Abstract
Esophageal pressure (Pes) is the closest surrogate for pleural pressure available in clinical practice. Pes closely reflects absolute values of pleural pressure in the dependent lung region and accurately tracks changes in pleural pressure due to ventilation.
Pes is useful to assess transpulmonary pressure and the risk of ventilator-induced lung injury. End-expiratory Pes could guide personalized positive end-expiratory pressure settings, while driving and end-inspiratory transpulmonary pressure measured with the elastance ratio method could help in avoiding overdistension.
In active patients, Pes swings are closely correlated with the respiratory effort, which is useful to avoid over and under assistance and the related risk of patient self-inflicted lung injury and diaphragm myotrauma.
Finally, patient-ventilator asynchronies are major determinants of patients’ outcome and can be more easily and precisely diagnosed and quantified when Pes monitoring is in place.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Esophageal Pressure as an Estimate of Pleural Pressure
In 1949, Buytendijk introduced the method of esophageal pressure measurement for studying the mechanics of active breathing (Buytendijk 1949). Being a collapsible empty cavity between the lungs and chest wall, the esophagus was thought to reflect pressures similar to the pleural cavity. Subsequent experimental findings confirmed that changes in pleural pressure (Ppl) were closely correlated to changes in esophageal pressure (Pes) (Dornhorst and Leathart 1952; Cherniack et al. 1955). Absolute values of pressures in the pleural space are often lower than in those measured in the esophagus, especially for gravitationally nondependent regions, but Pes measurements give good estimates of the effective Ppl in healthy, upright subjects (Washko et al. 2006; Fry et al. 1952). Moreover, recent studies have shown that Pes accurately reflects absolute Ppl values of the collapsed dependent lung regions in experimental acute lung injury and in cadavers (Yoshida et al. 2018).
Positioning of the Esophageal Balloon
Pes is measured with an air-filled catheter that terminates in a th/in balloon. The catheter is introduced through the nose or mouth and advanced until it reaches the stomach (approximately 50–55 cm from the nares in an adult). Then, the balloon is filled with a standard volume of air according to the manufacturer’s recommendations. This usually is 1–2 ml for smaller balloons and 3–4 ml for larger ones. The intragastric position of the esophageal balloon is confirmed by a positive pressure deflection during gentle external manual compression of the abdomen. The balloon then is withdrawn until cardiac artifacts appear, and there are negative inspiratory swings on the pressure tracings. This corresponds to placement in the lower third of the esophagus. Standard balloons are 10 cm long, and the catheter has multiple side holes within the balloon (Mauri et al. 2016).
Calibration
In 1982, Baydur and Colleagues described “the occlusion test” procedure for correct positioning of the esophageal balloon in active subjects (Baydur et al. 1982). The occlusion test allows subjects to perform static voluntary inspiratory efforts (glottis open) against a closed airway, and a comparison is made between the change in esophageal pressure (ΔPes) and the corresponding change in airway pressure (ΔPaw). In the lower third of the esophagus, approximately 10 cm above the gastroesophageal junction, and in the absence of airflow and change in lung volume, the ratio ΔPes/ΔPaw should be close to unity (1.0 ± 0.2). If ΔPes/ΔPaw is between 0.8 and 1.2, measurements of lung mechanics based on esophageal pressure changes are considered accurate and valid (Brochard 2014; Yoshida and Brochard 2018). It must be noted that esophageal balloon inflation plays an important role. When the balloon is inflated with too little air, the positive pressure in the esophagus on inspiration will empty the balloon, and the measured pressure will be an underestimate of Ppl. Conversely, if the balloon is inflated with too much air, it will distend the esophagus and stress the balloon so that the measured pressure will overestimate Ppl. The minimal non-stressed balloon volume should be used to measure Pes accurately; the range of appropriate filling volumes is catheter-specific and should be carefully checked before catheter positioning (Brochard 2014).
