Abstract
Interventional therapies are critical and integral parts of a multipronged strategy for pain relief and rehabilitation in patients with complex regional pain syndrome (CRPS). These therapies include intravenous drug infusion to a specific limb or as a systemic drug delivery route, sympathetic blocks, and neuromodulation modalities, such as peripheral nerve stimulation, dorsal root ganglion stimulation, spinal cord stimulation, and intrathecal drug delivery. This chapter focuses on the clinical evidence of efficacy of the interventional therapies for CRPS, as well as the essential skills in clinical practice of these interventions. There is level I evidence to support neuromodulation via dorsal root ganglion and spinal cord stimulation; level II evidence to support intrathecal drug therapy and sympathetic blocks; and substantial yet variable evidence to support peripheral nerve stimulation and intravenous therapies. It is imperative that interventional treatment is individualized, with an emphasis placed on improving quality of life and function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sympathetic block
- Spinal cord stimulation
- Dorsal root ganglion stimulation
- Peripheral nerve stimulation
- Intrathecal drug delivery
- Intravenous therapy
Introduction
Complex regional pain syndrome (CRPS) is a challenging biopsychosocial condition. As our understanding of the pathophysiology mechanisms underpinning CRPS evolves, so does therapeutic management. The optimal approach to CRPS treatment is multimodal and comprehensive. Previous chapters have discussed pharmacotherapy and physical therapy. Interventional therapies, such as nerve blocks and intravenous infusions, also have long held a role in pain relief, specifically in facilitating a patient’s participation in functional rehabilitation. This chapter discusses interventional approaches to the management of CRPS, including intravenous infusion therapies, sympathetic nerve blocks, and neuromodulation therapies. The latter approach includes peripheral nerve block and stimulation, dorsal root ganglion stimulation, spinal cord stimulation, and intrathecal drug delivery systems.
Intravenous Therapies
Compared with other interventional procedures discussed in this chapter, intravenous therapy is often less invasive and less costly. Intravenous regional blockade (IVRB) aims to block the sympathetic innervation or counter local neural inflammation in a single limb affected by CRPS.
First described in 1974, IVRB with guanethidine was performed for the treatment of CRPS type 1 [1]. The purpose of the technique was to facilitate the fixation of guanethidine to the tissue of the affected limb in order to displace norepinephrine at sympathetic nerve endings and prevent the reuptake of norepinephrine. This technique has since been utilized and explored with a variety of medications with low to moderate level evidence of support for clinical applications [2].
Description of IVRB
The patient is placed in the supine position and vital signs are monitored continuously. Sedation may or may not be utilized. A catheter is placed in a distal vein of the affected limb and another catheter is often placed in a vein of an unaffected limb for the purpose of providing sedation or resuscitation as needed. Once satisfactory intravenous access is established, the affected limb is elevated and/or elastic bandage is placed to facilitate venous drainage. A pneumatic double cuff tourniquet is used. The proximal cuff is inflated first followed by the distal cuff inflated to a pressure approximately 50–100 mmHg above the systolic blood pressure of the affected limb. After inflation, the desired solution is slowly injected into the catheter of the affected arm over about four minutes. After a period of time (typically 20–30 min), the pneumatic tourniquets are deflated intermittently and slowly over a period of five minutes to minimize adverse reactions, including dizziness, lightheadedness, and headache. Continuous monitoring of vital signs, including electrocardiogram, is maintained for about one hour after the block to detect adverse events related to any systemic absorption of the medication. Depending on the patient’s tolerance to the procedure, these treatments are often repeated every few days and/or weeks. Treatments are paired with careful evaluation of pain scores, edema, allodynia, temperature, and range of motion.
Intravenous infusion of different classes of medications, including but not limited to sympatholytic agents, anti-inflammatory medications, and N-Methyl-D-aspartate (NMDA) receptor antagonists, has been investigated as a treatment for CRPS [3]. Unlike an IVRB, an intravenous infusion does not utilize tourniquets and requires only intravenous access and continuous monitoring of vital signs.
Complications
Compared with other interventions discussed in this chapter, the IVRB technique has few major side effects but relatively frequent minor effects, including transient burning sensation with injection, nausea, dizziness , and lightheadedness, particularly after tourniquet deflation [2]. A major side effect is orthostatic hypotension requiring resuscitation and prolonged observation with continuous vital sign monitoring.
Intravenous Infusions and IVRB with Sympatholytic Agents
Animal models of CRPS have suggested a role for sympatholysis in mitigating symptoms of CRPS. One such study found a reduction in mechanical allodynia in rats with chronic post-ischemia pain after receiving both sympathetic vasoconstrictor antagonists as well as vasodilators [4]. As such, IVRB with sympatholytics, such as local anesthetics, guanethidine, clonidine, phentolamine, and beta blockers, have been investigated in patients suffering from CRPS.
Intravenous infusions of lidocaine have been studied in randomized controlled trials [5, 6]. In sixteen CRPS patients, Wallace et al. investigated the effect of various plasma concentrations of lidocaine on different thermal pain thresholds using neurosensory testing, compared with the placebo of diphenhydramine infusion [5]. They found that, at the highest studied plasma concentration of 3 mcg/ml, lidocaine significantly increased the hot pain thermal threshold in the painful area. In allodynic regions, intravenous lidocaine also produced a significantly decreased response to cool stimuli and stroking compared with placebo [5]. A separate randomized, placebo-controlled parallel study demonstrated that lidocaine at delivered concentrations of 5 mg/kg/hr was associated with significant relief of neuropathic pain compared with saline [6]. Both studies however studied the immediate effects of pain reduction and no long-term follow-up was performed.
Though initially introduced as a promising medication for IVRB in CRPS [1], recent systematic reviews have provided negative recommendations for IVRB with guanethidine based on literature evidence [2, 7]. Multiple randomized controlled trials found no significant sustained pain relief with guanethidine over placebo [2, 7,8,9,10]. Other agents explored for its potential sympatholytic mechanisms include clonidine, phenoxybenzamine, labetalol, ketanserin, and droperidol [2]. However, data supporting the use of these medications are limited and produce mixed results, thereby prompting at best a rating of 2B+ (individual cohort study or low-quality randomized controlled trials) (e.g., <80% follow-up) [2].
Intravenous Infusions and IVRB with Non-sympatholytic Agents
A number of studies found positive results in patients receiving IVRB with bisphosphonates [2]. In one prospective series of 27 patients, a single 60 mg dose of pamidronate was significantly more effective in reducing pain score, global assessment of disease severity score , and physical function at three-month follow-up compared to placebo [11]. A systematic review of four randomized trials found that bisphosphonates reduced intensity of pain at 4 and 12 weeks follow-up with rare adverse effects [12]. The bisphosphonates investigated were pamidronate [11, 13, 14], alendronate [13], and clondronate [14]. In these studies, bisphosphonate therapy (typically administered as a single dose which may or may not have been repeated over several days) was associated with reduced pain severity and swelling and increased range of motion. Furthermore, biochemical analyses found increased bone mineral content in the affected limb without significant changes in the unaffected limb [13]. While the data are limited, quality evidence seems to suggest a potential to reduce CRPS pain, particularly pain related to bone demineralization.
