Abstract
Single-domain antibodies (VHHs), in particular those engineered from the variable heavy-chain fragment (VHH gene) found in Camelidae heavy-chain antibodies, are the smallest fragments that retain the full antigen-binding capacity of the antibody with advantageous properties as drugs or targeting agents. The high stability of VHHs against high concentrations of organic solvents and extreme pHs and temperatures opens a wide range of applications for the detection of small molecules such as the development of more efficient methods of immobilization for biosensing purposes in addition to attachment sites in several immunotherapeutic constructs such as chimeric T cell therapy. VHHs, due to their unique 3D structure, have versatile abilities to inhibit or modulate enzyme activity, bind soluble factors, internalize cell membrane receptors, or block cytoplasmic targets. This chapter summarizes the applications and great potentials of VHHs as therapeutic tools or noninvasive in vivo molecular imaging agents. Moreover, the unique properties of VHHs are outlined like internalization, size, thermal and chemical stability, affinity, blood clearance, and labeling procedures. We highlight some already-reported therapeutic examples about VHHs being used for the treatment of several diseases such as cancer and several nanostructures which are used as molecular imaging or delivering tools.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Specific Characteristics of VHH
Antibodies are undoubtedly one of the crucial macromolecules in the immune system. Human antibodies are categorized based on the type of heavy chain, alpha (α), delta (δ), gamma (γ), epsilon (ε), and mu (μ) which give rise to IgA, IgD, IgG, IgE, and IgM, respectively. Among different types of antibodies, IgG plays an important role in passive immunotherapy for a wide span of maladies, especially cancer and autoimmunity. Human IgGs comprise four distinct polypeptide chains, two heavy chains and two light chains, H2L2 antibodies. The heavy chain is made of four domains, NH3–VH–CH1–CH2–CH3–COOH. The CH3 and CH2 establish antibody Fc, which interact with different immune effector cells, such as natural killer cells in antibody-dependent cell cytotoxicity (ADCC), or collaborate with serum protein such as complements in opsonization of invaders. There is no or little evidence regarding the interaction of IgG light chains with immune cells.
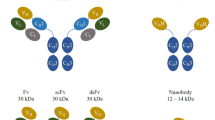
Unlike the heavy chain, the IgG light chain consists of two domains, CL1 and VL, which bind to their heavy chain counterpart and collectively shape the Fab fragment. The SDS-PAGE pattern of antibodies usually shows two bands, one heavy chain (50 kDa) and one light chain (25 kDa). The whole IgG molecule is gigantic, 150 kDa, with dimensions of 14.2 nm × 8.2 nm × 3.8 nm. The size of the IgG is a big issue in terms of antibody therapy and drug delivery. Whole IgG tumor penetration is low as the core of the tumor is extensively dense with minimal leakage. It is estimated that the penetration of monoclonal antibodies into the solid tumor is around 0.001–0.1% of injected dose (Marcucci et al., 2013; Thurber et al., 2008; Christiansen & Rajasekaran, 2004). Among the six aforementioned domains, only VH and VL engage together and generate scFv, which is the smallest fragment derived from H2L2 antibody with the ability to bind to the target of interest. Even though there are many products based on the scFv on the market, some disadvantages have been associated with this type of molecule. Firstly, the CDR3 length is comparatively small; hence it would not be able to recognize and bind to protein cavities or clefts. Secondly, due to reducing tumor microenvironments, scFvs disulfide bond may become broken, which significantly hamper the binding. All scFvs have two domains, VH and VL, which are usually locked together through a disulfide bond and a linker, usually glycin4serin. Thirdly, the scFv solubility is usually low. The residues in the interface of VH and VL are mostly composed of hydrophobic amino acids helping to keep two domains of scFv together through hydrophobic interactions. In prokaryotic expression, sometimes these hydrophobic amino acids of VH cannot thoroughly cover VL hydrophobic patches. Consequently, exposed hydrophobic amino acids cause extensive aggregation.
In cancer antibody therapy, antibody exerts its efficacy in three ways, first by blocking the receptor, and second by antibody-dependent cell cytotoxicity or ADCC. Upon binding to the target of interest, the natural killer cell binds to the Fc of the bound antibody through Fcγ receptor III a (CD16a) and kills the tumor. It is observed that cells with even a low density of receptor of interest are as good targets as cells overexpressing that receptor. The side effect of anti-HER2 antibody therapy attests to this claim. Cardiomyocytes express a very low level of the HER2 receptor. There are some reports of cardiotoxicity of Herceptin (anti-HER2 antibody) in breast cancer patients. This toxicity emanates from NK cell ADCC of cardiomyocyte and damages the heart tissue (Leemasawat et al., 2020; Herrmann, 2020; Padegimas et al., 2020). Last but not least, bound antibody can activate complement system to lyse the target cells.
In the early 1990s, scientists serendipitously came across a Camelidae IgG antibody with a different SDS-PAGE pattern (Hamers-Casterman et al., 1993). This pattern attested to the lack of a light chain. Later, this kind of antibody was named as Heavy-Chain only Antibody or HCAb with the molecular weight of 95 kDa. Further rummage into HCAbs revealed that lacking a light chain is not the only difference. This antibody also lacks the CH1 domain. Camelidae IgG has three subclasses, IgG1, IgG2, and IgG3. The IgG1 is identical to human IgGs with a heavy chain and light chain. But IgG2 and IgG3 are the ones called HCAb, solely composed of a heavy chain. The variable fragment of HCAb, which binds to the target of interest, is called the Variable of Heavy chain HCAb or VHH. Unlike scFv, VHH is composed of a single-domain antigen-binding fragment. For the very same reason, this molecule also is called either single-domain antibody (sdAb) or nanobody. VHH has many advantages over scFv. The most conspicuous advantage of VHH compared to the whole IgG is the petite size. The molecular weight of VHH is around 12–15 kDa with dimensions of 4 nm × 2.5 nm × 3 nm. Due to its small size, VHHs are able to penetrate the solid tumor, far more efficient than the whole antibody. VHHs are resistant to reducing tumor microenvironment. Besides, VHHs are very thermostable due to one or two intramolecular disulfide bonds (Muyldermans, 2020).
4.2 Structure of VHH
The secondary structure of VHH is very similar to both VH and VL domians of a conventional antibody. VHH consists of nine antiparallel β strands, A–B–C–C′–C″–D–E–F–G. These β strands are connected through several loops, three of the them participate in binding to the targets, H1, H2, and H3, which connects B–C, C′–C″, and F–G, respectively. From an immunological perspective, VHH structure includes four frameworks (FR 1–4) and three complementarity-determining regions (CDR), CDR 1–3 (Fig. 4.1). Frameworks are the platform of the molecule, which are conserved and work as a backbone of the entire molecule. This part of the molecule does not take part in binding directly but has a direct impact on the correct folding of CDRs. Hence the sequence of the frameworks could be effective on VHH affinity. The frameworks are connected via CDRs, which are hypervariable. This part of the molecule is responsible for binding specifically to the target of interest. Aligning immunological with biochemical secondary structure together, CDR1–3 are actually the loops 1–3 (H1, H2, H3). One of the disadvantages of VHH is inefficient binding to non-proteaceous targets, such as small molecules, and carbohydrates. By structural analysis of anti-methotrexate, anti-triclocarban, and anti-cortisol VHHs, Arabi-Ghahroudi et al. found out that the non-hypervariable loop between D and E β strands, within FR3, works as a CDR4 and plays an important role in binding to non-proteinaceous targets. They observe that this loop is 14 residues longer in anti-hapten VHHs compared to normal VHHs (Henry et al., 2019).
(a) Camelidae antibodies are devoid of the light chain. VHH, the variable of the heavy chain of HCAb, is the smallest natural binding moiety. VHH has an elongated CDR3, which is able to penetrate protein cavities and clefts. By comparing the sequence of VHH with human VH, four residues, denoted with green stars, are replaced in VHH by small and hydrophilic amino acids. These residues play an important role in VHH solubility. (b) Human antibody is composed of two heavy chains and two light chains. The variable domain is composed of two different domains, VH and VL, also called scFv. (c) The Fc region of antibody plays a vital role in cancer therapy. This domain interacts with natural killer cells through CD16 (FcγRIIIa) to exert ADCC. Besides, antibody-coated invaders are opsonized by the complement system which facilitates phagocytosis
As mentioned earlier, CDR3 connects F–G β strands, is longer than CDRs in convention antibodies. The length of CDR3 in VHH is on average 18 residues, but in humans and mice, VH CDR3 is 14 or 12 residues, respectively. Scientists found out CDR3 plays the most vital role in the affinity and specificity of the VHH. It is assumed that, during evolution, the length of CDR3 becomes longer to compensate for the absence of the light chain (Kubala et al., 2010). There are some reports that even VHH can reach enzyme active sites and either play an agonist or antagonist role. It is important to mention that CDR1 and CDR2 also form flat paratopes to bind to convex and concave epitopes. Hence, VHH can reach out to both protein cavities, clefts (through protruded CDR3) and convex, concave paratope (through CDR1 and CDR2), while VH and VL CDRs are shorter than VHH CDR3 and only recognize flat, convex, and concave epitopes. In another word, the epitopes that are inaccessible by scFv are accessible via protruded VHH CDR3 (Harmsen & De Haard, 2007).
But elongated CDR3 is not the only difference between VHH and VH. VHHs are very soluble proteins due to their hydrophilic amino acid composition. This feature makes VHH ideal for heterologous protein expression. As mentioned above, scFv solubility is lower than VHH. With a closer look at the VHH and VH structure and amino acid composition, it is observed that the second framework of VH is composed of hydrophobic amino acids. These amino acids form a hydrophobic patch that binds to the VL through hydrophobic interaction and stabilize the molecule. These hydrophobic amino acids are replaced by small and hydrophilic ones in VHH. The most notable substitutions are V37F/Y (in the strand C), G44E, L45R, and W47G (in the stand C′) (the exact number may be different based on the algorithm of antibody residues numbering). VHH is a very stable molecule. This stability is another distinction of VHH. Most of the VHH has an intramolecular disulfide bond to connect CDR1 (in camels) or CDR2 (in llamas) to CDR3 (Manglik et al., 2017). This bond helps to stabilize elongated CDR3. To produce disulfide-bonded VHH in a prokaryote, this molecule must be expressed and ushered to the periplasmic space. The E. coli cytoplasm environment is reducing and is not suitable for disulfide bond formation. But the periplasmic space is an oxidizing environment and also benefited from some foldase and chaperone to establish disulfide bonds. In the periplasmic space, DsbA and DsbB carry out the de novo disulfide bond formation and DsbC and DsbD proofread the bond. DsbA is a potent oxidase that oxidizes the cysteine residues in the periplasmic space. But the only oxidizing space and/or presence of DsbA is not enough for correct disulfide bond formation (Hatahet et al., 2010; Nguyen et al., 2011). DsbC is a V shape disulfide isomerase which ensures the correct S–S formation and folding. Besides, there are some engineered strains of E. coli with reducing cytoplasms, such as Origami B (DE3) (Kaplan et al., 2016) and SHuffle® (Lobstein et al., 2012). The advantage of SHuffle® is that DsbC is constitutively expressed in the cytoplasm. This ensures that the disulfide bond is established correctly, which is vital for protein folding and function. Based on our data, the expression of the SHuffle® is lower than BL21 Star (DE3) (Nikkhoi et al., 2017). The first reason is that due to the expression of some extra protein including DsbC, SHuffle® is metabolically more burdened than BL21 Star (DE3). The second reason is that the doubling time is SHuffle® in longer. It is noteworthy to mention that the inclusion body in BL21 Star (DE3) was higher than SHuffle®. Consequently, although the yield is lower in SHuffle®, the expressed VHH is soluble and in native conformation. In a recent study, scientists found out VHHs expressed in SHuffle® are heterogeneous in terms of disulfide bridge formation. This means the efficiency of disulfide bridge formation is not absolute, and still some proteins are expressed without an S–S bond. Conversely, VHH expressed in the periplasmic space revealed a more homogeneous disulfide-bonded protein (Chabrol et al., 2020).
