Abstract
Renal dysfunction, particularly acute kidney injury (AKI), is relatively common in patients with liver disease, but chronic kidney disease (CKD) is also a frequent co-morbidity in this patient population. Both pre-renal and intrinsic renal causes predominate, and defining the principal factor can be difficult. Sometimes the renal impairment can be a consequence of liver disease, as in the case of hepatorenal syndrome (HRS). On other occasions, there may be a common underlying aetiology as seen when a viral infection causes both a glomerulonephritis and hepatitis. This chapter focuses on the differential diagnoses of kidney injury in the context of liver disease, along with potential therapeutic options. There have also been some changes to the diagnostic criteria of HRS which are discussed in detail (Gines P, Schrier RW, N Engl J Med 361(13):1279–1290, 2009; Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al., Gut. 60(5):702–709, 2011).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Renal failure
- Acute kidney injury
- Chronic kidney disease
- Hepatorenal syndrome
- Cirrhosis
- Hepatitis
- Leptospirosis
- Combined liver and kidney transplantation
-
1.
To understand the causes and risk factors for hepatorenal syndrome and the interaction of AKI, CKD and chronic liver disease (CLD)
-
2.
To appreciate the differential diagnosis of hepatorenal diseases and the management of HRS
1 Definition and Classification of Renal Dysfunction in Cirrhosis
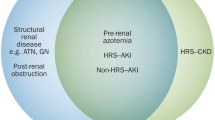
Traditionally when discussing renal impairment in the context of liver disease, a lot of focus has been put on HRS definition and diagnosis [3, 4]. However, there are other causes of renal dysfunction in this patient population, and HRS is often a diagnosis of exclusion. Definitions and classifications for AKI and CKD have also evolved over the years [5, 6] to include both rising creatinine and falling urine output. These were applied to cirrhotic patients in a 2015 update on the diagnosis and management of AKI by the International Club of Ascites (ICA) (◘ Table 36.1). Although the ICA adopts an AKI staging system based on changes in serum creatinine levels over 1 week, urine output was excluded as it was not felt to be relevant in patients with cirrhosis, many of whom are oliguric. The ICA classification has been validated in patients with cirrhosis where development of AKI is associated with increased mortality.
An estimated glomerular filtration rate (eGFR) <60 ml/min for >3 months is deemed to be the threshold for CKD in those with cirrhosis [7].
2 Definition and Classification of Hepatorenal Syndrome
Hepatorenal syndrome is a critically important cause of AKI in patients with cirrhosis. Previously, threshold values of at least a twofold increase in creatinine to a level >221 μmol/L were needed, but now HRS-AKI can be diagnosed when the patient has >/= stage 2 AKI and also meets the criteria detailed in ► Box 36.1. This change has not yet been updated in some guidelines [8, 9]. Hepatorenal syndrome was classically divided into types 1 and 2 depending on the severity and acuity of renal dysfunction (◘ Table 36.2a). Type 1 is now referred to as HRS type of AKI (HRS-AKI) and is an acute, rapidly progressive illness with a very poor prognosis without a liver transplant (LT). HRS type 2 is a less severe condition, traditionally defined using the same criteria as HRS type 1, but was more gradual in onset and had a creatinine threshold for diagnosis of >133 μmol/L. It is often characterised by diuretic-resistant ascites that is less amenable to pharmacological interventions. Hepatorenal syndrome type 2 is a specific form of CKD, and a new term, HRS type of chronic kidney disease (HRS-CKD), has been proposed [2, 4, 7, 10,11,12,13].
3 Incidence
The incidence of renal impairment depends on the aetiology. For example, post paracetamol overdose, the incidence of AKI is as high as 75%. Using the definitions outlined above, the incidence of AKI in hospitalised patients with cirrhosis is 19–54%. There is a broad differential, but HRS is the principal aetiology in 12–18% of cases. In 1993, a study showed that HRS occurred in 18% of patients with cirrhosis and ascites at 1 year and 39% at 5 years [14,15,16,17]. Chronic kidney disease occurs in about 1% of those with cirrhosis, and HRS-CKD occurs in between 16% and 61% of those with HRS [2, 12].
