Abstract
Schizophrenia (SZ) and bipolar disorder (BD) involve complex interactions between genetic and environmental factors, but underlying mechanisms are poorly understood. This review explains how human endogenous retroviruses (HERVs) may provide a missing link between environmental factors, genomics, and pathogenic pathways leading to clinical expression. HERVs represent a disregarded part of the human genome resulting from endogenization of retroviruses during evolution. HERVs can be activated by environmental triggers such as infectious agents. Elevated HERV-W transcriptional activity or HERV-W ENV antigenemia were reported in patients with SZ or BD. A recent study showed that HERV-W ENV affected synaptic surface dynamics of glutamatergic receptors and that psychotic behavior was induced in an animal model expressing HERV-W ENV protein in hippocampal neurons. A specific antibody targeting HERV-W ENV inhibited HERV-W ENV pathogenic effects in vitro and prevented abnormal behavioral signs in vivo. A lifelong scenario of interactions between environmental pathogens and HERV-W genes is now presented, which may decipher the actual development and course of such diseases. New therapeutic strategies targeting pathogenic and nonphysiological agonists are also suggested, which may allow treating or possibly preventing SZ or BD without impairing physiological functions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Schizophrenia
- Bipolar disorder
- Endogenous retrovirus
- HERV
- Inflammation
- Infection
- Genetics
- CNV
- Environment
- Perinatal
1 Introduction
Schizophrenia (SZ) and bipolar disorder (BD) are severe psychiatric disorders involving complex interactions between genetic and environmental factors [1,2,3,4,5].
Environmental factors, such as winter birth, urban environment, and maternal infection during pregnancy, in particular caused by Influenza virus, Herpesviruses or T. gondii, are associated with an increased risk for SZ and for BD [6,7,8,9]. Viruses or parasites have been associated with the pathogenesis of SZ or BD, but most studies were based on serology that essentially detects an immunological scar, i.e., specific immunoglobulin G antibody [10,11,12]. Nonetheless, though the period of infection can be debated, epidemiological data provide arguments for critical periods in life when they may have a significant impact, along with associated immune-mediated inflammation: (a) the perinatal period encompassing the embryonal development and the postnatal final steps of neurodevelopment and (b) the young adulthood period when infections with, e.g., Herpesviruses occur. These periods were further thought to correspond to the early acquisition of a “neurodevelopmental risk”, followed by some triggering effects of new infections and/or particular stress factors in teenagers or young adults [12,13,14,15,16,17]. As illustrated in Fig. 9.1, such a lifelong scenario may integrate perinatal “priming” event(s) and “boosting” events later in life, which could be synergistic or substitutable, with a critical period for teenagers and young adults.
Lifelong exposure to environmental factors impacting epigenetics and gene expression. Environmental triggers may influence gene expression but no direct link with disease was identified, nor directly targeted or involved genes. Epidemiological studies point to most critical periods of exposure to infectious factors, during the embryonal/postnatal period and during the teenage/young adult period. Black circles represent cumulated events having potentially impacted genomic regulation or structures. Colored stars on the drawing for DNA double helix represent potentially susceptible DNA sequences. Adapted from Rutten & Mills Sch. Bull. 2009
Genetic studies revealed a potential contribution of loci involved in the inflammatory/immune pathways, including the major histocompatibility complex region, in both SZ and BD [18,19,20,21], among other candidate genes [22, 23]. Structural genomic studies also highlighted significant modifications in psychotic patients, including copy number variations, deletions, or somatic modifications of the genomic DNA [24,25,26].
Nonetheless, the mechanisms possibly underlying interactions between genetic and environmental risk factors contributing to the clinical onset and/or to the progression of psychotic disorders remain to be understood. Moreover, the significant observations made in studies addressing different aspects from various disciplines should require a unifying, but missing, link to allow a global understanding.
Rather recently, the involvement of multicopy genetic elements of the human genome, such as Human Endogenous Retroviruses (HERVs), has been reported in SZ and BD [9, 13, 27].
