Abstract
The management of malignant lung lesions is complex and requires a multidisciplinary approach. Patients who are medically inoperable owing to comorbidities and/or poor pulmonary function have limited therapeutic options. Many factors are considered in choosing the appropriate therapy for maximum benefit to the patient while minimizing adverse effects. Important factors include goal of treatment, tumor location, tumor proximity to nearby vasculature or critical organs, and prior therapies. Careful consideration of these factors guides the multidisciplinary team into choosing one of several minimally invasive treatment options available for medically inoperable patients. Brachytherapy is an option that has the ability to overcome several limitations posed by other treatment alternatives, and it has been shown to be safe and effective. Specifically, interest in interstitial brachytherapy for treating malignant lung lesions is growing, and the University of California Los Angeles (UCLA) is the first institution in the United States to perform CT-guided interstitial HDR brachytherapy in this setting. In this chapter, we describe our institutional experience with this treatment technique for patients with either primary or metastatic pulmonary malignancies. The benefits and potential pitfalls to consider with interstitial HDR brachytherapy, clinical outcomes, and future directions are highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The lungs are a common site for primary and metastatic malignancies. Primary lung cancer is the second most common malignancy diagnosed in the United States and is the leading cause of cancer-related mortality with 5-year survival rates of less than 25% [1]. Lung cancer comprises two distinct subtypes, with 85% of lung cancers being non-small-cell lung cancer (NSCLC) and 15% being small-cell lung cancer (SCLC) [2]. NSCLC is further divided into three main histological subtypes—adenocarcinoma, squamous cell carcinoma, and large-cell (undifferentiated) carcinoma—each associated with its unique clinical presentation. SCLC is a high-grade neuroendocrine tumor and differs from NSCLC in its rapid growth and early development of disseminated metastasis. The majority of lung cancers are diagnosed in locally advanced or metastatic stages. Additionally, approximately 20–54% of extra-thoracic malignancies metastasize to the lungs thus making management of malignant lung lesions a common scenario faced by patients and oncologists [3].
The indications for treating primary or metastatic lung malignancies are evolving. First, malignant lung lesions can cause debilitating symptoms (such as cough, dyspnoea, hemoptysis, and airway obstruction) that greatly impact the quality of life. Palliation for obstructive tumors is of great value in this setting [4]. Secondly, the concept of treating all known sites of disease in patients with low metastatic disease burden (an “oligometastatic state”) has been shown to prolong survival [5,6,7]. Combining local therapies with more effective systemic treatments, such as targeted therapies and immunotherapy , is increasingly being adopted into clinical practice for patients with oligometastatic disease. Therefore, locoregional therapy such as external-beam radiation or brachytherapy can remain of great value in the future, for both clinical and research purposes.
Lung Brachytherapy
Many patients with malignant lung lesions present with poor performance status or limited cardiopulmonary reserve, which precludes them from surgery. Alternative therapeutic options for medically inoperable patients are heterogeneous, and the choice of therapy is made on a case-by-case basis in a multidisciplinary setting. Brachytherapy is a minimally invasive therapy that can be used for malignant lung lesions. This therapy delivers high doses of radiation directly to the target tumor in a highly conformal manner. Depositing such tumoricidal radiation doses ensures high local control, while the steep dose drop-off minimizes exposure of nearby critical organs to radiation. Most reported experiences of brachytherapy in the United States have been in one of two settings.
One such setting is the treatment of endoluminal lesions by introducing a radioisotope through a catheter carrying a flexible bronchoscope. While endobronchial brachytherapy has been used in conjunction with external-beam radiotherapy (EBRT) to provide more curative treatments, it has primarily been used for palliation. Several collaborative groups, including the American Brachytherapy Society and the American College of Radiology, have recommended that endobronchial brachytherapy be used for palliation in patients with symptoms secondary to obstructive endobronchial tumors, especially if the patients were previously treated by EBRT [8,9,10]. Objective tumor response rates have ranged from 78 to 87%, while subjective relief from obstructive symptoms has been reported in 66–92% of patients [11, 12]. While combining EBRT with endobronchial brachytherapy may improve local tumor control, a recent Cochrane meta-analysis showed no advantage in disease-free or overall survival [8]. High-dose-rate (HDR) or pulse-dose-rate (PDR) is recommended over low-dose-rate (LDR) brachytherapy for endobronchial treatments [10].
The second common setting for lung brachytherapy is intraoperative or adjuvant treatment after surgery to address areas of potential local recurrence (from sub-lobar lung resections, incomplete resection, or close surgical margins) by implanting several LDR radioactive seeds. These radioactive seeds can be implanted at various locations throughout the thoracic cavity, either directly into residual tumor or near high-risk areas including the suture line in a grid or mesh pattern [10]. Various radioisotopes are available for LDR interstitial brachytherapy, including iodine-125 (125I), palladium-103 (103Pd), and caesium-131 (131Cs). All sources are gamma emitters, with 103Pd and 131Cs having half-lives slightly shorter than that of 125I. Radioisotopes with shorter half-lives deposit dose more rapidly, which can make the treatment of late radiation-responding tissues (such as the lung) more efficacious [13]. However, the decision to use a particular radioisotope must be balanced against the risk of depositing dose quickly in surrounding critical normal structures. Smaller institutional retrospective series demonstrated lower than expected local recurrence rates with the addition of intraoperative or adjuvant LDR brachytherapy [14,15,16]. However, the American College of Surgeons Oncology Group (ACOSOG) Z4032 phase 3 prospective randomized trial of sub-lobar resection with or without LDR interstitial brachytherapy showed no benefit in local control or overall survival with the addition of brachytherapy [17]. Two- and 3-year local control rates were respectively 12.3% and 12.3% with sub-lobar resection alone, and 9.3% and 12.0% with the addition of brachytherapy (p = 0.47 and p = 0.96) with a hazard ratio for local recurrence of 1.01 (p = 0.98). The local recurrence rate overall was lower than expected (7.7%) from the sub-lobar “resection only” group, and it was postulated that the study was underpowered for finding a difference in its primary endpoint of local recurrence. Therefore, interstitial LDR brachytherapy used either intraoperatively or adjuvantly is not routinely recommended in the United States except within the context of a clinical trial.