NMDA receptor antagonists, such as ketamine and magnesium, have also been implicated as therapeutically beneficial when administered intravenously in patients with CRPS. A recent systematic review recommended intravenous ketamine infusion as a potential therapy for patients with CRPS refractory to other interventions [2]. Significant and sustained improvement across multiple pain domains were found in a randomized, double-blind placebo-controlled trial of 19 patients with CRPS [15]. The infusion dose of ketamine utilized was 50 mg/hr up to 200 mg/4 hr session [15]. A double-blind randomized placebo-controlled study of 60 patients with CRPS type I found a significant reduction in pain scores early in the follow-up period [16]. However, by week 12, there was no difference between placebo and ketamine [16]. Furthermore, there were no functional improvements in the ketamine group [16]. In contrast, studies investigating the efficacy of intravenous magnesium have produced contradictory results, and thus, magnesium is not recommended as a therapy for CRPS [2].
A small randomized, double-blinded crossover study of ten patients with unilateral lower extremity CRPS received IVRB with lidocaine and varying doses of ketorolac [17]. Significant pain reduction was observed in the ketorolac groups. However, the statistical difference was short-lived, lasting only one day after injection [17].
Finally, intravenous immunoglobulin (IVIG) therapy has been proposed as a potential therapy for long-standing CRPS based on data suggesting the involvement of the immune system. A randomized, double-blind placebo-controlled crossover study in 12 patients found a statistically significant reduction in pain intensity up to 19 days following a one-time dose of 0.5 g/kg IVIG, compared to saline [18]. The mean decrease in pain units was 1.55 [18].
Few studies exist comparing IVRB with more invasive interventions. Nascimento et al. compared IVRB using sympatholytic agents with a sympathetic ganglion block for CRPS type I [19]. Similar pain reduction results were found between the two groups: IVRB using 70 mg lidocaine with 30 mcg clonidine versus sympathetic ganglion block using 70 mg lidocaine [19]. Reductions in pain intensity and duration were observed after the first three iterations of each type of block but both groups failed to have further improvements thereafter [19]. This suggests that, at least for some CRPS patients, IVRB is an option for short-term pain relief to facilitate physical therapy.
Sympathetic Blocks
As previously discussed in earlier chapters, perturbations in the sympathetic nervous system have been implicated as an important mechanism in CRPS. Perhaps one of the most commonly described procedures in the management of CRPS, sympathetic blocks are utilized for diagnostic and therapeutic purposes. In the ascending pathway, such blocks aim to disrupt nociceptive as well as visceral and somatic afferent fibers. In addition, blockade of sudomotor, visceromotor, and vasomotor efferent fibers may be therapeutic for symptoms of CRPS. The sympathetic blocks used for CRPS are stellate ganglion block, upper thoracic sympathetic block, and lumbar sympathetic block.
Stellate Ganglion Block for Upper Extremity CRPS
In 80% of people, the stellate ganglion, also known as the cervicothoracic ganglion, is formed by the fusion of the inferior cervical ganglion and first thoracic ganglion. It is located at the level of C7, anterior to the C7 transverse process and posterior to the vertebral vessels. The stellate ganglion is located medial to the scalene muscles and lateral to the longus coli muscle, esophagus, and trachea. It is superior to the subclavian artery [20].
The stellate ganglion block (SGB) has been performed by landmark-based technique [20] and under fluoroscopy [21], CT [22], and ultrasound guidance [23]. In this chapter, fluoroscopy guidance and ultrasound guidance will be described.
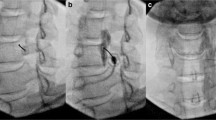
The patient is positioned supine with the head slightly hyperextended and rotated to the contralateral side. The C6–C7 level is identified by fluoroscopy in the anterior-posterior (AP) view. After sterile preparation and subcutaneous infiltration with a local anesthetic, a needle is inserted at the junction of the transverse process and corresponding C6 or C7 vertebral body. Contact is made with bone and an oblique view is obtained by fluoroscopy to assess needle position and ensure it is anterior to the intervertebral foramen (Fig. 9.1a). Once the needle position is adequate, 0.5 –1 ml of contrast dye is injected to confirm the correct needle tip position and to prevent intravascular or another off-target injection. The contrast dye should spread over the prevertebral sympathetic chain at C6-T1 (Fig. 9.1b). Thereafter, SGB is performed with injection of local anesthetic (often, 1% lidocaine or 0.25% bupivacaine) or a combination of local anesthetic and steroid (dexamethasone 10 mg, for instance) to prolong the blockade (Fig. 9.1c). When clinically indicated, neurolysis of the stellate ganglion can be performed using radiofrequency ablation. The specific radiofrequency protocol may differ between institutions.
Stellate ganglion block under fluoroscopy (a–c) or ultrasound (d–e) guidance. (a) The needle is placed at the base of the C6 transverse process in the oblique view. (b) In the anterior-posterior (AP) view, contrast is injected via extension tubing and spreads cephalad and caudad from C6. (c) Injection of local anesthetic and steroid in the AP view. (d) Ultrasound image of the stellate ganglion and its surrounding structures. (e) Needle and injectate targeting the stellate ganglion
In contrast to fluoroscopy, ultrasound imaging aims to identify the prevertebral fascia and allow for the precise deposition of local anesthetic just deep to the prevertebral fascia [23]. The sympathetic chain can be found between the longus colli muscle and longus capitis muscle (Fig. 9.1d–e). Proponents of this approach argue that ultrasound guidance increases the specificity of the procedure in blocking the sympathetic chain alone.
With the guidance of fluoroscopy or ultrasound, inadvertent injury to or injection of medications into nearby structures (i.e., vertebral artery, inferior thyroid vessels, carotid artery, vagus nerve , cervical nerve roots) may be avoided. It is noteworthy that simultaneous bilateral SGB should be avoided because it may compromise breathing by paralyzing the recurrent laryngeal nerves and the vocal cords, leading to airway obstruction. Recurrent laryngeal and phrenic nerve blocks are frequent side effects of SGB, due to local anesthetic diffusion from the area of the ganglion. Because diffusion of drug is required to obtain a satisfactory block, it is expected that these nerves will often be temporarily blocked.
Thoracic Sympathetic Block for Upper Extremity CRPS
While SGB has been frequently utilized for severe upper extremity pain, studies have found that SGB alone does not achieve sufficient sympatholysis [24]. In some patients, this is due to the direct projection of thoracic sympathetic ganglia to the brachial plexus, bypassing the cervical or stellate ganglia [25]. At the start of the procedure, the patient is placed in the prone position. The skin is infiltrated with a local anesthetic solution and prepared with disinfectant. Under fluoroscopic guidance [26], the spinal needle is inserted into the skin and advanced to the posterior third of the T2 vertebra. Contrast dye is injected and correct positioning is confirmed if the dye outlines the prevertebral sympathetic chain at T1–3. Thereafter, a local anesthetic solution is injected into the T2 sympathetic ganglion (Fig. 9.2).
