Abstract
Metastatic outcomes and survival have always been challenges in the management of uveal melanoma (UM). Since a gene expression profile (GEP) test was validated for UM, patient prognosis and management changed significantly. GEP is independently the most robust prognostic test for patients with UM improving physician’s ability to stratify need for surveillance testing and enrollment in adjuvant clinical trials. Recently, the discovery of preferentially expressed melanoma antigen (PRAME) has increased the predictive prognostic power of GEP identifying patient at high risk for development of metastases. The clinical applications of GEP in clinical practice have evolved since its validation to assist early clinical diagnosis and management of patients with UM.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Historically, one of greatest challenges in the management of uveal melanoma (UM) has been management of metastatic disease. Because UM is the most prevalent primary malignant intraocular tumor in adults and carries a significant risk of metastases, which has shown to be mostly unresponsive to available systemic therapy [1], very little progress has been made over the years in improving survival. Thus, researchers have searched for prognostic indicators (initially clinical [2], then pathological [3], chromosomal [4], and finally genomic [5, 6]) to identify patients at increased risk for developing such metastasis in order to optimize surveillance testing and early treatment of metastatic disease. New insights on molecular pathways have shown multiple events to be dysregulated during the multistep process of oncogenesis indicating potential novel therapeutic approaches with promising clinical applications [7].
It has been shown that prognosis of UM can be most accurately predicted by genetic profiling of a fine-needle aspiration biopsy (FNAB) aspirate from the primary tumor before treatment. Currently, research is also looking at next-generation sequencing, single-cell sequencing, and ancestry to further enhance the identification of high-risk patients for clinical trials that may lead to target-based therapies for metastatic disease and adjuvant therapy which aims to prevent metastatic disease [8, 9].
Historical Relevance of Gene Expression Profiling in UM Prognosis
Conceptually, cancer is believed to develop from a series of genomic aberrations. Conversely, it remains unclear when these metastases determining aberrations occur in the process of tumor evolution [8]. Prognostic assessment of UM was historically inaccurate likely because this tumor’s evolution was poorly understood. Several chromosomal abnormalities in UM have been used for prognostication, including loss of 1p, 3, 6q, 8p, and 9p and gain of 1q, 6p, and 8q. Various techniques have been investigated to detect these changes, including standard karyotyping, fluorescence in situ hybridization (FISH), comparative genomic hybridization (CGH), spectral karyotyping, microsatellite analysis (MSA), multiplex ligation-dependent probe amplification (MLPA), and single-nucleotide polymorphisms (SNPs) [4, 10]. While loss of chromosomal heterozygosity (LOH) was identified in 63% of tumors, loss of one copy of chromosome 3 (monosomy 3 or LOH3) occurs in 52% of all UMs and has shown to be the most prognostically significant of these chromosomal markers [6, 10]. The importance of monosomy 3 alone was misrepresented although some clinicians started using it as a prognostic marker [11]. Although cytogenetic alterations afforded an important step toward the development of accurate prognostic markers for uveal melanoma, they hold significant drawbacks in using this information in routine clinical practice. These methods were developed from uveal melanomas’ specimens obtained from enucleation that yields large amounts of tumor tissue. However, about 90% of uveal melanomas are now managed by plaque brachytherapy and not by enucleation, in which case the only way to obtain tumor tissue without severely damaging the patient’s vision is by needle biopsy. Unfortunately, the amount of tumor material obtained by needle biopsy is often insufficient for chromosomal assay techniques [10, 12, 13]. Further problems with chromosomal prognostic testing include sampling error resulting from intratumoral heterogeneity and the complicated combination of chromosomal changes and clinicopathologic information that are needed to maximize prognostic accuracy [11].
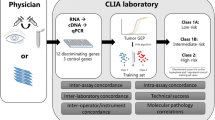
Clinical management of UM began to change with the discovery that molecular classification based on gene expression profiling (GEP) of the primary tumor was superior to monosomy 3 and clinicopathologic prognostic factors for predicting metastasis, and it is feasible even in small tumor aspirates [14]. GEP is strikingly different from chromosomal analysis because it provides a functional “snapshot” of the tumor’s microenvironment that is more consistent across the tumor [10, 15, 16]. The GEP test consists of reverse transcription (RT)-PCR-based assay comprising 12 discriminating genes and 3 control genes performed on a microfluidics platform used routinely in clinical practice on very small tumor samples from fine-needle aspiration biopsy (FNAB) [16]. GEP-based assignment of UMs to Class 1 (low risk for development of metastasis) or Class 2 (high risk for development of metastasis) was validated in a prospective, multicenter study [17] and is now routinely performed for clinical use in many centers [12, 18, 19].