4 Differential Diagnosis for Renal Dysfunction in Those with Liver Disease
This is broad and is summarised in ◘ Table 36.3. Getting the diagnosis correct has critical implications for patient management and prognosis. There is often a shared underlying aetiology causing both the renal and liver disease. Essentially, pre-renal and intrinsic renal causes predominate, and pre-existing renal conditions should always be considered, with HRS often a diagnosis of exclusion [1, 4, 15, 17,18,19].
5 Pathophysiology of HRS
Hepatorenal syndrome is a functional renal impairment characterised by a number of haemodynamic abnormalities. The pathogenic mechanisms outlined below probably integrate, and these are broadly illustrated in ◘ Fig. 36.1.
5.1 Peripheral Arterial Vasodilation
The peripheral arterial vasodilation seen with portal hypertension plays a key role in the pathogenesis of HRS. As the liver progressively fibroses, intrahepatic portal pressure increases, leading to splanchnic pooling. Nitric oxide release from the splanchnic vasculature endothelium also increases, due to portal hypertension-induced shear stress or bacterial translocation and cytokine-induced increased nitric oxide synthase activity, resulting in local vasodilation. These circulatory changes have been confirmed in studies where increased blood flow in the superior mesenteric artery was demonstrated compared with the femoral, correlating with the degree of liver dysfunction. The consequence of this splanchnic pooling is a reduction in effective arterial blood volume and vascular resistance [1, 4, 19].
5.2 Haemostatic Compensatory Mechanisms
To maintain homeostasis in response to the above, there is baroreceptor-mediated activation of the renin-angiotensin-aldosterone and sympathetic nervous systems with subsequent release of anti-diuretic hormone resulting in sodium and water retention. Baroreceptors are principally located in the aortic arch and carotid sinus; however, they are also present in other organs including the liver. Here, there is evidence for a hepatorenal baroreflex whereby afferent hepatic pressure sensors can influence renal blood flow, GFR and salt and water excretion via neurohormonal mechanisms that increase renal sympathetic activity. Renal blood flow may be preserved in the early stages of cirrhosis when local vasodilators such as prostaglandins and nitric oxide can overcome the vasoconstrictor effects, but as liver disease progresses, this equilibrium cannot be maintained, and renal hypoperfusion and HRS can ensue. Vasoconstriction is not isolated to the kidney but has been shown in other vascular beds too. However, the splanchnic vascular bed escapes the effects of the potent vasoconstrictors due to the local concentration of vasodilators. In addition to the factors already mentioned, an inadequate adrenal response to stress such as sepsis is also thought to play a role in the pathophysiology of HRS [1, 3, 19, 20].
5.3 Cirrhotic Cardiomyopathy
The increased sympathetic nervous system leads to a hyperdynamic circulation with tachycardia and increased cardiac output to overcome the decreased systemic vascular resistance and blood pressure. However, as liver disease progresses and when additional demands are placed on cardiac function, e.g. with infection, cardiac response may be inadequate despite the absence of known cardiac disease. This has been described as cirrhotic cardiomyopathy where there is reduced cardiac contractility, diastolic dysfunction and electrophysiological abnormalities. The pathophysiology of this blunted cardiac response may be due to some underlying cardiac hypertrophy and fibrosis, increased production of negatively inotropic mediators or functional changes in the cardiomyocyte plasma membrane properties. These changes are potentially reversible post-LT [19, 21].
6 Clinical Evaluation of Liver Patients with Renal Dysfunction
The key things to determine when assessing a patient include a history of any known renal and liver disease, the aetiology and chronicity of these illnesses including the presence of cirrhosis or signs of portal hypertension. Precipitating events such as a change in medications, haemorrhage, infection, recent diarrhoea or vomiting, or large volume paracentesis, should be ascertained.
Hepatorenal syndrome is an important cause to exclude and is characterised by a constellation of clinical features, outlined in ◘ Table 36.2. Most patients will give a long history of chronic liver disease, with ascites being a prominent feature. Depending on the speed of onset and severity of the renal dysfunction, HRS can be classified into HRS-AKI and HRS-CKD, as outlined above. The latter can suddenly progress to HRS-AKI after a precipitating event [2, 7].