Outside the United States, there is growing experience with percutaneous interstitial brachytherapy for the management of malignant pulmonary lesions, with relatively more data on interstitial LDR brachytherapy than on HDR. In a recent meta-analysis that analyzed the safety and efficacy of 125I brachytherapy combined with chemotherapy in 296 patients with advanced lung cancer from five randomized clinical trials, the addition of 125I was found to be safe and did not significantly increase the incidences of adverse effects, with the exception of pneumothorax (RR = 4.93, 95% CI 1.94–12.55, p < 0.001) [18]. It was also associated with improved overall response rates (RR = 1.85, 95% CI 1.54–2.22, p < 0.001) and disease control rate (RR = 1.19, 95% CI 1.10–1.29, p < 0.001). There was no significant difference in 2-year overall survival (RR = 1.30, 95% CI 0.72–2.37, p = 0.39). Recent retrospective series have also shown that permanent 125I interstitial brachytherapy led to disease-free and overall survival similar to those obtained by microwave ablation or second-line chemotherapy for patients who had pulmonary disease progression from first-line chemotherapy treatments; additional prospective studies in its utility for interstitial LDR brachytherapy are warranted [19, 20].
CT-guided interstitial HDR brachytherapy is, compared with LDR brachytherapy, a relatively new technique for treating pulmonary lesions, and it is practiced at UCLA. Few institutions have expertise with this technique, so reported experiences are few. It was initially introduced as a novel method for treating hepatic malignancies, and over recent years interest in applying this technique for treating malignant lung lesions has grown [21]. This method involves implanting an applicator percutaneously under CT guidance directly into a tumor of interest, and treating the tumor with an iridium-192 (192Ir) source. This will be described in more detail in subsequent sections. Data on early outcomes suggest that interstitial HDR brachytherapy can achieve high local control with a favorable safety profile in treating pulmonary lesions [22,23,24,25]. A summary of contemporary studies of LDR and HDR interstitial brachytherapy is provided in Table 13.1.
One of the earliest experience with CT-guided interstitial HDR brachytherapy was published by Ricke et al. on the basis of a Phase I trial with 15 patients [22]. Thirty malignant lung lesions with mean tumor diameter of 2 cm (range 0.6–11 cm) were treated with at least 20 Gy in a single fraction administered to the tumor surface. After median follow-up of >5 months, local tumor control was 97%. With the exception of one patient who experienced nausea post-procedurally, no patients developed acute adverse events such as pneumothorax, hemoptysis, or abscesses. Another report from Peters et al. reported a 1-year local control rate of 91% after treating 30 patients with 83 primary and secondary lung malignancies to at least 20 Gy in a single fraction administered to the clinical target volume on a prospective, non-randomized trial [23]. One (2%) patient experienced a major pneumothorax requiring a chest tube placement for 24 hours. Moreover, six (12%) patients experienced minor pneumothorax (managed conservatively) and three (6%) patients had nausea after a median follow-up interval of 9 months. Tselis et al. also reported high 2- and 3-year local control rates in a retrospective review of 55 patients treated for 60 malignant lung lesions [24]. Unlike in the two trials described above, patients in this cohort received a multi-fractionated regimen to a median total dose of 20 Gy (range 7–32 Gy). Approximately half of the patients received twice daily treatment fractions of (median) 6 Gy per fraction, while the rest of the patients received several once daily fractions of (median) 8 Gy per fraction. After 14 months of follow-up, estimated 2- and 3-year local rates were both 82% for metastatic tumors. Estimated 2- and 3-year local control for primary/locally recurrent intrathoracic lesions was 79% and 73%, respectively. Relative tumor volume reduction was related to local control according to univariate analysis. Furthermore, pneumothorax occurred in 11.7% of procedures, but only one (1.8%) patient required post-procedural drainage.
Interventional Percutaneous Ablative Therapies
CT-guided interstitial HDR brachytherapy has the potential to overcome some limitations of other therapies for medically inoperable patients. One class of therapies comprises of image guided thermal ablations. These include radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation. RFA employs electromagnetic energy to create oscillating electric field lines which ultimately induce frictional heating in tissue. It is best utilized to ablate small, peripheral tumors, and can be performed in an outpatient setting. However, 2-year local control rates have been variable, ranging from ~15- 76%, and relapse rates tend to increase for tumors larger than 3 cm because of the inability to ablate to the tumor edge [26,27,28,29,30]. Tumors close to central lung, mediastinum, diaphragm, and vascular structures are also not ideal for thermal ablation. Moreover, vasculature reduces the efficacy of thermal ablation by the “heat-sink effect” or the loss of thermal energy through convection in the circulation [31].
MWA also uses an electromagnetic source, but at higher wave frequencies to excite and oscillate water molecules within the tissue around a probe in order to ablate tumors [32]. Unlike RFA, it is less susceptible to the heat-sink effect and is thus potentially able to treat lesions near vasculature more effectively than RFA. Similar to RFA, MWA is limited to treating smaller lesions and is associated with risk of pneumothorax, hemoptysis, and post-procedural pain. There are fewer reported data on the use of MWA for pulmonary lesions than on RFA, but 2- and 3-year local control rates were respectively 64% and 56% in one large retrospective series [33]. Recurrence rates would also increase with tumors larger than 3 cm in diameter.
Alternatively, cryoablation utilizes freeze-and-thaw cycles to ablate small lesions by inducing intra and extracellular ice crystals that disrupt cellular membrane and processes. This therapy is well suited for smaller (<3 cm) tumors. It can also be safely applied for centrally located tumors because cellular architecture and collagenous tissue are preserved, and compared with thermal ablative techniques, it entails a lower risk of toxicity to major airways or mediastinal organs. The 2-year local progression-free survival rate in one large retrospective series of 210 tumors was 69% [34]. Additional interventional techniques are available to treat malignant endoluminal tumors, though these will not be the focus of this chapter. While quite effective under certain circumstances in controlling local growth, interventional percutaneous therapies have been associated with complication rates of pneumothorax, pleural effusion , and hemoptysis, as compared with percutaneous interstitial brachytherapy [31, 35]. Pneumothorax rates have reported to range from 11 to 67%, and chest tube insertions for drainage were required for 6–29% of patients after interventional therapies [36, 37].