2 HERVs Represent a Disregarded but Important Part of the Human Genome with Original Genetic Features
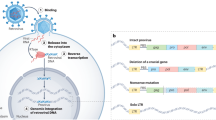
HERVs are multicopy families of polygenic structures, represent 8% of the human genome and have retained characteristics of retroviruses. They entered the genome of species from ancient germ-line infections by exogenous retroviruses that have integrated their DNA genomic copy (cDNA) into the DNA of such host cells. Integration of retroviral genome cDNA reverse-transcribed from the virion RNA is a common characteristic of retroviruses, mediated by their encoded enzymes, reverse transcriptase, and integrase. Thus, nonlethal infection and integration of a retroviral genome within a chromosomal region that may not affect embryonal and adult development following gametes fusion, can lead to a hereditary transmission to the offspring. Multiple similar events, called “endogenization”, with various retroviruses during evolution, led to multiple families of endogenous retroviruses (ERVs) within the genomes of species ending with numerous mutations, recombination events, and deletions within integrated sequences (provirus), as illustrated in Fig. 9.2.
Retroviral endogenizations and transmission to offspring. This illustration depicts the successive steps of retroviral endogenization in species, starting from infection of gametes, integration of a DNA retroviral copy (provirus) in a chromosome giving birth to a viable individual inheriting and retaining this copy in the DNA of all cells and transmitting it to its offspring. Throughout successive generations and species evolution, both endogenous retrotranspositions and re-infections of the germ line of certain individuals (as long as the exogenous strain is persisting in the environment) generate multiple and variable copy numbers in a final population. As shown in the lower panel, retroviral genomes integrated as proviruses are originally composed of two flanking long terminal regions of repeated sequences (LTR), with gag, pol, and env genes, respectively encoding viral capsid proteins, retroviral enzymes, and the envelope protein. They may undergo many somatic modifications during inheritance over generations and most elements are modified or inactivated. Nonetheless, few proviruses from various families may retain coding potential for proteins, if not for complete retroviral expression
HERVs, like other ERVs, belong to the superfamily of repeated and transposable elements (transposons, retrotransposons, and endogenous retroviruses) and altogether represent over 42% of the human genome. They were shown to have played a role in inter- and intra-species gene transmission between individuals. At the individual level, they are responsible of somatic modifications such as intracellular gene retro-transposition or recombination, and may undergo changes under selective environmental pressure or interactions with infectious pathogens.
HERVs are therefore components of the human genome that can be transmitted to subsequent generations through gametes, but have evolved differently from other host genes [28]. They have significant inter-individual copy number variations within the genome of healthy humans from different ethnic origins or simply between individuals [29,30,31,32], which would suggest inter-individual differences in potentially related genetic susceptibility.
3 HERVs in Schizophrenia and Bipolar Disorder
Interestingly, considering observed somatic rearrangements and copy number variation in psychotic patients’ DNA, HERVs may generate such genetic rearrangements linked to their properties of mobile genetic elements [33]. This could even be triggered by microbial agents activating the expression of certain HERV copies or families [34,35,36,37,38]. Interestingly, structural modifications in the major histocompatibility complex (MHC) C4 gene were associated with characteristics of HERVs [29] and numerous HERV sequences were also found in the chromosomal region corresponding to MHC class II genes [39, 40]. In addition, the role of HERV inserts in the regulation of schizophrenia-linked genes has been described [41] and genomic differences between affected and nonaffected homozygous twins identified a differentially amplified HERV copy from the twin with SZ [42].
Thus, HERVs may link many observed genetic features in psychoses such as schizophrenia, while providing an explanation for an underlying mechanism driven by these remnants of “mobile genetic elements” in the human genome, since still functionally interacting with environmental pathogens.
However, a potentially unifying role should not be limited to the genomic level, as HERV-encoded proteins with well characterized and relevant pathogenic mode of action [43] were shown to be expressed in SZ or BD [44,45,46,47,48,49].
Although most of the contemporary copies of HERVs were inactivated by mutations or deletions or silenced by epigenetic modifications, as schematized in Fig. 9.2, their plasticity and potential responsiveness to environmental triggers are of particular relevance for gene–environment interactions [50]. Under certain conditions, undisrupted HERV sequences or copies may be expressed and display viral protein properties [51,52,53].