On examination, volume assessment is key to the evaluation of patients with renal dysfunction. Hypotension and tachycardia are features of volume depletion and sepsis. A low mean arterial blood pressure <80 mmHg is also seen in HRS-AKI due to splanchnic pooling. This can also be precipitated by factors such as diuretics, paracentesis, sepsis or blood loss. The hypotension is typically accompanied by tachycardia, a manifestation of the hyperdynamic circulation. The haemodynamic changes are not always confined to the kidney, and other vascular beds may also be involved with a reduced cardiac output and encephalopathy in more severe cases. Stigmata of chronic liver disease will usually be evident in those with HRS. Clinical signs of an underlying infection such as peritonitis should be elucidated to enable prompt treatment. Impaired natriuresis is a feature of HRS, and an inability to excrete free water results in peripheral oedema and ascites, and this is typically diuretic resistant. Patients may develop oligo-anuria with a urine output <500 ml/day. Pulmonary oedema can occur in this setting but is not a typical feature of HRS. A bland urinary sediment is characteristic given the functional nature of the renal impairment in HRS, although patients with liver disease may have a number of possible causes for underlying CKD including glomerulonephritis, so haematuria, proteinuria and urinary casts should be excluded. Finally, the skin should be evaluated for signs of a vasculitis rash that can be seen in those with viral hepatitis-related cryoglobulinaemia [3, 4, 22].
7 Investigations
Renal function needs to be monitored carefully in those with liver disease, particularly when there is diuretic-resistant ascites, hyponatraemia, peritonitis or gastrointestinal haemorrhage. However, creatinine is a notoriously poor indicator of renal function in patients with cirrhotic liver failure due to poor nutrition, reduced hepatic creatinine production and muscle mass, leading to a delay in diagnosing and treatment based on the traditional creatinine threshold [1]. Commonly used eGFR equations overvalue true GFR, when compared to radio-isotopic methods potentially. This makes the application of the usual CKD stages based on eGFR alone problematic. However, despite these reservations, creatinine and eGFR are currently the easiest and most widely available tools for the assessment of renal function. Cystatin C is also problematic and influenced by clinical factors [13, 23, 24].
One of the most notable biochemical features of HRS is hyponatraemia. Water retention can exceed that of sodium, and so a dilutional hyponatraemia develops in about two-thirds of patients. This parameter can be useful in differentiating HRS from other aetiologies of renal impairment such as acute tubular necrosis. Natriuresis is impaired so one of the other classical findings in HRS is a urinary sodium <10 mmol/L in the context of a serum sodium <135 mmol/L and a urine osmolality that is greater than that of serum [8, 20, 22].
To help distinguish HRS from other parenchymal causes of renal impairment in cirrhotics, a number of tests can be useful (◘ Table 36.4a). A urinary protein-creatinine ratio should be performed if the dipstick is positive along with examination of urinary sediment for casts. If the proteinuria is found to be >500 mg/dL and there is microscopic haematuria (>50 urinary red cells per high-powered field) or any other clinical features to suggest parenchymal renal disease, then consider an alternative diagnosis. However, HRS can develop in the context of a pre-existing renal condition, so this must be taken into account. If no contraindications exist, a renal biopsy may be useful in this scenario to help determine the underling aetiology. This is particularly so if a combined liver and kidney transplant is being considered, as the degree of renal fibrosis will help predict renal prognosis post-LT and avoid unnecessary renal transplantation in those with HRS. The latter is characterised by a lack of significant parenchymal histological changes and typically recovers with LT alone.
As with all other causes of renal impairment, performing a renal ultrasound scan should be a priority to evaluate for evidence of parenchymal disease and to exclude obstruction.
A summary of some useful investigations for conditions that cause both renal and liver dysfunction is outlined in ◘ Table 36.4b [1,2,3,4, 13, 25].
8 Precipitating Factors, Prevention and Initial Therapy
Acute kidney injury is frequently triggered by complications such as peritonitis, acute alcoholic hepatitis and gastrointestinal haemorrhage. Hence, prompt diagnosis and effective treatment is imperative to prevent progression [7].
