Abstract
Apples are important from both a cultural and nutritional standpoint. The fruit produced from the cultivated apple, Malus × domestica Borkh., is grown in temperate regions throughout the world. Ornamental crabapple trees represent other Malus species and are generally valued for their landscape properties rather than their fruits. Genetic analyses of apple cultivars and species have revealed domestication pathways, pedigree relationships, as well as data that guide genebank collection management practices. Molecular information is also critical for breeding programs that are using new techniques to identify novel genetic combinations with enhanced biotic and abiotic stress resistance as well as desirable fruit quality and production traits. This chapter includes information about wild apple species, origins of cultivated apples, genetic assessments, as well as some basic information about tree phenology, architecture, and propagation methods.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
2.1 Introduction
Malus spp., belonging to the family Rosaceae, includes approximately 61 apple wild species and hybrids, traditionally taxonomically organized based on key morphological distinctions (Qian et al. 2006), and a single cultivated species, Malus × domestica Borkh. Worldwide, apples are one of the most economically important fruit crops (Bramel and Volk 2019). Fruit from M. × domestica is harvested for fresh consumption, cider, and processing uses, while other species of Malus are cultivated as either rootstocks or ornamentals. Wild Malus species are primarily found in temperate climates throughout much of the northern hemisphere, with China as a primary center of origin (Juniper and Mabberley 2006). Malus × domestica is proposed to have originated primarily from its progenitor, M. sieversii, which is native to Central Asia and Western China (Harris et al. 2002; Cornille et al. 2015, 2019).
2.2 Wild Malus Species and Hybrids
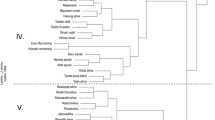
Most Malus species have a haploid genome with 17 chromosomes originating from genome duplication (Daccord et al. 2017). However, there is a range in genome size and ploidy among and within species (Höfer and Meister 2010; Chagné et al. 2015). Most dessert apple cultivars are either diploid or triploid, while wild species of Malus may be diploid, triploid, or tetraploid. Malus genera have been subdivided into eight sections, likely corresponding to their geographic origins (Fig. 2.1, Table 2.1).
Section Malus includes the cultivated apple, M. × domestica, along with its primary crop relatives including M. sieversii (Ledeb.) M. Roem., M. orientalis Uglitzk., and M. sylvestris Mill., as well as a number of other related species (Table 2.1). These three species, as well as M. prunifolia (Willd.) Borkh., share regions of nuclear and chloroplast sequences with M. × domestica (Fig. 2.2; Velasco et al. 2010; Nikiforova et al. 2013; Volk et al. 2015). Moreover, microsatellite, or simple sequence repeats (SSRs), markers have revealed that M. × domestica and M. sieversii form distinct genetic groups, with M. × domestica being a panmictic group (Cornille et al. 2012). Assessments of the wild apple genetic diversity using microsatellite markers have compared diversity among and within sampling locations in Kazakhstan (M. sieversii), the Caucasus region (M. orientalis), and Europe (M. sylvestris). It is found that the three wild species form distinct genetic groups in Eurasia but are only weakly genetically differentiated from each other (Richards et al. 2009; Cornille et al. 2013, 2015; Volk et al. 2008). Furthermore, data suggest that there is substantial gene flow among populations and species. These findings support the existence of wild Malus populations that are highly outcrossing (Richards et al. 2009) and that range contractions may have occurred during glacial periods in Europe (Cornille et al. 2013).

adapted from Volk et al. 2015)
Relationships among Malus × domestica and primary crop wild relatives based on chloroplast sequence data (
Sections Sorbomalus, Gymnomeles, and Yunnanensis are comprised of wild species native to China and the Far East (Fig. 2.1 and Table 2.1, USDA 2020; Zhi-Qin 1999; Volk et al. 2015; Yu 1988). An exception is M. fusca (Raf.) C.K. Schneid. (Section Sorbomalus), which is native to the western coast of North America, and it is suggested to have arrived in North America across the Bering Strait (Qian et al. 2006; Robinson et al. 2001; Routson et al. 2012).
Section Docyniopsis includes the only tropical apple species, M. doumeri (Bois) A. Chev., that grows in the wild in Southern China, and as far south as Vietnam. Moreover, M. leiocalyca S.Z. Huang and M. tschonoskii (Maxim.) C.K. Schneid. have also been assigned to this section (Robinson et al. 2001).
Each of sections Florentinae (M. florentina (Zucc.) C.K. Schneid., native to Southern Europe) and Eriolobus (M. trilobata (Poir.) C.K. Schneid., native to the Middle East and Southern Europe) has a single species. These are deemed as likely European relict species (Qian et al. 2008) that share common ancestries with North American Malus species in Section Chloromeles (Fig. 2.1, Forte et al. 2002). Ancestors of the species within Sections Florentinae, Eriolobus, and Chloromeles may have inhabited the northern landmass in the Cretaceous Period when current North America and Europe have been in close proximity, and have then diverged when these continents have separated (Forte et al. 2002).
Section Chloromeles consists of M. coronaria (L.) Mill., M. angustifolia (Aiton) Michx., and M. ioensis (Alph. Wood) Britton, these are found in eastern North America. Moreover, these appear to be the most distant species from M. × domestica and from Sections Malus and Sorbomalus based on assessments of chloroplast sequence data (Lo and Donoghue 2012; Robinson et al. 2001; Volk et al. 2015).