Available data on CT-guided HDR interstitial brachytherapy have shown consistently high local control rates irrespective of tumor volume, which is not often possible with image guided thermal ablation. One-year local control rates have been >90%, and 2-year control rates have been 79–82% [22,23,24]. Achieving high control rates for larger tumors is possible as several catheters can be implanted, and/or treatment time can be adjusted, to achieve optimal dosing. HDR brachytherapy also delivers the necessary tumoricidal doses in a non-homogenous manner, which is advantageous for boosting dose to intratumoral areas that may exhibit radioresistance from a hypoxic tumor microenvironment [38]. Furthermore, 3D computer-generated planning systems for radiation treatment allow radiation oncologists to optimize dose distribution before treatment delivery. Precise dose measurements cannot be performed during image guided thermal ablation as several factors cannot be accounted for at the time of delivery, including thermal conductivity, capacity, impedance, perfusion, and tissue inhomogeneity. The sharp dose fall-off in brachytherapy also makes it possible to manage pulmonary lesions, adjacent to major blood vessels and central/ultra-central regions. Finally, interstitial HDR brachytherapy has so far been associated with similar or lower rates of complications, especially of pneumothorax. While the exact mechanism of this trend is not entirely understood, it is hypothesized that it may be attributed to differences in biological effects between the two treatment methods. Cytotoxic effects from radiation often occur over weeks to months, which may cause less immediate structural changes, whereas interventional ablative techniques lead to instantaneous cell death and necrosis. With less immediate structural changes from radiation, there may be slower tissue reorganization, and this may mitigate the formation of air cavities and pneumothorax compared with interventional ablation methods [25, 39]. A summary of the use of image guided thermal ablative techniques is provided in Table 13.2.
Stereotactic Body Radiotherapy
Another class of therapies considered for malignant lung lesions is external-beam radiation. Stereotactic body radiotherapy (SBRT) is a specialized technique in which high doses of radiation are delivered to a target over five or fewer treatments. It is commonly employed for medically inoperable early-stage NSCLC and increasingly for oligometastatic pulmonary metastases. Much as in brachytherapy, the entirety of the tumor can be treated to tumoricidal doses in a highly conformal manner. Image guidance ensures reproducibility and accuracy during each treatment delivery. Unlike brachytherapy, SBRT treatment planning includes a safety margin of normal lung tissue around the target lesion to account for uncertainties in tumor localization from respiratory motion as well as systematic and random errors. Therefore, a relatively larger volume of lung tissue will be irradiated with external-beam radiation compared with brachytherapy.
Early studies evaluating SBRT in early-stage NSCLC and metastatic lung lesions demonstrated high local tumor control of >90% [40,41,42]. Long-term results of the RTOG 0236 study demonstrated that 5-year primary tumor failure was 7% [42]. However, disease progression outside the radiation field remained common and 5-year overall survival was 40%. Delivering higher dose per treatment confers an increased risk for long-term toxicities, including radiation pneumonitis, bronchial stenosis, hemorrhage, and respiratory failure, especially if radiation is directed toward central or ultra-central lesions that are close to critical structures including major airways, great vessels, and mediastinal structures (i.e., the heart, esophagus) [43,44,45,46]. In a phase 2 trial evaluating SBRT doses of 60–66 Gy in 3 fractions for early-stage NSCLC in medically inoperable patients, 2-year freedom from severe toxicity was 54% for patients with centrally located tumors compared with 83% for those with peripherally located tumors [44]. Since then, more acceptable toxicity rates have been found to be achievable by delivering the total radiation dose over a greater number (~7–12) of treatment fractions [47,48,49]. For example, NRG/RTOG 0813 was a dose-escalation trial for a 5-fraction SBRT regimen in central tumors to determine the maximum tolerated dose (MTD) that would yield a dose-limiting toxicity of <20%. The study concluded that the MTD was 12.0 Gy per fraction, and the associated probability of dose-limiting toxicity was 7.2% [50]. Treating central and especially ultra-central tumors remain challenging in the modern era. Several studies have reported severe and even fatal toxicities when SBRT was used to treat ultra-central pulmonary lesions, and special considerations for neighboring organs need to be taken into account during treatment planning [45, 46]. Interstitial HDR brachytherapy can be advantageous over SBRT in treating central and ultra-central tumors safely and effectively. Future dosimetric comparisons between SBRT and interstitial HDR brachytherapy would be of high interest.
Procedure
Interstitial Catheter Implantation
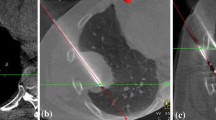
Interstitial catheter insertions are performed in collaboration with the Department of Interventional Radiology. Patients undergo a diagnostic chest CT. The tumor is located under CT guidance, and a mark is placed on the overlying skin by the interventional radiologist. The skin is sterilely prepared and local anesthetic (lidocaine 2% and bupivacaine 0.5%) is administered subcutaneously. A single 17-gauge coaxial introducer needle is inserted percutaneously through the marked location up to the pleura, where additional bupivacaine is injected. Serial CT scans are taken to confirm accurate needle trajectory as the needle tip is advanced to the distal edge of the lesion (Fig. 13.1). A single 4 Fr brachytherapy catheter is subsequently introduced through the needle sheath until its tip is coincident with the tip of the needle sheath (Fig. 13.2). Additional needles and catheters are introduced as needed to ensure adequate coverage and dosing of the tumor. Several catheter insertions are often considered when the tumor diameter is greater than 3 cm or near previously irradiated areas. The point at which the inner catheter leaves the outer coaxial introducer needle when their tips are coincident is marked on the catheter. The distances between the inner and outer Luer locks from the catheter and coaxial needle, respectively, are measured as well. These marks are used to confirm accurate placement of the brachytherapy catheter with respect to the coaxial needle during the treatment planning process. The brachytherapy catheters and coaxial needles are affixed to each other and to the patient’s skin with Mastisol liquid adhesive and Covidien or Transpore tape (Fig. 13.3).
The final position of a single 4 Fr brachytherapy catheter within percutaneous coaxial needle with both tips coincident with each other shown on the patient’s skin surface. A black mark is made on the brachytherapy catheter to ensure accurate placement during the treatment planning and delivery processes
Brachytherapy Planning and Treatment Delivery
Upon completion of catheter insertion, the patient undergoes a planning CT simulation scan using slice thickness of 2 mm. Acquired images are transferred to the treatment planning system (TPS). The radiation oncologist delineates the clinical target volume (CTV) on the CT planning simulation scan. The CTV includes the gross tumor volume (GTV) and suspicious areas shown on simulation or prior diagnostic chest scans. Critical nearby organs at risk (OAR) are contoured on each slice. The treatment catheter is reconstructed on the TPS. Inverse planning is utilized for a prescription dose to the CTV surface. Previous studies have shown significantly higher local tumor control when biologically effective doses (BED3) of greater than 100 Gy were delivered to the tumor [40]. A systematic review in 2013 also found that maintaining BED3 below 210 Gy, especially for centrally located tumors, would keep the risk of treatment-related death to 1.0% [48]. From our institutional experience, the median prescription dose was 21.5 Gy (range 15–27.5 Gy) in a single fraction, corresponding to median BED3 of 175.58 Gy. For a multi-fractionated regimen, the median dose prescribed was 24.75 Gy (range 24–25.5 Gy) in 2–3 fractions.
