Abstract
This chapter aims to explore the multiple interactions between diseases of the nervous system and the kidney. There are several important, multisystem diseases which affect both organs and may present initially to either nephrologist or neurologist. Similarly, renal diseases, renal replacement therapy and transplantation are associated with specific neurological complications, whilst neurological disorders and their treatments may impact on renal function. For the nephrologist, an awareness of the potential neurological sequalae of renal disease and its treatments (including drug toxicities) is vital to optimise clinical outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
1.
To identify multisystem diseases which have both renal and neurological manifestations
-
2.
To develop an awareness of why renal function maybe become impaired in the context of neurological disorders and their treatments.
-
3.
To outline the neurological complications of common renal diseases and their treatments and to develop a strategy for their prevention, investigation and treatment
1 Introduction
The kidney and nervous system may be mutually affected by systemic disease processes and also by the side effects of therapeutics directed towards each organ. The risk of stroke and small vessel disease is substantially increased in hypertensive patients with CKD, and there are other specific neurological complications that are strongly or exclusively associated with renal disease, such as PRES, uraemic encephalopathy, dialysis amyloid myelopathy and dialysis dementia. In addition, many patients with CKD will coincidentally have long-term neurological disorders requiring coordinated care, support for disability and advanced care planning for those with progressive neurological impairment. This chapter will explore diseases that may benefit from joint management between nephrologist and neurologist and delineate a practical approach to neurological presentations in renal patients.
2 Multisystem Diseases Affecting Both the Nervous System and Kidney
A variety of genetically determined and acquired systemic diseases exhibit both neurological and renal features. Clinically, the recognition of characteristic neurological features such as mononeuropathy multiplex in Churg-Strauss syndrome may aid swift diagnosis. Similarly, knowledge of the neurological associations of specific renal conditions may alert the nephrologist to possible causes of neurological dysfunction such as central pontine myelinolysis associated with over rapid correction of hyponatraemia (◘ Fig. 41.1), or facilitate pre-emptive screening, such as MRA in high-risk patients for cerebral aneurysm in ADPKD or cerebral tumours in tuberosclerosis complex (◘ Fig. 41.2).
Frequently, the consequences of neurological and renal involvement are chronic and debilitating and necessitate colleagues to provide integrated care, coordinated clinics and MDT working. ◘ Table 41.1 lists some of the many conditions that share both renal and neurological disease (◘ Table 41.1).
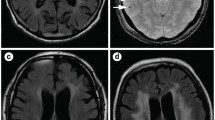
Axial MRI brain with contrast showing enhancing subependymal nodule in the anterior horn of the left lateral ventricle. Subependymal nodules are seen in nearly all tuberous sclerosis patients and calcify with age. They may give rise to supependymal giant cell astrocytomas (SEGAs) during childhood and adolescence
3 Neurological Diseases and Medications Which Affect the Kidney
3.1 Spinal Cord Disorders
Congenital and acquired spinal cord damage may lead to bladder dysfunction and indirectly to kidney disease. Risk of renal insufficiency increases with the following: indwelling urethral catheter, advanced age, time since spinal cord injury and vesicoureteric reflux from detrusor sphincter dyssynergia and raised bladder pressures. Patients are at risk of developing reflux nephropathy, hydronephrosis and pyelonephritis. Careful history and evaluation, including imaging, are vital for all patients with a neurogenic bladder. Renal failure in patients with spinal cord disease can be avoided by maintaining low post-void residual volumes, avoidance of indwelling catheters, care with nephrotoxic drugs and prompt treatment of sepsis. Most patients are managed with clean technique intermittent catheterization and oral anticholinergic medications. The UK NICE guidance suggests offering video-urodynamic investigations to people known to have high risk of renal complications (e.g. spina bifida and spinal cord injury) but not to those with low risk (e.g. most people with multiple sclerosis) [7]. Patients with spinal cord injury should be assessed and followed up by a urologist.
3.2 Rhabdomyolysis
Extensive muscle necrosis and release of intracellular muscle constituents resulting in AKI can be seen with a number of neurological conditions. Important neurological causes of rhabdomyolysis include metabolic and mitochondrial myopathies, inflammatory myopathies and excessive muscular activity, as seen in status epilepticus and dystonia. Seizures can also rarely cause rhabdomyolysis through a localised compartment syndrome. An underlying inherited metabolic or mitochondrial myopathy should be suspected if there is a history of recurrent episodes of rhabdomyolysis after exertion, fasting or viral illness; exercise intolerance with cramps, fatigue and pigmenturia; or family history of rhabdomyolysis or exercise intolerance. Helpful pointers include the type of exertional activity, presence or absence of interictal weakness or raised creatine kinase and second wind phenomenon. Patients with an underlying inflammatory myopathy, e.g. polymyositis or dermatomyositis, may present subacutely with features of systemic autoimmunity.
3.3 Medications
Medications used to treat neurological disease may adversely affect the kidney, especially in vulnerable patient groups.
