Abstract
Invasive non-native plants are changing ecosystems and native biodiversity, and modifying soil microbial feedback. The invasive species Prosopis juliflora (Sw.) D.C. (mesquite) has been introduced into several ecosystems, especially in tropical and subtropical regions, causing economic, ecological and health problems. This article reviews P. juliflora ecophysiological and reproductive attributes, such as phenology, vegetative, seed germination and dispersal, allelopathy and invasion mechanisms. We found that P. juliflora invasion has negative impacts on native biodiversity, ecosystem structure and function, bulk soil, seed bank, and hydrological cycle. We discuss P. juliflora as a ruminant food and for human use, and new management techniques. The easy naturalization P. juliflora in tropical regions has been explained by allelopathy, repeated flowering, vegetative propagation, production and dispersal of huge viable seeds. In particular, P. juliflora produces allelochemicals that are not produced in close relatives. Such chemicals have facilitative effects on associated vegetation in the native range, but have detrimental effects in the introduced range. Management strategies are presented to control P. juliflora invasion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Many human activities, such as agriculture, recreation, global trade and transportation have promoted both the intentional and accidental spread of species across their natural dispersal barriers (Kolar and Lodge 2001). A number of introduced species have been established and spread in the new range and are considered pests for agricultural, landscape and horticultural sector as they pose economic threats to these industries (Barbier et al. 2013). Several authors have considered the increasing number of introduced or invasive species as a major component of global change because of their potential to alter social-ecological systems (Richardson et al. 2011; Moodley et al. 2013; Potgieter et al. 2013; Shackleton et al. 2014). Biological invasions are recognized as one of the most important causes of ecosystem degradation, community structure, local species and biodiversity loss worldwide (Wardle et al 2001; Pyšek et al. 2012).
Prosopis L. is a genus with 44 species of medium-sized trees and shrubs belonging to family Fabaceae, subfamily Mimosoideae (Burkart 1976). Naranjo et al. (1984) and Hunziker et al. (1986) have reported the possibility of interspecific hybridization between Prosopis juliflora (Sw.) D.C. (Fabaceae) and P. pallida (Humb. & Bonpl. ex Willd.) Kunth, which led to the difficulty in differentiating the two taxa. Despite molecular studies were useful in differentiating the two species (Landeras et al. 2006; Sherry et al. 2011), such morphological similarities led to misidentification of Prosopis species and consequently all records of introduction of Prosopis need to be reevaluated. To avoid this problem, it is often referred to as the P. juliflora – P. pallida complex (Pasiecznik et al. 2001). Prosopis was found to occur as a native or introduced species in 129 countries worldwide mainly in the hot arid and semi-arid climatic regions of the world (Shackleton et al. 2014). The numerous goods and services provided by Prosopis have led to global introductions and have made some species important for local communities (Pasiecznik et al. 2001; Shackleton et al. 2014). At least 19 (invasive and weedy) of the 44 species in the genus Prosopis are known to generate benefits and costs, with the rest being primarily beneficial in their native ranges (Shackleton et al. 2014). It has been introduced to different parts of the world for different purposes, but it has become aggressive invader in most introduced ranges. For example, it was introduced to Sudan in 1917 to combat desertification and to provide fuelwood (Elfadl and Luukkanen 2006). Similarly, this species was brought to Lake Baringo, Kenya, in the 1980s to alleviate fuelwood shortage (Mwangi and Swallow 2005). Prosopis juliflora (Sw.) D.C. was introduced to India during late nineteenth century for the rehabilitation of sodic lands and to supply of fuelwood, fodder, timber, and fiber (Mishra et al. 2003; Sharma and Dakshini 1996) and into Ethiopia in the 1970s and 1980s mainly for soil and water conservation (Tegegn 2008). In fact, P. juliflora is present in IUCN’s new list of 100 world’s worst invasive alien species (Luque et al. 2014).
Management of the invaded species is inefficient in many areas due to lack of knowledge on key aspects of the invasive species. For proper management of P. juliflora, it is important to understand the reasons for introductions, benefits, costs, ecology and scales of invasions (Shackleton et al. 2014; Wilson et al. 2014). All of the used approaches for controlling mesquite were mainly to maximize its benefits and minimize its negative impacts (Wise et al. 2012). Patnaik et al. (2017) highlights the dual role of P. juliflora and itemizes the facts that make it a blessing in some contexts and a bane in other contexts with a more realistic view. Previous and recent reviews on genus Prosopis have focused on the special attributes that give plants competitive advantage, origin and systematics, spread and distribution, impacts, benefits and management (Patnaik et al. 2017 and Shackleton et al. 2014). This review article synthesizes all aspects of the taxonomy, current distribution, history of introduction and spread, ecological constrains (including preferred climate, substratum and habitats), ecophysiological responses to biotic and abiotic factors, biology (including phenology, vegetative and reproductive biology, seed germination and dispersal). Although, we review why Prosopis juliflora is invasive in many arid regions, where it was introduced while it is not invasive in its native distribution environment and areas. This will particularly help to find clues of its invasion mechanism (congeneric and biogeographic approach). Indicating potential trade-offs between economic benefits (ruminants feed and human uses) from biomass use and ecological impacts (aboveground, belowground, water and soil fertility) within a defined landscape, whilst also considering interventions in other habitats, will aid decision making, planners and risk managers about its better management in arid and semi-arid regions including Middle East and North Africa in a sustainable way.
7.2 Current Distribution and Status
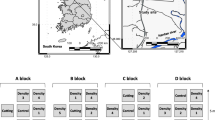
Most species of the genus Prosopis are native to the Americas, ranging from the southwestern USA, through Mexico and Central America into South America, as far as Argentina (Morgan et al. 2017). It was then introduced to Ethiopia, Kenya, Sudan, Eritrea, Iraq, Pakistan, India, Australia, South Africa, the Caribbean, the Atlantic Islands, Venezuela, Bolivia, Brazil, the Dominican Republic, El Salvador, Nicaragua, the United States (USA), and Uruguay (Abdulahi et al. 2017) where P. juliflora is an invasive weed. At present, Prosopis juliflora are distributed worldwide, they are more frequently invasive in Mediterranean, tropical and arid climates (Fig. 7.1). Furthermore, Table 7.1, demonstrate the Global distribution of Prosopis species.
7.2.1 Native Range: Climate, Soil and Habitats
P. juliflora is a shrub native to Mexico, South America and the Caribbean (Burkart 1976; Pasiecznik et al. 2001). It require at least 250 mm annual rainfall, but some have been found in areas with <100 mm (Morgan et al. 2017). Kaur et al. (2012) identified Venezuela as one of the native ranges of P. juliflora. Detrimental and beneficial mechanisms do not act in isolation from each other in nature. The relative importance of these two processes in a particular plant community determines the structure of that community (Callaway and Walker 1997). Many Prosopis species, in their native ranges, contribute with higher concentrations of organic matter, nitrogen, phosphorus and potassium beneath their canopies and behave as strong facilitators of other species (Tiedemann and Klemmedson 1973). In its native range in Venzuela, P. juliflora probably has much stronger facilitative effects on neighbours than other leguminous tree species (Larrea-Alcázar and Soriano 2008).
However, in the introduced range, P. juliflora has both facilitaive and allelopathic effects. However, the allelopathic effects of the litters of P. juliflora override its potential positive effects on soil fertility. For example, El-Keblawy and Abdelfatah (2013) assessed the impact of P. juliflora on the soil and associated flora in the natural habitats of the United Arab Emirates and concluded that this species has facilitative effects in relation to the available nutrients (i.e., most important macro-nutrients K, N and P were increased). In addition, P. juliflora increased the organic matter content, which would also increase the water holding capacity that would improve soil texture and increase soil moistures. However, the negative effect of P. juliflora on the associated flora indicates that the allelopathic effects of the litters may override its potential positive effects on soil fertility (El-Keblawy and Abdelfatah 2013). In India, Kaur et al. (2012) showed that P. juliflora form resource islands by accumulating total organic N and organic carbon in their rhizosphere soil. However, the allelochemicals produced by this species outweigh the facilitative by nutrient enrichment (Kaur et al. 2012).
The history and range of distribution of the invasive P. juliflora – P. pallida complex has been covered by several authors including Burkart (1976), Habit and Saavedra (1990a, b, c), Poynton (1990), Felker and Moss (1996) and Perry (1998). In their monograph about P. juliflora, Pasiecznik et al. (2001) described the environmental conditions where this complex grows and creates environmental problems. According to Pasiecznik et al. (2001), the P. juliflora – P. pallida complex has been recorded in most of the African countries.
7.2.2 Introduced Range: Climate, Soil and Habitats
In the introduced range P. juliflora can grow in a wide range of conditions ranging from sand dunes to clay soils; from saline to alkaline soils; from areas below 200 to more than 1500 m above sea level; and from 50 to 1500 mm mean annual rain fall (Pasiecznik et al. 2004; Zeila et al. 2004). It can also withstand and survive temperatures from as high as 50 °C (air temperature) and 70 °C (soil temperature) (Pasiecznik et al. 2004). The introduction of Prosopis species from the Americas started to Senegal in 1822, and then to Australia, Hawaii, India, Philippines, South Africa, Sri Lanka and Sudan in the late 1800s and early 1900s (Pasiecznik et al. 2001). However, reforestation programmes after major droughts in the Sahel encouraged the widespread introductions into Africa and Asia between the 1970s and 1990s (Shackleton et al. 2014). The reasons of the introductions of Prosopis include providing fodder, fuelwood and shade in the arid areas of South Africa and Australia. In addition, the introductions were for dune stabilization, afforestation and fuel wood supply, provision of and fodder in several African countries. In India and Middle Eastern countries, the introductions were for rehabilitating degraded soil, local greening, ornamental cultivation and soil stabilization (Shackleton et al. 2014).
Prosopis juliflora is a major invasive species in India, and has also invaded other regions throughout the world including Saharan and southern Africa, the Middle East, Pakistan, and Hawaii (USA) (Pasiecznik et al. 2001), where it appears to strongly suppress the species native to those regions (Kaur et al. 2012). Prosopis juliflora forms pure stands in its invaded range in India and Arabia (Figs. 7.1 and 7.2), and occurs in forests, wastelands and at the boundaries of crop fields. P. juliflora also occurs in saline habitats in Hawaii USA. Above all, the worst thing is its negative impacts on the ecosystem like forming impenetrable shrubby thickets, invading water courses, lowering the water-table and thus indirectly starving plants of other species of moisture and nutrients, creating what are known as ‘green deserts’, largely devoid of life, instead of meeting the stated objective (Abdulahi et al. 2017). Even in the arid lands of the Arabia, P. juliflora dominates areas with shallower water table (El-Keblawy et al. 2015). These functional properties of Prosopis and otherfoster its adaptability and support the invasion of thespecies across various agro-ecosystems includingwetlands, dry lands, and irrigated agricultural lands (Shiferaw et al. 2004).
The P. juliflora have typically invaded areas in Arabian Peninsula where water table is close to the ground, such as gravel deserts, open plains, sand sheets, wadis and edges of farms (El-Keblawy and Al-Rawai 2007; El-Keblawy and Abdelfatah 2013; El-Keblawy et al. 2015; Thomas et al. 2016). In Saudi Arabia, P. juliflorawas reported as a ruderal plant, mostly found inside the urban and suburban areas and has rarely seen among the native plant populations (Thomas et al. 2016). In the UAE, this species was reported in many natural habitats that have shallower water table. In addition, several farms of Ras Al Khaimah have been invaded and, consequently, ruined during the last 30 years (Ali El-Keblawy, unpublished data). Prosopis juliflora was identified as the most prominent invasive species in majority of lowlands of Saudi Arabia (Thomas et al. 2016).
This species was found as a troublesome exotic in the main inhabited island (Farasan Al-Kabir) in the Red Sea; approximately 12.5% of the total cover of inland vegetation is represented by P. juliflora. In addition, this species was found to be the most troublesome one in Najd, Central region of Saudi Arabia, which is considered as the most arid part of country; average annual rainfall is less than 100 mm. In this region, P. juliflora was reported as a ruderal plant, mostly found inside the urban and suburban areas and rarely seen among the native plant populations (Thomas et al. 2016). Careful examination of the P. juliflora plants showed the presence of P. juliflora growing mixed with P. pallida in most of the invaded habitat of the UAE and this complex is highly aggressive and coppices so well that it crowds out native vegetation (El-Keblawy and Al-Rawai 2007).The suitable habitat of P. juliflora is roadsides, but it is also planted in close spacing at an area of few hectares on sandy desert. Similarly, this species has been introduced to Qatar during 1950s and it grows around farmland as well as in depressions (Ahmad and Ismail 1996).
7.2.3 History of Introduction and Spread
Prosopis juliflora (Sw.) D.C. is native to south-west United States and north-west Mexico, and it is invasive in many tropical and subtropical regions, including North Africa and the Arab Gulf region (Abbas et al. 2016). P. juliflora was introduced to the UAE in the 1970s for greening deserts (El-Keblawy and Al-Rawai 2007; Tourenq and Shuriqi 2010). During the last four decades, this species has escaped the forests and currently is considered as a serious weed (Figs. 7.1 and 7.2). In Bahrain, scattered patches of young P. juliflora plants were recorded on coastal lowland. The presence of a huge single isolated P. julifloratree in the central plateau of Bahrain indicates the probability of its early introduction. In Ethiopia, Prosopis was introduced in the late 1970’s, or early 1980’s through collaborative efforts of governments and international development organizations to rehabilitate degraded soils, to supply firewood and fodder and to combat desertification (Berhanu and Tesfaye 2006; Rettberg and Müller-mahn 2012; Shackleton et al. 2014). Now a day, around one million hectares of Ethiopia are already covered by Prosopis (Abdulahi et al. 2017). In Egypt, P. juliflora was introduced intentionally in the Gebel-Elba National Park by local people of Old-Hala’ib. village for agroforestry purposes during 1980s. However, they found it as highly invasive, spreading rapidely, destroying the local flora (Abbas et al. 2016). At the moment, now, it has been spread to 116 Km Northwest of its introduced range in the wadis of Shalal, Mericowan, Sarara, Shab and Aibib (Shiferaw et al. 2004; Hundessa 2016).
In the arid and semi-arid areas of Kenya, the Prosopis species were first introduced to rehabilitate quarries near the coastal town of Mombasa with seed sourced from Brazil and Hawaii in the early 1970’s as a source of fuelwood and later, in the rehabilitation of degraded lands (Oduor and Githiomi 2013). In the Sudan, Prosopis spp. were introduced in many semi-arid areas, to combat desertification and provide fuel wood and fodder (Elfadl 1997a, b). However, this species have spread rapidly into fertile, productive areas, and irrigation and drainage channels (Morgan et al. 2017) (Fig. 7.3).
Prosopis juliflora and its congeneric species Prosopis cineraria are co-occurring in many habitats of the United Arab Emirates. A. Prosopis cineraria provided the shade for domestic and wild animals that left big amount of Prosopis juliflora seeds resulted in dense growing of their seedlings under the crown of native Prosopis cineraria (b) the dense competition between the two congeneric species, especially for water resources (c and d) the intense competition between the two species resulted in death of the native Prosopis cineraria
The highly invasive nature of P. juliflora is a result of several factors; it is extremely drought resistant, a quality mainly attributed to its deep taproot (Samuel et al. 2013). Riversand water canals also play a significant role in the dissemination of seeds to different areas. Swampareas, road sides and irrigation canals are highlyinvaded by Prosopis. This shows Prosopis establisheswell in areas where water is available and also wheresurface runoff water present for its seed dispersal. In Kenya, The banks of the Tana river, which is the largest river in Kenya have been invaded by Prosopis. Seeds could be dispersed by water, but the main dipsersal agent is the digestive system of most herbivores (Maundu et al. 2009)
7.3 Ecological Constraints
In the native range Prosopis juliflora exhibits different physiological responses to environmental variables, mainly temperature, rainfall and day length (Pasiecznik et al. 2001). P. juliflora can grow in arid and semi-arid regions because of its resistance to drought and heat. It thrives on all soil types under variable climatic conditions (Sawal 2004).
7.3.1 Climate
Prosopis juliflora is reported to tolerate an annual precipitation of 150 to 1670 mm, annual temperature of 20.3 °C to 28.5 °C, and pH around neutral (Ruskin 1980 and Larrea-Alcázar and Soriano 2008) but it can growth in areas with annual rainfall less than 100 mm as in the Central region of Saudi Arabia (Thomas et al. 2016) and UAE (El-Keblawy and Abdelfatah 2013). Given the fact that P. juliflora is evergreen and has fast growth aloo over the year, this is support the hypothesis that it relies manily on underground water as main source for life. P. juliflora is frost sensitive (Felker et al. 1982) and severe frost can cause stem and tree mortality in countries with cold weather. Consequently, there is doubt about the presence of this species in the Mediterranean climates of countries such as Morocco, Algeria, Tunisia, Libya and Egypt. For example, winter frosts occur over much of Egypt making it unsuitable for the P. juliflora – P. pallida complex unless it grow in sheltered or coastal locations (Burkart 1976). Similarly, Akrimi (1990) stated that cold sensitive P. juliflora was killed outright in Tunisia. Most of Prosopis species that are present in these countries are more likely be P. Glandulosa Torr., P. velutina Wooton, P. chilensis (Molina) Stuntz or hybrid forms from these species (Burkart 1976). In northern India, P. juliflora grows in regions that have high temperatures and high light intensities for most of the year. However, during the winter (December–January), the nocturnal temperatures fall to 2 °C, while during the summer (May–June), the temperatures of the day often exceed 45 °C (Shirke and Pathre 2004). P. juliflora shows a high capacity to adapt to theseextreme temperatures, especially, to the high temperaturesin summer.Similarly, in the UAE, where temperatures in summer can reach around 50 °C, P. juliflora grews nicely allover the year (El-Keblawy et al. 2015).