High target coverage was one of the primary goals during treatment planning and defined as 95% of the CTV to receive the full prescription dose (V100% ≥ 95%). Another dosimetric endpoint that was maximized when possible was the minimum dose that 90% of the tumor volume received (D90%). OAR dose tolerance limits outlined by AAPM Task Group 101 were given priority over treatment coverage [16]. The minimum dose to the most heavily irradiated 2 cc of OARs were also recorded. After plan approval and quality assurance checks, the patient is transported to the brachytherapy suite, where a 192Ir source is delivered using an HDR remote afterloader unit. Upon completing treatment, both the coaxial needle and brachytherapy catheter(s) are removed, with placement of a resorbable hydrogel to seal the pleural site of entry.
Clinical Cases
Case 1
A 51-year-old male with hepatocellular carcinoma secondary to hepatitis C treated by orthotopic liver transplantation presented to our clinic for possible brachytherapy for an enlarging lung metastasis. He developed several new lung nodules on surveillance CT chest in 2016, 3 years after his liver transplant. One dominant lung lesion located in the right middle lobe (RML) and in close proximity to the heart continued to enlarge throughout the year despite taking regorafenib.
During surveillance, the patient also developed chest pain due to myocardial infarction from stenosis in the mid-distal left anterior descending artery (LAD). Three bare metal stents were placed at the end of 2017. One month later, in 2018, he developed another stenosis in one of the diagonal branches of the LAD for which he underwent coronary angiography.
Meanwhile, the RML mass continued to enlarge, measuring 3.6 × 2.9 cm, and right pleural nodules consistent with pleural carcinomatosis developed (Fig. 13.4a). No other sites of metastatic disease in the abdomen or pelvis were noted. Aside from his recent myocardial infarction, the patient did not experience any pulmonary or airway-obstructive symptoms. Because systemic therapy options were limited for this patient in setting of his orthotopic liver transplant, a multi-disciplinary decision was made to switch systemic therapy to gain better systemic disease control while also to ablate the RML mass that had been refractory to prior systemic therapies. Given the tumor’s ultra-central location, large size, and the patient’s recent history of myocardial infractions, brachytherapy was recommended in a multi-disciplinary setting to treat this mass.
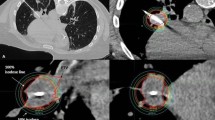
A 51-year-old male with metastatic hepatocellular carcinoma . (a) Ultra-central lesion located in the right middle lobe before interstitial HDR brachytherapy. This lesion measured 3.6 × 2.9 cm. (b) Resultant isodose distribution from treatment planning. The target lesion was 27.2 cc and 25 Gy dose in a single fraction was prescribed. The 100% isodose line is shown in light green, encompassing the entire CTV while avoiding the heart. (c) Solid and linear consolidation consistent with evolving response to radiation a year and a half after treatment. This lesion decreased in size to 2.3 × 2.3 cm, which was consistent with partial response according to the Response Evaluation Criteria in Solid Tumours (RECIST)
A single brachytherapy catheter was inserted into the tumor of interest during catheter implantation. The tumor was prescribed to 25 Gy in a single fraction. Figure 13.4b shows the isodose distribution of radiation treatment. The target volume measured 27.2 cc, and D90% = 101% (25.3 Gy). D2.0cc to normal ipsilateral lung (without CTV) was 193.7% (48.4 Gy). Likewise, D2.0cc of the heart was 20.0% (5 Gy). Serial follow-up CT scans of the chest with contrast were taken every 3 months. The nodule slowly decreased in size so that a year and a half after treatment the RML nodule measured 2.3 × 2.3 cm (Fig. 13.4c), which was consistent with partial response according to the Response Evaluation Criteria in Solid Tumours (RECIST).
The patient was followed every 3 months for a total of 15 months, and he did not experience any adverse sequelae of the radiation therapy. Unfortunately, he developed disease progression outside the irradiated area 3 months after brachytherapy treatment, and ultimately died 2 years later owing to respiratory failure from bilateral pleural effusions and multisystem organ failure. This case underscores the complexity of managing and palliating patients with metastatic disease. It also highlights the importance of a multi-disciplinary approach to management and the need for effective systemic therapies.
Case 2
A 65-year-old active smoker with medical history of early-stage renal cell carcinoma treated with nephrectomy and adrenalectomy developed multiple pulmonary metastasis soon after resection. Metastatic disease was temporarily controlled with pazopanib. Three years later disease progression was found, which manifested as increasing size of existing pulmonary nodules and a new left lower lobe interlobular septal thickening, raising concern of lymphangitic carcinomatosis. His systemic therapy was subsequently switched to nivolumab; and his pulmonary lesions remained stable for a year without additional sites of metastatic disease in the abdomen or pelvis. In light of the patient’s stable disease state, his favorable response to immunotherapy, and the limited number of available systemic therapy options should immunotherapy stop being effective, it was decided in a multi-disciplinary setting to treat some of the largest pulmonary lesions aggressively with local therapy. The patient did not present any obstructive airway symptoms. He was referred to our clinic for interstitial HDR brachytherapy to ablate four of the largest lesions with radiation in a staged approach over 6 months.
One ultra-central pulmonary lesion measuring 1.6 × 1.4 cm was located in the left lower lobe (LLL) and was adjacent to an enlarged basilar segmental lymph node measuring 1.7 × 1.5 cm. Given the close proximity to critical normal structures (such as the posterior pericardium, thoracic aorta, and basilar segmental arteries and bronchi), percutaneous HDR brachytherapy was recommended. A coaxial needle and a single brachytherapy catheter were introduced posteriorly in order to traverse the least amount of lung tissue (Fig. 13.5a). The patient underwent CT simulation, and the treatment planned was a prescribed dose of 21 Gy in a single fraction. Figure 13.5b shows the isodose curve of the final treatment plan. The target volume was 7.2 cc, and 95% of CTV met received full prescription dose (V100% = 95%) which met planning goals. D90% to the target was 122%. The treatment plan also met acceptable constraints on the dose to nearby OAR as well. D2.0cc to the aorta was 66.6% (14.0 Gy), D2.0cc to the heart/pericardium was 45.8% (9.6 Gy), and D2.0cc to normal ipsilateral lung was 179% (37.6 Gy). A representative dose-volume histogram (DVH) from this treatment is shown in Fig. 13.6a. Both coaxial needles and catheters were subsequently removed after treatment on the same day as implantation.
