Abstract
Artificial insemination is a key technique to increase reproductive efficiency and the genetic improvement in cattle. The first report of artificial insemination has been dated to more than 220 years ago; after that, many studies have been published, and this biotechnology is widely used in domestic animals. Despite the advances of embryo transfer programs in vivo and in vitro in cattle, artificial insemination still represents the main form of assisted reproduction in many herds, being one of the lowest cost strategies to improve genetic merit in the farms. Artificial insemination has many advantages compared to natural breeding, such as health benefits, e.g. control of infectious diseases; genetic improvement, e.g. use of genetically improved bulls; reproductive management improvements; and more stringent control of the zootechnical and economic features of the herd, e.g. standardized management and animals.
In addition, we highlight the main advances related to the use of artificial insemination in cattle, such as the development of other reproductive biotechniques, such as timed artificial insemination and resynchronization of estrus or ovulation. In addition, the sexed semen has evolved satisfactorily with promising results both in insemination and in embryo production. An important advance in artificial insemination is related to the hormonal control of the estrous cycle that allows the elaboration of highly efficient protocols for the synchronization of estrus and ovulation. The hormonal protocols have contributed to the dissemination of the artificial insemination by allowing the insemination of many females in a short period of time. Based on many studies evaluating timed artificial insemination protocols and early and accurate pregnancy diagnosis techniques, it was also possible to establish estrus and ovulation resynchronization strategies, which have greatly contributed to economic profitability. Finally, here we review many strategies associated with manipulation of the dominant follicle, estrus behavioral and antral follicle count have been elaborated and they represent an important progress to improve the use of timed artificial insemination in cattle. Therefore, this chapter aims to present the main advances of artificial insemination and timed artificial insemination, highlighting the technical parameters, advantages, and influencing factors, and to discuss practical and current strategies for the improvement of the herd via artificial insemination programs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cattle
- Estrous cycle
- Synchronization
- Artificial insemination
- Timed artificial insemination
- Resynchronization
- Sexed semen
- Pregnancy
- Antral follicle
- Fertility
1.1 Introduction
For definition artificial insemination (AI) is a procedure characterized by manually semen depositionin the female reproductive tract. This practice is considered the oldest reproductive biotechnology and is one of the more efficient strategies for genetically improving livestock and increasing their reproductive efficiency. Therefore, AI is still considered the most widely used biotechnology in the world due to its genetic, health and economic benefits for beef and dairy cattle.
The productive and reproductive performance of the herd are considered the main factors responsible for affecting the livestock economic scenario. However, reproductive traits have low heritability and can be highly affected by environmental, nutritional, and health factors. Thus, adequate management of all livestock sectors is requiredto increase productive and reproductive efficiency and achieve economic autonomy. Therefore, to use reproductive strategies with maximum efficiency, it is necessary to control influencing factors as well as ensure that the overall management is suitable for livestock production.
Conventional AI programs that using estrus detection and insemination procedure have been used for a long time and still represents the main form of reproduction in many herds, mainly for dairy cattle (Smith et al. 2018). However, low service rates due to estrus detection failures represents one of the main obstacles to improving reproductive efficiency for both beef and dairy cattle. This problem is most evident in Bos indicus herds that have a short duration of estrus and high percentage of estrus behavior that occurs at night in relation Bos taurus females (Pinheiro et al. 1998; Galina et al. 1996). In addition to the failures in the estrus detection and the particularities of estrus behavior, a high proportion of cows managed in tropical pastures face nutritional problems that intensify the postpartum anestrus, hindering the return of cyclicality and compromising the efficient use of conventional AI (Baruselli et al. 2004a, 2018).
On the other hand, timed artificial insemination (TAI) have emerged as an alternative to conventional AI programs, in addition to representing the most effective strategy for employment and dissemination of AI and its benefits, mainly for large-scale programs, because the TAI aims for efficient control of estrus and ovulation of all females destined for breeding. After the onset of programs for the synchronization of ovulation, the estrus detection was dispensed, and TAI became widely used because it allows ovulation control and insemination of large numbers of females in a predetermined amount of time. Many advantages have been added to the AI technique associated with synchronization of ovulation, such as elimination of estrus detection, improvement of livestock management, planning of services and financial resources, and improvement of pregnancy rates depending on categories and reproductive characteristics of the herd (Colazo and Mapletoft 2014; Lamb et al. 2010; Baruselli et al. 2004a). TAI protocols synchronize follicular recruitment and growth, regression of the corpus luteum (CL), and ovulation of the dominant follicle, improving reproductive performance because all females are inseminated regardless of their estrus behavior (Lamb and Mercadante 2016). Therefore, TAI programs have been used as a reproductive tool that better enables the expansion of AI in dairy and beef cattle.
After the first protocol of ovulation synchronization, a wide variety of protocols have been developed focusing on adjustmentsin female category to minimize management and cost and to obtain more reproductive efficiency. Among various hormonal combinations for TAI in cattle, the association of progesterone/progestin (P4) or gonadotropins with estrogen and prostaglandins (PGF2α) is noteworthy (Baruselli and Teixeira 2018; Baruselli et al. 2004a). Other reproductive strategies such as estrus or ovulation resynchronization have been successfully used to reduce the number of females that fail pregnancy after the first AI procedure. In addition, resynchronization has been used to increase pregnancy rates in the short time of the breeding season and reduces the use of bulls for natural breeding (Marques et al. 2015; Campos et al. 2013). This chapter aims to present the main advances of AI and TAI , highlighting mainly the technical parameters, advantages, and influencing factors and to discuss practical and current strategies for improving reproductive performance in cattle.
1.2 Artificial Insemination Program: Benefits and Advantages
Genetic improvement is considered one of the main benefits of using AI (Fig. 1.1). In cattle, the genetic improvement is achieved by the choice of proven bulls with desirable characteristics for enhancement in milk or meat production. In addition, AI also provides genetic gain in a short amount of time and low cost compared to natural breeding because semen storage is an economically viable alternative compared to purchase and keeping a bull with high zootechnynical potential in the herd (Baruselli et al. 2018).
Another benefit associated with the use of AI programs is the intensification and standardization of reproductive management of the herd for better zootechnical control of animals. Particularly, of this is important in tropical countries, because the majority of beef and dairy production comes from herds kept almost exclusively in grazing conditions. This strategy represents a low-cost production system; however, some management practices need some adjustments to achieve success after using reproductive biotechnologies. Thus, optimized management practices and better zootechnical control allows for more efficient production in addition to the selection of the most fertile animals and for discarding females with infertility (Marques et al. 2018).
Many infectious agents such as bacteria, viruses, protozoa, and fungi affect reproductive performance in cattle and can potentially be transmitted through natural breeding (Anderson 2007; Yoo 2010). Reproductive diseases such asBrucellosis, Leptospirosis, Campylobacteriosis, Trichomoniasis, Bovine viral diarrhea and Infectious bovine rhinotracheitis (Infectious pustular vulvovaginitis) and Trichomoniasis can provide severe consequences to reproductive performance and economic gain due to the increased occurrence of early and late embryonic loss and fetal death (Alfieri et al. 2019). In this context, another benefit of AI is herd sanity because of the elimination of natural breeding and the use of bulls that can carry contagious infectious diseases (Eaglesome and Garcia 1997). Thus, along with genetic improvement, AI aims to use semen commercialized by qualified companies, which select sires based on sanitary program analysis to control reproductive diseases.
The implementation of AI programs also allows for the reduction or elimination of bulls on farm (Marques et al. 2018), releasing pastures for other animal categories. Furthermore, replacing the natural breeding program to AI program can to avoiding the possibility of bull attacks (accidents) on workers involved in dairy activities, which is common. In addition, the insemination facilitates the selection of bulls with low expected progeny differences in birth weight, reducing the occurrence of dystocia due to the birth of smaller calves (Mee 2008).
In countries with a tropical climate, with high temperatures, the use of some bull breeds represents a great challenge for the breeder’s good performance in the natural breeding. The same situation can be observed in countries with cold climates and that intend to make use of bulls of Zebu breeds, which are often more adapted and regions with hot climates (Menegassi and Barcellos 2015). In this contex, the use of AI also allows for the use of bulls less adapted to some climatic conditions, i.e., it is common to observe a reduction in libido of some breeds of bulls when placed in high temperature environments. Therefore, AI enables the use of Bos taurus bulls for crossbreeding, which are less adapted to high temperatures and require more management. Furthermore, AI also favors heterosis by crossing Bos taurus and Bos indicus cattle (Buckley et al. 2014), which represents an interesting strategy due to the improvement in zootechnical performance, quality of progeny carcasses (Gama et al. 2013), parasitic resistance, adaptability, and good acceptance by consumers.
Finally, despite AI having many advantages for the herd, there are some limitations, such as the need for specialized labor, failure in applying biotechnology , and nutrition and sanitarian factors (Demetrio et al. 2007; Orihuela 2000). Understanding these limitations is necessary for implementation of strategies to enable the use of AI programs.
1.3 Physiology Aspects of the Estrous Cycle in Cattle
The estrous cycle reflects a cyclical pattern of ovarian activity that makes the female receptive to the male, favoring mating and further gestation (Forde et al. 2011). Domestic cattle (Bos indicus and Bos taurus) are considered annual poliestric animals and exhibit estrus behavior on average every 21 days, varying from 18 to 24 days depending on the number of waves of follicular growth (Figueiredo et al. 1997; Roche 1996; Ginther et al. 1989b). However, this cyclical behavior is only possible from puberty, when the female reached appropriate weight and age for each breed.
1.3.1 Puberty
The pubertal process is characterized by an activation of physiological events in the hypothalamic-adenohypophyseal-ovarian axis which culminate in reproductive maturity. In this process, the females undergo by a sequence of events that begins with the expression of estrus, followed by the occurrence of ovulation and the beginning of a luteal phase (Seneda et al. 2019; Atkins et al. 2013; Rawlings et al. 2003). The onset of puberty is variable according to age, weight, and subspecies of heifer, for example taurine animals have greater sexual precocity than zebuine animals. In Bos taurus, puberty can occur at approximately 6 to 12 months of age or when the heifer reaches 40 to 50% of the adult weight (Moran et al. 1989). However, in Bos indicus, puberty is achieved at approximately 15 to 18 months of age or close to 60 to 70% of the adult weight (Nogueira 2004). In addition, factors such as season of the year, growth rate, nutritional intake, social cues and treatment with exogenous progestins can change the age to the puberty.
In prepubertal females, the low concentration of estradiol produced in the gonads due a negative feedback mechanism prevents the hypothalamic preovulatory center from secreting adequate pulses of Gonadotropin-releasing hormone (GnRH; Amstalden et al. 2011). The absence of GnRH also inhibits the release of Follicle stimulating hormone (FSH) and Luteinizing hormone (LH) from the anterior pituitary, thus causing an absence of follicular waves and ovulation (Senger 1997). On the other hand, when the hypothalamus starts the GnRH neuron function (influenced mainly by nutritional control), there is a decrease in the negative feedback of estradiol, which leads to an increase in the frequency of release of the GnRH pulses (Amstalden et al. 2011; Kinder et al. 1995). In this context, the follicle growth occurs due to increased levels of intrafollicular estradiol and release of a GnRH discharge through of the center of the hypothalamus (Jainudeen and Hafez 2013). Subsequently, a GnRH discharge results in an increase in the tonic release of LH pulses that is the main endocrine factor that regulates the onset of puberty in heifers. Increased frequency of LH release increases the development of ovarian follicles that produce estradiol sufficient to induce behavioral estrus and a preovulatory peak of gonadotrophins (Foster et al. 2006; Kinder et al. 1995).
Commonly the first behavioral estrus is not preceded by significant concentrations of P4. Thus, the first luteal structure that forms disappears and puberty is reached only after the occurrence of the first behavioral estrus. From that, there is the ovulation and the development of a CL with a typical lifespan. Finally, in puberty, all components of the hypothalamic-adenohypophyseal-ovarian axis will be properly normalized so that reproductive activity occurs cyclically and regularly, and sexual maturity is achieved. The prepubertal heifers present a specific pattern of endocrine and ovarian follicular dynamics. The first ovulation usually occurs at 56 weeks of age, being usual non regular cycles at the beginning of ovarian activity. The short duration of ovulatory cycle was associated with low P4 concentrations and small CL (Evans et al. 1994).
In this context, it is important to emphasize that the occurrence of puberty is considered one starting point to the productive life of a female (Brumatti et al. 2011). Then, early attainment of puberty predicts anticipation of this process (Ferraz et al. 2018). However, studies have shown that the optimal age at first birth is 24 months (Haworth et al. 2008). Below this age range, it is improbable that heifers are of enough body size to express their full genetic potential, either for the production of milk throughout life or even to deliver and maintain a healthy calf. In addition, breeding costs are increased for heifers calving later than those heifers calving early (Boulton et al. 2017). Heifers calving later have lower chances of completing the first lactation (Brickell and Wathes 2011) and relatively low fertility with longer delivery intervals (Wathes et al. 2014). In this way, producers that breed late femalesmay be wasting resources with less likely to give birth in an ideal age or to survive beyond a first lactation in order to recover the costs of breeding (Boulton et al. 2017). In this context, studies have been developed with the purpose of generating alternatives to promote puberty as early as possible in heifers, without disrupting future productive and reproductive performance of these females (Seneda et al. 2019). Genetic selection by animals with greater sexual precocity and the use of hormonal protocols to induce puberty represent good alternatives for the selection of earlier and more productive matrices in the livestock (Gonzalez et al. 2020).
1.3.2 Neuroendocrine Control of the Estrous Cycle
The estrous cycle, a series of reproductive events and ovarian functions in cattle, such as recruitment, follicular growth, ovulation, luteinizing and luteolysis, are regulated by a perfect hormonal interaction among hormones from the hypothalamus (GnRH), anterior pituitary (FSH and LH), ovary (P4, estradiol, inhibins and oxytocin), and uterus (prostaglandin F2α – PGF2α). In addition, non-hormonal factors, such as insulin-like growth factors, also have an important function in ovarian follicular activity. The hormones act through a positive and negative feedback system for controlling estrous cycle, allowing reproductive events by specific control of ovary and uterus activity (Forde et al. 2011; Crowe 2008; Roche 1996).
GnRH is one of the main hormones involved in the estrous cycle control by stimulating the production and secretion of gonadotropins (Fig. 1.2). This hormone acting on the anterior pituitary or adenohypophysis to stimulate FSH and LH production and release. GnRH is produced in the nucleus of hypothalamus neurons and transported by axons to the median eminence, which is where it is stored (Yin and Gore 2010). Subsequently, GnRH is released and transported to the hypophysis by the portal hypothalamic-hypophyseal system, where GnRH binds to cell surface receptors and signals the release of FSH and LH (Vizcarra et al. 1999; Moenter et al. 1992; Keri et al. 1985). FSH is stored in secretory granules located in the cytoplasm for shorter periods, whereas LH is stored for longer periods during the estrous cycle (Farnworth 1995).
The GnRH functions depend of stage of the estrous cycle. At the beginning of the cycle, GnRH stimulates FSH secretion to follicular recruitment and initial growth of small follicles. At the end of the cycle GnRH stimulates LH secretion to induce final growth and ovulation of the dominant follicle (Crowe and Mullen 2013). The CL regression during the follicular phase of the estrous cycle results in basal P4 concentrations. So, there is a concomitant increase in estradiol concentration due to the development of a dominant follicle, which induces increasing release of GnRH and allows estrus behavior during which females are sexually receptive to mounts (Frandson et al. 2009). However, ovulation of the dominant follicle only occurs with basal P4 plasma concentration and LH pulse frequencies occurring every 40–70 min for 2–3 days (Roche 1996). The dominant follicle ovulates approximately 10 to 14 h after the end of the estrus behavior. After breakage of the preovulatory follicle, metaestrous is initiated and by 3 to 4 days is characterized by the formation of CL from the hemorrhagic corpus (Forde et al. 2011).
Immediately after ovulation, CL formation occurs from luteinization of granulosa and internal theca cells. This ovarian structure is responsible for P4 production, which is required for the diestrous phase or maintaining a pregnancy (Schams and Berisha 2004; Butcher et al. 1992). During the diestrous phase, P4 concentrations remain elevated, and recruited follicles continue to develop due to FSH being released from the pituitary. However, growing follicles are inhibited due to high levels of P4 during this phase, which does not allow appropriate frequencies and/or amplitudes of LH pulses due to negative feedback with this hormone. Therefore, the dominant follicle does not ovulate and undergoes atresia, gradually decreasing its diameter, during diestrous (Crowe 2008; Manikkam and Rajamahendran 1997; Savio et al. 1993; Taylor and Rajamahendran 1991). On the other hand, during the proestrous phase, P4 concentrations decrease due to CL regression in response to PGF2α uterine secretion; then, this hormonal condition (low P4 and high estradiol) allows for LH pulses and consequently ovulation (Fig. 1.3) (Crowe and Mullen 2013; Forde et al. 2011; Goff 2004).
Schematic description of the growth pattern of ovarian follicles during follicular dynamics [recruitment, selection, dominance, and atresia (luteal phase) or ovulation (follicular phase)] and secretion pattern of estradiol, Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), progesterone (P4), and prostaglandin F2α (PGF2α) during the estrous cycle in cattle
1.3.3 Estrous Cycle Phases and Estrus Behavior
The estrous cycle in cattle includes a normal length of 18 to 24 days with two distinct phases: (1) the luteal or progesteronic phase and (2) follicular or estrogenic phase. The luteal phase lasts 14 to 18 days and is the period that follows ovulation, being characterized by formation and CL presence (also known as metestrus and diestrus phases). The follicular phase extends from CL regression (luteolysis) until ovulation and lasts 4 to 6 days. This phase is also known as proestrous and estrus, being a required event for ovulation of dominant follicle. In this phase, the dominant follicle undergoes final maturation, and the ovulatory follicle releases the oocyte to fertilization at the uterine tube (Forde et al. 2011; Adams et al. 2008; Rawlings et al. 2003; Sunderland et al. 1994).
