Abstract
There have been recent encouraging reports about the development of vaccines for COVID-19. Given the scale and effects of this pandemic on public health and economies worldwide, there has been an unprecedented approach across the globe, leading to the emergence of vaccine candidates many times faster than the normal process would allow. This review gives up-to-date information as of November 28, 2020, on the latest developments in this area and covers the plans to roll out the most promising vaccines across the entire world to halt the spread of this devastating virus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the third virus from the betacoronavirus genus to cause serious illness and death in humans, following the appearance of SARS-CoV in 2002 and the Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 [1]. SARS-CoV-2 shows high similarity to bat coronaviruses and SARS-CoV [2]. This is consistent with the idea that these coronaviruses may have originated from bats [3]. Furthermore, SARS-CoV-2 was initially described close to when the same virus occurred in lung samples from two dead Malayan pangolins, suggesting that these species may be a natural reservoir of the virus [4, 5].
SARS-CoV-2 causes coronavirus disease 2019 (COVID-19), which was first reported in December 2019 in Wuhan, China [6]. It is typically characterized by high fever, dry cough, difficulty in breathing, severe atypical pneumonia, and other symptoms such as gastrointestinal difficulties, as well as loss of smell and taste [7, 8]. Severe cases are often marked by a cytokine storm in blood sample analyses and the appearance of ground glass opacities with consolidation on lung computed tomography (CT) imaging [9]. As of November 28, 2020, there have been 62,618,683 confirmed COVID-19 cases and 1,458,944 deaths reported globally [10]. At the present time, the daily rate of new cases and deaths is showing no signs of decrease (Fig. 18.1).
Given the scale and effects of this pandemic on public health and economies worldwide, there has been an unprecedented approach across the globe, to develop new treatment and vaccine candidates many times faster than the normal process allows [11,12,13,14].
As the most effective method for controlling the spread of COVID-19, this brief review focuses on the latest news in vaccine development. It will describe the main strategies involved in targeting the virus as well as the different methods involved in vaccine production. Finally, it will describe the main efforts that have already gone into fast-tracking dissemination of the top approved vaccine candidates around the globe.
2 The SARS-CoV-2 Spike Protein
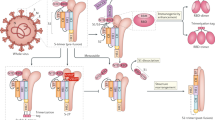
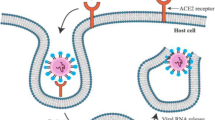
SARS-CoV-2 is a solitary strand RNA virus of approximately 30 kb and four main proteins, termed envelope, nucleocapsid, membrane, and spike. The virus gains entry into host cells through the concerted action of the transmembrane protease, serine 2 (TMPRSS2), and binding of the spike protein via the receptor binding domain to the angiotensin-converting enzyme 2 (ACE2) receptor (Fig. 18.2). This allows the virus to enter the cell by endocytosis and discharge the viral RNA into the cell cytosol. After this, the virus takes over the cellular machinery to reproduce itself and erupt from the cell via exocytosis, allowing the spread of the virus to other cells [15]. As the virus uses the spike protein for entering into host cells expressing ACE2 , most researchers all over the world are targeting this interaction in different ways in the development of potential vaccines. A schematic of the viral genome and the structure of the spike protein are shown in Fig. 18.3.
3 SARS-CoV-2 Vaccine Candidates in Phase 3 Clinical Trials
There are many vaccine candidates worldwide in the effort to control COVID-19 disease. Many of these are being rapidly progressed, considering the global emergency. As of November 28, 2020, 10 of these vaccines are now in phase 3 clinical trials and several are already showing promise. These are shown in Table 18.1.
3.1 AZD1222 (Covishield)
AstraZeneca, the University of Oxford and the Serum Institute of India are in Phase 3 with AZD1222 (Covishield), a study which is being carried out internationally, including in India and the USA [14]. The vaccine uses a weakened adenovirus that causes a cold in chimpanzees and genetically modified to express the genetic code of the SARS-CoV-2 spike protein . Once inside the body, the host cells produce the spike protein that primes an immune response against the virus (Fig. 18.4). If the vaccinated person encounters the real virus, their immune system will produce neutralizing antibodies.