7.3.2 Substratum
Prosopis juliflorais a facultative phreatophyte that has a higher tolerance to droughts and ability to access declining groundwater tables through its deeper root system.It survives and flourishes in heavy or sandy soils, as well as saline dry flats and it can tolerate grazing (Morgan et al. 2017). In the Lagunillas semiarid enclave in the Venezuelan Andes P. Juliflora is present on loamy clay soils with high concentrations of organic matter (4–5%), nitrogen (0.15–0.30%), potassium (0.4–1.1 ppm), magnesium (2.0–5.5 me/l) and calcium (30–45 me/1) (Larrea-Alcázar and Soriano 2008). Prosopis juliflora usually grows in non-saline soils in most of its native and introduced range, but it has been recorded to occur in saline habitats in Hawaii USA and Gujarat, India (Kaur et al. 2012). In the UAE, it is reported in both saline and non-saline hábitats as well as farms and disturbied sites (El-Keblawy and Al-Rawai 2007).
7.4 New Insights into Traits That Promote Invasion
7.4.1 Morphological Characteristics
The life history traits of invasive plants demonstrate an important feature that can help themselves in the invasion, adaptation and establishment in a new habitat or introduced ones. The morphological and agro-physiological characteristics include plant height, dispersal efficiency, compition potential and sprouting capacity (Gibson et al. 2011). However, the human and environmental factors are also a important contributor in the invasion and dispersal of exotic plant species into a new habitat (Castro-Díez et al. 2011). Prosopis juliflora (mesquite, vilayati babul, algarrobo) is a xerophytic evergreen and fast growing tree, native to frost-free tropical regions of Peru, Central America and the Caribbean, with long lateral shallow and deep taproot systems that are also able to fix nitrogen (Burkart 1976; Pasiecznik et al. 2001). Stem is green-brown, sinuous and twisted, up to 5–10 m in height, with strong axial thorns situated on both sides of the nodes, the bark is rough and light red in colour. Leaves are compound, bipinnate with one or sometimes two pairs of rachis, each having 12 to 25 pairs of green folioles (Silva 1986). Flowers are small (4–6 mm long, 1 cm long), produced in inflorescences of various sizes and shapes but generally in spike-like inflorescences (racemes) 9.5–16.5 cm long (Díaz 1995). Prosopis flowers are arranged in cylindrical racemes of about 300–400 flowers (Zaitoun et al. 2009), in clusters of 2–5, at the end of the branches. Flowers are hermaphrodite, sometimes sterile, actinomorphic and pentamerous (Burkart 1976). Fruit is a non-dehiscent pod, curved and about 4 mm thick, 1 cm wide and up to 15 cm in length made up of light yellow hardened epicarp, fleshy mesocarp and woody endocarp which contains seed (Silva 1986).
7.4.2 Reproductive Features
7.4.2.1 Phenology and Vegetative Growth Attributes
Prosopis trees generally initiate flowering and fruiting in the third or fourth year after germination. First flowering is dependent on optimal conditions and under drought conditions or very poor soils it can be considerable later (Pasiecznik et al. 2001). Prosopis species produce flowers every year increasing gradually up to 10–20 years and may be expected to continue at this high level for several decades. In the native range P. juliflora exhibits different physiological responses to environmental variables, mainly temperature, rainfall and day length (Pasiecznik et al. 2001), so variation in the onset of flowering is present between different populations due to climatic variation. Flowering is also variable within and between trees of the same population. Despite, flowering times are genetically controlled (Graham 1960; Pasiecznik et al. 2001). P. juliflora flowering season at the native range varies, with one or two periods of main flower production. It generally coincides with the wet season, from December to February and it is delayed from March to April and from July to September when there are two periods of flowering. Therefore, legume production generally overlaps with the end of the wet season improving seedling establishment or partly cover the dry season, ensuring pod consumption and seed dispersal by wild animals.
In the invaded area P. juliflora present a different multiple possibilities of flowering, associated not only to climatological conditions, but to evolutionary associations, extremely fast, with pollinator insects. In Punjab (India) P. juliflora showed one flowering period from January to May with earlier flowering time period with the increase in the temperature (Kaur et al. 2013) but in the ridge of Delhi (India) P. juliflora flowering occurs all year round and reaches its flower peak in April and September (Thakur 1989). Flowering almost continuous year round was observed in some invaded areas as Brasil or Haiti (Silva 1988; Timyan 1996). In the Arabian Peninsula, El-Keblawy and Al Rawai (2006) reported two episodes for flowering: autumn (November – Dec.) and (April – May). However, in moist year, the plants was observed in the flowering stage of the year around. There is controversy about time development of flowers in P. juliflora. Styles emerge from most flowers prior to anthesis and flowers remain in this state for some days (Pasiecznik et al. 2001). Anthesis occurs when flowers are fully open and accessible to pollinators. Flower maturation is variable in the same tree, often lower flowers in the raceme are fully developed than the upper ones that are still immature (acropetal succession). In general, the main pollen season took place in spring, the highest concentrations being recorded mainly in April, with a secondary peak in autumn, if any (Davis 1995 and Simpson et al. 1977). On the other hand, Perveen et al. (2014) studied the intradiurnal behaviour followed by P. juliflora pollen at Khairpur (Pakistan), where the maximum peak occurred near midday, at 11:00.
7.4.2.2 Prosopis juliflora and Pollinators
Pollinator assemblage structure can have important influences on floral evolution and reproductive interactions among plant species (Moeller and Tiffin 2005). Prosopis anthers have a glandular appendage that release an exudate containing protein and carbohydrates (Chaudhry and Vijayaraghavan 1992). Then, flowers produce copious amounts of nectar, as a nutritious reward for potential insect pollinators especially bee species (Simpson et al. 1977). In a geographical context, the spatial structure of variation in pollinator abundance and community composition can also have important implications for plant reproductive performance and ultimately floral evolution (Gomez et al. 2007). This entomophilia is extremely generalized, 77 insect visitors being reported in India by the former authors, Hymenoptera (bees and wasps) and Diptera (flies) being the main visitors.
P. juliflora is self-incompatible and receives insect pollinators and entomological visitors from a wide type of insect orders (Ward et al. 1977). P. juliflora has small size of flowers that ensures the contact of small insects with reproductive organs and there will be less chances of nectar robbing. Despite P. juliflora produces large number of inflorescences; the pollination success rate is significantly less. Based on inflorescence number/tree, according to reports of DeOliveira & Pires (1990), P. juliflora exhibited 29% pollination efficiency while it was dropped to 1.48%. Flowers of Prosopis are visited by different types of insects and more specifically from Hymenoptera (bees, wasps and Pepsis spp.), Coleoptera (Bruchidae, Lycidae, Tenebrionidae, and Scarabaeidae), Diptera (Syrphidae), and Lepidoptera (Noctuidae, Geometridae and various butterflies) (Simpson et al. 1977; Keys 1993).
Many plant species produces mass-flowers and mostly for floral display but usually they do not have enough food resources to transport to these points that help then to develop into fruit (Trueman & Wallace 1999). However, several plants produces significant number of seeds that are enough for that particular species to survive (Karron & Mitchell 2011). Other researchers demonstrated that there are several factors responsible for productivity and includes stigma reception, pollen release time, period and pollen viability. In this regard, ovary abortion, flower sterility and few pollen visitors are others major reasons for low pollination and productivity DeOliveira & Pires (1990). In case of P. juliflora that mostly grown in arid and semi arid regions with high summer temperature are another reason of low pollination. The insect pollinators are less mobile during midday (above 40 °C) that conincided with higher pollen production at midday (Goel and Behl 1995). There was also significant spatial variation observed among locations in pollinator assemblages in terms of species richness, diversity and dominance. The abundance-richness relationship is frequent in pollinator assemblages (Steffan-Dewenter et al. 2002),
According to studies of Sajjad et al. (2012), number of pods and germination rate of seeds rise with incease in its flower visitors. They concluded that flower visitor abundance was a better predictor of plant reproductive performance than species richness and the Shannon-Wiener index. We also found a significant spatial variation among locations in pollinator assemblages in terms of species richness, diversity and dominance. Diversity and identity (Gomez et al. 2010), and relative abundance of floral visitors (Sahli and Conner 2006) have been reported as the important predictors of plant reproductive success. The sensitivity of pollinator assemblages to predict plant reproductive success may vary with plant species and the pollination effectiveness of available pollinator species (Talavera et al. 2001).
7.4.2.3 Seed Production, Dispersal and Germination
The racemes work as reproduction units and the small size of the flower ensures contact of small insects with reproductive organs. Different maturation period in the reproductive organs and flowers development make P. juliflora plants self-incompatibles, depending of insects for seed setting (Sajjad et al. 2012). Obligate outcrossing leading to high genetic variability as an evolutionary mechanism for survival in zones with a high variability in rainfall, temperature and soil types. This trait, among others, allows to P. juliflora being an effective plant invader. By other hand, as was mentioned, long periods of asynchronous flower production would assume a long period of pollen release. However, due to P. juliflora is a tree and its flowers exhibit exerted stamens, its pollen grains present a secondary anemophily and can easily pass into the atmosphere and become airborne. Coat imposed dormancy is of wide occurrence in legumes and has been reported as a characteristic governing the weediness of P. juliflora (Morgan et al. 2017). In the field germination is favoured because of P. juliflora seeds pass through the digestive system of grazing sheep, goats and cows that might help in seed dissemination for long distances. In Ethiopia, it was observed that cattles are major source of seed disoersals followed by camels and goats (Shiferaw et al. 2004). Morgan et al. (2017) revealed that seeds collected from sheep’s droppings were free from the pericarp and displayed 65% germination and 60% emergence suggesting that soil seed bank contain seeds of P. juliflora ready to germinate when conditions are optimal or moved to a shallower depth by agricultural implements.
The seed traits such as seed coat, endosperm, pericarp and extrafloral organs prevent germination until they are removed or damaged (Finkelstein 2006). On average, fruits of P. juliflora (15–20 cm width, 10–40 cm length) possess 20 seeds/pod. The endocarp is divided into rigid, leathery segments with one brown, elliptical seed in each segment (Meyer et al. 1971). The seeds frequently have hard, impermeable seminal integuments (Manga and Sen 1995), which ensure dormancy and are well adapted to long-term survival (Tschirley & Martin 1960) and dispersal strategies (Bewley 1997). Several Environmental factors such as fire, temperature, salinity and light as well as their interaction might stimilate or retard the seed germination of Prosopis juliflora. Salinity stress of 400 mM NaCl at 40 °C and in 600 mM NaCl at 25 °C decreased the seed germination. Furthermore, light also stimulated the germination at low salinity (El-Keblawy et al. 2015). Autotoxicity due to dry leaves of P. juliflora also caused significant inhibition in germination, radicle and hypocotyl growth of mesquite sedes (Warrag 1995). Due to climate change and increase in soil temperature will results in inhibition or stimulation of certain plant seed germination (Ooi et al. 2009). Al-Rawahy and co-workers (2003), demonstrated that germination of both Prosopis juliflora and P. cineraria seeds were unaffected following exposure to 90 °C for 6 h.
Senthilkumar et al. (2005), documented that prosopis has also capacity to bioacumulate heavy and toxic metals in various plant organs. Prosopis accumulated significant quantity of Cd in roots than shoots. While, concentration of Cu was not much distinguished between roots or shoots. They concluded that animal grazing on heavy metal contaminated soil vegetation should be avoided because the plant foliage and pods can retain more toxic metals than other parts. Several diverse animanl species such as camels, goats, sheep, deer, horses, cattles, rodents, feral pigs, warthogs are also responsible for dispersal of Prosopis seeds from one place to other through their feces (Kneuper et al. 2003, Shiferaw et al. 2004). Shiferaw et al. (2004) reported germination rates for P. juliflora of 100% with mechanical scarification, 97–99% germination with sulfuric acid, 37% after passage through goats, 47% through warthogs, 15% through camels, 4% through cattle, and 21% with no treatment. However, El-Keblawy and Al-Rawai (2005), documented more than 80% germination success in unscarified seeds. A study conducted on anther glands of P. juliflora demonstrated that an exudates (composed of carbohydrates and proteins) was extreated through cuticular openings to exterior of glands and have function like insect and pollinators attractance (Chaudhry and Vijayaraghavan 1992).
The process of Endozoochory might be responsible for enhancing the germination through releasing the seeds from fruit and ruminants feces that can also serve as source of water and nutrients for seedling establishments. Local animals, cattles, goats, camals, mules and wild fauna (gazelles and coyotes) can play a significant role in dispersal of seeds from one to other places (Wronski et al. 2012; Alvarez et al. 2017). However, exposure f the seeds to animal gut microenvironment, stomach juices, chewing, and herbivores species also affect the quality of endozoochorous (Schupp (1993; Kneuper et al. 2003; Jaganathan et al. 2016). In this context, determining whether endozoochory is advantageous to plant germination requires examining the cumulative effects of gut passage, deposition in faeces and seed dispersal patterns on germination and viability. Thus, understanding the functional role of domestic and wild animal species in seed dispersal of invasive species is central to determining how biotic interactions could be affected by anthropogenic drivers.
7.5 Possible Mechanisms explaining the Invasive Ability of P. juliflora
The traditional, congeneric, and bio-geographical approaches have been used to examine the mechanism of invasive species success (Inderjit et al. 2008).
7.5.1 Traditional Approach
The traditional approach focuses on the fate, dose, replenishment, and effect of chemicals produced by invaders in the soil environment (Inderjit 2001; Hierro and Callaway 2003).
Allelopathy is mediated through the release of secondary metabolites that directly affects many physiological and biochemical reactions and thereby, influence the growth and development of neighboring plants (Lara-Núñez et al. 2006; Hussain et al. 2011; Hussain and Reigosa 2011). Allelopathy has been suggested as one of the mechanisms driving P. juliflora to become more abundant and competitively dominant in their introduced range than in their native range (Elfadl and Luukkanen 2006; El-Keblawy and Al-Rawai 2007; Inderjit et al. 2008 and Kaur et al. 2012) reducing significantly the number of annual plants under the canopy of P. juliflora. The plant has little or no autoallelopathic effect under field condition (EI-Keblawy and AI-Rawai 2006). This mechanism, combined with drought condition can inhibit other species and eliminate any kind of competition (Abdulahi et al. 2017).
7.5.2 Congeneric Approach
The congeneric, or phylogenetic, approach involves comparative studies of exotic species with natives in the same genus (Inderjit et al. 2008). Native plants typically do not share a co-evolutionary history with the exotic invasive species, and therefore greater allelopathic effects of the alien invasive, as compared the native congeneric in such ecosystems. The allelochemicals produced by the invaders are new to the native plant communities (i.e., novel weapons, Bais et al. 2003; Callaway and Ridenour 2004). Assessment of the impact of allelopathy of two Prosopis species on the germination and existing of the associated species helped in understating the role of allelopathy as a mechanism for invasion of P. juliflora in its new ranges (El-Keblawy and Abdelfatah 2013; Kaur et al. 2012). In both Arabia and India, the native P. cineraria is a slow growing tree and is beneficial for the growth and development of other species (Abdel Bari et al. 2007). It is rarely, if ever, seen as a weedy species and has not been successfully introduced into other parts of the world (Pasiecznik et al. 2001). In addition, P. cineraria had a facilitative rther tan determinetal effect on the asscoated flora (El-Keblawy and Abdelfatah 2013; Kaur et al. 2012). In the two regions, however,the introduced P. juliflora has invaded many habitats and residential area and significantly reduced the native flora diversity (El-Keblawy and Abdelfatah 2013; Kaur et al. 2012). In the introduced range of the UAE and India, both species produced allelochemcials. El-Keblawy and Abdelfatah (2015) assessed the impacts of allelopathy produced by P. juliflora and P. cineraria and soil properties on understory native plants in the arid deserts of the UAE. They found inhibitory effect for the aqueous extracts of fresh and old leaves of P. juliflora on the associated flora, but P. cineraria leaves and litter had positive effects on other native species. The quantity of the allelochemicals produced by the two species could help understaning their defferential effect on the assocted flora. Kaur et al. (2012, 2014) found that the amounts of phenolics and tryptophan produced by P. juliflora in India were significantly higher than those of P. cineraria. For example, Kaur et al. (2012) detected L-tryptophan in leaf leachates of both P. juliflora and P. cineraria, but the amounts were 73% higher in leaf leachate of the former than that of the latter. Similarly, Inderjit et al. (2008) compared soils collected from the rhizospheres of the two Prosopis species and found that soils beneath the exotic P. juliflora contained 63.2% higher concentrations of total phenolics than soil beneath the native P. cineraria. This result could explain the greater effect of P. juliflora litterson mortality of native Indian species, compared to litters from P. cineraria (Kaur et al. 2012). Similarly, aqueous extract of P. juliflora leaves showed negative effects on root growth of three common crop species of north-west India, whereas P. cineraria leaf leachate had positive effects (Kaur et al. 2012). Furthermore, Goel et al. (1989) reported greater allelopathic potential of P. juliflora leaf leachate and decomposing litter residues compared with P. cineraria.
7.5.3 Biogeographic Approach
The biogeographic approach studies ecological traits of species and ecological processes in native and non-native ranges. Exotic species bring chemicals novel for invaded communities that has potential to exhibit allelopathic effects due to naïve soil communities and sensitive neighbors (Callaway and Ridenour 2004, Inderjit et al. 2011). The ‘novel weapons hypothesis’ was posed by Callaway and Aschehoug 2000; also see Rabotnov 1982; Malik and Pellisier 2000; Ridenour and Callaway 2001) to study role of plant chemicals in ecological processes and evolutionary context (Inderjit et al. 2006, 2011). Several plant secondary metabolites (phenolics, flavonoids, glycosides, chalcones, cinnamic acid derivatives, Terpenoids, Coumarin, Saponins, and alkaloids) were reported from bark, leaves, stems, flowers, pods and sedes of P. juliflora (Table 7.2). Nakano et al. (2003), demlonstrated the presence of phenolics, tryptophan and juliflorine from P. juliflora foliage. Whereas P. juliflora has facilitative effect in its native range in Venezuela, it has an inhibitory effect in its introduced range.