4.3 VHH Cell Penetration
Due to the petite dimension, VHH can also be used to penetrate the cell as either agonist or antagonist of key enzymes, signaling molecules, or even viral enzymes inside cells. VHHs are unable to penetrate the cell without being fused to Cell-Penetrating Peptide (CPP). CPPs, 5–30 amino acids, are the sequence that gives rise to enhanced cellular uptake. One of the biggest disadvantages of CPP is that cargoes are delivered to any type of cell without discrimination (Böhmová et al., 2018). One of the efficient CPP is called penetratin. NS5B is pivotal RNA-dependent RNA polymerase which is crucial for the hepatitis C virus (HCV) replication. NS5B is a promising target in HCV therapy. Blocking this enzyme may end up suppressing virus replication. Hence, scientists developed a penetrable anti-NS5B VHH to block the RNA polymerase. To do so, anti-NS5B VHH was fused to penetratin to be able to enter the contaminated cells. Using this technique, they efficiently block the HCV replication inside the contaminated cells (Thueng-In et al., 2012). Cell-penetrating VHHs are ideal tools for tracing a molecule inside the cell. Chromobody, VHH fused to a fluorescent protein, is fully functional even in the reducing environment of cytoplasm. Li et al. used cell chromobody, anti-GFAP (glial fibrillary acidic protein) VHH fused to CPP and GFP, to track and image astrocytes (Li et al., 2012). In another study, a group of scientists used two cell-permeable chromobodies to show a protein-protein interaction. In this study, two VHHs against p53 and PCNA (proliferating cell nuclear antigen) were fused to arginine-rich CPP. They chose VHH since the molecular weight of the cargo of CPPs is a decisive factor in internalization efficiency. Using this technique, they were able to study the interaction of P53 and PCNA in the nucleus (Li et al., 2012).
4.4 Humanization of VHH
Humanization of antibodies is the best way to eliminate immune response when administering antibodies raised in different species. There are many humanized monoclonal antibodies with FDA approval, such as palivizumab, trastuzumab, natalizumab, and bevacizumab (-zumab suffix in antibody nomenclature denotes to humanized antibodies). To humanize the VHHs, the rule of thumb is that CDRs should be left intact. Any change to this part of VHHs may disrupting the affinity. The only parts that should be subjected to humanization are frameworks. It must be noted that the four residues in FR2, V37, G44, L45, and W47 are key for solubility, affinity, and stability; hence changing these residues must be done carefully. Totally there are ten amino acid difference between VHH and human VH, including four key amino acids mentioned above. Humanizing VHH should be neutral to affinity and stability. There is a report regarding changing amino acids in frameworks that may be detrimental to the folding of CDRs, especially CDR3, which is a consequence of loss of binding (Vincke et al., 2009). To circumvent this problem, humanization is partially done to prevent significant changes in affinity (Dong et al., 2020).
4.5 VHH-Based Therapeutic Systems
4.5.1 VHH-Drug Conjugates
One of the perks of VHHs is the ease of production and satisfactory penetration in solid tumors. These features make this molecule an ideal candidate for antibody-drug conjugate (ADC). In ADC, a drug is conjugated to an antibody binding to a tumor marker. The antibody moiety works as a vehicle to deliver cargo to the tumor microenvironment and target cells. Using this system, the cargo is localized to the tumor site and accumulates the therapeutic agent close to cancer cells. This system not only improves the efficiency of therapy but also curtails the side effects and toxicities.
There are many different compounds used to conjugate to an antibody to develop ADC. One of which is inhibitors, which block a vital biochemical process inside a cell. In 2015, Fang et al. generated ADC using VHH7, anti-MHC-II, and Mertansine (DM1) (Fang et al., 2016). The DM1 is a potent inhibitor of microtubule polymerization but with a narrow therapeutic window when used alone. A therapeutic window is a range of concentrations that the drug is effective with low toxicity. According to their results, DM1 toxicity was 20 times lower in HEK293 and HeLa (MHC-II negative cells) compared to A20 cells (MHC-II positive). Cucurmosin (CUS), extracted from pumpkin pulp, is a strong ribosome inhibitor. Deng et al. fused the anti-EGFR to CUS and showed improved targeted toxicity in HepG2 and A549 cell lines compared to SUC alone (Deng et al., 2017).
Enzymes also can be used in ADC. One of the widely used enzymes is urease, which converts urea to ammonia that is toxic for cancer cells (Tian et al., 2015). In a study, urease was conjugated to anti-vascular endothelial growth factor receptors (VEGFRs) VHH to deliver the enzyme to the tumor site. VEGFR is a hub receptor for angiogenesis in a tumor (Tian et al., 2017). Without angiogenesis, the tumor dies due to a lack of nutrients and oxygen. The results showed significant targeted toxicity in cells expressing VEGFR.
The other option to generate ADC is bacterial toxins. Pseudomonas exotoxin A, a bacterial toxin, fused to anti-CD7 VHH to induce apoptosis in CD7+ cells including Jurkat, CEM, T-cell acute lymphoblastic leukemia (T-ALL), and acute myeloid leukemia (AML) (Tang et al., 2016). In another study, PE38, also Pseudomonas exotoxin A (PE38), fused to anti-CD38 VHH (Wang et al., 2016). This immunotoxin showed great selective toxicity in several multiple myeloma cell lines. A similar study was also conducted by Li et al. using PE38 to VHH targeting vascular endothelial growth factor receptor 2 (VEGFR2) (Behdani et al., 2013).
4.5.2 VHH in Cancer Immune Cell Therapy
Adaptive immunotherapy is now the brightest road to contain cancer. Immunotherapy is a very broad field of study that is beyond the scope of this chapter. Although T cells are grouped as adaptive immunity and NK cells as innate immunity, both cells have a lot in common, especially in terms of immunotherapy (Mikkilineni & Kochenderfer, 2021). Both cells get activated upon encountering cancer cells (Galluzzi et al., 2020) and secretes perforin and granzyme B to kill tumor cells. Besides, both cells, upon activation, also secrete some cytokine and interleukin to boost the immune system to fight cancer more efficiently (Waldman et al., 2020). Here, we explain the recent advances in T and NK cell-based immunotherapy using VHH as a targeting moiety.
4.5.2.1 CAR T Cell Therapy
Chimeric antigen receptor (CAR) T cell has revolutionized adoptive immunotherapy. A chimeric antigen receptor comprises five distinct parts, 1) antigen-binding fragment, 2) spacer between antigen binding fragment and cell membrane, 3) transmembrane, 4) co-stimulatory, and 5) activator (CD3ζ) domain(s). Upon facing tumor cells, the antigen-binding domain engages the target of interest and an activatory signal relay to the immune cells. Co-stimulatory domains, such as CD28, OX40, 4-1BB, and many more, boost the signal to CD3ζ which subsequently activates T cells more robustly. Activated T cell secretes granzyme B and perforin into the immunological synapse. Perforins generate pores on the target cell which serves as a safe passage for granzyme B to get into the cell. Granzyme B activates Caspase cascades and subsequently leads to apoptosis that kills the cancerous cell. Here we mainly focus on CAR T cells having VHH as an antigen-binding domain. Utilizing CAR T cells has been very efficient in hematological malignancies, especially multiple myeloma. One of the important tumor markers in multiple myeloma is CD38. Anti-CD38-based CAR T cells therapy has been promising due to low side effects (Drent et al., 2016, 2017). VHH-based anti-CD38 CAR T cell was developed in 2018 by Chinese scientists (An et al., 2018). This engineered T cell was very efficient in secreting IL-2, TNF-α, and IFN-γ upon encountering the target cells. Besides, anti-CD38 CAR T cells showed little off-target toxicity.
Utilizing CAR T cells in solid tumors is not as efficient as in hematological malignancies. The reason is that solid tumors are quite dense and hard to penetrate. Most of the activated T cells are trapped on the edge of tumors. There are many undergoing pieces of research to increase the efficiency of solid tumor immunotherapy. In 2018, Munter et al. generated a dual CAR T cell. As an antigen-binding fragment, they used anti-HER2 and anti-CD20 VHH fused through Llama IgG2a hinge. The results showed that the bivalent VHH was able to bind to HER2 and CD20 simultaneously. Besides, the dual CAR T cells were able to kill both HER2+ and CD20+ cell lines (De Munter et al., 2018). The next important tumor biomarker is VEGFR2, which plays a central role in cancer progression and metastasis. This receptor facilitates angiogenesis which increases the nutrient and oxygen in the tumor site. Using anti-VEGFR2-based CAR T cells, not only VHH was able to bind and block this receptor but also kill the cells expressing this tumor biomarker (Hajari Taheri et al., 2019). Rajabzadeh et al. developed second-generation CAR T cells using anti-MUC1 VHH and CD28 as the co-stimulatory domain. According to their results, after co-culturing CAR T cell with MUC1+ cell lines, MCF-7 and T47D, the secretion of TNF-α and IFN-γ were increased several folds compared to co-culturing with untransduced T cell (Rajabzadeh et al., 2018, 2021). One of the disadvantages of monotherapy is that cancer cells may become resistant through different mechanisms, such as genetic drift. One approach to circumvent this dilemma is to use oligoclonal VHH as the binding domain. Oligoclonal VHH includes more than two nonoverlapping VHH binding to different epitopes, either on the same receptor, which is monovalent, or on the different receptors which are oligospecific. In 2014, Jamnani et al. generated an oligoclonal VHH-based CAR T cell using nonoverlapping anti-HER2 VHH. The results showed that oligoclonal VHH illustrated better toxicity toward HER2+ breast cancer cell line compared to monovalent CAR T cells (Jamnani et al., 2014). There are many studies using VHH-based CAR T cells in solid tumor immunotherapy such as breast cancer (Khaleghi et al., 2012; Iri-Sofla et al., 2011), prostate cancer (Hassani et al., 2019), and many more.
There is a new concept in CAR T cell therapy, so-called Universal CAR, or UniCAR. The difference between UniCAR to usual CAR lies in the extracellular antigen-binding fragment (Bachmann et al., 2018). In UniCAR the antigen-binding domain is against a unique epitope, not a tumor biomarker (Loureiro et al., 2018). Then the antibody, binding to the tumor biomarker, is fused to the epitope that is specific for UniCAR. The fused antibody is used to make a bridge between immune effector cells and target cells (Bachmann, 2019). One of the widely used epitopes in UniCAR is E5B9 which is not immunogenic and is not expressed on the surface of normal cells. The UniCAR is equipped with anti-E5B9 scFv, which binds to E5B9-tagged antibody to elicit an immune response. One of the leading scientists working in this field is Dr. Michael Bachmann. He has published several articles regarding VHH-based UniCAR. He generated a UniCAR expressing anti-E5B9 scFv as an extracellular binding moiety and then fused the anti-EGFR VHH to E5B9 tag. The fused VHH binds to the target of interest, EGFR, and UniCAR binds to E5B9 tag (Jureczek et al., 2020; Albert et al., 2017, 2018). Consequently, the effector immune cell can attack the target cell. The advantage of this system is that there is no need to develop new CAR T cells for different types of cancer. It is noteworthy to stipulate that transfecting and engineering an immune cell is very laborious, expensive, and time-consuming. Using UniCAR, different types of cancer can be treated just by changing the E5B9-tagged VHH or scFv (Bachmann et al., 2018).