In schizophrenia, a sequence homologous to a human endogenous retrovirus originally identified in MS and previously named “Multiple Sclerosis-associated Retroviral element” (MSRV), was identified from differential DNA amplification in homozygote twins discordant for the disease [42]. MSRV sequences later permitted to unravel a previously unknown HERV family, now named HERV-W [54,55,56]. MSRV/HERV-W proteins or elevated RNA levels were further detected in patients with schizophrenia from different world areas and in independent studies [45, 47, 49]. Significantly elevated HERV-W transcriptional activity, though with different expression patterns, was also reported in patients with SZ or BD [44].
The HERV-W family and copies corresponding to its MSRV element, have now consistently been shown to be activated by infectious agents [35, 57,58,59]. In particular, HERV-W elements have been reported to be activated by T. gondii [60] as well as by Influenza virus in human cell lines [37], which is relevant for environmental infections incriminated in elevated risk, for e.g., schizophrenia. Such pathogenic activation of HERV-W elements [45, 61,62,63] may result in the production of its envelope protein (HERV-W Env) that strongly stimulates a pro-inflammatory cascade through the TLR4 receptor pathway [64] and displays neurotoxicity [43].
At the neuronal level, altered NMDA receptor (NMDAR) signaling plays a central role in psychosis [65] and this glutaminergic system has been extensively described to be part of several key physiological processes, such as synapse maturation [61, 62], spinogenesis [63, 66], and learning/memory [67]. Moreover, the regulation of NMDA receptor signaling is highly dependent on the surface diffusion of the receptor and its dynamic anchoring within postsynaptic densities [68]. A recent study showed that the effect of HERV-W-encoded envelope protein (HERV-W ENV) on glutamatergic NMDAR signaling was not mediated through direct action on channel physiology, but on its trafficking in the plasma membrane at the level of neuronal synapses [43]. This showed a potent link between exposure to HERV-W ENV and NMDAR surface dynamics with a pattern of response that was unique to HERV-W Env compared to all other tested compounds, including LPS, another TLR4 agonist. The effect also uniquely targeted gluN2B and not gluN2A receptors. The fact that these effects were not observed under glia-free conditions also suggested the implication of glia-dependent pathways in this process. The study showed the potential impact of HERV-W env expression, beyond its pro-inflammatory effects, on neuroreceptor dysregulation as observed in psychoses such as schizophrenia and bipolar disorder. Moreover, these specific effects were reproduced using HERV-W ENV serum from patient.
Most interestingly, this study also showed that consistent psychotic behavior was induced in an animal model consisting in rats expressing HERV-W ENV protein in hippocampal neurons transfected with ENV gene expressing plasmids in vivo. In addition, a specific monoclonal antibody targeting HERV-W ENV not only inhibited HERV-W ENV pathogenic effects in vitro but also significantly prevented abnormal behavioral signs in antibody-treated rats using intraperitoneal injections in this animal model, versus mock-transfected controls. This not only confirmed the specificity of the neuropathogenic effects of HERV-W ENV and of its behavioral correlates in vivo, but importantly paves the way toward a new therapeutic avenue with antibodies neutralizing this HERV protein.
Thus, HERVs should not only provide a link with previously described genomic features of SZ or BD but also with the abnormal expression of pathogenic endogenous proteins activated by environmental factors, in particular HERV-W envelope protein with a now evidenced mode of action on the distribution of neuronal receptors at the synapse level. Therefore, the involvement of certain HERV elements appears consistent with the previously evoked missing link between known features of these psychotic diseases and it may provide a global understanding of their pathogenesis, with gene-environment interactions and resulting endogenous neuropathogenic effector molecules. A global scenario summarizing the hypothesis resulting from the present data on the role of HERV-W envelope protein is proposed in Fig. 9.3.
