Abstract
Understanding the mechanism of radiofrequency (RF) can optimize pain management and patient selection, prevent complications, and help design clinical trials in pain medicine. This chapter reviews the principles of nerve damage, physics of RF types, various mechanisms of action proposed for the RF, and areas of need for future studies.
The two main mechanisms of RF are ablation and creation of the electromagnetic field. In thermal RF, we have a circuit: the RF electrode, the pads, and the patient’s tissue as the therapeutic target. RF-induced interactions lead to heat production, which causes coagulation necrosis and tissue destruction, thereby relieving pain or burning the painful nerve.
The pulsed RF, unlike CRF, is a nondestructive method that generates an electromagnetic field and modulates pain signal, gene expression (C-Fos, CGRP, ATF3), and neuroinflammation. PRF can also act on DRG or spinal cord cells, minimize microglial activity (and neurotransmitters like BDNF, PI3K, p-ERK), and enhance endogenous opioids and regenerative mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mechanism of action
- Ablation
- Neuromodulation
- Electromagnetic
- Pain signal
- Gene expression
- Regenerative medicine
Why Knowing the Mechanism of Radiofrequency Is Important?
-
Patient: Doctor! How do these RF waves help relieve my pain?
-
Doctor: Well, sometimes it destroys your nerves, and most of the time, it doesn’t!
-
Patient: So, how does it calm my pain?
-
Doctor: “What does not kill you, makes you stronger!”
Radiofrequency (RF) waves are commonly utilized for pain relief in patients. RF ablation, or rhizotomy, is a minimally invasive procedure in pain management. RF waves ablate the damaged nerves or modulate them, to stop the transmission of pain [1]. Understanding the underlying mechanism of RF (ablation- non-ablation) can assist physicians to enhance their pain management practice and also better inform their patients.
Since RF ablation involves an electrical device, electrodes, and frequencies in RF, we need to understand how they affect the patient’s pain in order to enhance and optimize pain treatment. This basic mechanism helps us prevent unnecessary damage or ablation to the nerves, to decrease complications. By knowing the mechanism of electromagnetic stimulation more precisely, we can better perform the patient selection for RF, which improves the pain management outcome [2].
This knowledge also helps us design clinical trials in pain management via RF and combination therapies (different types of RF, RF adjunct therapy, etc.). Since we have limitations in designing pain management trials, and the ablation is sometimes irreversible, the design of complex pain studies based on RF’s primary mechanism is immensely valuable.
Although several studies have been performed on RF ablation, there is no general overview of different aspects of RF ablation in the literature. Accordingly, this chapter aims to provide a comprehensive review of various aspects of RF ablation, including the underlying phenomena, fundamental mechanisms, and areas of need for future studies.
Before explaining the specific effects of RF in pain relief, we must first describe the neurological basis of nerve injury, the physics of RF, and then the physiology of pain.
Review of the Neurological Base of Nerve Injury
The nervous system is divided into peripheral and central systems, and neurons are its building blocks. Each neuron is comprised of a dendrite (receptor), a cell body (containing the nucleus), and an axon that leads to axonal terminals. The axon is surrounded by myelin, a lipoprotein, which speeds up impulse transmission along the axon. “Ranvier nodes,” located at intervals of the myelin membrane and along the axon, increase nerve conduction velocity (Fig. 2.1).
In addition to neurons, other supporting cells, such as microglia, oligodendroglia, and Schwann cells, play specific roles in the nervous system. Microglia is a cellular macrophage that becomes more activated in response to injury. Myelin is made by Schwann cells in the PNS and by oligodendrocytes in the CNS. Schwann cell myelinate each axon separately and plays a vital role in neuron regeneration.
Nerve fibers are divided according to their size as well as whether or not they have myelin [3]:
-
1.
A-alpha fibers: The largest nerve fiber, with 6–15 microns in diameter. They are myelinated, transmitting sense of touch, vibration, and position.
-
2.
A-delta fibers: small, with a size of 3–5 microns in diameter, transmitting the sense of cold and pain.
-
3.
C fibers: small, with a size of 0.5–2 microns, transmitting the sense of warmth and pain.
There are various nerve terminals with particular usage, including free nerve endings, Meissner’s Corpuscles, Pacinian Corpuscles, and Merkel’s disks. Pain terminals mainly contain C and A-delta.
In addition to axons and myelin, there are various membranes within the structure of a peripheral nerve [4]. These structures are in order from smallest to largest as follows (Fig. 2.2):
-
1.
Endoneurium: surrounds myelinated axons and groups of unmyelinated axons.
-
2.
Perineurium: surrounds the fascicles (a set of axons)
-
3.
Epineurium: the outermost layer that surrounds the nerve trunk
Nerve damage has a different prognosis depending on the injury’s location and can cause sensory damage or weakness. According to the Seddon classification described for the degree of damage to peripheral nerves, these injuries listed below range from mild to severe [5] (Fig. 2.3):
-
1.
Neurapraxia: The mildest damage, which is a focal demyelination, and the axon is temporarily nonfunctional, but without structural damage. The distal axon to damage is intact, and its continuity is maintained. Wallerian degeneration (degeneration of a nerve’s distal aspects after the injury to the cell body or proximal portion of the axon, anterograde or orthograde degeneration) did not occur, and recovery was excellent (about 3–6 months). Examples of neurapraxia are “Saturday night radial nerve palsy” and “leg-crossing peroneal nerve palsy.”
-
2.
Axonotmesis: Grade 2 damage, where both myelin and axons are damaged, but the endoneurium and perineurium remain intact. Complete peripheral degeneration occurs, but the sheath and its supporting connective tissues are spared. Fragmentation of the axon and its myelin sheath can be observed.
-
3.
Neurotmesis : Cutting, third-degree damage, which is a complete neural separation. The epineurium and most connective tissue are lost.
There is another classification for nerve damage by Sunderland that was done to better understand spontaneous regeneration [6]. Sunderland divided the axonotmesis into three subcategories: second, third, and fourth degrees of peripheral nerve injury (PNI).
-
Second-degree PNI: Axonal discontinuity occurs, but the endoneurium, fascicular arrangement, and perineurium remain intact.
-
Third-degree PNI: Myelin, axon, and endoneurium are disrupted, but fascicular arrangement and perineurium remain intact.
-
Fourth-degree PNI: Only the epineurium remains intact.