As previously alluded to, NSAIDs inhibit renal perfusion and so should not be used in those with cirrhosis. Other drugs such as aminoglycosides and angiotensin-converting enzyme inhibitors should also be avoided, where possible. Radiological contrast should be administered with caution in those at risk of developing AKI. Typically, ascites is initially treated with fluid and sodium restriction, but diuretics especially aldosterone antagonists are frequently required. However, overzealous diuresis can have a negative impact on renal perfusion and hence precipitate AKI. Aldosterone antagonists can precipitate dangerous hyperkalaemia and so should be used with caution in those with poor renal function. Preventative strategies include regular monitoring of renal function for all those on diuretics [1, 19].
If renal function does deteriorate, then the first step is to correct intravascular volume depletion, preferably with 1 g/kg albumin per day. This acts as a circulatory expander and may also have antioxidant properties, so is the fluid of choice for resuscitation in all patients with AKI-HRS. Diuretic doses should be reduced or even stopped. In this scenario, the optimum treatment for ascites is paracentesis, with appropriate albumin support for those who require removal of large volumes of over 5 L (8 g/L of ascites drained). Without albumin, approximately 20% will develop HRS. Paracentesis may also relieve raised intra-abdominal pressure impeding renal venous return. There needs to be a low threshold for hospital admission in patients with deteriorating renal function aiming to restore renal perfusion. Some may require high dependency or intensive care unit support to facilitate close monitoring of vital signs and urine output. Adrenal insufficiency may be an exacerbating factor in some, and hydrocortisone administration may also have a role [1, 8, 9, 15].
In a third of cases, HRS is triggered by bacterial peritonitis and is associated with increased cytokine release. Therefore, rapid diagnosis and treatment of any sepsis, including peritonitis, is imperative. Along with antibiotic therapy, albumin administration has also been shown to decrease the risk of HRS from 30.6% to 8.3% compared with controls. This is felt to be due to an improvement in haemodynamics and renal perfusion along with antioxidant effects. For high-risk patients, the use of antibiotic prophylaxis with norfloxacin or ceftriaxone helps to reduce the risk of spontaneous bacterial peritonitis and HRS and improves survival [1, 8, 9, 19, 26, 27].
For those with CKD and liver disease, the key factors in patient management and prevention of progression include those mentioned above, but also attention needs to be given to the management of the underlying cause of the CKD (e.g. diabetes, hepatitis-related glomerulonephritis). Blood pressure should be controlled, and proteinuria minimised, where possible.
9 Treatment of AKI and AKI-HRS
If the preventative and initial management strategies outlined above fail and AKI develops secondary to HRS, then several therapies are available. The elimination of creatinine thresholds from the diagnostic criteria should allow for earlier intervention. The key treatment options in the management of HRS depend on the stage of AKI present (► Box 36.2) [7, 15].
9.1 Vasoconstrictors and Albumin
As splanchnic vasodilation rather than renal vasoconstriction is the initial circulatory derangement, vasoconstrictors are the pharmacological treatment of choice for HRS-AKI, improving renal function and patient survival. They have also been evaluated in HRS-CKD, but information there is limited. A number of agents have been shown to be effective, either alone or in combination with albumin, but terlipressin, an analogue of the vasopressin V1 receptor, is the most commonly used. A meta-analysis of 18 randomised controlled trials demonstrated that terlipressin resulted in reversal of HRS in 42% versus 15.4% in the placebo group. The relative risk of death was 0.63. It is important to evaluate cardiac risk prior to the initiation of these agents. Relapse after cessation of terlipressin is rare and usually responds to re-treatment. Alpha-1 adrenergic receptor agonists such as midodrine and noradrenaline can also be effective in reversing HRS. Noradrenaline has been compared to terlipressin, and both are equally effective in terms of renal recovery and patient mortality, although the former is less expensive and has fewer side effects. Octreotide is a glucagon inhibitor with vasoconstrictive effects on the splanchnic circulation. When given with midodrine, it has had a positive effect on renal haemodynamics, although benefits were inferior to terlipressin in a randomised controlled trial [1, 8, 13, 28, 29].