Costa et al. (2018), reported the ecological damage to ecosystems, stand stabilization and settlement of P. juliflora. The leaves, barks and roots (aqueous extracts) (125 g plant/500 ml of distilled water) inhibited the emergence and seedling growth of jurema-preta, Mimosa tenuiflora, native species of Caatinga and 100% root extract proved to be deleterious than all other extracts. Shah et al. (2018), in a two-year field experiments demonstrated the phytotoxicity of P. juliflora on weed control and yield of wheat. They found that aqueous extracts (0, 10, 20, 30 and 40% concentration of leaves, stems, and roots) reduced the weed density, biomass, leaf area index, leaf area duration, crop growth rate, net assimilation rate, chlorophyll contents, plant height, number of tillers, spike length, number of grains, 1000-grain weight, grain yield, biological yield, harvest index and grain protein content.
Prosopis juliflora is known to inhibit germination of seeds of other plants species that lie in its vicinity (Muturi et al. 2017; Shaik & Mehar 2015). It also discourages other species of plants to grow near it. It releases allelochemicals from its leaves, roots, as well as fruits to achieve this (Noor et al. 1995).Goel et al. (1989) found that leaf extracts as well as leaf leachates of P. juliflora carried allelochemicals, so did decaying leaves. These authors, as well as Chellamuthu et al. (1997), who studied the influence of P. juliflora leaf litter on the germination of seeds of other species, attributed the allelopathy to phenolic compounds present in P. juliflora. Al-Humaid and Warrag (1998) recorded suppression of seed germination and early growth of bermuda grass (Cynodon dactylon), and Kaur et al. (2014) of Brassica campestris, by aqueous extracts of Prosopis leaves. These studies indicate that P. juliflora foliage may contain water-soluble allelochemicals, which get leached to the ground as rain water falls on them and tikles down (Abbasi and Abbasi 2011). These chemicals were isolated by Nakano et al. (2002, 2003, 2004) and identified as syringin, (-) – lariciresinol, L- tryptophan, juliprosopine, juliprosine, and juliprosopinal. Among these, juloprosine derivatives exhibited the most pronounced allelopathy.
This indicates that allelopathy plays a significant role in shaping plant community structure. In its native range in Venezuela, P. juliflora appear to coexist with and facilitate large numbers of other native species (Kaur et al. 2012). In its non-native ranges, P. juliflora strongly suppress species native to those regions (Pasiecznik et al. 2001) and forms pure stands in India, Hawaii (USA) (Kaur et al. 2012) and the UAE (El-Keblawy and Al-Rawai 2007). Competition is another mechanism that would enable P. juliflora to replace native flora. Root density of P. juliflora was 3 cm of root/cm3 of soil in the upper 15 cm of the soil profile, dropping to less than 0.5 cm root/cm3 of soil at below 45 cm depth, and less than 0.2 cm root/cm3 of soil at 1.8 m depth (Jones et al. 1998). Hoshino et al. (2011) indicated that P. juliflora can detect even very tiny soil moisture and grow to various conditions. Some of the many adaptive abilities that allow P. juliflora to thrive under such conditions include ability of roots to adapt to a wide variety of soil conditions (Hoshino et al. 2011). Roots can grow upwards towards the soil surface to capitalize on little rainfall, but can also grow to depths of 80 m and extend laterally more than 30 m (Thorp and Lynch, 2001). Such high density of the superficial roots could enhance the competitive ability of P. juliflorato extract the limited nutrients and water resources of the arid deserts (El-Keblawy and Abdelfatah 2014). This could explain the high aggressive ability and how it could crowd out native vegetation in most invaded sites. It has been noticed that farmers in many places of the UAE just ruin their farms once they are invaded with this species.
Shirke et al. (2018) demonstrated that Prosopis juliflora has shown optimal physiological characteristics to adapt in monsoon season through its leaf architecture that exhibit maximum carbon fixation under moderate temperatures and a wide range of photosynthetic photon flux density. The leaves produced in spring were sensitive to very high temperature and others that develop during monsoon were sensitive to low temperatures causingsenescence in winter. Reinoso et al. (2004) found that salinity induced anatomical changes in roots (young and mature zones), hypocotyls, young stems, and leaflets, with small leaves, reduced cortex and nascular system. Salt stress was also lethal for stem and in hypocotyl size, diameter was reduced along with a reduction in secondary phloem. However, salinity stimulated the production of tannins in stem and leaflets of treated plants. They concluded that anatomical modifications in this species are related to metabolic adaptations, such as an early development of the endodermal barrier for ion exclusion, to allow survival in high salinity.
7.6 Impacts of Prosopis Invasions on Biophysical Features and Ecosystem Services
Propsopis juliflora can grow in different arid climates and substrates because of its resistance to drought and heat and it has many potential uses and impacts (Mendes 1986).
7.6.1 Uses and Positive Impacts
In some areas the benefits from Prosopis are regarded as a key income source for many households. For example, 44% of people in a village in Malawi replied on Prosopis products as a primary or supplementary source of income (Chikuni et al. 2004). Similarly, sale of charcoal and Prosopis pods for fodder have enhanced the local economy in some areas in Kenya by US$1.5 million per year (Choge et al. 2012). In India, Prosopis provides up to 70% of fuel wood needs for local households in some dry region villages (Pasiecznik et al. 2001). In Senegal, the positive aspects of P. juliflora are predominantly associated with regulatory services, such as soil erosion regulation, rehabilitation of sodic soils, flow regulation, and water purification (Tripathi and Singh 2010). The dense shrubs of P. juliflora stabilize the soil, regulate the flow of water, and promote the infiltration of water into the soil. The dense shrubs also provide physical protection to soils against wind erosion during dry periods and against heavy rains during the rainy seasons (Ayanu et al. 2015).
Prosopis juliflora increase organic matter and nutrients on soil beneath its canopy, and commonly used to improve soil physiochemical and biological properties (Vallejo et al. 2012).
Secondary products from P. juliflora includes honey, edible exudates gums, fibres, tannins, foliage for fodder, mulch, biopesticides and medicines, and other uses for wood and particle board, wood chips for energy generation, pods for ethanol production, galactomannan gums from the seeds and other specialist products (Wise et al. 2012; Oduor and Githiomi 2013; Haji and Mohammed 2013).
7.6.1.1 Pods Can Be Used as a Livestock Feed and for Making Human Foods
The pods of P. juliflora contain high levels of protein and are available for a minimum of 4–5 months in the UAE. This could be a good source for proteins for livestock. However, the low digestibility of the leaves and pods of P. juliflora is mainly associated with the presence of harmful substances, such as tannins, glucosinolate, cyanogens, alkaloids, and nitrates (Chaturvedi and Sahoo 2013; Leonard 2011). In some cases, some ruminants are spontaneously poisoned and intoxicated by pods of P. juliflora (Camara et al. 2011). In addition, the dried leaves of this species suppress feed intake and nutrient availability and shouldn’t be included in the feed of livestock (Chaturvedi and Sahoo 2013). An overview of use of Prosopis juliflora and its different organs in livestock feed was elaborated in Table 7.3.
P. juliflora was assayed as biosorbent due to their extremely rapid proliferation, massive growth, renewability, high biosorption capacity and low cost. Seed powder of the P. juliflora exhibit an ability to absorb Pb (II) from the contaminated environment (Jayaram and Prasad 2009). Biomass contributes a significant share of global primary energy consumption because liquid fuels produced from biomass contain no sulphur, thus avoiding SO2 emissions and also reducing emission of NOx (Kumar and Kotiya 2004). The P. juliflora was used as a potential renewable energy source and their large scale utilisation can represent one of the best strategies for their management. The fermentation of both acid and enzymatic hydrolysates, containing 18.24 g/L and 37.47 g/L sugars from P. juliflora, with Pichia stipitis and Saccharomyces cerevisiae produced 7.13 g/L and 18.52 g/L of ethanol with corresponding yield of 0.39 g/g and 0.49 g/g, respectively (Gupta et al. 2009).P. juliflora was used for extraction of energy precursors in the form of volatile fatty acids (VFAs). Patnaik et al. (2018) in a single one-pot step, were able to convert up to 10.7% of the total solids present in the Prosopis leaves to VFAs.
7.6.2 Negative Impacts
7.6.2.1 Aboveground Effects
Maundu et al. 2009 studied the negative aspects of P. juliflora onlocal livelihoods of three sites in Eastern Province of Kenya and reported 24 negative effects. The magnitude varied according to people’s sources of livelihood. The aspect that caught most attention in all sites was the thorn problems on humans. Several problems are related to animals that depended heavily on the pods. In addition, diarrhoea is happen among inexperienced goats that feed on the leaves for the first time. Furthermore, diarrhoea in goats was reported mainly in Loiyangalani town due to influx of pastoralists and their goats escaping drought and insecurity caused by raids for stealing animals. Encroachment was most serious in Baringo and Garissa sites where the species had displaced farmers from their crop farms, invaded areas used for grazing and browsing. Moreover, encroachment to paths, dwellings, water sources, farms and pastureland, constraining movement and other activities were reported as problems asscoaited with the introduction of the species.
7.6.2.1.1 Vegetation and Community Structure
Several reviews have shown deserts to be among the least-invaded ecosystems worldwide, at least in terms of the number of naturalized and invasive species (Lonsdale 1999). However, this species is expanding its range at an alarming rate and damaging native diversity and ecosystem health of the arid and hyper-arid regions. It discourages abundance, richness and growth of native species. P. juliflora often observed to form pure stands, and does not allow other species to grow beneath or around its canopies. Despite P. juliflora is a strong facilitator in the native range (Tiedemann and Klemmedson 1973; Kaur et al. 2012); it was found that the canopies of the invasive P. juliflora had far fewer understory species in the invaded areas than native congeners (Aggarwal et al. 1976). In the Arabian Peninsula the invasive has strong negative impacts on native species: number, richness, evenness, density and frequency of the associated native speciesdespite increases in the concentrations of some nutrients in subcanopy soil (El-Keblawy and Al-Rawai 2007). Especially important is that this depressive effect extended beyond the canopy-covered ground for dense sites. Old and dense sites of P. julifloraresulted in significantly lower density, frequency and diversity for most associated annual species (El-Keblawy and Al-Rawai 2007). Similarly, the growing of P. juliflora shrubs, as well as exotic Eucalyptus, in the forests of the UAE has also resulted in significant reductions in species diversity and abundance of understory species, compared to the native P. cineraria and Acacia arabica (El-Keblawy and Ksiksi 2005).
7.6.2.2 Belowground Effects
7.6.2.2.1 Soil Microorganisms
Soil microbial communities play an important role in the invasion success of exotic plant species (Inderjit and van der Putten 2010). Plant-soil feedbacks (PSFs) include plant-mediated changes in soil communities that affect the establishment and growth of plant species (van der Putten et al. 2013, Inderjit and Cahill 2015). Exotic invaders are known to culture soil biota that exert neutral or positive impacts on the invader compared to the negative impacts of soil biota cultured by native species on themselves (Callaway et al. 2004). The effect of P. juliflora on soil properties could be mediated through soil microbial flora. P. juliflora trees have the potential to establish a symbiotic relationship with N-fixing Rhizobium bacteria, which increase the N levels in the rhizosphere with root and nodule turnover (Reyes-Reyes et al. 2002; Perroni-Ventura et al. 2010). For example, a total of 150 bacterial strains were isolated from the root nodules of P. juliflora growing in soils collected from Marigat area of Kenya (Otieno et al. 2017). In addition, the microbial activity of P. juliflora as witnessed by the emission of CO2 was larger for soil sampled under canopy than outside it (Herrera-Arreola et al. 2007). Those authors indicated that addition of leaves increased production of CO2 and between 40% and 50% of the organic C of the leaves was mineralized. Prosopis has been recognized as a fertility island tree that significantly increase soil nutrient concentration under its canopy, and improve soil microbial activity and diversity (Abril et al. 2009; Vallejo et al. 2012). The organic matter inputs from leaf litter, fruit, and root exudates returned to the soil all could increase organic C and nutrients under canopy trees. Therefore, soil beneath the P. juliflora canopies had higher organic C, total N, nitrate, available P and lower bulk density than soil outside, improving chemical and physical soil quality, even in the arid deserts in which water is a limiting factor for decomposition process (Vallejo et al. 2012; El-Keblawy and Abdelfatah 2013; Kaur et al. 2012). This was attributed to organic matter build-up, higher biological activity, and improved soil structure (aggregation and porosity) favored by tree roots as well as fungal and actinomycetes hyphae. As P. juliflora produce allelochemcials that interfer with understory native plants and at the same time enhance soil and microbial activities, the relative importance of these two processes determines the structure of the plant community under and around the native and exotic trees (Callaway and Walker 1997).
7.6.2.2.2 Water and Soil Fertility
Several authors have considered the increasing number of introduced or invasive species as a major component of global change because of their potential to alter primary productivity, decomposition, hydrology, nutrient cycling, and natural disturbance regimes (Pyšek et al. 2012). The conversion of barren sand dunes in the arid deserts into dense thickets of P. juliflora can generate many environmental consequences through altering water and nutrient cycling, changing carbon storage and enhancing climate variability.
Considering that water is the limiting resource for the ecosystem in semi-arid and arid regions, hydrological response to P. juliflora invasionmay directly impact the availability of ecosystem services and consequently human wellbeing (Nie et al. 2012; Vaz et al. 2017). Groundwater, which is the main water source in semiarid regions, is severely and increasingly threatened. Consequently, the greater ability of P. juliflora to deplete the ground water would threaten the groundwater resource and consequently affect native plants. Nie et al. (2012) assessed hydrological consequences of P. juliflora invasionin the upper San Pedro watershed (U.S./Mexico) and found that the simulated average annual evapotranspiration increases with P. juliflora encroachment, leading to the decrease of annual water yield and percolation by 9.8% and 9.7%, respectively. Therefore, P. juliflora increase water demand from soil, especially in the arid regions, where the limited non-renewable groundwater is depleted and/or salinated (Murad et al. 2007, AlRukaibi 2010).The deep taproot system of Prosopis has been implicated in declining ground water tables in Hawaii (Richmond and Mueller-Dombois 1972). Similarly, the decline in the groundwater level in the island of Kahoolawe was attributed to the spread of P. pallida (Stearns 1940).
Dzikiti et al. (2017), documented the impact of removing Prosopis, co-occuring with indigenous trees, Vachellia karroo (Hayne) Banfi & Galasso on the groundwater characteristics in the Northern Cape (South Africa). They measured the water consumption of both tested species through stem sap flows and found that Prosopistranspire 5 times more water than V. karroo. Following the removal of invasive Prosopis from the area demonstrated that water table decline was significantly slow down. The P. juliflora has largely invaded alluvial floodplains in North Cape, uplands, and shrub lands in South Africa and competing with native species (Mucina and Rutherford 2006). The study suggests that clearing of invasive Prosopis would conserve groundwater in the arid parts of South Africa (Dzikiti et al. 2013). It has been observed the P. juliflora is mainly invading the lands that have shallower water tables in the UAE (El-Keblawy and Al-Rawai 2007). This tree relies mainly on ground water as a main source in the absence of rainfalls. After rainfall, the extensive superficial dense root system enables it to extract the available water, which should be at the expense of the associated native plants (Pasiecznik et al. 2001).
Several studies have assessed soil physical and chemical characters under P. juliflora canopies and in bare ground next to them and concluded that the growth of this species improves some soil physical and chemical properties. For example, Menezes et al. (2002) indicated that P. juliflorain semiarid northeastern Brazil significantly affected microclimate and the dynamics of litter and soil nutrients, and may contribute to increases in the cycling rate of nutrients in these systems. In addition, Garg and Singh (2003) have shown that nutrient concentrations (N, P, K, Ca and Mg) of P. juliflorastand were significantly greater than that of other woody species. Furthermore, Goel and Behl (1999) reported that P. juliflora plantation resulted in a marked decrease in soil pH and sodium content, and improved organic carbon, N, K and P concentrations of the soil. In the arid climate of the UAE, canopies of P. julifloraincreased the most important macro-nutrients K, N and P and the organic matter contents (El-Keblawy and Al-Rawai 2007; El-Keblawy and Abdelfatah 2014). The increase in organic content could increase the water holding capacity that would improve soil texture and increase soil moistures (El-Keblawy and Abdelfatah 2014). The effect of P. julifloraon soil properties could be mediated through soil microbial flora. For example, P. julifloratrees have the potential to establish a symbiotic relationship with N-fixing Rhizobium bacteria, which increase the N levels in the rhizosphere with root and nodule turnover (Reyes-Reyes et al. 2002; Perroni-Ventura et al. 2010). The organic matter inputs from leaf litter, fruit, and root exudates returned to the soil all could increase organic C and nutrients under canopy trees. Thwerefore, soil beneath the P. juliflora canopies had higher organic C, total N, nitrate, available P and lower bulk density than soil outside, improving chemical and physical soil quality, even in the arid deserts in which water is a limiting factor for decomposition process (Vallejo et al. 2012; El-Keblawy and Abdelfatah 2013; Kaur et al. 2012). This was attributed to organic matter build-up, higher biological activity, and improved soil structure (aggregation and porosity) favored by tree roots as well as fungal and actinobacteria hyphae.
7.7 Control Through Utilization
The high costs of P. juliflora eradication have argued several countries to follow a new and less expensive approach known as control through utilization. It has been argued that the negative impacts of P. juliflora invasion in Ethiopia are partially offset by provisioning of firewood and charcoal production. Wakie et al. (2016) have assessed the economic feasibility of selected P. juliflora eradication and utilization approaches that are currently practiced in Ethiopia. Their results showed that conversion of the infested area of P. juliflora to irrigated cotton reduces the spread of P. juliflora on farmlands and is economically feasible. In addition, managing P. juliflora infested lands for charcoal production with a four-year harvest cycle is also profitable (Wakie et al. 2016). However, the difficulties to control its rapid spread indicate that the threats it poses to ecosystem services, people’s livelihoods and lifestyles may exceed its benefits (Ayanu et al. 2015).