The most common interaction in clinical practice is probably nephrotoxicity due to crystal nephropathy and tubulointerstitial nephritis with intravenous aciclovir in patients being treated for encephalitis. Pre-hydration is recommended prior to infusion and renal impairment is generally reversible following discontinuation of therapy. Oral aciclovir and ganciclovir are both associated with lower risk (◘ Table 41.2).
4 Neurological Manifestations of Kidney Disease
Patients with CKD may develop central and peripheral neurological complications such as cognitive disorders [8], dementia [9], cerebrovascular diseases [10] and peripheral neuropathy. Conventional risk factors for vascular disease (such as old age, hypertension, diabetes, dyslipidaemia and atrial fibrillation) drive these neurological disorders in CKD through microvascular disease [11].
4.1 Encephalopathy
4.1.1 Uraemic Encephalopathy
Uraemic encephalopathy is seen in advanced kidney failure and demonstrates a fluctuating course. Early symptoms may be subtle and picked up on specific tests of attention, but these can progress to emotional lability, stupor, visual hallucinations, delirium, seizures and coma, if renal replacement therapy is not implemented in a timely manner. Seizures are usually bilateral tonic-clonic in nature, although focal motor seizures may also occur. In keeping with a metabolic encephalopathy, patients may present with multifocal myoclonus, asterixis, tremor and pyramidal signs. Focal signs, e.g. hemiparesis, may also be seen.
The onset and severity of uraemic encephalopathy broadly parallels the degree of renal impairment, but factors including age, coexisting comorbidity and speed and severity of renal dysfunction may influence the threshold at which symptoms present.
Biochemical changes, including alterations in water transport, brain oedema, disturbances of the blood-brain barrier, impaired synaptic function and changes in cerebral metabolism, have been implicated in the pathophysiology. Subsequent histopathological changes with extracellular deposition of amyloid protein in senile plaques occur, possibly mediated by aluminium from diet and phosphate binding drugs [12] Imaging studies may show cytotoxic oedema, particularly in subcortical grey and white matter, midbrain and mesial temporal lobes. Chronic renal impairment may be associated with cerebral atrophy. The EEG typically demonstrates prominence of slow waves, intermittent frontal rhythmic theta activity and paroxysmal, bilateral, high-voltage delta waves. Triphasic waves may appear in the frontal regions [13]. CSF analysis may show an aseptic meningitis and raised CSF protein of up to 1 g/l [14].
Uraemic encephalopathy typically improves with the initiation of renal replacement therapy, and improvement in neurological and cognitive function is normally appreciable within 48 hours of starting treatment, although subtle attentional, memory and perceptual deficits may persist. Failure to respond within this time frame should prompt investigations for an alternative diagnosis.
4.1.2 Hypertensive Encephalopathy
Hypertensive encephalopathy is characterised by cerebral oedema in the context of severe hypertension and reflects failure of cerebral autoregulation, leading to vascular damage and damage of the vascular endothelium. The clinical picture is often of gradually worsening headache, nausea and vomiting, cortical blindness and other visual symptoms, which, if left untreated, may progress to confusion, seizures and coma.
When imaging reveals oedema to the occipital and parietal lobes, the term PRES (posterior reversible encephalopathy syndrome) maybe applied [15]. Changes are seen both cortical and subcortical and may extend beyond a strictly posterior distribution (◘ Fig. 41.4). When primarily pontine abnormalities are observed, the term hypertensive brainstem encephalopathy is used [16]. Management of hypertensive crisis is covered elsewhere in this book, but gradual (avoiding precipitant falls) control of blood pressure and reduction or withdrawal of immunosuppressive agent are associated with good recovery.
4.1.3 Neuropathy
Uraemic polyneuropathy is common and seen in up to 70% adults with renal dysfunction [17]. The most common cause is due to the uraemic milieu found in CKD and typically develops below a GFR of 12 mL/min/1.73m2 [18]. This ‘uraemic polyneuropath’ is a symmetrical length-dependent axonal sensorimotor polyneuropathy with secondary segmental demyelination [19]. Patients present with loss of proprioception and vibration sense and absent ankle reflexes and progress to limb weakness. Motor nerve involvement with progressive distal atrophy and weakness and small fibre involvement may follow. Commencement of RRT, folic acid, vitamin B supplementation and neuropathic agents may help symptoms. Successful renal transplantation reverses autonomic dysfunction in all but the most severe neuropathies.
Where peripheral neuropathy is clinically out of proportion to the level of renal impairment, other causes including systemic vasculitides and diabetes should be considered.
Mononeuropathies are also common in patients with CKD. Carpal tunnel syndrome (CTS) is particularly prevalent, seen in 6–31 percent of patients with CKD [18] and caused by compression from local amyloid, calcific atherosclerosis, uraemic damage to median nerve, increased extracellular volume leading to nerve ischaemia and increased susceptibility to pressure palsies. CTS typically presents with pain or numbness along the distribution of the median nerve, often worse at night.
