Abstract
The most common diagnostic categories are chronic gingivitis and periodontitis. Many other conditions affect the periodontal tissues, including systemic conditions that have an oral involvement. Prognosis is a prediction of the probable outcome of a disease based on the patient’s present condition and the usual course of the disease as seen in similar situations. General prognostic factors include age, smoking and diabetes. Factors affecting individual tooth prognosis include attachment loss, probing depth, bone loss and root anatomy. A treatment plan should aim to produce a healthy and functional dentition that is aesthetically acceptable to the patient and within their physical and financial limits. Achieving a successful treatment result (PD 4 mm or less and no bleeding) will consequently improve the long-term prognosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Diagnosis
4.1.1 Terminology and Categorisation
The most common periodontal diagnoses to be made are gingivitis and periodontitis. Historically they were designated chronic (marginal) gingivitis and chronic (marginal) periodontitis. Both are chronic inflammatory conditions caused by host responses to persistent bacterial plaque challenge at and below the gingival margin. The qualifying term ‘marginal’ was used to denote the fact that the disease process started at the margin of the periodontal tissues. This is in contrast to apical periodontitis, which starts deep within the periodontal ligament due to trauma or because of non-vital pulp tissue.
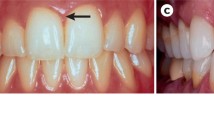
Gingivitis is inflammation of the marginal gingiva without loss of connective tissue attachment; that is, there has been no apical migration of the junctional epithelium and no bone loss (Fig. 4.1). It is therefore a reversible condition. A diagnosis of periodontitis means that there has been loss of attachment, that is, apical migration of the epithelial attachment, loss of inserting collagen fibres into the root surface and loss of bone (Fig. 4.2). Much of this loss of attachment may be irreversible, but the inflammation remains reversible. It is accepted that gingivitis is a precursor of periodontitis but that not all gingivitis progresses to periodontitis. An individual may have areas of gingivitis, periodontitis and healthy gingiva, but it is usual practice to assign the most advanced diagnostic category.
A 50-year-old patient with generalised moderate to severe (advanced) chronic periodontitis. (a) Clinical photograph of palatal view of maxillary posterior teeth showing very inflamed marginal tissue, recession and accumulation of plaque and calculus (previously subgingival). (b) Radiograph showing moderate to severe bone loss, large deposits of subgingival calculus and overhanging restoration margins. (c) Clinical charting showing moderate probing depths and recession
The clinical differentiation between gingivitis and periodontitis is especially important and is most difficult to make in the transitional stages. Clinical loss of attachment can be detected when the probe tip at the base of the crevice/pocket is contacting root surface, such that the probe tip can be felt to pass over and apical to the cement–enamel junction. Early bone loss (which follows loss of attachment) can be detected at interproximal sites on bitewing radiographs (normal distance from cement–enamel junction to bone crest is 1–2 mm). Simple features, such as whether any subgingival calculus visible on radiographs is located on the root surface or enamel, give important additional evidence as to whether attachment loss may have occurred.
By contrast, attachment loss is immediately apparent when gingival recession exposing the root surface has occurred. Gingival recession caused by toothbrush trauma in patients with thin gingivae and prominent roots should not be considered to be periodontitis even in the presence of gingival inflammation. It is a separate and distinct diagnostic entity requiring different management (see Chap. 9).
The diagnosis of periodontitis encompasses a wide range of disease entities, some of which fall neatly into specific categories whilst others do not. There have been many attempts to classify periodontal disease, resulting in changes in terminology that can lead to confusion. The disease categorisation system proposed in 1999 by the International Workshop for a Classification of Periodontal Diseases and Conditions (IWCPDC) was extremely complex and not helpful to most practitioners. The latest 2017 Classification of Periodontal Diseases has in some ways simplified the diagnostic categories but has introduced a classification of periodontitis that some may find complicated and unhelpful. It involves a staging process (disease severity) and grading process (mathematically taking account of the amount of disease divided by the age of the patient). The implementation of this classification in clinical practice has been facilitated by a publication in the British Dental Journal (BDJ 226,16-22,2019) and a flow chart at bsperio.org. We have persisted however with our simple attempt to categorise periodontitis under the section Disease Severity and provided a list of commonly recognised diagnostic entities in Table 4.1. This book has always focused and continues to focus on those conditions that are a major problem in general practice—chronic periodontitis, gingivitis and acute periodontal conditions. If a gingival condition looks unusual or the degree of periodontitis is very severe for the age of the patient, they may fall into one of the rarer categories or require systemic investigation and referral to a specialist (Fig. 4.3).