The signs of estrous behavior are manifested during the follicular phase and characterized by primary and secondary signs. Unrest, increased physical activity (vocalization and hyperactivity), swelling and reddening of the vulva, friendly contact with herd mates, smelling and licking the genitalia, clear mucus secretion, and attempts to mount constitute secondary signs. However, being receptive to mounting is the most reliable signal of estrus in cattle, which is considered the primary sign (Løvendahl and Chagunda 2010; Galina et al. 1982). Frequently, secondary signs precede (proestrous) estrus and may continue after females accept the mount . Therefore, the identification of secondary signals also assists in the process of identifying the estrus itself.
The estrogen is the main hormone responsible for estrus behavior, which is produced by the dominant follicle and signals the hypothalamus to induce the secondary signs and behavior of receptivity, an event necessary for successful mating. However, an increase in the estradiol concentration and a reduction of P4 concentration is required for manifestation of estrus behavior. This hormonal condition occurs at the end of the luteal phase, being induced by luteolysis between 15 and 17 days of the estrous cycle (Allrich 1994; Vailes et al. 1992). Thereafter, the P4 plasma level is reduced below 1 ng/mL, and this event is responsible for estrus return in nonpregnant females.
Estrus behavior duration and intensity can be affected by several factors, such as, age, production level, environmental factors, nutritional factors, health status, size of the sexually active group, presence of a bull, climatic factors (strong rain and wind), management of animals in pastures and stalls, and subspecies (Bos taurus versus Bos indicus) (Crowe 2008; Galina et al. 1996). In beef cattle, an average duration of 8 to 10 h has been reported (Crowe 2008; Bó et al. 2003). In dairy cattle, the average estrus duration is 8 to16 h in cows and 12 to 14 h in heifers, but this duration tends to decrease as milk production increases (Forde et al. 2011; Crowe 2008; Allrich 1994). This negative relation between milk production and duration of estrus has been reported in high-producing dairy cows (above 40 kg of milk per day) because there is a decreasing circulating estradiol level due to increased metabolism of this hormone (Wiltbank et al. 2006).
Differences in the characteristics of estrus between Bos taurus and Bos indicus females has been well reported (Pinheiro et al. 1998; Galina et al. 1996). In general, zebu cattle present a short duration of estrus and high rates of estrus expression at night, which makes it difficult to identify the estrus for conventional insemination program. Under Brazilian management conditions, the use of a radiotelemetry device in heifers has demonstrated a shorter average estrus duration for Bos indicus (12.9 h in Nelore) compared to that of Bos taurus (16.3 h in Angus), but no difference was found between Bos indicus x Bos taurus crossbred (12.4 h in Nelore x Angus) and Bos indicus (Mizuta 2003). In addition, it was reported that 53.8% of the estrus expression in Bosindicus (Nelore cows) begins at night (between 6 p.m. and 6 a.m.) with 30% of its beginning and finishing occurring at night (Pinheiro et al. 1998).
1.3.4 Events of the Ovarian Follicular Dynamics
Follicular dynamics is one of the most important events of ovarian physiology, being widely studied in Bos taurus and Bos indicus females (Cuervo-Arango et al. 2011; Mackey et al. 2000; Figueiredo et al. 1997; Ginther et al. 1989a). The follicular dynamics involve growth, development, and maturation of ovarian follicles that are essential processes for reproductive efficiency in farm animals. These dynamics events are totally dependent on the perfect functioning of the hypothalamus-pituitary-gonadal axis that acts through positive and negative feedback mechanisms to stimulate development of ovarian follicles by gonadotropins secretion (FSH and LH).
During the estrous cycle, 2 or 3 waves of follicular growth may be present, but only the last wave is considered an ovulatory wave (Figueiredo et al. 1997; Ginther et al. 1989b). Every wave consists of the recruitment of a group of small follicles (4 to 6 mm in diameter) by FSH stimulation. These follicles grow and future dominant follicle is selected, which suffers a more efficient action of FSH in relation to the other follicles of the wave. So, there is follicular divergence and a dominant follicle emerges, which undergoes atresia or ovulation depending on the circulating levels of P4 that may or may not allow the estrogen trigger on LH pulsatility (Atkins et al. 2013; Forde et al. 2011). Follicular waves are initially established during the prepubertal period; however, the dominant follicle undergoes atresia and does not ovulate. Therefore, only after puberty the ovarian follicular dynamics become a cyclic event characterized by estrus and ovulation (Atkins et al. 2013; Bergfeld et al. 1994).
In general, ovarian follicular development through a non-gonadotropin-dependent phase and a gonadotropin-dependent phase (Webb et al. 2004). During fetal life, for example, follicle development occurs for 3–4 months and the two phases are present. During the gonadotropin-dependent stage, follicular development occurs in waves (2–3 waves of growth per estrous cycle), being that each follicular wave comprises emergence, selection, dominance, and atresia or ovulation (Fig. 1.3) (Ginther et al. 1989a). Frequently, dairy cows have two waves and heifers, or beef cattle have three waves (Forde et al. 2011). In Bosindicus (Nelore), there is a predominance of two waves for cows (83.3%) and three waves for heifers (64.7%) (Figueiredo et al. 1997).
The emergence of the wave is the first event gonadotropin dependent, which is characterized by recruitment of a group of follicles (1 to 50 follicles ≥5 mm) that starts growth in response to increased FSH concentration (Sunderland et al. 1994). Follicular growth and cell proliferation in this period occur primarily by FSH stimulation, being characterized by the presence of FSH receptors located in the granulosa cells of follicles at day 3 of the follicle wave (Ginther et al. 2002; Richards et al. 1998). Following follicular development, the next important event is follicular selection, a mechanism that reducesthe number of recruited follicles to the ovulatory quota of each species, usually one follicle in bovine species (Sunderland et al. 1994). In this phase, there is a transient increase in FSH concentration, which induces an increase in the aromatase enzyme activity of granulosa cells responsible for conversion of androgen to estrogen (Hillier 1994). This process increases estrogen and inhibin concentrations of healthy follicles, assisting the selection process and gonadotropin secretion (Ginther et al. 2002).
Once the follicular selection process has been established, the dominant follicle diameter increases, and divergence is started when the largest follicle reaches an average diameter of 5.7 to 6.1 mm in Bosindicus (Sartorelli et al. 2005) and 8.0 to 8.5 mm in Bos taurus (Ginther et al. 1999). In sequence, the follicular dominance is started by growth of the dominant follicle of approximately 1 to 2 mm per day (Figueiredo et al. 1997; Knopf et al. 1989). Then, its diameter is enlarged due to an increase in the follicular fluid. This fluid contains high estrogen concentration, in addition to high levels of inhibin that is associated with negative feedback in the pituitary, reducing FSH levels to basal concentrations (Sunderland et al. 1994). However, due to the presence of LH receptors in the granulosa and theca cells, the dominant follicle becomes sensitive to LH, and even at a low FSH concentration, it continues to grow and accumulate estrogen in the follicular fluid (Xu et al. 1995).
With the growth of the dominant follicle, now being more stimulated by the action of LH than FSH, the destination of the dominant follicle is dependent on frequency and amplitude of the LH pulse (Forde et al. 2011). During the early luteal phase, LH pulsatility has a low amplitude (delta LH, 0.3 to 1.8 ng) and high frequency (20 to 30 pulses/24 h), and during the middle of the luteal phase, LH pulses have high amplitude (delta LH, 1.2 to 7.0 ng) and low frequency (6 to 8 pulses/24 h) (Rahe et al. 1980). This amplitude and frequency are still insufficient to promote final maturation and ovulation of the dominant follicle. Then, during the luteal phase of the estrous cycle, the dominant follicle undergoes atresia and all production of estrogen and inhibin decreases gradually, removing the negative feedback on the FSH secretion in the hypothalamus/pituitary. This event promotes new FSH secretion and the emergence of a second follicular wave (Atkins et al. 2013; Forde et al. 2011).
High estrogen production is an essential characteristic of the dominant follicle that largely depends on LH pulse frequency. In this phase, LH binding to its receptors on theca cells provides the conversion of cholesterol to testosterone through a series of catalytic reactions. Then, testosterone diffuses from theca cells to granulosa cells and is converted into estrogen by the aromatase enzyme (Hillier 1994; Sunderland et al. 1994). Estrogen has a local effect on follicular development and a systemic effect on the hypothalamus and hypophysis. During the follicular phase, when the P4 concentration is low, the estrogen produced by the preovulatory follicle induces an increase in the GnRH level in the hypothalamus, which promotes an increase in the amplitude and frequency of LH pulses that stimulates the final maturation and ovulation of the preovulatory follicle (Crowe and Mullen 2013; Sunderland et al. 1994). The maximum diameter of the preovulatory follicle is variable, being 10 to 14 mm in Bos indicus (Sales et al. 2012a; Figueiredo et al. 1997) and 14–20 mm in Bos taurus (Ginther et al. 1989a).
In addition, hormonal action intraovarian factors are also important to estrous cycle regulation, acting indirectly by altering estradiol synthesis or directly through negative feedback that controls the hypothalamus and pituitary gland (Forde et al. 2011). Although the acquisition of LH receptors by the granulosa cell layer is considered the main mechanism that promotes the follicular selection process (Lucy 2007), the bioavailability of the insulin-like growth factor (Rivera and Fortune 2003) and the presence of other growth factors also contributes to the development, proliferation, and steroidogenic capacity of the dominant follicle (Knight and Glister 2006).
1.4 Estrus Synchronization Program
Estrus synchronization is a hormonal strategy performed in a group of females with the aim of inducing all animals in the same phase of the estrous cycle. Using this hormonal strategy, management for estrus identification and insemination are facilitated. Two possible methods for estrus synchronization are available: (I) interrupting the activity of the luteal phase or (II) extending the CL length. A luteolytic agent such as PGF2α or its synthetic analogs (Table 1.1), frequentely are used to interruption of the luteal phase and then induce estrus. On the other hand, the duration of the luteal phase can be modified using a progestin treatment, which is similar to the endocrine activity of CL and prevents estrus behavior during treatment. In this case estrus synchronization occurs after removing the P4 source.
1.4.1 Use of Prostaglandins and Synthetic Analogs
Since the 1970s, a wide variety of studies have evaluated the effects of PGF2α and its synthetic analogs in domestic species (Odde 1990; Rajamahendran et al. 1976). A treatment with PGF2α in cattle decreases the P4 plasma concentration in 24 h after injection in 80 to 100% of the animals. However, morphological CL regression is gradual and can be observed by ultrasound approximately 24 to 48 h after treatment. Despite PGF2α inducing efficient luteolysis, estrus behavior can be observed for a long time due to the stage of development of dominant follicle at the time of application of PGF2α. If a dominant follicle is present at the time of application, the estrus behavior occurs in a short period, approximately 24 to 72 h after luteolytic agent application. Otherwise, only the dominant follicle of the next follicular wave may be considered, in this case there will be a longer interval until the occurrence of estrous behavior, up to 144 h (Kastelic et al. 1990). Therefore, the synchronization program with PGF2α requires careful practice for estrus observation in treated animals.
Despite the high efficiency of protaglandin causing luteolysis and inducing estrus, it is worth noting that its use only has an effect in the presence of a responsive CL. Therefore, for more efficient use of luteolytic agents, it is necessary to identify CL by transrectal palpation or ultrasound. A CL up to the 5th day of life after the ovulation is not responsive to luteolysis because the CL is still in formation. In the same way that a CL after the 15th day of the cycle also does not respond to the application because it may have already undergone natural luteolysis (Fernandes et al. 2006; Meidan and Levy 2002; Levy et al. 2000).
On the other hand, when CL is not evaluated, estrus synchronization with luteolytic agent can be performed by two applications of PGF2α with intervals of 11–14 days. The second application induces luteolysis in animals that do not respond to PGF2α at the first treatment, resulting in synchronization rates of 70 to 80% of females (Binelli et al. 2014; Machado et al. 2007; Baishya et al. 1980). Following this strategy, animals that manifest estrus after each application of PGF2α can be inseminated, or for greater concentration in the number of females in estrus it is recommended to inseminate all animals only after the second application.
Another option for application of PGF2α in AI programs is the detection of estrus for 5 to 7 consecutive days followed by AI , and in females that did not express estrus, CL identification and PGF2α application is perfomed for further estrus observation and insemination. The estrus observation can continue during the next 5 to 7 days, for a total of 12–14 days of work. These three protocols for the synchronization with PGF2α are illustrated in Fig. 1.4.
Currently the main synthetic analogs of PGF2α (Table 1.4) commercially available are dinoprosttromethamine, cloprostenol, and D-cloprostenol. These analogs are considered potent luteolytic agents used routinely in estrous cycle synchronization programs. Intramuscular administration of conventional doses (dinoprosttromethamine 25 mg, cloprostenol 500μg, D-cloprostenol 150–300μg) is the most used but also have tested other routes for reduction of doses. Half a dose by vulvar submucosa injection resulted in similar synchronization rates; however, application in the vulvar submucosa is a route less practiced, especially for a nonexperienced worker (Baryczka et al. 2018; Valldecabres-Torres et al. 2012; Chacur et al. 2010; Colazo et al. 2002).
Although estradiol esters (estradiol-17β, benzoate, cypionate and estradiol valerate) are capable of causing luteolysis, the luteolytic effect depends on the ester and dose used (Baruselli et al. 2004b). Of all available esters, estradiol valerate has the greatest potential for luteolysis; however, prostaglandins and their analogs are still more used to induce estrus in cyclic cows.
Commonly females in estrus are inseminated 12 h later, and conception rates are similar to those obtained in natural estrus. However, to facilitate the management of inseminations, females identified in estrus by the morning are inseminated in the late afternoon. Those already detected to be in estrus by the afternoon are inseminated in the morning of the next day. In addition to facilitating the management of the process, this strategy also results in better synchronization of oocyte and sperm viability.
1.4.2 Progesterone and Progestin
Since the 1950s, P4 and its synthetic analogs (progestins) have been used for estrous synchronization programs. The first studies involving P4 were carried out with formulations administered by an oral or injectable route for 14 to 21 days, producing an effective estrus synchronization rate but variable fertility (Hansel et al. 1966). These initial results were unsatisfactory, probably due to their use over a long time and the suboptimal concentration of P4, leading to the formation of persistent follicles containing low-quality oocytes (Fortune and Rivera 1999). In the last decade, the authors of this chapter have conducted a series of studies with injectable P4 formulations, showing promising results for ovarian follicular dynamics (Morotti et al. 2013a, b) and improved fertility of cows submitted to TAI program (Campos et al. 2016a, b). However, there is still no commercially available injectable formulation for use in estrous cycle synchronization programs. Natural P4 has been used in intravaginal devices, and synthetic P4 has been used in subcutaneous auricular implants (norgestomet) or oral use (melengestrol acetate). Despite the different forms of P4 presentation (Table 1.2), an intravaginal device is the most common for synchronization programs.
For the purpose of estrus synchronization, formulations containing P4 can be used to prolong the lutein phase of the estrous cycle or to simulate the effect of a CL, so the synchronization of the animals occurs when removing the source of P4. Although this strategy provides an effective synchronization of the females, in cyclic animals the result is more satisfactory when the application of a luteolytic agent is combined with the removal of the P4 source (Baruselli et al. 2004b). In addition to estrous cycle synchronization, P4 has also been used for cyclicity induction in prepubertal heifers (Gonzalez et al. 2020). This subject will be discussed further in another section of this chapter.
1.5 Ovulation Synchronization Program for Timed Artificial Insemination
For the elaboration of an efficient ovulation synchronization program, the hormonal protocol must combine three basic principles: (I) synchronization of the emergence of the follicular growth wave, (II) synchronic control of the progesteronic phase, and (III) synchronized ovulation induction.
Based on these principles, protocols for ovulation synchronization aim to control the growth of the follicular wave to regulate the luteal phase and induce ovulation in a fixed amount of time, avoiding the necessity of estrus detection for AI . For this purpose, some hormones such as P4, progestin, PGF2α, estradiol esters (estradiol-17β, benzoate, cypionate and estradiol valerate), equine chorionic gonadotropin (eCG), human chorionic gonadotropin (hCG), and GnRH are combined to provide in estrus and ovulation synchronization.
1.5.1 Synchronization of Follicular Wave Emergence
Progesterone combined with estrogen and/or GnRH is an efficient hormonal combination to synchronization in the emergence of a new wave of follicular growth. Treatment with P4 and estradiol esters on a random day of the estrous cycle (day 0) has been effective for promoting follicular atresia and the emergence of a new follicular wave at a fixed time in cattle (Bó and Baruselli 2014). Exogenous estradiol induces atresia of small follicles by decreasing FSH release, whereas P4 is used to suppress the release of LH and inhibit the activity of large follicles, which are dependent on this gonadotropin. Therefore, application of estrogen and P4 at the same time promotes atresia of all of the follicles (large, medium, and small) present in the ovary and a new wave emergence 2 to 4 days later (Baruselli et al. 2004b; Bó et al. 1995, 2003).
