Abstract
Exciting developments have been made in understanding antibody-mediated immunity, deepening understanding of antibody effector functions increasingly recognized as critical mechanisms of action beyond antigen recognition, and significantly broadening the evidence base for the importance of these effector mechanisms across diverse infectious and autoimmune diseases. Because these activities critically depend on the specific glycoforms present on a conserved site of the IgG Fc domain, relationships between the Fc glycosylation profiles of antigen-specific antibody pools and outcomes in infectious and autoimmune disease have begun to be defined, pointing to the key role of this posttranslational modification as a biomarker and mechanistic modifier of antibody-mediated immunity. Here we summarize studies evaluating the profiles and activities of antigen-specific antibodies elicited by infection and vaccination as well as within the context of allo- and autoimmunity, and consider current approaches to rational modification of Fc glycans in vivo.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Evidence of the critical importance of effector functions to antibody-mediated immunity (AMI) has been accumulating across studies of diverse infectious and autoimmune diseases. The ability to study and clinically leverage AMI was made a much simpler task after Kohler and Milstein’s (Köhler and Milstein 1975) discovery of an approach to generate consistent, reliable, and reproducible monoclonal antibody (mAb) preparations, which represented challenges to the use of polyclonal sera prevalent at that time. Advanced molecular methods in antibody cloning (Wang et al. 2019; Hunter et al. 2019; Kim et al. 2014; Winzeler and Wang 2013; Chon and Zarbis-Papastoitsis 2011) and engineering (Bruggeman et al. 2018; Dekkers et al. 2018; Crooks et al. 2018; Dekkers et al. 2016) have complemented the discovery of diverse Fc receptors (FcR) (Bournazos and Ravetch 2017; Castro-Dopico and Clatworthy 2016; Wu et al. 2014; Hirvinen et al. 2013; Nimmerjahn and Ravetch 2008a; Lazar et al. 2006; Hogarth 2002) and development of elegant knockout mouse models (Walsh et al. 2016; Verkoczy 2017; Stackowicz et al. 2020) to enable further basic science exploration and to support therapeutic optimization of AMI. In parallel, higher resolution means of profiling serum antibodies have accompanied these advances and greatly expanded the ability to interrogate polyclonal responses in serum and tissue. The high throughput of many of these profiling approaches has now turned the heterogeneity observed among polyclonal samples into a strength, providing means to interrogate the features and activities of antibodies that are associated with AMI.
2 Antibody Effector Functions
Antibodies play an important role in both effecting and regulating an immune response. They have the capacity to either amplify or dampen an inflammatory immune response based on their specificity, affinity, titer, isotype, and glycosylation profile. While the antigen-binding fragment (Fab) domain confers antigen specificity, the crystallizable fragment (Fc) domain is responsible for linking antigen recognition to downstream effector functions (Schroeder and Cavacini 2010). Antibodies can neutralize pathogens by directly binding through the Fab domain and occluding the binding of the pathogen or its toxins to cognate receptors. Such Fab-mediated antibody action is complemented by Fc domain engagement of complement proteins and FcR (Fig. 18.1). There are broadly two categories of FcRs—activating and inhibitory—that are ubiquitously expressed on human hematopoietic cells and play a key role in orchestrating potent antibody-mediated effector immune responses in the context of both protective immunity to pathogens and pathogenic immune responses to self (Fig. 18.2).
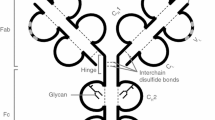
Antibody glycosylation sites in IgG. Glycosylation sites can be present in the Fab region (15–25% of IgGs), but are always present in the Fc region. There are distinct qualitative differences among the glycans commonly found at these sites, however, the importance of the glycans in mediating function via respective antibody domains is a shared characteristic. These glycans share the core heptasaccharide (dotted line in the inset) to which extensions of specific sugars are attached. Fc glycans tend to be heavily fucosylated whereas the Fab glycans have generally been observed to exhibit relatively high levels of sialylation. Adapted from Bondt et al. (2014)
The most widely studied Fc-mediated antibody effector functions are antibody-dependent cell-mediated cytotoxicity (ADCC) (Worley et al. 2018), antibody-dependent cellular phagocytosis (ADCP) (Gerber and Mosser 2001), and complement-dependent cytotoxicity (CDC) (Goldberg and Ackerman 2020). These activities are induced by engagement of the FcγRs on innate effector cells, or by soluble complement cascade initiators, such as C1q or Mannose Binding Lectin (MBL), by the Fc domain of antibodies that are bound to a target antigen. ADCC is characterized by FcγR engagement that causes the release of cytotoxic granules that contain perforin and granzyme, resulting in the killing of target cells (Smyth et al. 2005). FcγRIIIA-expressing Natural Killer (NK) cells are widely considered to be an important contributor to ADCC and are often assayed in vitro. However, in vivo, neutrophils, monocytes, and macrophages are also capable of driving ADCC and have been found to make important contributions to antibody mechanism of action (Smyth et al. 2005; van Erp et al. 2019).
ADCP or opsonophagocytosis is the uptake of immune complexes or antibody-coated antigens by phagocytic cells including monocytes, macrophages, dendritic cells (DCs), and others that express FcγRI, FcγRII, and/or FcαRI, each of which can mediate immune complex uptake (Li and Kimberly 2014). ADCP mediates clearance of immune complexes by trafficking them to the lysosomes for degradation and antigen processing for presentation on Major Histocompatibility Complex (MHC) molecules on the cell surface (Mantegazza et al. 2013). Previous work on influenza virus has shown that ADCP contributes to protection from infection in mice (Huber et al. 2001; He et al. 2017) and potentially plays a role in recovery from severe infections in humans (Vanderven et al. 2017; Ana-Sosa-Batiz et al. 2016). Associations between ADCP and improved outcomes in other disease settings such as HIV (Barouch et al. 2013, 2015), West Nile Virus (Vogt et al. 2011) in humans, and Respiratory Syncytial Virus (RSV) (Bukreyev et al. 2012) have also been recently established.
Besides ADCC and ADCP, antibodies can also induce complement activation. The complement cascade contributes to pathogen elimination either directly, by means of complement-dependent cytotoxicity (CDC), or indirectly, through phagocytic clearance of complement-coated targets and the induction of an inflammatory response (Goldberg and Ackerman 2020; van Erp et al. 2019; Grafals and Thurman 2019; Casadevall and Pirofski 2012). The complement cascade consists of a large number of distinct plasma proteins that react with one another to opsonize pathogens, inducing a series of inflammatory responses that help to fight infection (Noris and Remuzzi 2013). A number of complement proteins are proteases that are themselves activated by proteolytic cleavage (Dunkelberger and Song 2010). The terminal complement components assemble into the membrane attack complex (MAC), resulting in lysis of the pathogen-infected cell. Complement has been shown to have both protective and pathogenic effects in various disease conditions. In HIV (Barouch et al. 2013, 2015; Pittala et al. 2019), influenza (Co et al. 2014; Wu et al. 2015), and vaccinia (Benhnia et al. 2009) infection, antibody-mediated CDC has been shown to correlate or mechanistically contribute to antibody antiviral activity. Alternatively, complement-mediated activation has also been associated with disease severity (Nascimento et al. 2009; Churdboonchart et al. 1983; Füst et al. 1994). Lastly, the binding of complement-coated immune complexes to complement receptor 2 on B cells is reported to lower the B cell activation threshold, thereby promoting long-lived adaptive immunity and higher antibody levels (van Erp et al. 2019; Hebell et al. 1991; Gonzalez et al. 2010).
3 Immunomodulatory Antibody Activities
In contrast, anti-inflammatory effects of antibodies can help in alleviating severe immune damage. Based on this concept, administering intravenous immunoglobulin (IVIg) to treat inflammatory conditions such as autoimmune disease has found an important clinical application (Bayry 2016). While the underpinnings of IVIg mechanisms have yet to be clearly elucidated (Schwab and Nimmerjahn 2013), different mechanisms of action such as neonatal Fc receptor blockade resulting in accelerated clearance of autoantibodies (Li and Kimberly 2014), direct interaction with the inhibitory FcγRIIb (Nagelkerke and Kuijpers 2015), or occlusion of activating receptors and tempering the inflammatory effector responses (Nimmerjahn and Ravetch 2008b) have been proposed. However, since IVIg treatment is used to treat various diseases, it is likely that the mode of action differs per clinical setting.