Most research on Malus focuses heavily on the cultivated, consumed apple. While dessert apples have sweet fruit, crabapples represent their counterparts, wherein fruit is bitter, acidic, and in terms of human edibility, unappealing. The term crabapple could be used to describe nearly all species other than M. × domestica species. Generally, crabapples bear smaller fruit, although fruit size varies significantly (Cornille et al. 2014). In general, crabapple fruit size ranges from 10 to 50 mm (USDA 2020). Although poor in fruit quality, crabapples have a significant economic impact, particularly as ornamentals (Fiala 1994). Additionally, they are planted in commercial orchards as pollinizers. Many ornamental crabapples are either specific selections from Malus wild species or have developed as hybrids between two Malus species, as Malus species are interfertile/cross-compatible (Table 2.2).
2.3 Cultivar Origins
Desirable apple trees have been originally identified in their native settings. The first drivers of the domestication of apple are most probably fruit size and organoleptic traits (Yao et al. 2015; Duan et al. 2017). Malus × domestica is proposed to have arisen from the progenitor species M. sieversii (native to Western China, Kazakhstan, and other Central Asian countries). Wild M. sieversii trees exhibit a wide range of fruit diversity (Fig. 2.3), and these may have served as original sources for domestication events in China and Russia, as well as for western cultivars. Then, during its journey along the Silk Routes through the Caucasus region (native to M. orientalis) and Europe (native to M. sylvestris), M. sieversii underwent several hybridization events with local wild apples (Fig. 2.4; Cornille et al. 2019; Duan et al. 2017; Gao et al. 2015; Luby 2003; Peace et al. 2019). When Greeks and Romans brought apples into Europe, about 1,500 years ago, M. sieversii was introgressed to M. sylvestris, the European crab apple (Cornille et al. 2012, 2014, 2019).
Proposed routes of Malus domestication, with circled fruit denoting likely domestication lineages. Orange-colored arrows denote domestication routes of apples of European ancestry, while Black-colored arrows denote domestication route of apples in Russia, and Purple-colored arrows denote domestication of apples in East Asia
During its journey along the Silk Routes, desirable M. × domestica trees were vegetatively propagated locally to preserve desirable genetic combinations. Traits that were especially favored included lower acidity and higher sugar content, thus resulting in numerous cultivars with sweet or sub-acid fruits (Duan et al. 2017; Ma et al. 2018). Domestication, and recent breeding efforts, also changed fruit metabolic compositions and lowered levels of phenolic compounds influencing astringency, bitterness, and color (Lea and Arnold 1978; Sanoner et al. 1999; Khan et al. 2014), particularly as fruits were increasingly used for fresh and dessert consumption, in contrast to making alcoholic ciders. In Europe, Russia, and China, particular offsprings were named as cultivars and propagated.
Some apple cultivars are likely to be from the Roman period, including ‘Annurca’ and ‘Decio’. Moreover, there are many examples of Old-World apple cultivars, such as ‘Winter Pearmain’ (the year 1200), ‘Calville Blanc d’Hiver’ (1598), ‘Court Pendu Plat’ (1613), ‘Blenheim Orange’ (1740), ‘Antonovka’ (1826), and ‘Yellow Transparent’ (1850) (Fig. 2.5).
Watercolor paintings of apple fruits from Old World apple cultivars. a ‘Blenheim Orange’, United Kingdom, 1740; and b ‘Yellow Transparent’, Russia, 1850 (National Agricultural Library 2020)
Apple seeds and trees moved along as Europeans migrated to North America. Apple seeds were planted throughout North America, and in some cases, seedling trees were named, propagated, and thereafter referred to as cultivars. The plethora of seedling apple trees and hundreds of cultivars that were named led to the designation of North America as a likely secondary center of origin for apple cultivars. Early North American apple cultivars included ‘Roxbury Russet’ (1600), ‘Rhode Island Greening’ (1650), and ‘Esopus Spitzenburg’ (1735). Interestingly, molecular marker-based pedigree inferences identified pedigrees of cultivars derived from North American seedling trees (Muranty et al. 2020). For example, ‘Esopus Spitzenberg’ was found to be directly derived from the French cultivar ‘Reinette Franche’ (1510). By the mid-1800s, the most popular apple cultivar in the USA was ‘Ben Davis’, a cultivar identified from a North American seedling in about 1800 (Fig. 2.6).
