Abstract
The optic nerve head in highly myopic eyes is anatomically characterized by an axial elongation-associated enlargement of the optic nerve scleral canal with an elongation and thinning of the lamina cribrosa, leading to a reduction in the distance between the intraocular compartment with intraocular pressure and the retrolaminar compartment with the orbital cerebrospinal fluid pressure and to the exposure of the peripheral posterior lamina cribrosa surface to the cerebrospinal fluid space; a decrease in the spatial contrast between the height of the neuroretinal rim and the depth of the optic cup; a reduction in the color contrast between the pink color of the neuroretinal rim and the paleness of the cup; a shift of Bruch’s membrane opening in direction to the macula in medium myopic eyes, leading to an overhanging of Bruch’s membrane into the intrapapillary compartment on its nasal side, and a lack of Bruch’s membrane in the parapapillary temporal region (temporal parapapillary gamma zone); and an enlargement of Bruch’s membrane opening beyond an axial length of 26.0 to 26.5 mm, leading to a circular parapapillary gamma zone; elongation and thinning of the peripapillary scleral flange resulting in parapapillary delta zone and an extension of the cerebrospinal fluid space just behind the thinned peripapillary scleral flange; a decrease in the visibility of the peripapillary retinal nerve fiber layer due to the bright fundus reflectance and to a peripapillary retinoschisis. These axial elongation-associated histologic changes of the optic nerve head may be associated with an increase in the susceptibility for glaucomatous or glaucoma-like optic nerve damage with increasing axial length, even in the presence of a normal.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optic nerve head
- Optic disc
- Myopia
- High myopia
- Parapapillary atrophy
- Gamma zone
- Delta zone
- Lamina cribrosa
- Glaucoma
The optic nerve head (ONH) or “papilla nervi optici” is a defect in the wall of the posterior segment of the eye to allow the exit of the retinal nerve fibers and central retinal vein and the entrance of the central retinal artery. Simultaneously, it is part of the ocular wall and thus serves to keep up the difference between a higher pressure inside of the eye (so called intraocular pressure) and outside of the eye [1, 2]. The ONH can be regarded as a three-layered hole, with Bruch’s membrane opening (BMO) forming the inner layer, the hole in the choroid forming the middle layer, and the scleral canal forming the outer layer of the ONH [3]. The ONH can be divided into the intrapapillary region as all area within the scleral and choroidal canal and the parapapillary region as the area surrounding the intrapapillary region. If the scleral canal, covered by the lamina cribrosa, is taken for the definition of the bottom of the intrapapillary region, the ophthalmoscopically visible boundary of the ONH is the peripapillary ring. The latter is the ophthalmoscopical equivalent of the peripapillary border tissue of the choroid (Jacoby) which merges with the end of Bruch’s membrane (BM) on its inner side and which continues into the peripapillary border tissue of the scleral flange (Elschnig) on its outer side [3]. The peripapillary border tissue of the scleral flange itself is a continuation of the optic nerve pia mater.
1 Intrapapillary Region
1.1 Optic Disc
Ophthalmoscopically, the intrapapillary region is composed of the neuroretinal rim as the equivalent of the retinal nerve fibers and the central optic cup as the whole area not filled up by the optic nerve fibers [1, 2]. The optic disc is the sum of optic cup and neuroretinal rim. The area of the ONH shows a marked interindividual variability of about 1:7 within a normal non-highly myopic Caucasian population [1]. The ONH area additionally shows an inter-ethnic variability, with Caucasians having the smallest optic disc and Afro-Americans the largest discs [2]. As rule of thumb, the disc size increases with the decreasing distance to the equator. Within the non-highly myopic group, the disc size is slightly correlated with the refractive error and thus the size of the globe: the disc is smaller in hyperopic eyes, and it is slightly larger in medium myopic eyes. At a cutoff value of about −8 diopters of myopia or an axial length of 26.5 mm, the disc size increases more markedly with myopic refractive error and longer axial length. It leads to secondary or acquired macrodiscs in highly myopic eyes. These secondary macrodiscs have to be differentiated from primary macrodiscs in non-highly myopic eyes. Primary macrodiscs have a normal mostly circular shape, and their size is not markedly associated with refractive error or axial length. Primary macrodiscs are associated with large and relatively flat corneas and with large globe diameters in the horizontal and vertical direction, without much elongation of the sagittal globe diameter. Secondary macrodiscs in highly myopic eyes appear to have an elliptical or oval shape. This may at least partially be due to an ophthalmoscopical artifact, since the myopic elongation of the globe by the increase in the fovea-disc distance moves the ONH more to the nasal wall of the globe. By that, the ophthalmoscopic view onto the ONH is no longer en face or perpendicular, but it occurs in an oblique direction onto the ONH surface. This can lead to a seemingly oblique disc shape and an underestimation of the horizontal optic disc diameter. In non-highly myopic eyes, the disc size is correlated with the number of cones and rods of the retinal photoreceptors, the number of retinal pigment epithelium (RPE) cells, the number of retinal nerve fibers (and presumably retinal ganglion cells), and the number of lamina cribrosa pores and the total pore area [4, 5]. Since high myopia is an acquired condition and since the number of retinal cells does presumably not increase after birth, the relationship between disc size and retinal cell number may not be valid in highly myopic eyes. The variability in optic disc size is pathogenically important since optic disc drusen, pseudopapilledema, and non-arteritic anterior ischemic optic neuropathy occur almost exclusively in small optic discs, while congenital pits of the optic disc are more common in large optic nerve heads [2]. The prevalence of arteritic anterior ischemic optic neuropathy and of central retinal artery occlusion or central retinal vein occlusion is independent of the disc size [2]. It may imply that the frequency of disc drusen, pseudopapilledema, and non-arteritic anterior ischemic optic neuropathy may be lower in ethnic groups with large optic disc than in Caucasians with relatively small ONHs and the prevalence of these diseases may be lower in highly myopic eyes than in emmetropic eyes.