Mononeuropathies may also be related to diabetes or iatrogenic, attributable to vascular access surgery [20]; this is considered in ► Sect. 41.8.
The most commonly involved cranial nerve in uraemia is the vestibulocochlear nerve. Variable degrees of hearing loss can be seen, which reverse with dialysis or transplantation [21].
4.2 Myopathy
Proximal limb weakness and wasting are common in the uraemic state and usually develop when the glomerular filtration rate declines below 25 mL/min. In the absence of peripheral neuropathy, the knee jerks are preserved and may be brisk. Creatine kinase is usually normal and EMG shows a non-inflammatory myopathic picture. Muscle biopsy is non-specific with atrophy of type 2 muscle fibres [22]. The pathogenesis of uraemic myopathy is unclear but is likely multifactorial and related to uraemic toxins, vitamin D metabolic abnormalities, secondary hyperparathyroidism, insulin resistance, malnutrition, changes in mitochondrial metabolism and sedentary lifestyle. There is no specific treatment; it may be prevented with high-quality dialysis and effects ameliorated with aerobic exercise, management of secondary hyperparathyroidism and treatment of anaemia [23].
5 Stroke
Patients with CKD are at significantly increased risk of cerebrovascular morbidity and mortality. The risk of stroke is particularly increased in patients with CKD and AF, and the risk of stroke increases with falling GFR and increases with degree of proteinuria [24]. Patients with CKD may exhibit enhanced calcification in carotid plaques, which have greater risk of rupture and increased stroke severity due to impaired cerebral perfusion. It is important to consider specific diseases such as Fabry’s or mitochondrial cytopathies in young patients presenting with stroke.
Atrial fibrillation is a risk factor for ischaemic stroke; however, warfarin demonstrates significant lability in the context of haemodialysis, and its use is associated with a significantly elevated risk of intracranial bleeding [25, 26].
In the immediate post-stroke period, the haemodialysis patient will require specialised dialysis regimes to prevent haemorrhagic transformation or stroke extension due to hypoperfusion. Principles of care are to minimise the rate of change of urea and osmolality and to maintain cardiovascular stability. The dialysis prescription should reflect a combination of reduced blood and dialysate flow across a small surface dialyser and a shortened treatment session time [27]. Extra dialysis sessions may be necessary to ensure an adequate solute clearance and may need to continue for up to 2 weeks whilst the ischaemic brain injury stabilises.
Cerebral venous sinus thrombosis is a rare complication of nephrotic syndrome. In line with the more common types of venous thromboses, e.g. DVT and renal thrombosis, thrombosis typically occurs early on in the course of the disease. Risk is increased with severe hypoalbuminaemia and membranous nephropathy. Thrombosis is usually located in the superior sagittal sinus, and MR Venogram is the imaging modality of choice.
5.1 Cognitive Impairment and Dementia
CKD is associated with high rates of cognitive impairment, and the severity of CKD is associated with greater risk [8]. Each 10 mL/min/1.73m2 decrease in eGFR is associated with 11% increased prevalence of impairment [28]. There is increasing evidence that cognitive impairment is related to vascular disease manifest as cerebral microinfarcts, microhaemorrhages and accumulating white matter disease [29]. In addition to chronic hypertension, dyslipidaemia and diabetes, high levels of oxidative stress, high cystatin C levels and endothelial dysfunction contribute to accelerated small vessel disease and cognitive decline in chronic kidney disease.
Dialysis dementia is an important but now rare cause of cognitive deterioration. This is discussed in ► Sect. 41.8.
6 Common Neurological Presentations in the Renal Patient
6.1 Confusion
The differential diagnosis is vast in the CKD patient presenting with confusion and should be guided by the onset of symptoms, associated features and type of kidney disease.
Differential | Associated features |
|---|---|
Sepsis | |
Systemic | Delirium due to general sepsis: pneumonia, urinary sepsis, endocarditis Dialysis-related sepsis: peritoneal and haemodialysis |
CNS | Opportunistic organisms causing meningitis/encephalitis: fungal, viral, TB (◘ Figs. 41.5 and 41.6) Cerebritis, cerebral abscess, septic emboli |
Drugs | Opiate accumulation Hyponatraemia due to diuretics Hypoglycaemia due to insulin accumulation. Beta lactam toxicity Aciclovir-induced encephalopathy Erythropoietin causing hypertensive encephalopathy |
Metabolic | Uraemic encephalopathy Wernicke’s encephalopathy Electrolyte disturbance |
Vascular | Subdural haematoma Ischaemic stroke, intracerebral haemorrhage Cerebral venous sinus thrombosis PRES (related to hypertension, immunosuppressive medication) |
Inflammatory/autoimmune | Related to underlying systemic inflammation, e.g. SLE, Sjogren’s |
6.2 Seizures
In all patients presenting with seizures, particularly with focal neurology, a primary neurological diagnosis, such as stroke, SDH or intracranial infection, should be considered, given the increased risk of these conditions in renal patients. All causes of an encephalopathy listed above can potentially lead to seizures.