Human Endogenous retroviruses (HERV-W): the missing link? As previously presented, infectious agents were associated with increased risk for schizophrenia or bipolar disorder but, according to their tropism and corresponding periods of infection, they can activate HERV-W elements as already shown in vitro. They would however have a relevant impact in two different periods of life. The early perinatal infections would mostly involve influenza virus or Toxoplasma gondii, while viruses from the Herpesvirus family (e.g., Cytomegalovirus-CMV; Herpes simplex virus type 1 and type 2 -HSV-1 or 2) are commonly acquired in teenagers and young adults
4 Conclusion and Perspectives
In conclusion, a lifelong scenario of a detrimental interaction between infectious agents and HERV-W genes may decipher the actual development and course of schizophrenia or bipolar disorder. But, after the preclinical proof of concept provided with a monoclonal antibody neutralizing the pathogenic effects of HERV-W ENV in an animal model, further research and development followed by clinical studies are needed to find out if such a specific treatment strategy could neutralize the pathogenic effects of HERV-W ENV and/or reduce the expression of HERV-W, with appropriate endpoints in patients with schizophrenia or with bipolar disorder. Time may come when new therapeutic strategies targeting pathogenic and non-physiological agonists would allow treating, or possibly preventing, such psychiatric diseases without impairing physiological functions mediated by neuroreceptors and neurotransmitters.
References
Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry. 2014;5:48.
Misiak B, Stramecki F, Gaweda L, Prochwicz K, Sasiadek MM, Moustafa AA, et al. Interactions between variation in candidate genes and environmental factors in the etiology of schizophrenia and bipolar disorder: a systematic review. Mol Neurobiol. 2018;55(6):5075–100.
Mas S, Boloc D, Rodriguez N, Mezquida G, Amoretti S, Cuesta MJ, et al. Examining gene-environment interactions using aggregate scores in a first-episode psychosis cohort. Schizophr Bull. 2020;46(4):1019–25.
Kano SI, Hodgkinson CA, Jones-Brando L, Eastwood S, Ishizuka K, Niwa M, et al. Host-parasite interaction associated with major mental illness. Mol Psychiatry. 2020;25(1):194–205.
Sahu G, Malavade K, Jacob T. Cognitive impairment in schizophrenia: interplay of BDNF and childhood trauma? A review of literature. Psychiatr Q. 2016;87(3):559–69.
Hamdani N, Daban-Huard C, Godin O, Laouamri H, Jamain S, Attiba D, et al. Effects of cumulative herpesviridae and toxoplasma gondii Infections on cognitive function in healthy, bipolar, and schizophrenia subjects. J Clin Psychiatry. 2017;78(1):e18–27.
Burgdorf KS, Trabjerg BB, Pedersen MG, Nissen J, Banasik K, Pedersen OB, et al. Large-scale study of toxoplasma and cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019;79:152–8.
Parboosing R, Bao Y, Shen L, Schaefer CA, Brown AS. Gestational influenza and bipolar disorder in adult offspring. JAMA Psychiatry. 2013;70(7):677–85.
Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, et al. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res. 2012;136(1–3):128–36.
de Witte LD, van Mierlo HC, Litjens M, Klein HC, Bahn S, Osterhaus AD, et al. The association between antibodies to neurotropic pathogens and schizophrenia: a case-control study. NPJ Schizophr. 2015;1:15041.
Tanaka T, Matsuda T, Hayes LN, Yang S, Rodriguez K, Severance EG, et al. Infection and inflammation in schizophrenia and bipolar disorder. Neurosci Res. 2017;115:59–63.
Dickerson F, Jones-Brando L, Ford G, Genovese G, Stallings C, Origoni A, et al. Schizophrenia is associated with an aberrant immune response to epstein-barr virus. Schizophr Bull. 2019;45(5):1112–9.
Leboyer M, Tamouza R, Charron D, Faucard R, Perron H. Human endogenous retrovirus type W (HERV-W) in schizophrenia: a new avenue of research at the gene-environment interface. World J Biol Psychiatry. 2013;14(2):80–90.
Allswede DM, Yolken RH, Buka SL, Cannon TD. Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study. Lancet Psychiatry. 2020;7(3):254–61.
Thomas P, Bhatia T, Gauba D, Wood J, Long C, Prasad K, et al. Exposure to herpes simplex virus, type 1 and reduced cognitive function. J Psychiatr Res. 2013;47(11):1680–5.
Fruchter E, Goldberg S, Fenchel D, Grotto I, Ginat K, Weiser M. The impact of Herpes simplex virus type 1 on cognitive impairments in young, healthy individuals—a historical prospective study. Schizophr Res. 2015;168(1-2):292–6.