The plant biomass that is rich with high lignin content results in high biochar yields (Sohi et al. 2010). The major component of mesquite wood was lignin (63.96%), which makes it a potential source for biochar. Li et al. (2016) used the mesquite wood to produce three biochars with different pyrolysis conditions (no pyrolysis, 450 °C, or 750 °C). Among the three pyrolysis conditions, biochar pyrolyzed at 450 °C showed a moderate yield (40 wt%), the highest surface area (500 m2 g −1), the highest pore volume (1.02 cm3 g −1) and the highest CO2 uptake (26 mmol g−1) at 30 bar and 25 °C. When the pyrolysis was done at 700 °C, the yield of the activation reaction (63 wt%) was considerably higher. However, the CO2 uptake performance (13.9 mmol g−1) was much lower (Li et al. 2016). The yield of biochar produced by mesquite was 44.4% with pyrolysis temperature of 400 °C (Liu et al. 2016). The C content of the biochar depended on mesquite feedstock particle sizes; small particle sizes (<0.853 mm) produced higher C (68%), compared to larger particle sizes (1.70–2.00 mm) that produced (73%) (Liu et al. 2016). In addition, pH depended on particle sizes; small particle sizes (<0.853 mm) produced pH = 7.95, but larger particle sizes (1.70–2.00 mm) produced lower pH (7.41, Liu et al. 2016).The acidic nature of biochar produced from P. juliflora makes it perfect for nutrelizing the calcareous alkaline soils of the arid deserts.In addition, biochar can improve the physical and chemical properties of low quality sandy and marginal soils that devoid of nutrients and have very low water holding capacity (Cao et al. 2009). Many studies have shown that biochar, in different soils, is a useful resource to improve the physicochemical properties of soil, effectively maintain soil organic matter levels, increase fertilizer-use efficiency and increase crop production, particularly for long-term cultivated soils in subtropical and tropical regions (Lehmann et al. 2003).As biochar is a carbon-rich solid product of thermal stabilization of organic matter, it could be stored safely as a carbon source in soil, which could control Co2 emission and consequently is good solution for global warming.
7.8 Conclusion
The exotic invasive P. juliflora is threatening the ecosystem services and human well-being. The species has introduced to combat desertification in different places around the world, but became one of the factors causing land degradation. In the native range, it has very limited effects on ecosystem components and native plants, but came with their new weapons, such as new allelochemicals and may be some endophytic bacteria that helped them to have serious impacts on the native flora of the introduced range. P. juliflora invasions have led to changes in ecosystem services, ground water depletion, plant biodiversity loss and alteration in soil physicochemical properties. The widespread of P. juliflora and modification of its phenology to flower more than one episode per year enable it to cause serious health threat, such as allergies. A change in hydrological cycles associated with P. juliflora invasion has aggravated the drought episodes that will be further exacerbated due to future climate change scenarios. However, P. juliflora introduced several economic and environmental benefits in the introduced range, such as soil conservation, fuelwood, livestock fodder, timber, and fiber. It is important to calculate the beneficial and harmful impacts of P. juliflora and consequently take the decision whether to eradicate or conserve it. Allelochemicals produced by this species would affect the associated soil microbial communities, which in turn might have negative feedbacks for nutrient cycling, ecosystem processes and native vegetation. Further studies are needed to assess the impact of the introduced allelochemicals on local microbial communities. In the introduced range, where the plant has now become naturalized and became part of the flora, the threats posed by P. juliflora have been identified and control actions are currently implemented at both national and regional levels to reduce the deleterious effect of this species on the environment and human health. Regional regulations, prevention of expansion, knowledge platforms and environment protection laws are some of the crucial steps that needs to be undertaken for exotic species control measures and further spread and naturalization. In stipulations of the appraisal of native plant communities, functional traits should be assessed for understanding different mechanisms of controlling this plant.
Contribution of the Co-authors
M. Iftikhar Hussain: conceived the paper, obtained information, coordinate the review, wrote the first draft and edited the manuscript. Ross Shackleton: obtained information, wrote different sections and contributed to significant revision and organize the manuscript. Ali El-Keblawy: obtained information and contributed to draft and revise different sections. Luís González: contributed to draft, revise and organize the manuscript. M. Mar Trigo: obtained information and contributed to draft and revise different sections.
References
Abbas AM, Soliman WS, Mansour A et al (2016) Predicting the spatial spread of invasive Prosopis juliflora (SW.) D.C along environmental gradients in Gabel Elba National Park, Egypt. Int J Sci Eng Res 7:596–599
Abdel Bari E, Fahmy G, Al Thani N, Al Thani R, Abdel-Dayem M (2007) The Ghaf Tree Prosopis cineraria in Qatar. Qatar University Environmental Studies Centre, Doha
Abdillahi A, Aboud P, Kisoyan K, Coppock DL (2005) Agro-Pastoralists’ Wrath for the P. juliflora tree: the case of the II Chamus of Baringo District. Pastoral risk Management project, Kenya
Abdulahi MM, Ute JA, Regasa T (2017) Prosopis juliflora L: distribution, impacts and available control methods in Ethiopia. Trop Subtrop Agroecosyst 20(1)
Admasu D (2008) Invasive plants and food security: the case of Prosopis juliflora in the Afar region of Ethiopia. FARM-Africa and IUCN, Ethiopia, pp 1–13
Aggarwal RK, Gupta JP, Saxena SK, Muthana KD (1976) Studies on soil physico-chemical and ecological changes under twelve years old desert tree species of Western Rajasthan. Indian For 102:863–872
Agroforestree Database (AFTD) (2009) Prosopis. World Agroforestry Centre, Kenya
Ahmad R, Ismail S (1996) Use of Prosopis in Arab/Gulf states including possible cultivation with saline water in deserts. In: Felker P, Moss J (eds) Prosopis: semi-arid fuelwood and forage tree, vol 1. Building Consensus for the Disenfranchised. Center Semi-Arid Forest Resources, Kingsville, pp 41–1.52
Akrimi N (1990) Prosopis in Tunisia. In: Habit MA, Saavedra JC (eds) The current state of knowledge on Prosopis juliflora (Eds.) . FAO, Rome, pp 147–151
Al Abri AS, Al Ajmi DS, Al Halhali AS, Al Saqry NM, Forsberg NE, Kadim IT, Mahgoub O, Richie AR (2004) In: Salem B, Nefzaoui A, Morand-Fehr P (eds) Nutrition and feeding strategies of sheep and goats under harsh climates. CIHEAM, Zaragoza
Al Mutairi K, El-Bana M, Mansor M, Al-Rowaily S, Mansor A (2012) Floristic diversity, composition and environmental correlates on the arid Coralline Islands of the Farasan Archipelago, Red Sea, Saudi Arabia. Arid Land Res Manag 26:137–150
Al Rukaibi D (2010) Water resources of GCC countries. The Sustainable Resources Research Institute, Al-Riyadh
Al-Beitawi NA, Awawdeh FT, Khwaileh MM (2010) Preliminary study on Prosopis juliflora pods as unconventional feed ingredient in diets of broiler chicks. Anim Nutr Feed Technol 10:51–60
Al-Frayh A, Hasnain SM, Gad-elRab MO, Al-Turki T, Al-Mobeireek K, Al-Sedairy ST (1999) Human sensitization to Prosopis juliflora antigen in Saudi Arabia. Annals Saudi Med 19:331–336
Al-Humaid AI, Warrag MOA (1998) Allelopathic effects of mesquite (Prosopis juliflora) foliage on seed germination and seedling growth of bermudagrass (Cynodon dactylon). J Arid Environ 38(2):237–243
Ali AS, Tudsri S, Rungmekarat S, Kaewtrakulpong K (2012) Effect of feeding Prosopis juliflora pods and leaves on performance and carcass characteristics of Afar sheep. Kasetsart J Nat Sci 46:871–881
Al-Keblawy (2012) Impacts of native and exotic Prosopis species on native plants in aridlands of the UAE. International Conference on Ecology, Agriculture and Chemical Engineering December, Phuket, pp 18–19
Allam AM, Bakhashwain AA, Nagadi SA, Sallam SM (2012) Nutritive value assessment of some sub-tropical browses grown in arid region using in vitro gas production technique. J Food Agricult Environ 10:1339–1343
Almanza SG, Moya EG (1986) The uses of mesquite (Prosopis spp.) in the highlands of San Luis Potosi, Mexico. For Ecol Manag 16:49–56
Almaraz-Abarca N, da Graça Campos M, Ávila-Reyes J et al (2007) Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J Food Compos Anal 20:119–124
Al-Marzooqi W, Al-Kharousi K, Kadim IT, Mahgoub O, Zekri S, Al-Maqbaly R, Al-Busaidi M (2015) Effects of feeding Prosopis juliflora pods with and without exogenous enzyme on performance, meat quality and health of broiler chickens. Int J Poult Sci 14:76–88
Al-Rawahy SH, Al-Dhafri KS, Al-Bahlany SS (2003) Germination, growth and drought resistance of native and alien plant species of the genus Prosopis in the sultanate of Oman. Asian J Plant Sci 2:1020–1023
Al-Soqeer AA, Alsubaie QD, Motawei MI, Mousa HM, Ahmed M, Abdel-Salam AM (2017) Isolation and identification of allergens and biogenic amines of Prosopis juliflora genotypes. Electron J Biotechnol 30:24–32
Alvarez M, Leparmarai P, Heller G, Becker M (2017) Recovery and germination of Prosopis juliflora (Sw.) D.C. seeds after ingestion by goats and cattle. Arid Land Res Manag 31:71–80
Anderson S (2005) Spread of the introduced tree species Prosopis juliflora (Sw.) D.C. Sveriges lantbruksuniversitet: Institutionen för skoglig vegetationsekologi
Anderson LJ, van Klinken RD, Parr RJ, Climas R, Barton D (2006) Integrated management of hybrid mesquite: a collaborative fight against one of Australia’s worst woody weeds. Fifteenth Australian Weeds Conference, Adelaide
Ansley RJ, Pinchak WE, Teague WR, Kramp BA, Jones DL, Jacoby PW (2004) Long-term grass yield following chemical control of honey mesquite. J Range Manag 57:49–57
Ansley RJ, Boutton TW, Mirik M, Castellano MJ, Kramp BA (2010) Restoration of C4 grasses with seasonal fires in a C3/C4 grassland invaded by Prosopis glandulosa, a fire-resistant shrub. Appl Veg Sci 13:520–530
Araüjo AH, Cardoso PC, Pereira RA, Lima LM, Oliveira AS, Miranda MRA, Xavier-Filho J, Sales MP (2002) In vitro digestibility of globulins from cowpea (Vigna unguiculata) and xerophitic algaroba (Prosopis juliflora) seeds by mammalian digestive proteinases: a comparative study. Food Chem 78:143–147
Archer S (1989) Have southern Texas savannas been converted into woodlands in recent history? Am Nat 134:545–561
Argôlo LDS, Pereira MLA, Dias JCT, Cruz JFD, Del Rei AJ, Oliveira CASD (2010) Mesquite pod meal in diets of lactating goats: ruminal parameters and microbial efficiency synthesis. Rev Bras Zootec 39:541–548
Assarehzadegan MA, Khodadadi A, Amini A, Shakurnia AH, Marashi SS, Ali-Sadeghi H, Zarinhadideh F, Sepahi N (2015) Immunochemical Characterization of Prosopis Juliflora Pollen Allergens and Evaluation of Cross-Reactivity Pattern with the Most Allergenic Pollens in Tropical Areas. Iran J Allergy Asthma Immunol 14(1):74–82
Badano E, Pugnaire FI (2004) Invasion of Agave species (Agavaceae) in south-east Spain: invader demo- graphic parameters and impacts on native species. Divers Distrib 10:493–500
Bahre CJ, Shelton ML (1993) Historic vegetation change, mesquite increases, and climate in southeastern Arizona. J Biogeogr 20:489–504
Barbier EB, Knowler D, Gwatipedza J, Reichard SH (2013) An economic analysis of the invasive plant problem associated with the horticulture industry in North America. Invas Plant Ecol:259
Beggs PJ (2015) Environmental allergens: from asthma to hay fever and beyond. Curr Clim Change Rep 1:176–184
Beggs PJ, Bambrick HJ (2005) Is the global rise of asthma an early impact of anthropogenic climate change? Environ Health Perspect 113:915–919. https://doi.org/10.1289/ehp.7724
Behrendt H, Ring J (2012) Climate change, environment and allergy. Chem Immunol Allergy 96:7–14
Belton T (2008) Management strategy for Mexican thorn (Prosopis juliflora) on Ascension Island: an assessment of this species, and recommendations for management. RSPB, Bedfordshire
Benata H, Mohammed O, Noureddine B, Abdelbasset B, Abdelmoumen H, Muresu R, Squartini A, El Idrissi MM (2008) Diversity of bacteria that nodulate Prosopis juliflora in the eastern area of Morocco. Syst Appl Microbiol 31(5):378–386
Bener A, Safa W, Abdulhaik S, Lestringant GG (2002) An analysis of skin prick test reaction in asthmatics in a hot climate and desert environment. Allery Immunol 34:281–286
Berhanu A, Tesfaye G (2006) The Prosopis dilemma, impacts on drylands biodiversity and some controlling methods. J Drylands 1(2):158–164
Bestelmeyer BT (2005) Does desertification diminish biodiversity? Enhancement of ant diversity by shrub invasions in south-western USA. Divers Distrib 11:45–55
Bethune S, Griffin M, Joubert D (2004) National review of invasive alien species Namibia. Windhoek, Directorate of Environmental Affairs, Ministry of Environment and Tourism
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Bhatt SS, Chovatiya SG, Shah AR (2011) Evaluation of raw and hydrothermically processed Prosopis juliflora seed meal as supplementary feed for the growth of Labeo rohita fingerlings. Aquac Nutr 17:e164–e173
Bieberdord FW, Swinny B (1952) Mesquite and related plants in allergy. Ann Allergy 10:720
Bielory L, Lyons K, Goldberg R (2012) Climate change and allergic disease. Curr Allergy Asthma Rep 12:485–494
Bock CE, Kennedy L, Bock JH, Jones ZF (2007) Effects of fire frequency and intensity on velvet mesquite in an Arizona grassland. Rangel Ecol Manag 60:508–514
Bogino SM, Villalba R (2008) Radical growth and biological rotation age of Prosopis caldenia Burkart in Central Argentina. J Arid Environ 72:16–23
Bokrezion H (2008) The ecological and socio-economic role of Prosopis juliflora in Eritrea: an analytical assessment within the context of rural development in the Horn of Africa. PhD thesis, Johannes Gutenberg University, Mainz
Borokini TI, Babalola FD (2012) Management of invasive plant species in Nigeria through economic exploitation: lessons from other countries. Manage Biol Inv 3(1):45–55
Botswana Government (2009) Botswana fourth national report to the convention of biological diversity. Botswana Government, Gaborone
Bradley BA, Curtis CA, Chambers JC (2016) Bromus Response to Climate and Projected Changes with Climate Change. M.J. Germino et al. (eds.), Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US, Springer Series on Environmental Management. https://doi.org/10.1007/978-3-319-24930-8_9
Bragg LH, Bacon JD, McMillan C et al (1978) Flavonoid patterns in the Prosopis juliflora complex. Biochem Syst Ecol 6:113–116
Brown K (1991) Biology of Prosopis cineraria (Leguminosae) in the Sultanate of Oman. Phd in Science. Durham University, Durham
Brown JR, Archer S (1989) Woody plant invasion of grasslands: establishment of honey mesquite (Prosopis gnaldulosa var. glandulosa) on sites differing in herbaceous biomass and grazing history. Oecologia 80:19–26
Brown AF, Massey RE (1929) Flora of the Sudan. Thomas Murby and CO, p 376
Brown CJ, Macdonald IAW, Brown SE (1985) Invasive alien organisms in South West Africa. Foundation for Research Development, Namibia
