Abstract
Introduction: The knowledge in the field of nerve regeneration is incomplete; there is a constant input of information regarding better techniques, materials, and more importantly the use of regenerative medicine. The adipose-derived stem cell is a new tool in this field with promising results and clearly stated advantages, like ease of harvest and availability. Numerous studies have presented viable options regarding the use of the adipose-derived stem cell; one of the more demanding fields is nerve regeneration. One can say that nerve microsurgery has reached its potential and new techniques are necessary in order to obtain better results. The use of fat grafting can be an answer.
Material and method: An experimental model was used in order to understand the influence of the fat graft on nerve regeneration. Both sural nerves from 10 rats were transected and microsurgically coapted; on the left side, the sutured nerve was covered by processed fat from the inguinal region. Histology and ultrasound were used in order to quantify the results.
Result and discussions: The histology results, as well as the ultrasound study showed a positive influence of the adipose tissue graft in the process of nerve regeneration, especially regarding scar tissue quality and influencing the microenvironment around the nerve coaptation site.
Conclusions: Adipose tissue addition around neurorrhaphy has multiple positive effects on this regeneration, starting from its anti-inflammatory effect, stem cell source with differentiation potential for activated Schwann cells, to indirect action on the corresponding muscle.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Messages-
The adipose tissue proves to be a valuable resource of “renewable parts.”
-
Its implications in the nerve regeneration process are not yet clearly understood.
-
May help with scar formation—one of the main obstacles in the process of neuroregeneration.
-
The adipose tissue may be the resource much needed in forming new nerve tissue, most probably Schwann cells.
-
There is a need for further research in this domain.
1 Introduction
The nervous system is considered to be the most complex system of the body; due to its numerous functions, it alerts the body regarding internal and external changes and generates responses to effector organs. Complex traumas and major post-excisional defects in the upper limb may have an important functional impact. Reconstructive surgery has the task of restoring the function as close as possible to the initial one. Nervous lesions are an important part of this chapter and often the most difficult lesions to solve, being responsible for the existence of important sequelae. The clinical expression and type of lesion are given by the damaged structure; the different component structures of a peripheral nerve respond differently to the lesion and have different possibilities for regeneration. Repeated clinical observation led to a question: what causes mediocre results in peripheral nerve surgery, why are the results of primary neurorrhaphy sometimes unpredictable?
The literature lists a number of such causes, ranging from technical, material to physiological causes, both local and general (1). A possible answer would be that at this point peripheral nerve surgery, or nerve microsurgery, may have reached its limits, with the focus being automatically directed to new resources such as tissue regeneration. The post-lesion nervous regeneration, especially guided by Schwann cells, appears to be impinged by the appearance of scar tissue before the axonal growth. But scar tissue is normal in response to any injuries and is encountered throughout the body. Again, current clinical practice can provide answers and correlations. It has become common in recent years to use lipoaspirate (the remaining tissue together with the solution used in liposuction) to correct some volume deficiencies; it has also been used in areas with volume deficiency after scarring processes. The clinical observation was that the appearance of scarring improved considerably. Taking things further, why can lipoaspirate influence a scar? The presence of adipose-derived stem cells in this lipoaspirate has been demonstrated and their effects are multiple, from a local anti-inflammatory effect, to producing factors that can drive regeneration of different lines; these are of course incompletely understood and known processes. The study of the adipose-derived stem cell began with the reference article published by Zuk et al. in 2002 in Molecular Biology of the Cell (2). The ability of the adipocyte-derived stem cell to be pluripotent, not multipotent, as well as that derived from bone marrow, has been demonstrated. This observation has greatly extended the scope of applicability, given the ease with which this tissue can be harvested and processed, its availability and low morbidity. Also, there is a major difference in the quantity obtained after harvesting, if approximately one mesenchymal stem cell is obtained from the bone marrow from 25,000 to 100,000, 2% of the lipoaspirate cellularity is made up of stem cells derived from adipose tissue. The next step was isolation, which was initially done under laboratory conditions, but these processes are often lengthy and costly. Raposio’s study followed—he compared the classical method, based on collagenase, and a simpler, mechanical, centrifugal-based alternative (3). A series of initial effects were observed in surgical use, thus adipose tissue graft obtained via liposuction was used in augmentation of certain atrophic areas, implicitly also in scars and, as a result, in addition to the mechanical filling of the area, an improvement in the appearance of scars was also observed. Reference studies in this regard were undertaken by Coleman, who used the tumescent technique for harvesting and centrifugation by 3,000 rpm for 3 min (4).