In cyclic Bos taurus cows and heifers , the emergence of a new follicular wave occurred in 3 to 4 days after treatment with progestogen and 5 mg of estradiol-17β or progestin and 1 to 2.5 mg of estradiol benzoate (EB) (Martinez et al. 2005; Bó et al. 1995). Apparently, Bos indicus and Bos taurus females have similar intervals for the synchronization of the follicular wave. For example, in Brangus heifers treated with an intravaginal P4 device and 2 mg of EB the follicular wave emergence did not differ from wave emergence in Angus heifers (3.3 ± 0.6 versus 4.3 ± 0.2 days) (Bó et al. 2003). A similar time of emergence was also observed between Nelore and crossbred ½ Nelore x ½ Angus heifers (3.3 ± 0.6 versus 3.5 ± 0.1 days) (Carvalho et al. 2008). Heifers and Nelore cows receiving 2.0 mg of EB and insertion of an auricular implant of norgestomet presented intervals of 2.5 ± 0.2 days for a new wave of emergence (Sá Filho et al. 2011). However, doses of 2.5 and 5.0 mg of estradiol valerate (EV) resulted in intervals of wave emergence of 4.2 ± 0.3 and 6.1 ± 0.6 days in heifers and 3.1 ± 0.4 and 4.0 ± 0.5 days in cows, respectively (Bó and Baruselli 2014).
Another efficient alternative for the synchronization of follicular wave emergence is to associate P4 with GnRH analogs. A study carried with dairy cows (Bos taurus) in lactation evaluated the association of 2 mg of EB and CIDR versus 100μg of GnRH and CIDR and revealed follicular wave emergence at 4.8 ± 0.4 and 2.0 ± 0.2 days, respectively (Kim and Kim 2007). In high-yielding dairy cattle the emergence of a wave of follicular growth is a major challenge. In this context, studies show that the application of estrogen and GnRH analogs in association with the insertion of a P4 device appears to be more effective in synchronizing the emergence of the follicular growth wave than the isolated use of P4 and estrogen or P4 and GnRH. In addition, twice the dose of GnRH has been more effective in synchronizing the emergence of the wave in these animals (Silva 2020; Wiltbank and Pursley 2014). Despite the efficiency of both combinations, estrogen has a lower cost compared to that of GnRH.
1.5.2 Control of the Progesteronic phase
The progesteronic phase can be controlled efficiently by the use of a P4 device, by the use of luteolytic agents, and by the association of these, which is more effective for synchronization. The use of luteolytic agents is highly efficient for inducing estrus synchronization in cyclic females as previously discussed. However, the use of these agents in association with P4 devices has been more effective than their isolated uses. In this context, PGF2α or its synthetic analogs was proposed for increasing the fertility of cows submitted to synchronization with CIDR-B for 8–9 days associated with EB at the time of P4 device insertion (Macmillan and Peterson 1993a, b). Efficient control of the progesteronic phase has been reported with the removal of the P4 device between D6 and D9 (Bó and Baruselli 2014; Sá Filho et al. 2010a, b, 2011) and PGF2α application can be performed at any of the three times: (i) at the time of P4 removal, (ii) 24 to 48 h before P4 removal, or (iii) on day 0 at P4 device insertion (Bó et al. 2002; Macmillan and Peterson 1993a, b). Therefore, administration of luteolytic agents such as PGF2α before or at the end of P4 treatment is required to ensure luteolysis and adequate control of the progesteronic phase (Kastelic et al. 1999).
The time for application of the luteolytic agent has been widely studied in Bos indicus cattle. Initially, luteolysis was observed in 83.3% Nelore cows treated with 5 mg of EV between 2 and 7 days after treatment (Pinheiro et al. 1998). Therefore, due to the high percentage of induction of luteolysis using EV at the mentioned dose, it is not necessary to use PGF2α in protocols using progestin and EV at the beginning of hormonal treatment (Kastelic et al. 1999). The effects of the induction of luteolysiswith dinoprosttromethamine were investigated simultaneously or 48 h before P4 device removal. In this study, a higher P4 concentration (4.58 ± 0.21 ng/mL) was observed in cows treated simultaneously to the P4 device removal compared to those treated 48 h earlier (3.05 ± 0.21 ng/mL) (Peres et al. 2009). In addition, cows with early luteolysis improved the ovulation rate (77.0% versus 85.4%) and increased the pregnancy rate by aproximadely16%.
In summary, to obtain greater efficiency in TAI protocols, the addition of PGF2α 2 to 3 days before or at the end of P4 treatment is required to induce luteolysis in cyclic females. In a normal estrous cycle, this strategy increases the proestrous phase (low P4 and high estradiol), better preparing the uterus for pregnancy or allowing subsequent ovulation. However, it is possible that this anticipated luteolysis is not necessary when using more potent PGF2α analogs such as cloprostenol or D-cloprostenol.
1.5.3 Synchronized Induction of Ovulation
The use of ovulation inducers in association with P4 treatment has been proposed to reduce the dispersion of ovulation and improve the ovulation rate. In addition to the wave emergence, the use of estradiol esters [EB or estradiol cypionate (EC)] at the end of P4 treatment induces ovulation between 72 and 84 h after P4 removal in 75% of cows (Morotti et al. 2013a, b; Bó et al. 1995). Several hormones are used to induce ovulation in cattle, such as estradiol esters (estradiol-17β, EB, and EC), GnRH analogs, hCG, and LH. The main ovulatory mechanism associated with the use of ovulation inducers is related to the induction of an endogenous LH surge (Baruselli et al. 2004b). Estradiol esters are considered indirect inducers, and GnRH and LH are considered direct inducers. According to cost benefit analysis, the most used hormone for induction of ovulation in Bos indicus cattle is EB 24 h after P4 removal (1 mg) or EC along with device removal (0.5 mg in heifers to 1 mg in cows) (Torres et al. 2014). However, GnRH anologs can also be used for this purpose from the time of insemination up to 16 h before.
Frequently, within the TAI protocols the insemination is performed 48 h after P4 removal or approximately 8 to 12 h before the expected time for ovulation (Baruselli et al. 2004a, b; Hanlon et al. 1996). However, to reduce management practices, administration of estradiol esters was proposed to induce ovulation simultaneously or 24 h after removal of the P4 device. Similar results to those with EB for induction of ovulation were obtained with EC administration (Sales et al. 2012a). Similar intervals between CIDR removal and LH surge (54.6 ± 3.4 versus 59.3 ± 3.5 h) and between CIDR removal and ovulation (81.6 ± 5.0 versus 86.4 ± 4.8 h) were observed in heifers treated with 0.5 mg of EC 0 or 24 h after intravaginal device removal (Sales et al. 2012b). Application of EB or EC to induce ovulation after P4 removal resulted in similar (P > 0.05) follicular responses (ovulatory follicle diameter: 13.1 versus 13.9 mm), the interval from P4 device removal to ovulation (70.2 versus 68.5 h) and ovulation rate (77.8% versus 82.8%). In addition, the pregnancy per AI was similar (P > 0.22) between cows treated with EB (57.5%; 277/482) and EC (61.8%; 291/471) (Sales et al. 2012b). In another study, an interval of 45 h was observed between injection of 0.5 mg of EC simultaneously to P4 device removal and LH surge (Sales et al. 2015).
Other ovulation inducers, such as synthetic GnRH analogs, hCG, or LH, can be used. However, the use of LH is restricted to inducing ovulation in protocols for ovarian superovulation due to its high cost. The same limiting factor, cost, also has to be considered for hCG and GnRH, although GnRH has been widely used alone or in association with EC or EB in females that exhibit low or no estrus expression during TAI protocols (Rodrigues et al. 2019). Generally, indirect ovulation inducers are proconized in conventional TAI protocols, being that the ovulation is normally obtained by cheaper alternatives by the use of estrogens. On the oder hand, direct inductors are used either in specific situations, either for TAI or more often for superovulation protocols.
1.6 Strategies for Improving Fertility at TAI
TAI programs represent one of the most important advances in reproductive biotechniques in cattle. The main advantages of this reproductive tool are undoubtedly associated with ease of management, since the TAI protocol eliminated the practice of estrus identification and its low efficiency, contributed to a greater intensification in the use of AI , which made the technique better known and widespread in the world. In addition, TAI has increased the service rate to 100% of the synchronized animals, which significantly contributes to increasing the reproductive efficiency of the herd and the economic performance of the farm (Baruselli et al. 2018).
The goal of the TAI program is to inseminate all treated animals at the same time and to obtain pregnancy rates of approximately 40 to 60%. The best reproductive rates are achieved with well-managed herds, with high quality nutrition, good health, and semen quality and a well-trained team. Although good results can be obtained with TAI , a reduction of approximately 15 to 20% in the conception rate of Bos indicus cattle with a high rate of anestrous has been reported (Baruselli et al. 2004a; Fernandes et al. 2001).
Postpartum cows may undergo a prolonged anestrous period during which they do not show behavioral signs of estrus, which is decisive when reproduction is dependent on bull mating. In this context, anestrous postpartum represents one of the biggest challenges for beef breeding, mainly due to endocrine imbalance regarding adequate LH secretion during this period, which is considered the main factor for anestrous in Bos indicus cattle (Baruselli et al. 2004a, 2011). Additionally, many herds managed on tropical pastures may suffer nutritional restrictions due to the seasonality of forages, which may aggravate postpartum anestrus conditions. Therefore, due to characteristics of estrus expression, poor efficiency of estrus detection, and a high percentage of cows in postpartum anestrous (Stevenson et al. 2015; Lamb et al. 2010; Crowe 2008; Yavas and Walton 2000b), AI programs based on estrus detection present with low efficiency, mainly for tropical and subtropical herds (Baruselli et al. 2004a; Bó et al. 2003). On the other hand, TAI program represents one of the most efficient alternatives. However, even receiving a hormonal protocol for TAI , fertility may be affected if hormonal adjustments or management practices are not performed properly.
Administration of eCG and temporary calf removal (TCR) are strategies performed to provide appropriate gonadotropin support for growth and ovulation induction of the dominant follicle in females submitted to TAI (Barreiros et al. 2014; Campos et al. 2013; Sá Filho et al. 2014; Yavas and Walton 2000a, b). Practices such as this have been associated with TAI protocols and improved postpartum fertility because gonadotropin support provides an increase in follicular activity. However, the use of this gonadotropin support has been highly indicated mainly in animals with intense anestrus postpartum or that have a low body condition score (BCS) (Baruselli et al. 2004b).
eCG is a glycoprotein hormone produced by the corium of a pregnant mare with wide application in TAI protocols. This glycoprotein has a high molecular weight and has an excellent gonadotrophic support for follicular growth due its mixed bioactivity (at approximately 2/3 FSH and 1/3 LH) (Soumano et al. 1996; Murphy and Martinuk 1991). It can be used at the end of the hormonal protocol to provide adequate gonadotropic aid for dominant follicle growth, mainly in acyclic cows and heifers (Barreiros et al. 2014). A schematic representation of the pharmacological action and physiological variations of TAI protocols is shown in Fig. 1.5.
Diagrammatic representation of the protocol for the synchronization of ovulation and timed artificial insemination (TAI) in cattle. Treatment with progesterone (P4) for 8 days [ovulation induced by estradiol benzoate (EB, I) and estradiol cypionate (EC, II)] or 9 days [ovulation induced by EB (III) and EC (IV)]. D, day; P4, progesterone (1.0–1.9 g); EB (D0), 2 mg; EB (D9), 1 mg; PGF2α, 12.5 mg of dinoprost or 125–250μg of cloprostenol; EC, 1 mg; eCG, 300–400 IU equine chorionic gonadotropin
Many studies evaluated the effect of eCG administration or TCR use on the conception rate for TAI in cattle (Baruselli et al. 2004a, 2018; Prata et al. 2018; Barreiros et al. 2014; Campos et al. 2013; Sá Filho et al. 2014; Yavas and Walton 2000a, b). Initially, similar conception rate was observed after TAI between cyclic lactating Nelore cows that were submitted to TCR (50.5%) compared to control group (53.5%). However, there was a 22% increase in the conception rates of Bos indicus cows with high anestrous rate after the same treatment (Ereno et al. 2007). In addition, after TAI programs, females who do not become pregnant have hormone treatment-induced cyclicity and may result in pregnancy rates of approximately 60 to 65% in the first 45 days of the breeding season (Baruselli et al. 2004a). Therefore, eCG administration for acyclic cows has been recommended to provide a pregnancy rate of around 50%, which is similar to that observed in cyclic cows (Baruselli et al. 2004a; Kastelic et al. 1999).
The use of eCG has been proposed in cows with a high postpartum period (intense anestrus) and BCS < 3 to obtain conception rates around 50% (Baruselli et al. 2004a). However, an interaction between BCS and the postpartum period can also influence conception rates to TAI. Therefore, administration of eCG in cows among 30 to 60 days postpartum is recommended regardless of the BCS (2 to 3.5). Additionally, management using TCR for 56 h was effective for increasing the conception rate of cows submitted to TAI with BCS < 2.5 compared to cows that were of control group (48.2% versus 28.2%; P < 0.05) (Peña 2007).
Some studies were conducted to evaluate the effect of eCG and TCR after P4 device removal on the growth of the dominant follicle in Bos indicus cattle. Studies have shown increased dominant follicle diameter and increased ovulation rate (85%) in Nelore cows treated with GnRH associated with 48 h of TCR compared to cows that were injected with GnRH without TCR (51%) (Meneghetti and Vasconcelos 2001). A common effect of eCG and TCR on TAI protocols is associated with increased ovulation rates due to a greater synchronization of the preovulatory LH surge (Sá Filho et al. 2006; Cavalieri et al. 1997; Williams et al. 1996). The effects on dominant follicle growth are likely partly related to the percentage of cyclicality and the postpartum period at the beginning of the TAI protocol. It was reported that follicles with a high maximum diameter promote a linear increase in both the follicular diameter and conception rates in Bos indicus and Bos taurus cattle (Baruselli et al. 2018; Sá Filho et al. 2010a; Borsato et al. 2004).
The luteotrophic effect of eCG was already shown by an in increase in the P4 concentration (8.6 ± 0.4 versus 6.4 ± 0.4 ng/mL; P < 0.05) 12 days after TAI in Brangus cows (Baruselli et al. 2004a). In addition, the TCR practice also results in a higher concentration of P4 after TAI and provide a higher pregnancy rate especially in acyclic females. Thus, the importance of these gonadotrophic stimuli is remarkable because the interaction between the P4 concentration and early embryonic development are key events during the maternal recognition process of gestation (Mann and Lamming 2001).
1.6.1 TAI in Heifers: Reaching Sexual Maturity and Induction of Puberty
The age of puberty is one of the most important parameters to determine the occurrence of the first calving both in beef and dairy cattle. Although puberty is often associated with endocrine mechanisms, gene-environment interactions can also affect reproductive physiology in cattle. In heifers, follicular growth in the prepubertal period is characterized by the FSH secretion, occurrence of the follicular wave emergence and dominance follicular, but without the occurrence of ovulation (Wiltbank et al. 2002). Until puberty, endocrine mechanisms promote negative feedback on the hypothalamus, which decreases GnRH and LH pulsation, blocking the final growth of the dominant follicle and ovulation (Day et al. 1987). In this context, an interruption of inhibitory factors on the hypothalamus occurs only when the animal has adequate weight and body development, which allows for full ovarian activity (Seneda et al. 2019). Therefore, it is observed that nutrition can greatly affect the occurrence of puberty.
In general, zebuine and crossbred heifers experience delayed puberty approximately 15 to 27 months old and body weight around 280 to 300 kg (Teodoro et al. 1993). Taurine heifers start puberty approximately with 10 to 15 months and weight from 220 to 250 kg (Patterson et al. 1992). In the last weeks of the prepubertal period and soon after puberty, there is a modification in the size of the female genital system, possibly by increasing the concentration of P4 and estradiol, signaling the acquisition of sexual maturity. Then, puberty is the beginning, but not the fullness, of the activity of the reproductive female trait (Honaramooz et al. 2004). It is important to note that sexual maturity occurs with the full development of the reproductive system, as evidenced by the increase in the uterine size and tone, besides regular ovarian activity.
A combined analysis of the uterus and ovaries should always be considered before using early hormonal treatment programs for the synchronization of estrus and ovulation in heifers. Heifers with regular ovarian activity, but presenting uterine dimensions characteristic of the prepubertal period, generally have low pregnancy rates after AI . A specific pattern of uterine diameter to start reproduction has been studied, but comparative analysis among animals before and after puberty can provide standardization for both the diameter and the tone of the uterus.
A five-point reproductive tract scoring system (RTS) was developed to estimate the pubertal status and reproductive potential of beef heifers by transrectal palpation of the uterine horns, ovaries, and ovarian structures (Table 1.3) (Rosenkrans and Hardin 2003; Andersen et al. 1991; LeFever and Odde 1987) system is practical and highly accurate, and females with score I, II, or III are considered prepubertal and those with score IV or V are considered pubertal and respond better to reproductive practices.
Prior to the breeding season a puberty-inducing hormone treatment can be used for heifers that have not yet reached sexual maturity. P4 and estradiol-based hormonal protocols have been proposed to induce cyclicity in prepubertal heifers (Rasby et al. 1998). Although some mechanisms are not fully known, it seems that P4 sensitizes the hypothalamus, reducing estradiol receptors and inhibiting GnRH and LH pulsatility (Andersen et al. 1991). In addition to possible effects on the hypothalamus, 17β-estradiol and P4 treatment increases the expression of receptors for GnRH in the pituitary and improves LH synthesis and secretion (Looper et al. 2003). Therefore, P4 treatment for 10 to 14 days mimics a functional CL and consequently induces puberty, possibly by reducing estradiol receptors in the hypothalamus, which after P4 removal increases LH secretion and promotes ovulation. For greater efficiency, at the end of treatment with P4, 1 mg of EB is applied to increase the ovulatory potential of the dominant follicle (Gonzalez et al. 2020; Rodrigues et al. 2013).