Physics of Radiofrequency
Electromagnetic (EM) spectrums are a continuous spectrum of frequencies. These waves are made up of a combination of electric and magnetic fields oriented at 90 ° to each other.
This spectrum includes radio waves, infrared radiation, the visible spectrum, ultraviolet radiation, x-rays, and gamma-rays in the increasing order of frequency. Radio waves are at the beginning of this spectrum and include a range of 3 Hz to 300 GHz.
All EM waves (including RF) have the same physics, but their effects on the target tissue vary depending on their frequency and type of tissue. This difference can be used to design several therapeutic frequencies in distinct target tissues (nerves, joints, intervertebral disc).
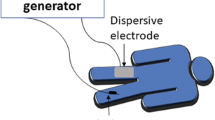
Overall, we need a circuit to apply RF ablation (Fig. 2.4). In this circuit, the RF electrode acts as a cathode, and the pads attached to the patient’s body act as an anode. The current as applied by the RF generator is transmitted from the cathode to the anode. The patient’s tissue is the therapeutic target, and subsequently, tissue conductivity in this circuit is crucial for energy transfer and ablation zone determination.
Circuit of RF. (Modified from Hong et al. [7])
We have a high-energy influx around the electrode’s tip due to its small cross section, and this energy is minimized as we move toward the pads. Therefore, most tissue damage has occurred around the cathode, and it is vital to select the appropriate location for the target.
In general, RF-induced interactions lead to heat production, which causes coagulation necrosis and tissue destruction, thereby relieving pain or burning the painful nerve [8]. Nevertheless, a few practical points in the RF mechanism are essential:
-
1.
Physics point of view: The RF electrode does not generate heat. The alternating EM field generated by the electrode creates an intense agitation in the adjusting molecules directly adjacent to the cathode. The molecules’ vibration also moves the next adjacent molecules in the direction of the applied RF current. Frictional energy lost in these molecules causes an increase in temperature and, consequently, coagulation necrosis in the tissue.
-
2.
The farther away from the RF cathode and the energy source, the less heat is generated in the molecules, and subsequently less tissue necrosis occurs (Fig. 2.5). Goldberg [9] formulates the amount of thermal lesion created by RF:
$$ \mathrm{Development}\ \mathrm{of}\ \mathrm{a}\ \mathrm{thermal}\ \mathrm{lesion}=\mathrm{inducedcoagulation}\ \mathrm{necrosis}=\left(\mathrm{energydeposited}\ast \mathrm{local}\ \mathrm{tissue}\ \mathrm{interactions}\right)\hbox{--} \mathrm{heat}\ \mathrm{loss}. $$ -
3.
In general, mammalian tissue is sensitive to heat. If heat is applied in a shorter time and with more intensity, more damage will be done. At 55 degrees, tissue destruction occurs in these tissues within 2 seconds, and at 100 degrees, evaporation and instantaneous death occur. At temperatures above 105 degrees, we will see boiling, evaporation, and carbonization.
RF cathode and its energy source. (Modified from Hong et al. [7])
If too much heat is applied to a tissue in a short time, it desiccates (becomes charred). Figure 2.5 shows the time needed for tissue death at various temperatures. Since the tissue adjacent to the electrode acts as the primary source of heat generation and transfer, it becomes a sleeve around the cathode and cannot transfer the generated energy if desiccated. This causes the ablation zone to become smaller, which is not desirable for treatment.
Therefore, in order to achieve a confident ablation zone, we must give the appropriate frequency at the desired time (e.g., raise each of the temperatures to 50–100 degrees, in 4–6 minutes).
Different Applications of Radiofrequency
There are several types of RF (thermal, pulsed, cooled), which will be discussed in more detail in the next chapter. However, in order to better understand the mechanism of action of RF types, we will give a brief explanation on how they work.
Thermal
In thermal (or conventional, continuous) RFA, a high-frequency current (500 kHz), creating a high temperature, leads to stimulation and ablation in the target tissue. Most CRFs use high temperatures of 60 C and 90 C for 90–120 seconds in clinical procedures, and we know that tissue destruction occurs at this temperature, which is the purpose of CRF [10, 11]. The severity of the lesion caused by CRF depends on the tissue temperature, the size of the electrode, and the length of time within which the procedure is performed.
In pain management, this heat causes a neurodestructive lesion in the small nerve and relieves the pain. The RF generator causes coagulation necrosis around the tip of the cannula by creating an alternating current [7]. The lesion is spherical, and its long axis is along the cannula tip. For this reason, the cannula must be parallel to the target nerve. Because the lesion is severely reduced by distance from the tip of the cannula [12], the lesions created by CRF are well circumscribed than other ablations (such as chemical neurolysis).
Pulsed
Pulsed RF, unlike CRF, is a nondestructive method that has been used extensively in pain management due to its minimizing nerve damage. Current in PRF is applied as high frequency but in short pulses, to the sensory nerve, joint, DRG, disc, etc. PRF pulses are given for a longer duration than continuous RFA, in repetitive intervals [1]. This generated electric field modulates pain signal, gene expression, and other relieving effects.
The PRF current is usually short (20 ms) and has a high-voltage burst (amplitude 45v), and then a silent phase (480 ms) occurs [13]. During the pulse, the oscillating frequency is 420 kHz. Intermittent pulses and long silent phase between pulses lead to heat reduction and keep the temperature below 42 degrees [14]. Consequently, tissue destruction does not occur, and complications such as neuritis, motor dysfunction, and deafferentation pain will be decreased [15, 16]. Although some mild damage around the PRF electrode has recently been reported, its effect is not clinically significant and detectable, and overall, PRF appears to be safe.
Cryoablation
Cooled radiofrequency ablation (CRFA) is a newer type of RFA that solves some of the problems of its predecessors, has a higher safety profile, and possesses long-term efficiency.
The difference between CRFA and other types of RFA (pulsed and thermal) is that it creates a larger local neuronal lesion [17]. Larger lesions increase the likelihood of successful treatment, especially if we have physiological variability of nerve location or complex innervation (like the knee).