More recently, the thoracic paravertebral block as an approach to achieving thoracic sympathetic blockade has been described and studied [27]. In comparison to SGB, a T2 paravertebral block significantly increased the incidence of temperature increase by at least 1.5 °C (primary outcome). Additionally, numeric rating scale scores were found to be significantly lower and satisfaction and block duration significantly higher in the paravertebral block group, compared with the group receiving SGB. However, only 20% of patients receiving SGB achieved the primary outcome of increasing the limb temperature of 1.5 °C, raising the question of whether the SGB was properly performed. In addition, the technical difference between the paravertebral block and the traditional thoracic sympathetic block (described above) was not clearly defined and the depth of the needle tip in the paravertebral block group was not clearly described. It is possible that the paravertebral block was in fact performed in a similar manner as the traditional thoracic sympathetic block. The use of a large volume of injectate (10 ml) for the paravertebral block further increased this possibility. Therefore, it remains to be determined whether the typical paravertebral block approach with the tip of the needle posterior to the posterior spinal line in lateral view is sufficient to achieve thoracic sympathetic block and therefore to replace the traditional thoracic sympathetic block approach (tip at the posterior third of the T2 vertebral body on lateral view). The significance of this difference is that the risk of pneumothorax associated with the traditional thoracic sympathetic block approach due to the unique anatomical features of the upper thoracic spine can be reduced by adopting the paravertebral block approach. Theoretically, T2 paravertebral block should be sufficient to block the upper thoracic sympathetic chain.
Lumbar Sympathetic Block for Lower Extremity CRPS
The lumbar sympathetic ganglia, the convergence of pre- and post- ganglionic fibers, are located at the anterolateral side of the lumbar vertebrae. The lumbar sympathetic block is performed under image guidance . Given the ease of use and efficiency, fluoroscopy, compared with CT and MRI, is most frequently utilized. More recently, ultrasound has emerged as a valuable tool [28].
Under fluoroscopic AP view, the L2–4 levels are identified with the patient in the prone position. Using sterile technique, the skin is infiltrated with local anesthetic and then a needle is advanced toward the anterolateral edge of the target L2 or L3 lumbar vertebra (Fig. 9.3a). The lateral view is then obtained to confirm the needle tip in the anterior two-thirds of the target lumbar vertebra . The needle is further advanced to the anterolateral margin of the vertebral body with the final position confirmed on all three standard views (AP, lateral, oblique) and with contrast dye injection. The contrast dye should outline over the prevertebral sympathetic chain at L2–4 (Fig. 9.3b). Finally, a local anesthetic is injected. A local anesthetic blockade may be followed up, if clinically indicated, with a more definitive block using radiofrequency ablation or neurolysis with phenol. The use of botulinum toxin has also been reported to prolong the blockade and therapeutic effects [29].
Outcomes
The efficacy of sympathetic blocks has undergone the scrutiny by the Cochrane Collaboration and its most recent systematic review was conducted in 2016. This Cochrane analysis considered randomized controlled trials that examined the outcomes of sympathetic blockade with local anesthetics in patients with CRPS compared to placebo versus no treatment versus alternative treatments [30]. At the time of the analysis in September 2015, a total of 12 studies were included with a combined patient population of 461 [30]. Despite a few studies reporting pain relief following either SGB or lumbar sympathetic blockade, taken as a whole, authors determined the level of evidence to be limited, low quality and sometimes conflicting, and concluded that sympathetic blockade has yet to be demonstrated superior to placebo in reducing pain in the long-term [30].
A recent cohort study of 225 patients in 2019 shed new light on the efficacy of sympathetic blocks in CRPS [31]. Many studies utilize an immediate increase in skin temperature (of at least 1.5 degrees Celsius) as a measure of completeness of the sympathetic block [24, 32]. In addition to skin temperature changes , a number of studies have investigated the degree and duration of pain relief associated with sympathetic blockade in the treatment of CRPS. The most recent retrospective cohort study found that 61% of its patients with CRPS had a greater than 50% pain reduction [31]. A majority of those experiencing pain relief reported a duration of relief 1–4+ weeks [31]. In contrast to conventional thought, this study also found no significant association between pre-procedure temperatures of the affected extremity and the pain reduction of sympathetic blockade , suggesting that temperatures were not predictive of successful outcome [31]. In addition, the study found that there was no difference in the success rate of spinal cord stimulation trials between patients with or without more than 50% pain relief after sympathetic blocks. It was concluded that sympathetic blocks may be therapeutic in patients with CRPS regardless of pre-procedure limb temperatures and that the effects of sympathetic blocks do not predict the success of spinal cord stimulation [31]. This study provided level II evidence in support of sympathetic blocks for CRPS in select patients [31].
Neuromodulation Therapies
Neuromodulation typically involves the implantation of a device to achieve long-term therapeutic benefit. Overlapping principles include basic indications, absolute contraindications, and preoperative considerations. The use of peripheral nerve block and/or stimulation, dorsal root ganglion (DRG) stimulation , spinal cord stimulation (SCS), and intrathecal drug delivery systems is warranted in patients with persistent CRPS symptoms despite reasonable attempts at conservative management with medication use and physical rehabilitation. While many would argue that neuromodulation should be considered sooner rather than later in a patient’s disease course to achieve longer lasting benefit, it is generally accepted that physical therapy and a trial of pharmacologic agents, including topical and oral agents, is a starting point.
Contraindications vary with each procedure. However, absolute contraindications most often include the following : preexisting infection at operative site, bacteremia and septicemia, hemodynamic instability, therapeutic anticoagulation without the ability to hold anticoagulants, allergy to procedure medications, and patient refusal.
Preoperative Considerations
Patient selection is key to the success of neuromodulation. Preoperative evaluation of the patient begins with a comprehensive history and physical exam, including a thorough review of relevant medical and psychiatric comorbidities. Prior to trial and permanent implantation of stimulators and intrathecal drug delivery systems , all patients are evaluated by a clinical psychologist/psychiatrist to identify factors that may lead to therapeutic failure, to address cognitive and behavioral concerns, and to set proper expectations for and from the patients.
Specific cardiopulmonary comorbidities will influence the sedation management and positioning of the patient during the procedure . Severe immunodeficiencies, including those caused by chemotherapy, may preclude implantation of permanent devices due to increased risk for infection. A careful review of medications is required and a coordinated plan must be made regarding the safety of withholding anticoagulant medications immediately prior to and after a procedure. Permanent implantation procedures require a dose of perioperative antibiotics to prevent surgical site infections. Choice of antibiotic will depend on the patient’s allergies though standard of care is usually a cephalosporin for adequate skin and soft tissue flora coverage. In addition to antibiotic use, proper sterile attire and surgical site skin prep with sterile drape are instrumental. All procedures should occur under continuous vital sign monitoring by a clinician and when appropriate, intravenous access should be established to permit resuscitation as needed by the clinician. When sedation is delivered, supplemental oxygen and noninvasive bag-valve-mask devices should also be available. Intubation is rarely needed for neuromodulation device implant surgeries.