The induction of cyclicity with the P4 device (previously used for 24 days) was tested in prepubertalNelore heifers and resulted in 85.6% of cyclicity in P4-treated heifers and 81.1% in heifers injected with 1 mg of EB at the time of device removal (Sá Filho et al. 2006). Cyclicity induction in Bos indicus heifers has been tested with different sources of P4. Inductions using a P4 device (fourth use) versus P4 injectable (150 mg, i.m.) were efficient for cyclicity induction (81.5% versus 86.3%; respectively; Fig. 1.6) (Gonzalez et al. 2020). A similar cyclicity rate was obtained in females treated with P4 and EB (64%) compared to those treated with P4 and EC (67.2%). However, after TAI, greater pregnancy /AI and pregnancy /treated were achieved with EC in relation to the EB (Sá Filho et al. 2015). It is worth highlighting that the authors observed maintenance of P4 concentrations above 1 ng/mL for 7 days in prepubertalNelore heifers in females treated with a new or reused intravaginal device for 24 days.
Diagrammatic representation of cyclicity induction protocols in prepubertal heifers. Treatment with progesterone (P4) for 10–12 days [P4 intravaginal device at 3rd or 4th use (I) or P4 injectable (II)]. EC, estradiol cypionate (0.5 mg); * P4 injectable, 150 mg; US, ultrasound evaluation for identification of corpus luteum (CL)
1.6.1.1 TAI in Bos indicus Heifers
TAI programs in heifers have resulted in high reproductive rates similar to those found in cows. However, Bos indicus heifers treated with a first-use P4 device (CIDR-B) and/or containing high P4 concentration can reduce the diameter of the preovulatory follicle and interfere with the conception rate for TAI (Dias et al. 2009; Carvalho et al. 2008). This occurs because a high P4 concentration can significantly suppress gonadotropin secretion, specifically LH, impairing the follicular growth rate (Dias et al. 2009; Kinder et al. 1996). Therefore, synchronization of ovulation in heifers is commonly performed with a lower circulating P4 concentration, employing a monodose P4 device, using a reused device (second or third use), by PGF2α anticipation (day 0 or 7), and/or employing eCG gonadotropic stimulation at device removal (Dias et al. 2009; Carvalho et al. 2008).
Although it provides a reduction in endogenous P4 concentration in cows, the reuse of intravaginal P4 devices in heifers increases the efficiency of TAI, reduces protocol costs, and results in similar conception rates to those of cows (Sales et al. 2011b; Peres et al. 2009). Another alternative for Bos indicus heifers is the use of norgestomet which does not compromise the follicular growth or pregnancy rate of TAI (Sá Filho et al. 2013, 2015). Figure 1.7 shows the principal hormone protocols for the synchronization of ovulation and TAI protocols for Bos indicus heifers.
Diagrammatic representation of protocols for the synchronization of ovulation and timed artificial insemination (TAI) in heifers. (I) Treatment with progesterone device (multidose intravaginal, 2nd or 3rd use; monodose intravaginal, 0.5–0.7 g; Ear implant, norgestomet) for 8–9 days. (II) Treatment with progesterone for 8–9 days with PGF2α injection at the beginning of the protocol. (III) Treatment with progesterone for 8–9 days with ½ PGF2α injected on day 0 and ½ PGF2α together with P4 device removal. P4, 0.5 and 0.7 g of progesterone; EB, 2 mg of estradiol benzoate; PGF2α, 12.5 mg of dinoprost or 125–250μg of cloprostenol; eCG, 300–400 IU of equine chorionic gonadotropin; EC, 1 mg of estradiol cypionate
1.7 Use of Sexed Semen for AI and TAI
The choice of the calf‘s sex in the livestock system is considered a determining factor in beef and dairy cattle. For example, a male calf has low or no zootechnical value in dairy farms compared to a female calf . However, a male calf has great importance for beef cattle farms due to its high potential production. In this context, sexed semen has been used in AI programs to increase efficiency in production systems and genetic improvement programs for better targeting sex-related characteristics (Baruselli et al. 2007; Weigel 2004).
Sexed semen has been highlighted in cattle breeding due to the benefits generated by its use (Seidel Jr et al. 1997, 1999). Among various advantages, sexed sperm can provide genetic improvements for milk and meat production, favoring expressions related to sex, decreased incidence of dystocia by the birth of female calves weighing around 2 kg less than males, better efficiency in the production of breeders for replacement (heifers or bulls), and increased number of calves when it is desired to increase herd size for production (Seidel Jr. 2007; Cerchiaro et al. 2007). In addition, advantages from the production of desired sex embryos (superovulation and in vitro fertilization) can be obtained, and the best performance using sexed semen is obtained with in vitro embryo production (Morotti et al. 2014; Pellegrino et al. 2016; Pontes et al. 2010).
Currently, flow cytometry is the most efficient technology for sexing sperm, and this approach is based on DNA differences between X and Y (Seidel Jr. 2007; Seidel Jr. and Garner 2002). In bulls, X sperm contains around 4% more DNA in their chromosome than Y sperm (Garner 2006), and such a difference allows sperm classification with an efficiency close to 95% for determining the desired sex (Morotti et al. 2014; Pontes et al. 2010). In general, the sexing process involves a preparation and adding the DNA-binding dye Hoechst 33342 in the ejaculated (Garner et al. 2013). In the sequence the mix of the ejaculate and DNA dye is analyzed in the flow cytometer that can make over 30,000 consecutive evaluations of individual sperm per second, sorting the ejaculate into three parts: X sperm, Y sperm, and waste (indiscriminate and dead sperm) (Seidel Jr. 2014). Therefore, even with high-speed spermatic sorting, producing sexed sperm straws with a conventional concentration (20 to 50 million sperm) is not viable or economical. For this reason, sexed semen was initially commercialized in straws with approximately 2 million sperm (Schenk et al. 2009; Weigel 2004).
In this context, many studies have proven that there is considerable variation in fertility among bulls (both sexed and non-sexed sperm) and that the use of low fertility bulls and low concentration dose may result in a marked reduction in fertility (DeJarnette et al. 2008, 2011; Andersson et al. 2004). Approximately 10 to 20% of bulls have low fertility with a conventional semen dose; therefore, in this case, a reduction in fertility of more than 10 to 20% is expected after using a dose of two million sperm (Schenk et al. 2009; Seidel Jr. and Schenk 2008a, b). However, there is evidence of a relationship between sexed and non-sexed semen. For example, bull fertility using low doses of non-sexed sperm is usually a good indicator of fertility using a low sexed sperm dose (Seidel Jr. 2014).
A comparison among conception rates in Holstein heifers and cows using sexed semen from different bulls and different insemination doses (2.1, 3.5, and 5.0 million sperm) recorded the best pregnancy rates in nulliparous heifers using the 5 million sperm dose (59.5% pregnancy rate) compared to those of the 2.1 and 3.5 million sperm doses (46.4% and 52.2%, respectively) (DeJarnette et al. 2008). Bull or sperm dose did not affect the conception rate among cows (27.0, 29.1, and 30.3% for the 2.1, 3.5, and 5.0 million sperm doses, respectively). This study indicated that using an increased sperm dose of some bulls could improve the conception rate in nulliparous heifers, whereas neither the bull nor the sexed sperm dose affected the conception rate in cows. Therefore, a high insemination dose is only advantageous for some bulls, and it is recommended to identify ahead of time the maximum bull fertility based on semen quality factors and perform tests with low insemination dose.
In addition, a lower sperm dose, sexed semen has lower fertility also due to a lot of damage caused during the sexing process (diluents, dye, classification pressure, freezing process, etc.). Using good management practice, pregnancy rates in cattle with sexed sperm are approximately 80% of those rates from a conventional semen dose, which represents a reduction of 10 to 20% in pregnancy rates compared to those of the conventional non-sexed semen dose (Butler et al. 2014; Seidel Jr. 2014). There is variation in the conception rates reported in the literature; however, there is a consensus regarding the conception rate of heifers after estrous detection. It is estimated that AI with sexed semen ranges from approximately 70 to 90% of those with conventional semen, depending mainly on the farm management conditions. Conception rates of 40 to 68% were reported for Holstein heifers after insemination with sexed semen and between 67 and 82% using conventional semen. Despite the lower fertility reported for sexed semen, adjustments of time of AI regarding the onset of estrous can improve conception rates (Seidel Jr. et al. 1999).
For AI programs using sexed semen frequentelyfemales are inseminated based on estrous detection after natural estrus or synchronization with PGF2α using two doses 14 days apart. Thus, insemination can be performed about 12 to 24 h after estrous detection (Seidel Jr. and Schenk 2008a, b) or about 80 to 82 h after the second administration of PGF2α (Kurykin et al. 2007). Using heat detectors (radiotelemetry, Heat Watch®) in Jersey heifers, AI with sexed semen was performed at different times considering the onset of estrous (12–16 h, 16–20 h, 20–24 h, and 24–30 h). In this study, higher conception rates were reported for females inseminated 16–24 h after the onset of estrous (average 53.7%) compared those inseminated after 12–16 h (37.7%); however, similar conception rates were observed compared to those of females inseminated 24–30 h after the onset of estrous (45.5%) (Sá Filho et al. 2010a, b). Based on estrous observation, an intravaginal P4 device can also be used from days 0 to 8 in association with PGF2α injection 24 h before device removal (Underwood et al. 2010a). This strategy facilitates the management of synchronization and estrus identification.
Other studies have also reported that using a high number of sexed sperm per insemination after estrous detection (2.1–4.2 million) in two stages with a 12 h interval, GnRH administration at the time of estrous detection (Sá Filho et al. 2010a, b), and the deposition site in the uterus (Kurykin et al. 2007) did not affect the conception rates in dairy heifers. On the other hand, conception rate is affected by number of services to which that female is submitted, first (55.3%a), second (46.1%a), or third services (34.8%b) (Sá Filho et al. 2010a, b), suggesting that fertility following AI with sexed semen tends to decrease with the number of services (DeJarnette et al. 2009). In this context, sexed semen is more appropriate for heifers (Butler et al. 2014; Seidel Jr. 2014), and its use is recommended in the first service postpuberty, followed by the use of conventional semen in the next AI . In addition, this strategy enables maximum fertility using sexed semen; it is possible to reduce dystocia (female calves cause fewer calving problems) and increase the proportion of females calves in heifers and primiparous around 65% (Weigel 2004).
Initially, low conception rates were reported for beef and dairy cattle submitted to TAI with sexed semen, but studies have contributed to promote satisfactory strategies. Heifers inseminated with sexed sperm between 55 and 56 h after CIDR removal and PGF2α injection had a 34% pregnancy rate, whereas those submitted to TAI between 67 and 68 h after CIDR removal presented a 49% pregnancy rate (Seidel Jr. and Schenk 2008a, b). Lactating dairy cows submitted to OvSynch protocol (GnRH, PGF2α, and GnRH) had similar pregnancy rates to TAI using a 10 million sexed sperm dose (43.9%), 2 million sexed sperm dose (40.5%), or 10 million non-sexed control sperm dose (55.6%) (Schenk et al. 2009).
Strategies have been developed to improve the reproductive performance of the herd after using sexed semen. The effect of time for AI (54 h after implant removal/16–18 h before ovulation versus 60 h after implant removal/10–12 h before ovulation) and semen [conventional (40 million sperm) versus sexed (2 million sperm)] were studied in 389 beef cows (Bos indicus) 30–60 days postpartum. In this study, conception rates were similar between TAI using sexed semen at 54 h (48.4%) and 60 h (55.1%) after norgestomet implant removal and among conventional (58.9%), sexed X (52.0%), and sexed Y semen (49.0%). However, 6 hours delay in TAI increased by 9% the conception rate of animals inseminated with sexed semen [54 h (37.4%) versus 60 h (46.4%)]. These data suggest that the most appropriate time to perform TAI with sexed semen is 60 h after P4 device removal (10–12 h before ovulation) (Souza et al. 2008). The same experimental design was tested in dairy cattle (Bos taurus) using ovulation synchronization with CIDR-B insertion, 2 mg of EB and PGF2α on day 0; CIDR-B removal and PGF2α on day 8; 1 mg of EB on day 9; and TAI on day 10 (54 or 60 h after CIDR-B removal). Similar results were obtained, demonstrating that a 6 h delay in TAI with sexed semen also increased conception rates in dairy cattle (Sales et al. 2011a).
These increased conception rates observed when using sexed semen after 54 or 60 h of device removal or 10–12 h before ovulation is justifiable because sexed sperm require less time for capacitation due to the flow cytometry process (Lu and Seidel Jr. 2004). In addition, ultrasonography evaluation to determine the diameter of the dominant follicle at the time of TAI with sexed semen has improved pregnancy rates. Using sexed semen for TAI , an effect of the type of semen [conventional (45.4%) versus sexed (54.2%)] and of the diameter of the dominant follicle at TAI [≥ 11 mm (57.9%) versus <11 mm (44.1%)] was observed on the pregnancy rates (Sá Filho et al. 2012).
Currently, sexing sperm has achieved important advances and process has been performed based on next-generation technologies (SexedULTRATM - Sexing Technologies, Navasota, TX), which provides sex-sorted semen that is commercially available for dairy and beef cattle. This sex-sorted semen is presented at a concentration of 4 × 106 spermatozoa per straw. In addition, this semen includes adjustments to the composition of the medium that include the prestaining seminal treatment, modifications in the staining medium itself, and freezing extenders, which contribute to greater balance and pH maintenance for prolonged times (Vishwanath and Moreno 2018; Thomas et al. 2017; de Graaf et al. 2014).
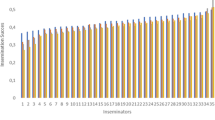
Sex-sorting from next-generation technologies was compared with the different methodologies of sex-sorted semen in Nelore cattle submitted to the TAI protocol. For all cows, the TAI protocol was similar as demonstrated in Fig. 1.8. In this study, the pregnancy rate for TAI (P < 0.0001) is promising for the current sexing methodology used (Table 1.4) (Baruselli et al. 2017).
Diagrammatic representation of the TAI protocol performed in Nelore cows inseminated with sexed-ultra (cows unpainted at the base of the tail/estrus expression) or conventional semen/non-sorted (cows painted at the base of the tail/no estrus expression). Treatment based on P4-estradiol and insemination performed 60 h after removal of the P4 device. P4, progesterone (0.6 g); EB, estradiol benzoate (2 mg); PGF2α, cloprostenol (0.526 mg); eCG, equine chorionic gonadotropin (300 IU); EC, estradiol cypionate (1 mg). (Adaptations from Marques et al. (2018))
Another study conducted in Brazil (Marques et al. 2018) analyzed the conception rates after TAI with sex-ultra semen in suckled Nelore cows (n = 281). In addition to testing different doses (4, 6, and 8 × 106 sperm from a single Nelore bull), this study evaluated the rational use of semen according to estrus behavior (sex-sorted to cows in estrus and non-sorted to cows with no estrus expression) after P4 removal (Fig. 1.8). The conception rate was similar among the cows inseminated with 4 (59.5%, 47/79), 6 (58.4%, 45/77), and 8 x 106 sperm (51 9%, 40/77). Cows in estrus showed 56.7% (123/233) and those not in estrus showed 33.3% (16/48) conception rates. Interestingly, this study highlights a strategy with high efficiency for the use of sex-sorted semen in commercial field conditions. It emphasizes the positive results achieved (pregnancy rate higher than 50%), which can be attributed to the new methodology of sexed semen and to the fact that this semen was only used in cows that expressed estrus, as indicated by unpainted tails.
Another important consideration about sexed semen is related to the time when sexing is performed based on freezing. Conventionally, sexed semen preparation begins with semen collection from bulls followed by sperm sex sorting (X and Y chromosomes) and freezing. Currently also is possible to obtaining sexed sperm from previously frozen doses (reverse-sorted semen, RSS), which represents a breakthrough in livestock management due to the possibility of its use in association with other biotechnologies. In addition, there is no need for the bull to be close to the sexing laboratory and allows for the sexing of freezing semen from bulls that have died. In this context, this technique can also be used as an alternative in AI (Underwood et al. 2010b) or IVF programs (Morotti et al. 2014) to preselect the sex of the offspring produced.
1.8 Resynchronization Program
Resynchronization is a reproductive strategy that aims to concentrate the breeding period from females that failed to become pregnant after receiving the AI procedure. Using resynchronization, non-pregnant females are quickly diagnosed and inseminated in the shortest possible time, reducing the service period and making the breeding season shorter. This strategy is very interesting because it is associated with a higher pregnancy rate from AI , reduction in the number of bulls to natural breeding after TAI or AI program, in addition to several benefits associated with the management of calves (Baruselli et al. 2018; Marques et al. 2015).
Insemination is a worldwide established biotechnology that provides economic returns by improving offspring production rates. However, this return is closely linked to strategies that can be associated with using this technique. Among the main factors that affect the productive performance of calves until weaning, there is the interaction between the month of birth and geographic location, such as variation in the calf weight at weaning. For example, in cattle from tropical countries Nelore calves born from August to October had high weaning weights (Bocchi and Albuquerque 2004), possibly due to a higher concentration of calving in the late winter and early spring after conception during months of high food availability. Therefore, a short breeding season aims to improve progeny performance. Furthermore, calves whose conceptions occurred in the first half of the breeding season had increased production rates, such as earlier slaughter, earlier reproductive life, and their mothers had a higher pregnancy rate at the end of the breeding season (Carneiro et al. 2012).