Fruits of historic North American apple cultivars. a ‘Roxbury Russet’ (1600 AD); b ‘Rhode Island Greening’ (1650 AD); c ‘Esopus Spitzenburg’ (1735 AD); and d ‘Ben Davis’ (1800 AD) (USDA 2020)
Centuries of domestication, selection, and propagation have resulted in a wide range of genetic diversity among apple cultivars. Some have bitter, phenolic traits desirable for hard cider production. Other cultivars have a wide range of fruit shapes and skin color, including stellar shape (e.g., ‘Api Etoilée’), ribbed apple (e.g., ‘Calville Blanc d’Hiver’), fully russeted fruit (e.g., ‘Renetta Grigia di Torriana’), or burgundy-colored apple mutants (e.g., ‘Bravo’™). Furthermore, new cultivars have enhanced biotic and/or abiotic stress resistance, as well as tree architectures that lend themselves to modern horticultural practices, including automated harvesting. Modern cultivars may also have an extremely long fruit shelf-life, particularly when stored under controlled atmosphere conditions (Stanger et al. 2018). In addition, modern cultivars and production practices result in high crop loads (i.e., number of fruits per tree), thus necessitating fruit thinning to avoid biennial bearing. Another innovation for breeding programs is the identification and use of wild apple species carrying desirable genes and alleles for disease resistance. For example, M. floribunda ‘821’ is a source for Vf (Rvi6) apple scab resistance allele for several commercial cultivars (Janick 2002).
Today, there are many well-known apple cultivars that are sold as specialty cultivars around the world, including those from Europe (‘Cox’s Orange Pippin’), Russia (‘Antonovka’, 1826), Japan (‘Fuji’, 1930s), Australia (‘Granny Smith’, 1868), New Zealand (‘Braeburn’, 1952; ‘Gala’, 1930s), and the USA ‘(Golden Delicious’, 1914; ‘Red Delicious’, 1880; ‘Honeycrisp’, 1991) (Fig. 2.7). Surprisingly, many of these well-known apple cultivars have been identified as chance seedlings or bred decades or even hundreds of years ago. Breeding programs continue to select for improved disease resistance and stress tolerance, fruit quality, and storage characteristics (Laurens et al. 2010).
Sometimes apple trees spontaneously mutate, and these mutations result in the observable change(s) that can be maintained following clonal propagation. These “sports” of a cultivar are nearly identical genetically to the original cultivar and are usually not detected using most genetic techniques. Sport variation ranges from mild to extreme. For example, ‘Red Delicious’ has a series of sports with various fruit red color pigmentation/or patterns, and ‘Sargeant Russet Golden’ is a russeted sport mutant of ‘Golden Delicious’ (Fig. 2.8; Gross et al. 2012). Another form of observed mutations, chimeras, is most often visible as sectional variations in apple fruit coloration. However, such chimeras are generally not maintained during clonal propagation.
Fruits of a ‘Golden Delicious’ and b ‘Sargeant Russet Golden’, a russetted sport of ‘Golden Delicious’ (USDA 2020)
2.4 Genetic Characterization
Genetic characterization of apple cultivars has long been grounded in the so-called pomological descriptions, based on accurate phenotypic observations of various fruit and tree traits (Juniper and Mabberley 2006). In his ‘Dictionnaire de Pomologie’, A. Leroy cites the first classifications performed by Greek writers such as Théophraste who subdivided Greek apple cultivars into six types according to their phenology or fruit taste (Leroy 1873). In modern times, biochemical analyses have been used to characterize sets of apple cultivars in addition to morphological traits. However, such biochemical analyses are more geared for purposes of description rather than classification.
Genetic characterization of apple cultivars began with isozyme analyses in the 1980s and 1990s (Weeden and Lamb 1985; Menendez et al. 1986; Korban and Bournival 1987; Battle et al. 1995). Subsequently, analyses of microsatellite/SSR markers have led to the collection and species diversity assessments, as well as for use in genetic fingerprinting for cultivar identity analyses. These markers can also be used to determine cultivar/genotype trueness-to-type, as well as identify duplications and sport-families within collections. Assessments have been performed for national apple genebank collections in France, the UK, the USA, and elsewhere (Lassois et al. 2016; Hokanson et al. 2001; Gross et al. 2012; Ordidge et al. 2018). Microsatellite assessments of collections have also facilitated international collection comparisons (Evans et al. 2011; Urrestarazu et al. 2016).
Genebank collections serve as reference sets of known cultivars to aid in the identification of historic fruit trees on public and private lands (Routson et al. 2009; Lassois et al. 2016; Magby et al. 2019). Recently, single nucleotide polymorphism (SNP) arrays and genomic sequencing have rapidly advanced our capacity to determine pedigree relationships, pursue marker-based selection breeding programs, and target specific cultivars/genotypes with alleles of particular interest to breeding programs (Cornille et al. 2019; Peace et al. 2019; Ordidge et al. 2018; Muranty et al. 2020; Howard et al. 2017; Baumgartner et al. 2016). The most frequently cited apple SNP arrays are the International RosBREED SNP Consortium (IRSC) Illumina Infinium 8K SNP array (Chagné et al. 2012), the Illumina Infinium 20K SNP array (Bianco et al. 2014), and the Affymetrix Axiom 487K SNP array (Bianco et al. 2016).
2.5 Tree Phenology and Architecture
Self-rooted apple trees grow to heights ranging between 2 and 20 m and have juvenile periods ranging between 4 and 12 years (Fischer 1994). Juvenility is a significant challenge for breeding and genetic research in apples. An early bearing can be induced by grafting seedling trees onto dwarfing rootstocks (Fazio et al. 2014). Transgenic approaches can drastically shorten generation cycles to 1 year or less (Flachowsky et al. 2011).