CAR T cells are not always used to attack tumor cells. In 2019, a group of scientists at MIT developed a new CAR T cell directed toward the tumor microenvironment (Xie et al., 2019). Through this strategy, not only engineered T cells were ushered to the tumor site but also trapped there and remained for a longer time (Xie et al., 2019, 2020). Besides, they engineered T cells to secrete anti-CD47 and anti-PD-1 VHH to overcome the immunosuppressive tumor microenvironment. The CD47 is a “don’t eat me” signal expressed by tumor cells to evade immune response (Eladl et al., 2020). The PD-1 is an inhibitory receptor expressed on T cells. When engaged to PD-L1, which is expressed on tumor cells, T cells become deactivated (Barclay et al., 2018; Havel et al., 2019). Hence, using the aforementioned strategy, T cells not only could be enriched in the tumor microenvironment but also escape the immune-suppressive tumor milieu.
4.5.2.2 CAR NK Cell Therapy
Unlike T cells, natural killer (NK) cells are part of the innate immune system, meaning NK cells recognize tumor cells or foreign invaders without undergoing somatic recombination (Shimasaki et al., 2020). One of the eye-catching advantages of NK cells is the lack of graft-versus-host disease (GVHD) response in patients (Guillerey et al., 2016). This means if an NK is administered to multiple patients, no toxic immune response will be raised against the estrange NK cells. This has helped the scientists to come up with an off-the-shelf immunotherapy system, which means the optimized NK cell can be prepared and administered to multiple patients without any host immune response. On the opposite side, GVHD is a big problem with T cell therapy. To address this concern, to prepare CAR T cell dose, PBMCs are collected from each patient, engineered by viral vectors to express CAR, and then reinfused to patients. This process makes the CAR T cells therapy pricey (Myers & Miller, 2021).
A group of scientists from Germany and the UK developed anti-CD38 VHH-based CAR NK. CD38 is NAD-hydrolyzing ectoenzyme, which is overexpressed in hematological malignancies, especially multiple myeloma. In this study, NK 92, an immortalized NK cell, was transduced virally to stably express CAR construct. Then, they used bone marrow samples of eight patients to test the CAR NK cell. The results were promising since anti-CD38 CAR NK was able to eliminate CD38+ cells from bone marrow samples (Hambach et al., 2020). In a similar study, anti-CD7 VHH-based CAR NK-cell was used for acute lymphoblastic leukemia (T-ALL) therapy. Using CAR NK cells to eradicate CD7+ T-ALL resulted in an elevated level of Granzyme B and interferon γ (IFN-γ) following co-culture of NK and T-ALL cells (Fig. 4.2).
Different strategies of adoptive immunotherapy. Chimeric antigen receptor (CAR) is a novel approach to direct NK and T cell activity toward specific cancer cells. Upon binding to the target of interest, CARs relay the activating signal to either NK or T cells. Consequently, after secretion of granzyme B and perforin, cancer cells start apoptosis. The second strategy to harness immune cell activity is a bivalent antibody. A bivalent antibody is composed of two antibodies, the first one binds to tumor-associated antigen (TAA) and the second one binds to either CD16 or CD3 on NK and T cells, respectively. The bivalent antibody works as a bridge between the target cell and immune effector cell. Upon immunological synapse formation, the effector cell lyses the tumor cells
4.5.3 Targeted Cancer Therapy by VHH-Armed Nanoparticles
One of the breakthroughs in cancer therapy developed almost two decades ago. The rapid expansion of using nanotechnology in medicine, nanomedicine, has opened a new and promising window in fighting cancer. Nanoparticles can be targeted toward tumor microenvironments by either passive or active targeting. Passive targeting means selective delivery of nanoparticles using a unique feature of the tumor microenvironment. There are many ways of passively targeting the drugs to the tumor site, one of which is enhanced permeation and retention (EPR). To expand very fast, tumors must be provided with sufficient amounts of nutrients and oxygen to generate enough energy (Lugano et al., 2020). To achieve this, cancer cells start establishing a neovascular vessel inside a solid tumor through a process called angiogenesis, which is fast and imperfect (Folkman, 2006). Newly established vessels have many pores in nanometer diameter, which is not seen in normal vessels (Fig. 4.3). These pores significantly increase the permeation of nanoparticles. It is observed that nanoparticles around 20–500 nm can pass through the pores in the leaky vessels but not in normal vessels (Kalyane et al., 2019). The other characteristic of a solid tumor is longer retention of drugs inside the tumor before being drained through the lymphatic system. The reason is that the solid tumor is tremendously dense in the core which causes the lymphatic vessels to collapse and block the drainage. It is noteworthy to mention longer retention does not apply to small molecule drugs since they are rapidly cleared from the blood through the renal system but encapsulating them inside a nanoparticle facilitate the delivery to the cancer cells. There are two FDA-approved nanoparticle-based drugs on the market, Doxil© and Caelyx©, which make most of the EPR effect. As the surface of most nanoparticles is hydrophobic, they tend to elicit immune response and aggregation (Osman et al., 2018). Attaching PEG on the surface of the nanoparticle makes the surface much more hydrophilic, which subsequently reduces immune response and aggregation. PEGylation also improves pharmacodynamic and pharmacokinetics features (Liu et al., 2018).
(a) Throughout the body, normal vessels are sealed; only nutrients and oxygen may pass through endothelial cells, from blood to tissue. But solid tumors demand more oxygen and nutrients, which leads to angiogenesis. Angiogenesis is the hallmark of all solid tumors. Newly established vessels are leaky; there are many nanodiameter-sized pores in these vessels, which are nanomedicine’s central dogma. The nanoparticle can go through these pores and get into a tumor microenvironment. As normal vessels are devoid of such pores, this is called passive targeting. (b) Nanoparticles also can be decorated with wide range of targeting moieties, such as VHH, to target and deliver the cargo of interest to specific tumor cells
Although nanoparticle delivery to peripheral tumor cells has been successful, delivery to the tumor core has been a big failure. Tumor cell proliferation rates are heterogeneous. The cells close to newly established vessels receive enough nutrients and oxygen, so they grow faster. The cells in the core are hypoxic/necrotic since the blood vessels in this area collapse due to condensed cells and high pressure. The penetration of nanoparticles is even hampered at the tumor core. There are some innovative approaches to boost the tumor perfusion, such as using bradykinin (kinin) (Qin et al., 2009; Emerich et al., 2001), nitric oxide (Ng et al., 2007; Sonveaux et al., 2005), peroxynitrite (Cooke & Davidge, 2002), prostaglandins (Emerich et al., 2001), vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) (Brown et al., 1993) and other cytokines. The shortcoming of all these approaches is a temporary increase in tumor perfusion. There are other approaches like radiation (Wang et al., 2009), ultrasound (Gee et al., 2001; Hoyt et al., 2015), hyperthermia (Song et al., 1984; Koning et al., 2010), and many more, but none of them are impeccable. The other problem associated with PEGylated nanoparticle delivery is that PEG can activate the complement system, which can further lower the efficiency of the delivery of therapeutic reagent to the tumor site (Pannuzzo et al., 2020).
The efficiency of passive targeting is around 20%. Encapsulation of toxic chemotherapeutic agents both reduces the toxicity and therapeutic effect. There is another layer of targeting called active targeting which refines the targeting efficiency. Active targeting is defined as decorating the surface of the nanoparticle with antibody/antibody fragment (Hervé-Aubert et al., 2020), VHH (D’Hollander et al., 2017; Wu et al., 2019), peptide (Sun et al., 2016), affibody (Akhtari et al., 2016; Jia et al., 2020), aptamer (Xu et al., 2016), and ligand (Xu et al., 2020) binding to cancer biomarkers. A biomarker is any feature to distinguish cancer cells from normal cells encompassing DNA, RNA, and protein (Lassere, 2008). For instance, some cancer cells express and/or overexpress a cell surface receptor. This receptor either is absent on normal cells or is expressed at a negligible level. Active targeting means aiming a nanoparticle to specific cells with specific characteristics (Slamon et al., 2001; Behr et al., 2001). Active targeting adds up some perks to passive targeting. Firstly, active targeting improves the accumulation of drugs in the tumor microenvironment. This could lead to further reduce the toxicity of the drug to undesired organs (Vong & Nagasaki, 2020). Secondly, targeted drug delivery would increase drug uptake which concludes in improved therapeutic characteristics (Huda et al., 2020). Finally, as the receptor engaged with surface-modified nanoparticles becomes internalized, it helps to reduce the density of cell surface oncogene receptors, such as HER2 (Li et al., 2016) (Fig. 4.3).