9.2 Transjugular Intrahepatic Portosystemic Shunt (TIPS)
Here, a metal stent is inserted to bridge the portal and central venous systems aimed at reducing portal hypertension. It is principally used in the treatment for refractory variceal bleeding and diuretic-resistant ascites. One study demonstrated an improvement in renal function in 75% of patients and a mean patient survival of 92 versus 12 weeks in those who underwent TIPS compared with a control group. Patients need to be carefully selected, as a TIPS can result in deterioration in those with severe liver failure, development of congestive cardiac failure or hepatic encephalopathy. In certain scenarios, TIPS does have a role, as in those with HRS and refractory ascites or as an adjunct to vasoconstrictors and albumin while awaiting LT. It may also be an option to prolong survival in those for whom transplantation is contraindicated [8, 13, 19, 30].
9.3 Renal Replacement Therapy (RRT) and Artificial Liver Support
End-stage renal failure can develop in both those with AKI and CKD complicating cirrhosis or fulminant hepatic failure. In this case, initiation of RRT and the modality of treatment need to be considered on a case-by-case basis.
Renal replacement therapy may be necessary as a bridge to LT where other treatments have failed. However, a recent study has shown 85% mortality at 6 months post-initiation of RRT in non-listed patients, so careful consideration should be given to initiation of this treatment in this patient group. There may be a role for a time-limited trial of RRT in these individuals if they are not critically ill. Post-transplant, complete renal recovery is usual in patients with HRS-AKI, even in those who have required RRT pre-operatively [31].
Indications for RRT are similar to those for other AKI populations including intractable hyperkalaemia, metabolic acidosis, uraemia and fluid overload. The RRT modality needs to be selected on an individual patient basis. Delivery of RRT can be difficult in those with liver failure for a number of reasons. Coagulopathy and thrombocytopaenia can make gaining vascular access a challenge. Another barrier to the use of intermittent haemodialysis is haemodynamic instability and hypotension. For this reason, continuous RRT is often favoured in patients with HRS-AKI as it allows for more gentle fluid removal, correction or hyponatraemia and other electrolyte disturbances and reduces the likelihood of raised intracranial pressure. Furthermore, the removal by continuous RRT of pro-inflammatory cytokines such as tumour necrosis factor and interleukins 1 and 6 may also be of potential benefit. However, there is no conclusive evidence to support continuous over intermittent therapies for all patients, and the modality should be decided on a case-by-case basis [1, 13, 19, 32].
Another technique that is available is extracorporeal albumin dialysis. This was developed to treat liver failure as a bridge to recovery or LT. The most widely used method is the molecular adsorbent re-circulating system, or MARS. Meta-analysis suggests a survival advantage in those with acute liver failure. Currently, these devices are not in widespread use [8, 13, 19, 33].
9.4 Transplantation
The prognosis for patients with HRS is dreadful, and a LT is the best treatment for a meaningful recovery. There is a clear benefit with LT compared with other therapies as it alleviates the underlying liver disease with a progressive improvement in the circulatory derangements post-transplantation, thereby usually restoring renal function. The negative impact of HRS on patient survival is highlighted by the fact that serum creatinine is a key variable in the Model for End-Stage Liver Disease (MELD) score, used to prioritise patients awaiting LT. The number of patients receiving combined liver and kidney transplants rose by 300% in the United States following the introduction of this score in 2002. However, a renal transplant is an inappropriate treatment for HRS unless they also meet the following suggested criteria. Although there are no standard criteria, some indications for combined liver and kidney transplantation are detailed in ◘ Table 36.5. Ideally, patients being considered for a combined transplant should undergo a renal biopsy, provided that it is safe to do so. The presence of >30% renal fibrosis prior to transplantation is likely to lead to a further decline in renal function with the introduction of calcineurin inhibitors post-LT and the development of post-operative AKI. Typically, between 12% and 80% of patients experience AKI in the post-LT period, depending on severity and the definition that is used. It is crucial that any decisions regarding single or dual transplantation are made jointly by the renal and liver teams and on a case-by-case basis [8, 13, 25, 34, 35].
9.5 Treatment of Hepatitis B and C in Renal Patients
In patients with glomerular disease, AKI or CKD due to underlying viral hepatitis, it is important to treat the underlying cause. Huge advances have been made in this area in recent times, particularly in relation to hepatitis C virus treatment. Previously, treatment of patients on dialysis or post-transplant with this infection was problematic or impossible because of intolerable side effects or increased risk of rejection. The timing of treatment and drug selection is complex and beyond the scope of this chapter, but guidelines have been published by the European Association for the Study of the Liver (EASL) diseases with details on how to treat those with CKD, on dialysis and pre- and post-renal transplantation [36, 37]. It is important to emphasise that some commonly used drugs need to be avoided or the dose reduced when treating patients with CKD or on dialysis.