A 65-year-old male with metastatic renal cell carcinoma . (a) Coaxial needle placement into an ultra-central lesion located in the left lower lobe (LLL) adjacent to posterior pericardium, thoracic aorta, and basilar segmental arteries and bronchi. (b) Representative isodose distribution. The target lesion was 7.2 cc and prescribed to 21 Gy in single fraction to the CTV surface. The 95%, 63.5%, and 50% isodose lines are shown in magenta (overlaps with yellow isodose line representing 91.6%), green, and light blue, respectively. (c) Coaxial needle placed in an ultra-central lesion located just superior to the first lesion. (d) Resultant isodose distribution. The volume measured was 7.6 cc, and 21 Gy in a single fraction was prescribed. The isodose distribution color scheme from (b) also applies here. (e) Two ultra-central lesions abutting the pericardium in the LLL treated with SBRT. (f) Isodose distribution resulting from SBRT prescribed to 50 Gy in 5 fractions. The isodose distribution color schema in (b) also applies here. (g) Coaxial needle placement into a peripheral LLL lesion in a previously irradiated area. (h) Resultant isodose distribution. The target was prescribed to receive 26 Gy in a single fraction. The thick red line outlines the clinical target volume. The 200% and 100% isodose lines are shown as thin red and green lines, respectively
A month later, a second LLL lesion just superior to the first treatment area measuring 3.2 × 2.6 cm was treated with interstitial HDR brachytherapy. This lesion was also in an ultra-central location, and adjacent to the descending thoracic aorta, pericardium, and left lower segmental arteries and bronchi. The lesion was prescribed to receive 21 Gy in a single dose to the CTV surface. Final treatment showed high conformality around the target lesion (Fig. 13.5c, d). The target volume was 7.6 cc, and planning goals for the CTV were also achieved: specifically, D90% was 102.5% to the target, D2.0cc = 41.7% (8.8 Gy) to the aorta, D2.0cc = 49.1% (10.3 Gy) to the heart/pericardium, and D2.0cc = 125.8% (26.4 Gy) to the ipsilateral normal lung. There was a small overlap from the first irradiated field occurring over a high-dose irradiated region.
A third large LLL ultra-central lesion that was more anteriorly positioned was targeted for brachytherapy a month later. However, the presence of calcifications in this particular nodule made placement of both coaxial needle and catheter difficult, and brachytherapy was aborted. Given its ultra-central location and tumor size, SBRT was considered to be the next best alternative. This lesion measured 1.7 × 1.1 cm, and an adjacent lesion measuring 1.0 × 0.7 cm lay just posterior to it (Fig. 13.5e). Both lesions were targeted during SBRT to a total of 50 Gy in 5 fractions (Fig. 13.5f). The total volume irradiated was 45.5 cc, which included additional margins added to the GTV to account for uncertainties in target location from respiratory motion, systematic patient set-up error, and random errors. All institutional and AAPM Task Group 101 OAR constraints for a 5-fraction SBRT regimen were met, including the V32Gy to the heart (2.4 cc) and V12.5Gy of normal lung (227.3 cc) (Fig. 13.6b).
Two months after SBRT, chest CT revealed new increased irregular airspace attenuation along the inferior aspect of the previously treated basilar left lower lobe nodule that was of concern for new regional pulmonary metastasis. After an additional month of close follow-up, the multi-disciplinary team believed that this area represented a new pulmonary lesion, and brachytherapy was pursued in order to spare as much normal lung tissue as possible since this area had been irradiated in the two previous brachytherapy procedures and SBRT. During brachytherapy catheter implantation, the patient developed a moderate pneumothorax in the left lung, requiring a chest tube insertion for 24 h. Despite this complication, the position of the single brachytherapy catheter was reconfirmed on CT. 26 Gy dose in a single fraction was prescribed for this lesion (Fig. 13.5g, h). CTV planning goals were met. CTV D90% was 138%. Dose constraints to OARs were also met: specifically, D2.0cc of normal left lung was 81.67% (21.2 Gy) and D2.0cc of adjacent rib was 24.3% (6.3 Gy).
Overall, the patient tolerated brachytherapy and SBRT well. Apart from the moderate pneumothorax that developed during one brachytherapy catheter placement, he did not experience any acute or long-term radiation-associated toxicities during 20 months of follow-up. On his 18-month post-treatment CT chest scan, there was mass-like consolidation, architectural distortion, and parenchymal bands in the area of the LLL basilar region from his first two brachytherapy treatments that were consistent with radiation-associated changes (Fig. 13.7a). The lesion treated with SBRT had a complete response and was replaced by patchy ground-glass opacifications (Fig. 13.7b). Finally, the fourth LLL lesion treated with brachytherapy also exhibited radiographic complete response with minimal surrounding pulmonary changes. No additional sites of metastatic disease developed, and the patient continues to be progression-free on maintenance nivolumab.
Responses to radiation treatments 18 months after completion of radiation treatment. (a) Radiographic response following interstitial HDR brachytherapy treatments. There is a slowly evolving mass-like consolidation with architectural distortions and parenchymal bands consistent with radiation-associated changes. (b) Radiographic response after SBRT. The lesion is no longer seen and is replaced by patchy ground-glass opacifications
Case 3
A 38-year-old male was diagnosed with metastatic (yT3N1M1) rectal cancer. He underwent neoadjuvant chemoradiation to the pelvis and total mesorectal excision low anterior resection (TME-LAR) . He subsequently developed disease progression with several metastatic liver and pulmonary nodules, and has since been on various lines of systemic chemotherapy. Pulmonary nodules were managed with several cryoablations and microwave ablations. He then presented at Radiation Oncology 5 years after his initial diagnosis for palliative treatment for a pulmonary lesion detected on surveillance CT causing right upper lobe (RUL) posterior segment airway occlusion that led to bronchial stenosis (Fig. 13.8a). Radiographic findings were associated clinically with productive cough and night sweats for several weeks.
A 38-year-old male with widely metastatic rectal adenocarcinoma who first underwent palliative endobronchial brachytherapy . (a) Right upper lobe lesion causing posterior segment airway obstruction in the background of radiographic changes from prior cryoablation. (b) Isodose distribution of endobronchial brachytherapy treatment. A dose of 20 Gy in 5 fractions was prescribed for the region. The 50% isodose line is represented in light blue
The patient underwent bronchoscopy , which showed complete obstruction of the RUL posterior segment airway by tumor. After biopsies had been obtained, the obstructing mass was cryoresected and the airway was dilated. Pathology from biopsy specimens confirmed metastatic, moderately differentiated adenocarcinoma with mucinous features consistent with a colorectal primary. He was referred to Radiation Oncology to discuss palliative endobronchial HDR brachytherapy to minimize the risk of recurrence in this region. He was counseled about the limitations of this procedure and informed that it would not adequately treat the outer parenchymal component of the obstructing tumor. The patient expressed the wish to be as aggressive as possible with his treatment and desired to pursue endobronchial brachytherapy.