Hjorthoj C, Starzer MSK, Benros ME, Nordentoft M. Infections as a risk factor for and prognostic factor after substance-induced psychoses. Am J Psychiatry. 2020;177(4):335–41.
Nudel R, Benros ME, Krebs MD, Allesoe RL, Lemvigh CK, Bybjerg-Grauholm J, et al. Immunity and mental illness: findings from a Danish population-based immunogenetic study of seven psychiatric and neurodevelopmental disorders. Eur J Hum Genet. 2019;27(9):1445–55.
International Consortium on Lithium G, Amare AT, Schubert KO, Hou L, Clark SR, Papiol S, et al. Association of polygenic score for schizophrenia and HLA antigen and inflammation genes with response to lithium in bipolar affective disorder: a genome-wide association study. JAMA Psychiatry. 2018;75(1):65–74.
Calabro M, Drago A, Sidoti A, Serretti A, Crisafulli C. Genes involved in pruning and inflammation are enriched in a large mega-sample of patients affected by Schizophrenia and Bipolar Disorder and controls. Psychiatry Res. 2015;228(3):945–9.
Avramopoulos D, Pearce BD, McGrath J, Wolyniec P, Wang R, Eckart N, et al. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS One. 2015;10(3):e0116696.
Forstner AJ, Fischer SB, Schenk LM, Strohmaier J, Maaser-Hecker A, Reinbold CS, et al. Whole-exome sequencing of 81 individuals from 27 multiply affected bipolar disorder families. Transl Psychiatry. 2020;10(1):57.
Nedic Erjavec G, Svob Strac D, Tudor L, Konjevod M, Sagud M, Pivac N. Genetic markers in psychiatry. Adv Exp Med Biol. 2019;1192:53–93.
Nishioka M, Bundo M, Iwamoto K, Kato T. Somatic mutations in the human brain: implications for psychiatric research. Mol Psychiatry. 2019;24(6):839–56.
Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–41.
Green EK, Rees E, Walters JT, Smith KG, Forty L, Grozeva D, et al. Copy number variation in bipolar disorder. Mol Psychiatry. 2016;21(1):89–93.
Balestrieri E, Matteucci C, Cipriani C, Grelli S, Ricceri L, Calamandrei G, et al. Endogenous retroviruses activity as a molecular signature of neurodevelopmental disorders. Int J Mol Sci. 2019;20:23.
Feschotte C, Gilbert C. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet. 2012;13(4):283–96.
Pani MA, Wood JP, Bieda K, Toenjes RR, Usadel KH, Badenhoop K. The variable endogenous retroviral insertion in the human complement C4 gene: a transmission study in type I diabetes mellitus. Hum Immunol. 2002;63(6):481–4.
Wildschutte JH, Williams ZH, Montesion M, Subramanian RP, Kidd JM, Coffin JM. Discovery of unfixed endogenous retrovirus insertions in diverse human populations. Proc Natl Acad Sci U S A. 2016;113(16):E2326–34.
Thomas J, Perron H, Feschotte C. Variation in proviral content among human genomes mediated by LTR recombination. Mob DNA. 2018;9:36.
Shin W, Lee J, Son SY, Ahn K, Kim HS, Han K. Human-specific HERV-K insertion causes genomic variations in the human genome. PLoS One. 2013;8(4):e60605.
Weckselblatt B, Rudd MK. Human structural variation: mechanisms of chromosome rearrangements. Trends Genet. 2015;31(10):587–99.
Sutkowski N, Chen G, Calderon G, Huber BT. Epstein-Barr virus latent membrane protein LMP-2A is sufficient for transactivation of the human endogenous retrovirus HERV-K18 superantigen. J Virol. 2004;78(14):7852–60.
Ruprecht K, Obojes K, Wengel V, Gronen F, Kim KS, Perron H, et al. Regulation of human endogenous retrovirus W protein expression by herpes simplex virus type 1: implications for multiple sclerosis. J Neuro-Oncol. 2006;12(1):65–71.
Nellaker C, Yao Y, Jones-Brando L, Mallet F, Yolken RH, Karlsson H. Transactivation of elements in the human endogenous retrovirus W family by viral infection. Retrovirology. 2006;3:44.