Seizures in the setting of end-stage renal disease may be related to complications of disease or treatment and may occur in earlier stages of disease due to metabolic disturbance or hypertension. Generalised seizures in the context of a uraemic encephalopathy are an indication for dialysis.
In patients with CKD, electrolyte disturbance is a common cause of seizures, particularly hyponatraemia and hypocalcaemia. Seizures generally occur with a sodium concentration below 115 mmol, although risk is increased if sodium falls precipitously. Acute hypocalcaemia as a complication of parathyroidectomy can be associated with bilateral tonic-clonic seizures, focal motor or absence seizures. Patients undergoing parathyroidectomy should be preloaded with 1.25 hydroxyvitamin D. Hypomagnesaemia should be corrected intravenously, alongside calcium replacement, as sulphate anions may bind calcium and aggravate hypocalcaemia.
Hypoglycaemia or hyperglycaemia in the context of suboptimal diabetic control or sepsis may precipitate seizures in patients with renal disease.
In the dialysis patient with epilepsy, timing of AED treatment may lead to subtherapeutic levels; levetiracetam, phenobarbital and primidone are water-soluble and poorly protein bound and therefore readily removed by dialysis. Dosing needs to be appropriately timed and supplemental doses given following dialysis. Marked fluctuations in serum levels may lead to loss of seizure control, and these AEDs are therefore best avoided. Phenytoin, carbamazepine and sodium valproate are poorly dialysed and require no changes. Levetiracetam, gabapentin and, to a lesser extent, topiramate are almost exclusively eliminated by the kidneys and should be avoided in CKD to prevent accumulation and toxicity. CKD and other hypoalbuminaemic states result in increased displacement of highly protein-bound drugs, e.g. phenytoin and valproate, and therefore free serum drug concentrations, if available, should be measured.
Convulsive seizures can also be directly related to complications of dialysis, e.g. dialysis disequilibrium syndrome, prolonged intradialytic hypotension or air embolism.
6.3 Movement Disorders
In the context of encephalopathy, patients may present with asterixis: multifocal, action-induced jerks. Uraemia also triggers spontaneous action myoclonus and stimulus-sensitive myoclonus, which can be treated with benzodiazepines. Water-electrolyte imbalance can cause dysfunction of the lower brainstem reticular formation with jerks accompanied by fasciculations, muscle twitches and seizures.
Because opiates depend on renal excretion, opiate toxicity is a common, characteristic and avoidable cause of twitching (often accompanied by confusion) in patients with significant renal impairment. Electronic prescribing linked to eGFR should prevent this, but anticipation of the risk when other teams are prescribing for patients with advanced CKD is important.
Common immunosuppressive drugs used in both renal transplant and renal vasculitis patients can cause movement disorders. Both tacrolimus and cyclosporin are associated with tremor and cerebellar ataxia and peripheral neuropathy.
Movement disorders may additionally result from structural pathology caused by vascular or metabolic complications, e.g. basal ganglia stroke and extrapontine myelinolysis.
Restless leg syndrome (RLS), the irresistible urge to move the lower limbs, particularly at night, is common in CKD patients and associated with significantly reduced quality of life. It is likely multifactorial due to anaemia, iron deficiency, hypercalcaemia and alterations of CNS dopamine and opioid activity. Dopamine-blocking medications such as metoclopramide are associated with increased rates of RLS. The symptoms can be treated through correction of iron [30] and exercise [31]. Clonazepam and gabapentin may improve symptoms [32], and dopamine agonists can be used but may lead to augmentation of symptoms. Short, daily dialysis also reduces prevalence, and renal transplant is associated with symptomatic improvement.
Cramping is a common complication of haemodialysis treatment. The causes are multifactorial but changes in plasma osmolality and extracellular fluid volume are thought to play a significant role. Initial measures should include minimising intradialytic weight gain and reducing ultrafiltration rate of dialysis. Conservative measures such as application of heat and massage to the local area may also be helpful. Pharmacological measures may include a trial of vitamin E [33], use of gabapentin [34] and short-acting benzodiazepines such as clonazepam.
6.4 Headache
Haemodialysis headache occurs in around 5% of HD patients and is probably related to intradialytic hypotension and changes in urea and magnesium concentrations. Patients develop bilateral throbbing or non-pulsatile headache lasting less than 4 hours, typically towards the end of haemodialysis sessions. Diagnosis requires at least two episodes of acute headache, which develop during dialysis session, worsen with dialysis and resolve within 72 hours of completing dialysis. The symptoms completely cease following transplantation. Commonly used renal drugs, e.g. alpha-1 blockers, directly acting vasodilators and calcium channel blockers may also contribute to headaches in patients with CKD.