Browning DM, Archer S, Asner G, McClaran MP, Wessman CA (2008) Woody plants in grasslands post-encroachment and stand dynamics. Ecol Appl 18:928–944
Burkart A (1976) A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). (Part 1 and 2). Catalogue of the recognized species of Prosopis. J Arnold Arborat 57: 219–249; 450–525
Busch M, Knight C, Hodara CNMK, Chaneton EME (2012) Rodent seed predation on tree invader species in grassland habitats of the inland Pampa. Ecol Res 27:369–376
Cadman A (1991) Airspora of Johannesburg and Pretoria, South Africa,1987/88. II. Meteorological relationships. Grana 30:181–183
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Cao X, Ma L, Gao B, Harris W (2009) Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ Sci Technol 43(9):3285–3291
Cardoso MB, Ladio AH, Lozada M (2012) The use of firewood in a Mapuche community in a semi-arid region of Patagonia, Argentina. Biomass Bioenergy 46:155–164
Cecchi L, D’Amato G, Ayres JG, Galan C, Forastiere F, Forsberg B, Gerritsen J, Nunes C, Behrendt H, Akdis C, Dahl R, Annesi-Maesano I (2010) Projections of the effects of climate change on allergic asthma: the contribution of aerobiology. Allergy 65:1073–1081
Chandrasekaran S, Swamy PS (Undated) Ecological and socio-economic impacts of Prosopis juliflora invasion into the semi-arid ecosystems in selected villages of Ramand district in Tamilnadu. Madurai Kamaraj University, Madurai
Chaturvedi OH, Sahoo A (2013) Nutrient utilization and rumen metabolism in sheep fed Prosopis juliflora pods and Cenchrus grass. Springer Plus 2:1–7
Chaudry B, Vijayaraghavan MR (1992) Structure and function of the anther gland in Prosopis juliflora (Leguminosae, Mimosoideae): a histochemical analysis. Phyton (Horn) 32:01–07
Chikuni MF, Dudley CO, Sambo EY (2004) Prosopis glandulosa Torry (Leguminosae-Mimosoidae) at Swang’oma, Lake Chilwa plain: a blessing in disguise. Malawi J Sci Technol 7:10–16
Choge SK, Chikami BN (2004) Experiences of Prosopis utilization and management from outside Kenya. In: Proceedings of the Workshop on Integrated Management of Prosopis Species in Kenya. KEFRI, Nairobi, Kenya
Choge SK, Ngujiri FD, Kuria MN, Busaka EA, Muthondeki JK (2002) The status and impact of Prosopis spp. in Kenya. KEFRI, Nairobi
Choge SK, Harvey M, Chesang S, Pasiecznik NM (2006) Cooking with Prosopis flour. Recipes tried and tested in Bango District. Kenya. Nairobi and Coventry, KEFRI and HDRA
Choge SK, Pasiecznik NM, Harvey M, Wright J, Awan SZ, Harris PJC (2007) Prosopis pods as human food, with special reference to Kenya. Water South Afr 33:419–424
Choge SK, Clement N, Gitonga M, Okuye J (2012) Status report on commercialization of Prosopis tree resources in Kenya, Technical report for the KEFRI/KFS Technical Forest Management and Research Liaison Committee. KEFRI, Nairobi
Cibichakravarthy B, Preetha R, Sundaram SP, Kumar K, Balachandar D (2012) Diazothrophic diversity in the rhizosphere of two exotic weed plants, Prosopis juliflora and Parthenium hysterophorus. World J Microbiol Biotechnol 28:605–613
Cienciala E, Centeio A, Balazek P, da Cruz Gomes Soares M, Russ R (2013) Estimation of stem and tree level biomass models for Prosopi juliflora/pallida applicable to multi-stemmed tree species. Trees 27:1061–1070
Coetzer W, Hoffmann JH (1997) Establishment of Neltumius arizonensis (Coleoptera: Bruchidae) on Mesquite (Prosopis species: Mimosaceae) in South Africa. Biol Control 10:187–182
Costa AC, Souchie EL, Teixeira MB, Resende O, Soares FAL, Saléh BB, Faria DJG, Soares MA, Ceballos DCJ, Martins EM (2018) Allelopathic effect of aqueous extracts of Prosopis juliflora (SW.) D.C. in the emergence and initial growth of Mimosa tenuiflora (WILLD.) Poiret seedlings. Conteúdo da revista. Capa 11(1)
Creamer CA, Filley TR, Olk DC, Stott DE, Dooling V, Boutton TW (2013) Changes to soil organic N dynamics with leguminous woody plant encroachment into grasslands. Biogeochemistry 113:307–321
Csurhes S (ed) (1996) Mesquite (Prosopis spp) in Queensland. Department of Natural Resources and Mines, Australia
da Silva CGM, Stamford TLM, de Andrade SAC, de Souza EL, de Araújo JM (2010) Production of ethanol from mesquite (Prosopis juliflora (SW) D.C.) pods mash by Zymomonas mobilis and Saccharomyces cerevisiae. Electron J Biotechnol 13:12–13
Davis OK (1995) Climate and vegetation patterns in surface samples from arid western U.S.A.: application to Holocene climatic reconstructions. Palynology 19:97–120
Dayal V (2007) Social diversity and ecological complexity: how an invasive tree could affect diverse agents in the land of the tiger. Environ Develop Eonom 12:553–571
De Andrade AA, Fabricante JR, de Oliveira FX (2010) Impactos da invasao de Prosopis juliflora (sw.) D.C.. (Fabaceae) sobre o estrato arbustivo-arboreo em areas de Caatinga no Estafo da Paraiba, Braazil. Maringá 32:249–255
de Dios V, Weltzin JF, Huxman TE, Williams DG (2012) Windows of opportunity for Prosopis velutina seeding establishment and encroachment in a semiarid grassland. Perspect Plant Ecolo Evol System 14:275–282
de Oliveira Moraes GS, de Souza EJ, Véras AS, de Paula Almeida M, da Cunha MV, Torres TR, Pereira GFC (2016) Total replacement of corn by mesquite pod meal considering nutritional value, performance, feeding behavior, nitrogen balance, and microbial protein synthesis of Holstein-Zebu crossbred dairy steers. Trop Anim Health Prod 48:1415–1420
De Oliveira VR, Pires IE (1990) Pollination efficiency of P. juliflora (Sw) D.C. in Petrolina, Pernambuco. In: Habit MA, Saavedra JC (eds) The current state of knowledge on P. juliflora. FAO, Rome
de Oliveira LSB, de Andrade LA, Fabricante JR, Gonçalves GS (2012) Structure of a Prosopis juliflora (Sw.) D.C.. Population established in a temporary riverbed in the microregion of Cariri in the Stat of Paraiba. Semina Ciências Agrárias Londrina 33(5):1769–1778
de Villalobos AE, Paláez DV, Elia OR (2005a) Factors related to establishment of Prosopis caldenia Burk. Seedlings in central rangelands of Argentina. Acta Oecol 27:99–106
de Villalobos AE, Paláez DV, Elia OR (2005b) Growth of Prosopis cladenia Burk: seedlings in central semi-arid rangelands of Argentina. J Arid Environ 61:345–346
de Villalobos AE, Peláez DV, Bóo RM, Mayor MD, Elia OR (2007) Effect of a postfire environment on the establishment of Prosopis caldenia seedlings in central semiarid Argentina. Austral Ecology 32:581–591
Dean WRJ, Anderson MD, Milton SJ, Anderson TA (2002) Avian assemblages in native Acacia and alien Prosopis drainage line woodland in the Kalahari, South Africa. J Arid Environ 51:1–19
DeLoach CJ (1984) Conflicts of interest over beneficial and undesirable aspects of Prosopis (Prosopis spp.) in the United States as related to biological control. Vancouver, Canada: 6th international symposium on biological control, 19–25 August 1984, pp 301–340
Demissie H (2009) Invasion of Prosopis juliflora (Sw.) D.C.. into Awash National Park and its impacts on plant species diversity and soil characteristics. Masters in Science. Addis Ababa University, Addis Ababa
Dhyani A, Arora N, Gaur SN, Jain VK, Sridhara S, Singh BP (2006) Analysis of IgE binding proteins of mesquite (Prosopis juliflora)pollencross-reactivitywithpredominant tree pollens. Immunobiology 211:733–740
Dhyani A, Arora N, Jain VK, Sridhara S, Singh BP (2007) Immunoglobulin E (IgE)-mediated cross-reactivity between mesquite pollen proteins and lima bean, an edible legume. Clin Exp Immunol 149:517–524
Diagne O (1996) Utilization and nitrogen fixation of Prosopis juliflora in Senegal. In: Felker P, Moss J (eds) Prosopis: Semiarid fuelwood and forage tree; Building consensus for the disenfranchised. Kingsville, Centre for Semi-Arid Forest Resources
Diaz A (1995) Los Algarrobos. Consejo Nacional de Ciencia y Tecnología (CONCYTEC). Lima, Perú, 207 pp
Díaz Celis A (1995) Los Algarrobos. CONCYTEC, Lima, Peru
Distel RA, Peláez DV, Bóo RM, Mayor MD, Elia OR (1996) Growth of Prosopis caldenia seedlings in the fields as related to grazing history of the site and in a greenhouse as related to different levels of competition from Stipa tenuis. J Arid Environ 32:251–257
Djoudi H, Brockhaus M, Locatelli B (2011) Once there was a lake: vulnerability to environmental changes in northern Mali. Reg Environ Chang. https://doi.org/10.1007/s10113-011-0262-5
dos Santos E, Pereira ML, Almeida PJ, Moreira JV, Souza AC, Pereira CA (2015) Mesquite pod meal in sheep diet: intake, apparent digestibility of nutrients and nitrogen balance. Acta Scientiarum. Anim Sci 37:55–59
Du Puy (1990) Prosopis juliflora (Sw.) D.C.. From Madagascar. Royal Botanic Gardens, Kew
Dudley BD, Flint Hughes R, Ostertag R (2014) Groundwater availability mediates the ecosystem effects of an invasion of Prosopis pallida. Ecol Appl 24(8):1954–1971
Dufgas WA, Mayeux HS (1991) Evaporation from rangeland with and without honey mesquite. J Range Manag 44:161–170
Dussart E, Peinetti R, Boninesegra JA (1997) Análisis del crecimiento de Prosopis caldenia (L) Burk. En relación con parametros ambientales y fuego. Reunión Argentina de Ecologia, Buenos Aires
Dutch Caribbean Biodiversity Explorer (D.C.BE) (2009) Prosopis. Government of the Netherlands Antilles, Netherlands Antilles
Dzikiti S, Schachtschneider K, Naiken V, Gush M, Moses G, Le Maitre DC (2013) Water relations and the effects of clearing invasive Prosopis trees on groundwater in an arid environment in the Northern Cape, South Africa. J Arid Environ 90:103–113
Eggemeyer KD, Schwinning S (2009) Biogeography of woody encroachment: why is mesquite excluded from shallow soils? Ecohydrology 2:81–87
Ehrenfeld JG, Kourtev P, Huang WZ (2001) Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol Appl 11:1287–1300
El Fadl MA (1997) Management of Prosopis juliflora for use in agroforestry systems in the Sudan, Tropical forestry reports 16. University of Helsinki, Helsinki
Elfadl MA (1997a) Management of Prosopis juliflora for use in agroforestry systems in the Sudan, Tropical forestry reports 16. University of Helsinki, Helsinki
Elfadl MA (1997b) Management of Prosopis juliflora for muse in agroforestry in the Sudan. Doctoral dissertation, PhD thesis. Department of forest ecology, Tropical Silviculture Unit, University of Helsinki, Finland
Elfadl MA, Luukkanen O (2006) Field studies on the ecological strategies of Prosopis juliflora in a dryland ecosystem 1: a leaf gas exchange approach. J Arid Environ 66(1):1–15
El-Keblawy A, Abdelfatah MA (2014) Impacts of native and invasive exotic Prosopis congeners on soil properties and associated flora in the arid United Arab Emirates 100–101:1–8
El-Keblawy A, Al-Rawai A (2005) Effect of salinity, temperature and light on germination of invasive Prosopis juliflor (Sw.) D.C. J Arid Environ 61(4):555–565
El-Keblawy A, Al-Rawai A (2007) Impacts of the invasive exotic Prosopis juliflora (Sw.) D.C. on the native flora and soils of the UAE. Plant Ecol 190:23–35
El-Keblawy A, Ksiksi T (2005) Artificial forests as conservation sites for native flora of the UAE. For Ecol Manag 213:288–296
Emlan JT (1986) Land-bird densities in matched habitats on six Hawaiian Islands: a test of resource regulation theory. Am Naturalist 127:125–139
Ethiopia Government (2002) Ethiopia-national plan for Prosopis – final draft. Ethiopia Government
Ethridge DE, Dahl BE, Sosebee RE (1984) Economic evaluation of chemical mesquite control using 2,4,5-T. J Range Manag 37:152–156
FAO (2004) The introduction of Prosopis spp. In the drylands of the world ledge of plight for biodiversity. Food and Agricultural Organization of the United Nations, Rome
FAO (2006) Problems posed by the introduction of Prosopis spp. in selected countries. Plant Production and Protection Division, Food and Agricultural Organization of the United Nations, Rome, pp 233–239
FARM-Africa (2008) Experiences on Prosopis management case of Afar region. FARM-Africa, Ethiopia
Felker P (1987) Recommendations for development of Prosopis in Pakistan. Centre for Semi-Arid Forest Resources, Texas
Felker P (2003) Management, use and control of Prosopis in Yemen. Yemen: Project Number: TCP/YEM/0169(A)
Felker P, Clark PR, Nash P, Osborn JF, Cannell GH (1982) Screening Prosopis (mesquite) for cold tolerance. For Sci 28:556–562
Finkelstein R (2006) Abscisic acid: a seed maturation and antistress signal. In: Taiz L, Zeiger E (eds) Plant physiology, 4th edn. Sinauer Associates, Sunderland, pp 539–558
Foroughbakhch R, Parra AC, Pinero JLH, Vázquez MAA, Estrada AR, Cardenas ML (2012) Wood volume production ans use of 10 woody species in semiarid zones of northeastern Mexico. Intl J For Res. https://doi.org/10.1155/2012/529829
Fosberg FR, Sachet M, Oliver R (1976) A geographical checklist of the Micronesian dicotyledonae. Micronesia 15:1–295
Gairola S, Mahmoud T, Shabana H, El-Keblawy A (2016) Growing knowledge about the floral diversity of United Arab Emirates: new additions and conservation through seed banking. Tribulus 24:136–143
Gallaber T, Merlin M (2010) Biology and impacts of Pacific Island invasive species. 6. Prosopis pallida and Prosopis juliflora (Algarroba, Mesquite, Kiawe) (Fabaceae). Pac Sci 64:489–526
Garg VK, Singh B (2003) Macronutrient dynamics and use efficiency in three species of short rotation forestry developed on sodic soils in North India. J Trop For Sci 15:289–302
Geesing D, Al-Khawlani M, Abba ML (2004) Management of introduced Prosopis species: can economic exploitation control and invasive species? Unasylva 217:36–44
Ghazaly UF (2006) Community-based management of invasive Prosopis juliflora in Egypt. GISP, Washington, DC
Ghazanfar SA (1996) Invasive Prosopis in the Sultanate of Oman. Alien 3:10
Girma M, Urge M, Animut G (2012) Ground Prosopis juliflora pods as feed ingredient in poultry diet: effects on nutrient intake, muscle fatty acid composition, sensory quality and hematology of broilers. Pak J Nutr 11:1014–1022
Global Biodiversity Information Facility (GBIF) (2013) Prosopis species lists. GBIF, Copenhagen
Global Invasive Species Database (ISSG) (2005) Prosopis. IUCN
Goel VL, Behl HM (1995) Propagation of Prosopis juliflora from rooted stem cuttings. Intl Tree Crops J 8:193–201
Goel U, Saxena DB, Kumar B (1989) Comparative study of allelopathy as exhibited by Prosopis juliflora swartz and Prosopis cineraria (L) Druce. J Chem Ecol 15:591–600
Gomez JM, Bosch J, Perfectti F, Fernandez J, Abdelaziz M (2007) Pollinator diversity affects plant reproduction and recruitments: the tradeoffs of generalization. Oecologia 153:597–605
Goslee SC, Havstad KM, Peters DP, Rango A, Schlesinger WH (2003) High-resolution images reveal rate and pattern of shrub encroachment over six decades in New Mexico, U.S.A. J Arid Environ 54:755–767
Graham JD (1960) Morphological variation in mesquite (Prosopis, Leguminosae) in the lowlands of northeastern Mexico. Southwest Nat:187–193
Grandtner MM (2005) Elsevier’s directory of trees. ELSEVIER, Amsterdam
Gritzner JA (1979) Environmental degradation in Mauritania: staff report. Board on Science and Technology for International Development, Commission on International Relations and National research Council, Mauritania
Gupta R, Sharma KK, Kuhad RC (2009) Separate hydrolysis and fermentation (SHF) of Prosopis juliflora, a woody substrate, for the production of cellulosic ethanol by Saccharomyces cerevisiae and Pichia stipitis-NCIM 3498. Bioresour Technol 100(3):1214–1220
Habit MA, Saavedra JA (1990a) The current state of knowledge on Prosopis juliflora. II international conference on Prosopis. Brazil 25–29 August. FAO, Rome
Habit MA, Saavedra JC (1990b) The current state of knowledge on Prosopis juliflora. FAO, Rome