4.5.3.1 Immunoliposome
The most objective of nanomedicine is characteristic mimetics in nanoparticles for decreasing cytotoxicity and a few side impacts in vitro and in vivo. Nanoparticles can overcome a few impediments in medicate organization such as quick clearance within the kidney and clearance through reticuloendothelial framework (RES) as well as factors such as drug plasma fluctuation, high toxicity of certain medicinal products, poor solubility of hydrophobic medicinal products, drug resistance, and the inactivation of certain medicinal products by acidic endolysosomal and tumor microenvironment, even ineffective drug penetration to the target tissue (Beltrán-Gracia et al., 2019). In order to increase the therapeutic effect, the arrival of nanotechnology has led to the discovery of many nano-based drug carriers, such as nanospheres, nanocapsules, polymeric nanoparticles, liposomes, and fullerenes (Eloy et al., 2017). Liposomes nanoparticles are the best example of cell membrane biomimetics, whereas lipids are main mammalian cell membrane compounds. In the 1960s, liposomes were first prepared, but it was known as a nanoparticle until the year 2000. Therefore, a fundamental target for the preparation of liposome nanoparticles is the types of phospholipids and cell membrane composition. As the activity and properties of liposomes depend on the physicochemical interactions between the different lipid species within the lipid composition. The presence of charges on the lipids, for example, is known to decrease the probability of aggregation and increase the overall efficiency of encapsulation (Eroğlu & İbrahim, 2020). Liposomes are used to encapsulate cosmetics, medications, and fluorescent detection reagents and to transport nucleic acids, peptides, and proteins in vivo to cellular sites as delivery devices. It is possible to bind targeting components such as antibodies to liposomal surfaces and use them to generate large antigen-specific complexes. So, over the past few years, liposomes have become a valuable method in drug delivery for the treatment of many diseases. Moreover, the biocompatibility and use of these nanoparticles can be improved by trapping other particle forms in the center of liposomes or between two layers of liposomes, such as iron oxide magnetic nanoparticles (Prasad et al., 2020), quantum dots (Sercombe et al., 2015), and polyethyleneimine (Patel, 2020). The development of bimodal nanoparticles with efficient imaging techniques and advanced responsive contrast agents for cell labeling and their clinical application have contributed to the advancement of these research activities (Prasad et al., 2020). In addition, liposomes are able to trap water-soluble drugs that otherwise do not easily move through the bilayer membrane and can also load lipophilic drugs into the lipid layers to make them dispersible in an aqueous medium. To date, liposomes have remained the most scientifically proven nano-carriers, owing to their improved bioavailability, biocompatibility, biodegradability, and low toxicity (due to their phospholipid content and high capacity for encapsulation) (Sercombe et al., 2015). Efforts have been made over the last four decades since the beginning of 1970 to maximize the stability of liposomes as well as to increase the capacity of encapsulation. In the year 1995, a revolution took place in which AmBisome® and Doxil® were approved for clinical trials (Patel, 2020). Over the past 30 years, active targeting by the use of bioconjugation techniques to bind peptides, antibodies, or aptamers to the liposome surface has been widely studied in order to enhance personalized medicine, despite passive targeting based on physical and chemical characteristics of liposomes. In this respect, the polar head group of lipids found in liposomes is very important because it enables the reactive group to be derivatized or chemically modified. Lipid activation may be achieved either prior to integration into the structure of the bilayer or after the intact liposome is formed. To activate the lipids in the liposome, sulfhydryl and amine-reactive crosslinking agents such as SPDP, MBS, SMPB, and SMCC might be used. The amine group on the phosphatidylethanolamine (PE) glycerol phosphate head is most widely found in liposome conjugates, while lipids such as PE and stearyl amine-containing amine groups can also be used in crosslinker nucleophilic reactions or modification reagents such as glutaraldehyde. In the same way, phosphatidylglycerol (PG) and carboxyl-containing phosphatidylserine (PS)-related aldehyde groups can be used to react with amine-containing ligands by reductive amination and carbodiimide reaction, respectively. The periodate-oxidation of carbohydrate or glycerol groups on lipid components accompanied by subsequent bonding with the amine-containing ligand requires reduction of amination-mediated conjugation (Khaleghi et al., 2017). The conjugation of thiolated VHHs (by 2-iminothiolane) to maleimide-PEG2000-DSPE of liposomes was performed covalently, according to Khaleghi et al. Targeted imaging of anti-HER2 VHH-conjugated magnetoliposomes for breast cancer was the objective of this research. Polyethylene glycol (PEG) may also be used to couple two molecules together as a spacer. In general, PEG spacers are heterobifunctional in nature in the sense that there are two groups at both ends that connect with the liposome and the attaching moiety. Alternatively, the liposomes may also be adjusted to hold SH groups that are guided by disulfide and thioether reactions to react with maleimide, vinyl sulfone, or orthopyridyl disulfide bearing PEG. In order to form such conjugate systems, such as ligand-Mal-PEG-DSPE conjugates, ligand-Hz-PEG-DSPE conjugates, ligand-amino-PEG-DSPE conjugatesm and ligand-carboxyl-PEG-DSPE conjugates, the other end of the PEG containing reactive groups could be used to bind the ligand to the shaped PEG lipid during liposome preparation or post-insertion (Khaleghi et al., 2017). In 2018, four types of non-overlapping monoclonal VHHs were developed by Nikkhoiee et al. to target the HER2 receptor ectodomain via methotrexate-loaded PEGylated liposomes. In this study, the functional assay of thiolated VHHs against HER2 antigen and flow cytometry against HER2 positive breast cancer cell lines were confirmed by ELISA (SK-BR-3, BT-474). The attachment was made according to the conjugates of ligand-Mal-PEG-DSPE (Fig. 4.4) (Nikkhoi et al., 2018). In 2018, doxorubicin-loaded PEGylated liposome nanoparticles targeted by anti-HER2 VHHs fragments suppressed HER2-positive breast cancer cell lines by Farasat et al. (2019). The VHHs can be a great tool for the targeted delivery of theranostic nanoparticles because of their small size and advanced physical characterization. Compared with trastuzumab targeted nanoparticles, the functional assay of VHH targeted nanoparticles showed more efficient penetration of internal cellular components and low side effects on the physical properties of nanoparticles, such as relaxivity, size, and stability (Khaleghi et al., 2016).
The procedure of methotrexate containing VHH-targeted immunoliposome. (1) Evaporation: The lipids must first be dissolved and blended into an organic solvent to ensure a homogeneous mixture of lipids when preparing liposomes with a mixed lipid composition. Usually, chloroform/methanol mixtures are used to perform this method. The aim is to obtain a clear lipid solution for full lipid mixing. Usually 20 mg lipid/ml organic solvent is prepared with lipid solutions, but higher concentrations can be used if lipid solubility and mixing are appropriate. (2) Hydration: By simply applying an aqueous medium (containing methotrexate) to the container of dry lipid and agitating, hydration of the dry lipid film/cake is achieved. Until adding to the dry lipid, the temperature of the hydrating medium should be above the temperature of the gel-liquid crystal transition (Tc or Tm) of the lipid with the highest Tc. The lipid suspension should be maintained above Tc throughout the hydration cycle after the addition of the hydrating medium. (3) Extrusion: Lipid extrusion is a technique in which a lipid suspension is forced to yield particles with a diameter close to the pore size of t thumbnail through a polycarbonate filter with a fixed pore size. (4) VHHs conjugation: The conjugation of thiolated VHHs (by 2-iminothiolane) to maleimide-PEG2000-DSPE of liposomes was performed covalently. (5) Gel filtration G50: The separation of conjugated immunoliposomes containing methotrexate was done by size exclusion chromatography
4.5.3.2 PAMMAM
Many different types of dendrimers are available, such as peptide dendrimers (PPI), poly(l-lysine) dendrimers, dendrimers of polyamidamine (PAMAM), PAMAM organosilicon dendrimers (PAMAMOS), etc. (Luong et al., 2016). Among these dendrimers, however, PAMAM has been widely studied as a carrier for the delivery of genes and therapeutic molecules. PAMAM dendrimers are a class of synthetic macromolecules with strongly branched and monodisperse, with well-defined structures and compositions. Their center is normally ethylenediamine, which is repeatedly added to methyl acrylate and ethylenediamine (EDTA) according to the desired number of generations, G0, G1, G2, G3, G4, etc. Various feature groups, including NH2, OH, CHO, COOMe, Boc, COONa, or CH3 groups, could be terminated by superficial divisions of PAMAM. Usually, the NH2 community is employed to bring genetic material into cells. Because of certain flexible PAMAM properties, such as non-immunogenicity, water-solubility, spherical shape, and controlled release of drugs, they are ideal carriers of genes and drugs for bimodal co-delivery systems. The efficacy of drug loading could be influenced by numerous factors, including functional surface groups, size, chemical structure, generation numbers, degree of PAMAM PEGylation, loaded drug molecular weight, pH, solvent form, and temperature (Fana et al., 2020). Without sacrificing the spherical geometry of PAMAM dendrimers in solution, the surface groups of PAMAM dendrimers provide a flexible attachment point for the conjugation of multiple therapeutic agents from anticancer drugs to image reporters. The most effective ligand for attacking cancer cells is potentially monoclonal antibodies by binding to a particular cognate antigen overexpressed on cancer cells (Abedi-Gaballu et al., 2018). Antibody conjugation to the dendrimer surface has also been used for tumor targeting, although the key drawback is the high molecular weight of antibodies (150 kDa). Folic acid (FA) is another well-studied cancer targeting ligand, and transferrin, low-density lipoprotein, integrins, and other adhesion molecules are favored in some studies. The functionality of PAMAM nanoparticles can as described above be affected by attached particle surface groups. The findings of selective PAMAM nanoparticles with small targeting modalities such as folic acid, ferritin, Aptamers, RGD, etc. are more advanced in many forms of research compared to traditional antibodies with enormous size and susceptibility to covalent conjugation (Li et al., 2018). For the active targeting of PAMAM nanoparticles, single-chain antibodies with small sizes and greater stability in various physical and chemical environments may be the correct option. There is a low risk of self-immunogenicity in repeated in vivo therapies due to the low immunogenicity of VHHs. In various delivery systems consisting of chemotherapeutic drugs (methotrexate, 5-FU, docetaxel, cisplatin, doxorubicin, paclitaxel), genes (DNA or RNA), and two forms of theranostic agents, PAMAM nanoparticles due to porous structures are acceptable. In order to prevent nuclease action, PAMAM dendrimers are able to create stable PAMAM-nucleic acid complexes. Via interactions with the major and minor grooves and the backbone of phosphate groups, the PAMAM dendrimers can strongly interact with DNA. In addition, the stability tests of the PAMAM/DNA complexes clearly showed that the PAMAM-G4 dendrimer was more stable than the others. Therapeutic nucleic acids are intended to activate or inhibit the expression of particular genes responsible for the biosynthesis and alteration of various proteins that can play a crucial role in the battle against a wide range of diseases such as cancer (Xiao et al., 2020). Jafari Iri Sofla et al. stabilize the targeted G5-PAMAM-dependent gene delivery mechanism against HER2-positive cancer cells by covalently binding anti-HER2 VHHs to the distal ends of polyethylene glycols in the framework of PAMAM-polyethylene glycol. In relation to condensed gene construction, this targeted PAMAM can effectively cause apoptosis, encoding a transcriptionally targeted truncated-Bid killer gene under the influence of the promoter of breast cancer-specific MUC1 (transmembrane glycoprotein mucin 1). Efficient targeted gene delivery by luciferase assay was seen in the cellular uptake assay and the apoptotic cells were measured by Annexin/PI flow cytometry (Jafari Iri Sofla et al., 2019).
4.5.3.3 PEI
There are many benefits to non-viral gene delivery agents over viral vectors, including their ability to directly target, inability to incorporate into the host genome, low immunogenicity, unrestricted transfection of DNA and ease of production/synthesis (Saqafi & Rahbarizadeh, 2019). Among non-viral vectors, due to its high DNA condensing and transfection ability, positively charged polyethyleneimine (PEI), a synthetic polymer, is most extensively evaluated. Successful electrostatic interactions with the anionic charges of DNA molecules can be encouraged by the strong cationic surface charge of PEI. The PEI polymer is ideal for the coupling of targeting ligands, including small peptides or antibodies, due to its large number of primary functional surface amine groups. Due to their negatively charged cell surface, positively charged nano-carriers such as PEI typically show higher cell surface binding and internalization skills. So, in the use of PEI nanoparticles, non-specific attachment is prevalent. This downside can be overcome by PEGylation and targeting of nanoparticles. While PEGylation increases the biocompatibility of PEI polycation, the transfection capacity of this nanoparticle is reduced. Targeting ligands should be used in the PEI-PEG copolymer to increase their specificity and gene transfection capacity in order to address this barrier. However, basic binding and cellular internalization may be influenced by the percentage of conjugation and the size of targeting agent molecules (Wagner et al., 2002). In 2018, Saqafi et al. prepared PEI nanoparticles targeting anti-HER2 VHH as compacting agents for the construction of the transcriptionally targeted tBid killer gene. In addition, as its tumor specificity and elevated protein expression capabilities in breast cancer have been confirmed, the MUC1 gene promoter has been used for transcriptional targeting. The conjugation of huge targeting agents such as traditional antibodies will promote immunological obstruction and induce self-immunogenicity, as with other delivery nanoparticles, the 25 kDa branched polyethyleneimine polymer conjugated with 15 kDa anti-HER2 VHHs has a low probability of immunological reactions compared to 150 kDa whole antibody (Saqafi & Rahbarizadeh, 2018).