But what is the mechanism of this difference in the size of the lesion? Traditional RFA probes operate at a set temperature of 80 degrees, and as described earlier, higher temperatures cause rapid burning of adjacent tissue and insufficient energy transfer to other tissues for larger ablation zones. However, in cooled RFA, water circulates about the RF probe and reduces its heat. Therefore, these internally cooled probes operate at 60 degrees set (20 degrees lower than traditional types), bringing the surrounding tissue heat to about 60 degrees. So, it causes more energy to be transferred in peripheral. The size of the lesion will be larger and deeper, and the pain relief will last longer [18].
Mechanism of Action of Radiofrequency
In this section, we describe the analgesic effects of different types of RF. It is noteworthy that despite numerous clinical studies on the effectiveness of RF types in pain management, the mechanism of action is still not generally agreed upon. This is especially true in the pulsed type.
Since the mechanisms proposed for RF in the treatment of pain are varied, we classified them based on the distinct factors for a better explanation. We also introduced the relevant gap of knowledge at the end of each section for further research.
Ablation Mechanism of Radiofrequency
Various chemical and physical methods (including thermal and electromagnetic) for ablation and resection/removal of innervation exist. In the thermal type, RF and cooled RF act mostly through the ablation mechanism, unlike pulsed RF, which leaves no damage or its destruction is negligible [1].
Nerve ablation disrupts axonal continuity. As a consequence of ablation, the distal nerve fibers to the lesion degenerate, a phenomenon called Wallerian degeneration . Wallerian degeneration causes a temporary interruption in a nerve cell, which causes a nociceptive block [19].
This nerve ablation only causes sensory or sympathetic degeneration, leaving no motor damage. According to the Sunderland classification, neural ablation causes third degree of peripheral nerve injury (PNI). In this type of injury, the axons, myelin, and endoneurium are damaged, but the rest of the neuron layers remain intact.
Nerve Regeneration and Pain Recurrence
Wallerian degeneration does not entirely interrupt the nerve cell, and it leaves the Schwann cell spared. Therefore, these Schwann cells allow the regeneration of axons in peripheral nerves. This nerve regeneration is suitable for patients with nerve damage, but in nerve ablation that we do in pain management, it is not desirable and causes the recurrence of pain that requires further procedures.
Nerve repair can begin very quickly after injury (30 minutes after). Its three main mechanisms are:
-
1.
Remyelination
-
2.
Sprout from the remaining healthy axons as lateral branches (especially in cases where less than 20% of the axons are damaged)
-
3.
Regeneration (especially in cases where more than 90% of axons are damaged) [20].
Schwann cells play a consequential role in nerve regeneration. They increase the synthesis of surface cell adhesion molecules (CAM) and prepare the basement membrane to regenerate. The NGF (nerve growth factor) receptors are increased on Schwann cells, causing sprouts and regeneration of axons [1].
Non-ablative Mechanisms of Radiofrequency
As mentioned, pulsed RF works in ways other than ablation. It has been shown that pain relief effect in thermal and pulsed RF in DRF stimulation is similar, without pulsed leaving a destructive lesion. Such studies have shown that the effect of pulsed RF is independent of the development of destructive lesions.
Table 2.1 described the non-ablative mechanism of RF.
Electromagnetic Fields
Most studies on the analgesic effect of PRF have focused on its neuromodulatory effect from its electromagnetic field [21]. PRF alters ynaptic transmission as well as neuron-specific gene expression thereby creating an alternating electrical field. The electromagnetic field created in PRF is a rapid electrical pulsation and has its intended biological effect on the target and the nerve [8, 22]. A popular theory for the mechanism of action of PRF is that it is a low electric field phenomenon that can induce long-term depression of synaptic transmission [23, 24].
Electromagnetic stimulation creates an electrical disruption for the transmission of sensory transitions, probably similar to the mechanism proposed in gate control theory [25]. This electric field disrupts the transmission of impulses in small, un-myelinated neurons, without destroying them. Interestingly, larger myelin-protected neurons remain unaffected.
Although pulsed-RF and continuous-RF follow basic physical principles, they differ in the space, time, and strength of the field that they create. PRF creates a stronger electric field than CRF, although the temperature generated and its destructive effect are far less. Tissue change by a strong electric field creates a more specific effect than heat energy. This electric field causes changes in tissue and charged molecular structures, causing them to distort, dislocate, and move [26].
Disrupt and Modulate Pain Signal Transmission Via Nerve Fibers
The electric field created by PRF around the sensory nerves can reduce the conduction of pain signals through the nerve fibers. PRF enhances various descending noradrenergic and serotonergic inhibitory pathways and performs its pain modulation [27]. In addition, electron microscopic studies show minor damage to the axonal microfilaments and the microtubules of pain-transmitting fibers after PRF. These changes were selectively observed, especially in smaller principal sensory neural fibers C and Ad, and less in larger non-pain-related sensory fibers, such as Aβ fibers [28]. The ultimate goal is to provide pain relief by selectively blocking the fibers that carry nociceptive signals from the joint or painful site.
For example, PRF can have several different analgesic effects [29]:
-
1.
Directly activate DRG or spinal cord cells
-
2.
Minimize microglial activity
-
3.
Enhancement of endogenous opioids, which inhibit the incoming nociceptive signal
-
4.
Inhibit the retrograde transport of neurotrophins in the posterior horn
The above items, as well as more theories, will be discussed later.
Microglia Activation
Microglia are macrophages of the central nervous system, which respond to pathological stimuli or anything disruptive of homeostasis [30]. After the damage to the nervous system, microglia are among the first cells to become activated and will remain so for several weeks. They switch to the active state with a series of cellular and molecular changes. These changes include morphological hypertrophy, proliferation, upregulated various genes, and increased expression of microglia characteristic markers, such as ionized calcium-binding adapter molecule 1 (Iba1) [31]. Considerable evidence has confirmed the critical role of spinal microglia in neuropathic pain. Behavioral pain responses are seen with a glial response at the dorsal horn [32].
By releasing various cytokines and chemokines involved in pain signaling, microglia play a substantial role in the development of chronic neuropathic pain, pain hypersensitization, and long-persistent pain [33]: Therefore, the downregulation of microglia can prevent the progression of chronic neuropathic pain. For example, intrathecal injection of microglia inhibitors has shown a significant impact on analgesic efficacy [34].
It has been reported that PRF application to DRG in rats with lumbar disc herniation may reduce microglia activity in the dorsal spinal horn [35]. Furthermore, the PRF application on DRG of rat models with neuropathic pain showed that the established mechanical hypersensitivity was relieved and the microglial activity in the spinal dorsal horn was strongly attenuated [36].