10 Patient and Renal Outcomes
Without a LT, patient survival with HRS is very poor. Median patient survival for those with HRS-AKI (formerly type 1) is usually as short as 2–4 weeks, while it is 6.7 months in those with HRS-CKD (formerly type 2). HRS-AKI remains an independent predictor of mortality irrespective of the MELD score, further highlighting the negative impact that HRS has on patient outcome [31, 38]. As previously mentioned, vasoconstrictor therapy and liver transplantation do have a positive influence on survival [1, 28]. However, even post-LT, patient survival at 1, 3 and 5 years is inferior in those with HRS compared with those without and survival is particularly poor in patients who remain on dialysis post-LT [39, 40].
The aetiology of renal failure is also important, as HRS is linked to increased mortality compared to other causes of renal failure. Three-month patient survival was 15% with HRS, significantly less than that seen with other causes of renal dysfunction [41]. However, if patients are RRT dependant, survival in those with HRS was not shown to be significantly different to those with a diagnosis of acute tubular necrosis [31].
Recovery of renal function following a LT alone is usual after 3–6 weeks, but it may take longer and is not guaranteed in all patients. Between 6% and 10% of patients remain dialysis dependant, and this figure has been reported to be as high as 25% compared with <1% in patients without HRS. Up to 42% of HRS patients continue to have some degree of CKD, but renal function declines in the non-HRS population too with 18% having an eGFR <15 ml/min at 5 years post-LT. This depends on a number of underlying risk factors including age, co-morbidities or pre-existing CKD. The use of calcineurin inhibitors may have further deleterious effects [39, 42, 43].
11 Conclusion
Renal dysfunction, including HRS, is a common and very serious complication of cirrhotic liver disease. Therapeutic advances have led to significant improvements in patient outcomes, and as such it is no longer always a terminal complication. However, without the option of LT, the prognosis remains grim for those with HRS-AKI, and the challenge for the nephrologist is the careful and rapid assessment of patients for reversible components and other causes for renal disease. New diagnostic criteria will help to facilitate this. It is critical to establish whether each patient with both renal and liver failure is suitable for a LT or whether a combined liver kidney transplant may be more appropriate in a small number of patients. Getting this right is likely to have a huge impact on the patient’s outcome.
Key Points of the Chapter
-
1.
There is a new approach to the diagnosis of hepatorenal syndrome (HRS).
-
2.
A new treatment algorithm has been introduced for the management of HRS type of acute kidney injury.
-
3.
HRS is a functional type of renal failure that is usually reversible post-liver transplant.
-
4.
Albumin and vasoconstrictors are key pharmacological treatment options, and without liver transplantation, prognosis remains very poor.
Tips and Tricks
-
1.
Be aware of the patients who are at risk of developing hepatorenal syndrome (HRS) and take steps to prevent it where possible.
-
2.
The creatinine threshold of 122 μmol/L for the diagnosis of HRS-AKI has been abandoned so treatment can commence earlier.
-
3.
Use albumin for fluid resuscitation.
-
4.
Consider other causes of AKI and CKD in patients with cirrhosis before diagnosing HRS, which is a diagnosis of exclusion.
Abbreviations: AKI acute kidney injury, CKD chronic kidney disease.
Chapter Review Questions
-
1.
What conditions cause both kidney and liver disease?
-
2.
How is hepatorenal syndrome now defined?
-
3.
Describe the types of hepatorenal syndrome.
-
4.
What is the approach to the treatment of acute kidney injury in patients with liver disease?
-
5.
What are some of the indications for combined liver and kidney transplantation?