Upon ophthalmoscopy, the optic disc has a slightly vertically oval form with the vertical diameter being about 7–10% larger than the horizontal one [1, 2]. The disc form is not correlated with age, gender, and body weight and height. Abnormal disc shapes may be divided into discs with a rotation around the vertical disc axis (“vertically rotated discs”), discs with a rotation around the horizontal disc axis (“horizontally rotated discs”), and discs with a rotation along the sagittal axis. In the case of vertically rotated optic discs, a passive movement of the ONH to the nasal ocular wall by the myopic enlargement of the posterior pole might play a role. It leads to an oblique view onto the ONH surface and a seemingly reduced horizontal disc diameter as an optical artifact in the two-dimensional ophthalmoscopical examination. Horizontally rotated discs were called “tilted discs.” The prevalence of horizontally rotated discs is independent of the refractive error, while vertically rotated discs are associated with high myopia. Horizontally rotated discs are significantly correlated with an increased corneal astigmatism and amblyopia. In contrast, vertically rotated discs in highly myopic eyes are not associated with an increased corneal astigmatism, since the changes associated with the development of high myopia occur mostly behind the equator and leave the cornea unchanged. According to a recent study, a major factor shaping the disc form may be a misalignment of the three ONH layers [6]. If the BMO shifts during the process of emmetropization or myopization into the temporal direction, the overhanging of BM on the nasal disc side leads to a vertically oval disc shape upon ophthalmoscopy. If the BMO shifts into the inferior direction, an overhanging of BM at the superior disc border occurs, leading to a horizontally oval disc shape upon ophthalmoscopy. If the BMO shifts into the nasal direction, a “situs inversus papillae” may develop, with a gamma zone on the nasal side and an exit of the retinal vessels first into the nasal direction before turning around into the temporal direction toward the macular region.
1.2 Neuroretinal Rim
The neuroretinal rim is the intrapapillary equivalent of the retinal nerve fibers and optic nerve fibers [1, 2]. As any biologic quantitative parameter, the neuroretinal rim size is not interindividually constant but shows, similar to the optic disc and cup, a high interindividual variability. The rim size is correlated with the optic disc area. The increase of rim area with enlarging disc area is most marked for eyes with no disc cupping, medium pronounced for eyes with a temporal flat sloping of the optic cup, and least marked in eyes with circular steep disc cupping. The correlation between rim area and disc area corresponds with the positive correlation between optic disc size and optic nerve fiber count. These associations are valid only for non-highly myopic eyes since high myopia develops after birth, while the number retinal nerve fibers do not increase after birth. Possible reasons for the interindividual size variability of the rim are differences in the nerve fiber count, ratio between formed and regressed retinal ganglion cell axons during embryogenesis, density of nerve fibers within the optic disc, lamina cribrosa architecture, diameters of retinal ganglion cell axons, proportion of glial cells on the whole intrapapillary tissue, and in other factors [2]. The nerve fibers within the neuroretinal rim are retinotopically arranged, with axons from ganglion cells close to the optic disc lying more centrally in the optic disc while axons from cells in the retinal periphery lie at the optic nerve head margin. It corresponds to the nerve fiber distribution in the retinal nerve fiber layer. Although not examined in highly myopic eyes, one may assume that the retinotopic arrangement of the retinal nerve fibers is preserved in highly myopic eyes.