The Covishield preclinical data showed a humoral and cellular immune response in all participants, and the phase 3 trial (NCT04516746) is underway with more 40,000 participants enrolled. In addition, an inhaled version is undergoing testing in 30 people. Interim analysis of the phase 3 results showed an efficacy of 70.4% across two dosing regimens for 131 of the cases. When a half dose was used for the initial injection and a full dose for the second, the effectiveness increased to 90%. However, further studies will be required, including the analysis of multiple age groups. Preliminary results from the phase 1/2 trial showed that the vaccine had an acceptable safety profile with most patients producing an antibody response after the first dose and all patients showing a response after the second [16]. The EMA Human Medicines Committee (CHMP) and Health Canada had initiated a rolling review of this vaccine candidate to minimize the amount of time for making conclusions on its safety and effectiveness, and the Australian Therapeutic Good Administration (TGA) has already taken the first step in the process for approval. In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) has also begun an accelerated review of Covishield.
Four million doses of the vaccine will be available in the UK by the end of 2020, assuming approval by the MHRA [17]. The UK government partially funded the development and, as of 2020, has preordered a total of 100 million doses to be shared between the four nations. Approximately 70 million of these will be administered by the end of March, 2021, which is hoped to be enough to vaccinate 35 million people. The remaining 30 million shots will be administered throughout 2021, which will be enough for another 15 million people. Furthermore, Astra Zeneca has estimated that it will produce 300 million doses around the world by the end of March, 2021. The Serum Institute of India has already produced 40 million doses and aims to increase this to 100 million by the end of December, and the overall aim is to produce one billion doses. Overall, the aim of Astra Zeneca and its global partners is to produce 3 billion doses by the end of 2021. Considering that this vaccine can be stored under refrigerated conditions, worldwide distribution should prove easier than other candidates below, which require more extreme freezer storage.
3.2 BNT162
Pfizer and BioNtech are currently running a phase 3 trial for BNT162, an mRNA-based vaccine [14]. This type of vaccine works by introduction of mRNA encoding the SARS-CoV-2 spike protein into a person’s body, which allows the person’s own cells to produce the spike protein that elicits an immune response (Fig. 18.5). On November 9th, they released interim results of 94 participants, which revealed that BNT162 showed greater than 90% efficacy in protecting volunteers from becoming infected by SARS-CoV-2 [18]. Their phase 1/2 data also showed robust immunogenicity of this candidate, [19, 20] and a phase 1 trial showed only a few adverse effects [21]. Pfizer and BioNTech have now received Food and Drug Administration (FDA) fast-tracking for two BNT162 candidates. BNT162b2 is now in a phase 2/3 safety study due to the robust immune response and high tolerability. The FDA is also considering expanding the Phase 3 trial to include as many as 44,000 participants, the European Medicines Agency (EMA) had already initiated a rolling review of BNT162b2, to potentially bring approval forward, and such reviews have also been submitted in Australia, Canada, Europe, Japan, and the UK. Pfizer and BioNTech plan to file for emergency use authorization so that the vaccine may begin rolling out in December. Based on current projections, Pfizer and BioNtech expect to produce 50 million doses in 2020 and up to 1.3 billion doses by the end of 2021, globally [22]. In addition, Australia has received provisional determination from the Therapeutic Goods Administration (TGA) and China is seeking approval as well via the Shanghai Fosun Pharmaceutical Group. Distribution of this vaccine might prove to be more difficult than the AstraZeneca/University of Oxford candidate since it requires freezing storage conditions.
3.3 mRNA-1273
Moderna is conducting a phase 3 study to test mRNA-1273 as a potential mRNA-based COVID-19 vaccine in the COVE trial of more than 30,000 participants at 100 clinical research sites in the USA [14]. Interim analysis of data regarding 95 participants who developed symptomatic COVID-19 disease released on November 16th showed an efficacy of 94.5% with no severe cases in the vaccinated group compared to 11 in the placebo group [23]. In addition, a phase 1 dose escalation study showed that the mRNA-1273 vaccine induced immune responses in all participants with no serious safety concerns [24]. On November 17th, Moderna Announced a supply agreement with the UK and the EMA began its rolling review process to facilitate distribution if mRNA-1273 is approved [25]. On November 25th, they announced an advanced purchase agreement with the European Commission for an initial 80 million doses of the vaccine [26]. In addition, the Medicines and Healthcare products Regulatory Agency (MHRA) initiated a rolling review to facilitate the approval process for the vaccine [27]. A similar process has begun in Switzerland via the Swissmedic regulator [28]. As above, this vaccine requires freezer storage conditions and therefore might prove difficult in worldwide distribution objectives.