4.6 VHH: The “Magic Bullet” for Molecular Imaging and Diagnosis
Therapeutic radiolabeled molecules such as mAbs, mAb fragments, peptides, or synthetic proteins that interact with tumor-associated membrane proteins are deployed through targeted radionuclide therapy (TRNT) and target both the primary tumor site and metastases. Molecular imaging integration can help to predict a good TRNT. To predict targeting and possible toxicity to healthy tissues, this theranostic approach aims to use an equivalent imaging compound. One mAb-based TRNT agent, anti-CD20mAb90Y-ibritumomab, is currently being commercially used to treat B cell non-Hodgkin lymphoma (Wagner et al., 2002). Peptide radionuclide receptor therapy (PRRNT) has been shown to be effective in neuroendocrine tumor patients and is currently being studied in prostate and pancreatic carcinomas (Iezzi et al., 2018). Camelid single-domain antibody (VHH) fragments, also known as VHHs or nanobodies, can resolve some of the problems associated with the use of other targeting vehicles, such as mAbs, for theranosis. VHHs have become useful theranosis drugs due to their smaller scale, high stability, and extremely precise targeting. In preclinical studies, VHHs targeting a variety of membrane-bound cancer biomarkers such as CEA (D’Huyvetter et al., 2017), EGFR (Iqbal et al., 2010), HER2 (Mir et al., 2020), and PSMA (Oliveira et al., 2013) were successfully assessed as in vivo theranostic tracers using a variety of radionuclides. In the treatment of HER2-overexpressing cancer, D’Huyvetter et al. developed a131I-labeled VHH as a theranostic medication. The results show the potential of SGMIB-2Rs15d for theranosis. A low radioactive SGMIB-2Rs15d initial scan enables patient selection and dosimetry calculations for subsequent therapeutic SGMIB-2Rs15d and may thus influence the outcome of therapy for HER2+ breast cancer (D’Huyvetter et al., 2017).
Li et al. prepared glial fibrillary acid protein (GFAP) and fluorescent protein (GFP) monomeric fusion VHHs to demonstrate that recombinant VHH-GFP complexes have substantial potential for a one-step fluorescent detection assay that can be used in brain imaging. These “fluobodies” explicitly labeled GFAP on murine brain parts and were able to cross the BBB and mark astrocytes in vivo with a simple version (pI = 9.3) of the fusion protein VHH-GFP (Li et al., 2012).
Iqbal et al. compared the ability of VHHs to bind single-domain antibody (EG2) (15 kDa), bivalent EG2-hFC (80 kDa) and pentavalent V2C-EG2 (128 kDa) using surface plasmon resonance and cell binding tests in vitro and in vivo using pharmacokinetics, biodistribution, optical imaging, and fluorescent microscopy studies for their binding affinities. The results showed that among the three constructs studied, the EG2-hFc VHH construct had the highest apparent affinity for EGFR/EGFRvIII receptors, the longest half-life of circulation, and the best glioblastoma-targeting properties, indicating that it can grow into a molecular imaging and/or therapeutic agent for EGFR-overexpressed tumors, including glioblastoma (Iqbal et al., 2010). For the diagnosis and stage of cancer, six imaging modalities can currently be used, namely, x-ray (computed tomography, CT), magnetic resonance imaging (MRI), single-photon emission tomography (SPECT), positron emission tomography (PET), ultrasound (US), and, more recently, optical imaging clinical evaluation (Mir et al., 2020).
4.6.1 Optical Imaging
Molecular imaging of cell surface markers has become an increasingly effective cancer imaging technique, which can be used for diagnosis, therapy response assessment, and surgical resection guidance. Recent developments in the production of targeted probes have prompted further advancements in Cat, SPECT, and, more recently, optical imaging as well. Recently, optical molecular imaging has gained a lot of interest because the probes used are non-radioactive, and in addition, recent camera systems allow high-resolution imaging, no ionizing radiation, so no need for workers protection; no radioactive decay of the probe, thus, longer stability. Limited sensitivity is one of the problems posed by optical molecular imaging, owing to limited light penetration into the tissue, which prohibits whole-body imaging applications. However, optical imaging is suitable for noninvasive identification of superficial tumors (e.g., breast or head and neck cancers) or endoscope-accessible tumors (e.g., lung cancer, tumors located in the gastrointestinal tract or abdominal cavity) (Oliveira et al., 2013).
4.6.1.1 Fluorescent Fusion VHH
Originally isolated from jellyfish, green fluorescent protein (GFP) is commonly used to observe the cellular location of proteins in cultured cell lines as a protein tag. In a large range of sources, including different A equorea species and reef corals, GFP variants have recently been discovered. The vivid, monomeric green fluorescent protein m Wasabi is a monomeric mTFP1 engineered variant originally derived from Clavularia sp. tetrameric cyan fluorescent protein cFP484. Coral. Coral. Li et al. used recombinant VHH directed against a particular marker of astrocytes, the human glial fibrillary acid protein (GFAP). Only simple VHHs (e.g., pI = 9.4) were able in vitro to cross the BBB (7.8 vs. 0% with pI = 7.7 for VHH). The findings showed that these simple VHHs are capable of crossing the BBB in vivo, diffusing into the brain tissue, penetrating astrocytes and explicitly labeling GFAP by intracarotid and intravenous injections into live mice (Li et al., 2012). For precise protein localization and molecular optical imagery, Shufeng Li et al. developed mWasabi fluorescent protein-binding VHHs. The bright, monomeric green fluorescent protein m Wasabi is a monomeric mTFP1 engineered version originally derived from the Clavularia sp. tetrameric cyan fluorescent protein cFP484. With coral. mWasabi is roughly 2 times brighter than EGFP. mWasabi is a true monomer and is most likely not to interfere with the function or localization of its fusion partner, unlike many fluorescent proteins widely used. With standard GFP filter sets, mWasabi can be easily detected and can be used as a direct substitute for EGFP or other GFPs. Nanobodies provide excellent alternatives to traditional antibodies, with reduced size, increased solubility, and higher development of less costly bacteria in their valued characteristics (Farasat et al., 2017). In addition, nanobodies are exceptionally high in thermostability and acid tolerance (maintaining intense heat and pH treatment functions) and can also be conveniently customized for their single domain format into a variety of constructs. It has few interactions with host proteins for which Wasabi are freely diffusible in the cytoplasm. For enhanced protein tagging and identification, mWasabi has proven to be very useful. mWasabi fusions are also more soluble than traditional GFP fusions, and blue and red fluorescent labels can be co-imaged. Nanobodies are also used to improve GFP fluorescence or to dim the signal from the fluorescence. The choice of camelid-derived single-domain antibodies (nanobodies) that modulate the conformation and spectral properties of the green fluorescent protein was reported by Kirchhofer et al. (GFP). More recently, as an approach to purifying the GFP from total protein extracts, anti-GFP nanobodies directly coupled to Sepharose produce a trap column-based purification system. A method that uses a YFP fusion-tag to produce recombinant proteins using suspension-cultured HEK293F cells was developed by Schellenberg et al. YFP is a dual-function tag that allows high-expressing clones to be directly visualized and fluorescence-based selected for rapid purification using a high-stringency high-affinity anti-GFP/YFP VHH support (Kirchhofer et al., 2010).
4.6.1.2 Near-Infrared/Fluorescent Fusion VHH
The foundation of optical imaging lies in the detection of fluorophore-emitted light, making it a cost-effective, non-radioactive cancer detection imaging modality, both in screening and intra-operative environments. The production of near-infrared (NIR) fluorophores is the subject of recent developments in optical imaging probes. Fluorophores that emit light in the spectrum’s NIR range (e.g., 700 and 800 nm) allow deeper tissue penetration than fluorophores that emit in the normal range of 400–600-nm (Bannas et al., 2014). These benefits are the result of lower absorption of light by the blood and other components of the tissue, as well as minimal autofluorescence of the tissue in this spectrum range. Optical molecular imaging requires high tumor specificity in addition to an effective NIR fluorophore and an imaging device capable of detecting the light emitted by this fluorophore, which can be achieved by using fluorescent probes targeting tumor-specific markers that are preferably (over) expressed strictly in cancerous tissues and not in normal tissues. Several biomarkers have been identified and cancer progression has been correlated with their (over) expression. In order to allow tumor targeting, various targeting moieties have been used, such as affibodies, peptides, traditional monoclonal antibodies (mAbs), or antibody fragments (De Meyer et al., 2014). VHHs penetrate successfully across the mass of the tumor and are maintained by the tumor. Due to the rapid accumulation of these nanobodies into the tumor and their rapid removal (1–2 h half-life), 2–4 h post-injection visualization is already possible (p.i.). In comparison, to accumulate in the tumor, mAbs can take more than 24–48 h and provide comparable contrast. For effective and accurate tumor detection through imaging, adequate signal (described as contrast or tumor-to-background ratio, i.e., T/B ratio) and a clear tumor delineation are necessary (Mir et al., 2020). Taking into account the heterogeneity of cancers (e.g., breast cancers), supplying an adequate signal for optical imaging of early-stage cancers can be a challenge for a specific probe. We proposed that two tumor-specific probes could be combined to increase the T/B ratio and thus promote the identification of tumors. In addition, by using dual-spectral imaging, knowledge on the degree of expression of various tumor markers within the same tumor can be obtained, which could speed up tumor characterization (De Meyer et al., 2014). Kijanka et al. investigated whether an optical imaging combination of two optical probes that explicitly identify two independent markers of breast cancer may enhance tumor detection. For this, VHH B9 targeting CAIX, which localizes to peri-necrotic regions of tumors, and VHH 11A4 targeting HER2, which is known to have a more homogeneous distribution across the tumor tissue, were used as a bimodal targeting agent. The findings showed that VHHs at earlier time points p.i. have better T/B ratios than traditional antibodies. VHHs grow faster in the tumor as a result of their small size, and the non-bound fraction is cleared faster. In reality, the first report on the phase I clinical trial of VHH targeting HER2 for the evaluation of HER2 expression in breast cancer by PET was published quite recently. In a phase II trial, the promising results obtained warrant further evaluation and highlight the potential of the VHHs as probes, even for whole-body imaging when combined with various modalities of imaging. The conjugation was performed using maleimide-modified fluorophores which bind directly to the C-terminal cysteine at the VHH to avoid any detrimental effects of fluorophore conjugation on the binding ability of the VHH. The findings showed that two tumor-specific VHHs bearing the same NIR fluorophore results were injected at a higher T/B ratio than a single tumor-specific VHH injection combined with an unrelated VHHH injection (Kijanka et al., 2016).