The shape of the neuroretinal rim follows the ISNT (inferior-superior-nasal-temporal) rule: it is usually wider at the inferior disc pole, followed by the superior disc pole, the nasal disc region, and smallest in the temporal disc sector (Fig. 12.1). While many normal eyes can have a wider rim superiorly than inferiorly, and while the rim width in the nasal region is of minor clinical importance, the most important part of the ISNT rule is the “T” in that more than 95% of normal eyes have the smallest rim part in the temporal 60° of the ONH. The ISNT rule is of importance for the early detection of glaucomatous optic nerve damage, and it is valid also in highly myopic eyes.
In non-highly myopic eyes, the rim shape (ISNT rule) is associated with the diameter of the retinal arterioles which are significantly wider in the inferotemporal arcade than in the superotemporal arcade; with the visibility and the thickness of the retinal nerve fiber layer which are significantly better detectable and thicker in the inferotemporal region than in the superotemporal region; with the location of the foveola about 0.5 inferior to the horizontal optic disc axis center; with the morphology of the lamina cribrosa with the largest pores and the least amount of inter-pore connective tissue in the inferior and superior regions as compared to the temporal and nasal sectors; and with the distribution of the thin and thick nerve fibers in the optic nerve just behind the ONH where the thin fibers from the foveal region are located in the temporal part of the nerve [2]. Although these relationships have not explicitly been examined in highly myopic eyes, one may assume that they prevail also in highly myopic eyes unless the position of the fovea has markedly changed during the process of axial elongation.
While the neuroretinal rim gets lost and changes its shape in glaucoma, rim size and shape remains mostly unchanged if non-glaucomatous optic nerve damage develops. These statements hold true for non-highly myopic eyes and for highly myopic eyes.
The delineation of the neuroretinal rim from the optic cup is more difficult in highly myopic eyes than in emmetropic eyes, since the spatial contrast between the height of the rim and the depth of the cup is reduced due to the myopic stretching of the ONH in high myopia; the spatial contrast additionally appears optically to be diminished due to the longer axis in the highly myopic eyes and the consequent reduction in the size of the image of posterior fundus structures. Furthermore, the color contrast between the neuroretinal rim and the optic cup is decreased since the rim in highly myopic eyes is markedly less pink than in emmetropic eyes. It has remained unclear whether this effect is due to a thinner nerve tissue in the highly myopic neuroretinal rim so that the underlying collagenous tissue of the lamina cribrosa can better gleam through and/or whether it may reflect a decrease in the density of blood capillaries or blood supply into the neuroretinal rim. These problems in outlining the border between neuroretinal rim and optic cup are one of the reasons for the difficulty in detecting glaucomatous or glaucoma-like optic nerve damage in highly myopic eyes. Other reasons are that the assessment of the retinal nerve fiber layer is markedly hampered by the bright peripapillary fundus reflectance in highly myopic eyes and by myopia-associated changes in the retinal nerve fiber layer, such as a peripapillary retinoschisis; that the fundus changes associated with myopic retinopathy are sufficient reasons for perimetric defects so that the role of perimetry for the detection of glaucoma is reduced; and that the intraocular pressure in the highly myopic type of primary open-angle glaucoma is often within the normal range.
1.3 Optic Cup
Parallel to the optic disc and the neuroretinal rim, also the optic cup shows a high interindividual variability. Large optic cups (macrocups) can be differentiated into primary macrocups which occur in primary macrodiscs and secondary or acquired macrocups. The latter can further be subclassified into secondary, highly myopic macrocups in highly myopic eyes with secondary macrodiscs due to the myopic stretching of the optic nerve head and into secondary macrocups which developed due to the glaucomatous loss of neuroretinal rim. In non-glaucomatous eyes including non-glaucomatous highly myopic eyes, the areas of the optic cup and disc are correlated with each other: the larger is the optic disc, the larger is the optic cup. Due to the vertically oval optic disc and the horizontally oval optic cup, the cup/disc diameter ratios in normal eyes are horizontally significantly larger than vertically. In less than 7% of non-glaucomatous eyes, the horizontal cup/disc ratio is smaller than the vertical one. It indicates that the quotient of the horizontal-to-vertical-cup/disc ratios is usually higher than 1.0. It is important for the diagnosis of glaucoma, in which, in the early to medium advanced stages, the vertical cup/disc diameter ratio increases faster than the horizontal one. It leads to an increase of the quotient of horizontal-to-vertical-cup/disc ratios to values lower than 1.0. This holds true also for the detection of glaucomatous optic nerve damage in highly myopic eyes. As ratio of cup diameter-to-disc diameter, the cup/disc ratios depend on the size of the optic disc and cup. The high interindividual variability in the optic disc and cup diameters explains the high physiological interindividual variability in cup/disc ratios ranging in the non-glaucomatous population between 0.0 and almost 0.9. It includes the highly myopic group.