Technical Pitfalls
Many factors can cause artifactual differences between Pes and regional Ppl. These include variations unrelated to Ppl, depending on postural artifacts, on the position of the balloon in the esophagus, or on the volume of air inside the balloon. Moreover, due to the effect of gravity, in an upright individual, Ppl at the base of the lung is greater (less negative) than at the apex. In the supine position, lung volume is decreased in the dependent zones, and the abdominal contents compress the dependent lung, resulting in an increased gravitational Ppl gradient (Hubmayr et al. 1983; Mead and Gaensler 1959). At low lung volumes, Ppl can even be locally positive (Washko et al. 2006). Furthermore, in the supine position, the esophagus is compressed by the overlying mediastinal structures, which potentially can increase Pes values. As reported by physiological studies, in the upright and prone positions, the lung’s inherent shape is close to that of its container, the gravitational Ppl gradient is low, and the lung compliance (CL) is high. Neither prone nor upright positions cause the compression of the dependent lung by the heart that occurs in the supine position. In summary, the pleural cavity is characterized by differences in regional pressures, while Pes reflects the pressure only corresponding to one region of the pleural space (Plataki and Hubmayr 2011).
Clinically Relevant Measures
The main clinical applications of Pes measurement are (Mauri et al. 2016):
-
1.
To estimate the transpulmonary pressure (PL)
-
2.
To assess patient’s effort when the respiratory muscles are active
-
3.
To monitor patient-ventilator interaction
Transpulmonary Pressure
Mechanical ventilation can, per se, cause lung injury. The so-called ventilator-induced lung injury (VILI) is a dysregulated inflammatory response due to an excessive volume and pressure load imposed on ventilated lung regions along with the cyclic opening and closing of distal airways and collapsed alveoli during tidal ventilation. VILI can result in worsening hypoxemia and multi-organ dysfunction, which increases the mortality of patients affected by hypoxemic respiratory failure (Grieco et al. 2017). Reducing VILI is a key goal in the management of acute respiratory distress syndrome (ARDS) ventilatory management. In a pioneering single-center study, Amato and Colleagues were the first to show a reduction in mortality in patients with ARDS when using a strategy based on achieving low tidal volume (Vt) (6 mL/Kg), high positive end-expiratory (PEEP), and low inspiratory driving pressures (< 20 cmH2O) (Amato et al. 2015). Driving pressure (DP) is the difference between the static airway pressure at the end of inspiration (plateau pressure, Pplat) and total positive end-expiratory pressure (PEEP). In turn, static compliance of the respiratory system (CRS) is the ratio between Vt and DP:
Thus, DP represents the Vt corrected for the patient’s CRS, and using DP as a safety limit may be more accurate than Vt to decrease VILI in ARDS patients. However, DP reflects a force acting on two different structures: lung and chest wall. Thus, because airway pressure (Paw) is the sum of pressures across both the lung and chest wall, the portion of the pressure applied to the lung varies widely, depending on chest wall characteristics. Lungs inflate and deflate in response to changes in transpulmonary pressure (PL), which is the pressure difference between the airway opening (Pao) and the pleural space:
In the absence of flow (i.e., during an end-inspiratory occlusion maneuver to obtain Pplat or an end-expiratory occlusion maneuver to measure total PEEP), Pao corresponds to the Paw measured by the ventilator at airway opening, which in turn is equal to the pressure inside the alveoli. The potential for damage to the lungs caused by mechanical ventilation depends on the magnitude of PL. (Mauri et al. 2016) The measurement of Pes as an estimate of Ppl can represent the only way to distinguish what fraction of Paw is applied to the lung and chest wall (Keller and Fessler 2014). In critically ill patients, chest wall elastance (ECW) is often increased by many factors including obesity, increased intra-abdominal pressure, the effect of drugs, and fluid overload, which cause chest wall edema (Brochard 2014; Gattinoni et al. 2004); in the presence of these alterations, Paw overestimates the pressure applied to the lungs and can limit optimal lung recruitment.