1.8.1 Estrus Resynchronization
A wide variety of hormonal treatments can be used for synchronization of estrus and ovulation in cattle, and this strategy allows for inseminating all treated animals, resulting in conception rates of approximately 50% and a service rate of 100% (Baruselli et al. 2004a, b, 2018). Using an ovulation synchronization protocol, synchronous estrus return is expected in those females who failed to conceive after the first TAI . Commonly, this estrus behavior is concentrated between 18 and 23 days later, and reproductive practices can be strategically combined at this time (Baruselli et al. 2004a, b; Cavalieri et al. 2004). In this estrus return, conventional AI can be used, or bulls can be rationally allocated together with cows for natural breeding from 10 to 15 days after TAI (Campos et al. 2013). This management results in high conception rates at the end of the breeding season (Torres-Júnior et al. 2009). Generally, visual estrous detection in Bos indicus cows results in low service rates (25%) due to a short estrous duration (<12 hours) and high incidence of estrous occurring at night (30%) in Nelore cows (Pinheiro et al. 1998; Galina et al. 1996). Furthermore, postpartum anestrous usually has a high incidence in Bos indicus cattle, especially if kept in regions with seasonal forages (Ayres et al. 2008). Therefore, difficulty in estrous detection in nonpregnant cows is a challenge for improving the number of pregnancies following AI because fewer than half of nonpregnant cows have become inseminated again (Campos et al. 2013).
The TAI program followed by estrous detection and subsequent AI usually results in 60 to 65% pregnancy rate in the first 45 days of the breeding season compared to 30% after natural mating and 20% after conventional AI . Failure in inseminating nonpregnant cows in a short period results in prolonged intervals among services and in delayed pregnancies (Baruselli et al. 2018; Marques et al. 2015; Campos et al. 2013; Galvao et al. 2007; Bartolome et al. 2005; El-Zarkouny, 2004). In this context, ovulation resynchronization is a strategy to increase the number of pregnancies in the first half of the breeding season. Therefore, the aim of ovulation resynchronization is to eliminate the estrous observation, increase the number of calves born by AI , and reduce the number of bulls needed.
1.8.2 Main Strategies for Ovulation Resynchronization
In general, hormonal treatments for ovulation resynchronization in cattle are similar to those previously described for the TAI protocol. One of the most common treatments for resynchronization involves the use of an intravaginal P4 device and EB, combined with application of PGF2α, EC and/or GnRH (Sá Filho et al. 2014; Campos et al. 2013; Galvao et al. 2007; El-Zarkouny, 2004). This hormonal combination allows for synchronization of the emergence of a new wave of follicular growth in nonpregnant females. The ovulation resynchronization can be started after confirming that the female is not pregnant (traditional resynch/30 days after 1st TAI ) (Marques et al. 2015) or before even knowing the female’s gestational status (early resynch/22–23 days after 1st TAI and super-early resynch/14 days after 1st TAI ) (Baruselli et al. 2018; Sá Filho et al. 2014; Campos et al. 2013).
Although TAI has shown satisfactory results, reductions of 15 to 20% in the conception rate are reported for cattle with high anestrous rate (Ayres et al. 2008; Baruselli et al. 2004a, b). In this context, administration of eCG in anestrous suckled cows has been recommended to provide pregnancy rates of approximately 50%, which is similar to those observed in cyclic cows (Barreiros et al. 2014; Kastelic et al. 1999). Therefore, the use of gonadotropic support (equine chorionic gonadotropin) or temporary calf removal (48–72 h) has been recommended at this time to final growth of the dominant follicle in resynchronization protocols of postpartum cattle (Campos et al. 2013).
Resynchronization 30 days (traditional resynch) was one of the first resynchronization strategies used, which consists in performed a pregnancy diagnosis from 28 to 32 days after the first TAI (Fig. 1.9). Therefore, only nonpregnant females are submitted to the second hormonal protocol, resulting in a 40-day interval between TAI (Marques et al. 2015; Bartolome et al. 2005). This resynchronization results in a conception rate of 50 to 60% for each TAI , and the final cumulative pregnancy rate can be over 80%.
Schematic representation of the protocol used for timed artificial insemination (TAI) and resynchronization of ovulation in nonpregnant cattle. I) Resynchronization 30 days/traditional resynch; II) Resynchronization 22–23 days/early resynch; III) Resynchronization 14 days/super-early resynch. P4 progesterone (0.5–1.9 g), EB estradiol benzoate (2 mg), EC estradiol cypionate (0.5 mg for heifers and 1 mg for cows), PGF2α prostaglandin (125–500μg), eCG equine chorionic gonadotropin (300–400 IU)
Another possibility for ovulation resynchronization (early resynch/22–23 days after 1st TAI ) is to start a second hormonal protocol 22–23 days after TAI without a previous pregnancy diagnosis. Therefore, all females initially treated for 1st TAI protocol receive a P4 intravaginal device and EB injection between 22 to 23 days regardless of gestational status (Sá Filho et al. 2014; Campos et al. 2013; Chebel et al. 2003). Upon P4 device removal (8–9 days from insertion), the pregnancy ultrasound evaluation is performed, and nonpregnant females are injected with PGF2α and an ovulation inducer, such as EC (Fig. 1.9).
The resynchronization program 14 days after 1st TAI (super-early resynch) anticipates insemination at 8 days in relation to resynch 22–23 and 16 days in relation to resynch 30, making the breeding season extremely short and a 14-day interval between TAIs. This resynch is initiated 14 days after the first insemination, being that all females receive a combination of treatments with intravaginal P4 (device) and 50–100 mg of injectable P4, IM. Then, on day 22, the diagnosis of pregnancy is made by assessing CL vascularization with color Doppler. Cows with low or absent vascularization are considered nonpregnant and continue resynchronization treatment with device removal, PGF2α, EC, and eCG, receiving TAI 48 h later (day 24). Cows with moderate CL or strong vascularity are considered pregnant and do not receive any hormonal treatment except reassessment with B-mode ultrasound (Baruselli et al. 2018).
The use of color Doppler ultrasonography to characterize the CL vascularization is essential for classifying female’s gestational status (Hassan et al. 2018). After the luteolysis process (approximately 14 to 17 days of the estrous cycle), the pregnant female maintains high vascularization in the CL, and therefore, the Doppler evaluation allows an indirect assessment of gestational status from the CL vascularization score. This technique allows for a much earlier pregnancy diagnosis than B-mode ultrasound identification. Nevertheless, females with vascularized CL need to be reevaluated with B-mode ultrasound 8 to 10 days later to visualize the gestational vesicle and detect eventual false positives (females classified as pregnant by the CL vascularization score but without a gestational vesicle) (Baruselli et al. 2017; Pugliesi et al. 2017; Siqueira et al. 2013).
Reproductive programs that allow insemination every 21 days would be a more ideal because they would have a 100% (21/21) service rate. In this context, resynch at 14 days is the strategy that most closely approximates this ideal service rate, being 87.5% (24/21) to resynch 14, 66% (32/21) to resynch 22–23, and 52.5% (40/21) to resynch 30 (Baruselli et al. 2018). However, the implementation of super-early resynch is more laborious and, for success, requires an ultrasound with Doppler function in addition to high professional experience.
1.8.3 Success Rate Using Resynchronization
Satisfactory results are achieved with resynchronization programs in cattle, which results with pregnancy rates of approximately 50% after each TAI . These strategies result in pregnancy rates of 80 to 90% during days 24 to 40 of the breeding season (according to resynch), contributing to greater genetic gain of the herd (more pregnant from AI ) besides reducing the number of bulls on the farm. In some situations, natural breeding (use of bulls) may be associated with resynchronization for 10–15 days after the 2nd or 3rd TAI until the end of the breeding season (Baruselli et al. 2018; Marques et al. 2015).
On the other hand, there is variation in conception according to the number of calving. Primiparous and secundiparous cows showed a reduction in the conception rate from the 1st to 2nd TAI (20%) compared to heifers and multiparous cows. This reduction is possibly associated with nutritional limitations and not the category’s own fertility. Conception rates among different categories of Nelore females were different (Fig. 1.10) in a study evaluating reproductive performance (Marques et al. 2015). At the end of the breeding season, heifers had a higher conception rate (85%) than those of primiparous (76%) and multiparous cows (78%). In this study, all animals received a source P4 (intravaginal device for the cows or ear implants for heifers) in combination with 2 mg of EB on day 0. All females received 250μg of cloprostenol, 300 IU of eCG, EC (1 mg to cows and 0.5 mg to heifers), and P4 removal on the eighth day. TAI was performed 48 hours after P4 removal. Thirty days after the 1st TAI , all females were evaluated by ultrasonography, and the nonpregnant bovines were resynchronized with the same hormonal treatment. The pregnancy rate was assessed by ultrasound 30 days after TAI .
Conception rates among different categories of Nelore females after the timed artificial insemination (1st TAI ) and ovulation resynchronization (2nd TAI ). (Adapted from Marques et al. (2015))
a-b: indicates statistical difference for categories in 1st TAI; A-B: indicates statistical difference for categories in 2nd TAI ; and α-β: indicates statistical difference for categories in 1st and 2nd TAI
Early resynch anticipates the 2nd TAI in 8–9 days, but both pregnant and nonpregnant cows receive a P4 device and 1–2 mg of EB between 19 and 23 days after the first TAI . In addition, to promote follicular atresia and synchronize follicular growth, estradiol esters can induce luteolysis (Vieira et al. 2014; Pinheiro et al. 1998). However, studies with Bostaurus (El-Zarkouny 2004; Chebel et al. 2003) and Bos indicus (Sá Filho et al. 2014; Campos et al. 2013) females reported no interruption of pregnancies obtained in the 1st TAI using GnRH or EB in the early resynchronization. There was no reduction in the conception rates in Nelore cows treated (54%) or untreated (47.8%) with 2 mg of EB 23 days after TAI (Campos et al. 2013). In addition, pregnancy losses were similar in Nelore females treated with 1.0 mg of EB (4.1%) compared to untreated females (2.0%) and maintained with bulls for natural mating (Sá Filho et al. 2014). Conception rates are similar to those obtained with resynch 30 days, as shown previously, resulting in approximately 50% in the 2nd TAI and a total of 75% of conception in 32–33 days of service (Sá Filho et al. 2014; Campos et al. 2013). In this resynch, variations in conception rates were also observed according to number of calving. Nonsuckled cows, heifers, and postpartum cows have a decrease of 20% in conception rates. Furthermore, primiparous cows showed a similar conception rate (40%) in two subsequent (Marques et al. 2015; Crepaldi et al. 2014; Sá Filho et al. 2014). In cows that did not become pregnant after the 1st TAI , a 2nd TAI (resynchronization of ovulation) may be performed 32 days later, and a 3rd TAI may be repeated, resulting in a total conception rate of 90% after three services in 64 days of a breeding season (Baruselli et al. 2018).
A similar pregnancy rate using resynch 22 versus resynch 14 was observed in the 1st synchronization (48% versus 53%) and resynchronization (56% versus 51%), respectively. Cumulative pregnancy rates after 32 and 24 days of the breeding season were also similar for resynch 22 (77%, 97/126) and resynch 14 (75%, 89/118). However, resynch 14 improved the service rate every 21 days with 66% for resynch 22–23 and 87.5% for resynch 14 (Baruselli et al. 2017).
In general, reduced conception rates are observed in the 2nd and 3rd TAI. In addition to the number of calving, it is necessary to consider other factors, such as BCS and health status of the herd. Reduction in the period of service for 62 to 80 days using three TAI provides a short calving interval and a considerable improvement in reproductive efficiency (with an average calving interval of less than 12 months). Pregnancy rates between 75 and 90% were reported for cattle submitted to resynchronization, reducing the numbers of bulls needed for natural matting or even not requiring them at all.
1.9 Dominant Follicle Manipulation During the TAI Protocol
The size of the dominant follicle at the time of TAI has been extensively investigated due to its influence on the reproductive behavior and performance of animals submitted to synchronization of ovulation. Although the TAI protocol aims for ovulation synchronization and does not require estrus detection, many studies have investigated the relationship of this behavior, diameter of dominant follicle and the fertility of animals undergoing TAI programs (Moraes et al. 2019; Morotti et al. 2013a, b, 2018a; Pfeifer et al. 2012, 2015; Sales et al. 2012a, b; Sá Filho et al. 2010a, b, 2011). In this context, dominant follicle size at the time of insemination has been widely studied in which the largest follicle diameter at TAI is positively associated with higher estrus expression, higher ovulation rate, larger size of CL, higher concentration of P4, and higher pregnancy probability (Moraes et al. 2019; Morotti et al. 2018a; Pfeifer et al. 2015).
1.9.1 Estrus Expression and Fertility in TAI
Considering that the follicular diameters may differ at the time of TAI and that this may determine a higher or less ovulatory potential according to follicle size (Gimenes et al. 2008), block TAI has been suggested as a possibility to improve fertility of females undergoing a TAI program. Block TAI can be applied as a strategy to increase the pregnancy rate in Nelore cows (Pfeifer et al. 2015). On day 10 of the TAI protocol, the blocks are divided according to the size of the dominant follicle; in this way, TAI is performed at different times in each group (Table 1.5). Performing TAI in a block of Nelore cows increased the pregnancy rate by 16.7% (65.5%; 129/203) compared to the group subjected to conventional TAI on day 10 (48.8%; 102/209; Pfeifer et al. 2015).
For better understanding of the relationship of dominant follicle size at TAI, estrus expression, and fertility, an estrus score can be performed based on the painting intensity remaining at the base of the tail. Normally, at the removal of the P4 device, all animals have the sacral and tail base region painted with marker stick, and at the time of TAI , the animals are classified into different scores (Nogueira et al. 2019). According to the proportion of paint removed, animals can be classified into (Fig. 1.11): score I, no or low paint removal (no estrus expression), score II, partial paint removal (up to 75%; low estrus expression), and score III, complete or > 75% paint removal (high estrus expression). This practical strategy allows for usefully identifying cows with greater estrus expression and consequently improving the pregnancy rates in TAI (score I, 40.0%; score II, 49.7%; and score III, 60.9%), allowing the cows with low score to be targeted for additional treatments aimed at improving pregnancy .
Scheme of the estrus expression intensity score during timed artificial insemination (TAI) using wax stick painting in the sacrococcygeal region. Commonly, paint management is performed at the time of removal of the intrvaginal progesterone device and the estrus score classification performed immediately before insemination. Cows with high estrus expression are conventionally inseminated and low estrus expression or no estrus expression receive insemination together with GnRH analogue application to potentialize the occurrence of ovulation
Even using TAI, the classification of the estrus score is interesting because scores I and II present lower reproductive performance. Based on this it was suggested application of 100μg of gonadorelin (GnRH group; n = 470) against 1 mL of saline (control group; n = 467; Rodrigues et al. 2019) to animals with I and II scores. Cows with score III (estrus group; n = 1347) received no additional treatment. The pregnancy rate was greater in the estrus group (57.09%; 769/1347) than in the control (36.18%, 169/467) and GnRH groups (45.95%, 216/470). However, GnRH injection increased pregnancy by approximately 10% in relation to the control. Therefore, using P4-estradiol-based TAI protocols, estrus expression can be efficiently monitored with painting on the sacrococcygeal region. Thus, GnRH application in cows with a low estrus score is a simple strategy that can increase the pregnancy rate in cattle.
1.9.2 Antral Follicle Count and Fertility in TAI
The antral follicle count (AFC) is a highly variable reproductive characteristic among the females of a herd, but with very high repeatability throughout the productive female life (Lima et al. 2020; Moraes et al. 2019; Jimenez-Krassel et al. 2017; Burns et al. 2005). For this reason, the AFC has become an interesting reproductive tool since it is closely related to the efficiency of reproductive biotechniques, in addition to the great possibility of affecting female fertility (Garcia et al. 2020; Morotti et al. 2018a, b). In general, AFC can be determined in a simple way through the ultrasound exam to quantify the number of follicles ≥3 mm in diameter and usually is performed on a random day of the estrous cycle of the cows. Therefore, considering the AFC variability, cows can be classified as low, intermediate, or high AFC according to the number of antral follicles present in the ovary during ultrasound evaluation (Silva-Santos et al. 2014; Burns et al. 2005).
The evaluation of the AFC is inserted in a very current context of the selection of females and that seems to be better established for the production of embryos (Garcia et al. 2020; Seneda et al. 2019; Santos et al. 2016; Silva-Santos et al. 2014). For example, for both in vivo production and in vitro production, the selection of donors with high AFC is positively associated with better reproductive performance in these biotechniques (Zangirolamo et al. 2018; Santos et al. 2016). However, relation between AFC and efficiency of the TAI program is not yet fully understood (Morotti et al. 2018a, b). In taurine cattle there are studies that show that AFC does not exert any influence, or it has been observed that high AFC is responsible for better reproductive performance (Evans et al. 2012; Ireland et al. 2010). However, the authors of this chapter have conducted a series of studies on this subject in zebuine cattle which reveal that low AFC appears to have better reproductive performance when subjected to TAI .
In this context, evaluating the ovarian follicular dynamics and fertility of Nelore cows submitted to TAI programs, Morotti et al. (2018a) revealed higher follicular diameters (Fig. 1.12) and pregnancy rates for females with low AFC compared to those with high counts (Table 1.6). In addition to these findings, other studies have also revealed an interaction of AFC with body condition score in Bosindicus (Moraes et al. 2019) and higher reproductive longevity and greater reproductive performance in Bos taurus with low AFC (Jimenez-Krassel et al. 2017).