7 Renal Treatments and Drugs with Neurological Side Effects
7.1 Dialysis
Dialysis complications are common but may present non-specifically [35]. Haemodialysis is more likely to be associated with haemodynamic instability than peritoneal dialysis and more commonly associated with headaches. Dialysis disequilibrium syndrome results from cerebral oedema triggered by more rapid falls in osmolality in serum than CNS. Urea gradient causes more water to move into CNS resulting in raised intracranial pressure. It is a particularly important consideration in patients starting HD with serum urea above 60 who are rapidly dialysed over short periods. Symptoms occur during or immediately after HD, including headache, vomiting, disorientation and progression to seizures, coma and even death. Prevention of these side effects is achieved by targeting a reduction of no more than 30% in serum urea in the first dialysis session. Metabolic derangement can also be seen with the rapid correction of chronic hyponatraemia with haemodialysis, leading to osmotic demyelination of the pons and extrapontine areas. This is usually manifested by altered consciousness and convulsions and can be avoided by lowering dialysate sodium, short duration HD with small surface area dialysers and low flow rate (◘ Fig. 41.1).
Intracerebral haemorrhage is a significant cause of morbidity and mortality in haemodialysis patients; risk factors include chronic uraemia leading to platelet dysfunction, intradialytic anticoagulation and hypertension. In one retrospective study, the prevalence of non-traumatic SDH in HD patients was 0.4% with incidence of 189 per 100,000 patients [36]. The presentation can be atypical with gait ignition failure, confusion and ataxia.
Although uncommon, it is important to consider treatable nutritional causes of confusion in dialysis patients, particularly chronic malnourished patients. Fortunately, thiamine is only lightly plasma protein bound and therefore not readily dialysed. However, classical presentation with ophthalmoplegia may be absent, and a high index of suspicion is required. There is also some evidence to suggest that high-flux HD patients are at increased risk of B12 deficiency through increased losses during dialysis and reduced dietary intake [37].
Dialysis amyloid myelopathy is a rarely observed complication where spinal cord compression develops following chronic haemodialysis. Myelopathy develops secondary to the formation of fibroligamentous rings caused by extradural amyloid derived from beta-2 microglobulin. A progressive myelopathy may require surgical treatment. Amyloid deposition is also seen in dialysis arthropathy, bone cysts and recurrent carpal tunnel syndrome.
Peripheral nerve injury is a risk associated with vascular access surgery required for dialysis. The proximity of median nerve to brachial artery makes it susceptible during the formation of brachiocephalic fistulae. Median nerve compression may result from haematoma and pseudoaneurysm during surgery or following repeating cannulation and require urgent management. Neuropathy of the median nerve occurs chronically in up to 10% of arteriovenous fistula surgery with ‘steal’ phenomenon and depleted blood supply causing axonal loss, particularly in the setting of diabetes or severe peripheral vascular disease. Management of an ischaemic monomelic neuropathy involves urgent ligation of the fistula.
Dialysis dementia was described in the 1970s and was strongly linked to prolonged exposure to aluminium in municipal water supplies and aluminium-containing phosphate binders. Symptoms start with mixed dysarthria and dysphasia and rapidly progress with more severe language dysfunction, myoclonic jerks, ataxia and seizures. Reduction of aluminium by means of reverse osmosis has markedly reduced the incidence of dialysis dementia. Given the availability of other non-aluminium phosphate binders now available, clinical guidelines advise against long-term use of aluminium-containing phosphate binders [20, 21]. Desferrioxamine is a chelating agent, which is used in the treatment of aluminium toxicity.
7.2 Renal Transplantation and the Immunosuppressed Patient
Almost one third of renal transplant recipients will develop neurological complications [38]. Surgical complications at the time of transplantation include neuropraxis of the femoral and lateral cutaneous nerve of the thigh from intraoperative retraction.
Drug-induced suppression of cell-mediated immunity renders patients at increased risk of opportunistic infections, but it is worth reiterating that such patients should be managed with due diligence and with a low threshold for excluding neurological infections due to their blunted inflammatory response to sepsis. In addition, calcineurin inhibitors have a significant burden of minor neurological symptoms. Tremor is said to occur in up to 40% patients on cyclosporine, sleep disturbance is common and less commonly patients may develop a sensory neuropathy and myopathy. Calcineurin inhibitors may be associated with a reversible posterior leukoencephalopathy syndrome. Early recognition, reduction or withdrawal of the immunosuppressive agent and institution of antihypertensive medication is associated with an excellent clinical outcome.
Rejection encephalopathy, usually seen within 3 months of transplantation, is characterised by headache, confusion and seizures in the context of systemic features of graft rejection. Cytokine release during the rejection process is thought to be implicated.
Post-transplant lymphoproliferative disorders may be driven by EBV, and cases with neurological involvement may present with headaches, seizures, altered mental state and focal neurological deficits. There is CNS involvement in 10–15% of cases. Prognosis depends on the extent of disease dissemination.