Peripheral Nerve Block and Stimulation
While several frameworks have been put forward to elucidate pain and its origins [33], the mechanism most frequently cited as the rationale behind the use of electrical stimulation is the gate control theory, first described by Melzack and Wall in 1965 [34]. Gate control theory proposes that non-painful sensory input, via large-diameter sensory fibers, closes the “gates” in the spinal cord dorsal horn laminae, thereby preventing transmission of painful input via small-diameter fibers. Thus, the patient would experience less pain. This theory provides a physiological explanation for how nociception may be modified by non-nociceptive stimulation, for example, rubbing or massaging a painful site. However, the true mechanisms of neuromodulation remain to be determined and are likely related to modulation of the conduction, transmission, and perception of pain signals, as well as processes involving non-neuronal cells in the spinal cord that contribute to central sensitization and chronification of pain. Neuromodulation techniques may alter the neurochemical components of the dorsal horn with a decrease in the excitatory neurotransmitters , aspartate, and glutamate, and an increase in the levels of inhibitory neurotransmitters, GABA, and glycine. Based on the above-described principle, techniques for electrical stimulation at both the peripheral and central nervous systems have been developed. We will begin our discussion with peripheral nerve stimulation.
Peripheral nerve stimulation (PNS) is the direct electrical stimulation of nerves outside of the central neuroaxis , such as the median nerve. Distinct from SCS, PNS aims to directly inhibit primary pain afferents, thereby replacing the pain experience with a more pleasant paresthesia.
Indication
The following patient selection criteria have been used for the consideration of PNS in CRPS, along with other chronic pain conditions [35]:
-
1.
Pain within a sensory distribution of a single peripheral nerve.
-
2.
Positive diagnostic peripheral nerve block.
-
3.
Exclusion of nerve entrapment neuropathies.
-
4.
Patient is free of major psychological or psychiatric disease.
Procedure
A PNS may be implanted percutaneously or under direct visualization. This chapter will discuss the implantation of PNS in a percutaneous fashion using ultrasound technology and a 14-gauge or 17-gauge needle. Equipment for the implantation consists of (1) an implantable PNS electrode with 8–16 contacts and (2) a pulse generator (battery), either implanted or external.
Positioning of the patient will depend on the target nerve, and repositioning during the surgery may be necessary . The patient’s skin is prepared in sterile fashion and relevant structures are identified using either ultrasound or fluoroscopy. Once the nerve is located, the skin is infiltrated with local anesthetic.
Similar to procedures for other electrical stimulation or devices, the implantation of a PNS is typically a two-stage procedure. The first stage trials the efficacy of electrical stimulation for pain relief via temporary implantation of an electrode near the target nerve. Next, the electrode is sutured in place and then connected to a temporary power source. The patient will then test the temporary peripheral nerve stimulator and assess for symptomatic pain relief. The patient will move ahead with the second stage if the relief is adequate. The temporary electrode will be explanted and replaced with a permanent electrode and typically with an implantable pulse generator (IPG) in a subcutaneous pocket. The permanent electrodes are anchored to the fascia with nonabsorbable suture. The implantable systems may last for up to 10 years or more (Fig. 9.4). More recently, research in PNS has produced devices that allow for pulse generators to communicate wirelessly to the in-situ electrode, thereby avoiding a second incision and foreign body. When a wireless system by Bioness or Stimwave is used, pulse generator implantation is not necessary. In the case of using the PNS system by SPRINT, the electrode is placed near the target nerve under ultrasound guidance and an external pulse generator is connected to the electrode. After about 60 days, the system is removed without incision.
Outcomes
Overall, there is little data on the long-term efficacy of PNS in the treatment of CRPS. One prospective study examined the efficacy of surgically placed plate-type electrodes on affected nerves in 30 patients [36]. About 63% of this cohort reported good or fair relief over a period of 2–4 years with an average reduction in pain from 8.3+/− 0.3 preimplantation to 3.5+/−0.4 at follow-up on a pain scale of 10. The authors of this study also report improvement in functional activity. One case report found success with peripheral median nerve stimulation for CRPS following multiple carpal tunnel release surgeries [37]. At 36 months, this patient reported good pain relief without the need for additional analgesics [37]. Thus, PNS has the potential to deliver focused stimulation to the target nerve that innervates the painful region of CRPS.
Complications
Potential complications include infection at the surgical site, PNS lead migration or tip erosion requiring explantation , hardware malfunction, pain over device, and tolerance/habituation to stimulation.
Spinal Cord Stimulation
For patients who do not respond to noninvasive conservative therapy, spinal cord stimulation (SCS) may be considered as an effective intervention. Traditionally, providers have utilized a multimodal approach centered on noninvasive therapy, including rehabilitation and analgesics . SCS may be considered an escalation of care and reserved for non-responders. More recent data suggest that delays in more definitive therapy may be associated with poorer outcomes, including limited improvements in functional status and mental health [38], and thus warrant earlier consideration of SCS in the care of patients with CRPS. Although the dorsal root ganglia (DRG) are part of the peripheral nervous system from an anatomical perspective, DRG stimulation is generally accepted as a form of SCS for regulatory and other reasons and is therefore discussed here in light of level I evidence for CRPS.
SCS has been utilized in a number of chronic pain syndromes, most commonly for CRPS and failed back surgery syndrome [39]. As a reversible intervention, SCS is programmed to deliver low voltage electrical stimulation to decrease pain sensation through implanted leads in the epidural space (Fig. 9.5). Classically, the gate control theory of pain proposed that pain relief arose from competitive inhibition of impulses from nociceptive neurons by SCS-mediated activation of large sensory nerve fibers. Neurophysiology studies in animal models of neuropathic pain have suggested potential biochemical bases for analgesia [40,41,42]. Electrical stimulation of dorsal columns has been associated with increased GABAergic activity and decreased release of glutamate and aspartate in the dorsal horn. As the latter are excitatory amino acids, it is thought that electrical stimulation mitigates nociceptive transmission via dampened excitatory activity [40]. Recordings of neuronal units in the dorsal horn in cats suggested inhibitory action in the dorsal horn via interneurons in or near the substantia gelatinosa [41].
Procedure
SCS therapy consists of two stages: the trial phase followed by permanent implantation should the trial be successful. Trials typically occur in the clinic setting, where under fluoroscopy, temporary electrodes are introduced into the epidural space in the cervical or thoracic region for upper or low extremity CRPS. First, the patient is positioned prone and standard monitors are applied. Sterile preparation is performed and the skin is infiltrated with local anesthesia. Under direct fluoroscopy, a Tuohy needle is introduced into the epidural space. The electrode is advanced until the tip is at the desired location . For treatment of upper extremity CRPS, the target is typically the superior aspect of the C4 vertebral body (Fig. 9.6). The T9–T12 vertebral bodies are typically targeted for the treatment of lower extremity CRPS (Fig. 9.7). For DRG stimulation, leads are placed in the lateral epidural space near the target DRG at levels from T10 to S2 for CRPS in the lower extremities, depending on the dermatomal target corresponding to the patient’s primary region of pain (Fig. 9.8). A special introducer is used to guide the placement of DRG leads, in addition to the needle for epidural access. Depending on the anatomical target, up to 16 contacts can be placed for SCS or DRG stimulation.