Diameter of dominant follicle (mm) during ovarian follicular dynamics in Nelore cows with consistently high (≥45 follicles; dashed line) or low (≤15 follicles; continuous line) antral follicle count synchronized with a taimedartifical insemination (TAI ) protocol. Values denoted using different Greek letters (α-β; punctual evaluation on day 4), lowercase letters (a-d; evaluations with 24-h intervals) or capital letters (A-B; evaluations with 12-h intervals) were different (P < 0.05). (Adapted from Morotti et al. (2018a))
In a study on the relationship between AFC and reproductive performance, and the pattern of expression of important genes for various cellular functions in Bos indicus cattle (Lima et al. 2020) showed promising data with positive influence of low AFC on fertility. In this study we found that very low AFC in Nelore cows resulted in a large dominant follicle diameter, a tendency to have higher P4 concentration and greater pregnancy rate in TAI program. In addition, Nelore heifers with low AFC exhibited oocytes and cumulus cells with a better expression patterns of genes linked to intercellular communication, meiotic control, epigenetic modulation, adaptation and cellular stress response and follicular growth. However, studies on the ovarian follicular population are current, it is a subject that has been widely investigated, and many aspects are not yet fully understood.
1.10 Considerations
Currently, reproductive biotechniques have achieved great technological advances that have contributed greatly to increasing the development of livestock. Artificial insemination certainly represents one of the most popular assisted reproductive techniques that contributes significantly to genetic improvementis, being easy to apply and has been considered one of the greatest potentials for expansion in cattle. In this context, many strategies have been developed to stimulate the use of artificial insemination programs. Alternatives to pharmacological control of the estrous cycle, the use of timed artificial insemination, insemination with sexed semen, ovulation resynchronization, and dominant follicle manipulation, are highly effective strategies that are currently indicated as reproductive practices in bee and dairy cattle. Finally, maintaining these advances and utilizing the strategies discussed herein are great challenges but are necessary to increase the productive and reproductive efficiency of livestock.
Abbreviations
- TAI :
-
Timed Artificial Insemination
- TCR:
-
Temporary calf removal
- AFC:
-
Antral follicle count
- AI:
-
Artificial Insemination
- BCS:
-
Body condition score
- CL:
-
Corpus luteum
- EB:
-
Estradiol benzoate
- EC:
-
Estradiol cypionate
- eCG:
-
equine chorionic gonadotropin
- EV:
-
Estradiol valerate
- FSH:
-
Follicle stimulating hormone
- GnRH:
-
Gonadotropin-releasing hormone
- hCG:
-
human chorionic gonadotropin
- IVF:
-
In vitro fertilization
- LH:
-
Luteinizing hormone
- P4:
-
Progesterone
- PGF2α:
-
Prostaglandins
- RSS:
-
Reverse-sorted semen
- RTS:
-
Reproductive tract scoring system
- US:
-
Ultrasound
References
Adams GP, Jaiswal R, Singh J, Malhi P (2008) Progress in understanding ovarian follicular dynamics in cattle. Theriogenology 69:72–80
Alfieri AA, Leme RA, Agnol AMD, Alfieri AF (2019) Sanitary program to reduce embryonic mortality associated with infectious diseases in cattle. Anim Reprod 16(3):386–393
Allrich RD (1994) Endocrine and neural control of estrus in dairy cows. J Dairy Sci 77:2738–2744
Amstalden M, Alves BR, Liu S, Cardoso RC, Williams GL (2011) Neuroendocrine pathways mediating nutritional acceleration of puberty: insights from ruminant models. Front Endocrinol 2:109
Anderson ML (2007) Infectious causes of bovine abortion during mid- to late-gestation. Theriogenology 68:474–486
Andersen KJ, Lefever DG, Brinks JS, Odde KG (1991) The use of reproductive tract scoring in beef heifers. Agri-Practice 12:19–26
Andersson M, Taponen J, Koskinen E, Dahlbom M (2004) Effect of insemination with doses of 2 or 15 million frozen-thawed spermatozoa and semen deposition site on pregnancy rate in dairy cows. Theriogenology 61:1583–1588
Atkins JA, Pohler KG, Smith MF (2013) Physiology and endocrinology of puberty in heifers. Vet Clin N Am Food Anim Pract 29:479–492
Ayres H, Martins CM, Ferreira RM, Mello JE, Dominguez JH, Souza AH, Valentin R, Santos IC, Baruselli PS (2008) Effect of timing of estradiol benzoate administration upon synchronization of ovulation in suckling nelore cows (Bos indicus) treated with a progesterone-releasing intravaginal device. Anim Reprod Sci 109:77–87
Baishya N, Ball PJH, Leaver JD, Pope GS (1980) Fertility of lactating dairy cows inseminated after treatment with cloprostenol. Br Vet J 136:227–239
Barreiros TR, Blaschi W, Santos GM, Morotti F, Andrade ER, Baruselli PS, Seneda MM (2014) D dynamics of follicular growth and progesterone concentrations in cyclic and anestrous suckling Nelore cows (Bos indicus) treated with progesterone, equine chorionic gonadotropin, or temporary calf removal. Theriogenology 81:651–656
Bartolome JA, Sozzi A, Mchale J, Melendez P, Arteche AC, Silvestre FT, Kelbert D, Swift K, Archbald LF, Thatcher WW (2005) Resynchronization of ovulation and timed insemination in lactating dairy cows, II: assigning protocols according to stages of the estrous cycle, or presence of ovarian cysts or anestrus. Theriogenology 63:1628–1642
Baruselli PS, Teixeira AA (2018) Inseminação Artificial em Tempo Fixo (IATF) em Bovinos. In: Morani ESC, Rodrigues LH, Rancoletta M (eds) Manual de Reprodução nas espécies domésticas. Editora MedVet, pp 147–166
Baruselli PS, Reis EL, Marques MO, Nasser LF, Bó GA (2004a) The use of hormonal treatments to improve reproductive performance of anestrous beef cattle in tropical climates. Anim Reprod Sci 82:479–486
Baruselli PS, Marques MO, Reis EL, Carvalho NAT, Carvalho JB (2004b) Manipulação Hormonal do estro e da ovulação. Curso à Distância de Manipulação do Ciclo Estral em Bovinos de Corte (Módulo 3), 1
Baruselli PS, Martins SAH, Gimenes CM, Sales LU, Ayres JNS, Andrade H, Raphael AFC, Arruda RP (2007) Sexed semen: artificial insemination and embryo transfer. Revista Brasileira de Reprodução Animal 31:374–381
Baruselli PS, Ferreira RM, Sales JNS, Gimenes LU, Sá Filho MF, Martins CM,..... Bó GA, et al (2011) Timed embryo transfer programs for management of donor and recipient cattle. Theriogenology 76(9):1583–1593
Baruselli PS, Ferreira RM, Colli MHA, Elliff FM (2017) Timed artificial insemination: current challenges and recent advances in reproductive efficiency in beef and dairy herds in Brazil. Anim Reprod 14:558–571
Baruselli PS, Ferreira RM, Sá Filho MF, Bó GA (2018) Review: using artificial insemination v. natural service in beef herds. Animal 12:45–52
Baryczka A, Barański W, Nowicki A, Zduńczyk S, Janowski T (2018) Effect of single treatment with cloprostenol or dinoprost on estrus and reproductive performance in anestrous dairy cows after service. Pol J Vet Sci 21:383–387
Bergfeld EG, Kojima FN, Cupp AS, Wehrman ME, Peters KE, Garcia-Winder M, Kinder JE (1994) Ovarian follicular development in prepubertal heifers is influenced by level of dietary energy intake. Biol Reprod 51:1051–1057
Binelli M, Sartori R, Vasconcelos JLM, Monteiro Jr. PLJ, Pereira MHCV, Ramos RS (2014) Fertility of fixed-time protocols in dairy cattle evolution in fixed-time: from synchronization of ovulation to improved fertility, 494–506
Bó GA, Baruselli PS (2014) Synchronization of ovulation and fixed-time artificial insemination in beef cattle. Animal 8:144–150
Bo GA, Adams GP, Caccia M, Martinez M, Pierson RA, Mapletoft RJ (1995) Ovarian follicular wave emergence after treatment with progestogen and estradiol in cattle. Anim Reprod Sci 39:193–204
Bó GA, Cutaia L, Tribulo R (2002) Tratamientos hormonales para inseminación artificial a tiempo fijo en bovinos para carne: algunas experiencias realizadas en Argentina. Segunda Parte. Taurus 15:17–32
Bó GA, Baruselli PS, Martinez MF (2003) Pattern and manipulation of follicular development in Bos indicus cattle. Anim Reprod Sci 78:307–326
Bocchi ALTRA, Albuquerque LG (2004) I Idade da vaca e mês de nascimento sobre o peso ao desmame de bezerros nelore nas diferentes regiões brasileiras. Acta Scientiarum. Anim Sci 26:475–482
Borsato EALJHE, Rubin KCP, Saut JP, Barreiros TRR, Seneda MM (2004) Relação entre o tamanho do folículo ovulatório e taxa de concepção em novilhas Bos taurus X Bos indicus submetidas à inseminação artificial em tempo fixo. Revista Brasileira de Reprodução Animal 28:137–142
Boulton AC, Rushton J, Wathes DC (2017) An empirical analysis of the cost of rearing dairy heifers from birth to first calving and the time taken to repay these costs. Animal 11(8):1372–1380
Brickell JS, Wathes DC (2011) A descriptive study of the survival of Holstein-Friesian heifers through to third calving on English dairy farms. J Dairy Sci 94(4):1831–1838
Brumatti RC, Ferraz JBS, Eler JP, Formigonni IB (2011) Desenvolvimento de índice de seleção em gado corte sob o enfoque de um modelo bioeconômico. Archivos de zootecnia 60(230):205–213
Buckley F, Lopez-Villalobos N, Heins BJ (2014) Crossbreeding: implications for dairy cow fertility and survival. Animal 8:122–133
Butcher RL, Reber JE, Lishman AW, Breuel KF, Schrick FN, Spitzer JC, Inskeep EK (1992) Maintenance of pregnancy in postpartum beef cows that have short-lived corpora lutea. J Anim Sci 70:3831–3837
Butler ST, Hutchinson IA, Cromie AR, Shalloo L (2014) Applications and cost benefits of sexed semen in pasture-based dairy production systems. Animal 8(Suppl 1):165–172
Burns DS, Jimenez-Krassel F, Ireland JL, Knight PG, Ireland JJ (2005) Numbers of antral follicles during follicular waves in cattle: evidence for high variation among animals, very high repeatability in individuals, and an inverse association with serum follicle-stimulating hormone concentrations. Biol Reprod 73(1):54–62
Campos JT, Marinho LS, Lunardelli PA, Morotti F, Seneda MM (2013) Resynchronization of estrous cycle with eCG and temporary calf removal in lactating Bos indicus cows. Theriogenology 80:619–623
Campos JT, Morotti F, Costa CB, Bergamo LZ, Seneda MM (2016a) Evaluation of pregnancy rates of Bos indicus cows subjected to different synchronization ovulation protocols using injectable progesterone or an intravaginal device. Semina: Ciências Agrárias 37(6):4149–4155
Campos JT, Morotti F, Bergamo LZ, Costa CB, Seneda MM (2016b) Pregnancy rate evaluation in lactating and non-lactating Nelore cows subjected to fixed-time artificial insemination using injectable progesterone. Semina: Ciências Agrárias 37(4):1991–1996
Carneiro LC, Silva JCC, Mendes GP, Ferreira IC, Santos RM (2012) Efeito do mês de parição na taxa de gestação subsequente e no peso ao desmamedos bezerros de vacas Nelore. Acta Sci Vet 40(2):1030
Carvalho JB, Carvalho NA, Reis EL, Nichi M, Souza AH, Baruselli PS (2008) Effect of early luteolysis in progesterone-based timed AI protocols in Bos indicus, Bos indicus x Bos taurus, and Bos taurus heifers. Theriogenology 69:167–175
Cavalieri J, Rubio I, Kinder J, Entwistle K, Fitzpatrick L (1997) Synchronization of estrus and ovulation and associated endocrine changes in Bos indicus cows. Theriogenology 47:801–814
Cavalieri J, Hepworth G, Fitzpatrick LA (2004) Comparison of two estrus synchronization and resynchronization treatments in lactating dairy cows. Theriogenology 62:729–747
Cerchiaro I, Cassandro M, Dal Zotto R, Carnier P, Gallo L (2007) A field study on fertility and purity of sex-sorted cattle sperm. J Dairy Sci 90:2538–2542
Chacur MGM, Ferreira Aurelio PT, Scalon O Jr, Inague L, Do Nascimento Kronka S, Scalon LF (2010) Effects of lower doses of cloprostenol intramuscular or into vulvar submucosa on estrus induction and pregnancy rates in Nelore cows. Semina: Ciencias Agrarias 31:451–458
Chebel RC, Santos JEP, Cerri RLA, Galvão KN, Juchem SO, Thatcher WW (2003) Effect of resynchronization with GnRH on day 21 after artificial insemination on pregnancy rate and pregnancy loss in lactating dairy cows. Theriogenology 60:1389–1399
Colazo MG, Mapletoft RJ (2014) A review of current timed-AI (TAI) programs for beef and dairy cattle. Can Vet J 55:772–780
Colazo MG, Martínez MF, Kastelic JP, Mapletoft RJ (2002) Effects of dose and route of administration of cloprostenol on luteolysis, estrus and ovulation in beef heifers. Anim Reprod Sci 15:47–62
Crepaldi GAFBG, Vieira LM, Sá Filho MF, Guerreiro BM, Baruselli PS (2014) Reproductive efficiency of Nelore females submitted to three consecutive FTAI programs with 32 days of interval between inseminations. Anim Reprod 11:355
Crowe MA (2008) Resumption of ovarian cyclicity in post-partum beef and dairy cows. Reprod Domest Anim 43(Suppl 5):20–28
Crowe MA, Mullen MP (2013) Regulation and function of gonadotropins throughout the bovine oestrous cycle
Cuervo-Arango J, Garcia-Rosello E, Garcia-Munoz A, Valldecabres-Torres X, Martinez-Ros P, Gonzalez-Bulnes A (2011) The effect of a single high dose of PGF2alpha administered to dairy cattle 3.5 days after ovulation on luteal function, morphology, and follicular dynamics. Theriogenology 76:1736–1743
Day ML, Imakawa K, Wolfe PL, Kittok RJ, Kinder JE (1987) Endocrine mechanisms of puberty in heifers. Role of hypothalamo-pituitary estradiol receptors in the negative feedback of estradiol on luteinizing hormone secretion. Biol Reprod 37:1054–1065
de Graaf SP, Leahy T, Vishwanath R (2014) Biological and practical lessons associated with the use of sexed semen. Reproduction in Domestic Ruminants, 8, 9th
DeJarnette JM, Nebel RL, Marshall CE, Moreno JF, Mccleary CR, Lenz RW (2008) Effect of sex-sorted sperm dosage on conception rates in holstein heifers and lactating cows. J Dairy Sci 91:1778–1785
DeJarnette JM, Nebel RL, Marshall CE (2009) Evaluating the success of sex-sorted semen in US dairy herds from on farm records. Theriogenology 71:49–58
DeJarnette JM, Leach MA, Nebel RL, Marshall CE, Mccleary CR, Moreno JF (2011) Effects of sex-sorting and sperm dosage on conception rates of Holstein heifers: is comparable fertility of sex-sorted and conventional semen plausible? J Dairy Sci 94:3477–3483
Demetrio DGB, Santos RM, Demetrio CGB, Vasconcelos JLM (2007) Factors affecting conception rates following artificial insemination or embryo transfer in lactating holstein cows. J Dairy Sci 90:5073–5082
Dias CC, Wechsler FS, Day ML, Vasconcelos JL (2009) Progesterone concentrations, exogenous equine chorionic gonadotropin, and timing of prostaglandin F(2alpha) treatment affect fertility in postpuberal Nelore heifers. Theriogenology 72:378–385
Eaglesome MD, Garcia MM (1997) Disease risks to animal health from artificial insemination with bovine semen. Rev Sci Tech 16:215–225
El-Zarkouny SZSJS (2004) Resynchronizing estrus with progesterone or progesterone plus estrogen in cows of unknown pregnancy status. J Dairy Sci 87:3306–3321
Ereno RL, Barreiros TRR, Seneda MM, Baruselli PS, Pegorer MF, Barros CM (2007) Taxa de prenhez de vacas Nelore lactantes tratadas com progesterona associada à remoção temporária de bezerros ou aplicação de gonadotrofina coriônica eqüina. Rev Bras Zootec 36:1288–1294
Evans AC, Adams GP, Rawlings NC (1994) Follicular and hormonal development in prepubertal heifers from 2 to 36 weeks of age. J Reprod Fertil 102:463–470
Evans ACO, Mossa F, Walsh SW, Scheetz D, Jimenez‐Krassel F, Ireland JLH,..... Ireland JJ, et al (2012) Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod Domestic Animals 47:31–37
Farnworth PG (1995) Gonadotrophin secretion revisited. How many ways can FSH leave a gonadotroph? J Endocrinol 145:387–395
Fernandes P, Teixeira AB, Crocci AJ, Barros CM (2001) Timed artificial insemination in beef cattle using GnRH agonist, PGF2alpha and estradiol benzoate (EB). Theriogenology 55:1521–1532