The correlation between these two clinical procedures was made and the logical subsequent question was what happens with primary neurorrhaphy, and implicitly with neuroregeneration, if we add processed fat? The present study has been developed with this in mind; experimental medicine will be used as follows in order to develop a model that can answer this question. Scientific literature contains numerous such studies of nerve regeneration and adipose tissue processing or various growth factors with influence on regeneration.
2 Material and Methods
2.1 The Adipose-Derived Stem Cell
The stem cell is the non-differentiated cell that can multiply and differentiate in all cell lines. There are two types of stem cells: the embryonic ones (derived from the internal mass of the blastocyst) and the adult ones, corresponding to postnatal life; the first have the ability to differentiate in all cell lines, meaning they are pluripotent, while the adult ones can only differentiate along the cell line from which they come from, depending on the embryonic origin (ectoderm, endoderm, mesoderm), meaning they are multipotent. The process through which a stem cell can differentiate by changing the origin line is called transdifferentiation (originated in the mesoderm, but differentiation done in endoderm-derived cells). Adult stem cells were described by McCullock and Till, in Ontario, in 1963, as self-renewing cells in the bone marrow of mice (5). Later, these cells, which were subsequently identified and called hematopoietic stem cells, were used in the therapy of severe bone marrow transplantation syndrome (Dicke and van Bekkum, 1973). In 1968, mesenchymal stem cells were introduced (Friedenstein, 1968). And in 1978 hematopoietic stem cells were also identified in the umbilical cord (Emerson, 1985). Reynolds and Weiss announce the identification of a neural stem cell population in the striatal layer of mice in 1992. In other words, the only stem cells with clinical potential were those found in the hematogenous bone marrow, having limited applicability, low cell count, and morbidity at harvest or in the umbilical cord, having the related ethical aspects. Almost 10 years will pass before Zuk and his collaborators describe a population of cells with regenerative potential isolated from the processed lipoaspirate tissue, in other words, from the residual tissue after liposuction (2).
The study of the adipose-derived stem cell continues with the reference article published by Zuk et al. in 2002 in Molecular Biology of the Cell (6). But the conversion of adipose tissue into other tissues has been observed before, for example, the bone calcifications that appear pathologically in lupus and Paget’s disease (Clarke, Williams 1975). Adipose tissue is macroscopically composed of lobules, which consist of 90% mature adipocytes and other cells forming the stromal vascular fraction: preadipocytes, fibroblasts, endothelial cells, vascular smooth muscle cells, monocytes, macrophages, lymphocytes, and adipose-derived stem cells. From a histological point of view, there are two types of adipose tissue, namely white and brown. White adipocytes are spherical, having a diameter between 30 and 70 micrometers. Depending on the amount of stored lipids, the nucleus is pushed to the periphery. The brown adipocytes are polygonal, having a central core and a diameter between 20 and 40 micrometers. There is also a clear distinction in vascularization, the brown ones being more vascularized and containing more mitochondria, with both of these aspects explaining the brown color. Structural differences also translate into functional differences: both store the lipids as a form of energy reserve, the white ones use it as a response to metabolic activity, while the brown ones use it in the production of heat—thermogenesis. In the human species, brown adipose tissue is found only in newborns and children, disappearing at maturity. The embryonic origin of adipose tissue is found in the mesoderm, thus explaining the possibility of a population of mesenchymal stem cells, similar to the one in the bone marrow. According to this principle, differentiation can be made on the same line, i.e., it can be differentiated in adipocytes, chondrocytes, osteocytes, or myocytes, elements shown in the article mentioned above (6). Subsequently, a series of studies appeared that also demonstrated the capacity for transdifferentiation; 2002 Safford and 2003 Ashjian demonstrate the emergence of ectodermal lines with neuronal-like differentiation (7), and the list continues with neuronal differentiation (Kang, 2004), oligodendrocytes (Safford, 2004) and Schwann cells (Kingham, 2007 and Xu, 2008). In terms of endodermal transdifferentiation, studies have not ceased to appear, and among the first ones have demonstrated hepatocyte formation (Seo, 2005 and Timper, 2006). Thus, the ability of the adipocyte-derived stem cell to be pluripotent, not multipotent, just as the one derived from bone marrow, has been demonstrated.