Mechanical allodynia and thermal hyperalgesia improved up to 14 days after a single PRF stimulation, associated with a significant reduction in Iba1 expression. PRF can suppress microglial activity, thereby creating nociceptive relief [37].
Gene Expression
One of the mechanisms of pulsed RF in pain management is its neuromodulatory effect, primarily through the alternation of gene expression, which will be described in the following items.
C-Fos
Neurophysiological studies have shown that PRF alters pain signaling at nerve synapses and induces electroporation [24]. RF-induced electromagnetic field alters C fiber transmission associated with greater c-Fos expression in the dorsal horn [38]. The c-Fos is an immediate-early gene used as an indirect marker of neuronal activity. C-Fos is most often expressed when neurons fire an action potential [39]. Increased expression of c-Fos has been suggested to activate some pain inhibitory mechanisms.
The formation and expression of c-Fos in the lamina during PRF treatment is one of its neuromodulation effects [23, 40]. In the study of Higuchi et al., pulsed RF was given to rat cervical DRG at 38 °C. Subsequently, c-Fos immunoreactivity in the superficial lamina [1 and 2] in the dorsal horn increased [41]. The formation of the c-Fos gene leads to the proliferation of a second messenger RNA and the production of a substance called pre-pro-dynorphin. The pre-pro-dynorphin belongs to the group of endogenous opioids and can increase endorphin production [42].
In confirmation of the above, it has been proven that antinociceptive effects are also applied in pulsed RF by enhancing pain inhibitory pathways . These pathways include the serotonergic, noradrenergic, and endogenous opioid pathways.
For more research: In most studies (such as Higuchi), elevated c-Fos was seen only in the pulsed-RF-treated group, not in animals treated with continued RF [41]; however, in some studies, this increase in c-Fos was seen in both CRF and PRF [43]. So, proving that this effect is only limited to pulsed RF or can be seen in continuous RF requires further study. In more recent studies, the causal relationship between the therapeutic effect of PRF and the increase in c-Fos has been questioned. More molecular evidence and more controlled studies are needed to prove this.
M-ENK
MET-encephalin is a peptide and neurotransmitter found in spinal cord neurons. M-ENK belongs to the endogenous opioid group, and its intravascular injection has not shown analgesic effects in either humans or rats [44]. It has generally been suggested that endogenous M-ENK expression is one of the mechanisms of the analgesic effect of RPF, similar to what occurs in spinal cord stimulation.
Various experiments in neuropathic pain have shown the analgesic effect of RPF on mechanical hypersensitivity, one of which is through the internal opioid pathways. A study in which PRF was applied to DRG revealed that the level of M-ENK in the dorsal horn of spinal cord was significantly elevated, indicating the effect of PRF on the CNS. At the same time, the mechanical threshold value in these rats had increased. This coincidence indicated that the application of PRF to the DRG reduces mechanical hypersensitivity, and it does so by modulating M-ENK expression in the dorsal horn in the spinal cord.
So, PRF could activate the endogenous analgesia system through nerve conduction in the spinal cord. This process increases the amount of M-ENK in the spinal cord to regulate nociceptive pain through synaptic mechanisms [45].
By the interaction of encephalin and opioid receptors on the cell surface, intracellular signal pathways are activated, an action which results in several conclusions: [1] Opioid receptors and membrane binding inhibitory channel are activated, and [2] opioid receptors connect to ion channels such as mu, delta, and kappa , which eventually inhibit neuronal excitability.
TNF-α, IL-6, IL-1
One of the mechanisms of PRF neuromodulation is by reducing the expression of proinflammatory cytokines, such as TNF-α, IL-6, and IL-1 . Proinflammatory cytokines are increased after nerve damage. For example, TNF-α has been shown to play a role as a pain modulation factor in the development and maintenance of neuropathic pain. TNF-α levels in the glial cell and nerve cell body also increase after chronic constriction injury (CCI)-induced neuropathic pain [46].
The properties of nerve roots in neuropathy are also closely related to cytokines such as TNF-α and COX-2. TNF-α induces the production of inflammatory neuropeptides (such as inflammatory neuropeptides) or increases their release from the dorsal horn [47].
The electric field generated in PRF, with its immunomodulatory effect, can alter immune cells and normalize the production of inflammatory cytokines [48]. In one study, 7 days after PRF stimulation on the spinal cord and sciatic nerve, the TNF-α immunoreactivity was decreased; additionally, mechanical allodynia and thermal hyperalgesia were improved. This study showed that PRF could relieve neuropathic pain by attenuating neuroinflammation at the molecular level [49].
It has also been shown that electric field therapy can induce the upregulation of adenosine A2A receptor density in human neutrophils. This upregulation appears to be associated with inhibition of catabolic cytokines such as TNF-α, IL-6, and IL-8 [49, 50]. The PRF electromagnetic field modulates and relieves pain neuroinflammatory conditions in two general ways:
-
1.
Decreased expression of proinflammatory cytokine genes, such as TNF-α, IL-6, and IL-1
-
2.
Increased expression of anti-inflammatory cytokine genes, such as GABAB-R1, Na/K ATPase, and 5-HT3r [2].
For future studies, it is suggested that the mechanism of injury-induced gene expression of neuroinflammatory conditions be investigated.
Calcitonin Gene-Related Peptide
One of the recently described ways for the analgesic effect of PRF is modulation in the expression of calcitonin gene-related peptide (CGRP) in the pain transmission pathway. CGRP is a 37-amino acid neuropeptide found in humans and rats [51]. CRGP plays a crucial role in transmitting synaptic pain information and uses two second-messenger pathways: protein-kinase-A along and protein-kinase-C. CGRP is also effective in creating and maintaining allodynia and hyperpathia [52].
CGRP is mainly synthesized in DRG, where primary sensory neurons are projected into the spinal dorsal horn. When peripheral nerve damage occurs, the spinal dorsal horn begins to release substances such as CGRP and P substance, which leads to the activation of glial cells; subsequently, several pain regulators are released, such as TNF-α, IL-6, and nerve growth factors involved in central sensitization [53].
It is suggested that PRF treatment can break this cycle by inhibiting CGRP expression, and this is one of the analgesic mechanisms of PRG. There is no definite consensus on CGRP changes in the nociception transduction pathway in neuropathic models; however, in most studies, after peripheral nerve injury, there is an increase in CGRP in the DRG, spinal cord, and its accumulation at the site of nerve injury [54, 55].