1.4 Histology of the Intrapapillary Region
The bottom of the intrapapillary region is formed by the lamina cribrosa which extends from the circular peripapillary scleral flange (Figs. 12.2 and 12.3) [7]. The peripapillary border tissue of the peripapillary scleral flange (Elschnig) is intertwined in a perpendicular manner with the scleral flange in its transition into the lamina cribrosa [3]. The lamina cribrosa may be compared with a multilayered structure of collagenous sheets with numerous pores. In the region of the neuroretinal rim, the retinal nerve fibers pierce through the lamina cribrosa pores, get myelinated just when leaving the lamina cribrosa and form the retrobulbar optic nerve. In the region of the optic cup, the lamina cribrosa pores appear to be sealed by connective tissue covering the lamina cribrosa. It has remained unclear whether this tissue sheet is watertight or whether it allows a leakage of intraocular fluid into the retrobulbar cerebrospinal fluid space. Clinical observations on accumulations of large pigmented particles at the bottom of the cup in eyes after pars plana vitrectomies may allow the speculation that the lamina cribrosa can function like a sieve allowing some leakage of fluid and keeping larger particles back. If that is the case, it would mean an additional outflow pathway of aqueous humor and would indicate that the cerebrospinal fluid just behind the globe may have another composition than in the apex of the orbit.
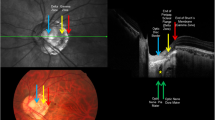
Electron micrograph of the inner surface of the lamina cribrosa after digestion of the retinal nerve fibers. White arrow, central retinal vessel trunk; red arrows, large lamina cribrosa pores in the inferior and superior disc region; blue arrows, small lamina cribrosa pores in the temporal and nasal disc region; the pores are generally larger closer to the optic disc margin; green stars, retrobulbar cerebrospinal fluid space
Photomicrograph of a normal optic nerve head in a medium myopic eye. Black arrows, lamina cribrosa of normal thickness; yellow arrow, end of Bruch’s membrane, leaving a parapapillary region free of Bruch’s membrane (“gamma zone”); red arrows, peripapillary scleral flange of normal thickness and length; blue arrows, pia mater of the optic nerve; green arrow, dura mater of the optic nerve; black star, retrobulbar cerebrospinal fluid space; white arrow, arterial circle of Zinn-Haller
In normal eyes, the lamina cribrosa has a hanging-mat-like shape. In eyes with advanced glaucomatous optic nerve damage, the lamina cribrosa gets condensed and thinned, and it changes its shape [6]. It develops a slight elevation in its central region, where the central retinal vessel trunk appears to stabilize the lamina cribrosa and a sectorial deepening in the inferior and superior peripheral regions. It leads to a W-shaped configuration.
The thickness of the lamina cribrosa was not significantly associated with the thickness of the cornea in a previous histologic study [8, 9]. In a parallel manner, corneal thickness was neither correlated with the thickness of the peripapillary scleral flange nor with the shortest distance between intraocular space and cerebrospinal fluid space. It suggested that an assumed relationship between central corneal thickness and glaucoma susceptibility could not be explained by a corresponding anatomy between corneal thickness and the histomorphometry of the optic nerve head [9, 10].
In highly myopic eyes, the lamina cribrosa is markedly thinned and elongated (Fig. 12.4) [10]. It has been speculated that these myopia-associated lamina cribrosa changes may be some of the reasons for the increased glaucoma susceptibility in highly myopic eyes [11]. The thinning of the lamina cribrosa leads to a shortening of the distance between the intraocular space with the intraocular pressure and the retrobulbar space with the orbital cerebrospinal fluid pressure. A decrease in the distance is geometrically associated with a steepening of the translamina cribrosa pressure gradient. Recent studies have discussed that the translamina cribrosa pressure difference (and gradient) more than the transcorneal pressure difference (so called intraocular pressure) is important for the physiology of the optic nerve head and may potentially play a role in the pathogenesis of glaucomatous optic neuropathy [12].
Studies by Anderson and later by Hernandez and coworkers and Quigley and colleagues assessed the elastic fibers in the lamina cribrosa and their replacement or augmentation by collagen fibers with increasing age or the development of glaucoma [13, 14]. Investigations by Burgoyne and coworkers addressed the remodeling of the lamina cribrosa in glaucomatous ONHs [15]. It has remained unclear so far whether the lamina cribrosa changes in high myopia are comparable to the age-related and glaucoma-associated changes in the connective tissue of the lamina.