Case Study
Case 1
A 53-year-old female was admitted with decompensated cirrhosis due to alcoholic liver disease. She was on the waiting list for liver transplantation but had deteriorating renal function and oliguria. She was disorientated and very oedematous with significant ascites despite high-dose loop diuretics and so was undergoing intermittent large volume paracentesis supported by albumin infusions. Her blood pressure was 100/70 mmHg, pulse rate 98 beats per minute and temperature 37.5°C. A dipstick urinalysis revealed trace proteinuria and blood and no casts were seen on microscopy. Significant lab results were as follows:
Selected laboratory parameters | At the time of initial renal review | On discharge from hospital |
|---|---|---|
Sodium (mmol/L) | 130 | 139 |
Potassium (mmol/L) | 4.8 | 4.5 |
Urea (mmol/L) | 35 | 8.1 |
Creatinine (μmol/L) | 204 | 79 |
Bilirubin (μmol/L) | 201 | 21 |
International normalised ratio | 1.7 | 1.1 |
Platelet count (×109/L) | 84 | 178 |
Urine protein-creatinine ratio (mg/mmol) | 47 | Not available |
Urinary sodium (mmol/L) | 19 | Not available |
She had a negative immunology and myeloma screen. A renal ultrasound was unremarkable. A diagnosis of HRS-AKI was made. The diuretics were stopped, and she was started on albumin and terlipressin intravenously. Despite this, there was little improvement clinically or biochemically, and she decompensated following an episode of sepsis, becoming more confused with haemodynamic instability. She was transferred to the intensive care unit where she was started on intravenous antibiotics for suspected bacterial peritonitis and continuous RRT. She improved significantly, and the antibiotics were stopped a week later. The encephalopathy also resolved, but she remained oliguric and so remained on continuous RRT. She underwent a liver transplant a week later which was without complications and made a full renal recovery. This case demonstrates the fulminant deterioration that can befall a patient with chronic liver disease and the urgency of treatment as well as the potential for good renal recovery when HRS is cured.
Case 2
A 27-year-old female presented to an accident and emergency department with a reduced level of consciousness, malaise and nausea. She had no significant past medical or surgical history of relevance and was not on any regular medications. The history revealed that she had taken a staggered, inadvertent paracetamol overdose over the preceding week for flu-like symptoms and musculoskeletal pain. Socially she drank 5 units of alcohol per week for the preceding 6 months but previously drank more heavily, up to 40 units a week. Her initial blood results are illustrated in the table below, and she also had a paracetamol level of 125 mg/L. She was commenced on acetylcysteine and intravenous fluids, transferred to the intensive care unit and intubated for a falling Glasgow Coma Scale. She was commenced on inotropes for haemodynamic instability and continuous renal replacement therapy for oliguric renal failure and metabolic acidosis. Her condition progressively deteriorated, and after discussion with the hepatology service, she was listed for a super urgent liver transplant. The liver transplant went ahead 2 days later, and the surgery was uncomplicated. She remained in the intensive care unit and on continuous RRT for another week before being commenced on intermittent haemodialysis. She was eventually discharged to the ward and continued to require dialysis for another week before this could be stopped. Her discharge bloods are indicated below. Renal recovery often lags behind hepatic recovery in paracetamol overdose not needing a liver transplant, but either way, in a young patient, the renal prognosis is likely to be good.
Selected laboratory parameters | Prior to liver transplant | On hospital discharge |
|---|---|---|
Sodium (mmol/L) | 131 | 135 |
Potassium (mmol/L) | 5.3 | 4.1 |
Urea (mmol/L) | 14.1 | 2.7 |
Creatinine (μmol/L) | 347 | 85 |
Albumin (g/l) | 25 | 28 |
Bilirubin (μmol/L) | 68 | 22 |
Aspartate aminotransferase (IU/L) | 11,487 | 35 |
Alanine transaminase (IU/L) | 8044 | 110 |
Lactate | 14 | |
pH | 7.01 | 7.35 |
International normalised ratio | 4.7 | 0.99 |
Haemoglobin | 9.1 | 9.7 |
Platelet count (×109/L) | 57 | 187 |
Urine protein-creatinine ratio (mg/mmol) | 58 | Urine dip negative |
References
Gines P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361(13):1279–90.
Wong F, Nadim MK, Kellum JA, Salerno F, Bellomo R, Gerbes A, et al. Working party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60(5):702–9.
Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–76.
Salerno F, Gerbes A, Gines P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56(9):1310–8.
KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements. 2012;2(1).
Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67(6):2089–100.
Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62(4):968–74.
EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53(3):397–417.
Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline on management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57(4):1651–3.