Because many DRG neurons begin to express CGRP after nerve damage, which is important in creating and maintaining pain behaviors, we can relieve pain by taking action against CGRP. In this regard, a new study has shown a decrease in expression CGRP in DRG after PRF application on the damaged sciatic nerve. In the study, after sciatic nerve ligation in rats, hyperalgesia and allodynia appeared, and CRGP mRNA and CRGP content in DRG increased. After PRF stimulation on DRG, ELISA, and RT-qPCR, studies showed that the proportion of CGRP-positive neurons in the DRG were reduced. This study showed that PRF could inhibit the transcription and translation of CGRP in the rat’s DRG, and this reduction in CGRP can alleviate pain behavior [52].
For further research: It is suggested that the role of the CGRP mechanism in post-PRF pain relief be investigated. The relationship between CGRP , pain behaviors, and PRF should be investigated in more follow-up studies.
ATF 3 (Is the Extended PRF Efficient?)
Activating translation factor 3 (ATF3) is a marker of cellular stress in various tissues. ATF 3 is used as a sensitive marker in neuronal response to injury, as well as in the neuropathy [56]. ATF 3 also increases in neurons and glial cells after axotomy. It is not expressed in healthy DRGs, but it is seen in axotomized DRG neurons [28]. As mentioned earlier, PRF is a nondestructive method and exerts its clinical effects through neuromodulation. Nevertheless, PRF application leads to ultrastructural changes in DRF cells as well as in sensory nociceptive axons. One of these changes is that by applying PRF to DRG, the amount of ATF 3 is upregulated. It is noteworthy that the increase was found only in small-diameter C and A-δ nociceptive fibers.
The story of ATF 3 is a bit different from the other markers mentioned earlier. So far, we have studied the mechanisms of pain relief after PRF. But in this section, we will answer these questions: according to molecular evidence and especially the amount of ATF3, does PRF application for a longer time provide more pain relief? If we stimulate the PRF for a longer time (e.g., 12 minutes), do we necessarily get a better therapeutic response than when we stimulate the PRF for a shorter time (e.g., 6 minutes)? Probably not!
A similar study was performed about extended PRF exposure times on mechanical allodynia in rats. First, an SNL nerve injury was created, and then PRF was applied to the DRG, after which antiallodynic effects were seen. Interestingly, the antiallodynic effects at 12-min PRF were not significantly different from 6-min PRF. On the other hand, the expression of ATF3 mRNA, as a marker for cell damage, was much higher in the 12-minute PRF than even the group without PRF treatment!
It was found that the amount of ATF3 mRNA was related to the PRF exposure time. Thus, the expression of ATF 3 in the naive group was very low, which indicates their intact neurons; however, the level of ATF3 mRNA in the sham group, PRF 6 minutes and PRF 12 minutes, was much higher. Finally, it was suggested that the extension of PRF exposure times did not increase the antiallodynic effect, but could also have neurolytic effects [57].
For further research : It is suggested that the optimum conditions for PRF treatment be determined, based on the molecular evidence and the mechanism of action of PRF. Also, further investigation of the side effects of PRF at the molecular level is suggested.
Neurotransmitter (BDNF, PI3K, and p-ERK)
In this section, we review three factors that are effective in the analgesic effect of PRF. These three substances, called neurotrophins , play their role as pain mediators/modulators:
-
1.
Brain-derived neurotrophic factor (BDNF)
-
2.
Phosphatidylinositol 3-kinase (PI3K)
-
3.
Phosphorylated extracellular signal-regulated kinase (p-ERK) [58].
By applying PRF to DRG in rats with neuropathic pain, the levels of these three substances (BDNF, PI3K, and p-ERK) are suppressed in the spinal cord.
Brain-derived neurotrophic factor (BDNF) is a secretory protein from the neurotrophin family. Neurotrophins are effective in the survival, growth, and differentiation of new neurons and synapses; however, after nerve damage, they have a devastating effect on the spinal cord. BDNF is associated with microglial neurons and is an important signaling molecule. It is involved in nociceptive processing in the spinal cord and pain processes in the peripheral and CNS. Nociceptor-derived BDNF is effective in inflammatory pain, and microglial-derived BDNF is effective in neuropathic pain [59].
Increased BDNF expression was found in the spinal cord after SNI. Enhanced BDNF was shown to induce nociceptive hypersensitivity, and inhibition of BDNF signal improved allodynia in rats with SNI. As Liu et al. reported, BDNF is effective in colitis-induced spinal central sensitization, and PI3K can mediate BDNF function in the spinal cord. PI3K can also enable p-ERK via second messengers’ pathways [60]. PI3K is a lipid kinase that acts as a membrane-embedded second messenger. The role of PI3K in refractory pain has been demonstrated. For example, plantar incision activates PI3K in the microglia, but inhibition of PI3K relieves pain-induced pain behaviors [61]. PI3K signaling has also been seen in bone cancer pain and also after SNI injury. PI3K-specific small-interfering RNA rat pain inhibited pain behaviors in bone cancer pain [62]. In that study, PI3K levels also increased after SNI injury.
ERK signaling pathway in microglia is involved in modulating different types of pain, and its inhibition can relieve pain. In microglia, ERK activity occurs after nerve damage, and inhibition of ERK can stop the spread of neuropathic pain. The p-ERK level was significantly upregulated after SNI. It was generally confirmed that microglia, BDNF, PI3K, and p-ERK are involved in developing chronic pain and pain sensitization. These substances are released in the spinal cord in a microglia-dependent manner.Since the application of PRF to DRG in rats with neuropathic pain reduces microglial activity, the studies concluded that PRF could regulate the release of BDNF, PI3K, and p-ERK in the spinal cord and subsequently reduce pain. After PRF application to ipsilateral DRG of the rats, mechanical allodynia and thermal hyperalgesia were reversed. In practice, this theory proved that the amount of these three substances decreased simultaneously after 6 minutes of PRF treatment for SNI.Therefore, it has been suggested that the application of PRF to DRG can reduce neuropathic pain by suppressing microglia and downregulated levels of BDNF, PI3K, and p-ERK in the spinal cord via microglia-dependent manner [37].