2 Parapapillary Region
2.1 Parapapillary Atrophy
Conventionally, the parapapillary region was divided into an alpha zone and beta zone (Fig. 12.5) [16, 17]. Alpha zone was defined by an irregular pigmentation and was detected in almost all eyes. On its outer side, alpha zone was adjacent to the retina, and on its inner side it was in touch with beta zone or, if beta zone was not present, with the peripapillary ring. Beta zone was ophthalmoscopically characterized by a visible large choroidal vessels and visible sclera and was found in about 25% of normal eyes and in a significantly higher proportion of glaucomatous eyes. In cross-sectional and in longitudinal studies, beta zone, not alpha zone, was associated with an increasing glaucomatous loss in neuroretinal rim and an increasing glaucomatous visual field loss. All highly myopic eyes had the (old) beta zone due to the myopic crescent surrounding highly myopic ONHs, independently whether there was glaucomatous optic nerve damage (Fig. 12.6) [18]. In contrast to glaucomatous optic neuropathy, non-glaucomatous optic nerve damage was not associated with an enlargement of beta zone.
Optic nerve head photograph of a highly myopic optic nerve head. Green arrows, parapapillary alpha zone; red arrows, parapapillary beta zone; black arrows, potentially the insertion line of the dura mater of the optic nerve into the posterior sclera; central to this line would be the peripapillary scleral flange; white arrows, peripapillary ring
In recent clinical and histological studies, however, the concept of (old) beta zone has been challenged [19,20,21]. In a histological study, it was differentiated between alpha zone characterized by the presence of BM with irregularly structured and pigmented RPE, beta zone defined as the presence of BM without RPE, gamma zone characterized by the lack of BM and normal thickness of the peripapillary scleral flange, and delta zone characterized by the lack of BM and a markedly elongated and thinned peripapillary scleral flange (Figs. 12.7 and 12.8). It was shown that beta zone (BM without RPE) was correlated with glaucoma but not markedly with globe elongation; that gamma zone (peripapillary sclera without overlying choroid, BM and deep retinal layers) was related with axial globe elongation and that it was mostly independent of glaucoma; and that delta zone was present only in highly axially elongated globes. Since the old beta zone definition included the histological new beta zone, gamma zone, and delta zone, and since gamma zone was not related with glaucoma, one may infer that a clinical differentiation between the new beta zone and gamma zone may increase the clinical diagnostic value of the redefined clinical beta zone (without gamma zone) for the diagnosis of glaucoma. The finding that beta zone was associated with glaucoma and that it was not profoundly associated with myopia suggested that the histological changes observed in histological beta zone, i.e., loss of RPE cells and photoreceptors and a closure of the choriocapillaris, may be related to the glaucomatous optic neuropathy. A recent study suggested that the beta zone may develop due to an intraocular pressure elevation-associated shifting of the parapapillary RPE on BM [22]. Interestingly, the region of BM with the underlying choriocapillaris occluded was significantly smaller than beta zone (defined as BM without RPE cells). One discussed that a complete loss of RPE cells may occur earlier than a complete closure of the choriocapillaris. It could indicate that a loss of RPE cells would lead to the closure of the choriocapillaris since an intact choriocapillaris depends on an intact RPE layer. One has to clearly keep in mind, however, that disturbances in the blood perfusion of the choriocapillaris may have first led to a damage and loss of the RPE, which could then lead to the complete choriocapillaris closure.
(a) Photomicrograph of a glaucomatous optic nerve head in a non-highly myopic eye. Black arrows, condensed thinned lamina cribrosa; blue arrows, pia mater of the optic nerve; red arrow, arterial circle of Zinn-Haller; yellow arrows, (1) end of Bruch’s membrane at the optic nerve head border, (2) end of photoreceptors, (3) end of retinal pigment epithelium layer, (4) end of regularly structured layer of the retinal pigment epithelium; black star, retrobulbar cerebrospinal fluid space. (b) Photomicrograph of a glaucomatous optic nerve head in a non-highly myopic eye (same eye as in Fig. 12.7a; higher magnification). Yellow arrows, (1) end of Bruch’s membrane at the optic nerve head border, (2) end of photoreceptors; black arrow, end of open choriocapillaris. (c) Photomicrograph of a glaucomatous optic nerve head in a non-highly myopic eye (same eye as in Fig. a; higher magnification). Yellow arrows, (2) end of photoreceptors, (3) end of retinal pigment epithelium layer; black arrow, open choriocapillaris. (d) Photomicrograph of a glaucomatous optic nerve head in a non-highly myopic eye (same eye as in Fig. a; higher magnification). Yellow arrows, (3) end of retinal pigment epithelium layer, (4) start of regularly structured retinal pigment epithelium; black arrow, large choroidal vessel
Interestingly, gamma zone was strongly associated with axial length with a steep increase beyond an axial length of 26.5 mm. The cutoff value of an axial length of 26.5 mm was similar to the cutoff values of about −8 diopters for the differentiation between medium myopia and high myopia as suggested in clinical studies. Parapapillary gamma zone has been discussed to develop in two steps. The first step consists of a temporal shift of the BMO during the process of moderate myopization, leading to an overhanging of BM into the intrapapillary compartment at the nasal side of the optic disc and to a lack of BM (i.e., gamma zone) in the temporal parapapillary region. In a second step, if the axial length exceeds 26.0 mm or 26.5 mm, the BMO enlarges, leading to a circular enlargement of gamma zone and appearance of gamma zone also in the nasal parapapillary region [6]. The basis for the notion of a temporal shift of the BMO, leading to a misalignment between the scleral canal and the BMO in eyes with moderate myopia, is the hypothesis that axial elongation occurs by new production of BM in the equatorial region and the sliding BM theory. Since BM is not firmly fixed with the sclera but separated from the sclera by the spongy choroid and the suprachoroidal cleft, one may assume that the spongy choroid allows a sliding or movement of BM in spatial relationship to the sclera. The “supertraction” of the retina and choroid to the temporal side with an overhanging of BM into the open area of the scleral opening of the ONH at the nasal disc side and a region on the temporal ONH side with no BM has already been described by Heine in 1899 [23]. Former ophthalmologists used to call Bruch’s membrane pushing into the nasal intrapapillary region a “supertraction” and a BM-free temporal parapapillary region a “distraction crescent.” The sliding of BM has also been observed in eyes after a marked reduction of IOP [24].