Li F, Nellaker C, Sabunciyan S, Yolken RH, Jones-Brando L, Johansson AS, et al. Transcriptional derepression of the ERVWE1 locus following influenza A virus infection. J Virol. 2014;88(8):4328–37.
Charvet B, Reynaud JM, Gourru-Lesimple G, Perron H, Marche PN, Horvat B. Induction of proinflammatory multiple sclerosis-associated retrovirus envelope protein by human herpesvirus-6A and CD46 receptor engagement. Front Immunol. 2018;9:2803.
Kulski JK, Gaudieri S, Inoko H, Dawkins RL. Comparison between two human endogenous retrovirus (HERV)-rich regions within the major histocompatibility complex. J Mol Evol. 1999;48(6):675–83.
Andersson G, Svensson AC, Setterblad N, Rask L. Retroelements in the human MHC class II region. Trends Genet. 1998;14(3):109–14.
Suntsova M, Gogvadze EV, Salozhin S, Gaifullin N, Eroshkin F, Dmitriev SE, et al. Human-specific endogenous retroviral insert serves as an enhancer for the schizophrenia-linked gene PRODH. Proc Natl Acad Sci U S A. 2013;110(48):19472–7.
Deb-Rinker P, Klempan TA, O’Reilly RL, Torrey EF, Singh SM. Molecular characterization of a MSRV-like sequence identified by RDA from monozygotic twin pairs discordant for schizophrenia. Genomics. 1999;61(2):133–44.
Johansson EM, Bouchet D, Tamouza R, Ellul P, Morr A, Avignone E, et al. Human endogenous retroviral protein triggers deficit in glutamate synapse maturation and behaviors associated with psychosis. Sci Adv. 2020;6(29):eabc0708.
Perron H, Hamdani N, Faucard R, Lajnef M, Jamain S, Daban-Huard C, et al. Molecular characteristics of human endogenous retrovirus type-W in schizophrenia and bipolar disorder. Transl Psychiatry. 2012;2:e201.
Perron H, Mekaoui L, Bernard C, Veas F, Stefas I, Leboyer M. Endogenous retrovirus type W GAG and envelope protein antigenemia in serum of schizophrenic patients. Biol Psychiatry. 2008;64(12):1019–23.
Huang W, Li S, Hu Y, Yu H, Luo F, Zhang Q, et al. Implication of the env gene of the human endogenous retrovirus W family in the expression of BDNF and DRD3 and development of recent-onset schizophrenia. Schizophr Bull. 2010;37(5):988–1000.
Huang WJ, Liu ZC, Wei W, Wang GH, Wu JG, Zhu F. Human endogenous retroviral pol RNA and protein detected and identified in the blood of individuals with schizophrenia. Schizophr Res. 2006;83(2–3):193–9.
Karlsson H, Schroder J, Bachmann S, Bottmer C, Yolken RH. HERV-W-related RNA detected in plasma from individuals with recent-onset schizophrenia or schizoaffective disorder. Mol Psychiatry. 2004;9(1):12–3.
Karlsson H, Bachmann S, Schroder J, McArthur J, Torrey EF, Yolken RH. Retroviral RNA identified in the cerebrospinal fluids and brains of individuals with schizophrenia. Proc Natl Acad Sci U S A. 2001;98(8):4634–9.
Perron H, Lang A. The human endogenous retrovirus link between genes and environment in multiple sclerosis and in multifactorial diseases associating neuroinflammation. Clin Rev Allergy Immunol. 2010;39(1):51–61.
Duperray A, Barbe D, Raguenez G, Weksler BB, Romero IA, Couraud PO, et al. Inflammatory response of endothelial cells to a human endogenous retrovirus associated with multiple sclerosis is mediated by TLR4. Int Immunol. 2015;27(11):545–53.
Rolland A, Jouvin-Marche E, Saresella M, Ferrante P, Cavaretta R, Creange A, et al. Correlation between disease severity and in vitro cytokine production mediated by MSRV (multiple sclerosis associated retroviral element) envelope protein in patients with multiple sclerosis. J Neuroimmunol. 2005;160(1-2):195–203.