Habit MA, Saavedra JC (eds) (1990c) The current state of knowledge on Prosopis juliflora. FAO, Rome
Haji J, Mohammed A (2013) Economic impact of Prosopis juliflora on agropastoral households of Dire Dawa Administration, Ethiopia. Afr J Agric Res 8(9):768–779
Hall M, Llewellyn OA, Miller AG, Al-Abbasi TM, Al-Wetaid AH, Al-Harbi RJ, Al-Shammari KF (2010) Important plant areas in the Arabian Peninsula: 2. Farasan Archipelago. Edinb J Bot 67(2):189–208
Harding GB (1987) The status of Prosopis as a weed. Plant Protection Research Institute, Uitenhage
Harding GB, Bate GC (1991) The occurrence of invasive Prosopis species in the north- western cape, South Africa. S Afr J Sci 87:188–192
Harfi HH, Alsaeed AH (2011) Allergenicity to allergins like Prosopis juliflora and date tree pollens in Saudi Arabia. Kuwait Med J 43:109–112
Hasan MK, Alam AKAA (2006) Land degradation situation in Bangladesh and the role of Agroforestry. J Agricult Rural Develop 4:19–25
Havstad KM, James D (2010) Prescribed burning to affect a state transition in a shrub-encroached desert grassland. J Arid Environ 74:1324–1328
Hawaii Ecosystem at Risk Project (1998) Prosopis pallida (Fabaceae). Map of estimated distribution on Molokai. Hawaii Ecosystem at Risk Project, Hawaii
Hawke PR, Meadows ME (1989) Winter airspora spectra and meteorological conditions in Cape Town, South Africa. Grana 28:187–192
Heiss S, Fischer S, Muller WD, Weber B, Hirschwehr R, Spitzauer S et al (1996) Identification of a 60 kD cross-reactive allergen in pollen and plant derived food. J Allergy Clin Immunol 98:938–947
Heitschmidt RK, Ansley RJ, Dowhower SL, Jacoby PW, Price DL (1988) Some observations from the excavation of honey mesquite root systems. J Range Manag 41:227–231
Hennessy JT, Gibbens RP, Tromble JM, Cardenas M (1983) Vegetation changes from 1935 to 1980 in mesquite dunelands and former grasslands of southern New Mexico. J Range Manag:370–374
Henschel JR, Parr T (2010) Population changes of alien invasive plants in the Lower Kuiseb River. DINTERIA No. 31, Windhoek, pp 5–17
Hierro JL, Callaway RM (2003) Allelopathy and exotic plant invasion. Plant Soil 256:29–39
Hintsa K, Balehegn M, Birhane E (2015) Utilization of pods of Prosopis juliflora, an invasive tree, as a replacement to concentrate feed for goats in Ethiopia. Livest Res Rural Dev 27:9
Hoffman MT, Sonnenberg D, Hurford JL, Jagger BW (1995) Ecology and management of Riemvasmaak’s natural resources. National Botanical Institute, Cape Town
Hoffmann JH, Impson FAC, Moran VC (1993) Competitive interactions between two Burchid species (Algarobius spp.) introduced into South Africa for biological control of mesquite weeds 9Prosopis spp. Biol Control 3:215–220
Hollister EB, Schadt CW, Palumbo AV, Ansley JR, Boutton TW (2010) Structural and functional diversity of soil bacteria and fungal communities following woody plant encroachment in the southern Great Planes. Soil Biol Biochem 42:1816–1824
Hoshino B, Yonemori M, Manayeva K, Karamalla A, Yoda K, Suliman M, Elgamri M, Nawata H, Mori Y, Yabuki S, Aida S (2011, July). Remote sensing methods for the evaluation of the mesquite tree (Prosopis juliflora) environmental adaptation to semi-arid Africa. In: Geoscience and Remote Sensing Symposium (IGARSS), 2011 IEEE International. IEEE, pp 1910–1913
Hoshino B, Karamalla A, Abd Elbasit MAM, Manayeva K, Yoda K, Suliman M, Elgamri M, Nawata H, Yasuda H (2012) Evaluating the invasion strategic of Mesquite (Prosopis juliflora) in eastern Sudan using remotely sensed technique. J Arid Land Stud 22:1–4
Hundessa N (2016) Distribution and socio-economic impacts of Prosopis juliflora in East Shewa and West Arsi zones, Ethiopia. Intl J Afr Asian Stud 24:31–41
Hunziker J-H, Saidman BO, Naranjo CA, Palacios RA, Poggio L, Burghardt AD (1986) Hybridization and genetic variation of Argentina species of Prosopis. For Ecol Manag 16:301–316
Hussain MI, Reigosa MJ (2011) Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching and heat energy dissipation in three C3 perennial species. J Exp Bot 62:4533–4545
Hussain SS, Ahmed M, Siddiqui MF, Wahab M (2010) Threatened and endangered native plants of Karachi. Int J Biol Biotechnol 7(3):259–266
Hussain MI, González L, Souto C, Reigosa MJ (2011) Ecophysiological responses of native plants to phytotoxic effect of Acacia melanoxylon R. Br. Agrofor Syst 83:149–166. https://doi.org/10.1007/s10457-011-9433-0
Ibrahim F (2004) No place like home: history, politics and mobility among a pastoral nomadic community in western India. Nomadic Peoples 8:168–190
Ibrahim M, Nadir M, Ali A et al (2013) Phytochemical analyses of Prosopis juliflora Swartz D.C. Pak J Bot 45:2101–2104
Immerzeel P, Schols HA, Voragen AGJ, de Vries SC (2004) Different arabinogalactan proteins are present in carrot (Daucus carota) cell culture medium and in seeds. Physiol Plant 122:181–189
Inderjit (2001) Soils: environmental effect on allelochemical activity. Agron J 93:79–84
Inderjit S, Callaway TR, Pollock RM, Kaur JL (2008) Allelopathy and plant invasions: traditional, congeneric, and biogeographical approaches. Biol Invasions 10:875e890
Inderjit EH, Crocoll C, Bajpai D, Kaur R, Feng Y, Silva C, Carreo’n JT, Valiente-BanuetA GJ, Callaway RM (2011) Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 92:316–324
Invasive Species Compendium (CABI) (2005) Prosopis. CABI, Oxfordshire
IPCC. Summary for policymakers. In: Parry ML, Canziani OF, Palutikof JP, van der Linden
Issa S, Dohai B (2008) GIS analysis of invasive Prosopis juliflora dynamics in two selected sites from the United Arab Emirates. Can J Pure Appl Sci 2:235–242
Jackson RB, Banner JL, Jobbágy EG, Pockman WT, Wall DH (2002) Ecosystem carbon loss with woody plant invasion of grasslands. Nature 418(6898):623–626
Jaganathan GK, Yule K, Liu B (2016) On the evolutionary and ecological value of breaking physical dormancy by endozoochory. Perspect Plant Ecolo Evol System 22:11–22
Jayaram K, Prasad MNV (2009) Removal of Pb (II) from aqueous solution by seed powder of Prosopis juliflora D.C. J Hazard Mater 169(1–3):991–997
Jensen AM, Hajej MS (2001) The road of hope: control of moving sand dunes in Mauritania. Unasylva 207:31–36
Jhala YV (1993) Predation on blackbuck by wolves in the Velavadar National Park, Gujarat, India. Conserv Biol 7:874–881
Johns RE, Lee JS, Agahian B, Gibbons HL, Reading JC (1986) Respiratory effects of mesquite broiling. J Occup Med 28:1181–1184
Johnston MC (1962) The North American mesquites Prosopis Sect. Algarobia (Leguminosae). Brittonia 14(1):72–89
Jones M, Sinclair F, Grime V (1998) Effect of tree species and crown pruning on root length and soil water content in semi-arid agroforestry. Plant Soil 201:–197
Joshi EB, Joshi PN, Jain BK (2012) Phytosociological study and management action for the invasive weed : Prosopis juliflora (SW.) D.C.. in Tapkeshwari Hill Ranges in the Kachchh Island, Gujarat, India. J Non-Timber Forest Prod 19:29–36
Jyrkama MI, Sykes JF (2007) The impact of climate change on spatially varying groundwater recharge in the grand river watershed (Ontario). J Hydrol 338(3):237–250
Kahi CH, Ngugi RK, Mureithi SM, Ng’ethe JC (2009) The canopy effects of Prosopis juliflora (D.C..) and Acacia tortillis (hayne) trees on herbaceous plants species and soil physio-chemical properties in Njemps Flats, Kenya. Trop Subtrop Agroecosyst 10:441–449
Karron JD, Mitchell RJ (2011) Effects of floral display size on male and female reproductive success in Mimulus ringens. Annals Bot. https://doi.org/10.1093/aob/mcr193
Kaur R, Gonzáles WL, Llambi LD, Soriano PJ, Callaway RM, Rout ME et al (2012) Community impacts of Prosopis juliflora invasion: biogeographic and congeneric comparisons. PLoS One 7(9):e44966
Kaur G, Singh BP, Nagpal AK (2013) Phenology of some phanerogams (trees and shrubs) of Northwestern Punjab, India. J Bot 2013
Kazmi JH (2009) Ecological and Socio-economic evaluation of the use of Prosopis juliflora for bio-char production in Pakistan. Drynet, Pakistan
Kedzie-Webb SA, Sheley RL, Borkowski JJ, Jacobs JJ (2001) Relationships between Centaurea maculosa and indigenous plant assemblages. Western North Am Nat 61:43–49
Keys RN (1993) Mating systems and pollination biology of velvet mesquite (Prosopis velutina Wooton). PhD dissertation, The University of Arizona. Tucson, AZ
Khan I, Marwat KB, Khan IA, Ali H, Dawar K, Khan H (2011) Invasive weeds of southern districts of Khyber Pakhtunkhwa –Pakistan. Pakistani J Weed Sci Res 17(2):161–174
Khider MM, Al Abjar ZA, Eltigani A (2011) Studies on ecology and biology of some insect pests of the mesquite trees Prosopis juliflora in Sudan. Egypt J Biol Pest Contr 21:353–359
Killian S, McMichael J (2004) The human allergens of mesquite (Prosopis juliflora). Clini Mol All 2(1):8
Kim SH, Park HS, Jang JY (2011) Impact of meteorological variation on hospital visits of patients with tree pollen allergy. BMC Public Health 11:890
Kneuper CL, Scott CB, Pinchak WE (2003) Consumption and dispersion of Mesquite seeds by ruminants. J Range Manag 56:255–259
Knox RB, Suphioglu C, Taylor P, Desai R, Watson HC, Peng JL, Bursill LA (1997) Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy 27(3):246–251
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204
Kreuter UP, Amestoy HE, Kothmann MM, Ueckert DN, McGinty WA, Cummings SR (2005) The use of brush management methods: a Texas landowner survey. Rangel Ecol Manag 58:284–291
Kueffer C, Lavergne C (2004) Case studies on the status of invasive woody plant species in the western Indian ocean. FAO, Rome
Kumar ASHWANI, Kotiya AMIT (2004) Some Potential Plants for Bio-energy. Biomass for energy industry and climate protection. WIP-Munich, pp 180–183
Landeras G, Alfonso M, Pasiecznik NM, Harris PJC, Ramirez L (2006) Identification of Prosopis juliflora and Prosopis pallida accessions using molecular markers. Biodivers Conserv 15(5):1829–1844
Lara-Núñez A, Romero-Romero T, Blancas V, Ventura JL, Anaya AL, Cruz-Ortega R (2006) Allelochemical stress cause inhibition of growth and oxidative damage in Lycopersicon esculentum. Plant Cell Environ 29:2009–2016
Larrea-Alcázar DM, Soriano PJ (2008) Columnar cacti-shrub relationships in an Andean semiarid valley in Western Venezuela. Plant Ecol 196:153–161
Lasalle JC (1962) El increment de la masa forestall del caldén (Prosopis caldenia Burk). Rev Forestal Argentina 6:44–50
Latorre F, Perez CF (1997) One year of airborne pollen sampling in Mar del Plata (Argentina). Grana 36:49–53
Lauenstein DAL, Fernanández ME, Verga AR (2013) Drought stress tolerance of Prosopis chilensis and Prosopis flexuosa species and their hybrids. Trees 27:285–296
Laxén J (2007) Is Prosopis a curse of a blessing? – an ecological-economic analysis of the invasive alien tree species in Sudan. Doctorate of Science dissertation, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki
Leão TCC, de Almeida WR, de Sá Dechoum M, Ziller SR (2011) Espécies exόticas invasoras: no Nordeste do Brasil. CEPAN Instituto Hόrus, Brazil
Lee SG, Russel EJ, Bingham RL, Felker P (1992) Discovery of thornless, non-browsed, erect tropical Prosopis in 3-year-old Hatian progeny trials. For Ecol Manag 48:1–13
Lemlem N (2003) Mapping of Prosopis juliflora using remote sensing and GIS – case srudy of Marigat Division, Baringo District. Bachelor of Science in Surveying. University of Nairobi, Nairobi
Lewis WH (1986) Airborne Pollen of the Neotropics. Grana 25:75–83
Lewis WH, Elvin Lewis MPF (2004) Medical Botany. Wiley, Plants Affecting Human Health. Economic Botany
Liao C, Peng R, Luo Y, Zhou X, Wu X, Fang C, Li B (2008) Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol 177(3):706–714
Little EL, Wadsworth FH (1964) Common trees of Porto Rico and the Virgin Islands. Department of Agriculture, Forest Services, Washington, DC
Lloyd J, Mannan RW, Destefano S, Kirkpatrick C (1998) The effects of mesquite invasion on a southeastern Arizona grassland bird community. Wilson Bull 110:403–408
López-Portillo J, Montana C (1999) Spatiall distribution of Prosopis glandulosa var. torreyana in vegetation strips of the southern Chihuahuan Desert. Acta Oecol 20:197–208
Lorence DH, Wagner WL (2013) Flora of the Marquesas Islands. National Tropical Botanical Garden and the Smithsonian Institution
Lucas SK, Buckley CE (1989) Quantitative studies of cutaneous hypersensitivity. The prevalence of epicutaneous flare reactions to allergenic pollen extracts. J Allergy Clin Immunol 84:465–474
Luque GM, Bellard C, Bertelsmeier C, Bonnaud E, Genovesi P, Simberloff D, Courchamp F (2014) The 100th of the world’s worst invasive alien species. Biol Invasions 16(5):981–985
Lynes BC, Campell SD (2000) Germination and viability of mesquite (Prosopis pallida) seed following ingestion and excretion by feral pigs (Sus scrofa). Tropical Grasslands 34:125–128
Mabrouk H, Hilmi E, Abdullah M (2008) Nutritional value of Prosopis juliflora pods in feeding nile tilapia (Oreochromis niloticus) fries. Arab Gulf J Scientific Res 26:49–62
MacKee HS (1994) Catalogue des plantes introduites et cultivées en Nouvelle-Calédonie. Muséum Natural d’Historie, Paris
Mahgoub O, Kadim IT, Johnston EH, Srikandakumar A, Al-Saqri NM, Al-Abri AS, Ritchie A (2005a) The use of concentrate containing Meskit (Prosopis juliflora) pods and date palm by-products to replace commercial concentrate in diets of Omani sheep. Anim Feed Sci Technol 120:33–41
Mahgoub O, Kadim IT, Forsberg NE, Al-Ajmi DS, Al-Saqry NM, Al-Abri AS, Annamalai K (2005b) Evaluation of meskit (Prosopis juliflora) pods as a feed for goats. Anim Feed Sci Technol 121:319–327
Makkar HPS, Singh B, Negi SS (1990) Tannin levels and their degree of polymerisation and specific activity in some agro-industrial by-products. Biol Wastes 31:137–144
Malhotra S, Misra K (1981a) 3,30-di-O-methylellagic acid 4-Orhamnoside from the roots of Prosopis juliflora. Phytochemistry 20:2043–2044
Malhotra S, Misra K (1981b) Ellagic acid 4-O-rutinoside from pods of Prosopis juliflora. Phytochemistry 20:2439–2440
Malhotra S, Misra K (1981c) An ellagic acid glycoside from the pods of Prosopis juliflora. Phytochemistry 20:860–861
Malhotra S, Misra K (1983) New flavanones from Prosopis juliflora roots. Planta Med 47:46–48
Malik P, Singh AB, Babu CR, Gangal SV (1990) Head-high, airborne pollen grains from different areas of metropolitan Delhi. Allergy 45:298–305
Mandu P, Kibet S, Morimoto Y, Imbumi M, Adeka R (2009) Impact of Prosopis juliflora on Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodiversity 2(3):33–50
Manga VK, Sen DN (1995) Influence of seed traits on germination in Prosopis cineraria (L.) MacBride. J Arid Environ 31:371–375
Mann HS, Shankarnarayan KA (1980) The role of Prosopis cineraria in an agropastoral system in Western Rajasthan. Browse in Africa. International Livestock Centre for Africa, Addis Ababa, pp 437–442
Mari A (2008) When does a protein become an allergen? Searching for a dynamic definition based on most advanced technology tools. Clin Exp Allergy 38:1089–1094. PMID: 18477011; Bergmann KC, Ring J. (2014) History of allergy. Karger AG, Basel, pp 172–192
Maundu P, Kibet S, Morimoto Y, Imbumi M, Adeka R (2009) Impact of Prosopis julifloraon Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodiversity 10:33–50
Maundu P, Kibet S, Morimoto Y, Imbumi M, Adeka R Impact of Prosopis juliflora on Kenya’s semi-arid and arid ecosystems and local livelihoods. Biodiversity 10:33–50
Mazibuko DM. 2012. Phylogenetic relationships of Prosopis in South Africa: an assessment of the extent of hybridization, and the role of genome size and seed size in the invasion dynamics. MSc thesis, Stellenbosch University, Stellenbosch