7.3 Medications Commonly Used in Nephrology and Dosing Considerations
Medication | Neurological complication | Considerations in prescribing/dosing |
|---|---|---|
Penicillins and cephalosporin-related antibiotics | Seizures | Only at high doses. Dose reduction in severe renal impairment. Penicillins removed by HD/HDF but not by PD |
Gentamicin | Aminoglycoside antibiotic associated with ototoxicity and vestibular failure. Ototoxicity is more prevalent in those patients receiving a prolonged course of treatment | Extending the dose interval to >24 hours. Regular monitoring of serum levels and dose adjustments |
Erythropoietin | Hypertensive encephalopathy, seizures | Less common with lower doses of EPO prescribed. Avoid EPO in severe and uncontrolled hypertension |
Meperidine | Seizures | Caused by accumulation of toxic metabolite, normeperidine |
Metoclopramide | Seizures | Decrease seizure threshold. No change in dosing required. May increase blood ciclosporin levels |
Theophylline | Seizures | Seizures in overdose. Dose reduction not required in renal impairment. 50% of dose removed by HD |
Ertapenem/meropenem | Seizures | Competitive inhibition of GABA receptors. Dose reduction necessary in renal impairment. Give after dialysis |
Aciclovir | Seizures | Dose reduction required in renal impairment. Interactions with tacrolimus, mycophenolate and ciclosporin |
Carbamazepine | Seizures | Seizures in overdose. Dose reduction not required in renal failure. May reduce ciclosporin levels and effect of corticosteroids |
Lithium | Seizures | Seizures in overdose. Lithium should be preferably avoided in renal impairment. Otherwise, close monitoring is necessary. Loop and thiazide diuretics reduce lithium excretion. |
Gabapentin/pregabalin | Vertigo/sedation/ataxia | Dose reduction required in renal impairment. Dialysed. Give after haemodialysis on dialysis days |
Levetiracetam | Vertigo, nausea, diplopia | Dose reduction required in renal impairment. Readily dialysed, therefore 250–500mgs supplemental dose needed post dialysis |
Benzodiazepines | Sedation | Active metabolites are renally excreted. Dose reduction advised in renal impairment. Not removed by HD or PD |
Opioids | Sedation and twitching | Active metabolites of morphine and diamorphine accumulate in renal impairment. Fentanyl is predominantly metabolised in the liver to the inactive metabolite norfentanyl and maybe preferable to morphine derivatives |
Calcineurin inhibitors (cyclosporine/tacrolimus) | Tremor (fine resting or action), headaches, paraesthesia, mood changes, seizures, ataxia, motor deficits, posterior reversible encephalopathy syndrome | May be present even with therapeutic levels |
Corticosteroids | Proximal myopathy, anxiety, psychosis, headache, fever and lethargy on withdrawal | Prednisolone metabolism accelerated by carbamazepine, barbiturates and phenytoin. |
OKT3 monoclonal antibody | Aseptic meningitis, encephalopathy | Increases cyclosporine levels |
Sunitinib | Posterior reversible encephalopathy syndrome [39] | Case reports only |
8 Mental Health in Kidney Disease
Psychiatric illness is common among patients with end-stage renal failure, particularly those on haemodialysis compared with those treated conservatively or post transplantation. The psychiatric disorders most frequently observed include affective disorders (particularly depression), anxiety, suicide, organic brain disease such as dementia and delirium, drug-related disorders such as alcoholism, psychoses and personality disorders [40]. Steroids and other immunomodulating drugs can result in insomnia, mood swings, mania, behavioural problems, severe depression and psychosis in 5–18% patients [41].
Depression is under-recognised and undertreated in CKD. Patients with CKD are 1.5–3 times more likely to require inpatient psychiatric treatment than patients with other chronic disorders. Contributing factors may include the following: the feeling of total dependency on a dialysis machine, restrictions on diet and fluid, poor quality of life, reduced employment and limited functional capacity. Untreated depression may impact on treatment compliance, nutritional status, impaired immune function and increased suicide rates. Patients may require psychotropic drugs and psychotherapy and benefit from a MDT approach. Some psychotropic drugs require dose changes in both CKD and dialysis patient, e.g. paroxetine can lead to seizures at higher doses, venlafaxine may cause hypertension at higher doses and mirtazapine can be sedating. Lithium is the treatment of choice for bipolar disease but can be nephrotoxic, associated with nephrogenic diabetes and CKD due to chronic interstitial nephritis and distal renal tubular acidosis. Progression of kidney disease can occur despite discontinuation of lithium.
Tips and Tricks
EEG: The EEG will show triphasic waves and diffuse slow activity in dialysis dementia, metabolic encephalopathy and seizures (◘ Fig. 41.7). In acute uraemic encephalopathy, EEG changes are usually more severe and may help to distinguish from non-convulsive status epilepticus. There may be focal dysfunction in HSV encephalitis and tacrolimus-related encephalopathy.
LP: Lumbar puncture is essential for the diagnosis of encephalopathy. The CSF in uraemia will show pleocytosis and raised protein concentration in 50% patients, and protein may also be raised in PRES. Lymphocytic pleocytosis would point towards a viral encephalitis.