The parapapillary zones gamma and delta are associated with changes in the deep layers of the macula in highly myopic eyes [25]. Highly myopic eyes can show defects in BM in the macular region as demonstrated in recent histologic and clinical studies [25,26,27,28]. These defects in BM are associated with a complete lack of RPE and choriocapillaris and a marked reduction of photoreceptors and large choroidal vessels. The existence of such macular BM holes is strongly associated with axial length and with the parapapillary gamma zone and delta zones. In a recent study, a larger size of the papillary BMO was associated with a lower prevalence of macular BM defects [6].
The parapapillary zones alpha, beta, gamma, and delta as defined and described in histological studies can also be visualized and analyzed clinically by using enhanced depth imaging of optical coherence tomography [21]. In a recent clinical study, gamma zone was significantly associated with longer axial length, longer vertical disc diameter, older age, and absence of glaucoma, while beta zone was associated with longer axial length and presence of glaucoma. It shows that gamma zone and beta zone can clinically be differentiated from each other and that the differentiation is clinically useful [21, 29].
2.2 Peripapillary Border Tissue of the Choroid and Peripapillary Scleral Flange
The choroid and the peripapillary scleral flange are separated from the intrapapillary compartment and the lamina cribrosa by the peripapillary border tissue of the choroid (Jacoby) and the peripapillary border tissue of the scleral flange (Elschnig), respectively [3]. In a histomorphometric study, a thicker choroidal border tissue (mean: 68.8 ± 35.7 μm) was correlated with shorter axial length, and a longer choroidal border tissue (mean: 531 ± 802 μm) was associated with longer axial length. Correspondingly, the cross-sectional area of the choroidal border tissue was not related with axial length. A thicker scleral flange border tissue (mean: 83 ± 21 μm) was associated with the presence of glaucoma as was the thickness of the optic nerve pia mater [3]. Since the border tissues connect BM with the lamina cribrosa, these findings may be of interest for the biomechanics of the optic nerve head in general and in particular in high myopia.
2.3 Peripapillary Scleral Flange
The peripapillary scleral flange originates in the inner half of the posterior sclera and continues into the lamina cribrosa, the thickness of which is almost identical with the thickness of the peripapillary scleral flange (Figs. 12.3, 12.8, and 12.9) [19, 20]. While the inner 50% of the posterior sclera form the peripapillary scleral flange, the outer 50% of the sclera merge with the dura mater of the retrobulbar optic nerve. The peripapillary scleral flange is the part of the sclera between the optic nerve border (defined as optic nerve head scleral canal or the anterior continuation of the pia mater) and the point where the optic nerve dura mater merges with the sclera. The peripapillary scleral flange thus forms the anterior roof of the orbital cerebrospinal fluid space. The peripapillary scleral flange may also serve for functional dynamic purposes: the pulse wave in the orbital cerebrospinal fluid space and the pulse wave in the eye (“ocular pulse”) may have their maxima at slightly different time points. This would lead to a fluctuating change in the translamina cribrosa pressure difference and consequently to an undulating movement of the lamina cribrosa in sagittal direction. The peripapillary scleral flange could function here as a flange similar to the flange or a hinge in a door swinging in and out.