In general, inflorescences develop on first-year fruiting shoots (i.e., 2-year-old wood), brindles (medium shoots), or spurs (compact shoots). Bourse shoots (short vegetative shoots) develop proximally from the inflorescence meristem and terminate in vegetative or inflorescence buds (Costes et al. 2006). Floral bud development is initiated after full-bloom when terminal meristems either remain vegetative or commit to flowering (Foster et al. 2003). The development of floral primordia is influenced by genetic, physiological, and environmental factors (Koutinas et al. 2010). Flowers develop from inflorescences (consisting of 5–7 flowers) with a distinctive king flower. Flowers are complete, typically arranged with five petals, ranging from white to red in color. Malus trees are either deciduous or semi-deciduous with an alternate leaf arrangement. Leaves are typically serrulate elliptical to ovate. Fruit is a pome ranging from 3 to 12 cm in diameter, usually with five carpels, a red, yellow, or green exocarp, and typically a white mesocarp. The persistent calyx may be open or closed, pedicle length is typically 1.5 cm but ranges from 0.5 to 4.0 cm (USDA 2020).
Growth habits of apple trees can be classified into distinct categories based on the positioning of fruits and branches, with columnar and weeping representing the two extremes. High-density planting favors more compact columnar and spur types to maximize production and sustainability. The genetic characteristics that contribute to distinct architectural traits have been identified (Kenis and Keuleman 2007; Segura et al. 2009; Okada et al. 2020).
2.6 Propagation
Wild Malus trees propagate by seeds that are often spread by animals. Malus species, including M. × domestica, are self-incompatible (Igic et al. 2008). They exhibit gametophytic self-incompatibility, which is determined by the multigenic S-locus with two major components (Kubo et al. 2010; Pratas et al. 2018). One of these two components is a pistil specificity S-RNase that inhibits pollen tube growth from self-pollen, and the second consists of S-pollen specificity F-box proteins that recognize and degrade non-self S-RNases (Kubo et al. 2010; Pratas et al. 2018).
Due to self-incompatibility, apple trees must be pollinated by a different cultivar/genotype as a pollen source. Therefore, seeds collected from these fruits are hybrids, and if germinated, they grow into seedlings that are not true-to-type to their maternal parent as they are highly heterozygous. A notable exception is when seeds are derived asexually from maternal tissues and are true-to-type through apomixis. Apomixis is observed in Malus species, including M. hupehensis (Pamp.) Rehder and M. toringo (Siebold) de Vriese.
Cultivar-specific allelic combinations are maintained through asexual propagation techniques, with grafting as the most common propagation method. Either dormant or summer buds are budded/grafted onto rootstocks, thereby resulting in a two-genotype compound tree system, consisting of a combination of a rootstock (below-ground) and a scion cultivar (above-ground) (Fig. 2.9). This clonal propagation method dates back approximately 4000 years ago (Zohary 2000), and it has been used for centuries to maintain identities of unique apple selections. Remarkably, ‘Winter Pearmain’ fruit consumed today has the same genetic profile as that of fruit grown in 1200 AD. In some cases, somatic mutations may accumulate as a result of asexual propagation methods, but the rate(s) of incidence of such mutations has not been well documented.
Until the mid-1800s, rootstocks were derived from seeds, and germinating seedlings were grown simply for use in the vegetative propagation of scion cultivars. Modern apple clonal rootstocks have transformed apple production worldwide, as these have been selected for particular desirable traits that they confer to scions, including disease resistance and dwarfing, fruit quality, tree habit, nutrient assimilation, and tolerance to biotic and abiotic stress (Marini and Fazio 2018; also see Chap. 6 in this volume). Modern rootstocks are vegetatively propagated using mound layering or in vitro tissue culture systems (Fig. 2.9, Teixeira da Silva et al. 2019).
2.7 Conclusions
Malus species have critical importance in world agriculture, yet the genetic relationships among wild species, their contributions to apple domestication, and cultivar pedigrees are currently under investigation and will be elucidated. Genomic approaches are being used to assess the diversity of wild species, as well as those of important and desirable cultivars. These wild species and targeted cultivars are critical for current and future breeding efforts to enhance nutritional content, productivity, resistance to various biotic and abiotic stress, as well as sustainably of apple produced for future generations.