NCS/EMG: Nerve conduction studies show predominantly axonal neuropathy in uraemic neuropathy although sensory, and motor conduction velocities are often reduced. CKD patients may develop GBS type neuropathy with conduction block, slowing and prolonged F wave latencies. EMG in uraemic myopathy is usually normal.
Neurotoxicity with some drugs such as opiates and aciclovir in severe CKD is very predictable, and local education on dose reduction/avoidance or electronic prescribing should significantly reduce this risk.
Chapter Review Questions
-
1.
Should all ADPKD patients be screened to exclude intracerebral aneurysms?
-
2.
Which of the ANCA-associated vasculitides is most commonly associated with neurological complications, and what is the most common neurological complication?
-
3.
Can suprasacral spinal cord injuries cause urological dysfunction? Which patients should be screened?
-
4.
What is the mechanism of aciclovir-induced AKI?
-
5.
What are the cognitive features of uraemic encephalopathy?
Answers
-
1.
No. Low level evidence, based on expert consensus, currently recommends no systematic screening and targeted screening in patients with good life expectancy who have a family history of aneurysm or haemorrhage, with previous rupture, high-risk profession or anxiety.
-
2.
Churg-Strauss syndrome has the highest incidence of neurological involvement. Most commonly, this is a peripheral neuropathy, with 80% of patients showing signs of mononeuropathy multiplex.
-
3.
Yes. Patients with suprasacral spinal cord injury often have detrusor overactivity and high bladder pressures through detrusor sphincter dyssynergia. The UK NICE guidance suggests offering video-urodynamic investigations to people known to have high risk of renal complications, e.g. spinal cord injury or spina bifida.
-
4.
AKI develops due to obstructive nephropathy, with intratubular precipitation of crystals. It may be preventable with appropriate volume expansion prior to administration.
-
5.
Attentional deficits in the early stages with progress to delirium and then decreased level of consciousness.
Case Study
Case 1
A 54-year-old lady who had undergone a simultaneous kidney and pancreas transplantation 5 months previously and who was maintained on tacrolimus and mycophenolate mofetil immunosuppression presented to the emergency department with a 3-day history of progressive worsening headache, confusion and disorientation. Blood pressure was 170/100 and physical examination revealed bilateral pyramidal signs, cortical blindness, dysphasia and clonus. Blood tests were unremarkable. EEG was consistent with a metabolic encephalopathy and MRI was consistent with PRES. Lumber puncture was unremarkable. Antihypertensive treatment was commenced, and tacrolimus was switched to sirolimus. The patient made a slow but complete recovery over 6 weeks. Blood pressure at 6 weeks was 140/60 after the withdrawal of her antihypertensive medication.
Case 2
A 74-year-old lady who had been on maintenance haemodialysis for many years reported a progressively worsening, unpleasant sensation in both her legs over the preceding months. The sensation was worse at night or after any long period of lying down. The sensation was improved by movement but was causing significant sleep deprivation and a marked deterioration in her quality of life. Her partner commented that whilst asleep, she exhibited involuntary, jerking movements of the legs. Neurological examination was unremarkable. Blood tests demonstrated a Hb of 120 g/L in the context of a ferritin of 50. Markers of dialysis adequacy were satisfactory. A diagnosis of restless legs and periodic limb movements was postulated. The patient was advised to restrict her caffeine intake and to take regular exercise. She received treatment with intravenous iron and was started on 25 mg pregabalin, which was doubled 1 month later. Within 2 months, her symptoms had significantly improved and her sleep pattern had returned to normal.
Case 3
An 80-year-old gentleman receiving maintenance haemodialysis complained of increasing somnolence, fatigue and dizziness. Collateral history confirmed unsteadiness and clumsiness at home and also slurring of speech at times. Physical examination revealed depressed reflexes, past pointing bilaterally and evidence of mild dysarthria. The patient was deemed too unsteady to return home and was admitted for investigation and observation. Subsequent review of his repeat prescription demonstrated that gabapentin 300 mg tds had been commenced some 3 weeks previously when the patient had consulted a physician with regard to neuropathic pain along the distribution of his sciatic nerve. The dose and frequency of gabapentin was reduced to 300 mg after every dialysis session. The neurological signs and symptoms gradually improved over the subsequent 10 days, and the patient was discharged home.
References
Chapman AB, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88(1):17–27.
Malik MT, Kazmi SJ, Turner S. Teaching NeuroImages: Intradural, intramedullary spinal cord metastasis from primary renal cell carcinoma. Neurology. 2018;90(10):e911–2.
Sharma K, et al. Leber's congenital amaurosis with nephropathy. Indian J Ophthalmol. 1994;42(2):83–4.
Nishino H, et al. Neurological involvement in Wegener's granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol. 1993;33(1):4–9.
Sehgal M, et al. Neurologic manifestations of Churg-Strauss syndrome. Mayo Clin Proc. 1995;70(4):337–41.
Finsterer J, Scorza FA. Renal manifestations of primary mitochondrial disorders. Biomed Rep. 2017;6(5):487–94.