As previously mentioned, GEP was developed to be used ideally on fresh tumor samples obtained from FNAB [20]. However, GEP can also be tested on formalin-fixed, paraffin-embedded tissue (Fig. 6.1) [18]. Once the genetic material is extracted, RNA samples quantified using the Nanodrop 1000 spectrophotometer are converted to cDNA using the High-Capacity cDNA Reverse Transcription kit. The technique used for GEP testing of UM has been described in great detail [10]. Separate from its prognostic value, GEP has provided critical insights into the pathobiology of UM. It has been shown that GEP of Class 1 tumors closely resembles that of normal uveal melanocytes and low-grade uveal melanocytic tumors, whereas GEP of Class 2 tumors shows reduced expression of melanocytic genes and instead resembles the transcriptome of primitive neural/ectodermal stem cells.
After the GEP test became available commercially, clinicians started using it routinely to determine the frequency of surveillance testing since clinical evidence confirmed that most of UM metastases occurred in patients with Class 2 tumors. However, a small number of Class 1 tumors were retrospectively identified to also develop delayed metastases. Further investigation based on a retrospective analysis of expression data from the 12-gene classifier on Class 1 tumors that metastasized revealed a subgrouping of Class 1 tumors into “1A” and “1B” based on the expression of two of these genes (CDH1 and RAB31). Class 1A tumors had low CDH1/RAB31 expression while Class 1B tumors had high expression. This subgrouping has been used as a provisional indicator of Class 1 patients who may be at increased risk of metastasis [18]. The further pursuit to recognize additional more accurate biomarkers for metastasis in Class 1 tumors led to a genome-wide integrated transcriptomic and chromosomal analysis in a cohort of Class 1 tumors. The cancer-testis-antigen PRAME (preferentially expressed antigen in melanoma) was shown to be a biomarker for increased metastatic risk in Class 1 tumors. This finding showed that PRAME provides additional discriminating power among Class 1 patients and provides a potential pathway for stratification of patients for clinical trials involving adjuvant and targeted therapies [19, 21].
Practical Clinical Application of GEP in UM
After the GEP assay was validated, it was transitioned from a high-density microarray platform to a 15-gene (quantitative) qPCR-based assay that is now performed in a College of American Pathologists (CAP)-accredited Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory routinely on tumor aspirates and on archival formalin-fixed specimens [18, 22].
Multiple groups have published their findings after the GEP test for uveal melanoma became commercially available [18, 19, 23]. The GEP assay remains the only prospectively validated tool that can be used for routine clinical prognostic testing of UM and for stratifying patients into high or low risk for development of metastasis [17]. The reliable results and accessible logistics have made the commercially available Decision-DX UM® (Castle Biosciences, Inc.) the most used prognostic test for uveal melanoma in the United States [22].
Because most patients with a class 2 tumor will develop detectable metastasis within 3 to 5 years after primary tumor diagnosis despite successful treatment of the primary tumor, enrolling these patients into clinical trials at the time of primary tumor diagnosis can hypothetically reduce the number of patients needed to treat and the length of follow-up needed to detect a difference in outcomes. The identification of BAP1 and other driver mutations as well as PRAME expression, all of which strongly associated with tumor prognosis, may soon lead to the discovery of new targeted therapies for clinical trials [9].
By and large, GEP is used to assess clinically diagnosed uveal melanoma at the time of (or immediately prior to) treatment to access individual risk for development of metastases and tailor surveillance testing in order to allow early detection of tumor spread and timely management as seen on Fig. 6.2. Patients with a Class 1 PRAME-negative tumor (or Class 1A) are recommended to have annual surveillance tests consisting of liver and lung imaging that may range from abdominal ultrasound and chest X-ray to CT of the chest and abdomen for at least 5 years. Patients with Class 1 PRAME-positive tumor (or Class 1B) should have biannual surveillance testing similar to other Class 1 patients also for at least 5 to 7 years. Patients with Class 2 tumor (independent of their PRAME status) are suggested to have surveillance imaging consisting of MRI of the abdomen every 3–4 months and chest X-ray every 6 months.
Curiously, clinicians started to use the test as a “diagnostic surrogate” to recommend treatment after the prospective validation of GEP. This unexpected use of the test was driven by the need ocular oncologists have to confirm early tumor diagnosis, the size overlap between small melanomas and large nevi, and the fact that GEP testing needs fewer cells than cytology to yield a conclusive test result [12, 24]. Despite personal clinical preferences, most clinicians follow a certain patter illustrated on Fig. 6.3. When an indeterminate uveal tumor is detected, it prompts 1 out of 3 options, observation for documented tumor progression, diagnostic FNAB to determine management, or treatment (that may concur with a prognostic FNAB). If FNAB is performed, cytology and GEP test may be obtained. If cytology is performed, it is used as the first diagnostic point. In cases which the FNAB yield renders an inconclusive cytology assessment (benign cells or insufficient cellular aspirate) or if cytology is not performed, GEP is solely used as a surrogate diagnostic test to recommend treatment. Patients with high-risk tumor (Class 2/PRAME positive or negative) and those with moderate risk (Class 1B and/or PRAME-positive) are treated promptly. Those with low risk (Class 1A/PRAME-negative) may or may not be treated depending on tumor location and risk for vision loss, patient age, and overall health. This information allows deferral of treatment and safe observation of patients for tumor progression and malignant transformation.