Mc Kay F, Gandolfo D, Witt ABR (2012) Biology and host range of Coelocephalapion gandolfoi Kissinger (Brentidae), a promosing candidate for the biological control of invasive Prosopis species (Legumiosae) in South Africa. Afr Entomol 20:281–291
McClaran MP, Angell DL (2006) Long-term vegetation response to mesquite removal in Desert Grassland. J Arid Environ 66:686–697
Medeiros MA, Riet-Correa F, Dantas FP, Santos JR, Medeiros RM (2014) Teratogenic effects of Prosopis juliflora in rats and analysis of the toxicity of the pods. Pesqui Vet Bras 34:1089–1093
Medina AA, Dussart EG, Estelrich HD, Morici EA (2000) Reconstrucción de la historia del fuego en un bosque de Prosopis caldenia (Burk.) de Arizona, south of San Luis Province. Multequina 9:91–98
Mehar SK (2011) Assessment of effect of Prosopis juliflora litter extract on seed germination and growth of rice. Food Sci Qual Manage 2:9–18
Mendes BV (1986) Potential offered by Prosopis juliflora (SW) D.C. pods in the Brazilian semi arid region. The current state of knowledge onProsopis juliflora. II International Conference on Prosopis recife, 25–29 August, Brazil, pp 61–62
Menezes RSC, Salcedo IH, Elliott ET (2002) Microclimate and nutrient dynamics in a silvopastoral system of semi- arid northeastern Brazil. Agrofor Syst 56
Merga Y (2012) Assessing the impact of Prosopis juliflora (SW.) D.C.. invasion on livelihood of pastoralists: the case of fentale woreda, east showa zone of Oromia. MSc thesis, Haramaya University, Haramaya, Ethiopia
Meyer RE, Morton HL, Haas RH, Robison ED, Riley TE (1971) Morphology and anatomy of honey mesquite. USDA Technology Bulletin, Washington, DC
Miller C, Darlow A (2008) A reiview of the Ascension Island action plan for SAIS Project. RSPB
Miranda RQ, Oliveira MTP, Correia RM, Almedia-Cortez JS, Pompelli MF (2011) Germination of Prosopis juliflora(Sw) D.C. seeds after scarification treatments. Plant Species Biol 26:186–192
Mishra A, Sharma SD, Gupta MK (2003) Soil rehabilitation through afforestation: evaluation of the performance of Prosopis julifl- ora, Dalbergia sissoo and Eucalyptus tereticornis plantations in a sodic environment. Arid Land Res Manag 17(3):257–269
Moeller DA, Tiffin P (2005) Genetic diversity and the evolutionary history of plant immunity genes in two species of Zea. Mol Biol Evol 22:2480–2490
Mohammadi A, Nasr J, Rahmatnejad E, Dashtizadeh M, Golshahi A (2013) Performance and carcass quality of broiler chickens in response to Prosopis juliflora seed (PJS) as a by-product. J Agricult Technol 9:317–322
Moodley D, Geerts S, Richardson DM, Wilson JRU (2013) Different traits determine introduction, naturalization and invasion success in woody plants: Proteaceae as a test case. PLoS One 8:75–78
More D, Whisman L, Whisman B, Jordan-Wagner D (2002) Identification of specific IgE to mesquite wood smoke in individuals with mesquite pollen allergy. J Allergy Clin Immunol 110:814–816
Morgan AM, Hamdoun AM, Bashir NH (2017) Studies on Seed Germination and Seedling Emergence of Mesquite, Prosopis juliflora (Swartz) D.C.. in Sudan Gezira. Univ J Agricult Res 5(2):159–163
Mosweu S, Munyati C, Kabnda T, Setshongo M, Muzila M (2013) Prosopis L. invasion in the south-western region of Botswana: the perceptions of rural communities and management options. Natural Resour 4:496–505
Muller, G.C., Junnila, A., Traore, M.M., Traore, S.F., Doumbia, S., Sissoko, F., Dembele, S.M., Schlein, Y., Arheart, K.L.., Revay, E.E., Kravchenko, V.D., Witt, A. and Beier, J.C. 2017. The invasive shrub Prosopis julifloraenhances the malaria parasite transmission capacity of Anopheles mosquitos: a habitat manipulation experiment
Munzbergova Z, Ward D (2002) Acacia trees as keystone species in Negev desert ecosystems. J Veg Sci 13:227e236
Murad AA, Al Nuaimi H, Al Hammadi M (2007) Comprehensive assessment of water resources in the United Arab Emirates (UAE). Water Resour Manag 21(9):1449–1463
Muturi GM, Mohren GMJ, Kimani JN. (2009) Prediction of Prosopis species invasion in Kenya using geographical information system techniques
Muturi GM, Poorter L, Mohren GMJ, Kigomo BN (2013) Ecological impacts of Prosopis species invasion in Turkwel riverine forest, Kenya. J Arid Environ 92:89–97
Muzila M, Setshogo MP, Moskei B, Morapedi R (2011) An assessment of Prosopis L. in the Bokspits area south-western Botswana, based on morphology. Afr J Plant Sci Biotechnol 5:75–80
Mwangi M, Swallow B (2005) Invasion of Prosopis juliflora and local livelihoods: case study from the Lake Baringo area of Kenya. ICRAF Working Paper – no. 3. World Agroforestry Centre, Nairobi
Mwangi E, Swallow B (2008) Prosopis juliflora invasion and rural livelihoods in the Lake Baringo Area of Kenya. Conserv Soc 6(2):130–140
Mworia JK, Kinyamario JL, Omari JK, Wambua JK (2011) Patterns of seed dispersal and establishment of invader Prosopis juliflora in the upper floodplain of Tana River, Kenya. Afr J Range Forage Sci 28:35–41
Nahonyo CL, Mwasumbi LB, Msuya CA, Masao CA, Suya TB, Shing’weda C (2005) NGEZI – vumawimbi forest reserves biodiversity inventory report. Department of Zoology and Marin Biology, University of Dar es Salam, Dar es Salam
Nakano H, Fujii Y, Suzuki T, Yamada K, Kosemura S, Yamamura S, Hasegawa K (2001) A growth-inhibi- tory substance exuded from freeze-dried mesquite (Prosopis juliflora (Sw.) D.C..) leaves. Plant Growth Regul 33:165–168
Naranjo CA, Poggio L, Enus-Zeiger S (1984) Phenol chromatography, morphology and cytogenetics in three species and natural hybrids of Prosopis (Leguminosae, Mimosoideae). Plant Syst Evol 144:257–276
Naseeruddin S, Yadav KS, Sateesh L, Manikyam A, Desai S, Rao LV (2013) Selection of the best chemical pretreatment for lignocellulosic substance Prosopis juliflora. Bioresour Technol 136:542–549
National Weeds Strategy Executive Committee (2001) Mesquite (Prosopis Speceis) strategic plan. National Weeds Strategy Executive Committee, Launceston
Naylor RL (1996) Invasions in agriculture: assessing the cost of the golden apple snail in Asia. Ambio 25:443–448
Ndhlovu T, Milton-Dean SJ, Esler KJ (2011) Impact of Prosopis (mesquite) invasion and clearing on the grazing capacity of semiarid Nama Karoo rangeland, South Africa. Afr J Range Forage Sci 28:129–137
Nee’ Shukla RV, Misra K (1981) Two flavonoid glycosides from the bark of Prosopis juliflora. Phytochemistry 20:339–340
Neudecker P, Schweimer K, Nerkamp J, Scheurer S, Vieths S, Sticht H et al (2001) Allergic cross-reactivity made visible: solution structure of the major cherry allergen Pru av 1. J Biol Chem 276:22756–22763
Nie W, Yuan Y, Kepner W, Erickson C, Jackson M (2012) Hydrological impacts of mesquite encroachment in the upper San Pedro watershed. J Arid Environ 82:147–155
Niguse H, Amare F (2016) Distribution and Socio-economic Impacts of Prosopis juliflora in East Shewa and West Arsi Zones, Ethiopia. Intl J Afr Asian Stud 24:31–41
Njoroge E, Sirmah P, Mburu F, Koech E, Mware M, Chepkwony J (2012) Preference and adoption of farmer field school (FFS) Prosopis juliflora management practices: experiences in Baringo District, Kenya. Forest Stud China 14(4):283–290
Nolte KR, Fulbright TE (1997) Plant, small mammal and avian diversity following control of honey mesquite. J Range Manag 50:205–212
Northern Territory Government (2012) Weed management plan for mesquite (Prosopis species). Northern Territory Government, Palmeton
Novey HA, Roth M, Wells ID (1977) Mesquite pollen: an aeroallergen in asthma and allergic rhinitis. J Allergy Clin Immun 59:359–363
Obeidat BS, Shdaifat MM (2013) Partial substitution of barley grain with Prosopis juliflora pods in lactating Awassi ewes’ diets: effect on intake, digestibility, and nursing performance. Small Rumin Res 111:50–55
Obeidat BS, Abdullah AY, Al-Lataifeh FA (2008) The effect of partial replacement of barley grains by Prosopis juliflora pods on growth performance, nutrient intake, digestibility, and carcass characteristics of Awassi lambs fed finishing diets. Anim Feed Sci Technol 146:42–54
Odero-Waitituh JA, King’ori AM, Guliye AY (2016) Effect of replacing maize with milled mature pods of Prosopis juliflora on performance of finishing broiler chicken. Livestock Res Rural Develop 28:Article No. 22
Oduor NM, Githiomi JK (2013) Fuel-wood energy properties of Prosopis juliflora and Prosopis pallida grown in Baringo District, Kenya. Afr J Agric Res 8(21):2476–2481
Ojaveer H, Galil BS, Minchin D, Olenin S, Amorim A, Canning-Clode J et al (2014) Ten recommendations for advancing the assessment and management of non-indigenous species in marine ecosystems. Mar Policy 44:160–165
Ooi MKJ, Auld TD, Denham AJ (2009) Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Glob Chang Biol 15:2375e2386
Ordman D (1950) Prosopis tree as a cause of seasonal hay fever and asthma in South Africa. S Afr Med J 33:12
Osmond R (2003) Best practice manual, mesquite: control and management options for mesquite (Prosopis spp.) in Australia. National Weeds Programme and Queensland Department of Natural Resources and Mines, Queensland
Page AR, Lacey KL (2006) Economic impact assessment of Australia weed biological control. CRC for Australian Weed Management, Australia
Palacios RA (2006) Los mezquites Mexicanos: biodiversidad y distrabucion geografica. Bull Bot Soc Argentina 41:99–121
Pandey CN, Pandey R, Bhatt JR. (2012) Prosopis juliflora (Swartz) D.C.: management dilemmas and regulatory issues in Gujarat. In: Bhatt et al (eds) Invasive alien plants: an ecological appraisal for the Indian subcontinent. CAB International
Panetta FD, Carstairs SA (1989) Isozymic discrimination of tropical Australian populations of mesquite (Prosopis spp.): implications for biological control. Weed Res 29:157–165
Pasiecznik NM, Peñalvo López E (In review) 25 year results from an arid zone tree species elimination trial in Almeria, Spain, and an invasive risk assessment of the exotic species introduced. Biodiversity and Conservation
Pasiecznik NM, Felker P, Harris PJ, Harsh L, Cruz G, Tewari JC, Cadoretm K, Maldonado LJ (2001) The ‘Prosopis Juliflora’-‘Prosopis Pallida’ complex: a monograph, vol 172. HDRA, Coventry
Pasiecznik NM, Harris PJC, Smith SJ (2004) Identifying tropical Prosopis species: a field guide. HDRA, Coventry
Pasiecznik NM, Choge SK, Muthike GM, Chesang S, Fehr C, Bakewell-Stone P, Wright J, PJC H (2006) Putting knowledge on Prosopis into use in Kenya. Pioneering advances in 2006. KEFRI and HDRA, Nairobi/Coventry
Patnaik P, Abbasi T, Abbasi SA (2017) Prosopis (Prosopis juliflora): blessing and bane. Trop Ecol 58(3):455–483
Patnaik P, Abbasi T, Abbasi SA (2018) Extraction of energy precursors in the form of volatile fatty acids (VFAs) from the xerophyte prosopis (Prosopis juliflora), In Advances in Health and Environment Safety (pp. 255–261). Springer, Singapore
Pauli G (2000) Evolution in the understanding of cross-reactivities of respiratory allergens: the role of recombinant allergens. Int Arch Allergy Immunol 123:183–195
Peinetti R, Sosa A, Kin A, Cerqueira E (1997) Modelo de simulación del banco de semillas del caldén (Prosopis caldenia) Reunión Argentina de Ecologia 48:98
Pereira TCJ, Pereira MLA et al (2013) Mesquite pod meal in diets for Santa Inês sheep: Ingestive behavior. Acta Scientiarum Anim Sci 35:201–206
Perera ANF, Pasiecznik NM (2005) Using invasive Prosopis to improve livelihoods in Sri Lanka. HDRA, Coventry
Perroni-Ventura Y, Montaña C, García-Oliva F (2010) Carbon–nitrogen interactions in fertility island soil from a tropical semi-arid ecosystem. Funct Ecol 24:233–242
Perveen A, Zeb S, Khan M, Qaiser M (2014) Seasonal fluctuations of airborne pollen grains count and its correlation with climatic factors from Khairpur; Sindh, Pakistan. Pak J Bot 46(1):299–306
Pickup AR (1999) Ascension Island Management Plan. Report from RSPB and Birdlife International
Pinto-Ruiz R, Gomez-Castro H, Guevara-Hernandez F, Hernandez-Sanchez D, Ruiz-Sesma B (2014) Preference and lngestive behavior of sheep fed on tropical tree fruits. Revista Cientifica-Facultad De Ciencias Veterinarias 24:158–163
Polley HW, Johnson HB, Mayeux JH (1994) Increasing CO2: comparitive responces of the C4 grass schizachyrium and grassland invader Prosopis. Ecology 75:976–988
Potgieter LJ, Richardson DM, Wilson JRU (2013) Casuarina: biogeography and ecology of an important tree genus in a changing world. Biol Invasions 16:609–633
Poynton RJ (1990) The genus Prosopis in southern Africa. South Afr For J 152:62–66
Poynton RJ (2009) Tree planting in southern Africa, volume 3: other genera. Department of Agriculture, Forestry and Fisheries, Pretoria
Prado Silva RH, de Rezende ASC, da Silva Inácio DF (2016) Pectin-rich by-products in feeding horses – a review. Cogent Food Agricult 2:1193925. https://doi.org/10.1080/23311932.2016.1193925
Prieur-Richard AH, Lavorel S, Linhart YB, Dos Santos A (2002) Plant diversity, herbivory and resistance of a plant community to invasion in Mediterranean annual communities. Oecologia 130:96–104
Pyšek P, Jarošík V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vilà M (2012) A global assessment of invasive plant impacts on resident species, communities and ecosystems: the interaction of impact measures, invading species’ traits and environment. Glob Chang Biol 18(5):1725–1737
Qasem JR (2007) Chemical control of Prosopis farcta (Banks and Sol.) Macbride in the Jordan Valley. Crop Prot 26:572–575
Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J Allergy Clin Immunol 2008; 121: 847–852. e7. PMID: 18395549
Ræbild A, Diallo BO, Graudal L, Dao L, Sanou J (2003) Evaluation of a species and provenance trial of Prosopis at Gonsé, Nurkina Faso. FAO, Rome
Rashid M, Abbas SH, Rehman A (2014) The status of highly alien invasive plants in Pakistan and their impact on the ecosystem: a review. Innovare J Agricult Sci 2:1–4
Reddy CS, Rangaswamy M, Pattanaik C, Jah CS (2009) Invasion of alien species in wetland of Samaspur Bird Sanctuary, Uttar Pradesh, India. Asian J Water Environ Pollut 6:43–50
Reinoso H, Sosa L, Ramírez L, Luna V (2004) Salt-induced changes in the vegetative anatomy of Prosopis strombulifera (Leguminosae). Can J Bot 82(5):618–628
Rettberg S, Müller-Mahn D (2012) Human-environment interactions: the invasion of Prosopis juliflora in the Drylands of Northeast Ethiopia. In: Mol L, Sternberg T (eds) Changing deserts – integrating people and their environments. Whitehorse Press, Cambridge, pp 297–316
Richardson DM, Carruthers J, Hui C, Impson FAC, Miller JT, Robertson MP, Rouget M, Le Roux JJ, Wilson JRU (2011) Human-mediated introductions of Australian Acacias—a global experiment in biogeography. Divers Distrib 17:771–787
Riet-Correa F, de Andrade FR, Carvalho FK, Tabosa IM, Galiza GJ, Bernardino JN, Simões SV, Medeiros RM (2012) Use of Prosopis juliflora pods as food for sheep and goats. Pesqui Vet Bras 32:987–989
Ring J, Akdis C, Behrendt H, Lauener RP, Schäppi G, Akdis M, et al. Davos declaration: allergy as a global problem. Allergy 2012; 67: 141–143. PMID: 22235793
Robinson TP, van Klinken RD, Metternicht G (2008) Spatial and temporal rates and patterns of mesquite (Prosopis species) invasions in Western Australia. J Arid Environ 72:175–188
Ruiz TG, Zaragoza SR, Cerrato RF (2008) Fertility islands around Prosopis laevigata and pachycereus hollianus in the drylands of Zapotitlán Salinnas, Mexico. J Arid Environ 72:1202–1212
Ruskin FR (1980) Firewood crops. Shrub and tree species for energy production. National Academy of Sciences, Washington, D.C.