Two different methods have been proposed to estimate PL from Pes (Mauri et al. 2016). The first method is based on the absolute value of Pes as a surrogate for absolute Ppl. By this method, PL is directly calculated as follows:
That is,
This “static” PL decreases progressively from nondependent to dependent lung region in the supine position, because of gravitational edema and reduction of lung volume. At very low lung volume, such as what occurs in ARDS, Ppl can be locally positive in the dependent lung, and thus, PL can be a negative value. This phenomenon exposes the unstable, collapsed alveoli that needs to be repeatedly opened and re-collapsed with each tidal breath and an increased shunt fraction. In the presence of closed airway and flooded or atelectatic lung, raising PEEP until PL becomes positive at end-expiration could avoid cyclic alveolar recruitment and de-recruitment and assure that airways remain open. In a phase-2 randomized controlled trial, Talmor et al. assigned patients with ARDS to undergo mechanical ventilation with PEEP adjusted to obtain positive end-expiratory absolute PL values (intervention group) or according to the ARDS Network PEEP/FIO2 titration tables (control group) (Talmor et al. 2008). The primary endpoint was the improvement in oxygenation. The intervention group had both significantly improved oxygenation and respiratory system compliance. Moreover, these improvements were achieved without elevating transpulmonary pressure at end-inspiration above the physiologic range. PL during end-inspiratory occlusion never exceeded 24 cmH2O. More recently, the same group conducted a larger multicenter randomized controlled trial comparing PEEP set by positive transpulmonary pressure at end-expiration vs. a high PEEP/FiO2 table method. The study enrolled 200 patients, but the results weren’t as encouraging: the mortality and ventilation days didn’t differ as well as most secondary endpoints. However, the average PEEP and end-expiratory transpulmonary pressure were similar in the two groups, questioning whether the study really compared two different strategies for PEEP titration (Beitler et al. 2019). Moreover, PL measured from absolute Pes does not assure that VILI is prevented in lung regions that are not near to the esophagus. This is particularly true in ARDS in which the lung involvement is heterogeneous and not symmetric. In ARDS, it’s reasonable to expect that in lung regions below the level of esophageal balloon, Ppl will be underestimated, whereas in lung regions above the level of esophageal balloon, Ppl will be lower than Pes. Even if PEEP is titrated to optimize the lung volume at the level of the esophagus, lung regions elsewhere likely are under- or overinflated (Talmor and Fessler 2010).
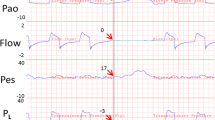
The second method is based on the assumption that the ratio of lung elastance (EL) to respiratory system elastance (ERS) determines the fraction of DP needed to expand the lung. Physiologically, EL/ERS is around 0.5, but in ARDS, it ranges from 0.2 to 0.8. The ratio of lung elastance to respiratory system elastance (EL/ERS) may be used to calculate transpulmonary pressure and guide the “open lung” ventilation approach. In this method, PL is calculated as follows:
where ERS is calculated as the DP/Vt in liter ratio at the set PEEP level and ∆PL is the true indicator of inspiratory lung stress (Fig. 33.1). In a case series of patients with severe ARDS and who were candidates for ECMO, Grasso et al. increased end-inspiratory PL as calculated by the elastance ratio method up to the physiologic threshold of 25 cmH2O, and this improved oxygenation and prevented the use of ECMO without signs of barotrauma (Grasso et al. 2012).
Patient’s Effort When the Respiratory Muscles Are Active
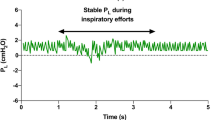
The main goal of assisted mechanical ventilation is to permit spontaneous breathing but with a reduction of the patient’s work of breathing (WOB). Compared to controlled mechanical ventilation, spontaneous breathing has some advantages: oxygenation is generally better because of recruitment of the juxta-diaphragmatic lung region, respiratory muscle atrophy is avoided, and the breathing pattern is variable (“noisy”), unlike the monotonous ventilator pattern. However, uncontrolled patient efforts which correspond to negative Pes deflections (Fig. 33.2) can cause additional lung injury. This is known as patient self-inflicted lung injury (P-SILI). (Li et al. 2017) Because of the large variability in the negative inspiratory Ppl that can occur with large variability in patient efforts, airway pressure delivered by the ventilator is a poor indicator of the inspiratory stress determined by the ΔPL.