4 Induction and Regulation of Antigen-Specific Antibodies
During an immune response, B cells are stimulated to mature and to undergo class switch recombination (CSR) resulting in genetic modification of the IgH locus and selection of the antibody isotype and subclass to be secreted (Stavnezer and Schrader 2014). Just as B cells undergo rounds of somatic hypermutation over the course of affinity maturation as they migrate in and out of regions in the germinal center, CSR can occur in rounds with repeated switching to downstream types (Mesin et al. 2016). This heterogeneity in the amino acid sequence of both variable fragment (Fv) and Fc regions is coupled to further functional diversification via incorporation of one of the >30 possible glycoforms (Jennewein and Alter 2017) in the conserved N-linked glycosylation motif. While multiple isotypes are glycosylated in the Fc, we will focus on glycosylation in the context of the four IgG subclasses (Vidarsson et al. 2014).
In the past 20 years, the role of antibody glycosylation as an important parameter modulating the potency of effector functions has been firmly established through advances in monoclonal antibody research and development, as well as in studies of natural immune responses in the context of infectious and autoimmune disease. Here we focus on recent research considering the glycosylation of antigen-specific antibodies in these settings.
5 Importance of Ab Glycosylation
The Fc domain contains a consensus N-linked glycosylation site that is typically occupied by a heptasaccharide core structure consisting of four N-acetylglucosamine (GlcNAc) and three mannose moieties that form a biantennary complex (Liu 2015). Additional glycosylation features such as fucose, galactose, sialic acid, and GlcNAc can be added later to the core structure to produce over 30 distinct glycovariants. As both heavy chains are glycosylated, a single IgG molecule can have a diverse array of glycosylation heterogeneity (Jefferis 2009). Nuclear magnetic resonance (NMR) studies have shown that variability in the glycans at this conserved position has a profound effect on the hinge region conformation (Yamaguchi et al. 2006). Similarly, interactions with Fcγ and other IgG and glycan receptors are entirely dependent on or modified by glycan composition and conformation, thus the type of glycan occupying this site modifies antibody effector function (Saunders and Conceptual 2019). Unlike genetically templated factors that impact IgG activity, such as Fv sequence and Fc subclass, antibody glycosylation is remarkably varied, resulting in a high level of microheterogeneity that facilitates the fine tuning of antibody function (Alter et al. 2018a). These dynamic changes in antibody glycosylation can have a subtle or profound effect in their interactions or downstream functions.
6 Typical Serum IgG Fc Glycan Composition
Given the importance of IgG Fc glycans, the composition of serum antibodies has been evaluated in a number of populations, providing insight into changes associated with age, sex, hormone levels, and disease status. Nonetheless, “typical” compositions have been articulated among healthy individuals (Fig. 18.1), and deviations from this profile suggest active processes regulating this posttranslational modification at multiple levels.
Serum IgG Fc is typically overwhelmingly fucosylated (>90%) (Gudelj et al. 2018). However, skewed glycosylation variants, produced by chemoenzymatic modifications or expressed in engineered cells, have been produced that lack this fucose moiety, and as a result exhibit significantly improved effector function. For example, an afucosylated form of an anti-CD20 IgG1 showed a 50-fold improvement in binding to FcγRIIIa and enhanced ADCC activity (Shields et al. 2002). Later, structural studies found that the fucose on the Fc glycan clashes with a GlcNAc2 group of an FcγRIIIa glycan, thereby providing a structural rationale to the improved ADCC activity of afucosylated antibody (Ferrara et al. 2011).
About 10% of all circulating IgGs in healthy human adults exhibit bisected Fc glycans (Gudelj et al. 2018), which have been shown previously to relate to ADCC activity (Hodoniczky et al. 2005). However, this amplification in ADCC, caused by the increased engagement of the FcγRIII, is believed predominantly to be due to the indirect role of bisection in decreasing fucosylation, rather than a direct consequence of its presence in the antibody structure (Shinkawa et al. 2002).
Similarly, agalactosylated, monogalactosylated, and digalactosylated glycan structures account for approximately 35%, 35%, and 15% of circulating IgG Fc-glycans, respectively (Gudelj et al. 2018). A prominent bias towards agalactosylated antibodies has been observed in people with active autoimmune and inflammatory diseases (Parekh et al. 1989; Tomana et al. 1992; Rademacher et al. 1994; Decker et al. 2016), however, a clear consensus on cause or consequence is yet to be achieved (Alter et al. 2018a). Furthermore, there are conflicting reports on the role of galactosylated antibodies in mediating proinflammatory activities, with some reports observing the presence of galactosylation on the IgGs to enhance the ADCC and complement binding (C1q) in vitro (Nimmerjahn et al. 2007; Peschke et al. 2017; Thomann et al. 2015; Tsuchiya et al. 1989), while others have noted a dampening of an inflammatory response by highly galactosylated immune complexes (Karsten et al. 2012). A lack of correlation between the presence or absence of galactosylation on IgGs and corresponding in vivo activity has also been reported (Nimmerjahn et al. 2007), suggesting that the consequences of variable galactosylation may be best investigated per disease model and per antibody.
Lastly, approximately 10% of circulating IgG Fc is sialylated (Gudelj et al. 2018). Sialylated IgG Fc is associated with an anti-inflammatory profile of antibodies in mouse models, in which neuraminidase-treated, asialylated pooled human IgG (IVIg) has been observed to abrogate the normally anti-inflammatory activity of IVIg (Kaneko et al. 2006). However, this mechanism of action remains controversial in humans. Discrepant observations have been made as to the ability of IgG to interact with the candidate receptor proposed on the basis of mouse studies (Anthony et al. 2008; Temming et al. 2019), and sialylated IgG has shown slightly elevated binding to activating FcγR and C1q, and associated effector functions (Dekkers et al. 2017; Subedi and Barb 2016), which would suggest a greater inflammatory capacity.
7 Variations in Ab Glycoprofiles
Deviations from these “typical” profiles have been associated with diverse physiological and immunological states. For example, changes in total serum IgG Fc glycosylation are observed in early life (Cheng et al. 2019), in adolescence (Gudelj et al. 2018; de Haan et al. 2016), and in association with hormonal status (Ercan et al. 2017), as well as more gradual changes during immune senescence (Krištić et al. 2013), across a broad range of glycoforms and constituent sugar moieties. In the context of ongoing inflammation, such as observed in chronic infection (Moore et al. 2005) or autoimmunity (Parekh et al. 1985), global IgG Fc glycosylation is often modified, showing reduced galactose and sialic acid content (Lastra et al. 2009).
Beyond approaches to evaluate these global changes, the role of Fc glycans in antibody function has also motivated the development of robust methods to define the glycosylation profiles of antigen-specific antibodies (ASA) purified from serum. Early questions about ASA fractions related to whether they are typically composed of IgG Fc glycovariants with similar prevalence to those observed for total serum IgG, and if not, whether glycoprofiles vary by pathogen, antigen, and epitope specificity.
8 ASA Glycosylation in Infectious Disease
In the context of responses to the HIV envelope protein among chronically infected individuals, HIV envelope glycoprotein-specific antibodies were found to exhibit reduced galactosylation, fucosylation, and sialylation (Ackerman et al. 2013), even when compared to global serum IgG Fc glycan profiles that were shifted in these same directions as compared to uninfected and acutely infected individuals (Moore et al. 2005). Among ASA, galactosylation levels correlated with Ab-dependent inhibition of viral infection and replication and were consistent with glycosyltransferase and glycosidase expression in peripheral B cells (Ackerman et al. 2013). Perhaps surprisingly, these global and HIV-specific plasma IgG Fc glycan changes were not resolved by either antiretroviral drug therapy or in the context of spontaneous virus control. Subsequent studies have shown the contribution of HIV-specific IgG glycans to predicting HIV-specific antibody effector functions (Alter et al. 2018b) and vaccine efficacy (Vaccari et al. 2016; Ackerman et al. 2018).