Perron H, Jouvin-Marche E, Michel M, Ounanian-Paraz A, Camelo S, Dumon A, et al. Multiple sclerosis retrovirus particles and recombinant envelope trigger an abnormal immune response in vitro, by inducing polyclonal Vbeta16 T-lymphocyte activation. Virology. 2001;287(2):321–32.
Perron H, Garson JA, Bedin F, Beseme F, Paranhos-Baccala G, Komurian-Pradel F, et al. Molecular identification of a novel retrovirus repeatedly isolated from patients with multiple sclerosis. The Collaborative Research Group on Multiple Sclerosis. Proc Natl Acad Sci U S A. 1997;94(14):7583–8.
Blond JL, Beseme F, Duret L, Bouton O, Bedin F, Perron H, et al. Molecular characterization and placental expression of HERV-W, a new human endogenous retrovirus family. J Virol. 1999;73(2):1175–85.
Perron H, Perin JP, Rieger F, Alliel PM. Particle-associated retroviral RNA and tandem RGH/HERV-W copies on human chromosome 7q: possible components of a ‘chain-reaction’ triggered by infectious agents in multiple sclerosis? J Neuro-Oncol. 2000;6(Suppl 2):S67–75.
Perron H, Bernard C, Bertrand JB, Lang AB, Popa I, Sanhadji K, et al. Endogenous retroviral genes, herpesviruses and gender in multiple sclerosis. J Neurol Sci. 2009;286(1–2):65–72.
Lafon M, Jouvin-Marche E, Marche PN, Perron H. Human viral superantigens: to be or not to be transactivated? Trends Immunol. 2002;23(5):238–9; author reply 9.
Perron H, Suh M, Lalande B, Gratacap B, Laurent A, Stoebner P, et al. Herpes simplex virus ICP0 and ICP4 immediate early proteins strongly enhance expression of a retrovirus harboured by a leptomeningeal cell line from a patient with multiple sclerosis. J Gen Virol. 1993;74(Pt 1):65–72.
Frank O, Jones-Brando L, Leib-Mosch C, Yolken R, Seifarth W. Altered transcriptional activity of human endogenous retroviruses in neuroepithelial cells after infection with Toxoplasma gondii. J Infect Dis. 2006;194(10):1447–9.
Kelsch W, Li Z, Wieland S, Senkov O, Herb A, Gongrich C, et al. GluN2B-containing NMDA receptors promote glutamate synapse development in hippocampal interneurons. J Neurosci. 2014;34(48):16022–30.
Frank RA, Komiyama NH, Ryan TJ, Zhu F, O’Dell TJ, Grant SG. NMDA receptors are selectively partitioned into complexes and supercomplexes during synapse maturation. Nat Commun. 2016;7:11264.
Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, et al. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73(3):511–22.
Rolland A, Jouvin-Marche E, Viret C, Faure M, Perron H, Marche PN. The envelope protein of a human endogenous retrovirus-W family activates innate immunity through CD14/TLR4 and promotes Th1-like responses. J Immunol. 2006;176(12):7636–44.
Tamminga CA, Zukin RS. Schizophrenia: evidence implicating hippocampal GluN2B protein and REST epigenetics in psychosis pathophysiology. Neuroscience. 2015;309:233–42.
Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400.
Baez MV, Cercato MC, Jerusalinsky DA. NMDA receptor subunits change after synaptic plasticity induction and learning and memory acquisition. Neural Plast. 2018;2018:5093048.
Li B, Otsu Y, Murphy TH, Raymond LA. Developmental decrease in NMDA receptor desensitization associated with shift to synapse and interaction with postsynaptic density-95. J Neurosci. 2003;23(35):11244–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Perron, H., Leboyer, M. (2021). Human Endogenous Retrovirus as Missing Link in the Global Etiopathogenesis of Schizophrenia and Bipolar Disorder. In: Berk, M., Leboyer, M., Sommer, I.E. (eds) Immuno-Psychiatry. Springer, Cham. https://doi.org/10.1007/978-3-030-71229-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-71229-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-71228-0
Online ISBN: 978-3-030-71229-7
eBook Packages: MedicineMedicine (R0)