This observation has greatly extended the scope of applicability, given the ease with which this tissue can be harvested and processed, its availability and low morbidity.
There is also a major difference in the amount obtained after harvesting. If approximately one mesenchymal stem cell is obtained from the bone marrow from 25,000 to 100,000, 2% of the lipoaspirated adipose tissue cellularity consists of adipose-derived stem cells (8). Thus, the existence of such a cell population with multipotent properties has been demonstrated. It remained to establish effective methods of isolation, ways of influencing certain cell lines and, last but not least, clinical applicability in solving certain pathologies.
2.2 Isolation
Isolation and extraction were performed initially under laboratory conditions; this involves repeated washing with phosphate buffer solution in order to separate erythrocytes and incubation with type I collagenase 0.075% (9) 37 °C for one hour, the infranatant is separated, followed by centrifugation at 1200 rpm for 10 min, resuspended in ammonium chloride, then again centrifugation and repeated scrubbing to form the stromal vascular fraction and, in the end, culture on fetal bovine serum. The whole process takes about 2 h. Another variant, more cost-effective, is the enzymatic digestion with trypsin (1). But these processes are often lengthy and costly. In addition, the use of animal collagen for isolation and subsequently for clinical application may be accompanied by the appearance of undesirable effects such as skin ulcers, nerve, tendon or ligament injuries, but also allergic reactions. No studies have been developed to evaluate the presence of the remaining collagenase.
A series of initial effects were observed in surgical use, thus the liposuction graft was used to augment certain atrophic areas as, implicitly also in scars, and the result, in addition to the mechanical filling of the area, was also an observed improvement in the appearance of scars. Reference studies in this regard were undertaken by Coleman, who used the tumescent technique for harvesting and centrifugation at 3000 rpm for 3 min (4). Then followed Raposio’s study (3) that compared the classical method, based on collagenase, with a simpler, more mechanical variant. Here is how he proceeded: the fat was obtained through classical liposuction using a blunt cannula attached to the 10 mL Luer-Lock syringe; the lipoaspirate tissue was further subjected to laminar flow (to avoid contamination) to a vibration of 6000 for 6 min, followed by centrifugation at 1600 rpm for 6 min. The infranatant was collected, and, through flow cytometry, the presence of adipose tissue-specific antigens (AdSC) was examined: CD34APC, CD45APC-CY7, CD73PE, CD31FITC, and CD90APC. The results were evaluated qualitatively and quantitatively, and the presence of AdSC in a considerable amount was established; namely, in 80 mL of fat, 5 × 105 cells were detected, approximately 5% of the total cells, the remaining 95% being blood-derived cells and endothelial cells. There have been differences and multiple studies regarding centrifugation speed. A study done by Kurita et al. in 2008 compares the effects of different centrifugation rates on adipose tissue transfer and derived stem cell viability; non-centrifuged and centrifuged concoctions are used at 400, 700, 1200, 3000, and 4200 × g for 3 min. Both the centrifuged concoction composition and the reaction of the athymic mice post-1 mL injection of the solution were observed. The conclusions were: the centrifugation process is beneficial for separating and concentrating adipose-derived stem cells, but the rate should be limited to 1200 × g (3000 rpm) for optimal results (10).