National Clinical Guideline Centre (UK). Urinary incontinence in neurological disease: management of lower urinary tract dysfunction in neurological disease. London: National Clinical Guideline Centre (UK); 2012.
Kurella M, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16(7):2127–33.
Seliger SL, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15(7):1904–11.
Chillon JM, Massy ZA, Stengel B. Neurological complications in chronic kidney disease patients. Nephrol Dial Transplant. 2016;31(10):1606–14.
Zoccali C, et al. The systemic nature of CKD. Nat Rev Nephrol. 2017;13(6):344–58.
Candy JM, et al. Aluminium accumulation in relation to senile plaque and neurofibrillary tangle formation in the brains of patients with renal failure. J Neurol Sci. 1992;107(2):210–8.
Brenner RP. The electroencephalogram in altered states of consciousness. Neurol Clin. 1985;3(3):615–31.
Raskin NH. Neurological aspects of renal failure. In: Aminoff MJ, editor. Neurology and general medicine. 1st ed. New York: Churchill Livingstone; 1989.
Hinchey J, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500.
Kitaguchi H, et al. A brainstem variant of reversible posterior leukoencephalopathy syndrome. Neuroradiology. 2005;47(9):652–6.
Herbert H, Schaumburg ARB, Thomas PK. Disorders of peripheral nerves. Philadelphia: F.A. Davis; 1991.
Krishnan AV, Kiernan MC. Uremic neuropathy: clinical features and new pathophysiological insights. Muscle Nerve. 2007;35(3):273–90.
Raskin NH, Fishman RA. Neurologic disorders in renal failure (first of two parts). N Engl J Med. 1976;294(3):143–8.
Dyck PJ, et al. Segmental demyelination secondary to axonal degeneration in uremic neuropathy. Mayo Clin Proc. 1971;46(6):400–31.
Fraser CL. Neurological manifestations of the uremic state. In: Arieff A, Griggs R, editors. Metabolic brain dysfunction in systemic disorders. Philadelphia: Lippincott Williams and Wilkins; 1992. p. 500.
Bautista J, et al. Dialysis myopathy. Report of 13 cases. Acta Neuropathol. 1983;61(1):71–5.
Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107(1):1–16.
Go AS, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–9.
Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50(3):433–40.
Vazquez E, et al. Ought dialysis patients with atrial fibrillation be treated with oral anticoagulants? Int J Cardiol. 2003;87(2–3):135–9; discussion 139–41.
Davenport A. Practical guidance for dialyzing a hemodialysis patient following acute brain injury. Hemodial Int. 2008;12(3):307–12.
Kurella Tamura M, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52(2):227–34.
Helmer C, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77(23):2043–51.
Allen RP, et al. Evidence-based and consensus clinical practice guidelines for the iron treatment of restless legs syndrome/Willis-Ekbom disease in adults and children: an IRLSSG task force report. Sleep Med. 2018;41:27–44.
Aukerman MM, et al. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2006;19(5):487–93.
Winkelman JW, et al. Practice guideline summary: treatment of restless legs syndrome in adults: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87(24):2585–93.
Roca AO, et al. Dialysis leg cramps. Efficacy of quinine versus vitamin E. ASAIO J. 1992;38(3):M481–5.
Serrao M, et al. Gabapentin treatment for muscle cramps: an open-label trial. Clin Neuropharmacol. 2000;23(1):45–9.
Karunaratne K, et al. Neurological complications of renal dialysis and transplantation. Pract Neurol. 2018;18(2):115–25.
Power A, et al. High but stable incidence of subdural haematoma in haemodialysis--a single-centre study. Nephrol Dial Transplant. 2010;25(7):2272–5.
Chandna SM, et al. Low serum vitamin B12 levels in chronic high-flux haemodialysis patients. Nephron. 1997;75(3):259–63.
Patchell RA. Neurological complications of organ transplantation. Ann Neurol. 1994;36(5):688–703.
Duchnowska R, et al. Severe neurological symptoms in a patient with advanced renal cell carcinoma treated with sunitinib. J Oncol Pharm Pract. 2013;19(2):186–9.
Kimmel PL, et al. Psychiatric illness in patients with end-stage renal disease. Am J Med. 1998;105(3):214–21.
Cerullo M. Corticosteroid-induced mania: Prepare for the unpredictable. Curr Psychiatry 2006 [cited 2016 22/6/16]; Available from: https://www.mdedge.com/psychiatry/article/62206/mental-health/corticosteroid-induced-mania-prepare-unpredictable.
Patient Information and Guidelines
NICE clinical guideline CG148: urinary incontinence in neurological disease: assessment and management. August 2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nagy, A., Dingley, G., Liu, R. (2022). The Nervous System and the Kidney. In: Harber, M. (eds) Primer on Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-76419-7_41
Download citation
DOI: https://doi.org/10.1007/978-3-030-76419-7_41
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76418-0
Online ISBN: 978-3-030-76419-7
eBook Packages: MedicineMedicine (R0)