For this reason, the difference between peak airway pressure and PEEP in pressure-target mode with active triggering cannot be taken as an approximation of driving pressure. Furthermore, in the injured, “solid-like” lung, the inspiratory Ppl swing is localized more in dependent regions and with the higher regional stress can damage lung tissues (Yoshida et al. 2013, 2016). Negative, vigorous Ppl swings increase transmural vascular pressure and favor lung edema and worse hypoxemia. Hence, monitoring Pes swings in ARDS patients with assisted mechanical ventilation is highly recommended. According to the PLUG working group recommendations in these patients, end-inspiratory dynamic PL should be less than 20–25 cmH2O with inspiratory Peo from muscle effort in the 5–10 cmH2O range (Mauri et al. 2016).
Patient-Ventilator Interaction
As widely reported in literature, poor patient-ventilator interaction worsens lung injury, causes discomfort and dyspnea, increases the need for sedative and paralytic agents, prolongs mechanical ventilation and intensive care unit length of stay, and increases the likelihood of respiratory muscle injury and the need for a tracheostomy (Murias et al. 2016). As reported by Blanch et al., in a large, prospective, non-interventional observational study, respiratory asynchronies are associated with mortality, although further studies are needed to know whether an elevated asynchrony index is just a marker of the severity or whether it constitutes a cause of higher mortality (Blanch et al. 2015).
Major asynchronies can be classified as follows (Mellott et al. 2014):
-
1.
Diaphragmatic muscle contractions triggered by ventilator insufflations constitute a form of patient-ventilator interaction referred to as “entrainment.” This phenomenon is frequent in deeply sedated patients, and it is called reverse triggering, because the insufflation triggers respiratory muscle contraction. Reverse triggering results in breath stacking and high Vt and increased oxygen consumption. It is usually very difficult to recognize in critically ill patients, but it becomes easy to recognize if respiratory muscle activity is monitored by the measurement of Pes.
-
2.
Ineffective efforts are recognized by a negative Pes swing without ventilator pressurization. It can be caused by the presence of auto-PEEP and/or excessive support, and it is aggravated by the trigger setting being set too low (if pressure-based) or too high (if flow-based).
-
3.
Double triggering is the result of the ventilator inspiratory time (Ti) being shorter than the patient’s inspiratory time. The patient’s effort then continues after the first cycle and can trigger a second ventilator breath. In this case, a single Pes deflection can persist for two or even three breaths delivered by the ventilator.
-
4.
Auto-triggering is defined as a cycle delivered by the ventilator without inspiratory effort by the patient. It is often due to an air leak, cardiac artifacts, or secretions. Auto-triggering is recognized by the absence of negative Pes swing preceding a mechanical breath.
-
5.
Premature cycling is defined as a cycle in which the ventilator’s inspiratory time, Ti, is shorter than the neural Ti. This is seen as the duration of Pes deflection being longer than the ventilator’s inspiratory time.
-
6.
Delayed cycling occurs when the machine Ti is longer than the neural Ti (i.e., longer than the duration of Pes deflection).
Understanding how to place an esophageal balloon, the limitations of the measurements, and potential artifacts can lead to the improved matching of ventilator settings to patient’s physiology and safer management of ventilated patients. Monitoring Pes allows the early detection of asynchronies, which can help physicians optimize ventilatory settings and to titrate sedation. Esophageal pressure allows better titration of PEEP by identifying positive transpulmonary pressure at the end of expiration and/or excessive transpulmonary pressure at end-inspiration. Finally, it may especially have a value by avoiding potentially large transpulmonary pressures due to uncontrolled patient efforts.
References
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55.
Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126(5):788–91.
Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, Loring SH, Talmor D. EPVent-2 Study Group. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019. https://doi.org/10.1001/jama.2019.0555.
Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Luján M, García-Esquirol O, Chacón E, Estruga A, Oliva JC, Hernández-Abadia A, Albaiceta GM, Fernández-Mondejar E, Fernández R, Lopez-Aguilar J, Villar J, Murias G, Kacmarek RM. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015;41(4):633–41.
Brochard L. Measurement of esophageal pressure at bedside: pros and cons. Curr Opin Crit Care. 2014;20(1):39–46.