He began endobronchial brachytherapy treatment 2 weeks after his cryoresection. 20 Gy over 5 treatment fractions was administered to the post-cryoresection region in the RUL posterior segment airways. Figure 13.8b shows the resultant isodose lines from his last treatment fraction. Note that the 50% isodose line did not encompass the entire obstructing tumor, primarily at the superior and posterior tumor edges. The patient’s cough soon resolved and he had no further issues with breathing. Within a few weeks, he resumed walking 6 miles per day and was able to go on vacation without any issues.
Unfortunately, subsequent surveillance scans showed increase in size of the RUL mass (measuring 4.0 × 3.6 cm) with persistent obliteration of the subsegmental airway and increased obstruction of the anterior and superior subsegmental airways. The patient again expressed the wish for aggressive treatment to optimize his quality of life. In multi-disciplinary discussion, it was agreed to palliate this persistently enlarging RUL mass with interstitial brachytherapy, given its close proximity to the right bronchus that was previously irradiated.
The patient underwent CT-guided interstitial HDR brachytherapy 8 months after his endobronchial brachytherapy . Owing to the size of the tumor and its close proximity to a previously irradiated critical structure, three brachytherapy catheters were implanted into the RUL mass (Fig. 13.9a). He was prescribed 21 Gy in a single fraction. This dose was kept lower than what was considered needed to achieve adequate tumor control in order to minimize the risk of damage from re-irradiation of surrounding normal structures. Figure 13.9b, c show the isodose curves and DVH from this treatment. The CTV shown in red is the achieved planning target goal (V95% = 110%). The CTV D90% was 126.4%. D2.0cc of the previously irradiated right bronchi was 49.3% (10.4 Gy).
A 38-year-old male with widely metastatic rectal adenocarcinoma who underwent palliative interstitial HDR brachytherapy for local tumor progression after endobronchial brachytherapy. (a) Three coaxial needles, and therefore brachytherapy catheters, were implanted in right upper lobe lung metastasis. (b) Isodose distribution. The lesion was prescribed to 21 Gy in a single fraction. (c) Dose-volume histogram of the resulting treatment. Red represents the CTV, blue is the right bronchus, and green is the right normal lung
Six months after interstitial brachytherapy treatment, postradiation changes were noted in surveillance CT scans. During this period, the patient had developed transient chest pain and nonproductive cough, with radiographic evidence of new airspace consolidation of the middle and lower margins of the irradiated area consistent with postradiation pneumonitis (Fig. 13.10a). He was treated with prednisone and symptoms resolved within a few weeks. Approximately 1 year after the brachytherapy, the patient’s disease unfortunately progressed and he developed several new pulmonary and brain metastases. The RUL mass had continued to grow during this time and now extended to the right lower lobe and mediastinum. It was associated with satellite nodules extending into the right major fissure the right mainstem, upper and lower lobe airways (Fig. 13.10b). This case highlights the difficult decisions that must be made during palliative interstitial brachytherapy treatments to balance optimum tumor control while minimizing normal tissue injury, especially in a location near previously irradiated tissue.
Follow-up scans after palliative interstitial HDR brachytherapy for a 38-year-old male with widely metastatic rectal adenocarcinoma. (a) Transient development of airspace consolidation near the irradiated site occurring 3 months after treatment. Findings were associated with chest pain and non-productive cough, altogether consistent with radiation pneumonitis. The patient was treated with prednisone, and symptoms resolved in a few weeks. (b) Radiographic findings 1 year after brachytherapy treatment. CT chest scan demonstrated slow interval disease progression, now involving portions of the right lower lobe, right main-/upper/lower lobe airways, and mediastinum. There are associated satellite nodules extending into the right major fissure
Clinical Outcomes
At our institution, 37 malignant lung lesions from 25 patients were treated with CT-guided interstitial HDR brachytherapy from September 2015 to August 2019. Common lung histologies were renal cell carcinoma (24%), NSCLC (20%), and soft-tissue sarcoma (20%). Twenty (80%) patients had received at least one prior treatment for their malignant lung lesion, including systemic therapy (72%), interventional procedure(s) such as cryoablation (32%), or radiotherapy (24%). Five (20%) patients had not received any prior therapy. Of the 37 treated lesions, 22 (88%) were metastatic lesions, 2 (8%) were primary NSCLC, and 1 (4%) was locally recurrent NSCLC. Altogether, 78% of lesions were located in either an ultra-central or a central location. Twenty-two (88%) of patients received a single fraction to a median total dose of 21.5 Gy (range 15–26 Gy). For the three patients (12%) receiving multi-fraction radiation treatment, the median dose was 24 Gy (range 20–25.5 Gy) with a range of 2–5 fractions.
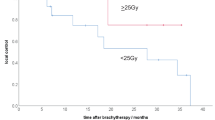
After median follow-up of 19 months (range 3–48 months), 3 (14%), 9 (41%), and 9 (41%) patients experienced respectively complete response, partial response, and stable disease on follow-up imaging. Only one patient developed local progression of a right upper lobe lesion, as detailed in the previous section (Case 3). Two- and 3-year local control rates were both 90% on a per-patient basis, and 96% on a per-lesion basis. 52% of patients developed systemic disease progression outside the irradiated area after treatment. 80% of the patients were alive at last follow-up, and 2- and 3-year overall survival rates were both 67%. Four patients developed grade 1 and 2 acute toxicities: specifically, two patients developed grade 2 pneumonitis treated with steroids, and 1 patient developed a pneumothorax during catheter implantation which required an overnight chest tube insertion. No patient developed late treatment-related toxicities. One patient with metastatic colorectal cancer experienced mild dyspnoea on exertion 5 months after brachytherapy treatment, but the etiology was attributed to be multi-factorial given his smoking history and prior treatments (several resections and microwave ablations) for lung metastases.
Future Directions
Treatment paradigms for primary NSCLC and metastatic disease arising from many different primary sites are rapidly evolving. As newer systemic agents demonstrate improved efficacy in controlling advanced-stage solid tumors, locoregional therapies such as CT-guided interstitial HDR brachytherapy could play an increasing role in the future for the management of malignant pulmonary lesions. Further, understanding how local therapy interplays with systemic therapies will become increasingly important and can inform strategic combinations of these.