2.3 Differentiation, Effects, Applicability
After the discovery of the adipose-derived stem cell, reported by Zuk (2), studies referring to its differentiation and implicit clinical applicability began to appear. Most were performed under laboratory conditions (both in vitro and in vivo on laboratory animals) and under the influence of growth factors, and the clinical involvement was relatively modest by comparison. Depending on the differentiation line, there are several studies: for endodermal development, the studies conducted by Seo (2005) and Temper (2006) demonstrate the ability to form pancreatic tissue and hepatic tissue. And the list can continue with all cell lines, namely many types of tissues: adipose (Mauney 2007), bone (Cowan 2004, Dudas 2006, Yoon 2007), cartilaginous (Dragoo 2003, Guilak 2004), striated muscle tissue (Bacou 2004, Goudenege 2009), smooth muscle tissue (Rodriguez 2006), uroepithelial (Jack 2005), vascular (Miranville 2005, Heydarkhan-Hagvall 2008, Froehlich 2009, Okura 2009), hematopoietic (Cousin 2003, Puissant 2005), epithelial tissue (Brzoska 2005, Jeong 2009). In parallel, research has also focused on applicability in various pathologies, via laboratory animal studies: intervertebral disc repair (Hsu 2008), spinal cord injuries (Ryu 2009), glioblastoma treatment (Josiah 2010), Huntington’s disease therapy (Lee 2009), multiple sclerosis (Riordan 2009), stroke (Kim 2007), urinary incontinence (Lin 2010), erectile dysfunction (Lin 2009), diabetes (Lin 2009), ischemia (Kondo 2009), rheumatoid arthritis (Gonzalez-Rey 2010), epithelial regeneration (Trottier 2008), and tendon repair (Uysal, Mizuno 2009). The transition to clinical trials was made in 2004 through the case study published by Lendeckel et al., where bone graft with addition of adipose stem cells was used to treat an extensive craniofacial bone defect. Later, it was followed by a phase II clinical study on Crohn’s disease (Garcia-Olmo 2009), a clinical study on urinary incontinence (Yamamoto 2009) and organ transplant rejection (Fang 2007). Of course, the ethical aspect of these studies is shadowed by the risk of malignant transformation, by stem cell pluripotency and, in particular, by the use of growth factors; it is difficult to appreciate how these cells will differentiate and when they will stop.
The field of peripheral nerve regeneration has also been approached from the perspective of the adipose stem cell. The Safford-led team uses a combination of butylated hydroxyanisole, potassium chloride, valproic acid, forskolin, hydrocortisone, and insulin to obtain Schwann-like cells expressing the following markers: GFAP (fibril glial acid protein), MAP2 (microtubule-associated protein 2) and Beta 2 tubulin (11). When specific growth factors such as PDGF (platelet-derived growth factor) or bFGF (fibroblast growth factor) were added, changes in cell morphology and expression of specific markers for the Schwann cell, S100, GFAP , P75, and Beta 3 tubulin (Kingham 2007 and diSumma 2010) were observed. Several experimental models based on a peripheral nervous defect have been established in order to demonstrate the ability of nerve regeneration; the defect was resolved by the use of nerve ducts (natural or synthetic) to which adipose stem cells have been added. The results were contradictory, there being a possible interaction between the cells and the fabric of the duct. Another study, conducted by Lopatina et al., collects fat from the inguinal region of the rat, uses enzymatic digestion and induction towards nerve differentiation with retinoic acid; the model of injuries to which it applies is the crushing and subsequent application of matrigel containing culture cells; the study’s final analysis is based on the usage of peroneal functional index, histology, and PCR. The study determines the expression of genes encoding certain enzymes, cytoskeleton proteins, laminar adhesion molecules, myelin components (Krox20 gene), concluding that transplantation of adipose-derived stem cells stimulates nerve regeneration by two main means: angiogenesis (by VEGF, FGF), and brain-derived neurotrophic factor (BDNF) secretion (12). This release of neurotrophic factors may act on the remaining Schwann cells (also activated by angiogenesis), causing a response supporting regeneration and nerve repair.
Adipose tissue has long been ignored by researchers, anatomists, and physicians, but over the last two decades we can say that it has undergone a transition from being a simple form of energy storage to an important endocrine organ that plays a role in metabolism and immunity. Naturally, the question that follows is why does adipose tissue have such a vast and important supply of pluripotent stem cells, what is its actual role?