4.6.2 PET/CT/SPECT
The noninvasive quantitation and visualization of tumors in vivo is made possible by molecular imaging techniques, commonly used in the clinic, and VHHs have become promising, small-sized high-affinity tracers. In both single-photon emission computed tomography (SPECT) and positron emission tomography, nuclear imaging probes associated with VHHs have been evaluated (PET). Nanobodies have a higher penetration rate in tissues compared with monoclonal antibodies and can be cleared easily via the kidney. Nanobodies are now used extensively in molecular imaging (De Meyer et al., 2014; Bala et al., 2019). Radiolabeled VHHs penetrate tumors and tissues effectively and bind biomarkers very easily and very precisely expressed on cells, whereas unbound VHH is rapidly cleared from non-target organs and tissues. Broos K and his team have carried out noninvasive SPECT/CT imaging murine tumor models with positive PD-L1 expression using 99mTc-labeled PD-L1-specific VHHs; when coupled to a diagnostic radionuclide, this allows generating high contrast images as quickly as 1 h after tracer administration, for example (Bridoux et al., 2020). The 68Ga-labeled anti-HER2 VHH 2Rs15d probe, developed to screen candidates who qualify for treatment with antiHER2 therapeutics, is the most advanced VHH under clinical evaluation. Via conjugation of a residualized prosthetic agent that was synthesized by copper-catalyzed azide-alkyne cycloaddition, Zhou et al. evaluated a HER2-VHH 2Rs15d labeled with 18F. There is great interest in developing improved methods for labeling fast-clearing biomolecules with 18F that can be performed efficiently under physiological conditions because of the short half-life, widespread availability, and favorable radiation dosimetry properties of 18F (Zhao et al., 2016). A fusion VHHs called Nb6 (relative molecular mass is 77837.357 Da) was prepared by Jiang et al., which are two anti-hPD-L1VHHs connected to Fcc6 (Huang et al., 2019). In addition, the I-124 radio-label PD-L1 Nb6 was stated by Huang HF et al. to be useful for non-invasive PET imaging in osteosarcoma tumor mode (Huang et al., 2019). Molecular imaging of immune checkpoints was the subject of Lecocq et al. Nanobodies, the smallest functional fragments of camelid heavy-chain-only antibodies, were produced in this research to noninvasively evaluate the expression of mouse LAG-3 using single-positron emission computed tomography (SPECT)/CT imaging. Injection of 99mTechnetium-labeled nanobodies in healthy mice demonstrated specific absorption in peripheral immune organs, such as the spleen and lymph nodes, not found in knock-out mice of the LAG-3 gene. In addition, using SPECT/CT, VHH uptake could be visualized and compared to the existence of LAG-3 as evaluated in flow cytometry and immunohistochemistry. The diagnostic ability of nanobodies was further confirmed by SPECT/CT scans of tumor-bearing mice (Lecocq et al., 2019). Verhelle et al. showed that 99mTc can be labeled with VHHs raised against the 8-kDa amyloidogenic gelsolin peptide and used in vivo as amyloidogenic gelsolin imaging agents. In addition, there has been some evidence that this can be accomplished with a low background signal and high specificity. This and other VHH-based imaging agents can be helpful in drug screening research and clinical application, especially in situations where their limits are revealed by current imaging platforms (Verhelle et al., 2016).
4.7 Conclusion
In short, this chapter describes the role of VHHs in various fields of biotechnology, such as diagnostics and therapy. Single-domain antibodies are soluble, stable with unique attachment flexibility and deep penetration capabilities in solid tumors. An effective tiny protein in molecular imaging with non-invasive in vivo imaging due to rapid kidney clearance. In addition, VHHs can fold independently so that many forms of conjugation with dyes, peptides, radioisotopes as tracing elements or attachment as targeting agents to the surface of nanoparticles have no effect on the 3D structure of VHHs so that many tags can be fused in their tertiary structure, such as His-tag or even fluorescent labels such as green fluorescent protein (GFP). Compared to traditional antibodies, the use of VHHs for therapeutic applications, blocking the interaction between the target and the corresponding receptor, or disrupting the signal transduction cascade caused after their detection shows many advantages, the most powerful distinction being the small size and simple 3D structure of VHHs. In addition, VHHs in the bacterial host can be produced on a large scale with good yields. VHHs are smaller (15 kDa) than standard fragments of IgGs (150 kDa) or their corresponding fragments of Fab (55 kDa) or scFv (28 kDa) that can be chemically or recombinantly prepared. In most commercial diagnostic studies, VHHs can be a good alternative to traditional antibodies for their tolerance and stability over long periods of time until immobilized in solid support. Fortunately, nanomedicine will enhance the clinical and diagnostic skills and capabilities of VHHs. The integration of VHHs into various nanotechnology systems, such as nanocarriers or biosensors, would strengthen some of the disadvantages observed in the currently elevated VHHs, such as the decrease in renal clearance or the effective refolding and functionalization of the transducer, in order to improve sensing affinity. In addition, to further maximize their solubility, VHHs may be encapsulated, adding drugs or radio labels for therapy.
References
Abedi-Gaballu, F., Dehghan, G., Ghaffari, M., Yekta, R., Abbaspour-Ravasjani, S., Baradaran, B., et al. (2018). PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Applied Materials Today, 12, 177–190.
Akhtari, J., Rezayat, S. M., Teymouri, M., Alavizadeh, S. H., Gheybi, F., Badiee, A., et al. (2016). Targeting, bio distributive and tumor growth inhibiting characterization of anti-HER2 affibody coupling to liposomal doxorubicin using BALB/c mice bearing TUBO tumors. International Journal of Pharmaceutics, 505(1–2), 89–95.
Albert, S., Arndt, C., Feldmann, A., Bergmann, R., Bachmann, D., Koristka, S., et al. (2017). A novel nanobody-based target module for retargeting of T lymphocytes to EGFR-expressing cancer cells via the modular UniCAR platform. Oncoimmunology, 6(4), e1287246.
Albert, S., Arndt, C., Koristka, S., Berndt, N., Bergmann, R., Feldmann, A., et al. (2018). From mono-to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo. Oncotarget, 9(39), 25597.
An, N., Hou, Y. N., Zhang, Q. X., Li, T., Zhang, Q. L., Fang, C., et al. (2018). Anti-multiple myeloma activity of nanobody-based anti-CD38 chimeric antigen receptor T cells. Molecular Pharmaceutics, 15(10), 4577–4588.
Bachmann, M. (2019). The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunology Letters, 211, 13–22.
Bachmann, D., Aliperta, R., Bergmann, R., Feldmann, A., Koristka, S., Arndt, C., et al. (2018). Retargeting of UniCAR T cells with an in vivo synthesized target module directed against CD19 positive tumor cells. Oncotarget, 9(7), 7487.
Bala, G., Crauwels, M., Blykers, A., Remory, I., Marschall, A. L., Dübel, S., et al. (2019). Radiometal-labeled anti-VCAM-1 nanobodies as molecular tracers for atherosclerosis–impact of radiochemistry on pharmacokinetics. Biological Chemistry, 400(3), 323–332.
Bannas, P., Well, L., Lenz, A., Rissiek, B., Haag, F., Schmid, J., et al. (2014). In vivo near-infrared fluorescence targeting of T cells: Comparison of nanobodies and conventional monoclonal antibodies. Contrast Media & Molecular Imaging, 9(2), 135–142.
Barclay, J., Creswell, J., & León, J. (2018). Cancer immunotherapy and the PD-1/PD-L1 checkpoint pathway. Archivos Espanoles de Urologia, 71(4), 393–399.
Behdani, M., Zeinali, S., Karimipour, M., Khanahmad, H., Schoonooghe, S., Aslemarz, A., et al. (2013). Development of VEGFR2-specific nanobody Pseudomonas exotoxin A conjugated to provide efficient inhibition of tumor cell growth. New Biotechnology, 30(2), 205–209.
Behr, T., Behe, M., & Wörmann, B. (2001). Trastuzumab and breast cancer. The New England Journal of Medicine, 345(13), 995–996.
Beltrán-Gracia, E., López-Camacho, A., Higuera-Ciapara, I., Velázquez-Fernández, J. B., & Vallejo-Cardona, A. A. (2019). Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnology, 10(1), 11.
Böhmová, E., Machová, D., Pechar, M., Pola, R., Venclíková, K., Janoušková, O., et al. (2018). Cell-penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiological Research, 67, S267–SS79.
Bridoux, J., Broos, K., Lecocq, Q., Debie, P., Martin, C., Ballet, S., et al. (2020). Anti-human PD-L1 nanobody for immuno-PET imaging: Validation of a conjugation strategy for clinical translation. Biomolecules, 10(10), 1388.
Brown, L. F., Berse, B., Jackman, R. W., Tognazzi, K., Manseau, E. J., Senger, D. R., et al. (1993). Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Research, 53(19), 4727–4735.
Chabrol, E., Stojko, J., Nicolas, A., Botzanowski, T., Fould, B., Antoine, M., et al. (2020). VHH characterization. Recombinant VHHs: Production, characterization and affinity. Analytical Biochemistry, 589, 113491.
Christiansen, J., & Rajasekaran, A. K. (2004). Biological impediments to monoclonal antibody–based cancer immunotherapy. Molecular Cancer Therapeutics, 3(11), 1493–1501.
Cooke, C.-L. M., & Davidge, S. T. (2002). Peroxynitrite increases iNOS through NF-κB and decreases prostacyclin synthase in endothelial cells. American Journal of Physiology-Cell Physiology, 282(2), C395–C402.
D’Hollander, A., Jans, H., Velde, G. V., Verstraete, C., Massa, S., Devoogdt, N., et al. (2017). Limiting the protein corona: A successful strategy for in vivo active targeting of anti-HER2 nanobody-functionalized nanostars. Biomaterials, 123, 15–23.
D’Huyvetter, M., De Vos, J., Xavier, C., Pruszynski, M., Sterckx, Y. G., Massa, S., et al. (2017). 131I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clinical Cancer Research, 23(21), 6616–6628.
De Meyer, T., Muyldermans, S., & Depicker, A. (2014). Nanobody-based products as research and diagnostic tools. Trends in Biotechnology, 32(5), 263–270.
De Munter, S., Ingels, J., Goetgeluk, G., Bonte, S., Pille, M., Weening, K., et al. (2018). Nanobody based dual specific CARs. International Journal of Molecular Sciences, 19(2), 403.
Deng, C., Xiong, J., Gu, X., Chen, X., Wu, S., Wang, Z., et al. (2017). Novel recombinant immunotoxin of EGFR specific nanobody fused with cucurmosin, construction and antitumor efficiency in vitro. Oncotarget, 8(24), 38568–38580.
Dong, J., Huang, B., Jia, Z., Wang, B., Kankanamalage, S. G., Titong, A., et al. (2020). Development of multi-specific humanized llama antibodies blocking SARS-CoV-2/ACE2 interaction with high affinity and avidity. Emerging Microbes & Infections, 9(1), 1034–1036.
Drent, E., Groen, R. W., Noort, W. A., Themeli, M., van Bueren, J. J. L., Parren, P. W., et al. (2016). Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica, 101(5), 616–625.
Drent, E., Themeli, M., Poels, R., de Jong-Korlaar, R., Yuan, H., de Bruijn, J., et al. (2017). A rational strategy for reducing on-target off-tumor effects of CD38-chimeric antigen receptors by affinity optimization. Molecular Therapy, 25(8), 1946–1958.
Eladl, E., Tremblay-LeMay, R., Rastgoo, N., Musani, R., Chen, W., Liu, A., et al. (2020). Role of CD47 in hematological malignancies. Journal of Hematology & Oncology, 13(1), 1–14.
Eloy, J. O., Petrilli, R., Trevizan, L. N. F., & Chorilli, M. (2017). Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids and Surfaces B: Biointerfaces, 159, 454–467.
Emerich, D. F., Dean, R. L., Snodgrass, P., Lafreniere, D., Agostino, M., Wiens, T., et al. (2001). Bradykinin modulation of tumor vasculature: II. Activation of nitric oxide and phospholipase A2/prostaglandin signaling pathways synergistically modifies vascular physiology and morphology to enhance delivery of chemotherapeutic agents to tumors. Journal of Pharmacology and Experimental Therapeutics, 296(2), 632–641.
Eroğlu, İ., & İbrahim, M. (2020). Liposome–ligand conjugates: A review on the current state of art. Journal of Drug Targeting, 28(3), 225–244.
Fana, M., Gallien, J., Srinageshwar, B., Dunbar, G. L., & Rossignol, J. (2020). PAMAM dendrimer nanomolecules utilized as drug delivery systems for potential treatment of glioblastoma: A systematic review. International Journal of Nanomedicine, 15, 2789.
Fang, T., Duarte, J. N., Ling, J., Li, Z., Guzman, J. S., & Ploegh, H. L. (2016). Structurally defined αMHC-II nanobody–drug conjugates: A therapeutic and imaging system for B-cell lymphoma. Angewandte Chemie International Edition, 55(7), 2416–2420.