These and other early studies have firmly established that ASA can differ from total serum IgG in their glycosylation states. As methods for analysis of ASA have advanced, analysis of ASA targeting different proteins has become increasingly feasible but not yet common. To the extent studies have addressed multiple target antigens, there has been some evidence for consistent glycoforms across distinct specificities and other cases in which different antigen-specificities, or even different epitope-specificities within the same protein have shown distinct profiles. For example, in tuberculosis, distinct ASA IgG Fc glycan profiles for two different antigen types were reported to show similar glycan profiles to each other, but with striking decreases in fucose and increases in galactose, sialic acid, and bisecting GlcNac as compared to total serum IgG Fc (Lu et al. 2020). In contrast, Wang et al. reported that the abundance of sialylation and fucosylation among influenza hemagglutinin-specific (HA) IgG differed depending on specificity of the Fab domain. Antibodies to the HA globular head were significantly more sialylated and fucosylated than those directed against the HA stem domain (Wang and Ravetch 2019), though it may be important to keep in mind that the globular head functions as a sialic acid-binding protein.
One of the most interesting examples of the effect of ASA Fc glycosylation comes from the setting of flavivirus infection. This family of viruses has been associated with a phenomenon called Antibody-Dependent Enhancement (ADE), in which virus-specific antibodies increase infection of FcγR-bearing target cells. Among these, dengue is a mosquito-borne pathogen caused by four distinct but closely related dengue virus (DENV) types. Recovery from infection is believed to typically provide immunity against infection from the same type. However, cross-type immunity is partial and temporary. Subsequent (secondary) infection by another serotype is associated with an increased risk of developing severe dengue via ADE (Katzelnick et al. 2017; Guzman et al. 2013). While prior work has shown that waning antibody titer is associated with severe disease upon secondary exposure (Katzelnick et al. 2017), recent work has highlighted the potential importance and clinical impact of the glycosylation of dengue-specific antibodies. As perhaps the most elegant setting in which to evaluate ADE, severe disease of neonates is associated with the level of passively transferred maternal dengue-specific antibody that is afucosylated, resulting in dengue hemorrhagic fever or dengue shock syndrome (Wang et al. 2017; Thulin et al. 2020; Khandia et al. 2018). This potent ADE response is thought to manifest via non-neutralizing, dengue-specific antibodies that exhibit increased affinity to the activating FcγRIIIA receptor.
In the context of coronavirus disease 2019 (COVID-19), Fc glycans of IgG antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) envelope spike and nucleocapsid proteins differ from those of total serum IgG (Larsen et al. 2020). In this study, these profiles were observed to differ between spike and capsid, and multiple studies have observed that spike-specific IgG Fc afucosylation is correlated to disease severity (Larsen et al. 2020; Chakraborty et al. 2020), with some evidence that they may contribute to pathology via inducing inflammatory responses from macrophages (Hoepel et al. 2020). Global serum IgG glycans have also been reported to diverge according to COVID-19 severity, with decreased bisecting GlcNAc observed in multiple cohorts (Petrovic et al. 2020). The role of IgG Fc glycosylation of ASA remains to be defined in many more infectious disease settings. Like in COVID-19, HIV, and other settings in which ASAs have been profiled, intriguing observations regarding differences in global IgG Fc glycosylation abound—such as in meningococcal sepsis (Haan 2018), visceral leishmaniasis (Haan 2018; Gardinassi et al. 2014), and tuberculosis (Lu et al. 2016, 2020)—and have been found to relate to disease status or outcomes.
9 ASA Glycosylation in Allo/Autoimmunity
Rheumatoid arthritis (RA) is a common systemic inflammatory autoimmune disease in which joint synovium is affected by a dysregulated immune system. RA is typically associated with serological evidence of systemic autoimmunity as indicated by the presence of autoantibodies in serum and synovial fluid (Coutant 2019; Song and Kang 2010). Instead of being characterized by specific reactivity to a particular autoantigen, RA is associated with antibodies reactive against a wide spectrum of autoantigens, which can make the etiology of disease progression in RA patients very different. Among various autoantigens targeted in RA, anti-citrullinated protein antibodies (ACPA) have been identified as a useful marker in diagnosis (Coutant 2019) and predicting whether undifferentiated arthritis will progress to RA (Forslind et al. 2004). ACPA are associated with an increased risk of developing bone erosions (Rönnelid et al. 2005; Rycke et al. 2004), suggesting their potential to contribute to joint pathology. Like total serum IgG, long known to show decreased galactosylation, ACPA are observed to exhibit further reduction in sialylation and galactosylation (Scherer et al. 2010; Ohmi et al. 2016), though there is some evidence that the IgG subclasses may differ from each other in this regard (Lundström et al. 2014). Reinforcing the controversy regarding the potentially conflicting roles of sialylated IgG in different species, but supporting the role of glycoengineered ASA as therapeutic interventions, sialylated ACPA have been shown to reduce arthritis pathology in a mouse model (Ohmi et al. 2016).
Whether ACPA are a cause or consequence of RA status remains controversial, but they have been reported to activate effector cells via FcγR (Clavel et al. 2008), whose allotypic and copy number variation have sometimes but not always been observed to associate with RA status and severity (Thabet et al. 2009; Kastbom and Ahmadi 2005; Nieto et al. 2000; Radstake et al. 2003). Further, several longitudinal studies have observed that galactosylation and sialylation levels of ACPAs decreased shortly before symptom onset in patients who had ACPA but no evidence of RA at baseline (Pfeifle et al. 2017; Harre et al. 2015; Rombouts et al. 2015), suggesting the potential value of measuring the level of ACPA galactosylation/sialylation as a biomarker to predict the risk of progression from pre-clinical disease to chronic inflammatory disease. Beyond differences between ACPA and total IgG Fc glycosylation, differences in ACPA Fc glycan profiles have also been noted between individuals with and without rheumatoid factor, and between serum and synovial fluid (Scherer et al. 2010). While some have interpreted these differences to potentially relate to active alteration of Fc glycans in affected joints, the lack of differences in total serum and synovial fluid IgG1 agalactosylation suggests that alternative mechanisms may be at play. To this end, ACPA-secreting plasma cells have been reported to exist in synovial fluid (Rodríguez-Bayona et al. 2007), suggesting the possibility that differences in systemic versus synovial ACPA Fc glycosylation may be driven by differences associated with plasma cells in the synovium and elsewhere.
Beyond these alterations in Fc glycosylation, ACPA have more recently been reported to exhibit striking glycosylation of their Fab domains. Unlike total IgG, a majority of ACPA variable domains are glycosylated (Lloyd et al. 2018; Hafkenscheid et al. 2019; Hafkenscheid et al. 2017). Unlike their Fc domains, these APCA Fab glycans are overwhelmingly sialylated (Hafkenscheid et al. 2017). Variable domain glycosylation has also been reported to modify antigen binding among ACPA (Rombouts et al. 2016), suggesting the potential for antibody glycosylation in both variable and crystallizable domains to contribute to RA pathogenesis.
Functional consequences of variations in the profile of ASA have also been reported in fetal or neonatal allo-immune thrombocytopenia (FNAIT). In this disease condition, fetal allo-antigens induce production of maternal antibodies that are then transported across the placenta and drive lysis of fetal cells. While allotypic variation of a variety of maternal fetal antigens is possible, the best studied is that of rhesus D (RhD) antigen incompatibility. Curiously, this incompatibility, which resulted in hemolytic disease in 1% of babies born through the 1940s, 40% of which would die as a result (Bowman 2003), is treated by administration of IVIG from RhD-sensitized donors. While like IVIG used in other indications, the precise mechanisms of this intervention remain unclear; prevention of sensitization, immunomodulatory effects, and accelerated clearance of endogenous maternal IgG have all been proposed as candidate mediators. To this end, the RhD-specific antibodies in at least one commercial product show increased galactosylation and sialylation relative to the entire mixture of antibodies in that product (Winkler et al. 2013), suggesting their potential immunosuppressive character. Evaluations of the mechanism of action have been hampered by the difficulty in recapitulating protective effects of polyclonal RhD IgG with monoclonal antibodies. The difference in the effect of polyclonal versus monoclonal antibody infusions may relate to differential glycosylation of RhD-specific fraction or entire pool, differences in affinity and avidity, altered red blood cell clearance capacity, or other factors, but have led to observations of alternatively enhanced or inhibited maternal sensitization, leaving many unanswered questions (Kumpel 2007; Kumpel et al. 1995). To this end, it has been recently reported that RhD-specific monoclonal antibodies varied in their ability to clear RhD+ target cells and prevent alloimmunization, dependent on their fucosylation status and associated ADCC activity (Kumpel et al. 2020). Similarly, in the context of seropositive mothers, IgG Fc fucosylation of RhD-specific antibodies have been found to correlate with ADCC activity and low fetal neonatal hemoglobin levels (Kapur et al. 2014a).