Sahli HF, Conner JK (2006) Characterizing ecological generalization in plant-pollinator systems. Oecologia 148:365–372
Sajad G, Sefidi K (2012) Comparison of sustainable forest management (SFM) trends at global and country levels: case study in Iran. J For Res 23(2):311–317
Sajjad A, Saeed S, Bashir MA (2012) Spatial variation in pollinator communities and reproductive performance of Prosopis juliflora (Fabaceae). J Pollinat Ecol 8(9):59–66
Salazar PC, Navarro-Cerrillo RM, Cruz G, Villar R (2018) Intraspecific leaf functional trait variability of eight Prosopis pallida tree populations along a climatic gradient of the dry forests of northern Peru. J Arid Environ
Samuel G (2010) Allelopathic effects of the invasive Prosopis juliflora on selected native plant species at Middle Awash, Southern Afar Rift of Ethiopia. MSc thesis, Addis Ababa University, Addis Ababa Ethiopia
Samuel M, Christopher M, Tibangayuka K, Moffat S, Mbaki M (2013) Prosopis L. Invasion in the South-Western Region of Botswana: the perceptions of rural communities and management options. Nat Resour 4:496–505
Satheesh Kumar S, Vittal BPR (1998) A preliminay survey of airborne pollen in Madras city. Aerobiologia 14:69–73
Sato T (2013) Beyond water-intensive agriculture: expansion of Prosopis juliflora and its growing economic use in Tamil Nadu, India. Land Use Policy 35:283–292
Sawal RK, Ratan R, Yadav SBS (2004) Mesquite (Prosopis juliflora) pods as a feed resource for livestock-A review. Asian Australas J Anim Sci 17:719–725
Schachtschneider K, February E (2013) Impact of Prosopis invasion on a keystone tree species in the Kalahari Desert. Plant Ecol 214:597–605
Schupp EW (1993) Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 107:15–29
Seid MJ (2012) Household perception about Prosopis juliflora and its effect on pastoral livelihood diversification strategy: the case of Gewane District in Afar Regional State, Ethiopia. Intl J Agricult Sci Res 2:21–51
Senthilkumar P, Prince WS, Sivakumar S, Subbhuraam CV (2005) Prosopis juliflora—a green solution to decontaminate heavy metal (Cu and Cd) contaminated soils. Chemosphere 60:1493–1496
Shackleton RT, Le Maitre DC, Pasiecznik NM, Richardson DM (2014) Prosopis: a global assessment of the biogeography, benefits, impacts and management of one of the world’s worst woody invasive plant taxa. AoB Plants 6:1–18
Shackleton RT, Le Maitre DC, Richardson DM (2015) Stakeholder perceptions and practices regarding Prosopis (mesquite) invasions and management in South Africa. Ambio 44:569–581
Shah RH, Baloch M, Khan AA, Ijaz M, Zubair M (2018) Bioherbicidal assessment of aqueous extracts of mesquite (Prosopis juliflora) on weeds control and growth, yield and quality of wheat. Planta Daninha 36
Shapaka TN, Cunningham PL, Joubert DF (2008) Invasive alien plants in the Daan Viljoen Game Park, vol 30. DINTERIA, Windhoek, pp 19–32
Sharma IK (1981) Ecological and economic importance of Prosopis juliflora in the Indian Thar Desert. J Taxon Bot 2:245–248
Sharma R, Dakshini KMM (1996) Ecological implications of seed characteristics of the native Prosopis cineraria and the alien P. juliflora. Vegetation 124:101–105
Sharma R, Dakshini KMM (1998) Intergration of plant and soil charecteristics and the ecological success of two Prosopis species. Plant Ecol 139:63–69
Sharmila S, Rebecca Jeyanthi L, Saduzzaman M (2013) Biodegradation of tannery effluent using Prosopis juliflora. Int J ChemTech Res 5:2186–2192
Shea KM, Truckner RT, Weber RW, Peden DB (2008) Climate change and allergic disease. J Allergy Clin Immunol 122:443–453
Shiferaw H, Teketay D, Nemomissa S, Assefa F (2004) Some biological characteristics that foster the invasion of Prosopis juliflora (Sw.) D.C.. at Middle Awash Rift Valley Area, north-eastern Ethiopia. J Arid Environ 58:135–154
Shimles A, Getachew M (2016) Contemporary Prosopis dilemma: perception of inhabitants of Sabian Kebele (Goro) towards invasive tree Prosopis juliflora, Eastern Ethiopia. J Agricult Biotechnol Sustain Develop 8(1):1–6
Shirke PA, Pathre UV (2004) Influence of leaf-to-air vapour pressure deficit (VPD) on the biochemistry and physiology of photosynthesis in Prosopis juliflora. J Exp Bot 55(405):2111–2120
Shirke PA, Pathre UV, Sane PV (2018) Adaptation strategies of two leaf cohorts of Prosopis juliflora produced in spring and monsoon. Photosynthetica:1–10
Shivpuri DN, Parkash D (1967) A study in allergy to Prosopis juliflora (Kabuli keekar). Ann Aller 25:643–648
Sigh A, Singh PK (2009) An ethnobotanical study of medicinal plants in Chandauli District of Uttar Pradesh, India. J Ethnopharmacol 121:324–329
Silva S (1986) Prosopis juliflora (SW) D.C. in Brazil. The current state of knowledge on Prosopis juliflora. II international conference on Propopis recife, 25–29 August, Brazil, pp 29–51
Silva S (1988) Prosopis juliflora (Sw) D.C. Brazil. In The Current State of Knowledge on Prosopis juliflora. (Eds.) M. A. Habit and J. C. Saavedra. FAO, Rome
Silva JHVD, Silva MBD, Silva ELD, Jordão Filho J, Ribeiro MLG, Costa FGP, Dutra Júnior WM (2003) Energia metabolizável de ingredientes determinada com codornas japonesas (Coturnix coturnix japonica). Rev Bras Zootec 32(6):1912–1918
Silva AMM, Silva AR, Pinheiro AM et al (2007) Alkaloids from Prosopis juliflora leaves induce glial activation, cytotoxicity
Silva VDA, Pitanga BPS, Nascimento RP et al (2013) Juliprosopine and Juliprosine from Prosopis juliflora leaves induce mitochondrial damage and cytoplasmic vacuolation on cocultured glial cells and neurons. Chem Res Toxicol 26:1810–1820
Silva TRM, Chung S, de Araújo TAT, de Azevedo KSP, dos Santos MC, Bicudo ÁDA (2015) Replacing corn by mesquite meal (Prosopis juliflora) in diets for juvenile Nile tilapia reared in low temperature. Revista Brasileira de Ciências Agrárias (Agrária) 10:460–465
Simpson BB (1977) Mesquite, its Biology in Two Desert Shrub Ecosystems. Dowden, Hutchinson and Ross, Stroudsberg, Pennsylvania, USA
Simpson BB, Neff JL, Moldenke AR (1977) Prosopis flowers as a resource. Mesquite: its biology in two desert scrub ecosystems. Simpson B. B. Ed. Dowden, Hutchinson and Ross, Stroudsburg, Pennsylvania
Singh S (2012) Phytochemical analysis of different parts of Prosopis juliflora. Intl J Curr Pharmaceut Res 4:59–61
Singh AB, Mathur C (2012) An aerobiological perspective in allergy and asthma. Asia Pac Allergy 2:210–222
Singh G, Shukla S (2012) Effect of Prosopis julifora (D.C..) tree on under canopy resources, dicersity and productivity of herbaceous vegetation in Indian desert. Arid Land Res Manag 26:151–165
Skolmen RG (Undated) Prosopis pallida (Humb. And Bonpl. Ex Willd.) H.B.K. Kiawe
Smit P (2004) Prosopis: a review of existing knowledge relevant to Namibia. J Scientific Soc 52:13–40
Smith KR, Woodward A, Campbell-Lendrum D, Chadee DD, Honda Y, Liu Q, et al. 2014. Human health: impacts, adaptation, and co-benefits. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al. (eds) Climate Change 2014: impacts, adaptation, and vulnerability Part A: global and sectoral aspects contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 709–754
Sohrabi S, Gherekhloo J, Mohassel MHR, Ghanbari A, Mahalati MN. 2011. Cardinal temperatures of three invasive weeds in Iran. 3rd International Symposium on Weeds and Invasive Plants, Acuna
Song U, Mun S, Ho CH, Lee EJ (2012) Responses of two invasive plants under various microclimate conditions in the Seoul metropolitan region. Environ Manag 49:1238–1246
Steele P, Breithaupt J, Labrada R (2008) Proceedings Expert Consultation (no. 4): increases food security control and management of Prosopis. FAO, Ethiopia
Steenkamp HE, Chown SL (1996) Influence of dense stands of an exotic tree Prosopis glandulosa Benson, on a savanna dung beetle (Coleoptera: Scarabeidae) assemblage in southern Africa. Biol Conserv 78:305–311
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 121:432–440
Stromberg JC, Wilkins SD, Tress JA (1993) Vegetation-hydrology models: implications for management of Prosopis velutina (velvet mesquite) riparian ecosystems. Ecol Appl 2:307–314
Tabosa IM, Riet-Correa F, Barros SS, Summers BA, Simoes SVD, Medeiros RMT, Nobre VMT (2006) Neurohistologic and ultrastructural lesions in cattle experimentaly intoxicated with the plant Prosopis juliflora. Vet Pathol 43:695–701
Talavera S, Bastida F, Ortiz PL, Arista M (2001) Pollinator attendance and reproductive success in Cistus libanotis L. (Cistaceae). Int J Plant Sci 162:343–352
Teague WR, Ansley RJ, Kreuter UP, Pinchak WE, McGrann JM (2001) Economics of managing mesquite in northern Texas: a sensitivity analysis. J Range Manage 54:553–560
Teague WR, Grant WE, Kreuter UP, Diaz-Solis H, Dube S, Kothmann MM, Pinchak WE, Ansley RJ (2008) An ecological economic simulation model for assessing fire and grazing management effects on mesquite rangelands in Texas. Ecol Econ 64:611–624
Tegegn G (2008) Experiences on Prosopis management case of Afar region. FARM-Africa, London (September), ARM-Africa, London
Terink W, Immerzeel WW, Droogers P (2013) Climate change projections of precipitation and reference evapotranspiration for the Middle East and Northern Africa until 2050. Intl J Climatol 33(14):3055–3072
Tesoriere L, Butera D, Allegra M et al (2005) Distribution of betalain pigments in red blood cells after consumption of cactus pear fruits and increased resistance of the cells to ex vivo induced oxidative hemolysis in humans. J Agric Food Chem 53:1266–1270
Tessema YA (2012) Ecological and Economic Dimensions of the paradoxical invasive species – Prosopis juliflora and policy changes in Ethiopia. J Econ Sustain Develop 3:62–71
Thakur IS (1986) Prosopis juliflora pollen allergen induced hypersensitivity and anaphylaxis studies in guinea pigs. Biochem Int 13:915–912
Thakur IS (1989) Fractionation and analysis of allergenicity of allergens from Prosopis juliflora pollen. Int Arch Allergy Immunol 90(2):124–129
Thakur IS (1991) Purification and characterization of the glycoprotein allergen from Prosopis juliflora pollen. Biochem Int 23(3):449–459
Thakur IS, Sharma JD (1985) Isolation and characterization of allergens of Prosopis juliflora pollen grains. Biochem Int 11(6):903–912
Thomas J, El-Sheikh MA, Alfarhan AH, Alatar AA, Sivadasan M, Basahi M, Al-Obaid S, Rajakrishnan R (2016) Impact of alien invasive species on habitats and species richness in Saudi Arabia. J Arid Environ 127:53–65
Thorp JR, Lynch RA (2001) The determination of weeds of national significance. National Weeds Strategy Executive Committee, Launceston, 234 pages
Throop HL, Archer SR (2008) Srub (Prosopis velutina) encroachment in a semidesert grassland: special-temporal changes in soil organic carbon and nitrogen pools. Glob Chang Biol 14:2420–2431
Tiedemann AR, Klemmedson JO (1973) Nutrient availability in desert grassland soils under Mesquite (Prosopis juliflora) trees and adjacent open areas. Soil Sci Soc Am J 37:107–111
Tilstone GH, Pasiecznik NM, PJC H, Wainwright SJ (1998) The growth of multipurpose tree species in the Almeria province of Spain and its relationship to native plant communities. Intl Tree Crops J 9(4):247–259
Timyan J (1996) BWA YO: important trees of Hati. South-East Consortium for International Development, Washington, DC
Torell LA, McDaniel KC (1986) Optimal timing of investements to control honey mesquite. J Range Manag 39:378–382
Tourenq C, Shuriqi MK (2010) Snapshot on introduced invasives in a desertic country, the United Arab Emirates. Aliens 30:49–51
Truman ST, Wallace HM (1999) Pollination and resource constraints on fruit set and fruit size of Persoonia rigida (Proteaceae). Ann Bot 83:145–155
Tschirley FH, Martin SC (1960) Germination and longevity of velvet mesquite seed in soil. J Range Manag 13:94–97
Valenta R, Steinberger P, Duchêne M, Kraft D (1996) Immunological and structural similarities among allergens: prerequisite for a specific and component-based therapy of allergy. Immunol Cell Biol 74:187–194
Vallejo VD, Arbeli Z, Tera’n W, Lorenz N, Dick RP, Roldan F (2012) Effect of land management and Prosopis juliflora (Sw.) D.C. trees on soil microbial community and enzymatic communities in intensive silvopastoral systems of Colombia. Agric Ecosyst Environ 150:139–148
Van den Berg EC (2010) Detection, quantification and monitoring Prosopis spp. in the Northern Cape Province of South Africa using Remote Sensing and GIS. MSc thesis, North-West University, Potchefstroom
van der Linden PJ, Hanson CE (2007) Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 7–22
van Klinken R (2012) Prosopis spp. – mesquite. In: Julien M, McFadyen R, Cullen J (eds) Biological control of weeds in Australia. CSIRO, Melbourne, Australia
van Klinken ED, Burwell CJ (2005) Evidence from a gelechidd leaf-tier on mesquite (Mimosaceae: Prosopis) that semi-concealed Lepidopteran biological control agents may not be at risk from parasitism in Australian rangelands. Biol Control 32:121–129
van Klinken RD, White AJ (2011) Overcoming seasonally fluctuating resources: Bruchid predation of mesquite (Prosopis) seed in dung. Biol Control 59:361–365
van Klinken RD, Graham J, Flack LK (2006) Population ecology of hybrid mesquite (Prosopis species) in Western Australia: how does it differ from native range invasions and what are the implications for impacts and management? Biol Invasions 8:727–741
van Klinken RD, Shepherd D, Parr R, Robinson TP, Anderson L (2007) Mapping mesquite (Prosopis) distribution and desnnity using visual areal surveys. Rangel Ecol Manag 60:408–416
van Wilgen BW, Forsyth GG, Le Maitre DC, Wannenburgh A, Kotze DF, van den Berg E, Henderson L (2012) An assessment of the effectiveness of a large, national-scale invasive alien plant control strategy in South Africa. Biol Conserv 148:28–38
Vaz AS, Kueffer C, Kull CA, Richardson DM, Vicente JR, Kühn I, Schröter M, Hauck J, Bonn A, Honrado JP (2017) Integrating ecosystem services and disservices: insights from plant invasions. Ecosyst Ser 23:94–107
Villagra PE, Boninsegna JA, Alvarez JA, Cony M, Cesca E, Villalba R (2005) Dendroecology of Prosoois flexuosa woodlands in the Monte desert: implications for their management. Dendrochronologia 22:209–213
Villagra PE, Vilela A, Giordano C, Alvarez JA (2010) Ecophysiology of Prosopis species from the arid lands of Argentina: what do we know about adaptation to stressful environments? In: Desert plants. Springer, Berlin/Heidelberg, pp 321–340
Vitousek PM (1986) Biological invasions and ecosystem properties: can species make a difference?. In Ecology of biological invasions of North America and Hawaii. Springer, New York, pp 163–176
Vitousek PM, D’Antonio CM, Loope LL, Westbrooks R (1996) Biological invasions as global environmental change. Am Scientist 84(5):468–478
Vitousek PM, Dantonio CM, Loope LL, Rejmanek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16
Wakie TT, Laituri M, Evangelista PH (2016) Assessing the distribution and impacts on Prosopis juliflora through participatory approaches. Appl Geogr 66:132–143
Walkie TT, Hoag D, Evangelista PH, Luizza M, Laituri M (2016) Is control through utilization a cost effective Prosopis juliflora management strategy? J Environ Manag 168:74–86
Walter K (2011) Prosopis, an alien amoung the sacred trees of south India. Dissertation. University of Helsinki, Helsinki
Ward CR, O’Brien CW, O’Brien LB, Foster DE, Huddleston EW (1977) Annotated checklist of New World Insects associated with Prosopis (Mesquite). Technical bulletin, vol 1557. United States Department of Agriculture, USA
Wardle DA, Bardgett RD, Callaway RM, Van der Putten WH (2011) Terrestrial ecosystem responses to species gains and losses. Science 332(6035):1273–1277
Warrag MOA (1995) Autotoxic potential of foliage on seed germination and early growth of mesquite (Prosopis juliflora). J Arid Environ 31(4):415–421
Warren A, Holechek J, Cardenas M (1996) Honey mesquite influence on Chihuahuan desert vegetation. J Range Manag 49:46–52
Wassel GM, Rizk AM, Abdel-Bary EF (1972) Phytochemical investigation of Prosopis juliflora D. C. I. Flavonoids and free sugars. Qual Plant Mater Veg 22:119–121
Wayne P, Forster S, Connelly J, Bazzaz FA, Epstein PR (2002) Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2 enriched atmospheres. Ann Allergy Asthma Immunol 88:279–282
Weber JC, Larwanou M, Abasse TA, Kalinganire A (2008) Growth and survival of Prosopis africana provenances tested in Niger and related rainfall gradients in the West African Sahel. For Ecol Manag 256:585–592
White F (1962) Forest and Flora of Northern Rhodesia. Oxford University Press, Oxford
Wiggens IR, Proter M, Anderson EF (1971) Flora of the Galápagos Islands. Stanford University Press, Stanford
Wise RM, van Wilgen BW, Le Maitre DC (2012) Costs, benefits and management options for an invasive alien tree species: the case of mesquite in the Northern Cape, South Africa. J Arid Environ 84:80–90
Wojtusik T, Felker P, Russel EJ (1993) Cloning the erect, thornless, non-browsed nitrogen fixing trees of Haiti’s principal fuelwood species (Prosopis juliflora). Agroferest Syst 21:293–300
Wronski T, Mosfer AN, Plath M (2012) Endemic Farasan Gazelle (Gazella gazella farasani) enhances the dispersal of invasive Prosopis juliflora on Farsan Islands, Saudi Arabia. Revue d’_ecologie – La Terre et la Vie 67:329–337
Yasin M, Animut G (2014) Replacing cottonseed meal with ground Prosopis juliflora pods; effect on intake, weight gain and carcass parameters of Afar sheep fed pasture hay basal diet. Trop Anim Health Prod 46:1079–1085
Zachariades C, Hofmann JH, Roberts A (2011) Biological control of mesquite (Prosopis species) (Fabaceae) in South Africa. Afr Entomol 19:402–415
Zaitoun S, Al-Ghzawi AA, Samarah N, Mullen R (2009) Pod production of Prosopis juliflora (Sw.) D.C.. As affected by supplementary and honeybee pollination under arid conditions. Acta Agric Scand 59:349–356
Zeila AA, Mwangi E, Swallew B (2004) Prosopis juliflora: boon or bane for dryland agroforestry. The Prunus Tribune, Jan–March
Ziello C, Sparks TH, Estrella N, Belmonte J, Bergmann KC, Bucher E, Brighetti MA, Damialis A, Detandt M, Galan C, Gehrig R, Grewling L, Gutierrez Bustillo AM, Hallsdottir M, Kockhans-Bieda MC, De Linares C, Myszkowska D, Paldy A, Sanchez A, Smith M, Thibaudon M, Travaglini A, Uruska A, Valencia-Barrera RM, Vokou D, Wachter R, de Weger LA, Menzel A (2012) Changes to airborne pollen counts across Europe. PLoS One 7:e34076
Zimmermann HG (1991) Biological control of Prosopis, Prosopis spp. (Fabaceae), in South Africa. Agric Ecosyst Environ 37:175–186
Zollner D (1986) Sand dune stabilization in central Somalia. Forest Ecol Manage 16:223–232
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hussain, M.I., Shackleton, R., El-Keblawy, A., González, L., Trigo, M.M. (2021). Impact of the Invasive Prosopis juliflora on Terrestrial Ecosystems. In: Lichtfouse, E. (eds) Sustainable Agriculture Reviews 52. Sustainable Agriculture Reviews, vol 52. Springer, Cham. https://doi.org/10.1007/978-3-030-73245-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-73245-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-73244-8
Online ISBN: 978-3-030-73245-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)