Cervical spinal cord stimulator implantation under fluoroscopy for upper extremity CRPS type I. Needle entry at T2–3 with tips of the leads at C4. (a) AP view of dual lead placement, one in the lateral posterior epidural space and the other in the mid-lateral posterior epidural space. C5 is denoted in the image. (b) Lateral view of lead placement in the posterior epidural space
Intraoperative testing to determine stimulation overlap with subjects’ painful areas is conducted during implantation. Of note, there is no need for intraoperative testing if HF10 (high-frequency (10 kHz) stimulation) by Nevro is used as it is a paresthesia-free mode of stimulation. Depending on the technology, the patient may or may not experience paresthesia in the area covering pain when the electrode is activated. Following satisfactory placement, the Tuohy needle (and introducer for DRG leads) is withdrawn and an external stimulator is connected to the trial electrode. The patient is instructed on how to proceed with the trial stimulation at home over the next 5–10 days. A successful trial is often defined as a 50% or greater reduction in pain.
For the permanent implantation, the patient presents to an operating room where either monitored anesthesia care or general anesthesia is induced. The patient is positioned prone and prepared using the sterile technique. Occasionally, the patient may be positioned in the lateral decubitus position. Fluoroscopy is used to mark anatomical landmarks and decision as to which level of interlaminar space to access is made by the operator. Prior to incision, a local anesthetic is infiltrated into the skin and subcutaneous tissue. A midline longitudinal incision is made and dissection down to the fascia and supraspinous ligament is performed with careful attention to hemostasis using electrocautery . The operator may continue to inject local anesthetic along the desired path of the Tuohy needle. The epidural space is then accessed using the paramedian approach with a Tuohy needle, through which, an electrode is introduced into the epidural space. Both leads may be placed at the same interlaminar space using a right and left paramedian approach. Under live fluoroscopy guidance, one lead at a time is advanced cephalad until the tip of the electrode is at a satisfactory position. As each lead contains a wired stylet, some degree of lead steering is possible (Figs. 9.6a and 9.7a). A lateral fluoroscopy view may then be obtained to ensure that both leads are in the posterior epidural space (Figs. 9.6b and 9.7b). Both leads are then fixed using small anchors into the deep fascial tissue with further securement using nonabsorbable sutures. After fixation, another fluoroscopic image is obtained to ensure that the leads had not migrated during the fixation process. With the help of an introducer, DRG leads are placed in the lateral epidural space near the target DRG. Fluoroscopy images are taken to confirm lead location underneath the pedicle of respective vertebra in AP view and in the foramen in the lateral view (Fig. 9.9). The introducing sheath is then retracted back to the epidural space and an S-shape curve of the lead is made in the epidural space to relieve the strain. The epidural needle and the introducer are removed without dislodging the electrode.
SCS electrodes can also be placed surgically through laminectomy. In such cases, paddle leads are used for stability to prevent lead migration. This approach can be advantageous for lead placement in the cervical region, where lead migration is more common. A disadvantage of paddle lead is that revision/replacement can be more challenging when lead fracture occurs.
The IPG is then implanted by a single incision. The location of the IPG is typically at the buttock for both thoracic and cervical stimulation. There are occasions when the IPG may be placed in the subclavicular area or mid-axillary line for cervical leads or abdominal wall for thoracic leads. After an appropriately sized pocket is created, the generator is inserted and the leads carefully tunneled from the anchor site to the pocket using a tunneling device. The leads are then connected to the generator. The IPG is secured with nonabsorbable sutures to the subcutaneous fascia. Once again, fluoroscopic images are obtained to confirm lead positioning. Fascia and skin are meticulously closed. The patient emerges and is brought to the recovery unit.
Complications
Potential device-related complications of SCS implantation include lead migration, lead fracture, and IPG dysfunction. Biological complications include epidural hematoma, spinal cord or peripheral nerve injury, postdural puncture headaches , surgical site infection, and pain at the pocket of the IPG. Most commonly reported side effects include paresthesia in other locations and pain or irritation from the leads or IPG [43].
Outcomes
Recent studies have provided level I evidence to support DRG stimulation for CRPS [44]. The efficacy of SCS for CRPS has been demonstrated by numerous case reports, few randomized controlled trials , and systematic reviews. The first prospective randomized controlled trial compared two arms: physical therapy (PT) only versus SCS + PT [45]. Patients enrolled in the trial had CRPS involving either an upper or lower extremity for at least 6 months. Those randomized to the SCS + PT arm only received a permanent implantation if the trial was successful, defined as a reduction in pain intensity by at least 50% prior to randomization or if the patient rated the global perceived effect of treatment as at least a 6 (“much improved”) on a 7-point scale. Of the 36 patients who received a trial, 24 moved on to receive a permanent implantation. In an intention-to-treat analysis, the SCS + PT group had a statistically and clinically significant reduction in pain intensity at six months compared with the PT group. Those actually receiving the implantation also reported an improvement in health-related quality of life. These effects were maintained at two-year follow-up in a subsequent study [43]. By five years, pain scores were similar among the two arms however 95% of the patients who had received an SCS implantation reported that they would repeat treatment for the same results [46]. A prospective case series of 19 patients at two centers found significant improvement in pain level (Visual analog scale scores, McGill Pain Rating Index) and in sickness impact profile [47]. A systematic review including the aforementioned randomized controlled trial as well as 25 case studies and one cost-effectiveness study found level I evidence for SCS as an effective intervention for CRPS [48]. A more recent systematic review included a total of 19 studies and found high-level evidence for the use of SCS for CRPS with respect to outcomes of perceived pain relief, pain score improvement, quality of life, and patient satisfaction [49].
Intrathecal Drug Delivery Therapy
Pain management through intrathecal delivery systems began as early as the 1980s and was approved by the U.S. Food and Drug Administration in 1991. Implantable intrathecal systems are used for malignant and non-malignant chronic pain refractory to medical therapy including failed back surgery syndrome, spinal cord injury-induced spasticity, CRPS, and chronic pancreatitis . The goal is to provide a targeted approach to drug delivery, which is especially beneficial to those patients who have been dose limited by medication side effects. Similar to other interventional procedures, absolute contraindications include anticoagulation with the inability to discontinue anticoagulants, coagulopathies, cerebrospinal fluid outflow obstruction, intracranial hypertension, and systemic infection or infection at the site of insertion . Medication choice varies and depends in part on the mechanism of pain. Several medications have been studied, including opioids, baclofen, local anesthetics, clonidine, glycine, and ziconotide.
Procedure
A basic intrathecal drug delivery system consists of 1) indwelling catheter, 2) implanted pump containing a reservoir of drug, and 3) external controller. The catheter is placed percutaneously into the intrathecal space, and the implanted pump is most often placed in a subcutaneous pocket in the abdomen.