References
Battle I, Alston FH, Evans KM (1995) The use of the isoenzymic marker gene Got-1 in the recognition of incompatibility S alleles in apple. Theor Appl Genet 90:303–306
Baumgartner IO, Kellerhals M, Costa F, Dondini L, Pagliarani G, Gregori R, Tartarini S, Leumann L, Laurens F, Patocchi A (2016) Development of SNP-based assays for disease resistance and fruit quality traits in apple (Malus × domestica Borkh.) and validation in breeding pilot studies. Tree Genet Genomes 12:35. https://doi.org/10.1007/s11295-016-0994-y
Bianco L, Cestaro A, Sargent DJ, Banchi E, Derdak S, Di Guardo M, Salvi S, Jansen J, Viola R, Gut I, Laurens F, Chagné D, Velasco R, van de Weg E, Troggio M (2014) Development and validation of a 20K single nucleotide polymorphism (SNP) whole genome genotyping array for apple (Malus × domestica Borkh). PLoS ONE 9(10). https://doi.org/10.1371/journal.pone.0110377
Bianco L, Cestaro A, Linsmith G, Muranty H, Denancé C, Théron A, Poncet C, Micheletti D, Kerschbamer E, Di Pierro EA, Larger S, Pindo M, Van de Weg E, Davassi A, Laurens F, Velasco R, Durel C-E, Troggio M (2016) Development and validation of the Axiom Apple 480K SNP genotyping array. Plant J 86:62–74. https://doi.org/10.1111/tpj.13145
Bramel PJ, Volk G (2019) A global strategy for the conservation and use of apple genetic resources. Global Crop Diversity Trust. Bonn, Germany. https://doi.org/10.13140/RG.2.2.34072.34562
Chagné D, Crowhurst RN, Troggio M, Davey MW, Gilmore B, Lawley C, Vanderzande S, Hellens RP, Kumar S, Cestaro A, Velasco R, Main D, Rees JD, Iezzoni A, Mockler T, Wilhelm L, van de Weg E, Gardiner SE, Bassil N, Peace C (2012) Genome-wide SNP detection, validation, and development of an 9K SNP array for apple. PLoS ONE 7(2). https://doi.org/10.1371/journal.pone.0031745
Chagné D, Kirk C, Whitworth C, Erasmuson S, Bicknell R, Sargent DJ, Kumar S, Troggio M (2015) Polyploid and aneuploid detection in apple using a single nucleotide polymorphism array. Tree Genet Genomes 11:94. https://doi.org/10.1007/s11295-015-0920-8
Cornille A, Gladieux P, Smulders MJM, Roldán-Ruiz I, Laurens F, Le Cam B, Nersesyan A, Clavel J, Olonova M, Feugey L, Gabrielyan I, Zhang X-G, Tenaillon MI, Giraud T (2012) New insight into the history of domesticated apple: secondary contribution of the European wild apple to the genome of cultivated varieties. PLoS Genet 8(5): e1002703. https://doi.org/10.1371/journal.pgen.1002703
Cornille A, Giraud T, Bellard C, Tellier A, Le Cam B, Smulders MJM, Kleinschmit J, Roldan-Ruiz I, Gladieux P (2013) Postglacial recolonization history of the European crabapple (Malus sylvestris Mill.), a wild contributor to the domesticated apple. Mol Ecol 22:2249–2263
Cornille A, Giraud T, Smulders MJM, Roldán-Ruiz I, Gladieux P (2014) The domestication and evolutionary ecology of apples. Trends Genet 30:57–64
Cornille A, Feurtey A, Gélin U, Ropars J, Misvanderbrugge K, Gladieux P, Giraud T (2015) Anthropogenic and natural drivers of gene flow in a temperate wild fruit tree: a basis for conservation and breeding programs in apples. Evol Appl 8:373–384
Cornille A, Antolín F, Garcia E, Vernesi C, Fietta A, Brinkkemper O, Kirleis W, Schlumbaum A, Roldán-Ruiz I (2019) A multifaceted overview of apple tree domestication. Trends Plant Sci 24:770–782. https://doi.org/10.1016/j.tplants.2019.05.007
Costes E, Lauri P, Regnard J (2006) Analyzing fruit tree architecture: implications for tree management and fruit production. In: Janick J (ed) Horticultural reviews. John Wiley & Sons, pp 1–62
Daccord N, Celton J-M, Linsmith G, Becker C, Choisne N, Schijlen E, van de Geest H, Bianco L, Micheletti D, Velasco R, Di Pierro EA, Gouzy J, Rees DJG, Guérif P, Muranty H, Durel C-E, Laurens F, Lespinasse Y, Gaillard S, Aubourg S, Quesneville H, Weigel D, van de Weg E, Troggio M, Bucher E (2017) High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat Genet 49:1099–1106. https://doi.org/10.1038/ng.3886
Duan N, Bai Y, Sun H, Wang N, Ma Y, Li M, Wang X, Jiao C, Legall N, Mao L, Wan S, Wang K, He T, Feng S, Zhang Z, Mao Z, Shen X, Chen X, Jiang Y, Wu S, Yin C, Ge S, Yang L, Jiang S, Xu H, Liu J, Wang D, Qu C, Wang Y, Zuo W, Xiang L, Liu C, Zhang D, Gao Y, Xu Y, Xu K, Chao T, Fazio G, Shu H, Zhong GY, Cheng L, Fei Z, Chen X (2017) Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat Commun 8:249. https://doi.org/10.1038/s41467-017-00336-7