Despite questions as to mechanism, RhD+ serum IgG has all but eliminated pregnancy loss and neonatal death from RhD incompatibility in much of the world. In contrast, other less frequently observed incompatibilities have no effective preventative interventions. For a number of these antigens, maternal antibody titer is a poor indicator of pathology, and in some of these settings, variation in ASA-Fc glycosylation has been investigated for its predictive value. Here, more mixed results as to the importance of glycosylation profiles of fetal antigen-specific antibodies have been observed. As compared to RhD-specific antibodies, those recognizing red blood cell antigens K, c, and E were less distinct from total plasma IgG Fc glycans than those recognizing RhD, but nonetheless, afucosylation of Kell (K)-specific antibodies and high galactosylation and sialylation of anti-c antibodies were correlated with severe anemia of the fetus (Sonneveld et al. 2016a). In a small follow up study of maternal K-specific antibodies, IgG1 and IgG3 fractions were shown to exhibit similar glycoform prevalences, and while the previously observed relationship between afucosylation and disease severity did not meet an arbitrary significance threshold of p = 0.05, galactose content was shown to correlate with disease severity (Sonneveld et al. 2018).
Beyond red blood cell alloantigens, Fc glycoforms of human platelet antigen 1a (HPA-1a)-specific antibodies have been analyzed. Like other maternal alloantibody responses, HPA-1a-specific antibodies show markedly decreased levels of fucosylation as compared to total serum IgG1 (Kapur et al. 2014b). These significantly less fucosylated anti-HPA-1a antibodies showed enhanced phagocytosis of platelets on account of higher binding affinity to FcγRIIIa and FcγRIIIb, but not to FcγRIIa, compared with antibodies with a high amount of Fc fucose. Most critically, the extent of HPA-1a-specific antibody Fc fucosylation was shown to correlate with clinical disease severity. In a follow-up study, stability of ASA Fc glycans was defined and correlations between bleeding severity and fucose, galactose, and antibody titer were observed (Sonneveld et al. 2016b). Similarly, Jo1 anti-histidyl tRNA synthetase autoantibodies, which are observed in idiopathic inflammatory myopathy and anti-synthetase syndrome, have demonstrated similar reductions in galactose, sialic acid, and fucose, with glycoprofiles relating to disease status (Fernandes-Cerqueira et al. 2018).
Collectively, auto- and alloimmune responses have supported the importance of Fc glycans of ASA to diverse antigens. These observations have motivated investigation of deglycosylated IgGs to prevent FNAIT (Bakchoul et al. 2013), and sialylated ACPA to treat RA (Ohmi et al. 2016). While similar evaluation of alloantibodies in the setting of organ transplant has proven challenging, the role of effector functions is well established, with assessment of complement deposition associated with transplant- or donor-specific antibodies (DSA) forming part of the basis for evaluation of suitability of transplant (Zeevi et al. 2013; Mohan et al. 2012; Stegall et al. 2011; Lefaucheur et al. 2010), and enzymatic Fc restriction of serum IgG showing potential in reducing transplant loss associated with DSA positive organ recipients (Jordan et al. 2017).
10 In vivo Fc Glycan Programming
The importance of IgG Fc glycans to Ab biology in vivo has motivated a number of interventions that take advantage of this dependence. Beyond glycoengineering of therapeutic antibodies to optimize their activity, sophisticated new approaches are being explored to control antibody activity. These include leveraging B cell-independent sialylation (Jones et al. 2016) by administration of exogenous galactosyl and sialyltransferase in order to accomplish in vivo sialylation and thereby ameliorate autoimmune disease (Pagan et al. 2018). Similarly, changes in sialyltransferase expression induced by estrogen therapy suggest alternatives to exogenous enzyme therapy (Engdahl et al. 2018).
As opposed to extending IgG Fc glycans, glycan restriction is also being employed toward the same goal of reducing autoimmunity. Glycosidase therapy, most notably EndoS from S. pyogenes, the same organism that expresses the IgG protease IdeS used to disarm HLA alloantibodies in kidney transplant, has been investigated in diverse autoimmune conditions in animal models. These settings include IgG-driven thrombocytopenia purpura (Collin et al. 2008), collagen autoimmunity (Hirose et al. 2012), anti-neutrophil cytoplasmic autoantibody-mediated glomerulonephritis (van Timmeren et al. 2010), and autoimmune hemolysis (Allhorn et al. 2010). Challenges to clinical translation remain, including the consequences of globally eliminating effector function non-specifically, as well as the induction of anti-enzyme antibodies, but recent translation of the Fc protease IdeS suggests that these barriers may be surmountable (Collin and Bjorck 2017).
Other possibilities, such as the ability to vaccinate to drive specific inflammatory or anti-inflammatory antibody responses, also exist. A future in which allergen therapy leverages B cell transcriptional programs to not only undergo CSR toward less inflammatory IgG4 molecules but also toward anti-inflammatory glycans comes to mind, as has been shown to lessen allergic reactions in a mouse model using a recombinant glycoengineered antibody (Epp et al. 2017). To this end, Vestrheim et al. considered four distinct bacterial and viral vaccines and observed that the IgG subclass that dominated the response exhibited a temporal increase in galactosylation and sialylation for most vaccinees (Vestrheim et al. 2014). Other studies have observed this effect only within the ASA fraction (Selman et al. 2012).
With a more nuanced perspective, Larsen et al. compared and contrasted ASA targeting enveloped and non-enveloped viral pathogens and found decreased fucose content that is consistent with responses to infection by enveloped viruses, though to varying extents (Larsen et al. 2020). Natural infection, at least in the case of Hepatitis B Virus, was found to better induce afucosylated IgG1 as compared to immunization with a protein subunit vaccine. In contrast, attenuated Mumps virus vaccination induced a similar level of IgG1 afucosylation as natural infection. A study considering HIV-specific IgG Fc glycans observed that vaccination was able to overcome the normally observed variations in total serum IgG associated with geography (Mahan et al. 2016). ASA showed similar glycosylation patterns for a given vaccine, but distinct vaccine regimens resulted in distinct ASA glycosylation profiles.
These and complementary observations related to difference in induction of the IgG subclasses mediated by distinct antigen, pathogen, or vaccine stimuli suggest the existence of “rules” regulating the CSR and glycosylation processes in B cells. While refined insight into these pathways continues to develop, using an in vitro B-cell culture system resembling the in vivo T-cell-dependent antibody production, Wang et al. showed that B-cells secreted variably glycosylated IgG1 when stimulated with TLR ligands, metabolites, and cytokines (Wang et al. 2011). Indeed, because the antibody Fc domain itself can regulate responses by antigen-presenting cells and B-cells, manipulation of Fc glycans in the context of immune complex vaccines has been used to intentionally influence subsequent Ab induction/maturation (Lofano et al. 2018).
11 Summary and Future Perspectives
Distinctly different global IgG and ASA Fc profiles have been observed in both infectious disease and auto- and alloimmune settings. Studying the Fc glycosylation profile of ASA presents an excellent opportunity to understand the mechanistic underpinnings and the in vivo regulation of the diverse adaptive immune processes that define protective and pathological humoral responses. To this end, many unanswered questions remain.