2.4 Experimental Model
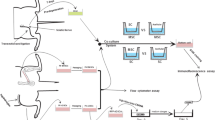
In order to evaluate the possible effects of the processed fat graft in the nerve regeneration process, we have developed an experimental model. The chosen study animal was the Wistar rat, a total of 10 animals were used. The nerve chosen for the experimental study was the sciatic nerve, due to its diameter corresponding to a microsurgical suture and anatomical topography, a relatively easy dissection exposure. In order to minimize the effect of other factors than the applied method, it was decided to use both sciatic nerves from the same animal, applying two different methods. In the right sciatic nerve, there was a microsurgical end-to-end neurorrhaphy, and in the left sciatic nerve, after the neurorrhaphy was completed in the same manner, the sutured nerve was wrapped around by a fragment of adipose tissue harvested from the inguinal region, due to the lack of donor site in the Wistar rat (an alternative would have been the peritoneum). Following incision and dissection in the inguinal region, a fragment of fat tissue covering the femoral vessels was harvested by surgical excision (between 3 and 5 g). Further the tissue was processed by washing with saline and centrifugation for 3 min at 3000 rotations per min (Fig. 38.1), obtaining the fat graft. A pre-established protocol was divided into three consecutive steps: stage 0 (day 0), when the microsurgical part was completed, stage 1 (day 30), when the effects of applied and sampled methods from the first 5 animals were assessed and stage 2 (day 70), when the effects were evaluated again and samples from the last 5 animals were taken; all animals were subsequently euthanized. The tracking and quantification of the results was accomplished by two means: the evaluation of the motor response of the gastrocnemius muscle—distal and innervated by the sciatic nerve, while observing the evolutionary diameter of this muscle via musculoskeletal ultrasound (13), as well as histological evaluation of the treated nerve fragment, along with a fragment of muscle distal to injuries. From the histological point of view, several types of staining methods were used: common (hematoxylin-eosin, Masson’s trichrome), histochemical (Gordon-Sweet and Luxol Fast blue arginine impregnation), but also several immunohistochemical (GFAP, NFAP, Prox1, AC133, OCT3 / 4, PGP 9.5, CD34, and MCT).
3 Results
The first analysis recorded was the ultrasound (Fig. 38.2), observing the diameter of the muscles corresponding to the injured nerve, quantifying the atrophy and its regeneration. There was a better evolution in the left gastrocnemius muscle, adjacent to the sural nerve treated by suture and addition of processed adipose tissue compared to the right one; the recovered percentage difference is more significant in the first group (4 weeks). Subsequently, a comparison was made between the ultrasound and the histological evaluation, with statistically significant correlations (Table 38.1).
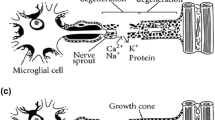
After that the morphological analysis of sutured nerve fragments and adjacent muscle was performed by hematoxylin-eosin staining, Masson’s trichrome, and argentic impregnation. The following conclusions have been drawn: adipose tissue adhesion around neurorrhaphy had an antifibrotic and anti-inflammatory effect, the addition of minimally processed adipose tissue stimulates the growth of the Schwann cell proliferation cone, it causes a hypervascularization of the microenvironment around the neurorrhaphy site, which could enhance normal nerve regeneration (Fig. 38.3).
The histochemical methods used demonstrate the stimulation of not only the growth of the Schwann cell proliferation cone, but also the stimulation of the intraneuronal synthesis of neurofibrils that have previously invaded the Schwann cell guiding cone in the treated group, compared to the group untreated with adipose tissue. The morphological and histochemical studies of specimens harvested at 10 weeks demonstrate the ability of self-adherence and fixation of adipose tissue processed around the neurorrhaphy site, having a possible impact on the surgical technique.
The following study theme consisted of the variability of GFAP (glial fibrillary acidic protein) and NFAP (neurofilament-associated protein) expression in nerve traumatic lesions. We observed a series of GFAP-positive cells situated between the nerve and the fat graft, emerging from the latest (Fig. 38.4). The GFAP reaction has reached its maximum at 4 weeks, unlike the NFAP reaction, which peaked at 10 weeks, but showing an incomplete process of nerve regeneration, needing yet a remodeling phase (14). Further, PGP 9.5 (protein gene product) expression was observed in the regenerated nerve. At 10 weeks on the treated part, the density of the PGP 9.5 was higher than that on the contralateral part, probably induced by the presence of the fat graft with its adipose-derived stem cells.