Evans K, Patocchi A, Rezzonico F, Mathis F, Durel C, Fernández-Fernández F, Boudichevskaia A, Dunemann F, Stankiewicz-Kosyl M, Gianfranceschi L, Komjanc M, Lateur M, Madduri M, Noordijk Y, van de Weg WE (2011) Genotyping of pedigreed apple breeding material with a genome-covering set of SSRs: trueness-to-type of cultivars and their parentages. Mol Breed 28:535–547
Fazio G, Wan Y, Kviklys D, Romero L, Adams R, Strickland D, Robinson T (2014) Dw2, a new dwarfing locus in apple rootstocks and its relationship to induction of early bearing in apple scions. J Am Soc Hortic Sci 139:87–98. https://doi.org/10.21273/JASHS.139.2.87
Fiala JL (1994) Flowering crabapples. The genus Malus. Timber Press, Portland, OR. 273p
Fischer C (1994) Shortening of the juvenile period in apple breeding. In: Schmidt H, Kellerhals M (eds) Progress in temperate fruit breeding: proceedings of the eucarpia fruit breeding section meeting held at Wädenswil/Einsiedeln, Switzerland from August 30 to September 3, 1993. Springer Netherlands, Dordrecht, pp 161–164. https://doi.org/10.1007/978-94-011-0467-8_32
Flachowsky H, Le Roux P-M, Peil A, Patocchi A, Richter K, Hanke M-V (2011) Application of a high-speed breeding technology to apple (Malus x domestica) based on transgenic early flowering plants and marker-assisted selection. New Phytol 192:364–377. https://doi.org/10.1111/j.1469-8137.2011.03813.x
Forte AV, Ignatov AN, Ponomarenko VV, Dorokhov DB, Savelyev NI (2002) Phylogeny of Malus (apple tree) species inferred from the morphological traits and molecular DNA analysis. Russ J Genet 38:1150–1160
Foster T, Johnston R, Seleznyova A (2003) A morphological and quantitative characterization of early floral development in apple (Malus × domestica Borkh.). Ann Bot 92:199–206. https://doi.org/10.1093/aob/mcg120
Gao Y, Liu F, Wang K, Wang D, Gong X, Liu L, Richards CM, Henk AD, Volk GM (2015) Genetic diversity of Malus cultivars and wild relatives in the Chinese National Repository of Apple Germplasm Resources. Tree Genet Genomes 11:106. https://doi.org/10.1007/s11295-015-0913-7
Gross B, Volk GM, Richards CM, Forsline P, Fazio G, Chao C-T (2012) Identification of “duplicate” accessions within the USDA-ARS National Plant Germplasm System Malus collection. J Am Soc Hort Sci 137:333–342
Harris SA, Robinson JP, Juniper BE (2002) Genetic clues to the origin of the apple. Trends Genet 18:426–430
Höfer M, Meister A (2010) Genome size variation in Malus species. J Bot 2010. https://doi.org/10.1155/2010/480873
Hokanson SC, Lamboy WF, Szewc-McFadden AK, McFerson JR (2001) Microsatellite (SSR) variation in a collection of Malus (apple) species and hybrids. Euphytica 118:281–294
Howard NP, van de Weg E, Bedford DS, Peace CP, Vanderzande S, Clark MD, Teh SL, Cai L,
Igic B, Lande R, Kohn JR (2008) Loss of self-incompatibility and its evolutionary consequences. Int J Plant Sci 169:93–104. https://doi.org/10.1086/523362
Janick J (2002) History of the PRI apple breeding program. Acta Hortic 55–60. https://doi.org/10.17660/ActaHortic.2002.595.7
Juniper BE, Mabberley DJ (2006) The Story of the Apple, Timber Press, Inc.
Kenis K, Keuleman J (2007) Study of tree architecture of apple (Malus × domestica Borkh.) by QTL analysis of growth traits. Mol Breed 19:193–208. https://doi.org/10.1007/s11032-006-9022-5
Khan SA, Tikunov Y, Chibon PY, Maliepaard C, Beekwilder J, Jacobsen E, Schouten HJ (2014) Metabolic diversity in apple germplasm. Plant Breed 133:281–290. https://doi.org/10.1111/pbr.12134
Korban SS, Bournival BL (1987) Catalase, esterase and peroxidase enzymes in seeds and leaves of Malus domestica Borkh. Scient Hortic 32:213–219
Koutinas N, Pepelyankov G, Lichev V (2010) Flower induction and flower bud development in apple and sweet cherry. Biotech Biotechnol Equip 24:1549–1558. https://doi.org/10.2478/V10133-010-0003-9
Kubo K, Entani T, Takara A, Wang N, Fields AM, Hua Z, Toyoda M, Kawashima S, Ando T, Isogai A, Kao T, Takayama S (2010) Collaborative non-self recognition system in S-RNase–based self-incompatibility. Science 330:796–799. https://doi.org/10.1126/science.1195243
Lassois L, Denancé C, Ravon E, Guyader A, Guisnel R, Hibrand-Saint-Oyant L, Poncet C, Lasserre-Zuber P, Feugey L, Durel CE (2016) Genetic diversity, population structure, parentage analysis and construction of core collections in the French apple germplasm based on SSR markers. Plant Mol Biol Rep 34:827–844. https://doi.org/10.1007/s11105-015-0966-7
Laurens F, Aranzana MJ, Arus P, Bassi D, Bonany J, Corelli L, Durel CE, Mes J, Pascal T, Patocchi A, Peil A, Quilot B, Salvi S, Tartarini S, Troggio M, Vecchietti A, Velasco R, van de Weg E (2010) Review of fruit genetics and breeding programmes and a new European initiative to increase fruit breeding efficiency. Acta Hortic 929:95–102