Excitatory Amino Acids (EAAs)
Another mechanism proposed for the analgesic effect of PRF is through inhibition of excitatory amino acids. Excitatory amino acids (EAAs) are essential neurotransmitters of the central and peripheral nervous systems, involved in modulating peripheral inflammation and the transmission of peripheral pain in the spinal cord [63]. Glutamate and aspartate are among the EEAs. The activation of glutamate receptors is involved in central hypersensitivity [64].
Inhibitory amino acids, including glycine and g-aminobutyric acid, also act to counteract the effects of EAAs, for example, by inhibiting nociceptive input and modulating the level of pain transmission [65]. The role of EEA , citrulline (a marker for nitric oxide synthesis), and glycine in thermal and tactile after peripheral inflammation, has been demonstrated [66].
It has been shown that applying PRF to DRG reduces mechanical allodynia, spinal EAAs (glutamate and aspartate), and citrulline concentration. PRF was able to reduce experimentally induced inflammatory pain with spinal dorsal horn modulation, suppress EAAs-citrulline release, and alter glutamate receptor activity [67].
Overall, given that some PRF target tissues do not have nerve tissue (such as intra-articular PRF), neurophysiological theories alone cannot suffice to find the analgesic mechanism of PRF, making the role of novel mechanisms , such as EAA, more critical.
Regenerative Mechanism
Another mechanism proposed for PRF, especially in the intra-articular type, is the cartilage-protective or regenerative mechanism. Laboratory studies show that electrical stimulation can increase chondrocyte proliferation and matrix synthesis [68]. A study by Fini et al. suggested that pulsed electric fields have several effects, including an anabolic effect on chondrocytes, catabolic cytokine blockage, and inhibition of inflammatory processes in osteoarthritis [69]. These studies need further investigation in vivo.
A review article in 2019 examines the effects of electromagnetic fields on cartilage. In vivo, research has shown that EM can protect the chondrocyte form, increase DNA synthesis, and increase GAG proliferation and synthesis in human cartilage. In vivo studies have also shown that the EM field can improve osteoarthritis, increase PG synthesis, and counteract catabolic activity [70].
Overall, it has been shown that EM stimulation can preserve articular cartilage morphology, improve joint mobility, and reduce joint pain.
For further studies: Research on the mechanism of action of PRF may need to be more focused on other cell lines, such as the joint, cartilage, and bone. It should also not be limited to the pathways of pain transmission.
IGF-2
Insulin-like growth factor 2 (IGF2) is a protein involved in prenatal growth and development and the growth and proliferation of various tissue cells [71]. The role of IGF 2 in pain has not been confirmed but is being investigated as a new target in nerve injury-induced pathological pain [72].
PRF , which is applied immediately after nerve injury, has been shown to have a more significant inhibitory effect of mechanical allodynia than delayed PRF (14 days after injury). This effect of immediate PRF is achieved through the downregulation of IGF 2 and reduction of phosphorylation of ERK1/2. This reduction is mainly in microglial cells in the spinal dorsal horn [73].
Therefore, further study to determine the time to optimize RF using IGF 2 is recommended.
Cellular and Histological Changes in RA
In an animal study, by applying continuous RF at 67 °C to DRG, changes such as mitochondrial degeneration and the loss of nuclear membrane integrity were observed. These changes were not seen in PRF [74]. In another study, which performed continuous RF and PRF at 42 °C, no significant structural changes were seen except for transient endoneural edema and collagen deposition [75].
The research on axonal ultrastructural changes after PRF has shown changes in mitochondrial membranes and appearance, as well as disorganized microfilaments and microtubules [76]. Another similar study in PRF for 120 seconds showed just separation in myelin configuration in damaged myelinated axons [15]. These histological changes in PRF are probably due to the high transmembrane potentials generated and the tissue being exposed to electrical current.
In general, by calculating the electric field generated, and in vitro studies, PRF has been shown to cause definite tissue changes. These changes can also relieve neuropathic pain in animal models in vivo [23]. In addition to the histological and ultrastructural axonal findings that occur after the PRF application, there is a convincing biochemical basis for PRF mechanisms, which has been described in earlier sections.
Concluding Remarks
In this chapter, with a review of the principles of nerve damage and the physics of RF types, we described the various mechanisms of action proposed for RF. The two main mechanisms of RF are ablation and creation of the electromagnetic field. These therapeutic effects were mediated by neuronal modulation in pain signal transmission via nerve fibers, changes in gene expression, and changes in cytokines and neurotransmitters. The direct and indirect effects of RF on nerve fibers, microglia, and chondrocytes were also discussed.
In the decades since the application of RF in pain management, much research has focused on determining the outcome of RF in various areas, rather than finding its mechanism of action. Many advances have been made in basic science and pain-related translation research. It is clear that using this new knowledge window has enabled novel researches to determine the accurate mechanism of action of RF easier and more possible. We can do a more optimized patient selection, approach selection, and pain management via these findings. For these goals, in addition to the research questions posed in each section, longitudinal studies with longer follow-up, as well as closer contact of pain physician with pain scientists and researchers, are recommended.
References
Choi EJ, Choi YM, Jang EJ, Kim JY, Kim TK, Kim KH. Neural ablation and regeneration in pain practice. Korean J Pain. 2016;29(1):3–11.
Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013;16(5):E601–13.
Varga I, Mravec B. Chapter 8 - Nerve Fiber Types. In: Tubbs RS, Rizk E, Shoja MM, Loukas M, Barbaro N, Spinner RJ, editors. Nerves and nerve injuries. San Diego: Academic Press; 2015. p. 107–13.
Reina MA, Sala-Blanch X, Arriazu R, Machés F. Chapter 7 - microscopic morphology and ultrastructure of human peripheral nerves. In: Tubbs RS, Rizk E, Shoja MM, Loukas M, Barbaro N, Spinner RJ, editors. Nerves and nerve injuries. San Diego: Academic Press; 2015. p. 91–106.
Biso GMNR, Munakomi S. Neuroanatomy, Neurapraxia. StatPearls Publishing, Treasure Island; 2020.
Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(4):491–516.
Hong K, Georgiades C. Radiofrequency ablation: mechanism of action and devices. J Vasc Interv Radiol. 2010;21(8 Suppl):S179–86.