Abbreviations
- ACPA:
-
Anti-citrullinated protein antibodies
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- ADE:
-
Antibody-dependent enhancement
- AMI:
-
Antibody-mediated immunity
- ASA:
-
Antigen-specific antibody
- CDC:
-
Complement-dependent cytotoxicity
- COVID-19:
-
Coronavirus disease 2019
- CSR:
-
Class switch recombination
- DC:
-
Dendritic cell
- DENV:
-
Dengue virus
- EndoS:
-
Endoglycosidase S
- Fab:
-
Fragment antigen-binding
- Fc:
-
Fragment crystallizable
- FcR:
-
Fc receptor
- FNAIT:
-
Fetal or neonatal allo-immune thrombocytopenia
- Fv:
-
Fragment variable
- GlcNAc:
-
N-acetylglucosamine
- HPA-1a:
-
Human platelet antigen 1a
- IdeS:
-
IgG digesting enzyme S
- IgG:
-
Immunoglobulin G
- IVIg:
-
Intravenous immunoglobulin
- K:
-
Kell
- mAb:
-
Monoclonal antibody
- MAC:
-
Membrane attack complex
- MBL:
-
Mannose-binding lectin
- MHC:
-
Major histocompatibility complex
- NK:
-
Natural killer
- HIV:
-
Human immunodeficiency virus
- RA:
-
Rheumatoid arthritis
- RhD:
-
Rhesus D
- RSV:
-
Respiratory syncytial virus
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
References
Ackerman ME et al (2013) Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 123:2183–2192. https://doi.org/10.1172/JCI65708
Ackerman ME et al (2018) Route of immunization defines multiple mechanisms of vaccine-mediated protection against SIV. Nat Med 24:1590–1598. https://doi.org/10.1038/s41591-018-0161-0
Allhorn M et al (2010) The IgG-specific endoglycosidase EndoS inhibits both cellular and complement-mediated autoimmune hemolysis. Blood 115:5080–5088. https://doi.org/10.1182/blood-2009-08-239020
Alter G, Ottenhoff THM, Joosten SA (2018a) Antibody glycosylation in inflammation, disease and vaccination. Semin Immunol 39:102–110. https://doi.org/10.1016/j.smim.2018.05.003
Alter G et al (2018b) High-resolution definition of humoral immune response correlates of effective immunity against HIV. Mol Syst Biol 14:e7881. https://doi.org/10.15252/msb.20177881
Ana-Sosa-Batiz F et al (2016) Influenza-specific antibody-dependent phagocytosis. PLoS One 11:e0154461. https://doi.org/10.1371/journal.pone.0154461
Anthony RM, Wermeling F, Karlsson MCI, Ravetch JV (2008) Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci 105:19571–19578. https://doi.org/10.1073/pnas.0810163105
Bakchoul T et al (2013) Inhibition of HPA-1a alloantibody-mediated platelet destruction by a deglycosylated anti–HPA-1a monoclonal antibody in mice: toward targeted treatment of fetal-alloimmune thrombocytopenia. Blood 122:321–327. https://doi.org/10.1182/blood-2012-11-468561
Barouch DH et al (2013) Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155:531–539. https://doi.org/10.1016/j.cell.2013.09.061
Barouch DH et al (2015) Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 349:320–324. https://doi.org/10.1126/science.aab3886
Bayry J (2016) Lupus pathogenesis: role of IgE autoantibodies. Cell Res 26:271–272. https://doi.org/10.1038/cr.2016.12
Benhnia MR-E-I et al (2009) Heavily isotype-dependent protective activities of human antibodies against vaccinia virus extracellular virion antigen B5▿. J Virol 83:12355–12367. https://doi.org/10.1128/jvi.01593-09
Bondt A et al (2014) Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol Cell Proteomics 13:3029–3039. https://doi.org/10.1074/mcp.M114.039537
Bournazos S, Ravetch JV (2017) Fcγ receptor function and the design of vaccination strategies. Immunity 47:224–233. https://doi.org/10.1016/j.immuni.2017.07.009
Bowman J (2003) Thirty-five years of Rh prophylaxis. Transfusion 43:1661–1666. https://doi.org/10.1111/j.0041-1132.2003.00632.x
Bruggeman CW et al (2018) IgG Glyco-engineering to improve IVIg potency. Front Immunol 9:2442. https://doi.org/10.3389/fimmu.2018.02442
Bukreyev A, Yang L, Collins PL (2012) The secreted G protein of human respiratory syncytial virus antagonizes antibody-mediated restriction of replication involving macrophages and complement. J Virol 86:10880–10884. https://doi.org/10.1128/jvi.01162-12
Casadevall A, Pirofski, L.-a. (2012) Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 11:447–456. https://doi.org/10.1016/j.chom.2012.04.004
Castro-Dopico T, Clatworthy MR (2016) Fcγ receptors in solid organ transplantation. Curr Transplant Rep 3:284–293. https://doi.org/10.1007/s40472-016-0116-7
Chakraborty S et al (2020) Proinflammatory IgG Fc structures in patients with severe COVID-19. Nat Immunol. https://doi.org/10.1038/s41590-020-00828-7
Cheng HD et al (2019) IgG Fc glycosylation as an axis of humoral immunity in childhood. J Allergy Clin Immunol 145:710–713.e719. https://doi.org/10.1016/j.jaci.2019.10.012
Chon JH, Zarbis-Papastoitsis G (2011) Advances in the production and downstream processing of antibodies. New Biotechnol 28:458–463. https://doi.org/10.1016/j.nbt.2011.03.015
Churdboonchart V, Futrakul P, Bhamarapravati N (1983) Crossed immunoelectrophoresis for the detection of split products of the third complement in dengue hemorrhagic fever: I. Observations in patients’ plasma*. Am J Tropical Med Hyg 32:569–576. https://doi.org/10.4269/ajtmh.1983.32.569
Clavel C et al (2008) Induction of macrophage secretion of tumor necrosis factor α through Fcγ receptor IIa engagement by rheumatoid arthritis–specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum 58:678–688. https://doi.org/10.1002/art.23284
Co MDT et al (2014) Relationship of preexisting influenza hemagglutination inhibition, complement-dependent lytic, and antibody-dependent cellular cytotoxicity antibodies to the development of clinical illness in a prospective study of A(H1N1)pdm09 influenza in children. Viral Immunol 27:375–382. https://doi.org/10.1089/vim.2014.0061
Collin M, Bjorck L (2017) Toward clinical use of the IgG specific enzymes IdeS and EndoS against antibody-mediated diseases. Methods Mol Biol 1535:339–351. https://doi.org/10.1007/978-1-4939-6673-8_23
Collin M, Shannon O, Bjorck L (2008) IgG glycan hydrolysis by a bacterial enzyme as a therapy against autoimmune conditions. Proc Natl Acad Sci USA 105:4265–4270. https://doi.org/10.1073/pnas.0711271105
Coutant F (2019) Pathogenic effects of anti-citrullinated peptide antibodies in rheumatoid arthritis – role for glycosylation. Joint Bone Spine 86:562–567. https://doi.org/10.1016/j.jbspin.2019.01.005
Crooks ET et al (2018) Glycoengineering HIV-1 Env creates ‘supercharged’ and ‘hybrid’ glycans to increase neutralizing antibody potency, breadth and saturation. PLoS Pathog 14:e1007024. https://doi.org/10.1371/journal.ppat.1007024
de Haan N, Reiding KR, Driessen G, van der Burg M, Wuhrer M (2016) Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J Proteome Res 15:1853–1861. https://doi.org/10.1021/acs.jproteome.6b00038
Decker Y et al (2016) Abnormal galactosylation of immunoglobulin G in cerebrospinal fluid of multiple sclerosis patients. Mult Scler J 22:1794–1803. https://doi.org/10.1177/1352458516631036
Dekkers G et al (2016) Multi-level glyco-engineering techniques to generate IgG with defined Fc-glycans. Sci Rep 6:36964. https://doi.org/10.1038/srep36964
Dekkers G et al (2017) Decoding the human immunoglobulin G-glycan repertoire reveals a Spectrum of Fc-receptor- and complement-mediated-effector activities. Front Immunol 8:877. https://doi.org/10.3389/fimmu.2017.00877
Dekkers G, Rispens T, Vidarsson G (2018) Novel concepts of altered immunoglobulin G galactosylation in autoimmune diseases. Front Immunol 9:553. https://doi.org/10.3389/fimmu.2018.00553
Dunkelberger JR, Song W-C (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20:34–50. https://doi.org/10.1038/cr.2009.139