Lea AGH, Arnold GM (1978) The phenolics of ciders: bitterness and astringency. J Sci Food Agric 29:478–483
Leroy A (1873) Dictionnaire de pomologie: contenant l’histoire, la description, la figure des fruits anciens et des fruits modernes les plus généralement connus et cultivés. Imprimerie Lachèse, Belleuvre et Dolbeau, Paris
Lo EYY, Donoghue MJ (2012) Expanded phylogenetic and dating analyses of the apples and their relatives (Pyreae, Rosaceae). Mol Phylogenet Evol 63:230–243
Luby JJ (2003) Taxonomic classification and brief history. In: Ferree DC, Warrington IJ (eds) Apples: Botany, Production and Uses. CAB Intl, Wallingford, UK. p 1–14
Ma B, Yuan Y, Gao M, Li C, Ogutu C, Li M, Ma F (2018) Determination of predominant organic acid components in Malus species: correlation with apple domestication. Metabolites 8:74. https://doi.org/10.3390/metabo8040074
Magby J, Volk G, Henk A, Miller S (2019) Identification of historic homestead and orchard apple cultivars in Wyoming. HortSci 54:8–16
Marini RP, Fazio G (2018) Apple rootstocks. In: History, Physiology, Management, and Breeding. Hortic Rev 45:197–312
Menendez RA, Larsen FE, Fritts R (1986) Fingerprinting apple cultivars by electrophoretic isozyme banding patterns. J Environ Hort 4:101–107
Muranty H, Denancé C, Feugey L, Crépin JL, Barbier Y, Tartarini S, Ordidge M, Troggio M, Lateur M, Nybom H, Paprstein F, Laurens F, Durel CE (2020) Using whole-genome SNP data to reconstruct a large multi-generation pedigree in apple germplasm. BMC Plant Biol 20:2. https://doi.org/10.1186/s12870-019-2171-6
National Agricultural Library (2020) USDA Pomological Watercolor Collection. https://usdawatercolors.nal.usda.gov/pom/about.xhtml Accessed 28 Aug 2020
Nikiforova SV, Cavalieri D, Velasco R, Goremykin V (2013) Phylogenetic analysis of 47 chloroplast genomes clarifies the contribution of wild species to the domesticated apple maternal line. Mol Biol Evol 30:1751–1760
Okada K, Wada M, Takebayashi Y, Kojima M, Sakakibara H, Nakayasu M, Mizutani M, Nakajima M, Moriya S, Shimizu T, Abe K (2020) Columnar growth phenotype in apple results from gibberellin deficiency by ectopic expression of a dioxygenase gene. Tree Physiol 40:1205–1216. https://doi.org/10.1093/treephys/tpaa049
Ordidge M, Kirdwichai P, Baksh MF, Venison EP, Gibbings JG, Dunwell JM (2018) Genetic analysis of a major international collection of cultivated apple varieties reveals previously unknown historic heteroploid and inbred relationships. PLoS ONE 13. https://doi.org/10.1371/journal.pone.0202405
Peace DP, Bianco L, Troggio M, Van de Weg E, Howard NP, Cornille A, Durel CE, Myles S, Migicovsky Z, Schaffer R, Costes E, Fazio G, Yamane H, van Nocker S, Gottschalk C, Costa F, Chagné D, Zhang X, Patocchi A, Gardiner S, Hardner C, Kumar S, Laurens F, Bucher E, Main D, Jung S, Vanderzande S (2019) Apple whole genome sequences: recent advances and new prospects. Hortic Res 6:59. https://doi.org/10.1038/s41438-019-0141-7
Pratas MI, Aguiar B, Vieira J, Nunes V, Teixeira V, Fonseca NA, Iezzoni A, van Nocker S, Vieira CP (2018) Inferences on specificity recognition at the Malus × domestica gametophytic self-incompatibility system. Sci Rep 8:1–17. https://doi.org/10.1038/s41598-018-19820-1
Qian G-Z, Liu L-F, Tang G-G (2006) A new section in Malus (Rosaceae) from China. Ann Bot Fenn 43:68–73
Qian G-Z, Liu L-F, Hong D-Y, Tang G-G (2008) Taxonomic study of Malus section Florentinae (Rosaceae). Bot J Linn Soc 158:223–227
Richards CM, Volk GM, Reeves PA, Reilley AA, Henk AD (2009) Selection of stratified core sets representing wild apple (Malus sieversii). J Am Soc Hort Sci 134:228–235
Robinson JP, Harris SA, Juniper BE (2001) Taxonomy of the genus Malus Mill. (Rosaceae) with emphasis on the cultivated apple Malus Domestica Borkh. . Plant Syst Evol 226:35–58
Routson KJ, Reilley AA, Henk AD, Volk GM (2009) Identification of historic apple trees in the Southwestern United States and implications for conservation. HortSci 44:589–594
Routson KJ, Volk GM, Richards CM, Smith SE, Nabhan GP, de Echeverria VW (2012) Genetic variation and distribution of pacific crabapple. J Am Soc Hort Sci 137:325–332
Sanoner P, Guyot S, Marnet N, Molle D, Drilleau JF (1999) Polyphenol profiles of French cider apple varieties (Malus domestica sp.). Agric Food Chem 47:4847–4853