The length of the scleral flange increases with axial length and decreases with the thickness of the flange [17, 18]. The increased length of the flange in highly myopic eyes leads to an extension of the orbital cerebrospinal fluid space into the retrobulbar peripapillary region. At this location, the cerebrospinal fluid is separated from the vitreous cavity just by a thin peripapillary scleral flange (as thin as 50 μm), retinal nerve fibers, and the retinal inner limiting membrane. It has remained unclear, whether, and if yes, in which layer, a watertight shed exists between the intraocular compartment and the compartment of the extended peripapillary cerebrospinal fluid space. Considering that the highly myopic scleral flange is not covered on its inner side by Bruch membrane and RPE, one may speculate whether there may be some leakage of fluid through the retinal nerve fiber layer and the water permeable scleral tissue. This fluid leakage would reduce the intraocular pressure and lead to a different composition of the cerebrospinal fluid in the retrobulbar region as compared to the apex of the orbit. It has also remained unclear so far, whether the extension of the retrobulbar cerebrospinal spinal fluid space into the parapapillary region has pathophysiological consequences. One may speculate whether the very thin peripapillary scleral flange in highly myopic eyes may act in a similar manner as open fontanelles do in babies. The scleral flange may undulate as consequence of the ocular pulse and may thus lead to a change in the ocular pulse.
In highly myopic eyes, the peripapillary scleral flange elongates from about 500 μm to 5 mm by a factor of 10. Simultaneously, the flange thins from about 500 μm to 50 μm by a factor of 1/10. Considering the peripapillary scleral flange as biomechanical anchor for the lamina cribrosa, the myopia-associated stretching and thinning of the flange may be one of the reasons for the increased glaucoma susceptibility in highly myopic eyes. The biomechanical consequence of a thin parapapillary sclera in the highly myopic eyes may further be aggravated by the finding that the highly myopic eyes did not have the normal composition of the parapapillary anatomy. In the highly myopic eyes as in contrast to the non-highly myopic eyes, the parapapillary retina is composed of retinal nerve fiber layer (or its remnants) only, without elements of any other retinal layer, without parapapillary Bruch membrane or choroid. The implications of a missing Bruch membrane as stable element in the whole architecture of the retina-choroid complex and the consequence of missing choroidal vessels at the border of highly myopic optic nerve head have remained unknown so far.
The elongation of the peripapillary scleral flange in delta zone of highly myopic eyes is associated with an increased distance between the peripapillary arterial circle of Zinn-Haller and the optic disc border. The arterial circle of Zinn-Haller is usually located close to the merging point of the dura mater with the posterior sclera [30]. Since the arterial circle of Zinn-Haller supports the blood vessels in the optic nerve head, in particular in the lamina cribrosa, one may speculate whether the tenfold increase in the distance between the arterial circle and the lamina cribrosa may lead to a malperfusion of the lamina cribrosa. Anatomical studies which specifically examined the communicating vessels between the arterial circle of Zinn-Haller and the tissue of the lamina cribrosa in highly myopic eyes have been missing so far.
The thinning of the scleral flange in highly myopic eyes is paralleled by myopia-associated changes of the sclera in other fundus regions. Examining formalin-fixed human globes, a recent histomorphometric study showed that in non-axially elongated eyes with an axial length of ≤26 mm, the sclera was thickest at the posterior pole (0.94 ± 0.18 mm), followed by the perioptic nerve region (0.86 ± 0.21 mm), the midpoint between posterior pole and equator (0.65 ± 0.15 mm), the limbus (0.50 ± 0.11 mm), the ora serrata (0.43 ± 0.14 mm), the equator (0.42 ± 0.15 mm), and finally the peripapillary scleral flange (0.39 ± 0.09 mm) [31]. In axially elongated eyes, scleral thinning occurred at and posterior to the equator, being more marked closer to the posterior pole and the longer the axial length was. Scleral thickness anterior to the equator did not markedly differ between the highly myopic eyes and the non-highly myopic eyes. Within the anterior and posterior segment, respectively, scleral thickness measurements were correlated with each other. Posterior scleral thickness was correlated with the lamina cribrosa thickness. Scleral thickness measurements at any location of examination were not significantly correlated with corneal thickness or with age, gender, and presence of absolute secondary angle-closure glaucoma [31].
References (Further References Are Found in These Citations)
Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29:1151–8.
Jonas JB, Budde WM, Panda-Jonas S. Ophthalmoscopic evaluation of the optic nerve head. Surv Ophthalmol. 1999;43:293–320.
Jonas RA, Holbach L. Peripapillary border tissue of the choroid and peripapillary scleral flange in human eyes. Acta Ophthalmol. 2020;98:e43–e9.
Jonas JB, Schmidt AM, Müller-Bergh JA, Schlötzer-Schrehardt UM, Naumann GOH. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–8.
Panda-Jonas S, Jonas JB, Jakobczyk M, Schneider U. Retinal photoreceptor count, retinal surface area, and optic disc size in normal human eyes. Ophthalmology. 1994;101:519–23.
Zhang Q, Xu L, Wei WB, Wang YX, Jonas JB. Size and shape of Bruch’s membrane opening in relationship to axial length, gamma zone and macular Bruch’s membrane defects. Invest Ophthalmol Vis Sci. 2019;60:2591–8.