The appearance of the gingiva in this patient with pemphigoid is quite unlike that seen in gingivitis or periodontitis. The shiny red inflammation extends beyond the mucogingival junction. There is a large fluid-filled blister (bulla) in the lower incisor region that has formed following minor trauma;—this will burst to leave an ulcer like the one seen in the upper left incisor region
4.2 Disease Progression
The 2017 Classification of Periodontal Diseases proposes three grades of progression, slow, moderate and rapid. This is based upon the % bone loss at the worst affected site divided by the patient’s age. This cannot provide an accurate or indeed even helpful assessment even if it does produce a numerical value. In reality, the best you may be able to achieve is to label severe disease in a younger person as rapid, mild disease in an older patient as slow and the remainder somewhere in between as moderate!
Historically, periodontitis that appeared to be more severe for the age of the patient was given a diagnosis of “rapidly progressive” or “aggressive” periodontitis. The diagnosis of aggressive periodontitis was relatively uncommon, affecting 0.1–1% of patients depending upon the population and strictness of application of required criteria.
Although it may be helpful if there are historical data from previous examination charts or radiographs, details of any previous treatment, prediction of disease progression or stability are fraught with difficulty. As with many chronic inflammatory conditions, it is likely that periodontitis undergoes periods of activity and relative quiescence (in addition to periods of improvement and stability induced by episodes of treatment). The periods of activity may represent an increase in the amount of local inflammatory infiltrate without a change in the level of the connective tissue attachment or actual destruction of the connective tissue attachment to the root surface. Resolution of this inflammation may produce an apparent improvement in clinical attachment level, resulting from reduced penetration of the probe at the base of the pocket, but will not be due to any improvement in the actual connective tissue attachment level. Routine clinical probing measurements and radiographs are unable to detect small increments of change. It has been suggested that probing attachment level changes of 2 mm and over is required for the clinician to be more certain of progression. Small changes in probing depth may be attributable to measurement error.
Monitoring of probing depth alone will often fail to detect disease progression because apical movement of the gingival margin may accompany attachment loss at the depth of the pocket. Sophisticated radiographic techniques such as digitised subtraction radiography can detect small changes in crestal height and density, provided that the series of radiographs are strictly comparable.
There have been diagnostic test kits to detect or predict disease progression. In general they rely upon sampling individual tooth sites which are chosen as either representative of the overall periodontal status or the sites most likely to deteriorate. They are based upon detection of either bacterial species associated with periodontitis or components of inflammation (e.g. neutrophil enzymes, prostaglandins, tissue breakdown products). There is a problem of validating tests against an acceptable ‘gold standard’, which at the present time has to be clinical measurement with all its attendant inaccuracies. The cost-effectiveness, specificity and sensitivity of these tests are not good enough to recommend their use in everyday practice.
4.3 Disease Severity or Staging
It is useful to subdivide chronic periodontitis into severity/staging categories and whether the disease is localised to a few teeth or generalised. Severity can be based upon the amount of attachment loss or bone loss (Table 4.2), and it is also helpful to categorise pocket probing depths (Table 4.3).