Solé C, Pose E, Solà E, Ginès P. Hepatorenal syndrome in the era of acute kidney injury. Liver Int. 2018;38(11):1891–901.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266.
Bucsics T, Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5(2):127–37.
Nadim MK, Kellum JA, Davenport A, Wong F, Davis C, Pannu N, et al. Hepatorenal syndrome: the 8th international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16(1):R23.
Fagundes C, Barreto R, Guevara M, Garcia E, Solà E, Rodríguez E, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59(3):474–81.
Angeli P, Rodríguez E, Piano S, Ariza X, Morando F, Solà E, et al. Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut. 2015;64(10):1616–22.
Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105(1):229–36.
Betrosian AP, Agarwal B, Douzinas EE. Acute renal dysfunction in liver diseases. World J Gastroenterol. 2007;13(42):5552–9.
Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362(9398):1819–27.
Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1(5):1066–79.
Oliver JA, Verna EC. Afferent mechanisms of sodium retention in cirrhosis and hepatorenal syndrome. Kidney Int. 2010;77(8):669–80.
Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57(2):268–78.
Cardenas A. Hepatorenal syndrome: a dreaded complication of end-stage liver disease. Am J Gastroenterol. 2005;100(2):460–7.
Gonwa TA, Jennings L, Mai ML, Stark PC, Levey AS, Klintmalm GB. Estimation of glomerular filtration rates before and after orthotopic liver transplantation: evaluation of current equations. Liver Transpl. 2004;10(2):301–9.
Puthumana J, Ariza X, Belcher JM, Graupera I, Ginès P, Parikh CR. Urine interleukin 18 and lipocalin 2 are biomarkers of acute tubular necrosis in patients with cirrhosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(7):1003–13.e3.
Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK). Am J Transplant. 2008;8(11):2243–51.
Cardenas A, Gines P, Uriz J, Bessa X, Salmeron JM, Mas A, et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: incidence, clinical course, predictive factors, and short-term prognosis. Hepatology. 2001;34(4 Pt 1):671–6.
Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11(2):123–30.e1.
Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51(2):576–84.
Wang H, Liu A, Bo W, Feng X, Hu Y. Terlipressin in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(16):e0431.
Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, et al. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47(2):288–95.
Allegretti AS, Parada XV, Eneanya ND, Gilligan H, Xu D, Zhao S, et al. Prognosis of patients with cirrhosis and AKI who initiate RRT. Clin J Am Soc Nephrol. 2018;13(1):16–25.
Davenport A. Continuous renal replacement therapies in patients with liver disease. Semin Dial. 2009;22(2):169–72.
He GL, Feng L, Duan CY, Hu X, Zhou CJ, Cheng Y, et al. Meta-analysis of survival with the molecular adsorbent recirculating system for liver failure. Int J Clin Exp Med. 2015;8(10):17046–54.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464–70.
Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, et al. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant. 2016;16(3):758–66.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–98.
Alessandria C, Ozdogan O, Guevara M, Restuccia T, Jimenez W, Arroyo V, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41(6):1282–9.
Ruiz R, Barri YM, Jennings LW, Chinnakotla S, Goldstein RM, Levy MF, et al. Hepatorenal syndrome: a proposal for kidney after liver transplantation (KALT). Liver Transpl. 2007;13(6):838–43.
Davis CL, Feng S, Sung R, Wong F, Goodrich NP, Melton LB, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7(7):1702–9.
Martin-Llahi M, Guevara M, Torre A, Fagundes C, Restuccia T, Gilabert R, et al. Prognostic importance of the cause of renal failure in patients with cirrhosis. Gastroenterology. 2011;140(2):488–96. e4
Marik PE, Wood K, Starzl TE. The course of type 1 hepato-renal syndrome post liver transplantation. Nephrol Dial Transplant. 2006;21(2):478–82.
Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349(10):931–40.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
O’Riordan, A., Ware, T. (2022). Hepatology and the Kidney. In: Harber, M. (eds) Primer on Nephrology. Springer, Cham. https://doi.org/10.1007/978-3-030-76419-7_36
Download citation
DOI: https://doi.org/10.1007/978-3-030-76419-7_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76418-0
Online ISBN: 978-3-030-76419-7
eBook Packages: MedicineMedicine (R0)