Fernandes CAC, Figueiredo ACS, Oliveira ER, Oba E, Vasconcelos TD (2006) Eficiency of different pgf2α analogous in the pospartum perido of dairy cows. In: World Buiatrics Congress, 2006, Nice, France. WBC, Nice
Ferraz MVC, Pires AV, Santos MH, Silva RG, Oliveira GB, Polizel DM, Biehl MV, Sartori R, Nogueira GP (2018) A combination of nutrition and genetics is able to reduce age at puberty in Nelore heifers to below 18 months. Animal 12(3):569–574
Figueiredo RA, Barros CM, Pinheiro OL, Soler JM (1997) Ovarian follicular dynamics in Nelore breed (Bos indicus) cattle. Theriogenology 47:1489–1505
Forde N, Beltman ME, Lonergan P, Diskin M, Roche JF, Crowe MA (2011) Oestrous cycles in Bos taurus cattle. Anim Reprod Sci 124:163–169
Fortune J, Rivera G (1999) Folículo dominante persistente em bovinos: aspectos básicos e aplicados. Arquivos da Faculdade de Veterinária UFRGS 27:22–34
Foster DL, Jackson LM, Padmanabhan V (2006) Programming of GnRH feedback controls timing puberty and adult reproductive activity. Mol Cell Endocrinol 25:254–255
Frandson RD, Wilke WL, Fails AD (2009) Anatomy and physiology of farm animals. Wiley
Galina CS, Calderon A, Mccloskey M (1982) Detection of signs of estrus in the charolais cow and its Brahman cross under continuous observation. Theriogenology 17:485–498
Galina CS, Orihuela A, Rubio I (1996) Behavioural trends affecting oestrus detection in zebu cattle. Anim Reprod Sci 42:465–470
Galvao KN, Santos JE, Cerri RL, Chebel RC, Rutigliano HM, Bruno RG, Bicalho RC (2007) Evaluation of methods of resynchronization for insemination in cows of unknown pregnancy status. J Dairy Sci 90:4240–4252
Gama LT, Bressan MC, Rodrigues EC, Rossato LV, Moreira OC, Alves SP, Bessa RJB (2013) Heterosis for meat quality and fatty acid profiles in crosses among Bos indicus and Bos taurus finished on pasture or grain. Meat Sci 93:98–104
Garcia SM, Morotti F, Cavalieri FLB, Lunardelli PA, de Oliveira Santos A, Membrive CMB,..... Seneda MM et al (2020) Synchronization of stage of follicle development before OPU improves embryo production in cows with large antral follicle counts. Animal Reprod Sci 221:106601
Garner DL (2006) Flow cytometric sexing of mammalian sperm. Theriogenology 65:943–957
Garner DL, Evans KM, Seidel GE (2013) Sex-sorting sperm using flow cytometry/cell sorting. Methods Mol Biol 927:279–295
Gimenes LU, Sá Filho MF, Carvalho NA, Torres-Junior JR, Souza AH, Madureira EH, Trinca LA, Sartorelli ES, Barros CM, Carvalho JB, Mapletoft RJ, Baruselli PS (2008) Follicle deviation and ovulatory capacity in Bos indicus heifers. Theriogenology 69:852–858
Ginther OJ, Kastelic JP, Knopf L (1989a) Composition and characteristics of follicular waves during the bovine estrous cycle. Anim Reprod Sci 20:187–200
Ginther OJ, Knopf L, Kastelic JP (1989b) Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J Reprod Fertil 87:223–230
Ginther OJ, Bergfelt DR, Kulick LJ, Kot K (1999) Selection of the dominant follicle in cattle: establishment of follicle deviation in less than 8 hours through depression of FSH concentrations. Theriogenology 52:1079–1093
Ginther OJ, Bergfelt DR, Beg MA, Kot K (2002) Role of low circulating FSH concentrations in controlling the interval to emergence of the subsequent follicular wave in cattle. Reproduction 124:475–482
Goff AK (2004) Steroid hormone modulation of prostaglandin secretion in the ruminant endometrium during the estrous cycle. Biol Reprod 71:11–16
Gonzalez SM, Assunção IEG, Duarte J, Oliveira CHA, Guedes TA, Morotti F, Seneda MM, Andrade ER (2020) Induction of puberty in Bos indicus heifers in the western Amazon region. Semina: Ciências Agrárias 41(5 Suppl):2153–2162
Hanlon DW, Williamson NB, Wichtel JJ, Steffert IJ, Craigie AL, Pfeiffer DU (1996) The effect of estradiol benzoate administration on estrous response and synchronized pregnancy rate in dairy heifers after treatment with exogenous progesterone. Theriogenology 45:775–785
Hansel W, Donaldson LE, Wagner WC, Brunner MA (1966) A comparison of Estrous cycle synchronization methods in beef cattle under feedlot conditions. J Anim Sci 25:497–503
Hassan M, Arshad U, Bilal M, Sattar A, Avais M, Bollwein H, Ahmad N (2018) Luteal blood flow measured by Doppler ultrasonography during the first three weeks after artificial insemination in pregnant and non-pregnant Bos indicus dairy cows. J Reprod Dev 65(1):29–36
Haworth GM, Tranter WP, Chuck JN, Cheng Z, Wathes DC (2008) Relationships between age at first calving and first lactation milk yield, and lifetime productivity and longevity in dairy cows. Vet Rec 162(20):643–647
Hillier SG (1994) Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 9:188–191
Honaramooz A, Aravindakshan J, Chandolia RK, Beard AP, Bartlewski PM, Pierson RA, Rawlings NC (2004) Ultrasonographic evaluation of the pre-pubertal development of the reproductive tract in beef heifers. Anim Reprod Sci 80:15–29
Ireland JJ, Smith GW, Scheetz D, Jimenez-Krassel F, Folger JK, Ireland JLH,..... Evans ACO, et al (2010) Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Müllerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod Fertil Dev 23(1):1–14
Jainudeen MR, Hafez ESE (2013) Reproduction in farm animals. Raven Press, Somerset
Jimenez-Krassel F, Scheetz DM, Neuder LM, Pursley JR, Ireland JJ (2017) A single ultrasound determination of≥ 25 follicles≥ 3 mm in diameter in dairy heifers is predictive of a reduced productive herd life. J Dairy Sci 100(6):5019–5027
Kastelic JP, Knopf L, Ginther OJ (1990) Effect of day of prostaglandin F2α treatment on selection and development of the ovulatory follicle in heifers. Anim Reprod Sci 23:169–180
Kastelic JP, Olson WO, Martinez M, Cook RB, Mapletoft RJ (1999) Synchronization of estrus in beef cattle with norgestomet and estradiol valerate. Can Vet J 40:173–178
Keri G, Horvath A, Nikolics K, Teplan I (1985) In the central role of GnRH in the regulation of reproductive functions: mechanism of action and practical results. J Steroid Biochem 23:719–723
Kim IH, Kim UH (2007) Comparison of the effect of estradiol benzoate plus progesterone and GnRH on the follicular wave emergence and subsequent follicular development in CIDR-treated, lactating dairy cows with follicular cysts. Anim Reprod Sci 98:197–203
Kinder JE, Bergfeld EGM, Wehrman ME, Peters KE, Kojima FN (1995) Endocrine basis for puberty in heifers and ewes. J Reprod Fertil 49:393–407
Kinder JE, Kojima FN, Bergfeld EG, Wehrman ME, Fike KE (1996) Progestin and estrogen regulation of pulsatile LH release and development of persistent ovarian follicles in cattle. J Anim Sci 74:1424–1440
Knight PG, Glister C (2006) TGF-β superfamily members and ovarian follicle development. Reproduction 132:191–206
Knopf L, Kastelic JP, Schallenberger E, Ginther OJ (1989) Ovarian follicular dynamics in heifers: test of two-wave hypothesis by ultrasonically monitoring individual follicles. Domest Anim Endocrinol 6:111–119
Kurykin J, Jaakma U, Jalakas M, Aidnik M, Waldmann A, Majas L (2007) Pregnancy percentage following deposition of sex-sorted sperm at different sites within the uterus in estrus-synchronized heifers. Theriogenology 67:754–759
Lamb GC, Mercadante VR (2016) Synchronization and artificial insemination strategies in beef cattle. Vet Clin N Am Food Anim Pract 32(2):335–347. https://doi.org/10.1016/j.cvfa.2016.01.006. Epub 2016 Apr 29
Lamb GC, Dahlen CR, Larson JE, Marquezini G, Stevenson JS (2010) Control of the estrous cycle to improve fertility for fixed-time artificial insemination in beef cattle: a review. J Anim Sci 88:E181–E192
LeFever DG, Odde KG (1987) Predicting reproductive performance in beef heifers by reproductive tract evaluation before breeding. CSU Beef Res Rep:13–15
Levy N, Kobayashi S, Roth Z, Miyamoto A, Meidan R (2000) Administration of prostaglandin F2α during the early bovine luteal phase does not alter the expression of ET-1 and its type a receptor: a possible cause for corpus luteum refractoriness. Biol Reprod 63:377–382
Lima MA, Morotti F, Bayeux BM, Rezende RG, Botigelli RC, De Bem THC, Fontes PK, Nogueira MFG, Meirelles FV, Baruselli PS, Silveira JC, Perecin F, Seneda MM (2020) Ovarian follicular dynamics, progesterone concentrations, pregnancy rates and transcriptional patterns in Bos indicus females with a high or low antral follicle count. Sci Rep 10:19557
Looper ML, Vizcarra JA, Wettemann RP, Malayer JR, Braden TD, Geisert RD, Morgan GL (2003) Influence of estradiol, progesterone, and nutrition on concentrations of gonadotropins and GnRH receptors, and abundance of mRNA for GnRH receptors and gonadotropin subunits in pituitary glands of beef cows. J Anim Sci 81:269–278
Løvendahl P, Chagunda MGG (2010) On the use of physical activity monitoring for estrus detection in dairy cows. J Dairy Sci 93:249–259
Lu KH, Seidel GE Jr (2004) Effects of heparin and sperm concentration on cleavage and blastocyst development rates of bovine oocytes inseminated with flow cytometrically-sorted sperm. Theriogenology 62:819–830
Lucy MC (2007) The bovine dominant ovarian follicle. J Anim Sci 85(13 Suppl):89–99
Machado R, Barbosa RT, Bergamaschi MA, Figueiredo RA (2007) A inseminação artificial em tempo fixo como biotécnica aplicada na reprodução dos bovinos de corte. In Embrapa Pecuária Sudeste-Artigo em anais de congresso (ALICE). In: SEMANA DO ESTUDANTE, 18, 2007, São Carlos, SP. Palestras. Embrapa Pecuária Sudeste, São Carlos, p 2007
Mackey DR, Sreenan JM, Rochet JF, Diskin MG (2000) The effect of progesterone alone or in combination with estradiol on follicular dynamics, gonadotropin profiles, and estrus in beef cows following calf isolation and restricted suckling. J Anim Sci 78:1917–1929
Macmillan KL, Peterson AJ (1993a) Current advances in the manipulation of reproductive function in domestic animals a new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus. Anim Reprod Sci 33:1–25
Macmillan KL, Peterson AJ (1993b) A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchonization, increasing pregnancy rates and the treatment of post-partum anoestrus. Anim Reprod Sci 33:1–25
Manikkam M, Rajamahendran R (1997) Progesterone-induced atresia of the proestrous dominant follicle in the bovine ovary: changes in diameter, insulin-like growth factor system, aromatase activity, steroid hormones, and apoptotic index. Biol Reprod 57:580–587
Mann GE, Lamming GE (2001) Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 121:175–180
Marques MO, Morotti F, Silva CB, Ribeiro-Júnior M, da Silva RCP, Baruselli PS, Seneda MM (2015) Influence of category−heifers, primiparous and multiparous lactating cows−in a large-scale resynchronization fixed-time artificial insemination program. J Vet Sci 16:367–371
Marques MO, Morotti F, Lorenzetti E, Bizarro-Silva C, Seneda MM (2018) Intensified use of TAI and sexed semen on commercial farms. Anim Reprod 15(3):197–203
Martinez MF, Kastelic JP, Bo GA, Caccia M, Mapletoft RJ (2005) Effects of oestradiol and some of its esters on gonadotrophin release and ovarian follicular dynamics in CIDR-treated beef cattle. Anim Reprod Sci 86:37–52
Mee JF (2008) Prevalence and risk factors for dystocia in dairy cattle: a review. Vet J 176:93–101
Meidan R, Levy N (2002) Endothelin-1 receptors and biosynthesis in the corpus luteum: molecular and physiological implications. Domest Anim Endocrinol 23:287–298
Menegassi SRO, Barcellos JOJ (2015) Aspectos Reprodutivos do Touro: Teoria e Prática. Agrolivros, Guaíba, 280p
Meneghetti MVER, Vasconcelos JLM (2001) Efeito da remoção temporária dos bezerros nos folículos dominantes e na taxa de ovulação ao primeiro GnRH em protocolos de sincronização em vacas Nelore em anestro. Revista Brasileira de Reprodução Animal 25:286–288
Mizuta K (2003) Estudo comparativo dos aspectos comportamentais do estro e dos teores plasmáticos de LH, FSH, progesterona e estradiol que precedem a ovulação em fˆemeas bovinas Nelore (Bos taurus indicus), Angus (Bos taurus taurus) e Nelore × Angus (Bos taurus indicus × Bos taurus taurus). Tese de Doutorado em Reprodução Animal – Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo., 98 p
Moenter SM, Brand RC, Karsch FJ (1992) Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 130:2978–2984
Moraes FLZ, Morotti F, Costa CB, Lunardelli PA, Seneda MM (2019) Relationships between antral follicle count, body condition, and pregnancy rates after timed-AI in Bos indicus cattle. Theriogenology 136:10–14
Moran C, Quirke JF, Roche JF (1989) Puberty in heifers: a review. Anim Reprod Sci 18:167–182
Morotti F, Campos JT, Seneda MM (2013a) Fixed-time artificial insemination using injectable progesterone: ovarian follicular dynamics and pregnancy rates of Nelore cows (Bos indicus) with and without a corpus luteum. Semina: Ciências Agrárias 34:3867–3875
Morotti F, Campos JT, Oliveira RE, Seneda MM (2013b) Ovarian follicular dynamics of Nelore (Bos indicus) cows subjected to a fixed-time artificial insemination protocol with injectable progesterone. Semina: Ciências Agrárias 34:3859–3866
Morotti F, Sanches BV, Pontes JH, Basso AC, Siqueira ER, Lisboa LA, Seneda MM (2014) Pregnancy rate and birth rate of calves from a large-scale IVF program using reverse-sorted semen in Bos indicus, Bos indicus-taurus, and Bos taurus cattle. Theriogenology 81:696–701
Morotti F, Moretti R, Santos GM, Silva-Santos KC, Cerqueira PHR, Seneda MM (2018a) Ovarian follicular dynamics and conception rate in Bos indicus cows with different antral follicle counts subjected to timed artificial insemination. Anim Reprod Sci 188:170–177
Morotti F, Zangirolamo AF, da Silva NC, da Silva CB, Rosa CO, Seneda MM (2018b) Antral follicle count in cattle: advantages, challenges, and controversy. Anim Reprod 14(3):414–420
Murphy BD, Martinuk SD (1991) Equine chorionic gonadotropin. Endocr Rev 12:27–44
Nogueira GP (2004) Puberty in south American Bos indicus (zebu) cattle. Anim Reprod Sci 82-83:361–372
Nogueira E, Silva MR, Silva JCB, Abreu UPG, Anache NA, Silva KC,..... Rodrigues WB, et al (2019) Timed artificial insemination plus heat I: effect of estrus expression scores on pregnancy of cows subjected to progesterone–estradiol-based protocols. Animal 13(10):2305–2312
Odde KG (1990) A review of synchronization of estrus in postpartum cattle. J Anim Sci 68:817–830
Orihuela AN (2000) Some factors affecting the behavioural manifestation of oestrus in cattle: a review. Appl Anim Behav Sci 70:1–16
Patterson DJ, Corah LR, Brethour JR, Higgins JJ, Kiracofe GH, Stevenson JS (1992) Evaluation of reproductive traits in Bos taurus and Bos indicus crossbred heifers: relationship of age at puberty to length of the postpartum interval to estrus. J Anim Sci 70:1994–1999
Pellegrino CA, Morotti F, Untura RM, Pontes JH, Pellegrino MF, Campolina JP, Seneda MM, Barbosa FA, Henry M (2016) Use of sexed sorted semen for fixed-time artificial insemination or fixed-time embryo transfer of in vitro-produced embryos in cattle. Theriogenology 86:888–8893
Peña DM (2007) Estrategias de destete temporario y tratamientos con ecg en protocolos de inseminación artificial a tiempo fijo (IATF) con dispositivos con progesterona en vacas cruza cebú con cria. Magíster en Ciencias Agropecuárias – Faculdad de Ciências Agropecuárias, Universidad Nacional de Córdoba., 37 p
Peres RF, Claro I Jr, Sá Filho OG, Nogueira GP, Vasconcelos JL (2009) Strategies to improve fertility in Bos indicus postpubertal heifers and nonlactating cows submitted to fixed-time artificial insemination. Theriogenology 72:681–689
Pfeifer LFM, Leal SDCBDS, Schneider A, Schmitt E, Corrêa MN (2012) Effect of the ovulatory follicle diameter and progesterone concentration on the pregnancy rate of fixed-time inseminated lactating beef cows. Revista Brasileira de Zootecnia 41(4):1004–1008
Pfeifer LFM, Castro NA, Melo VTO, Neves PMA, Cestaro JP, Schneider A (2015) Timed artificial insemination in blocks: a new alternative to improve fertility in lactating beef cows. Animal Reprod Sci 163:89–96