3.4 Sputnik V
The Gamaleya Research Institute in Russia and Health Ministry of the Russian Federation are carrying out a phase 3 trial of 40,000 participants to evaluate a heterologous adenoviral vector-based vaccine against SARS-CoV-2, in Russia, Belarus, and the United Arab Emirates [14]. The vaccine was announced as 92% effective in an interim analysis of data from 20 participants. The Health Ministry has already approved Sputnik V although no trial data have been published as of November 27. This decision has been criticized as there are no data on safety and efficacy. However, two small phase 1/2 trials suggest that the vaccine induced a strong humoral and cellular immune response with a good safety profile [29, 30]. In light of this, a preliminary presubmission of the vaccine has been made in Brazil.
3.5 Other Candidates
Several other candidates are also in phase 3 studies, although none of these have reported efficacy data as of November 27, 2020 [14]. This includes the CanSino Biologics (China) vaccine that incorporates the adenovirus type 5 vector . They are carrying out a phase 3 study in Russia (500 participants across multiple study centers) as well as another phase 3 study including up to 40,000 participants internationally. An inactivated SARS-CoV-2 vaccine (CoronaVac) from the Chinese company Sinovac Life Sciences is being trialed in phase 3 studies in Brazil. Phase 1/2 trials of 743 volunteers showed that CoronaVac had a good safety and immunogenicity profile [31]. Sinopharm and the Wuhan Institute of Virology are carrying out a phase 3 trial in Peru, Morocco, and the United Arab Emirates using an inactivated COVID-19 vaccine candidate [14]. The vaccine has shown a good neutralizing antibody response in Phase 1/2 trials [32].
Bharat Biotech and the National Institute of Virology in India are in phase 3 studies with another inactivated vaccine called Covaxin of 26,000 participants [14]. Phase 1/2 and phase 3 trials are also underway. Johnson & Johnson is conducting a phase 3 trial with 30,000 volunteers called ENSEMBLE 2 using their recombinant spike protein JNJ-78436735 vaccine [14]. The preclinical data showed good immunogenicity and suggested protection against severe disease [33, 34]. Finally, the USA company Novavax will begin a phase 3 study of a recombinant spike protein nanoparticle vaccine candidate called NVX-CoV2373 in the UK, in up to 10,000 participants [14]. In a phase 1 study, participants who received the vaccine developed an antibody response at multiple doses with a favorable safety profile [35].
4 Conclusions and Future Perspectives
A number of vaccine candidates for SARS-CoV-2 have now shown promise in interim analyses of phase 3 clinical trials. These were produced in record time compared to the normal process of vaccine production, and procedures have already been put in place around the world to manufacture and distribute the most efficacious of these in anticipation rapid approval. This is critical as every day that passes without a vaccine for this disease results in substantial costs at both the public health and economic levels, worldwide. However, the knowledge that we have gained over the past several months about COVID-19 and the systems we have put in place to identify, manufacture, and distribute new treatments and vaccines will also provide important insights and strategic measures to successfully control future epidemics and pandemics.