Martin DC, Willis ML, Mullinax LA, Clarke NL, Homburger JA, Berger IH. Pulsed radiofrequency application in the treatment of chronic pain. Pain Pract. 2007;7(1):31–5.
Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174(2):323–31.
Shanthanna H, Chan P, McChesney J, Paul J, Thabane L. Assessing the effectiveness of ‘pulse radiofrequency treatment of dorsal root ganglion’ in patients with chronic lumbar radicular pain: study protocol for a randomized control trial. Trials. 2012;13(1):52.
Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12(1):37–41.
Filippiadis D, Velonakis G, Mazioti A, Konstantos C, Brountzos E, Kelekis N, et al. Intra-articular application of pulsed radiofrequency combined with viscosupplementation for improvement of knee osteoarthritis symptoms: a single centre prospective study. Int J Hyperth. 2018;34(8):1265–9.
Mata J, Valentí P, Hernández B, Mir B, Aguilar JL. Study protocol for a randomised controlled trial of ultrasound-guided pulsed radiofrequency of the genicular nerves in the treatment of patients with osteoarthritis knee pain. BMJ Open. 2017;7(11):e016377.
Ding Y, Li H, Hong T, Zhao R, Yao P, Zhao G. Efficacy and safety of computed tomography-guided pulsed radiofrequency modulation of thoracic dorsal root ganglion on herpes zoster neuralgia. Neuromodulation. 2019;22(1):108–14.
Tun K, Cemil B, Gurcay AG, Kaptanoglu E, Sargon MF, Tekdemir I, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72(5):496–500; discussion 1
Abbott Z, Smuck M, Haig A, Sagher O. Irreversible spinal nerve injury from dorsal ramus radiofrequency Neurotomy: a case report. Arch Phys Med Rehabil. 2007;88(10):1350–2.
Malik K, Benzon H, Walega D. Water-cooled radiofrequency: a Neuroablative or a neuromodulatory modality with broader applications? Case Rep Anesthesiol. 2011;2011:263101.
Oladeji LO, Cook JL. Cooled radio frequency ablation for the treatment of osteoarthritis-related knee pain: evidence, indications, and outcomes. J Knee Surg. 2019;32(1):65–71.
Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–79.
Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119(9):1951–65.
Abejón D, Reig E. Is pulsed radiofrequency a neuromodulation technique? Neuromodul Technol Neural Interface. 2003;6(1):1–3.
Sluijter ME, van Kleef M. Pulsed radiofrequency. Pain Med. 2007;8(4):388–9. author reply 90-1
Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir. 2011;153(4):763–71.
Cosman ER Jr, Cosman ER Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005;6(6):405–24.
Bartsch T, Goadsby PJ. Central mechanisms of peripheral nerve stimulation in headache disorders. Prog Neurol Surg. 2011;24:16–26.
Colini-Baldeschi G. Evaluation of pulsed radiofrequency denervation in the treatment of chronic facetjoint pain: an observational study. Anesth Pain Med. 2012;1(3):168–73.
Hagiwara S, Iwasaka H, Takeshima N, Noguchi T. Mechanisms of analgesic action of pulsed radiofrequency on adjuvant-induced pain in the rat: roles of descending adrenergic and serotonergic systems. Eur J Pain. 2009;13(3):249–52.
Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain (London, England). 2006;10:171–6.
Yao P, Hong T, Zhu Y-q, Li H-x, Wang Z-b, Ding Y-y, et al. Efficacy and safety of continuous radiofrequency thermocoagulation plus pulsed radiofrequency for treatment of V1 trigeminal neuralgia: a prospective cohort study. Medicine. 2016;95(44):e5247.
Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10(4):217–24.
Masuda T, Tsuda M, Yoshinaga R, Tozaki-Saitoh H, Ozato K, Tamura T, et al. IRF8 is a critical transcription factor for transforming microglia into a reactive phenotype. Cell Rep. 2012;1(4):334–40.
Chen G, Zhang Y-Q, Qadri YJ, Serhan CN, Ji R-R. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100(6):1292–311.
Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716(1-3):106–19.
Fang H, Zhang H-H, Yang B-X, Huang J-L, Shun J-L, Kong F-J, et al. Cdk5 contributes to inflammation-induced thermal hyperalgesia mediated by the p38 MAPK pathway in microglia. Brain Res. 1619;2015:166–75.
Cho HK, Cho YW, Kim EH, Sluijter ME, Hwang SJ, Ahn SH. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: laboratory investigation. J Neurosurg Spine. 2013;19(2):256–63.
Park H-W, Ahn S-H, Son J-Y, Kim S-J, Hwang S, Cho Y-W, et al. Pulsed radiofrequency application reduced mechanical hypersensitivity and microglial expression in neuropathic pain model. Pain Med (Malden, Mass). 2012;13:1227–34.
Xu X, Fu S, Shi X, Liu R. Microglial BDNF, PI3K, and p-ERK in the spinal cord are suppressed by pulsed radiofrequency on dorsal root ganglion to ease SNI-induced neuropathic pain in rats. Pain Res Manag. 2019;2019:1–15.
Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;10(2):171–6.
Bogduk N. Pulsed radiofrequency. Pain Med. 2006;7(5):396–407.
Imani F. Using pulsed radiofrequency for chronic pain. Anesth Pain Med. 2012;1(3):155–6.
Higuchi Y, Nashold BS Jr, Sluijter M, Cosman E, Pearlstein RD. Exposure of the dorsal root ganglion in rats to pulsed radiofrequency currents activates dorsal horn lamina I and II neurons. Neurosurgery. 2002;50(4):850–5; discussion 6
Fipp J, Louw A, Joosten E, Kessels A, Honig W, Dederen PJWC, et al. Pulsed and Continous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology. 2005;102:125–31.
Van Zundert J, de Louw AJ, Joosten EA, Kessels AG, Honig W, Dederen PJ, et al. Pulsed and continuous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology. 2005;102(1):125–31.
Li Y, Lefever MR, Muthu D, Bidlack JM, Bilsky EJ, Polt R. Opioid glycopeptide analgesics derived from endogenous enkephalins and endorphins. Future Med Chem. 2012;4(2):205–26.
Wu B, Ni J, Zhang C, Fu P, Yue J, Yang L. Changes in spinal cord met-enkephalin levels and mechanical threshold values of pain after pulsed radio frequency in a spared nerve injury rat model. Neurol Res. 2012;34(4):408–14.