Prior to permanent implantation, the patient undergoes a trial to determine whether or not medications delivered intrathecally would alleviate pain. Such trials may take many forms, including single or repeated injections of medication into the intrathecal space and/or an inpatient trial of continuous infusion via intrathecal catheter.
For the permanent implantation of an intrathecal pump, the patient is brought to the operating room. The procedure can be performed under general anesthesia, regional anesthesia, or local anesthesia with sedation. The advantage of regional and local anesthesia is the ability of the patient to provide any direct feedback during implantation, thereby potentially preventing nerve injury. Following satisfactory induction of anesthesia, the patient is placed in the lateral decubitus position and prepared in a sterile fashion. Using fluoroscopy, the L3–4 intervertebral space is identified. A 3- to 4-cm vertical skin incision is made over the L3–4 space and dissection is performed from skin to lumbodorsal fascia, taking care to ensure hemostasis with electrocautery . Blunt dissection is then used to extend laterally along the lumbodorsal fascia plane to create space for excess catheter length and the anchor. Using a paramedian approach and under fluoroscopic guidance, a 14-gauge Tuohy needle is advanced toward the intrathecal space (Fig. 9.10a). Access to the space is confirmed radiographically and with return of cerebral spinal fluid (CSF) (Fig. 9.10b). After stylet removal, the catheter is inserted into the Tuohy needle and advanced to the desired vertebral level under fluoroscopy. A guidewire in the catheter facilitates cephalad advancement. After satisfactory positioning of the catheter dorsal to the spinal cord, a purse-string nonabsorbable suture is made around the Tuohy needle. The Tuohy needle and catheter guidewire are then removed carefully without retraction or shearing of the catheter. The purse-string suture is tightened to prevent a CSF leak. The catheter position is verified with fluoroscopy before anchoring to the lumbodorsal fascia with a small anchoring device which accompanies the pump.
Intrathecal pump implant under fluoroscopy. (a) Catheter placement through a spinal needle entry at L2–3 in AP view. (b) Lateral view showing the tip of the catheter (white arrow) at T9 in the intrathecal space just behind the spinal cord. (c) Pump placement within the right abdominal wall in AP view
Once the catheter is secure, attention is then turned to creating a pocket for the intrathecal pump. Prior to presenting to the operating room, the physician identifies and marks an ideal position on the patient’s abdomen , taking care to avoid the belt line and the costal margin. An 8 cm horizontal incision is made and then dissected to a depth of 1.5 cm. This incision represents the middle of the pocket. With careful blunt dissection, a pocket is then created which approximates the size of the pump. The intrathecal catheter is then tunneled laterally to the pump pocket using a tunneling device. Four non-absorbable sutures are made at the four corners of the pocket. The pump, which had been filled and primed with medication, is connected to the pump and inserted into the pocket. The pump is secured to the external abdominal fascia by tightening the four sutures (Fig. 9.10c). All incisions are then irrigated and closed.
Complications
Immediately after the procedure, complications may include CSF leak and/or postdural puncture headache. In the days to weeks to years following implantation, potential complications are hematoma, seroma, or infection surrounding the implant. Erosion through the skin may also occur . Device-related complications include displacement, kink, or fractures of the catheter, as well as pump failure. Medication-related complications vary with the type of medication selected for the intrathecal drug delivery system. For example, though less frequent than oral or intravenous delivery of opioids, intrathecal opioids can still lead to respiratory depression, sedation, and constipation. Other complications include the formation of inflammatory mass around the tip of the catheter and possible overdose or withdrawal due to malfunction of the pump or human error during medication preparation or refill.
Pharmacologic Agents
A variety of studies have examined many different types of medications and combinations of medications for intrathecal pharmacologic management of chronic pain. At present, only three medications are approved by the FDA for use in intrathecal pumps: morphine, baclofen, and ziconotide. For the treatment of CRPS, studies have examined the efficacy and safety of clonidine and adenosine [50], baclofen [51, 52], methylprednisolone [53], glycine [54], bupivacaine [55], opioids [56], and ziconotide [57]. Combination of medications has also been studied, such as opiate plus local anesthetic [58].
The Polyanalgesic Consensus Conference (PACC) panel of experts have developed guidelines based on current research, with the most recent update published in 2017 [59]. This panel presented best practices for the use of intrathecal infusion of medications to treat patients with chronic refractory pain, including CRPS. Various treatment statements were ranked by the quality of the evidence, degree of recommendation, and strength of consensus among the panel members.
Through this systematic methodology, the conclusion that intrathecal clonidine decreases pain scores, allodynia, hyperalgesia, and mean arterial blood pressure in CRPS patients was determined to have high-quality evidence, strong recommendation, and strong consensus amongst panel members [44]. In their secondary analysis, investigators found a significant decrease in pain scores over time with intrathecal clonidine infusion [50]. Clonidine is an alpha2 adrenergic agonist and has been found in basic research to inhibit the activation of glial cells, ultimately inhibiting the production of proinflammatory cytokines [60].
Ziconotide, an antagonist of presynaptic N-type calcium channels in the dorsal horn of the spinal cord [61], is a first-line therapy and FDA approved for the intrathecal management of neuropathic and nociceptive pain . The PACC strongly recommends with high-quality evidence that intrathecal therapy with ziconotide be utilized for cancer- and noncancer-related pain [59]. In a series of patients with CRPS, pain scores as well as edema, skin abnormalities, and mobility were found to be markedly improved with ziconotide therapy [57].
Baclofen is also highly recommended but specifically for the indication of spasticity associated with chronic pain [59]. A single-blind , placebo-run-in, dose-escalation study in 36 CRPS patients found significant improvement in dystonia scores, pain disability, and quality of life at 12-month follow-up after implantation of an intrathecal pump administering continuous baclofen [62]. Forty-two patients with CRPS and dystonia symptoms received baclofen via intrathecal pump and investigators found a significant improvement in multiple dimensions of pain, including global intense pain, sharp pain , dull pain, and deep pain [63]. Unlike the symptom of dystonia, however, the degree of pain improvement did seem to plateau after about 6 months of follow-up [63]. A randomized-controlled, double-blind crossover study found no differences in fast versus slower infusion rates of baclofen on dystonia and pain [52]. In fact, there was an increase in adverse events with a faster infusion rate, which included headache, drowsiness, short-term amnesia, and light-headedness [52].
Local anesthetics, steroids, and other medications have been studied. A randomized, double-blind placebo-controlled cross-over study found no improvement in pain or dystonia with intrathecal glycine in CRPS patients [54]. Similarly, methylprednisolone, delivered as a single 60 mg dose, was ineffective in improving pain intensity in a double-blind, randomized controlled trial of 20 CRPS patients [53]. Intrathecal bupivacaine monotherapy was trialed in a woman with CRPS of her lower extremity, whose condition was refractory to local blocks and SCS [55]. Initial trials found intrathecal morphine to offer minimal relief [55]. Clonidine was trialed thereafter and found to provide excellent pain relief for several days however was limited by significant adverse events, including headaches, weakness, and hypotension [55]. A trial of bupivacaine ensued and produced complete pain relief with minimal perineal anesthesia and extremity motor block at an infusion of 3 mg/day with additional self-administered boluses [55].