Intraoral radiographs of a young person aged 16 years with localised severe bone loss consistent with a diagnosis of molar/incisor severe/ very severe periodontitis. This case is typical of cases previously termed localised aggressive or localised juvenile periodontitis with involvement restricted to incisors and first molars
The decision whether to describe the disease as localised or generalised is quite difficult and somewhat arbitrary. It may be decided that localised denotes that less than a third or half of the teeth are affected. The description ‘generalised’ does not have to mean that all teeth are affected, but one would expect the majority of teeth to be involved. Interestingly, the 1999 and 2017 classifications use more or less than 30% of sites involved to discriminate localised/generalised disease. In some cases only the molar teeth are involved, and this can be used as a descriptor—molar periodontitis. Application of these qualifying terms to the diagnosis allows a better description of the disease category, for example, ‘generalised moderate periodontitis’ or ‘localised severe periodontitis’.
4.4 Prognosis
In much the same way as we assign diagnoses (general and tooth specific), it is good practice to estimate prognoses for the dentition and individual teeth. Proposing a prognosis is very much an estimate or forecast and is a prediction of the probable outcome of a disease based on the patient’s present condition and the usual course of the disease as seen in similar situations. Prognosis in periodontics is commonly applied to the likely outcome with appropriate treatment, which is dependent upon:
-
Patient compliance.
-
Disease severity.
-
Clinical experience and skill in treating similar patterns/severity of disease in other patients.
Factors which affect periodontal prognosis can conveniently be divided into those that affect the overall prognosis (general factors) and those that are more tooth specific.
4.5 General Factors Affecting Prognosis
The most commonly taught general factors that affect prognosis are:
-
Age.
-
Genetics.
-
Tobacco smoking.
-
Diabetes.
-
Stress.
-
Other significant medical factors.
-
Oral hygiene, plaque and patient compliance.
-
Presence of bacterial pathogens.
4.5.1 Age
Age and disease severity are used commonly to estimate the patient’s susceptibility. It is easy to recognise that an elderly person with gingivitis has no significant susceptibility to periodontitis (see Fig. 4.1). They may be described as ‘resistant’ and the prognosis is excellent. Similarly, it is easy to categorise a young patient (say under 35 years) with moderate or severe periodontitis as being highly susceptible. Logically, severe disease in the younger patient should indicate a poor prognosis. However, in many cases, appropriate treatment of this type of patient produces a very favourable response and a high degree of stability. This type of patient may therefore be assessed following treatment as having a more favourable prognosis than initially assigned. Prediction of this type of response is difficult even for the experienced specialist periodontist.
4.5.2 Genetics
Periodontitis may have a familial basis and a significant genetic susceptibility, particularly younger patients with significant disease. Many patients, however, report a family history of periodontitis—‘my parents lost their teeth through gum disease’—and in some cases they may be aware of siblings being affected. The problem with establishing familial patterns in adult periodontitis is that it was thought to be such a common condition that a family history was almost inevitable. With overall improvements in the periodontal status of the population, it is now obvious that perhaps only 10–20% of the adult population will suffer from periodontitis that is severe enough to result in tooth loss. Far fewer individuals (0.1–1%) may be in the highly susceptible category of disease. More recent studies on identical and non-identical twins indicate that much of the susceptibility to periodontitis is genetically based. There has been a rapid growth in interest, therefore, in the potential of genetic susceptibility testing in individuals who suffer from significant levels of periodontitis. Patients should be told that there is accumulating evidence of genetic susceptibility to periodontitis but that it is not a single gene defect and that there are likely to be a large number of contributing minor genetic variations. There is interest in genetic polymorphisms in a number of genes encoding cytokine proteins (e.g. IL-1, TNF). Small variations in these genes would not compromise the overall health of the individual but may cause an excess local production of pro-inflammatory cytokines that would invoke greater tissue destruction.
In addition, genetics has to be considered in the inheritance of tooth morphology. Unfavourable root forms and short roots will adversely affect prognosis (see ‘Factors affecting individual tooth prognosis’).