Engdahl C et al (2018) Estrogen induces St6gal1 expression and increases IgG sialylation in mice and patients with rheumatoid arthritis: a potential explanation for the increased risk of rheumatoid arthritis in postmenopausal women. Arthritis Res Ther 20:84. https://doi.org/10.1186/s13075-018-1586-z
Epp A et al (2017) Sialylation of IgG antibodies inhibits IgG-mediated allergic reactions. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2017.06.021
Ercan A et al (2017) Estrogens regulate glycosylation of IgG in women and men. Jci Insight 2:e89703. https://doi.org/10.1172/jci.insight.89703
Fernandes-Cerqueira C et al (2018) Patients with anti-Jo1 antibodies display a characteristic IgG Fc-glycan profile which is further enhanced in anti-Jo1 autoantibodies. Sci Rep 8:17958. https://doi.org/10.1038/s41598-018-36395-z
Ferrara C et al (2011) Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci 108:12669–12674. https://doi.org/10.1073/pnas.1108455108
Forslind K et al (2004) Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis 63:1090. https://doi.org/10.1136/ard.2003.014233
Füst G et al (1994) Neutralizing and enhancing antibodies measured in complement-restored serum samples from HIV-1-infected individuals correlate with immunosuppression and disease. AIDS 8:603–610. https://doi.org/10.1097/00002030-199405000-00005
Gardinassi LG et al (2014) Clinical severity of visceral leishmaniasis is associated with changes in immunoglobulin G Fc N-glycosylation. MBio 5:e01844–e01814. https://doi.org/10.1128/mbio.01844-14
Gerber JS, Mosser DM (2001) Stimulatory and inhibitory signals originating from the macrophage Fcγ receptors. Microbes Infect 3:131–139. https://doi.org/10.1016/s1286-4579(00)01360-5
Goldberg BS, Ackerman ME (2020) Antibody-mediated complement activation in pathology and protection. Immunol Cell Biol 98:305–317. https://doi.org/10.1111/imcb.12324
Gonzalez SF et al (2010) Complement-dependent transport of antigen into B cell follicles. J Immunol 185:2659–2664. https://doi.org/10.4049/jimmunol.1000522
Grafals M, Thurman JM (2019) The role of complement in organ transplantation. Front Immunol 10:2380. https://doi.org/10.3389/fimmu.2019.02380
Gudelj I, Lauc G, Pezer M (2018) Immunoglobulin G glycosylation in aging and diseases. Cell Immunol 333:65–79. https://doi.org/10.1016/j.cellimm.2018.07.009
Guzman MG, Alvarez M, Halstead SB (2013) Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 158:1445–1459. https://doi.org/10.1007/s00705-013-1645-3
Haan N (2018) Differences in IgG Fc glycosylation are associated with outcome of pediatric Meningococcal sepsis. MBio 9:e00546–e00518. https://doi.org/10.1128/mbio.00546-18
Hafkenscheid L et al (2017) Structural analysis of variable domain glycosylation of anti-citrullinated protein antibodies in rheumatoid arthritis reveals the presence of highly sialylated glycans. Mol Cell Proteomics 16:278–287. https://doi.org/10.1074/mcp.m116.062919
Hafkenscheid L et al (2019) N-linked glycans in the variable domain of IgG anti–citrullinated protein antibodies predict the development of rheumatoid arthritis. Arthritis Rheumatol 71:1626–1633. https://doi.org/10.1002/art.40920
Harre U et al (2015) Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun 6:6651. https://doi.org/10.1038/ncomms7651
He W et al (2017) Alveolar macrophages are critical for broadly-reactive antibody-mediated protection against influenza A virus in mice. Nat Commun 8:846. https://doi.org/10.1038/s41467-017-00928-3
Hebell T, Ahearn JM, Fearon DT (1991) Suppression of the immune response by a soluble complement receptor of B lymphocytes. Science 254:102–105. https://doi.org/10.1126/science.1718035
Hirose M et al (2012) Enzymatic autoantibody glycan hydrolysis alleviates autoimmunity against type VII collagen. J Autoimmun 39:304–314. https://doi.org/10.1016/j.jaut.2012.04.002
Hirvinen M et al (2013) Fc-gamma receptor polymorphisms as predictive and prognostic factors in patients receiving oncolytic adenovirus treatment. J Transl Med 11:1–12. https://doi.org/10.1186/1479-5876-11-193
Hodoniczky J, Zheng YZ, James DC (2005) Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol Prog 21:1644–1652. https://doi.org/10.1021/bp050228w
Hoepel W et al (2020) Anti-SARS-CoV-2 IgG from severely ill COVID-19 patients promotes macrophage hyper-inflammatory responses. Biorxiv 2020.2007.2013.190140. https://doi.org/10.1101/2020.07.13.190140
Hogarth PM (2002) Fc receptors are major mediators of antibody based inflammation in autoimmunity. Curr Opin Immunol 14:798–802. https://doi.org/10.1016/s0952-7915(02)00409-0
Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW (2001) Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol 166:7381–7388. https://doi.org/10.4049/jimmunol.166.12.7381
Hunter M, Yuan P, Vavilala D, Fox M (2019) Optimization of protein expression in mammalian cells. Curr Protoc Protein Sci 95:e77. https://doi.org/10.1002/cpps.77
Jefferis R (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8:226–234. https://doi.org/10.1038/nrd2804
Jennewein MF, Alter G (2017) The immunoregulatory roles of antibody glycosylation. Trends Immunol 38:358–372. https://doi.org/10.1016/j.it.2017.02.004
Jones MB et al (2016) B-cell-independent sialylation of IgG. Proc Natl Acad Sci USA 113:7207–7212. https://doi.org/10.1073/pnas.1523968113
Jordan SC et al (2017) IgG endopeptidase in highly sensitized patients undergoing transplantation. New Engl J Med 377:442–453. https://doi.org/10.1056/nejmoa1612567
Kaneko Y, Nimmerjahn F, Ravetch JV (2006) Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313:670–673. https://doi.org/10.1126/science.1129594
Kapur R et al (2014a) Low anti-RhD IgG-Fc-fucosylation in pregnancy: a new variable predicting severity in haemolytic disease of the fetus and newborn. Br J Haematol 166:936–945. https://doi.org/10.1111/bjh.12965
Kapur R et al (2014b) A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 123:471–480. https://doi.org/10.1182/blood-2013-09-527978
Karsten CM et al (2012) Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 18:1401–1406. https://doi.org/10.1038/nm.2862
Kastbom A, Ahmadi A (2005) Söderkvist, P. & Skogh, T. The 158V polymorphism of fc gamma receptor type IIIA in early rheumatoid arthritis: increased susceptibility and severity in male patients (the Swedish TIRA*TIRA is a Swedish acronym for ‘early invention in rheumatoid arthritis’ and is a multicentre cooperation between rheumatology units in southeastern Sweden. project). Rheumatology 44:1294–1298. https://doi.org/10.1093/rheumatology/kei010
Katzelnick LC et al (2017) Antibody-dependent enhancement of severe dengue disease in humans. Science eaan6836. https://doi.org/10.1126/science.aan6836
Khandia R et al (2018) Modulation of dengue/Zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in Zika virus infection. Front Immunol 9:597. https://doi.org/10.3389/fimmu.2018.00597
Kim H-Y, Stojadinovic A, Izadjoo MJ (2014) Affinity maturation of monoclonal antibodies by multi-site-directed mutagenesis. Methods Mol Biol Clifton N J 1131:407–420. https://doi.org/10.1007/978-1-62703-992-5_24
Köhler G, Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495–497. https://doi.org/10.1038/256495a0
Krištić J et al (2013) Glycans are a novel biomarker of chronological and biological ages. J Gerontol Ser 69:779–789. https://doi.org/10.1093/gerona/glt190
Kumpel BM (2007) Efficacy of RhD monoclonal antibodies in clinical trials as replacement therapy for prophylactic anti-D immunoglobulin: more questions than answers. Vox Sang 93:99–111. https://doi.org/10.1111/j.1423-0410.2007.00945.x