Pinheiro OL, Barros CM, Figueiredo RA, Do Valle ER, Encarnação RO, Padovani CR (1998) Estrous behavior and the estrus-to-ovulation interval in nelore cattle Bos indicus with natural estrus or estrus induced with prostaglandin F2α or norgestomet and estradiol valerate. Theriogenology 49:667–681
Pontes JHF, Silva KCF, Basso AC, Rigo AG, Ferreira CR, Santos GMG, Sanches BV, Porcionato JPF, Vieira PHS, Faifer FS, Sterza FAM, Schenk JL, Seneda MM (2010) Large-scale in vitro embryo production and pregnancy rates from Bos taurus, Bos indicus, and indicus-taurus dairy cows using sexed sperm. Theriogenology 74:1349–1355
Prata AB, Drum JN, Melo LF, Araujo ER, Sartori R (2018) Effect of different chorionic gonadotropins on final growth of the dominant follicle in Bos indicus cows. Theriogenology 111:52–55
Pugliesi G, Rezende RG, Silva JCB, Lopes E, Nishimura T K, Baruselli PS,..... Binelli M, et al (2017) Uso da ultrassonografia Doppler em programas de IATF e TETF em bovinos. Rev Bras Reprod Anim 41(1):140–150
Rahe CH, Owens RE, Fleeger JL, Newton HJ, Harms PG (1980) Pattern of plasma luteinizing hormone in the cyclic cow: dependence upon the period of the cycle. Endocrinology 107:498–503
Rajamahendran R, Bedirian KN, Lague PC, Baker RD (1976) Luteolytic activity of a synthetic prostaglandin and PGF2alpha in heifers. Prostaglandins 11:143–153
Rasby RJ, Day ML, Johnson SK, Kinder JE, Lynch JM, Short RE, Wettemann RP, Hafs HD (1998) Luteal function and estrus in peripubertal beef heifers treated with an intravaginal progesterone releasing device with or without a subsequent injection of estradiol. Theriogenology 50:55–63
Rawlings NC, Evans AC, Honaramooz A, Bartlewski PM (2003) Antral follicle growth and endocrine changes in prepubertal cattle, sheep and goats. Anim Reprod Sci 78:259–270
Richards JS, Russell DL, Robker RL, Dajee M, Alliston TN (1998) Molecular mechanisms of ovulation and luteinization. Mol Cell Endocrinol 145:47–54
Rivera G, Fortune J (2003) Proteolysis of insulin-like growth factor binding proteins-4 and-5 in bovine follicular fluid: implications for ovarian follicular selection and dominance. Endocrinology 144:2977–2987
Roche JF (1996) Control and regulation of folliculogenesis – a symposium in perspective. Rev Reprod 1:19–27
Rodrigues ADP, Peres RFG, Lemes AP, Martins T, Pereira MHC, Day ML, Vasconcelos JLM (2013) Progesterone-based strategies to induce ovulation in prepubertal Nellore heifers. Theriogenology 79(1):135–141
Rodrigues WB, Silva AS, Silva JCB, Anache NA, Silva KC, Cardoso CJT, Garcia WR, Sutovsky P, Nogueira E (2019) Timed artificial insemination plus heat II: gonadorelin injection in cows with low estrus expression scores increased pregnancy in progesterone/estradiol-based protocol. Animal 13:2313–2318
Rosenkrans KS, Hardin DK (2003) Repeatability and accuracy of reproductive tract scoring to determine pubertal status in beef heifers. Theriogenology 59(5–6):1087–1092
Sá Filho M, Reis E, Ayres H, Gimenes L, Peres A, Carvalho C, Carvalho J, Araújo C, Baruselli P (2006) Effect of oestradiol valerate or benzoate on induction of a new follicular wave emergence in Bos indicus cows and heifers treated with norgestomet auricular implant. Reprod Fertil Dev 18:289
Sá Filho MF, Crespilho AM, Santos JE, Perry GA, Baruselli PS (2010a) Ovarian follicle diameter at timed insemination and estrous response influence likelihood of ovulation and pregnancy after estrous synchronization with progesterone or progestin-based protocols in suckled Bos indicus cows. Anim Reprod Sci 120:23–30
Sá Filho MF, Ayres H, Ferreira RM, Nichi M, Fosado M, Campos Filho EP, Baruselli PS (2010b) Strategies to improve pregnancy per insemination using sex-sorted semen in dairy heifers detected in estrus. Theriogenology 74:1636–1642
Sá Filho MF, Baldrighi JM, Sales JN, Crepaldi GA, Carvalho JB, Bó GA, Baruselli PS (2011) Nduction of ovarian follicular wave emergence and ovulation in progestin-based timed artificial insemination protocols for Bos indicus cattle. Anim Reprod Sci 129:132–139
Sá Filho MF, Girotto R, Abe EK, Penteado L, Campos Filho EP, Moreno JF, Sala RV, Nichi M, Baruselli PS (2012) Optimizing the use of sex-sorted sperm in timed artificial insemination programs for suckled beef cows. J Anim Sci 90:1816–1823
Sá Filho MF, Penteado L, Siqueira GR, Soares JG, Mendanha MF, Macedo GG, Baruselli PS (2013) Timed artificial insemination should be performed early when used norgestomet ear implants are applied for synchronizing ovulation in beef heifers. Theriogenology 80:642–647
Sá Filho MF, Marques MO, Girotto R, Santos FA, Sala RV, Barbuio JP, Baruselli PS (2014) Resynchronization with unknown pregnancy status using progestin-based timed artificial insemination protocol in beef cattle. Theriogenology 81:284–290
Sá Filho MF, Nasser LFT, Penteado L, Prestes R, Marques MO, Freitas BG, Monteiro BM, Ferreira RM, Gimenes LU, Baruselli PS (2015) Impact of progesterone and estradiol treatment before the onset of the breeding period on reproductive performance of Bos indicus beef heifers. Anim Reprod Sci 160:30–39
Sales JN, Neves KA, Souza AH, Crepaldi GA, Sala RV, Fosado M, Campos Filho EP, De Faria M, Sá Filho MF, Baruselli PS (2011a) Timing of insemination and fertility in dairy and beef cattle receiving timed artificial insemination using sex-sorted sperm. Theriogenology 76:427–435
Sales JNS, Neves KAL, Souza AH, Crepaldi GA, Sala RV, Fosado M, Filho EPC, De Faria M, Filho MFS, Baruselli PS (2011b) Timing of insemination and fertility in dairy and beef cattle receiving timed artificial insemination using sex-sorted sperm. Theriogenology 76:427–435
Sales JN, Carvalho JB, Crepaldi GA, Cipriano RS, Jacomini JO, Maio JR, Souza JC, Nogueira GP, Baruselli PS (2012a) Effects of two estradiol esters (benzoate and cypionate) on the induction of synchronized ovulations in Bos indicus cows submitted to a timed artificial insemination protocol. Theriogenology 78:510–516
Sales JNS, Carvalho JBP, Crepaldi GA, Cipriano RS, Jacomini JO, Maio JRG, Souza JC, Nogueira GP, Baruselli PS (2012b) Effects of two estradiol esters (benzoate and cypionate) on the induction of synchronized ovulations in Bos indicus cows submitted to a timed artificial insemination protocol. Theriogenology 78:510–516
Sales JNS, Carvalho JBP, Crepaldi GA, Soares JG, Girotto RW, Maio JRG, Souza JC, Baruselli PS (2015) Effect of circulating progesterone concentration during synchronization for fixed-time artificial insemination on ovulation and fertility in Bos indicus (Nelore) beef cows. Theriogenology 83:1093–1100
Santos GMG, Silva-Santos KC, Barreiros TRR, Morotti F, Sanches BV, de Moraes FLZ,..... Seneda MM, et al (2016) High numbers of antral follicles are positively associated with in vitro embryo production but not the conception rate for FTAI in Nelore cattle. Animal Reproduct Sci 165:17–21
Sartorelli ES, Carvalho LM, Bergfelt DR, Ginther OJ, Barros CM (2005) Morphological characterization of follicle deviation in Nelore (Bos indicus) heifers and cows. Theriogenology 63:2382–2394
Savio JD, Thatcher WW, Morris GR, Entwistle K, Drost M, Mattiacci MR (1993) Effects of induction of low plasma progesterone concentrations with a progesterone-releasing intravaginal device on follicular turnover and fertility in cattle. J Reprod Fertil 98:77–84
Schams D, Berisha B (2004) Regulation of corpus luteum function in cattle-an overview. Reprod Domest Anim 39:241–251
Schenk JL, Cran DG, Everett RW, Seidel GE Jr (2009) Pregnancy rates in heifers and cows with cryopreserved sexed sperm: effects of sperm numbers per inseminate, sorting pressure and sperm storage before sorting. Theriogenology 71:717–728
Seidel GE Jr, Allen CH, Johnson LA, Holland MD, Brink Z, Welch GR, Graham JK, Cattell MB (1997) Uterine horn insemination of heifers with very low numbers of nonfrozen and sexed spermatozoa. Theriogenology 48:1255–1264
Seidel GE Jr (2007) Overview of sexing sperm. Theriogenology 68:443–446
Seidel GE Jr (2014) Update on sexed semen technology in cattle. Animal 8(Suppl 1):160–164
Seidel GE Jr, Garner DL (2002) Current status of sexing mammalian spermatozoa. Reproduction 124:733–743
Seidel GE Jr, Schenk JL (2008a) Pregnancy rates in cattle with cryopreserved sexed sperm: effects of sperm numbers per inseminate and site of sperm deposition. Anim Reprod Sci 105:129–138
Seidel GE Jr, Schenk JL (2008b) Pregnancy rates in cattle with cryopreserved sexed sperm: effects of sperm numbers per inseminate and site of sperm deposition. Anim Reprod Sci 105:129–138
Seidel GE Jr, Schenk JL, Herickhoff LA, Doyle SP, Brink Z, Green RD, Cran DG (1999) Insemination of heifers with sexed sperm. Theriogenology 52:1407–1420
Seneda MM, Morotti F, Zangirolamo AF, Silva NC, Sanches TK, Blaschi W, Barreiros TRR (2019) Antral follicle population in prepubertal and pubertal heifers. Reprod Fertil Dev 31:10–16
Senger PL (1997) Pathways to pregnancy and parturition. Raven Press, Redmond
Silva LO (2020) Physiological responses of Bos taurus and Bos indicus cows submitted to hormonal treatments for estrous cycle synchronization. Master’s dissertation, Escola superior de Agricultura Luiz de Queiroz, University of São Paulo, Piracicaba. https://doi.org/10.11606/D.11.2020.tde-06102020-183501. Retrieved 2021-01-23, from www.teses.usp.br
Silva‐Santos KC, Santos GMG, Koetz Júnior C, Morotti F, Siloto LS, Marcantonio TN,..... Seneda MM, et al (2014) Antral Follicle Populations and Embryo Production–In Vitro and In Vivo–of B os indicus–taurus Donors from Weaning to Yearling Ages. Reproduct Domestic Animals 49(2):228–232
Siqueira LGB, Areas VS, Ghetti AM, Fonseca JF, Palhao MP, Fernandes CAC, Viana JHM (2013) Color Doppler flow imaging for the early detection of nonpregnant cattle at 20 days after timed artificial insemination. J Dairy Sci 96(10):6461–6472
Smith MF, Geisert RD, Parrish JJ (2018) Reproduction in domestic ruminants during the past 50 yr: discovery to application. J Anim Sci 96:2952–2970
Soumano K, Silversides DW, Doize F, Price CA (1996) Follicular 3 beta-hydroxysteroid dehydrogenase and cytochromes P450 17 alpha-hydroxylase and aromatase messenger ribonucleic acids in cattle undergoing superovulation. Biol Reprod 55:1419–1426
Souza AHSJNS, Crepaldi GA, Teixeira AA, Baruselli PS (2008) Effect of type of semen (sexed vs non-sexed) and time of AI (60h vs 64h) on pregnancy rates of postpartum Nelore cows inseminated in a fixed time. Anim Reprod 6:224
Stevenson JS, Hill SL, Bridges GA, Larson JE, Lamb GC (2015) Progesterone status, parity, body condition, and days postpartum before estrus or ovulation synchronization in suckled beef cattle influence artificial insemination pregnancy outcomes. J Anim Sci 93:2111–2123
Sunderland SJ, Crowe MA, Boland MP, Roche JF, Ireland JJ (1994) Selection, dominance and atresia of follicles during the oestrous cycle of heifers. J Reprod Fertil 101:547–555
Taylor C, Rajamahendran R (1991) Follicular dynamics and Corpus luteum growth and function in pregnant versus nonpregnant dairy cows. J Dairy Sci 74:115–123
Teodoro RLMJC, Regazzi AJ, Lemos AM, Freitas AF (1993) Características associadas com a maturidade sexual em vacas mestiças Holandês:Gir e vacas do cruzamento tríplice Jersey ou Suíço x Holandês:Gir. Revista da Sociedade Brasileira de Zootecnia 22:488–494
Thomas JM, Locke JWC, Vishwanath R, Hall JB, Ellersieck MR, Smith MF, Patterson DJ (2017) Effective use of SexedULTRA™ sex-sorted semen for timed artificial insemination of beef heifers. Theriogenology 98:88–93
Torres JR Jr, Penteado L, Sales JN, Sá Filho MF, Ayres H, Baruselli PS (2014) A comparison of two different esters of estradiol for the induction of ovulation in an estradiol plus progestin-based timed artificial insemination protocol for suckled Bos indicus beef cows. Anim Reprod Sci 151:9–14
Torres-Júnior JRS, Melo WO, Elias AKS, Rodrigues LS, Penteado L, Baruselli PS (2009) Considerações técnicas e econômicas sobre reprodução assistida em gado de corte. Revista Brasileira de Reprodução Animal 33(1):53–58
Underwood SL, Bathgate R, Ebsworth M, Maxwell WM, Evans G (2010a) Pregnancy loss in heifers after artificial insemination with frozen-thawed, sex-sorted, re-frozen-thawed dairy bull sperm. Anim Reprod Sci 118:7–12
Underwood SL, Bathgate R, Maxwell WM, Evans G (2010b) Birth of offspring after artificial insemination of heifers with frozen-thawed, sex-sorted, re-frozen-thawed bull sperm. Anim Reprod Sci 118:171–175
Vailes LD, Washburn SP, Britt JH (1992) Effects of various steroid milieus or physiological states on sexual behavior of Holstein cows. J Anim Sci 70:2094–2103
Valldecabres-Torres X, García-Roselló E, García-Muñoz A, Cuervo-Arango J (2012) Effects of d-cloprostenol dose and corpus luteum age on ovulation, luteal function, and morphology in nonlactating dairy cows with early corpora lutea. J Dairy Sci 95:4389–4395
Vieira LMSFMF, Pugliesi G, Guerreiro BM, Cristaldo MA, Batista EOS, Freitas BG, Carvalho FJ, Guimaraes LHC, Baruselli PS (2014) Resynchronization in dairy cows 13 days after TAI followed by pregnancy diagnosis based on corpus luteum vascularization by color doppler. Anim Reprod 11:378
Vishwanath R, Moreno JF (2018) Semen sexing–current state of the art with emphasis on bovine species. Animal 12(s1):s85–s96
Vizcarra JA, Wettemann RP, Morgan GL (1999) Influence of dose, frequency, and duration of infused gonadotropin-releasing hormone on secretion of luteinizing hormone and follicle-stimulating hormone in nutritionally anestrous beef cows. Domest Anim Endocrinol 16:171–181
Wathes DC, Pollott GE, Johnson KF, Richardson H, Cooke JS (2014) Heifer fertility and carry over consequences for life time production in dairy and beef cattle. Animal 8(s1):91–104
Webb R, Garnsworthy PC, Gong JG, Armstrong DG (2004) Control of follicular growth: local interactions and nutritional influences. J Anim Sci 82(E-Suppl):E63-74
Weigel KA (2004) Exploring the role of sexed semen in dairy production systems. J Dairy Sci 87:120–130
Williams GL, Gazal OS, Vega GAG, Stanko RL (1996) Mechanisms regulating suckling-mediated anovulation in the cow. Anim Reprod Sci 42:289–297
Wiltbank MC, Pursley JR (2014) The cow as an induced ovulator: timed AI after synchronization of ovulation. Theriogenology 81(1):170–185
Wiltbank MC, Gümen A, Sartori R (2002) Physiological classification of anovulatory conditions in cattle. Theriogenology 57:21–52
Wiltbank M, Lopez H, Sartori R, Sangsritavong S, Gumen A (2006) Changes in reproductive physiology of lactating dairy cows due to elevated steroid metabolism. Theriogenology 65:17–29
Xu Z, Garverick HA, Smith GW, Smith MF, Hamilton SA, Youngquist RS (1995) Expression of follicle-stimulating hormone and luteinizing hormone receptor messenger ribonucleic acids in bovine follicles during the first follicular wave. Biol Reprod 53:951–957
Yavas Y, Walton JS (2000a) Induction of ovulation in postpartum suckled beef cows: a review. Theriogenology 54:1–23
Yavas Y, Walton JS (2000b) Postpartum acyclicity in suckled beef cows: a review. Theriogenology 54:25–55
Yin W, Gore AC (2010) The hypothalamic median Eminence and its role in reproductive aging. Ann N Y Acad Sci 1204:113–122
Yoo HS (2010) Infectious causes of reproductive disorders in cattle. J Reprod Dev 56(Suppl):S53–S60
Zangirolamo AF, Morotti F, da Silva NC, Sanches TK, Seneda MM (2018) Ovarian antral follicle populations and embryo production in cattle. Anim Reprod 15(3):310–315
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Morotti, F., Lorenzetti, E., Seneda, M.M. (2021). Artificial Insemination Program in Cattle. In: Yata, V.K., Mohanty, A.K., Lichtfouse, E. (eds) Sustainable Agriculture Reviews 54. Sustainable Agriculture Reviews, vol 54. Springer, Cham. https://doi.org/10.1007/978-3-030-76529-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-76529-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76528-6
Online ISBN: 978-3-030-76529-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)