Segura V, Cilas C, Costes E (2009) Dissecting apple tree architecture into genetic, ontogenetic and environmental effects: mixed linear modelling of repeated spatial and temporal measures. New Phytol 178:302–314. https://doi.org/10.1111/j.1469-8137.2007.02374.x
Stanger MC, Steffens CA, Soethe C, Moreira MA, do Amarante CVT, Both V, Brackmann A, (2018) Phenolic compounds content and antioxidant activity of ‘Galaxy’ apples stored in dynamic controlled atmosphere and ultralow oxygen conditions. Postharv Biol Technol 144:70–76
Teixeira da Silva JA, Gulyás A, Magyar-Tábori K, Wang M-R, Wang Q-C, Dobránszki J (2019) In vitro tissue culture of apple and other Malus species: recent advances and applications. Planta 249:975–1006. https://doi.org/10.1007/s00425-019-03100-x
Urrestarazu J, Denancé C, Ravon E, Guyader A, Guisnel R, Feugey L, Poncet C, Lateur M, Houben P, Ordidge M, Fernandez-Fernandez F, Evans KM, Paprstein F, Sedlak J, Nybom H, Garkava-Gustavsson L, Miranda C, Gassmann J, Kellerhals M, Suprun I, Pikunova AV, Krasova NG, Torutaeva E, Dondini L, Tartarini S, Laurens F, Durel CE (2016) Analysis of the genetic diversity and structure across a wide range of germplasm reveals prominent gene flow in apple at the European level. BMC Plant Biol 16:130. https://doi.org/10.1186/s12870-016-0818-0
USDA (2020) National genetic resources program. Germplasm Resources Information Network (GRIN). USDA ARS Natl Germplasm Resour Lab, Beltsville, MD. https://www.ars-grin.gov/npgs/index.html. Accessed 28 Aug 2020
Velasco R, Zharkikh A, Affourtit J, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Dhingra A, Cestaro A, Kalyanaraman A, Fontana P, Bhatnagar SK, Troggio M, Pruss D, Salvi S, Pindo M, Baldi P, Castelletti S, Cavaiuolo M, Coppola G, Costa F, Cova V, Ri AD, Goremykin V, Komjanc M, Longhi S, Magnago P, Malacarne G, Malnoy M, Micheletti D, Moretto M, Perazzolli M, Si-Ammour A, Vezzulli S, Zini E, Eldredge G, Fitzgerald LM, Gutin N, Lanchbury G, Macalma T, Mitchell JT, Reid J, Wardell B, Kodira C, Chen Z, Desany B, Niazi F, Palmer M, Koepke T, Jiwan D, Schaeffer S, Krishnan V, Wu C, Chu VT, King ST, Vick J, Tao Q, Mraz A, Stormo A, Stormo K, Bogden R, Ederle D, Stella A, Vecchietti A, Kater MM, Masiero S, Lasserre P, Lespinasse Y, Allan AC, Bus V, Chagné D, Crowhurst RN, Gleave AP, Lavezzo E, Fawcett JA, Proost S, Rouzé P, Sterck L, Toppo S, Lazzari B, Hellens RP, Durel C-E, Gutin A, Bumgarner R, Gardiner SE, Skolnick M, Egholm M, Van de Peer Y, Salamini F, Viola R (2010) The genome of the domesticated apple (Malus × domestica Borkh.). Nat Genet 42:833–839. https://doi.org/10.1038/ng.654
Volk GM, Richards CM, Reilley AA, Henk AD, Reeves PA, Forsline PL, Aldwinckle HS (2008) Genetic diversity of wild Malus orientalis from Turkey and southern Russia. J Am Soc Hort Sci 133:383–389
Volk GM, Henk AD, Baldo A, Fazio G, Chao CT, Richards CM (2015) Chloroplast heterogeneity and historical admixture within the genus Malus. Am J Bot 102(7):1198–1208
Weeden NF, Lamb RC (1985) Identification of apple cultivars by isozyme phenotypes. J Am Soc Hort Sci 110:509–515
Yao JL, Xu J, Cornille A, Tomes S, Karunairetnam S, Luo Z, Bassett H, Whitworth C, Rees-George J, Ranatunga C, Snirc A, Crowhurst R, de Silva N, Warren B, Deng C, Kumar S, Chagné D, Bus VG, Volz RK, Rikkerink EH, Gardiner SE, Giraud T, MacDiarmid R, Gleave AP (2015) A microRNA allele that emerged prior to apple domestication may underlie fruit size evolution. Plant J 84:417–427
Yu TC (1988) Taxonomy of fruit trees in China. Pome Fruits. 1. Malus. Translated from Kuo C-K, Heueh FL, Ghang TP, Lei JX. F’in Xuo Lei. 1979. Pei-ching: Hung yeh eh-u. pa she. pp 87–122
Zhi-Qin Z (1999) The apple genetic resources in China: The wild species and their distributions, informative characteristics and utilization. Genet Resour Crop Evol 46:599–609
Zohary D, Hopf M (2000) Domestication of plants in the Old World, 3rd edn. Oxford University Press, New York, 316 p
Acknowledgements
We thank Katheryn Chen for providing the illustrations in Fig. 2.9. USDA is an equal opportunity provider, employer, and lender.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply
About this chapter
Cite this chapter
Volk, G.M., Cornille, A., Durel, CE., Gutierrez, B. (2021). Botany, Taxonomy, and Origins of the Apple. In: Korban, S.S. (eds) The Apple Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-74682-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-74682-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74681-0
Online ISBN: 978-3-030-74682-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)