Intrathecal opioids have been utilized for pain management for as long as intrathecal drug therapy has been approved. Compared with systemically delivered opioids, intrathecal opioids typically confer the advantage of fewer side effects. An early prospective series of 15 patients with reflex sympathetic dystrophy following spinal surgery found excellent pain relief in a little more than half of its patients and good-to-fair pain relief in the remaining study population over a 44-month follow-up period [56]. The studies discussed thus far tracked patients, on average, over a 1- to 2-year time frame. Herring et al. sought to better understand the long-term outcomes of intrathecal drug delivery systems in patients with CRPS at a single institution who had at least four years of continuous follow-up [64]. They found that intrathecal opioid dose was not associated with long-term decreases in oral opioid consumption; ziconotide was associated with a decrease in oral opioid intake over the four-year follow-up; and bupivacaine was associated with an increase in oral opioid intake [64].
Concluding Remarks
In summary, interventional therapy is a critical component of multidisciplinary and multimodal management of CRPS, particularly for refractory cases. There is level I evidence to support DRG stimulation and SCS, level II evidence to support intrathecal drug therapy and sympathetic blocks, and substantial and variable evidence to support PNS and intravenous therapies with specific treatment regimens. It is important to emphasize a comprehensive and holistic approach to the management of CRPS based on the biopsychosocial model of patient-centered care. When interventional therapies are indicated, it is essential for practicing physicians to have the training and competence to appropriately select suitable candidates, proficiently perform the procedures, closely monitor patients’ outcomes, and promptly identify and manage potential complications.
References
Hannington-Kiff JG. Intravenous regional sympathetic block with guanethidine. Lancet. 1974;1(7865):1019–20.
Xu J, Yang J, Lin P, Rosenquist E, Cheng J. Intravenous therapies for complex regional pain syndrome. Anesth Analg. 2016;122(3):843–56.
Xu J, Herndon C, Anderson S, Getson P, Foorsov V, Harbut RE, Moskovitz P, Harden RN. Intravenous ketamine infusion for complex regional pain syndrome: survey, consensus, and a reference protocol. Pain Med. 2019;20(2):323–34.
Xanthos DN, Coderre TJ. Sympathetic vasoconstrictor antagonism and vasodilatation relieve mechanical allodynia in rats with chronic Postischemia pain. J Pain. 2008;9(5):423–33.
Wallace MS, Ridgeway BM, Leung AY, Gerayli A, Yaksh TL. Concentration-effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000;92(1):75–83.
Tremont-Lukats IW, Hutson PR, Backonja M-M. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22(3):266–71.
van Eijs F, Stanton-Hicks M, Van Zundert J, Faber CG, Lubenow TR, Mekhail N, van Kleef M, Huygen F. 16. Complex regional pain syndrome. Pain Pract. 2010;11(1):70–87.
Ramamurthy S, Hoffman J. Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia: a randomized, double-blind study. Guanethidine Study Group Anesth Analg. 1995;81(4):718–23.
Jadad AR, Carroll D, Glynn CJ, McQuay HJ. Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double-blind crossover study. J Pain Symptom Manag. 1995;10(1):13–20.
Livingstone JA, Atkins RM. Intravenous regional guanethidine blockade in the treatment of post-traumatic complex regional pain syndrome type 1 (algodystrophy) of the hand. J Bone Joint Surg Br. British Editorial Society of Bone and Joint Surgery. 2002;84(3):380–6.
Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004;5(3):276–80.
Brunner F, Schmid A, Kissling R, Held U, Bachmann LM. Biphosphonates for the therapy of complex regional pain syndrome I – Systematic review. Eur J Pain. European Federation of Chapters of the International Association for the Study of Pain. 2009;13(1):17–21.
Adami S, Fossaluzza V, Gatti D, Fracassi E, Braga V. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis. BMJ Publishing Group Ltd. 1997;56(3):201–4.
Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevilacqua M, Bettica P, Sinigaglia L. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27(6):1477–83.
Schwartzman RJ, Alexander GM, Grothusen JR, Paylor T, Reichenberger E, Perreault M. Outpatient intravenous ketamine for the treatment of complex regional pain syndrome: a double-blind placebo controlled study. Pain Interl Assoc Stud Pain. 2009;147(1–3):107–15.
Sigtermans MJ, van Hilten JJ, Bauer MCR, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain Inter Assoc Stud Pain. 2009;145(3):304–11.
Eckmann MS, Ramamurthy S, Griffin JG. Intravenous regional ketorolac and lidocaine in the treatment of complex regional pain syndrome of the lower extremity: a randomized, double-blinded, crossover study. Clin J Pain. 2011;27(3):203–6.
Goebel A, Baranowski A, Maurer K, Ghiai A, McCabe C, Ambler G. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152(3):152–8.
Nascimento MSA, Klamt JG, Prado WA. Intravenous regional block is similar to sympathetic ganglion block for pain management in patients with complex regional pain syndrome type I. Braz J Med Biol Res. 2010;43(12):1239–44.
Carron H, Litwiller R. Stellate ganglion block. Anesth Analg. 1975;54(5):567–70.
Abdi S, Zhou Y, Patel N, Saini B, Nelson J. A new and easy technique to block the stellate ganglion. Pain Physician. 2004;7(3):327–31.
Erickson SJ, Hogan QH. CT-guided injection of the stellate ganglion: description of technique and efficacy of sympathetic blockade. Radiology. 1993;188(3):707–9.
Narouze S. Ultrasound-Guided Stellate Ganglion Block: Safety and Efficacy. Curr Pain Headache Rep. 38 ed. 2014;18(6):S55.
Hogan QH, Taylor ML, Goldstein M, Stevens R, Kettler R. Success rates in producing sympathetic blockade by paratracheal injection. Clin J Pain. 1994;10(2):139–45.
Ramsaroop L, Partab P, Singh B, Satyapal KS. Thoracic origin of a sympathetic supply to the upper limb: the “nerve of Kuntz” revisited. J Anat. 2001;199(Pt 6):675–82.
de Rocha RO, Teixeira MJ, Yeng LT, Cantara MG, Faria VG, Liggieri V, Loduca AA, Müller BM, ACMS S, de Andrade DC. Thoracic sympathetic block for the treatment of complex regional pain syndrome type I: a double-blind randomized controlled study. Pain. 2014;155(11):2274–81.
Kim YH, Kim SY, Lee YJ, Kim ED. A prospective, randomized cross-over trial of T2 paravertebral block as a sympathetic block in complex regional pain syndrome. Pain Physician. 2019;22(5):E417–24.
Liu Y, Ke X, Wu X, Mei W. Ultrasound-guided lumbar plexus block in supine position. Anesthesiology. 2018;128(4):812.
Carroll I, Clark JD, Mackey S. Sympathetic block with botulinum toxin to treat complex regional pain syndrome. Ann Neurol. 2009;65(3):348–51.
O'Connell NE, Wand BM, Gibson W, Carr DB, Birklein F, Stanton TR. Local anaesthetic sympathetic blockade for complex regional pain syndrome. Cochrane pain, palliative and supportive care group, editor. Cochrane Database Syst Rev. 2016;17(3):193–60.