4.5.3 Tobacco Smoking
Smoking is the most important environmental risk factor for periodontitis and is one that can be eliminated. In the 1950s it was first associated with acute necrotising ulcerative gingivitis and by the 1980s was firmly associated with chronic periodontitis. Early reports suggested that the main reason was the fact that smokers had poorer oral hygiene, but this is not invariably the case. Current evidence suggests that most of the increased susceptibility to periodontitis is due to the systemic effects of smoking on the inflammatory, immune and healing responses (Fig. 4.6). Nicotine is the major addictive component of tobacco and does have profound physiological effects. However, tobacco smoke contains in excess of 4000 constituents, many of which are capable of inducing direct cellular damage. Smoking is therefore a risk factor—a characteristic associated with development of disease in the first place – and an adverse prognostic factor. It is worth noting at this point that nicotine replacement therapy is a useful adjunct in quit smoking programmes and does not include the vast array of harmful constituents. The same is mostly true of e-cigarettes and vaping which have helped many people quit. The question remains as to whether the various constituents added to the vaping liquids are harmful in the long term, to both general and oral health.
Periodontal disease in smokers is characterised by:
-
More severe disease.
-
More attachment loss (may manifest as deeper pockets or more recession).
-
More bone loss.
-
More furcation involvement.
-
-
Higher rate of disease progression.
-
More tooth loss.
-
Less favourable response to treatment.
All these factors , but most importantly the last one, contribute to poorer prognosis. The poorer response to treatment (non-surgical, surgical, regenerative, mucogingival/plastic) is demonstrated by less favourable reductions in probing depth, less gain in attachment level and less alteration in bleeding response. As far as the healing response is concerned, it is well known that bone and soft tissue healing is compromised in smokers. Much of this response, however, may be conditioned by the pre-existing clinical status of the gingival tissues in heavy smokers, in that they tend to have:
-
More fibrotic gingivae.
-
Less marginal redness/bleeding.
-
Less bleeding on probing.
-
Less gingival crevicular fluid flow.
These characteristics indicate a reduction in the inflammatory response (Fig. 4.6), which is not simply due to the vasoconstrictive effect of nicotine but also to more complex effects of smoke constituents on endothelium, neutrophils, lymphocytes, etc. (Fig. 4.7). It is also possible that some individuals will have a genetic periodontal susceptibility that links to a tobacco smoking susceptibility and places them in a very high-risk category.
Clinical (a) and radiographic (b) appearance of an untreated smoker (20 cigarettes/day for 25 years) with generalised severe periodontitis. There is little obvious marginal inflammation but generalised recession. Probing depths of 6–8 mm were widespread, with less gingival bleeding than one would expect in a non-smoker
All smoking patients should therefore be made aware of the negative impact on their periodontal status, advised to quit and given the necessary support. This is dealt with in more detail in Chap. 5.
4.5.4 Diabetes
Diabetes, both type 1 and type 2 , is another important risk factor that will compromise the overall periodontal prognosis. Diabetes has wide-ranging and complex effects on metabolism, the vascular system and the immune system giving rise to poorer healing and an impaired host response. The effect on periodontitis is more of a problem in patients who have poorly controlled diabetes and who suffer from other well-known complications of this disease (e.g. retinopathy, vascular disease). In contrast to smoking, diabetic patients are more likely to exhibit an increased level of periodontal inflammation. It is also possible that severe periodontitis may compromise diabetic control. This complex relationship is dealt with in more detail in Chap. 3, ‘Periodontal disease and systemic health’.
4.5.5 Stress
Stress affects the general well-being of people and may have a negative impact on oral health care and treatment. The complex effects on the immune system may increase susceptibility to periodontitis. These important issues are dealt with in Chaps. 3 and 5.
4.5.6 Other Significant Medical Factors
There are many diseases that compromise the immune response and will affect periodontal susceptibility/prognosis. Most are relatively rare, but there are serious conditions affecting leukocytes that include:
-
Neutropenias.
-
Leukaemias (Fig. 4.8).
-
Leukocyte adhesion deficiency.
-
Lazy leukocyte syndrome .
These disease presentations are now classified as periodontal disease as a manifestation of systemic disease. Most of these individuals will be receiving appropriate medical management and treated in specialist units. Occasionally, the dentist may be the first clinician to suspect a systemic disease because of the unusual clinical presentation.