There is currently no certified phenotypic assessment of the expression of certain markers specific to this stage of nerve regeneration and therefore there is also a lack of actual assessment of the events that are involved in the pathological regeneration of a nerve. A specific phenotype was detected: AC133 positive 3+, 3/4 +/−, and Prox1 +/−, characterize the untreated group at 4 and at 10 weeks. Instead, for the treated group, at week 10, an Ac133 −, Prox1 3+ and Oct 3/4 + phenotype is identified, unlike week 4 for the treated group, where we noticed Ac133+, Prox1 with 3+ and Oct 3/4 with 3+. It has also been observed that nerve regeneration by addition of adipose tissue also influences the reactivity of satellite muscle cells, which tend to express a phenotype similar to those identified in the nerve repair cone but also perineural fat.
A special and interesting study consisted of assessing the presence and possible roles of mast cells in nerve regeneration. This was done by Luxol fast blue staining and mast cell tryptase evaluation (MCT). The findings were the following: mast cells are directly involved in nerve regeneration, their number being stimulated by adipose tissue addition; accumulation of mast cells compared to normal nervous tissue has an early onset at 4 weeks after neurorrhaphy and progressively increases at 10 weeks; mast cells influence not only nerve regeneration but also the stimulation of muscle function, as evidenced by the statistically significant correlation found between the diameter of the gastrocnemius muscle and the number of mast cells in the group treated with addition of adipose tissue; the increase in the number of mast cells in the group treated by addition of adipose tissue at 10 weeks—both inside the nerve fibers, as well as around the nerve, suggests that the adipose tissue is a source of mast cells with direct involvement in stimulating nerve regeneration.
Last but not least, the nerve angiogenesis process was evaluated in regeneration by CD34 expression; it has been shown that vascular microdensity plays a major role in the regeneration of the peripheral nerve and that peripheral nerve regeneration angiogenesis is independent of mast cell activation and activation of mast cell tryptase. This study demonstrated once more that the addition of adipose tissue proved to be effective in stimulating the nerve regeneration process, but mainly by its anti-inflammatory effect, obtained partly by inhibiting the subpopulation of mast cell that have this inflammatory effect.
4 Discussion
GFAP is not the most common marker used for nerve regeneration, but it represents the reactive Schwann cell. A recent study presented that the Schwann-like stem cells, derived from the adipose tissue were positive for GFAP (15), this fact correlated with our own findings.
PGP 9.5 has a higher sensitivity in detecting tissues with neuroectodermal origin then neuron-specific enolase. It was found to be useful for identifying mesenchymal cells in adipose tissue with differentiation to the neuronal line. Furno et al. used in an experimental model the PGP 9.5 expression and adipose tissue, they demonstrated that the microenvironment created could determine differentiation of the adipose-derived mesenchymal stem cells into progenitor neuronal cells, capable of expressing PGP 9.5, but also nestine and MAP 2 (16), corelating with our own findings. This is one of the few studies in which the expression of the PGP 9.5 is correlated with the adipose-derived stem cells, suggesting a role for the latest in stimulating the nerve regeneration process. DiSumma evaluated the presence of PGP 9.5 in an experimental model, using fibrine, Schwann cells, and adipose-derived stem cells, he obtained the same degree of nerve regeneration in two settings, one by using mesenchymal stem cells (medullary origin) and the other by using adipose tissue (17). A correlation between expression of PGP 9.5 in adipose tissue, correspondent muscle, regenerative nerve, and ultrasound data could be a useful marker for assessing motor function recovery in patients with peripheral nerve injuries.