Leung L, Cahill CM. TNF-alpha and neuropathic pain--a review. J Neuroinflammation. 2010;7:27.
Cho H, Kang J, Kim S-Y, Choi M-J, Hwang S, Cho Y-W, et al. Changes in neuroglial activity in multiple spinal segments after caudal epidural pulsed radiofrequency in a rat model of lumbar disc herniation. Pain Phys. 2016;19:E1197–E209.
Chua N, Vissers K, Sluijter M. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications – a review. Acta Neurochir. 2010;153:763–71.
Lee J-B, Byun J-H, Choi I-S, Kim Y, Lee JS. The effect of pulsed radiofrequency applied to the peripheral nerve in chronic constriction injury rat model. Ann Rehabil Med. 2015;39(5):667–75.
Varani K, Gessi S, Merighi S, Iannotta V, Cattabriga E, Spisani S, et al. Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol. 2002;136(1):57–66.
Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–142.
Ren H, Jin H, Jia Z, Ji N, Luo F. Pulsed radiofrequency applied to the sciatic nerve improves neuropathic pain by down-regulating the expression of calcitonin gene-related peptide in the dorsal root ganglion. Int J Med Sci. 2018;15:153–60.
Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–84.
Hirose K, Iwakura N, Orita S, Yamashita M, Inoue G, Yamauchi K, et al. Evaluation of behavior and neuropeptide markers of pain in a simple, sciatic nerve-pinch pain model in rats. Eur Spine J. 2010;19:1746–52.
Zheng L-F, Wang R, Xu Y-z, Yi X-N, Zhang J-W, Zeng Z-C. Calcitonin gene-related peptide dynamics in rat dorsal root ganglia and spinal cord following different sciatic nerve injuries. Brain Res. 2008;1187:20–32.
Hunt D, Raivich G, Anderson P. Activating transcription factor 3 and the nervous system. Front Mol Neurosci. 2012;5:7.
Arakawa K, Kaku R, Kurita M, Matsuoka Y, Morimatsu H. Prolonged-duration pulsed radiofrequency is associated with increased neuronal damage without further antiallodynic effects in neuropathic pain model rats. J Pain Res. 2018;11:2645–51.
Siniscalco D, Giordano C, Rossi F, Maione S, de Novellis V. Role of neurotrophins in neuropathic pain. Curr Neuropharmacol. 2011;9(4):523–9.
Sikandar S, Minett MS, Millet Q, Santana-Varela S, Lau J, Wood JN, et al. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141(4):1028–39.
Liu M, Kay JC, Shen S, Qiao L-Y. Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation. 2015;12(1):151.
Xu B, Mo C, Lv C, Liu S, Li J, Chen J, et al. Post-surgical inhibition of phosphatidylinositol 3-kinase attenuates the plantar incision-induced postoperative pain behavior via spinal Akt activation in male mice. BMC Neurosci. 2019;20(1):36.
Jin D, Yang J-P, Hu J-H, Wang L-N, Zuo J. MCP-1 stimulates spinal microglia via PI3K/Akt pathway in bone Cancer pain. Brain Res. 2014;1599:158–67.
Westlund KN. Chapter 9 the dorsal horn and hyperalgesia. Handb Clin Neurol. 2006;81:103–25.
Liu XJ, Salter MW. Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci. 2010;32(2):278–89.
Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28(5):633–8.
Lin CR, Wang CH, Wu P, Wen ZH, Buerkle H, Yang LC. Apraclonidine attenuates the increases in spinal excitatory amino acid release in rats with adjuvant-induced inflammation. Anesth Analg. 2002;94(3):701–5; table of contents.
Yang CH, Chen KH, Huang HW, Sheen-Chen SM, Lin CR. Pulsed radiofrequency treatment attenuates increases in spinal excitatory amino acid release in rats with adjuvant-induced mechanical allodynia. Neuroreport. 2013;24(8):431–6.
Hiemer B, Krogull M, Bender T, Ziebart J, Krueger S, Bader R, et al. Effect of electric stimulation on human chondrocytes and mesenchymal stem cells under normoxia and hypoxia. Mol Med Rep. 2018;18(2):2133–41.
Fini M, Giavaresi G, Carpi A, Nicolini A, Setti S, Giardino R. Effects of pulsed electromagnetic fields on articular hyaline cartilage: review of experimental and clinical studies. Biomed Pharmacother. 2005;59(7):388–94.
Vaca-González JJ, Guevara JM, Moncayo MA, Castro-Abril H, Hata Y, Garzón-Alvarado DA. Biophysical stimuli: a review of electrical and mechanical stimulation in hyaline cartilage. Cartilage. 2019;10(2):157–72.
Röttgering B, Szuhai K. Insulin-like growth factor 2 in physiology, cancer, and cancer treatment. OBM Genet. 2019;3:1.
Lewitt MS, Boyd GW. The role of insulin-like growth factors and insulin-like growth factor-binding proteins in the nervous system. Biochem Insights. 2019;12:1178626419842176.
Yeh C-C, Sun H-L, Huang C-J, Wong C-S, Cherng C-H, Huh BK, et al. Long-term anti-Allodynic effect of immediate pulsed radiofrequency modulation through Down-regulation of insulin-like growth factor 2 in a neuropathic pain model. Int J Mol Sci. 2015;16(11):27156–70.
Erdine S, Yucel A, Cimen A, Aydin S, Sav A, Bilir A. Effects of pulsed versus conventional radiofrequency current on rabbit dorsal root ganglion morphology. Eur J Pain. 2005;9(3):251–6.
Podhajsky RJ, Sekiguchi Y, Kikuchi S, Myers RR. The histologic effects of pulsed and continuous radiofrequency lesions at 42 degrees C to rat dorsal root ganglion and sciatic nerve. Spine. 2005;30(9):1008–13.
Erdine S, Bilir A, Cosman ER, Cosman ER Jr. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9(6):407–17.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vahedifard, F., Malinowski, M., Chakravarthy, K. (2021). Mechanism of Action of Radiofrequency Ablation. In: Deer, T.R., Azeem, N. (eds) Essentials of Radiofrequency Ablation of the Spine and Joints. Springer, Cham. https://doi.org/10.1007/978-3-030-78032-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-78032-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78031-9
Online ISBN: 978-3-030-78032-6
eBook Packages: MedicineMedicine (R0)