4.5.7 Oral Hygiene, Plaque and Patient Compliance
Some clinicians attempt to make a judgement of patient susceptibility based on the degree of destruction and the amount of plaque and calculus present. This is far more difficult than it would first appear because once a patient has established periodontitis, it provides an ideal environment for increased plaque growth, retention and calcification. The presence of large amounts of plaque and calculus may, however, be helpful in predicting a dramatic response in the tissues following its removal during the initial stages of treatment. It is also difficult to estimate the effect of the observed level of oral hygiene performance on prognosis at an initial diagnostic appointment, as the degree and consistency of subsequent improvement achieved by patients vary enormously. In general terms, patients who attain and maintain a good level of plaque control will respond well to treatment and have a better prognosis than those who fail to achieve it.
4.5.8 Presence of Bacterial Pathogens
This is dealt with in more detail in Chap. 2, which asserts that the types of bacteria present are probably more important than the quantity of plaque. The presence of certain pathogenic species (e.g. Porphyromonas gingivalis, Prevotella intermedia, Tannerella forsythia, Treponema denticola, Fusobacterium nucleatum) indicates higher risk, and consequent failure to eliminate them leads to a poorer prognosis. However, detection of these bacteria and antibiotic sensitivity testing are not widely available. It is expensive and labour intensive, tends to be site specific (how many and which sites should be sampled?) and has yet to be confirmed of clinical benefit in the treatment of chronic periodontitis.
4.6 Factors Affecting Individual Tooth Prognosis
It is good practice to assign a diagnosis and prognosis to individual teeth as an aid to establishing a treatment plan. It is helpful to establish prognostic categories, such as:
-
Good/favourable.
-
Questionable/doubtful.
-
Hopeless.
-
Irrational to treat.
A tooth with a good prognosis would be expected to be retained for a long period of time with routine treatment and to be kept indefinitely with appropriate treatment/maintenance. Long-term retention of a questionable tooth is very dependent on an effective treatment response and patient compliance. The ‘hopeless’ category implies that the tooth is not amenable to treatment and should be extracted, providing the patient consents to this recommendation. Early extraction of teeth considered to be ‘hopeless’ for periodontal reasons is considered when:
-
There is progression of disease to the point where there is insufficient periodontal ligament/bone support remaining.
-
The pattern of disease, combined with complex/unfavourable root anatomy, is such that the tooth is considered to be untreatable.
Formulation of a subsequent definitive treatment plan may indicate further extractions for strategic reasons or because the tooth is ‘irrational to treat’. For example, clinicians are more likely to advise loss of third or second molars because:
-
Root morphology is often unfavourable.
-
They may be the worst affected teeth.
-
Access to the tooth by clinician and patient is difficult.
-
Loss will not usually produce an aesthetic compromise.
-
There will only be a small functional compromise.
The factors presented in Table 4.4 are useful for assigning a prognosis to an individual tooth.
4.7 Treatment Planning
Having established the diagnoses and prognoses an initial treatment plan should be determined, together with definitive treatment options, for presentation to the patient. This will also be dependent on:
-
Patient motivation and ability to carry out proper plaque control.
-
Patient motivation to quit smoking.
-
Operator skill and knowledge.
-
Availability of treatment techniques.
The treatment plan should aim to produce a healthy and functional dentition that is aesthetically acceptable to the patient and within their physical and financial limits. In the majority of patients suffering from relatively early stages of periodontitis, the treatment plan will be very simple and one that you have proposed and carried out many times, for example, basic non-surgical periodontal care and replace defective restorations. By contrast, some patients with advanced periodontal disease and complex restorative problems will require a very different strategy. Figure 4.9 provides a scheme on which to base these more complex treatment planning decisions.
-
1.
Start by gathering sufficient information to arrive at valid diagnoses (e.g. recurrent caries, endodontic lesions, defective crown margins/bridges, mild/moderate/severe periodontitis).
-
2.
With the clinical information and appropriate radiographs, assign prognoses to individual teeth and an overall prognosis for the dentition.