Stem cells play an important role in nerve regeneration, unfortunately, the phenotype of these cells is not characterized; paradoxically, not even markers for neural cells that are expressed in the early stages of development of the central and peripheral nervous system and are not used as markers for assessing cells with stem potential that appear early in the development of the central and peripheral nervous system. Of these markers, Prox1 and OCT3/4 appear early in the primitive neuroepithelium cells as well as in the neuroepithelial cells of the neural ridge from which the peripheral nervous system, and implicitly the peripheral nerves, develop. The generic marker for cells with stem potential is AC133 (CD133), but its disadvantage is that it is not specific for stem cells with potential to differentiate into Schwann cells, useful in nerve regeneration. In contrast, CD133 has proven to be a factor that potentiates peripheral nerve regeneration after injury through the ability of these cells to stimulate bridge formation between the two nerve heads, most likely by differentiating them into Schwann cells (18). Regarding the phenotype that was detected in our study, especially in the fat graft surrounding the nerve coaptation site, one cannot release the hypothesis that the adipose-derived stem cells found can differentiate into nerve fibers, further research must clarify this.
In the peripheral nervous system, the implication of mast cells is currently linked to autoimmune diseases; it looks like mast cells from the nerve are strongly linked to neurotransmission changes and to inflammatory processes (19). The adipose tissue proved to be a good reservoir of mast cells, having a positive effect on nerve regeneration.
5 Clinical Application
Although several experimental models proved to be successful, fat grafting and nerve surgery are not used often in clinical practice. The reasons for this can be: a lack of protocol regarding tissue processing or manipulation, a lack of correct and precise indication towards nerve surgery, when this technique should be applied. So far, in the literature, the indication is scarcely used, most of the studies are conducted using an experimental model, one of the most common procedures that involves nerve conduits made from several materials filled with processed adipose tissue. In a recent study published by Zimmermann et al., they used fat grafting in combination with stromal vascular fraction as an mechanical barrier in order to block the end-neuroma formation of a nerve stump; accordingly to our research, this proved to improve angiogenesis, reduce inflammation and fibrosis, preventing the recurrence of a painful end-neuroma; although the addition of fat graft stimulates the growth cone of the axon, the regeneration process they described is a more organized one, the limitation of the study is the limited number of patients (20). Once again, the fat graft has been used for its mechanical effect, as well as for its rich content in adipose-derived stem cells. Our own experience is somewhat similar to those described in the literature. Most of the indications were for its mechanical properties. The recurrent carpal tunnel syndrome was the main indication, after several attempts of decompression; in most cases, we observed abundant scar tissue formation, around the nerve, necessitating neurolysis of the median nerve and further more application of fat graft in the carpal tunnel with improvement of the symptomatology and no further surgery being needed. Similar indications were after major trauma of the forearm and hand; in the second procedure when neurolysis and tenolysis were necessary, fat graft was applied, but again for its mechanical properties, with excellent results. So far, one can say that there is reluctance towards using the fat-grafting technique in nerve surgery for clinical purpose.
6 Conclusions
-
Nerve regeneration is a multistep process that includes a series of particular cell and molecular events specific to this type of regeneration
-
Adipose tissue addition around neurorrhaphy has multiple positive effects on this regeneration, starting from its anti-inflammatory effect, stem cell source with differentiation potential for activated Schwann cells, to indirect action on the corresponding muscle.
-
Stem cells involved in nerve regeneration have a versatile phenotype that varies depending on the time elapsed since neurorrhaphy and adipose tissue addition.
-
In the first 4 weeks, the regeneration by addition of adipose tissue is active, characterized by the persistence of the Schwann cell immature phenotype; the regeneration process is incomplete at 10 weeks, but unlike the first stage, the nerve maturation is predominant, a fact proven also by the diameter increase of the muscle in the adipose tissue treated group at 10 weeks.
-
Angiogenesis is mandatory in nerve regeneration, being stimulated by adipose tissue addition; in addition to endothelial cells, mast cells play an important role in nerve regeneration, their number increasing in the adipose tissue group (which suggests that it can be a source of mast cells).
-
The decrease of mast cell tryptase in the adipose tissue group and in the second part of regeneration (weeks 5–10) supports the anti-inflammatory effect. Most likely, the increase in the total number of mast cells is determined by their attractiveness through a range of factors secreted at the injury site, and their most likely role is to synthesize NGF, a fact that will later need to be proven, as it is not part of the scope of this study.