The immune system is widely recognized as playing a central role in cancer development, progression, and treatment. In particular, there has been increasing interest in the role of radiation as an in situ vaccination for immune activation, with numerous preclinical and clinical studies highlighting the importance of anti-tumor lymphocytes [51]. Indeed, absolute lymphocyte count has been found to correlate with clinical outcomes (i.e., survival) across various histologies [51].
In addition to the growing body of literature that supports immune-activating functions of radiation, radiation can act as a double-edged sword to exert immunosuppression by several mechanisms, including depletion of lymphocytes by direct damage to DNA [52, 53]. Lymphocytes are known to be very radiosensitive, with doses as low as 1 Gy leading to destruction [53]. As a result, lymphocytes can be depleted by virtue of their transit through the vasculature of the irradiated field. This is of particular relevance in the context of the lung, where the neighboring heart circulates 100% of the total blood volume through the pulmonary vasculature [51, 54].
With increasing use of immunotherapy across various histologies in the up-front and salvage settings, lymphocyte preservation may be of increasing importance in optimizing the efficacy of immunotherapy [55, 56]. Numerous factors—such as lung V5, larger radiation portals, conventional fractionation, unintentional radiation to lymphoid organs, and the heart—can contribute to lymphopenia, suggesting that careful selection of radiation technique can help spare circulating lymphocytes [51, 54, 57,58,59,60].
Brachytherapy for lung lesions is well-positioned as a radiation modality to spare circulating lymphocytes, owing to its unique dosimetry, with sharp dose fall-off outside the target. There are limited studies evaluating brachytherapy compared with external-beam treatment, as it relates to lymphopenia, and studies by our group based on experience at UCLA are underway.
Conclusion
UCLA is one of the first institutions in the United States to gain experience with CT-guided interstitial HDR brachytherapy for the treatment and management of malignant lung lesions. Acquiring experience with this technique was made possible with the support of a multi-disciplinary team, including colleagues from interventional radiology, thoracic surgery, and medical oncology. Through this technique, we have been able to achieve high 2- and 3-year local tumor control rates of 96%, despite 78% of patients having central or ultra-central lesions. The majority of our patients did not develop any acute or late toxicities. Among those who developed acute toxicities, all adverse events were of grade 1–2 and self-limiting. Our experience with CT-guided interstitial HDR brachytherapy shows promising long-term safety and clinical efficacy. It can be an attractive treatment option to consider during the multi-disciplinary management of malignant lung lesions, especially for those in precarious locations close to critical organs or those that cannot be treated adequately with other alternative management options.
Key Points
-
Management of malignant lung lesions is complex and should be done in a multi-disciplinary setting.
-
There are several minimally invasive treatment options that are available for medically inoperable patients, but used under specific clinical conditions.
-
CT-guided interstitial HDR brachytherapy is safe and effective.
-
CT-guided interstitial brachytherapy should especially be considered for central or ultra-central lesions and for lesions located near previously irradiated areas.
-
The role of locoregional therapies such as brachytherapy will expand in the future as treatment paradigms for advanced-stage solid cancers evolve.
References
Howlander N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER cancer statistics review. https://seer.cancer.gov/csr/1975_2017/. Accessed 2020.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Mohammed TL, Chowdhry A, Reddy GP, et al. ACR Appropriateness Criteria® screening for pulmonary metastases. J Thorac Imaging. 2011;26(1):W1–3.
Skowronek J. Brachytherapy in the treatment of lung cancer—a valuable solution. J Contemp Brachytherapy. 2015;7(4):297–311.
Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–82.
Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37(18):1558–65.
Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–8.
Reveiz L, Rueda JR, Cardona AF. Palliative endobronchial brachytherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2012;12:Cd004284.
Rosenzweig KE, Chang JY, Chetty IJ, et al. ACR appropriateness criteria nonsurgical treatment for non-small-cell lung cancer: poor performance status or palliative intent. J Am Coll Radiol. 2013;10(9):654–64.
Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy. 2016;15(1):1–11.
Kelly JF, Delclos ME, Morice RC, Huaringa A, Allen PK, Komaki R. High-dose-rate endobronchial brachytherapy effectively palliates symptoms due to airway tumors: the 10-year M. D. Anderson cancer center experience. Int J Radiat Oncol Biol Phys. 2000;48(3):697–702.
Guarnaschelli JN, Jose BO. Palliative high-dose-rate endobronchial brachytherapy for recurrent carcinoma: the University of Louisville experience. J Palliat Med. 2010;13(8):981–9.
Armpilia CI, Dale RG, Coles IP, Jones B, Antipas V. The determination of radiobiologically optimized half-lives for radionuclides used in permanent brachytherapy implants. Int J Radiat Oncol Biol Phys. 2003;55(2):378–85.
Wernicke AG, Parikh A, Yondorf M, et al. Lung-conserving treatment of a pulmonary oligometastasis with a wedge resection and 131Cs brachytherapy. Brachytherapy. 2013;12(6):567–72.
Mutyala S, Stewart A, Khan AJ, et al. Permanent iodine-125 interstitial planar seed brachytherapy for close or positive margins for thoracic malignancies. Int J Radiat Oncol Biol Phys. 2010;76(4):1114–20.
Huo X, Wang H, Yang J, et al. Effectiveness and safety of CT-guided (125)I seed brachytherapy for postoperative locoregional recurrence in patients with non-small cell lung cancer. Brachytherapy. 2016;15(3):370–80.
Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Impact of brachytherapy on local recurrence rates after sublobar resection: results from ACOSOG Z4032 (Alliance), a phase III randomized trial for high-risk operable non-small-cell lung cancer. J Clin Oncol. 2014;32(23):2456–62.
Qiu H, Ji J, Shao Z, et al. The efficacy and safety of Iodine-125 brachytherapy combined with chemotherapy in treatment of advanced lung cancer: a meta-analysis. J Coll Physicians Surg Pak. 2017;27(4):237–45.
Wang Z-M, Lu J, Liu T, Chen K-M, Huang G, Liu F-J. CT-guided interstitial brachytherapy of inoperable non-small cell lung cancer. Lung Cancer. 2011;74(2):253–7.
Liu B, Wang Y, Tian S, Hertzanu Y, Zhao X, Li Y. Salvage treatment of NSCLC recurrence after first-line chemotherapy failure: Iodine-125 seed brachytherapy or microwave ablation? Thorac Cancer. 2020;11(3):697–703.
Ricke J, Wust P, Stohlmann A, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58(5):1496–505.
Ricke J, Wust P, Wieners G, et al. CT-guided interstitial single-fraction brachytherapy of lung tumors: phase I results of a novel technique. Chest. 2005;127(6):2237–42.