Kumpel BM et al (1995) Human Rh D monoclonal antibodies (BRAD-3 and BRAD-5) cause accelerated clearance of Rh D+ red blood cells and suppression of Rh D immunization in Rh D-volunteers. Blood 86:1701–1709
Kumpel BM et al (2020) Anti-D monoclonal antibodies from 23 human and rodent cell lines display diverse IgG Fc-glycosylation profiles that determine their clinical efficacy. Sci Rep 10:1464. https://doi.org/10.1038/s41598-019-57393-9
Larsen MD et al (2020) Afucosylated immunoglobulin G responses are a hallmark of enveloped virus infections and show an exacerbated phenotype in COVID-19. Biorxiv 2020.2005.2018.099507. https://doi.org/10.1101/2020.05.18.099507
Lastra GC, Thompson SJ, Lemonidis AS, Elson CJ (2009) Changes in the galactose content of IgG during humoral immune responses. Autoimmunity 28:25–30. https://doi.org/10.3109/08916939808993842
Lazar GA et al (2006) Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA 103:4005–4010. https://doi.org/10.1073/pnas.0508123103
Lefaucheur C et al (2010) Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 21:1398–1406. https://doi.org/10.1681/asn.2009101065
Li X, Kimberly RP (2014) Targeting the Fc receptor in autoimmune disease. Expert Opin Ther Tar 18:335–350. https://doi.org/10.1517/14728222.2014.877891
Liu L (2015) Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J Pharm Sci 104:1866–1884. https://doi.org/10.1002/jps.24444
Lloyd KA et al (2018) Variable domain N-linked glycosylation and negative surface charge are key features of monoclonal ACPA: implications for B-cell selection. Eur J Immunol 48:1030–1045. https://doi.org/10.1002/eji.201747446
Lofano G et al (2018) Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci Immunol 3:eaat7796. https://doi.org/10.1126/sciimmunol.aat7796
Lu LL et al (2016) A functional role for antibodies in tuberculosis. Cell 167:433–443.e414. https://doi.org/10.1016/j.cell.2016.08.072
Lu LL et al (2020) Antibody Fc glycosylation discriminates between latent and active tuberculosis. J Infect Dis. https://doi.org/10.1093/infdis/jiz643
Lundström SL et al (2014) IgG antibodies to cyclic Citrullinated peptides exhibit profiles specific in terms of IgG subclasses, Fc-Glycans and a Fab-peptide sequence. PLoS One 9:e113924. https://doi.org/10.1371/journal.pone.0113924
Mahan AE et al (2016) Antigen-specific antibody glycosylation is regulated via vaccination. PLoS Pathog 12:e1005456. https://doi.org/10.1371/journal.ppat.1005456
Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS (2013) Presentation of phagocytosed antigens by MHC class I and II. Traffic 14:135–152. https://doi.org/10.1111/tra.12026
Mesin L, Ersching J, Victora GD (2016) Germinal center B cell dynamics. Immunity 45:471–482. https://doi.org/10.1016/j.immuni.2016.09.001
Mohan S et al (2012) Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol 23:2061–2071. https://doi.org/10.1681/asn.2012070664
Moore JS et al (2005) Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19:381–389. https://doi.org/10.1097/01.aids.0000161767.21405.68
Nagelkerke SQ, Kuijpers TW (2015) Immunomodulation by IVIg and the role of Fc-gamma receptors: classic mechanisms of action after all? Front Immunol 5:674. https://doi.org/10.3389/fimmu.2014.00674
Nascimento EJM et al (2009) Alternative complement pathway deregulation is correlated with dengue severity. PLoS One 4:e6782. https://doi.org/10.1371/journal.pone.0006782
Nieto A et al (2000) Involvement of Fcγ receptor IIIA genotypes in susceptibility to rheumatoid arthritis. Arthritis Rheum 43:735–739. https://doi.org/10.1002/1529-0131(200004)43:4<735::aid-anr3>3.0.co;2-q
Nimmerjahn F, Ravetch JV (2008a) Fcgamma receptors as regulators of immune responses. Nat Rev Immunol 8:34–47. https://doi.org/10.1038/nri2206
Nimmerjahn F, Ravetch JV (2008b) Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 26:513–533. https://doi.org/10.1146/annurev.immunol.26.021607.090232
Nimmerjahn F, Anthony RM, Ravetch JV (2007) Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci 104:8433–8437. https://doi.org/10.1073/pnas.0702936104
Noris M, Remuzzi G (2013) Overview of complement activation and regulation. Semin Nephrol 33:479–492. https://doi.org/10.1016/j.semnephrol.2013.08.001
Ohmi Y et al (2016) Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun 7:11205. https://doi.org/10.1038/ncomms11205
Pagan JD, Kitaoka M, Anthony RM (2018) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172:564–577 e513. https://doi.org/10.1016/j.cell.2017.11.041
Parekh RB et al (1985) Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature 316:452–457. https://doi.org/10.1038/316452a0
Parekh R et al (1989) A comparative analysis of disease-associated changes in the galactosylation of serum IgG. J Autoimmun 2:101–114. https://doi.org/10.1016/0896-8411(89)90148-0
Peschke B, Keller CW, Weber P, Quast I, Lünemann JD (2017) Fc-Galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol 8:646. https://doi.org/10.3389/fimmu.2017.00646
Petrovic T et al (2020) Composition of the immunoglobulin G glycome associates with the severity of COVID-19. Glycobiology. https://doi.org/10.1093/glycob/cwaa102
Pfeifle R et al (2017) Regulation of autoantibody activity by the IL-23–TH17 axis determines the onset of autoimmune disease. Nat Immunol 18:104–113. https://doi.org/10.1038/ni.3579
Pittala S et al (2019) Antibody Fab-Fc properties outperform titer in predictive models of SIV vaccine-induced protection. Mol Syst Biol 15:e8747. https://doi.org/10.15252/msb.20188747
Rademacher TW, Williams P, Dwek RA (1994) Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc Natl Acad Sci 91:6123–6127. https://doi.org/10.1073/pnas.91.13.6123
Radstake TRDJ et al (2003) Role of Fcgamma receptors IIA, IIIA, and IIIB in susceptibility to rheumatoid arthritis. J Rheumatol 30:926–933
Rodríguez-Bayona B, Pérez-Venegas JJ, Rodríguez C, Brieva JA (2007) CD95-mediated control of anti-citrullinated protein/peptides antibodies (ACPA)-producing plasma cells occurring in rheumatoid arthritis inflamed joints. Rheumatology 46:612–616. https://doi.org/10.1093/rheumatology/kel395
Rombouts Y et al (2015) Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 74:234. https://doi.org/10.1136/annrheumdis-2013-203565
Rombouts Y et al (2016) Extensive glycosylation of ACPA-IgG variable domains modulates binding to citrullinated antigens in rheumatoid arthritis. Ann Rheum Dis 75:578. https://doi.org/10.1136/annrheumdis-2014-206598
Rönnelid J et al (2005) Longitudinal analysis of citrullinated protein/peptide antibodies (anti-CP) during 5 year follow up in early rheumatoid arthritis: anti-CP status predicts worse disease activity and greater radiological progression. Ann Rheum Dis 64:1744. https://doi.org/10.1136/ard.2004.033571
Rycke LD et al (2004) Rheumatoid factor and anticitrullinated protein antibodies in rheumatoid arthritis: diagnostic value, associations with radiological progression rate, and extra-articular manifestations. Ann Rheum Dis 63:1587. https://doi.org/10.1136/ard.2003.017574
Saunders, Conceptual O (2019) Approaches to modulating antibody effector functions and circulation half-life. Front Immunol 10:1296. https://doi.org/10.3389/fimmu.2019.01296
Scherer HU et al (2010) Glycan profiling of anti–citrullinated protein antibodies isolated from human serum and synovial fluid. Arthritis Rheum 62:1620–1629. https://doi.org/10.1002/art.27414
Schroeder HW, Cavacini L (2010) Structure and function of immunoglobulins. J Allergy Clin Immunol 125:S41–S52. https://doi.org/10.1016/j.jaci.2009.09.046