Farasat, A., Rahbarizadeh, F., Ahmadvand, D., & Yazdian, F. (2017). Optimization of an anti-HER2 nanobody expression using the Taguchi method. Preparative Biochemistry and Biotechnology, 47(8), 795–803.
Farasat, A., Rahbarizadeh, F., Ahmadvand, D., Ranjbar, S., & Khoshtinat, N. S. (2019). Effective suppression of tumour cells by oligoclonal HER2-targeted delivery of liposomal doxorubicin. Journal of Liposome Research, 29(1), 53–65.
Folkman, J. (2006). Angiogenesis. Annual Review of Medicine, 57(1), 1–18.
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L., & Kroemer, G. (2020). Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nature Reviews Clinical Oncology, 17(12), 725–741.
Gee, M. S., Saunders, H. M., Lee, J. C., Sanzo, J. F., Jenkins, W. T., Evans, S. M., et al. (2001). Doppler ultrasound imaging detects changes in tumor perfusion during antivascular therapy associated with vascular anatomic alterations. Cancer Research, 61(7), 2974–2982.
Guillerey, C., Huntington, N. D., & Smyth, M. J. (2016). Targeting natural killer cells in cancer immunotherapy. Nature Immunology, 17(9), 1025–1036.
Hajari Taheri, F., Hassani, M., Sharifzadeh, Z., Behdani, M., Arashkia, A., & Abolhassani, M. (2019). T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy. IUBMB Life, 71(9), 1259–1267.
Hambach, J., Riecken, K., Cichutek, S., Schütze, K., Albrecht, B., Petry, K., et al. (2020). Targeting CD38-expressing multiple myeloma and Burkitt lymphoma cells in vitro with nanobody-based chimeric antigen receptors (Nb-CARs). Cell, 9(2), 321.
Hamers-Casterman, C., Atarhouch, T., Muyldermans, S., Robinson, G., Hammers, C., Songa, E. B., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363(6428), 446–448.
Harmsen, M., & De Haard, H. (2007). Properties, production, and applications of camelid single-domain antibody fragments. Applied Microbiology and Biotechnology, 77(1), 13–22.
Hassani, M., Hajari Taheri, F., Sharifzadeh, Z., Arashkia, A., Hadjati, J., van Weerden, W. M., et al. (2019). Construction of a chimeric antigen receptor bearing a nanobody against prostate a specific membrane antigen in prostate cancer. Journal of Cellular Biochemistry, 120(6), 10787–10795.
Hatahet, F., Nguyen, V. D., Salo, K. E., & Ruddock, L. W. (2010). Disruption of reducing pathways is not essential for efficient disulfide bond formation in the cytoplasm of E. coli. Microbial Cell Factories, 9(1), 67.
Havel, J. J., Chowell, D., & Chan, T. A. (2019). The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nature Reviews Cancer, 19(3), 133–150.
Henry, K. A., Hussack, G., Kumaran, J., Gilbert, M., MacKenzie, C. R., Sulea, T., et al. (2019). Role of the non-hypervariable FR3 D-E loop in single-domain antibody recognition of haptens and carbohydrates. Journal of Molecular Recognition, 32(11), e2805.
Herrmann, J. (2020). Adverse cardiac effects of cancer therapies: Cardiotoxicity and arrhythmia. Nature Reviews Cardiology, 17(8), 474–502.
Hervé-Aubert, K., David, S., Lautram, N., Passirani, C., Chourpa, I., Aubrey, N., et al. (2020). Targeted nanomedicine with anti-EGFR scFv for siRNA delivery into triple negative breast cancer cells. European Journal of Pharmaceutics and Biopharmaceutics, 157, 74–84.
Hoyt, K., Umphrey, H., Lockhart, M., Robbin, M., & Forero-Torres, A. (2015). Ultrasound imaging of breast tumor perfusion and neovascular morphology. Ultrasound in Medicine & Biology, 41(9), 2292–2302.
Huang, H.-F., Zhu, H., Li, G.-H., Xie, Q., Yang, X.-T., Xu, X.-X., et al. (2019). Construction of anti-hPD-L1 HCAb Nb6 and in situ 124I labeling for noninvasive detection of PD-L1 expression in human bone sarcoma. Bioconjugate Chemistry, 30(10), 2614–2623.
Huda, S., Alam, M. A., & Sharma, P. K. (2020). Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. Journal of Drug Delivery Science and Technology, 60, 102018.
Iezzi, M. E., Policastro, L., Werbajh, S., Podhajcer, O., & Canziani, G. A. (2018). Single-domain antibodies and the promise of modular targeting in cancer imaging and treatment. Frontiers in Immunology, 9, 273.
Iqbal, U., Trojahn, U., Albaghdadi, H., Zhang, J., O’Connor-McCourt, M., Stanimirovic, D., et al. (2010). Kinetic analysis of novel mono-and multivalent VHH-fragments and their application for molecular imaging of brain tumours. British Journal of Pharmacology, 160(4), 1016–1028.
Iri-Sofla, F. J., Rahbarizadeh, F., Ahmadvand, D., & Rasaee, M. J. (2011). Nanobody-based chimeric receptor gene integration in Jurkat cells mediated by PhiC31 integrase. Experimental Cell Research, 317(18), 2630–2641.
Jafari Iri Sofla, F., Rahbarizadeh, F., Ahmadvand, D., Nomani, A., & Vernet, E. (2019). Anti–HER2 single domain antibody-conjugated dendrimers for targeted delivery of truncated-bid transgene to breast cancer cells. Journal of Bioactive and Compatible Polymers, 34(1), 39–57.
Jamnani, F. R., Rahbarizadeh, F., Shokrgozar, M. A., Mahboudi, F., Ahmadvand, D., Sharifzadeh, Z., et al. (2014). T cells expressing VHH-directed oligoclonal chimeric HER2 antigen receptors: Towards tumor-directed oligoclonal T cell therapy. Biochimica et Biophysica Acta (BBA)-General Subjects, 1840(1), 378–386.
Jia, D., Yang, Y., Yuan, F., Fan, Q., Wang, F., Huang, Y., et al. (2020). Increasing the antitumor efficacy of doxorubicin liposomes with coupling an anti-EGFR affibody in EGFR-expressing tumor models. International Journal of Pharmaceutics, 586, 119541.
Jureczek, J., Feldmann, A., Bergmann, R., Arndt, C., Berndt, N., Koristka, S., et al. (2020). Highly efficient targeting of EGFR-expressing tumor cells with UniCAR T cells via target modules based on Cetuximab®. OncoTargets and Therapy, 13, 5515–5527.
Kalyane, D., Raval, N., Maheshwari, R., Tambe, V., Kalia, K., & Tekade, R. K. (2019). Employment of enhanced permeability and retention effect (EPR): Nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Materials Science and Engineering: C, 98, 1252–1276.
Kaplan, O., Zarubova, J., Mikulova, B., Filova, E., Bártová, J., Bačáková, L., et al. (2016). Enhanced mitogenic activity of recombinant human vascular endothelial growth factor VEGF121 expressed in E. coli origami B (DE3) with molecular chaperones. PLoS One, 11(10), e0163697.
Khaleghi, S., Rahbarizadeh, F., Ahmadvand, D., Rasaee, M. J., & Pognonec, P. (2012). A caspase 8-based suicide switch induces apoptosis in nanobody-directed chimeric receptor expressing T cells. International Journal of Hematology, 95(4), 434–444.
Khaleghi, S., Rahbarizadeh, F., Ahmadvand, D., Malek, M., & Madaah Hosseini, H. R. (2016). The effect of superparamagnetic iron oxide nanoparticles surface engineering on relaxivity of magnetoliposome. Contrast Media & Molecular Imaging, 11(5), 340–349.
Khaleghi, S., Rahbarizadeh, F., Ahmadvand, D., & Hosseini, H. R. M. (2017). Anti-HER2 VHH targeted magnetoliposome for intelligent magnetic resonance imaging of breast cancer cells. Cellular and Molecular Bioengineering, 10(3), 263–272.
Kijanka, M. M., van Brussel, A. S., van der Wall, E., Mali, W. P., van Diest, P. J., van Bergen En Henegouwen, P. M., et al. (2016). Optical imaging of pre-invasive breast cancer with a combination of VHHs targeting CAIX and HER2 increases contrast and facilitates tumour characterization. EJNMMI Research, 6(1), 14.
Kirchhofer, A., Helma, J., Schmidthals, K., Frauer, C., Cui, S., Karcher, A., et al. (2010). Modulation of protein properties in living cells using nanobodies. Nature Structural & Molecular Biology, 17(1), 133.
Koning, G. A., Eggermont, A. M., Lindner, L. H., & ten Hagen, T. L. (2010). Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharmaceutical Research, 27(8), 1750–1754.
Kubala, M. H., Kovtun, O., Alexandrov, K., & Collins, B. M. (2010). Structural and thermodynamic analysis of the GFP: GFP-nanobody complex. Protein Science, 19(12), 2389–2401.
Lassere, M. N. (2008). The Biomarker-Surrogacy Evaluation Schema: A review of the biomarker-surrogate literature and a proposal for a criterion-based, quantitative, multidimensional hierarchical levels of evidence schema for evaluating the status of biomarkers as surrogate endpoints. Statistical Methods in Medical Research, 17(3), 303–340.
Lecocq, Q., Zeven, K., De Vlaeminck, Y., Martens, S., Massa, S., Goyvaerts, C., et al. (2019). Noninvasive imaging of the immune checkpoint LAG-3 using nanobodies, from development to pre-clinical use. Biomolecules, 9(10), 548.
Leemasawat, K., Phrommintikul, A., Chattipakorn, S. C., & Chattipakorn, N. (2020). Mechanisms and potential interventions associated with the cardiotoxicity of ErbB2-targeted drugs: Insights from in vitro, in vivo, and clinical studies in breast cancer patients. Cellular and Molecular Life Sciences, 77(8), 1571–1589.
Li, T., Bourgeois, J. P., Celli, S., Glacial, F., Le Sourd, A. M., Mecheri, S., et al. (2012). Cell-penetrating anti-GFAP VHH and corresponding fluorescent fusion protein VHH-GFP spontaneously cross the blood-brain barrier and specifically recognize astrocytes: Application to brain imaging. The FASEB Journal, 26(10), 3969–3979.
Li, J. Y., Perry, S. R., Muniz-Medina, V., Wang, X., Wetzel, L. K., Rebelatto, M. C., et al. (2016). A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell, 29(1), 117–129.
Li, J., Liang, H., Liu, J., & Wang, Z. (2018). Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. International Journal of Pharmaceutics, 546(1–2), 215–225.
Liu, S., Pan, J., Liu, J., Ma, Y., Qiu, F., Mei, L., et al. (2018). Dynamically PEGylated and borate-coordination-polymer-coated polydopamine nanoparticles for synergetic tumor-targeted, chemo-photothermal combination therapy. Small, 14(13), 1703968.
Lobstein, J., Emrich, C. A., Jeans, C., Faulkner, M., Riggs, P., & Berkmen, M. (2012). SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microbial Cell Factories, 11(1), 753.
Loureiro, L., Feldmann, A., Bergmann, R., Koristka, S., Berndt, N., Arndt, C., et al. (2018). Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells. Blood Cancer Journal, 8(9), 1–6.
Lugano, R., Ramachandran, M., & Dimberg, A. (2020). Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cellular and Molecular Life Sciences, 77(9), 1745–1770.