Jonas JB, Mardin CY, Schlötzer-Schrehardt U, Naumann GOH. Morphometry of the human lamina cribrosa surface. Invest Ophthalmol Vis Sci. 1991;32:401–5.
Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci. 2003;44:5189–95.
Jonas JB, Holbach L. Central corneal thickness and thickness of the lamina cribrosa in human eyes. Invest Ophthalmol Vis Sci. 2005;46:1275–9.
Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004;45:2660–5.
Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility. The Beijing Eye Study. Ophthalmology. 2007;114:216–20.
Ren R, Jonas JB, Tian G, Zhen Y, Ma K, Li S, Wang H, Li B, Zhang X, Wang N. Cerebrospinal fluid pressure in glaucoma. A prospective study. Ophthalmology. 2010;117:259–66.
Hernandez MR. Ultrastructural immunocytochemical analysis of elastin in the human lamina cribrosa. Changes in elastic fibers in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1992;33:2891–903.
Quigley EN, Quigley HA, Pease ME, Kerrigan LA. Quantitative studies of elastin in the optic nerve heads of persons with primary open-angle glaucoma. Ophthalmology. 1996;103:1680–5.
Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, Downs JC. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:681–90.
Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18.
Jonas JB, Naumann GOH. Parapapillary chorio-retinal atrophy in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989;30:919–26.
Jonas JB, Gusek GC, Naumann GOH. Optic disk morphometry in high myopia. Graefes Arch Clin Exp Ophthalmol. 1988;226:587–90.
Jonas JB, Jonas SB, Jonas RA, Holbach L, Panda-Jonas S. Histology of the parapapillary region in high myopia. Am J Ophthalmol. 2011;152:1021–9.
Jonas JB, Jonas SB, Jonas RA, Holbach L, Dai Y, Sun X, Panda-Jonas S. Parapapillary atrophy: Histological gamma zone and delta zone. PLoS One. 2012;7:e47237.
Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: Beta zone and gamma zone. Invest Ophthalmol Vis Sci. 2013;54:2013–8.
Wang YX, Jiang R, Wang NL, Xu L, Jonas JB. Acute peripapillary retinal pigment epithelium changes associated with acute intraocular pressure elevation. Ophthalmology. 2015;122:2022–8.
Heine L. Beiträge zur Anatomie des myopischen Auges. Arch Augenheilkd. 1899;38:277–90.
Panda-Jonas S, Xu L, Yang H, Wang YX, Jonas SB, Jonas JB. Optic disc morphology in young patients after antiglaucomatous filtering surgery. Acta Ophthalmol. 2014;92:59–64.
Jonas JB, Ohno-Matsui K, Spaide RF, Holbach L, Panda-Jonas S. Macular Bruch’s membrane holes in high myopia: associated with gamma zone and delta zone of parapapillary region. Invest Ophthalmol Vis Sci. 2013;54:1295–30.
Ohno-Matsui K, Jonas JB, Spaide RF. Macular Bruch membrane holes in highly myopic patchy chorioretinal atrophy. Am J Ophthalmol. 2016;166:22–8.
Ohno-Matsui K, Jonas JB, Spaide RF. Macular Bruch membrane holes in choroidal neovascularization-related myopic macular atrophy by swept-source optical coherence tomography. Am J Ophthalmol. 2016;162:133–9.
Fang Y, Jonas JB, Yokoi T, Cao K, Shinohara K, Ohno-Matsui K. Macular Bruch’s membrane defect and dome-shaped macula in high myopia. PLoS One. 2017;12:e0178998.
Manalastas PIC, Belghith A, Weinreb RN, Jonas JB, Suh MH, Yarmohammadi A, Medeiros FA, Girkin CA, Liebmann JM, Zangwill LM. Automated beta zone parapapillary area measurement to differentiate between healthy and glaucoma eyes. Am J Ophthalmol. 2018;191:140–8.
Jonas JB, Jonas SB. Histomorphometry of the circular arterial ring of Zinn-Haller in normal and glaucomatous eyes. Acta Ophthalmol. 2010;88:e317–22.
Vurgese S, Panda-Jonas S, Jonas JB. Sclera thickness in human globes and its relations to age, axial length and glaucoma. PLoS One. 2012;7:e29692.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jonas, J.B., Panda-Jonas, S. (2021). The Optic Nerve Head in High Myopia/Abnormalities of the Intrapapillary and Parapapillary Region. In: Spaide, R.F., Ohno-Matsui, K., Yannuzzi, L.A. (eds) Pathologic Myopia. Springer, Cham. https://doi.org/10.1007/978-3-030-74334-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-74334-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74333-8
Online ISBN: 978-3-030-74334-5
eBook Packages: MedicineMedicine (R0)