Cheng J, Salmasi V, You J, Grille M, Yang D, Mascha EJ, Cheng OT, Zhao F, Rosenquist RW. Outcomes of sympathetic blocks in the management of complex regional pain syndrome. Anesthesiology. 2019;131(4):883–93.
Schürmann M, Gradl G, Wizgal I, Tutic M, Moser C, Azad S, Beyer A. Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clin J Pain. 2001;17(1):94–100.
Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5–12.
Melzack R, Wall PD. Pain mechanisms: a new theory. Science. American Association for the Advancement of Science. 1965;150(3699):971–9.
Nayak R, Banik RK. Review article current innovations in peripheral nerve stimulation. Pain Res Treat. Hindawi. 2018:1–5.
Hassenbusch SJ, Stanton-Hicks M, Schoppa D, Walsh JG, Covington EC. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 2nd ed. 1996;84(3):415–23.
Mirone G, Natale M, Rotondo M. Peripheral median nerve stimulation for the treatment of iatrogenic complex regional pain syndrome (CRPS) type II after carpal tunnel surgery. J Clin Neurosci Elsevier Ltd. 2009;16(6):825–7.
Kumar K, Rizvi S, Bnurs SB. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 4 ed. 2011;69(3):566–80.
Xu J, Liu A, Cheng J. New advancements in spinal cord stimulation for chronic pain management. Curr Opin Anaesthesiol. 2017;30(6):710–7.
Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95.
Dubuisson D. Effect of dorsal-column stimulation on gelatinosa and marginal neurons of cat spinal cord. J Neurosurg. 1989;70(2):257–65.
Linderoth B, Foreman RD. Physiology of spinal cord stimulation: review and update. Neuromodulation: Technology at the Neural Interface. 1999;2(3):150–64.
Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years' follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13–8.
Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail N. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months. Pain. 2017;158(4):669–81.
Kemler MA, Barendse GA, van Kleef M, de Vet HC, Rijks CP, Furnée CA, van den Wildenberg FA. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618–24.
Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, van Kleef M. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. American Association of Neurological Surgeons. 2008;108(2):292–8.
Oakley JC, Weiner RL. Spinal cord stimulation for complex regional pain syndrome: a prospective study of 19 patients at two centers. Neuromodulation: Technology at the Neural Interface. John Wiley & Sons, Ltd. 1999;2(1):47–50.
Taylor RS, Buyten J-P, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10(2):91.
Visnjevac O, Costandi S, Patel BA, Azer G, Agarwal P, Bolash R, Mekhail NA. A comprehensive outcome-specific review of the use of spinal cord stimulation for complex regional pain syndrome. Pain Pract. 2nd ed. 2016;17(4):533–45.
Rauck RL, North J, Eisenach JC. Intrathecal clonidine and adenosine. Pain. 2015;156(1):88–95.
van Hilten BJ, van de Beek WJ, Hoff JI, Voormolen JH, Delhaas EM. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):625–30.
van der Plas AA, Marinus J, Eldabe S, Buchser E, van Hilten JJ. The lack of efficacy of different infusion rates of intrathecal baclofen in complex regional pain syndrome: a randomized, double-blind, crossover study. Pain Med. 2011;12(3):459–65.
Munts AG, van der Plas AA, Ferrari MD, Teepe-Twiss IM, Marinus J, van Hilten JJ. Efficacy and safety of a single intrathecal methylprednisolone bolus in chronic complex regional pain syndrome. Eur J Pain. European Federation of International Association for the Study of Pain Chapters. 2010;14(5):523–8.
Munts AG, van der Plas AA, Voormolen JH, Marinus J, Teepe-Twiss IM, Onkenhout W, van Gerven JM, van Hilten JJ. Intrathecal glycine for pain and dystonia in complex regional pain syndrome. Pain. Inter Assoc Study of Pain. 2009;146(1–2):199–204.
McRoberts WP, Apostol C, Haleem A. Intrathecal bupivacaine monotherapy with a retrograde catheter for the management of complex regional pain syndrome of the lower extremity. Pain Physician. 2016;19(7):E1087–92.
Kanoff RB. Intraspinal delivery of opiates by an implantable, programmable pump in patients with chronic, intractable pain of nonmalignant origin. J Am Osteopath Assoc. 1994;94(6):487–93.
Kapural L, Lokey K, Leong MS, Fiekowsky S, Stanton-Hicks M, Sapienza-Crawford AJ, Webster LR. Intrathecal Ziconotide for complex regional pain syndrome: seven case reports. Pain Pract. 2009;9(4):296–303.
Hagedorn JM, Atallah G. Intrathecal management of complex regional pain syndrome: a case report and literature. Scandinavian J Pain. Elsevier B.V. 2017;14:110–2.
Deer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S, De Andrés JA, Erdek M, Patin D, Grider JS, Doleys DM, Jacobs MS, Yaksh TL, Poree L, Wallace MS, Prager J, Rauck R, DeLeon O, Diwan S, Falowski SM, Gazelka HM, Kim P, Leong M, Levy RM, McDowell G, McRoberts P, Naidu R, Narouze S, Perruchoud C, Rosen SM, Rosenberg WS, Saulino M, Staats P, Stearns LJ, Willis D, Krames E, Huntoon M, Mekhail N. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation: Technology at the Neural Interface. 1st ed. 2017;20(2):96–132.
Guevara-López U, Aldrete JA, Covarrubias-Gómez A, Hernández-Pando RE, López-Muñoz FJ. Absence of histological changes after the Administration of a Continuous Intrathecal Clonidine in Wistar rats. Pain Pract. 2009;9(2):122–9.
Zamponi GW, Striessnig J, Koschak A, Dolphin AC. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Sibley DR, editor. Pharmacol Rev. 2015;67(4):821–70.
van Rijn MA, Munts AG, Marinus J, Voormolen JHC, de Boer KS, Teepe-Twiss IM, van Dasselaar NT, Delhaas EM, van Hilten JJ. Intrathecal baclofen for dystonia of complex regional pain syndrome. Pain. Inter Assoc Study Pain. 2009;143(1–2):41–7.
van der Plas AA, van Rijn MA, Marinus J, Putter H, van Hilten JJ. Efficacy of intrathecal baclofen on different pain qualities in complex regional pain syndrome. Anesth Analg. 2013;116(1):211–5.
Herring EZ, Frizon LA, Hogue O, Mejia JU, Rosenquist R, Bolash RB, Machado AG, Nagel SJ. Long-term outcomes using intrathecal drug delivery systems in complex regional pain syndrome. Pain Med. 2018;20(3):515–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shin, C., Cheng, J. (2021). Interventional Treatment of Complex Regional Pain Syndrome. In: Lawson, E.F., Castellanos, J.P. (eds) Complex Regional Pain Syndrome. Springer, Cham. https://doi.org/10.1007/978-3-030-75373-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-75373-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75372-6
Online ISBN: 978-3-030-75373-3
eBook Packages: MedicineMedicine (R0)