Peters N, Wieners G, Pech M, et al. CT-guided interstitial brachytherapy of primary and secondary lung malignancies: results of a prospective phase II trial. Strahlenther Onkol. 2008;184(6):296–301.
Tselis N, Ferentinos K, Kolotas C, et al. Computed tomography-guided interstitial high-dose-rate brachytherapy in the local treatment of primary and secondary intrathoracic malignancies. J Thorac Oncol. 2011;6(3):545–52.
Sharma DN, Rath GK, Thulkar S, Bahl A, Pandit S, Julka PK. Computerized tomography-guided percutaneous high-dose-rate interstitial brachytherapy for malignant lung lesions. J Cancer Res Ther. 2011;7(2):174–9.
Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491–8.
de Baère T, Aupérin A, Deschamps F, et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 2015;26(5):987–91.
Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol. 2008;15(6):1765–74.
Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastases: preliminary report. Radiology. 2004;230(1):125–34.
Huang L, Han Y, Zhao J, et al. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg. 2011;39(3):348–51.
Jones GC, Kehrer JD, Kahn J, et al. Primary treatment options for high-risk/medically inoperable early stage NSCLC patients. Clin Lung Cancer. 2015;16(6):413–30.
Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(Suppl 1):S69–83.
Healey TT, March BT, Baird G, Dupuy DE. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28(2):206–11.
Yashiro H, Nakatsuka S, Inoue M, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol. 2013;24(6):813–21.
Bi N, Shedden K, Zheng X, Kong F. Comparison of the effectiveness of radiofrequency ablation with stereotactic body radiation therapy in inoperable stage I non-small cell lung cancer: a systemic review and meta-analysis. Pract Radiat Oncol. 2013;3(2 Suppl 1):S19.
Hiraki T, Gobara H, Fujiwara H, et al. Lung cancer ablation: complications. Semin Interv Radiol. 2013;30(2):169–75.
Welch BT, Brinjikji W, Schmit GD, et al. A national analysis of the complications, cost, and mortality of percutaneous lung ablation. J Vasc Interv Radiol. 2015;26(6):787–91.
Ruggieri R, Naccarato S, Nahum AE. Severe hypofractionation: non-homogeneous tumour dose delivery can counteract tumour hypoxia. Acta Oncol (Stockholm, Sweden). 2010;49(8):1304–14.
Manning MA, Zwicker RD, Arthur DW, Arnfield M. Biologic treatment planning for high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):839–45.
Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100.
Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–6.
Timmerman RD, Hu C, Michalski J, et al. Long-term results of RTOG 0236: a phase II trial of stereotactic body radiation therapy (SBRT) in the treatment of patients with medically inoperable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):S30.
Nguyen KNB, Hause DJ, Novak J, Monjazeb AM, Daly ME. Tumor control and toxicity after SBRT for ultracentral, central, and paramediastinal lung tumors. Pract Radiat Oncol. 2019;9(2):e196–202.
Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–9.
Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with "ultracentral" non-small cell lung cancer. J Thorac Oncol. 2016;11(7):1081–9.
Sebastian NT, Xu-Welliver M, Williams TM. Stereotactic body radiation therapy (SBRT) for early stage non-small cell lung cancer (NSCLC): contemporary insights and advances. J Thorac Dis. 2018;10(Suppl 21):S2451–s2464.
Li Q, Swanick CW, Allen PK, et al. Stereotactic ablative radiotherapy (SABR) using 70 Gy in 10 fractions for non-small cell lung cancer: exploration of clinical indications. Radiother Oncol. 2014;112(2):256–61.
Senthi S, Haasbeek CJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol. 2013;106(3):276–82.
Murrell DH, Laba JM, Erickson A, Millman B, Palma DA, Louie AV. Stereotactic ablative radiotherapy for ultra-central lung tumors: prioritize target coverage or organs at risk? Radiat Oncol (London, England). 2018;13(1):57.
Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 2019;37(15):1316–25.
Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51.
Golden EB, Marciscano AE, Formenti SC. Radiation therapy and the in situ vaccination approach. Int J Radiat Oncol Biol Phys. 2020;108(4):891–8.
Sellins KS, Cohen JJ. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol (Baltimore, MD: 1950). 1987;139(10):3199–206.
Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89(5):1084–91.
Karantanos T, Karanika S, Seth B, Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol. 2019;21(2):206–12.
Pike LRG, Bang A, Mahal BA, et al. The impact of radiation therapy on lymphocyte count and survival in metastatic cancer patients receiving PD-1 immune checkpoint inhibitors. Int J Radiat Oncol Biol Phys. 2019;103(1):142–51.
Crocenzi T, Cottam B, Newell P, et al. A hypofractionated radiation regimen avoids the lymphopenia associated with neoadjuvant chemoradiation therapy of borderline resectable and locally advanced pancreatic adenocarcinoma. J Immunother Cancer. 2016;4:45.
Wu G, Baine MJ, Zhao N, Li S, Li X, Lin C. Lymphocyte-sparing effect of stereotactic body radiation therapy compared to conventional fractionated radiation therapy in patients with locally advanced pancreatic cancer. BMC Cancer. 2019;19(1):977.
Chen D, Patel RR, Verma V, et al. Interaction between lymphopenia, radiotherapy technique, dosimetry, and survival outcomes in lung cancer patients receiving combined immunotherapy and radiotherapy. Radiother Oncol. 2020;150:114–20.
Liu J, Zhao Q, Deng W, et al. Radiation-related lymphopenia is associated with spleen irradiation dose during radiotherapy in patients with hepatocellular carcinoma. Radiat Oncol (London, England). 2017;12(1):90.
Yue TH, Xing W. (125)I Seed brachytherapy combined with single-agent chemotherapy in the treatment of non-small-cell lung cancer in the elderly: a valuable solution. Onco Targets Ther. 2020;13:10581–91.
Wang H, Lu J, Zheng X-T, et al. Oligorecurrence non-small cell lung cancer after failure of first-line chemotherapy: computed tomography-guided (125)I seed implantation vs. second-line chemotherapy. Front Oncol. 2020;10:470.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yoon, S.M., Deng, J., Wong, K., Lee, A., Venkat, P., Chang, A.J. (2021). CT-Guided Interstitial HDR Brachytherapy for Malignant Lung Lesions: Experience from University of California Los Angeles. In: Mohnike, K., Ricke, J., Corradini, S. (eds) Manual on Image-Guided Brachytherapy of Inner Organs. Springer, Cham. https://doi.org/10.1007/978-3-030-78079-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-78079-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78078-4
Online ISBN: 978-3-030-78079-1
eBook Packages: MedicineMedicine (R0)