Schwab I, Nimmerjahn F (2013) Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 13:176–189. https://doi.org/10.1038/nri3401
Selman MHJ et al (2012) Changes in antigen-specific IgG1 Fc N-glycosylation upon influenza and tetanus vaccination. Mol Cell Proteomics 11:M111.014563. https://doi.org/10.1074/mcp.M111.014563
Shields RL et al (2002) Lack of Fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740. https://doi.org/10.1074/jbc.m202069200
Shinkawa T et al (2002) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473. https://doi.org/10.1074/jbc.m210665200
Smyth MJ et al (2005) Activation of NK cell cytotoxicity. Mol Immunol 42:501–510. https://doi.org/10.1016/j.molimm.2004.07.034
Song YW, Kang EH (2010) Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. Qjm Int J Med 103:139–146. https://doi.org/10.1093/qjmed/hcp165
Sonneveld ME et al (2016a) Antigen specificity determines anti-red blood cell IgG-Fc alloantibody glycosylation and thereby severity of haemolytic disease of the fetus and newborn. Br J Haematol. https://doi.org/10.1111/bjh.14438
Sonneveld ME et al (2016b) Glycosylation pattern of anti-platelet IgG is stable during pregnancy and predicts clinical outcome in alloimmune thrombocytopenia. Br J Haematol. https://doi.org/10.1111/bjh.14053
Sonneveld ME et al (2018) Fc-glycosylation in human IgG1 and IgG3 is similar for both total and anti-red-blood cell anti-K antibodies. Front Immunol 9:129. https://doi.org/10.3389/fimmu.2018.00129
Stackowicz J, Jönsson F, Reber LL (2020) Mouse models and tools for the in vivo study of neutrophils. Front Immunol 10:3130. https://doi.org/10.3389/fimmu.2019.03130
Stavnezer J, Schrader CE (2014) IgH chain class switch recombination: mechanism and regulation. J Immunol 193:5370–5378. https://doi.org/10.4049/jimmunol.1401849
Stegall MD et al (2011) Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients: terminal complement inhibition decreases antibody-mediated rejection. Am J Transplant 11:2405–2413. https://doi.org/10.1111/j.1600-6143.2011.03757.x
Subedi GP, Barb AW (2016) The immunoglobulin G1 N-glycan composition affects binding to each low affinity Fc gamma receptor. MAbs 8:1512–1524. https://doi.org/10.1080/19420862.2016.1218586
Temming AR et al (2019) Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep 9:9995. https://doi.org/10.1038/s41598-019-46484-2
Thabet MM et al (2009) Contribution of Fcγ receptor IIIA gene 158V/F polymorphism and copy number variation to the risk of ACPA-positive rheumatoid arthritis. Ann Rheum Dis 68:1775. https://doi.org/10.1136/ard.2008.099309
Thomann M et al (2015) In vitro Glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity. PLoS One 10:e0134949. https://doi.org/10.1371/journal.pone.0134949
Thulin NK et al (2020) Maternal anti-dengue IgG fucosylation predicts susceptibility to dengue disease in infants. Cell Rep 31:107642. https://doi.org/10.1016/j.celrep.2020.107642
Tomana M, Schrohenloher RE, Reveille JD, Arnett FC, Koopman WJ (1992) Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol Int 12:191–194. https://doi.org/10.1007/bf00302151
Tsuchiya N et al (1989) Effects of galactose depletion from oligosaccharide chains on immunological activities of human IgG. J Rheumatol 16:285–290
Vaccari M et al (2016) Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med 22:762–770. https://doi.org/10.1038/nm.4105
van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB (2019) Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol 10:548. https://doi.org/10.3389/fimmu.2019.00548
van Timmeren MM et al (2010) IgG glycan hydrolysis attenuates ANCA-mediated glomerulonephritis. J Am Soc Nephrol 21:1103–1114. https://doi.org/10.1681/ASN.2009090984
Vanderven HA et al (2017) Fc functional antibodies in humans with severe H7N9 and seasonal influenza. Jci Insight 2:e92750. https://doi.org/10.1172/jci.insight.92750
Verkoczy L (2017) Chapter five humanized immunoglobulin mice models for HIV vaccine testing and studying the broadly neutralizing antibody problem. Adv Immunol 134:235–352. https://doi.org/10.1016/bs.ai.2017.01.004
Vestrheim AC et al (2014) A pilot study showing differences in glycosylation patterns of IgG subclasses induced by pneumococcal, meningococcal, and two types of influenza vaccines. Immun Inflamm Dis 2:76–91. https://doi.org/10.1002/iid3.22
Vidarsson G, Dekkers G, Rispens T (2014) IgG subclasses and Allotypes: from structure to effector functions. Front Immunol 5:520. https://doi.org/10.3389/fimmu.2014.00520
Vogt MR et al (2011) Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcγ receptor and complement-dependent effector mechanisms. J Virol 85:11567–11580. https://doi.org/10.1128/jvi.05859-11
Walsh NC et al (2016) Humanized mouse models of clinical disease. Annu Rev Pathol Mech Dis 12:187–215. https://doi.org/10.1146/annurev-pathol-052016-100332
Wang TT, Ravetch JV (2019) Functional diversification of IgGs through Fc glycosylation. J Clin Invest 129:3492–3498. https://doi.org/10.1172/jci130029
Wang J et al (2011) Fc-glycosylation of IgG1 is modulated by B-cell stimuli. Mol Cell Proteomics 10:M110.004655. https://doi.org/10.1074/mcp.m110.004655
Wang TT et al (2017) IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science 355:395–398. https://doi.org/10.1126/science.aai8128
Wang Q et al (2019) Design and production of bispecific antibodies. Antibodies 8:43. https://doi.org/10.3390/antib8030043
Winkler A, Berger M, Ehlers M (2013) Anti-rhesus D prophylaxis in pregnant women is based on sialylated IgG antibodies. F1000research 2(169). https://doi.org/10.12688/f1000research.2-169.v1
Winzeler A, Wang JT (2013) Culturing hybridoma cell lines for monoclonal antibody production. Cold Spring Harbor Protocols 2013:pdb.prot074914. https://doi.org/10.1101/pdb.prot074914
Worley MJ et al (2018) Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods 457:41–52. https://doi.org/10.1016/j.jim.2018.03.007
Wu J et al (2014) Functional Fcgamma receptor polymorphisms are associated with human allergy. PLoS One 9:e89196. https://doi.org/10.1371/journal.pone.0089196
Wu Y et al (2015) A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat Commun 6:7708. https://doi.org/10.1038/ncomms8708
Yamaguchi Y et al (2006) Glycoform-dependent conformational alteration of the Fc region of human immunoglobulin G1 as revealed by NMR spectroscopy. Biochim Biophys Acta Bba – Gen Subj 1760:693–700. https://doi.org/10.1016/j.bbagen.2005.10.002
Zeevi A et al (2013) Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Hear Lung Transplant 32:98–105. https://doi.org/10.1016/j.healun.2012.09.021
Acknowledgments
The authors thank Chanc VanWinkle Orzell for copyediting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding
This study was funded in part by the National Institutes of Health NIAID and NIGMS (grant number R01AI131975).
Conflict of Interest
PB declares he has no conflict of interest. MEA declares that she has no conflict of interest.
Ethical Approval
This chapter does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bharadwaj, P., Ackerman, M.E. (2021). Glycosylation of Antigen-Specific Antibodies: Perspectives on Immunoglobulin G Glycosylation in Vaccination and Immunotherapy. In: Pezer, M. (eds) Antibody Glycosylation. Experientia Supplementum, vol 112. Springer, Cham. https://doi.org/10.1007/978-3-030-76912-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-76912-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76911-6
Online ISBN: 978-3-030-76912-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)