The chosen field of study proved to be surprising and abounding in results. It initiated the opening of new horizons with regard to the research and improvement of peripheral nerve regeneration, with possible direct implications in current clinical practice.
References
Zack-Williams SDL, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells. 2015;7(1):51–64.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Raposio E, Caruana G, Bonomini S, Libondi G. A novel and effective strategy for the isolation of adipose-derived stem cells: minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr Surg. 2014;133(6):1406–9.
Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108s–20s.
Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 197(4866):452–4. Bibcode:1963Natur.197..452B. https://doi.org/10.1038/197452a0.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. https://doi.org/10.1091/mbc.E02-02-0105.
Safford KM, Hicok KC, Safford SD, Halvorsen YD, Wilkison WO, Gimble JM, Rice HE. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun. 2002;294(2):371–9.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–4.
Rodbell M. Metabolism of isolated fat cells. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375–80.
Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, Yoshimura K. Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg. 2008;121(3):1033–41.
Safford KM, Safford SD, Gimble JM, Shetty AK, Rice HE. Characterization of neural-glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol. 2004;187:319–28.
Lopatina T, Kalinina N, Kragyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899.
Nijhuis TH, de Boer SA, Wahegaonkar AL, Bishop AT, Shin AY, Hovius SE, Selles RW. A new approach to assess the gastrocnemius muscle volume in rodents using ultrasound; comparison with the gastrocnemius muscle index. PLoS One. 2015;10(7):e0133944. https://doi.org/10.1371/journal.pone.0133944.
Bloancă V, Ceauşu AR, Jitariu AA, Barmayoun A, Moş R, Crăiniceanu Z, Bratu T. Adipose tissue graft improves early but not late stages of nerve regeneration. In Vivo. 2017;31(4):649–55.
Salazar MF, Tena Suck ML, Rembao Bojórquez D, Salinas LC. Intraventricular neurilemmoma (Schwannoma): shall GFAP immunostaining be regarded as a histogenetical tag or as a mere histomimetical trait? Case Reports in Pathol. 2016;2016:2494175. https://doi.org/10.1155/2016/2494175.
Lo Furno D, Pellitteri R, Graziano AC, Giuffrida R, Vancheri C, Gili E, Cardile V. Differentiation of human adipose stem cells into neural phenotype by neuroblastoma- or olfactory ensheathing cells-conditioned medium. J Cell Physiol. 2013;228(11):2109–18.
di Summa PG, Kingham PJ, Raffoul W, Wiberg M, Terenghi G, Kalbermatten DF. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63(9):1544–52. https://doi.org/10.1016/j.bjps.2009.09.012.
Ohtsubo S, Ishikawa M, Kamei N, Kijima Y, Suzuki O, Sunagawa T, Higashi Y, Masuda H, Asahara T, Ochi M. The therapeutic potential of ex vivo expanded CD133+ cells derived from human peripheral blood for peripheral nerve injuries. J Neurosurg. 2012;117(4):787–94.
Alhelal MA, Palaska I, Panagiotidou S, Letourneau R, Theoharides TC. Trigeminal nerve stimulation triggers oral mast cell activation and vascular permeability. Ann Allergy Asthma Immunol. 2014;112(1):40–5.
Zimmermann S, Fakin RM, Giesen T, Giovanoli P, Calcagni M. Stromal vascular fraction-enriched fat grafting for the treatment of symptomatic end-neuromata. J Vis Exp. 2017;129 https://doi.org/10.3791/55962.).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bloanca, V., Crainiceanu, Z., Bratu, T., Agovino, A., Cimpean, A.M. (2022). The Role of Adipose Tissue Graft on Nerve Regeneration from the Perspective of the Adipose-Derived Stem Cell. In: Kalaaji, A. (eds) Plastic and Aesthetic Regenerative Surgery and Fat Grafting. Springer, Cham. https://doi.org/10.1007/978-3-030-77455-4_38
Download citation
DOI: https://doi.org/10.1007/978-3-030-77455-4_38
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-77454-7
Online ISBN: 978-3-030-77455-4
eBook Packages: MedicineMedicine (R0)