Luong, D., Kesharwani, P., Deshmukh, R., Amin, M. C. I. M., Gupta, U., Greish, K., et al. (2016). PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomaterialia, 43, 14–29.
Manglik, A., Kobilka, B. K., & Steyaert, J. (2017). Nanobodies to study G protein–coupled receptor structure and function. Annual Review of Pharmacology and Toxicology, 57, 19–37.
Marcucci, F., Bellone, M., Rumio, C., & Corti, A. (2013). Approaches to improve tumor accumulation and interactions between monoclonal antibodies and immune cells. MAbs, 5(1), 34–46. Taylor & Francis.
Mikkilineni, L., & Kochenderfer, J. N. (2021). CAR T cell therapies for patients with multiple myeloma. Nature Reviews Clinical Oncology, 18(2), 71–84.
Mir, M. A., Mehraj, U., Sheikh, B. A., & Hamdani, S. S. (2020). Nanobodies: The “magic bullets” in therapeutics, drug delivery and diagnostics. Human Antibodies, 28(1), 29–51.
Muyldermans, S. (2020). A guide to: Generation and design of nanobodies. The FEBS Journal. https://doi.org/10.1111/febs.15515
Myers, J. A., & Miller, J. S. (2021). Exploring the NK cell platform for cancer immunotherapy. Nature Reviews Clinical Oncology, 18(2), 85–100.
Ng, Q.-S., Goh, V., Milner, J., Stratford, M. R., Folkes, L. K., Tozer, G. M., et al. (2007). Effect of nitric-oxide synthesis on tumour blood volume and vascular activity: A phase I study. The Lancet Oncology, 8(2), 111–118.
Nguyen, V. D., Hatahet, F., Salo, K. E., Enlund, E., Zhang, C., & Ruddock, L. W. (2011). Pre-expression of a sulfhydryl oxidase significantly increases the yields of eukaryotic disulfide bond containing proteins expressed in the cytoplasm of E. coli. Microbial Cell Factories, 10(1), 1–13.
Nikkhoi, S. K., Rahbarizadeh, F., & Ahmadvand, D. (2017). Oligo-clonal nanobodies as an innovative targeting agent for cancer therapy: New biology and novel targeting systems. Protein Expression and Purification, 129, 115–121.
Nikkhoi, S. K., Rahbarizadeh, F., Ahmadvand, D., & Moghimi, S. M. (2018). Multivalent targeting and killing of HER2 overexpressing breast carcinoma cells with methotrexate-encapsulated tetra-specific non-overlapping variable domain heavy chain anti-HER2 antibody-PEG-liposomes: In vitro proof-of-concept. European Journal of Pharmaceutical Sciences, 122, 42–50.
Oliveira, S., Heukers, R., Sornkom, J., Kok, R. J., & van Bergen En Henegouwen, P. M. (2013). Targeting tumors with nanobodies for cancer imaging and therapy. Journal of Controlled Release, 172(3), 607–617.
Osman, G., Rodriguez, J., Chan, S. Y., Chisholm, J., Duncan, G., Kim, N., et al. (2018). PEGylated enhanced cell penetrating peptide nanoparticles for lung gene therapy. Journal of Controlled Release, 285, 35–45.
Padegimas, A., Clasen, S., & Ky, B. (2020). Cardioprotective strategies to prevent breast cancer therapy-induced cardiotoxicity. Trends in Cardiovascular Medicine, 30(1), 22–28.
Pannuzzo, M., Esposito, S., Wu, L.-P., Key, J., Aryal, S., Celia, C., et al. (2020). Overcoming nanoparticle-mediated complement activation by surface PEG-pairing. Nano Letters, 20(6), 4312–4321.
Patel, V. (2020). Liposome: A novel carrier for targeting drug delivery system. Asian Journal of Pharmaceutical Research and Development, 8(4), 67–76.
Prasad, R., Jain, N. K., Yadav, A. S., Chauhan, D. S., Devrukhkar, J., Kumawat, M. K., et al. (2020). Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near-infrared light mediated cancer therapy. Communications Biology, 3(1), 1–14.
Qin, L.-J., Gu, Y.-T., Zhang, H., & Xue, Y.-X. (2009). Bradykinin-induced blood–tumor barrier opening is mediated by tumor necrosis factor-α. Neuroscience Letters, 450(2), 172–175.
Rajabzadeh, A., Hamidieh, A. A., & Rahbarizadeh, F. (2018). Cytotoxic function of chimeric antigen receptor (CAR) T cells redirected by anti-Muci nanobody. Biology of Blood and Marrow Transplantation, 24(3), S474.
Rajabzadeh, A., Rahbarizadeh, F., Ahmadvand, D., Kabir, S. M., & Hamidieh, A. A. (2021). A VHH-based anti-MUC1 chimeric antigen receptor for specific retargeting of human primary T cells to MUC1-positive cancer cells. Cell Journal, 22(4), 502.
Saqafi, B., & Rahbarizadeh, F. (2018). Specific targeting of human epidermal growth factor receptor 2 (HER2) overexpressing breast cancer cells by polyethylene glycol-grafted polyethyleneimine modified with anti-HER2 single-domain antibody. Journal of Bioactive and Compatible Polymers, 33(1), 17–37.
Saqafi, B., & Rahbarizadeh, F. (2019). Polyethyleneimine-polyethylene glycol copolymer targeted by anti-HER2 nanobody for specific delivery of transcriptionally targeted tBid containing construct. Artificial Cells, Nanomedicine, and Biotechnology, 47(1), 501–511.
Sercombe, L., Veerati, T., Moheimani, F., Wu, S. Y., Sood, A. K., & Hua, S. (2015). Advances and challenges of liposome assisted drug delivery. Frontiers in Pharmacology, 6, 286.
Shimasaki, N., Jain, A., & Campana, D. (2020). NK cells for cancer immunotherapy. Nature Reviews Drug Discovery, 19(3), 200–218.
Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001). Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. The New England Journal of Medicine, 344(11), 783–792.
Song, C. W., Lokshina, A., Rhee, J. G., Patten, M., & Levitt, S. H. (1984). Implication of blood flow in hyperthermic treatment of tumors. IEEE Transactions on Biomedical Engineering, 1, 9–16.
Sonveaux, P., Kaz, A. M., Snyder, S. A., Richardson, R. A., Cárdenas-Navia, L. I., Braun, R. D., et al. (2005). Oxygen regulation of tumor perfusion by S-nitrosohemoglobin reveals a pressor activity of nitric oxide. Circulation Research, 96(10), 1119–1126.
Sun, Y., Kang, C., Yao, Z., Liu, F., & Zhou, Y. (2016). Peptide-based ligand for active delivery of liposomal doxorubicin. Nano Life, 6(03n04), 1642004.
Tang, J., Li, J., Zhu, X., Yu, Y., Chen, D., Yuan, L., et al. (2016). Novel CD7-specific nanobody-based immunotoxins potently enhanced apoptosis of CD7-positive malignant cells. Oncotarget, 7(23), 34070.
Thueng-In, K., Thanongsaksrikul, J., Srimanote, P., Bangphoomi, K., Poungpair, O., Maneewatch, S., et al. (2012). Cell penetrable humanized-VH/VHH that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS One, 7(11), e49254.
Thurber, G. M., Schmidt, M. M., & Wittrup, K. D. (2008). Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Advanced Drug Delivery Reviews, 60(12), 1421–1434.
Tian, B., Wong, W. Y., Hegmann, E., Gaspar, K., Kumar, P., & Chao, H. (2015). Production and characterization of a camelid single domain antibody–urease enzyme conjugate for the treatment of cancer. Bioconjugate Chemistry, 26(6), 1144–1155.
Tian, B., Wong, W. Y., Uger, M. D., Wisniewski, P., & Chao, H. (2017). Development and characterization of a camelid single domain antibody–urease conjugate that targets vascular endothelial growth factor receptor 2. Frontiers in Immunology, 8, 956.
Verhelle, A., Van Overbeke, W., Peleman, C., De Smet, R., Zwaenepoel, O., Lahoutte, T., et al. (2016). Non-invasive imaging of amyloid deposits in a mouse model of AGel using 99m Tc-modified nanobodies and SPECT/CT. Molecular Imaging and Biology, 18(6), 887–897.
Vincke, C., Loris, R., Saerens, D., Martinez-Rodriguez, S., Muyldermans, S., & Conrath, K. (2009). General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. Journal of Biological Chemistry, 284(5), 3273–3284.
Vong, L. B., & Nagasaki, Y. (2020). Nitric oxide nano-delivery systems for cancer therapeutics: Advances and challenges. Antioxidants, 9(9), 791.
Wagner, H. N., Wiseman, G. A., Marcus, C. S., Nabi, H. A., Nagle, C. E., Fink-Bennett, D. M., et al. (2002). Administration guidelines for radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-labeled anti-CD20 monoclonal antibody. Journal of Nuclear Medicine, 43(2), 267–272.
Waldman, A. D., Fritz, J. M., & Lenardo, M. J. (2020). A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nature Reviews Immunology, 20(11), 651–668.
Wang, J., Wu, N., Cham, M. D., & Song, Y. (2009). Tumor response in patients with advanced non–small cell lung cancer: Perfusion CT evaluation of chemotherapy and radiation therapy. American Journal of Roentgenology, 193(4), 1090–1096.
Wang, Y., Fan, Z., Shao, L., Kong, X., Hou, X., Tian, D., et al. (2016). Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications. International Journal of Nanomedicine, 11, 3287.
Wu, T., Liu, J., Liu, M., Liu, S., Zhao, S., Tian, R., et al. (2019). A nanobody-conjugated DNA nanoplatform for targeted platinum-drug delivery. Angewandte Chemie International Edition, 58(40), 14224–14228.
Xiao, T., Li, D., Shi, X., & Shen, M. (2020). PAMAM dendrimer-based nanodevices for nuclear medicine applications. Macromolecular Bioscience, 20(2), 1900282.
Xie, Y. J., Dougan, M., Jailkhani, N., Ingram, J., Fang, T., Kummer, L., et al. (2019). Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proceedings of the National Academy of Sciences, 116(16), 7624–7631.
Xie, Y. J., Dougan, M., Ingram, J. R., Pishesha, N., Fang, T., Momin, N., et al. (2020). Improved antitumor efficacy of chimeric antigen receptor T cells that secrete single-domain antibody fragments. Cancer Immunology Research, 8(4), 518–529.
Xu, G., Yu, X., Zhang, J., Sheng, Y., Liu, G., Tao, W., et al. (2016). Robust aptamer–polydopamine-functionalized M-PLGA–TPGS nanoparticles for targeted delivery of docetaxel and enhanced cervical cancer therapy. International Journal of Nanomedicine, 11, 2953.
Xu, Y., Wu, H., Huang, J., Qian, W., Martinson, D. E., Ji, B., et al. (2020). Probing and enhancing ligand-mediated active targeting of tumors using sub-5 nm ultrafine iron oxide nanoparticles. Theranostics, 10(6), 2479–2494.
Zhao, J., Zhou, M., & Li, C. (2016). Synthetic nanoparticles for delivery of radioisotopes and radiosensitizers in cancer therapy. Cancer Nanotechnology, 7(1), 1–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khaleghi, S., Khoshtinat Nikkhoi, S., Rahbarizadeh, F. (2021). Camelid Single-Domain Antibodies for Targeting Cancer Nanotheranostics. In: Saravanan, M., Barabadi, H. (eds) Cancer Nanotheranostics. Nanotechnology in the Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-74330-7_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-74330-7_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-74329-1
Online ISBN: 978-3-030-74330-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)