapy of U87 human glioma in nude rat using liposome-delivered photofrin. Lasers Surg. Med. 22, 74–80 (1998)
Son, K.J., et al.: Photosensitizing hollow nanocapsules for combination cancer therapy. Angew. Chem. 123, 12174–12177 (2011)
Banerjee, R., et al.: Nanomedicine: magnetic nanoparticles and their biomedical applications. Curr. Med. Chem. 17, 3120–3141 (2010)
Tu, H.L., et al.: In vitro studies of functionalized mesoporous silica nanoparticles for photodynamic therapy. Adv. Mater. 21, 172–177 (2009)
Gandra, N., et al.: Photosensitized singlet oxygen production upon two-photon excitation of single-walled carbon nanotubes and their functionalized analogues. J. Phys. Chem. C. 113, 5182–5185 (2009)
Wang, C., Tao, H., Cheng, L., Liu, Z.: Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 32, 6145–6154 (2011)
Duan, X., Chan, C., Lin, W.: Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. 58, 670–680 (2019)
Joffre, O., Nolte, M.A., Spörri, R., Sousa, C.R.E.: Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 (2009)
Kono, H., Rock, K.L.: How dying cells alert the immune system to danger. Nat. Rev. Immunol. 8, 279 (2008)
Scaffidi, P., Misteli, T., Bianchi, M.E.: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 418, 191 (2002)
Obeid, M., et al.: Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54 (2007)
Nestle, F.O., et al.: Vaccination of melanoma patients with peptide-or tumorlysate-pulsed dendritic cells. Nat. Med. 4, 328 (1998)
Pardoll, D.M.: The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 12, 252 (2012)
Chen, W.R., Adams, R.L., Carubelli, R., Nordquist, R.E.: Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer Lett. 115, 25–30 (1997)
Li, X., et al.: Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol. Ther. 10, 1081–1087 (2010)
Yata, T., et al.: DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 146, 136–145 (2017)
Zhou, F., et al.: Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials. 33, 3235–3242 (2012)
Tao, Y., Ju, E., Ren, J., Qu, X.: Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials. 35, 9963–9971 (2014)
Guo, L., et al.: Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 8, 5670–5681 (2014)
Han, Q., et al.: CpG loaded MoS 2 nanosheets as multifunctional agents for photothermal enhanced cancer immunotherapy. Nanoscale. 9, 5927–5934 (2017)
Li, L., et al.: An endogenous vaccine based on fluorophores and multivalent immunoadjuvants regulates tumor micro-environment for synergistic photothermal and immunotherapy. Theranostics. 8, 860 (2018)
Kumar, P., Srivastava, R.: IR 820 dye encapsulated in polycaprolactone glycol chitosan: Poloxamer blend nanoparticles for photo immunotherapy for breast cancer. Mater. Sci. Eng. C. 57, 321–327 (2015)
Wang, C., et al.: Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 26, 8154–8162 (2014)
Chen, Q., et al.: Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016)
Castano, A.P., Mroz, P., Hamblin, M.R.: Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 6, 535 (2006)
Cecic, I., Parkins, C.S., Korbelik, M.: Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 74, 712–720 (2001)
Gollnick, S.O., Vaughan, L., Henderson, B.W.: Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 62, 1604–1608 (2002)
Korbelik, M., Sun, J., Posakony, J.J.: Interaction between photodynamic therapy and BCG immunotherapy responsible for the reduced recurrence of treated mouse tumors. Photochem. Photobiol. 73, 403–409 (2001)
Marrache, S., et al.: Immune stimulating photoactive hybrid nanoparticles for metastatic breast cancer. Integr. Biol. 5, 215–223 (2012)
Lu, K., et al.: Chlorin-based nanoscale metal–organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc. 138, 12502–12510 (2016)
Duan, X., et al.: Photodynamic therapy mediated by nontoxic core–shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J. Am. Chem. Soc. 138, 16686–16695 (2016)
Wang, D., et al.: Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 16, 5503–5513 (2016)
Idris, N.M., et al.: In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 18, 1580 (2012)
Xu, J., et al.: Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 11, 4463–4474 (2017)
Chen, Q., et al.: Intelligent albumin–MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 28, 7129–7136 (2016)
Huang, Y., et al.: Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl. Acad. Sci. 109, 17561–17566 (2012)
Yang, G., et al.: Hollow MnO 2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 8, 902 (2017)
Lan, G., et al.: Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 140, 5670–5673 (2018)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, Q., Liu, Z. (2021). Nanoparticle-Based Phototherapy in Combination with Checkpoint Blockade for Cancer Immunotherapy. In: Vo-Dinh, T. (eds) Nanoparticle-Mediated Immunotherapy. Bioanalysis, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-030-78338-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-78338-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78337-2
Online ISBN: 978-3-030-78338-9
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)