References
Malik YA (2020) Properties of coronavirus and SARS-CoV-2. Malays J Pathol 42(1):3–11
Tabibzadeh A, Esghaei M, Soltani S, Yousefi P, Taherizadeh M, Safarnezhad Tameshkel F et al (2020) Evolutionary study of COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as an emerging coronavirus: phylogenetic analysis and literature review. Vet Med Sci. https://doi.org/10.1002/vms3.394. Online ahead of print
Gentles AD, Guth S, Rozins C, Brook CE (2020) A review of mechanistic models of viral dynamics in bat reservoirs for zoonotic disease. Pathog Glob Health, 1–19. https://doi.org/10.1080/20477724.2020.1833161. Online ahead of print
Liu P, Chen W, Chen JP (2019) Viral metagenomics revealed sendai virus and coronavirus infection of malayan pangolins (Manis javanica). Viruses 11(11):pii: E979. https://doi.org/10.3390/v11110979
Zhang T, Wu Q, Zhang Z (2020) Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr Biol 30(7):1346–1351.e2
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al (2020) Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med 382(18):1708–1720
Lovato A, de Filippis C (2020) Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J 99(9):569–576
Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S (2020) Smell and taste dysfunction in patients with SARS-CoV-2 infection: a review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol 38(2):69–77
Li J, Yan R, Zhai Y, Qi X, Lei J (2020) Chest CT findings in patients with coronavirus disease 2019 (COVID-19): a comprehensive review. Diagn Interv Radiol. https://doi.org/10.5152/dir.2020.20212. Online ahead of print
Chugh H, Awasthi A, Agarwal Y, Gaur RK, Dhawan G, Chandra R (2020) A comprehensive review on potential therapeutics interventions for COVID-19. Eur J Pharmacol:173741. https://doi.org/10.1016/j.ejphar.2020.173741. Online ahead of print
Tsai SC, Lu CC, Bau DT, Chiu YJ, Yen YT, Hsu YM et al (2020) Approaches towards fighting the COVID-19 pandemic (Review). Int J Mol Med. https://doi.org/10.3892/ijmm.2020.4794. Online ahead of print
COVID-19 Studies from the World Health Organization database. https://clinicaltrials.gov/ct2/who_table
COVID-19 vaccine tracker. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
Rawat K, Kumari P, Saha L (2020) COVID-19 vaccine: a recent update in pipeline vaccines, their design and development strategies. Eur J Pharmacol, 173751. https://doi.org/10.1016/j.ejphar.2020.173751. Online ahead of print
Oxford COVID Vaccine Trial Group, Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S et al (2020) Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396(10249):467–478
Coronavirus: Oxford vaccine to be rolled out within weeks, and 70 million doses by Easter. Two-dose candidate found to be up to 90 per cent effective in preventing Covid-19. https://www.independent.co.uk/news/health/vaccine-oxford-covid-latest-doses-b1760353.html
Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S et al (2020) Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586(7830):589–593
Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M et al (2020) COVID-19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature 586(7830):594–599
Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A et al (2020) Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. NEJMoa2027906. https://doi.org/10.1056/NEJMoa2027906. Online ahead of print
Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN et al (2020) An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med 383(20):1920–1931
https://ec.europa.eu/commission/presscorner/detail/en/IP_20_2200
Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, Dzharullaeva AS et al (2020) Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 396(10255):887–897
Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al (2020) Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. S1473-3099(20)30843-4. https://doi.org/10.1016/S1473-3099(20)30843-4. Online ahead of print
Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z et al (2020) Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 324(10):951–960
Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J et al (2020) Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 586(7830):583–588
Tostanoski LH, Wegmann F, Martinot AJ, Loos C, McMahan K, Mercado NB et al (2020) Ad26 vaccine protects against SARS-CoV-2 severe clinical disease in hamsters. Nat Med 26(11):1694–1700
Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S et al (2020) Phase 1-2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. NEJMoa2026920. https://doi.org/10.1056/NEJMoa2026920. Online ahead of print
Added Note
As of March 11, 2021, the integrated effort to develop vaccines for COVID-19 has progressed rapidly with the authorization and distribution of more than one dozen vaccines around the world. In fact, several countries have now vaccinated more than 25% of their populations, such as Israel, United Arab Emirates, United Kingdom, Bahrain, United States of America, Chile and Serbia. To aid this process, the WHO is leading a global alliance called COVAX, which aims to accelerate the development and manufacturing of COVID-19 vaccines. This will facilitate access to all countries, prioritizing frontline healthcare workers, older adults, and those with underlying conditions in order to protect those most at risk of serious disease. The ultimate objective is to help the entire world in ending this devastating pandemic.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guest, P.C., Ozanne, S.E. (2021). The Worldwide Effort to Develop Vaccines for COVID-19. In: Guest, P.C. (eds) Identification of Biomarkers, New Treatments, and Vaccines for COVID-19. Advances in Experimental Medicine and Biology(), vol 1327. Springer, Cham. https://doi.org/10.1007/978-3-030-71697-4_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-71697-4_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71696-7
Online ISBN: 978-3-030-71697-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)