Buytendijk JH. Intraesophageal pressure and lung elasticity [thesis]. Groningen: University of Groningen; 1949.
Cherniack RM, Farhi LE, Armstrong BW, Proctor DF. A comparison of esophageal and intrapleural pressure in man. J Appl Physiol. 1955;8(2):203–11.
Dornhorst AC, Leathart GL. A method of assessing the mechanical properties of lungs and air-passages. Lancet. 1952;2(6725):109–11.
Fry DL, Stead WW, Ebert RV, Lunin RI, Wells HS. The measurement of intraesophageal pressure and its relationship to intrathoracic pressure. J Lab Clin Med. 1952;40:664–73.
Gattinoni L, Chiumello D, Carlesso E, Valenza F. Bench-to-bedside review: chest wall elastance in acute lung injury/acute respiratory distress syndrome patients. Crit Care. 2004;8(5):350–5.
Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM. ECMO criteria for influenza a (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med. 2012;38(3):395–403.
Grieco DL, Chen L, Brochard L. Transpulmonary pressure: importance and limits. Ann Transl Med. 2017;5(14):285.
Hubmayr RD, Walters BJ, Chevalier PA, Rodarte JR, Olson LE. Topographical distribution of regional lung volume in anesthetized dogs. J Appl Physiol. 1983;54:1048–56.
Keller SP, Fessler HE. Monitoring of oesophageal pressure. Curr Opin Crit Care. 2014;20(3):340–6.
Li HL, Chen L, Brochard L. Protecting lungs during spontaneous breathing: what can we do? J Thorac Dis. 2017;9(9):2777–81.
Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J, PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine). Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42(9):1360–73.
Mead J, Gaensler EA. Esophageal and pleural pressures in man, upright and supine. J Appl Physiol. 1959;14:81–3.
Mellott KG, Grap MJ, Munro CL, Sessler CN, Wetzel PA, Nilsestuen JO, Ketchum JM. Patient ventilator asynchrony in critically ill adults: frequency and types. Heart Lung. 2014;43(3):231–43.
Murias G, Lucangelo U, Blanch L. Patient-ventilator asynchrony. Curr Opin Crit Care. 2016;22(1):53–9.
Plataki M, Hubmayr RD. Should mechanical ventilation be guided by esophageal pressure measurements? Curr Opin Crit Care. 2011;17(3):275–80.
Talmor DS, Fessler HE. Are esophageal pressure measurements important in clinical decision-making in mechanically ventilated patients? Respir Care. 2010;55(2):162–72; discussion 172-4.
Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–104.
Washko GR, O’Donnell CR, Loring SH. Volume-related and volume-independent effects of posture on esophageal and transpulmonary pressures in healthy subjects. J Appl Physiol (1985). 2006;100(3):753–8.
Yoshida T, Brochard L. Esophageal pressure monitoring: why, when and how? Curr Opin Crit Care. 2018;24(3):216–22.
Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, Tucci MR, Zin WA, Kavanagh BP, Amato MB. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med. 2013;188(12):1420–7.
Yoshida T, Roldan R, Beraldo MA, Torsani V, Gomes S, De Santis RR, Costa EL, Tucci MR, Lima RG, Kavanagh BP, Amato MB. Spontaneous effort during mechanical ventilation: maximal injury with less positive end-expiratory pressure. Crit Care Med. 2016;44(8):e678–88.
Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, Morais CCA, Gomes S, Costa ELV, Cardoso PFG, Charbonney E, Richard JM, Brochard L, Kavanagh BP. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med. 2018;197(8):1018–26.
Authors’ Contribution
NC, FDC, and TM conceived and drafted the text; all authors approved the final draft of the report.
Conflicts of Interest
None
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Corcione, N., Corte, F.D., Mauri, T. (2021). Measurement of Pleural Pressure. In: Magder, S., Malhotra, A., Hibbert, K.A., Hardin, C.C. (eds) Cardiopulmonary Monitoring. Springer, Cham. https://doi.org/10.1007/978-3-030-73387-2_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-73387-2_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73386-5
Online ISBN: 978-3-030-73387-2
eBook Packages: MedicineMedicine (R0)