-
(a)
Decide which teeth have a ‘hopeless’ prognosis and what form of initial replacement will be required if they are extracted (nothing, provisional denture/bridge). Consider what impact further loss of questionable teeth would have.
-
(b)
Try to visualise/define the desired end point of treatment. Consider what definitive treatments will be required to reach the desired end point and how predictable they are (e.g. periodontal surgery, root resection, fixed bridge, implant-supported prosthesis). It is helpful if there is good evidence in the literature to support the treatment modalities under consideration.
-
(a)
-
3.
Discuss with the patient his/her expectations. Provide the patient with information about the status of his/her teeth and the treatment possibilities.
-
(a)
Carry out any emergency treatment/stabilisation.
-
(b)
Decide whether any specialist opinions/treatment may be required (e.g. endodontic/prosthodontic/orthodontic).
-
(a)
-
4.
Propose one or several solutions to his/her problems. Describe advantages and disadvantages to various approaches and the estimated degree of predictability. Outline costs of various options. If necessary allow time for reflection by patient and yourself. You may wish to discuss the plans with colleagues or refer for a second opinion. The patient may wish to discuss proposals with his/her friends/relatives or seek further opinions.
-
5.
Patient decides on final treatment plan and there is agreement to proceed. Consent is obtained.
Be prepared to modify the treatment plan if circumstances change.
Further Reading
Systematic Reviews/Reviews
Albandar JM, Susin C, Hughes FJ. Manifestations of systemic diseases and conditions that affect the periodontal attachment apparatus: case definitions and diagnostic considerations. J Clin Periodontol. 2018;45(Suppl 20):S171–89.
Caton JG, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions—introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(Suppl 20):S1–8.
Lang NP, Suvan JE, Tonetti MS. Risk factor assessment tools for the prevention of periodontitis progression a systematic review. J Clin Periodontol. 2015;42(Suppl. 16):S59–70.
Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45(Suppl 20):S149–S16.
Background/Historical Literature
Fardal Ø, Grytten J, Martin J, Houlihan C, Heasman P. Using prognostic factors from case series and cohort studies to identify individuals with poor long‐term outcomes during periodontal maintenance. J Clin Periodontol. 2016;43:789–96.
Greenstein G, Caton J. Periodontal disease activity: a critical assessment. J Periodontol. 1990;61:543–52.
Huynh-Ba G, Lang NP, Tonetti MS, Salvi GE. The association of the composite IL-1 genotype with periodontitis progression and/or treatment outcomes: a systematic review. J Clin Periodontol. 2007;34:305–17.
Jeffcoat M. Radiographic methods for detection of progressive alveolar bone loss. J Periodontol. 1992;63:367–72.
Jeffcoat MK, Reddy MS. Progression of probing attachment loss in adult periodontitis. J Periodontol. 1991;62:185–9.
Lang NP, Nyman S, Senn C, Joss A. Bleeding on probing as it relates to probing pressure and gingival health. J Clin Periodontol. 1991;18:257–61.
Listgarten MA. Periodontal probing: what does it mean? J Clin Periodontol. 1980;7:165–76.
Loe H, Anerud A, Boysen H. The natural history of periodontal disease in man: prevalence, severity and extent of gingival recession. J Periodontol. 1992;63:489–95.
Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors—tobacco smoking. J Clin Periodontol. 2005;32:180–95.
Schäfer AS, Jepsen S, Loos BG. Periodontal genetics: a decade of genetic association studies mandates better study designs. J Clin Periodontol. 2011;38:103–7.
Socransky SS, Haffajee AD, Goodson JM, Lindhe J. New concepts of destructive periodontal disease. J Periodontol. 1984;11:21–32.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Palmer, R., Floyd, P. (2021). Diagnosis, Prognosis and Treatment Planning. In: Palmer, R., Floyd, P. (eds) Periodontology. BDJ Clinician’s Guides. Springer, Cham. https://doi.org/10.1007/978-3-030-76243-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-76243-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-76242-1
Online ISBN: 978-3-030-76243-8
eBook Packages: MedicineMedicine (R0)