Clinical application of gene expression profiling (GEP) in clinically indeterminate uveal melanocytic lesion. In these cases, cytology and GEP (or GEP alone) are used to indicate tumor management. If cytology is performed, it is the first diagnostic point. If cytology is inconclusive or not performed, GEP is solely used as a surrogate diagnostic test to recommend treatment. *Until documented tumor growth prompts treatment or repeat biopsy
Future Clinical Applications of GEP in UM
Research into molecular prognostic testing in uveal melanoma continues to advance as new technologies are becoming available. Rigorous research is essential to guarantee accuracy and reproducibility of any assay. While there are other tests used currently around the world, the great variability in methodology and quality requires critical assessment to identify the most accurate, accessible, and cost-effective test to move research forward with clinical trials for high-risk patients in the adjuvant and metastatic settings. Similar to the standard for other forms of medical genetic testing, centralized testing facilities are necessary to achieve high quality-control standards and provide worldwide access to this technology [10]. Continued effort is pointing to new classes of compounds, including MEK, protein kinase C, histone deacetylase inhibitors, and more recently Lag 3 inhibitor that may be tested as adjuvant therapy for high-risk patients identified as Class 2, as well as in the setting of advanced disseminated disease [25]. Consensus in methodology and multi-institutional collaborations are critical to achieve these goals and provide reliable and timely progress in managing patients with uveal melanoma.
References
Augsburger JJ, Correa ZM, Shaikh AH. Quality of evidence about effectiveness of treatments for metastatic uveal melanoma. Trans Am Ophthalmol Soc. 2008;106:128–35; discussion 35-7.
Augsburger JJ, Gamel JW. Clinical prognostic factors in patients with posterior uveal malignant melanoma. Cancer. 1990;66(7):1596–600.
Augsburger JJ, Correa ZM, Trichopoulos N. Prognostic implications of cytopathologic classification of melanocytic uveal tumors evaluated by fine-needle aspiration biopsy. Arq Bras Oftalmol. 2013;76(2):72–9.
Correa ZM. Assessing prognosis in Uveal melanoma. Cancer Control. 2016;23(2):93–8.
Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012;47(3):246–53.
Harbour JW. Genomic, prognostic, and cell-signaling advances in uveal melanoma. Am Soc Clin Oncol Educ Book. 2013:388–91.
Cai L, Paez-Escamilla M, Walter SD, Tarlan B, Decatur CL, Perez BM, et al. Gene expression profiling and PRAME status versus tumor-node-metastasis staging for prognostication in uveal melanoma. Am J Ophthalmol. 2018;195:154–60.
Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun. 2018;9(1):116.
Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134(7):728–33.
Harbour JW. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile. Methods Mol Biol. 2014;1102:427–40.
Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin Cancer Res. 2010;16(24):6083–92.
Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):131–5.
Correa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol. 2016;162:20–7.e1.
Worley LA, Onken MD, Person E, Robirds D, Branson J, Char DH, et al. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res. 2007;13(5):1466–71.
Schopper VJ, Correa ZM. Clinical application of genetic testing for posterior uveal melanoma. Int J Retina Vitreous. 2016;2:4.
Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64(20):7205–9.
Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, et al. Collaborative ocular oncology group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–603.
Harbour JW, Chen R. The DecisionDx-UM gene expression profile test provides risk stratification and individualized patient care in uveal melanoma. PLoS Curr. 2013;5.
Schefler AC, Koca E, Bernicker EH, Correa ZM. Relationship between clinical features, GEP class, and PRAME expression in uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2019;257(7):1541–5.
Correa ZM, Augsburger JJ. Indications for fine needle aspiration biopsy of posterior segment intraocular tumors. Am J Ophthalmol. 2019;207:45–61.
Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22(5):1234–42.
Plasseraud KM, Cook RW, Tsai T, Shildkrot Y, Middlebrook B, Maetzold D, et al. Clinical performance and management outcomes with the DecisionDx-UM gene expression profile test in a prospective multicenter study. J Oncol. 2016;2016:5325762.
Demirci H, Niziol LM, Ozkurt Z, Slimani N, Ozgonul C, Liu T, et al. Do largest basal tumor diameter and the American joint committee on cancer’s cancer staging influence prognostication by gene expression profiling in choroidal melanoma. Am J Ophthalmol. 2018;195:83–92.
Augsburger JJ, Correa ZM, Trichopoulos N, Shaikh A. Size overlap between benign melanocytic choroidal nevi and choroidal malignant melanomas. Invest Ophthalmol Vis Sci. 2008;49(7):2823–8.
Kuznetsoff JN, Owens DA, Lopez A, Rodriguez DA, Chee NT, Kurtenbach S, et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol Cancer Res. 2020;19(2):215–22.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Correa, Z.M. (2021). Gene Expression Profiling in the Management of Uveal Melanoma. In: Bernicker, E.H. (eds) Uveal Melanoma. Springer, Cham. https://doi.org/10.1007/978-3-030-78117-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-78117-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78116-3
Online ISBN: 978-3-030-78117-0
eBook Packages: MedicineMedicine (R0)