Abstract
This work consists of a literature review that aims to evaluate the effects and efficacy of two drugs used to treat individuals with COVID-19: chloroquine (CQ) and hydroxychloroquine (HCQ). Both drugs have demonstrated antiviral activity against the new coronavirus in in vitro tests. However, there is currently no strong evidence from well-designed controlled studies of COVID-19 therapies tested in humans. In addition, the evidence on the effects of drugs on patients is extremely limited. The search for evidence was made from five chosen databases, and after applying the criteria for inclusion and exclusion of articles, 5 publications were obtained, which represented the basis for the construction of this work. We concluded that, in relation to CQ, no favorable outcomes were found in relation to its use. High doses of this drug can increase lethality due to the prolongation of the QT interval, and lower doses could not estimate evident benefits in infected patients. Assessing the results of HCQ, it can be concluded that more studies are needed to effectively use this drug in the treatment of individuals with COVID-19.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In December 2019, a new pneumonia caused by a previously unknown pathogen emerged in Wuhan, the capital of Hubei province in China [1]. The causative agent, identified from throat swab samples collected from individuals linked to the city's seafood market, was named Coronavirus 2 of the Severe Acute Respiratory Syndrome (SARS-CoV-2) [1, 2].
The new coronavirus has a high spreading power, as by the end of January 2020 the virus had already spread to 18 other countries. Then, the World Health Organization (WHO) declared the disease pandemic, called Coronavirus Disease 2019 (COVID-19), which became a global threat to public health [3].
The most prevalent manifestations among patients with COVID-19, according to research published in the PubMed database, are: fever (67–89%), cough (43–81%), dyspnea (31–55%) and myalgia (3–44%). In addition, 20.3% of patients with COVID-19 require admission to intensive care units and 13.9% of hospitalized patients had fatal results [4].
According to a study by the Center for Diseases Control and Prevention in the United States, based on data from China, mortality is higher in older adults, particularly those with serious underlying health conditions. Approximately 80% of deaths in China occurred among adults aged ≥60 years, and only one (0.1%) death occurred in a person aged ≤19 years [3].
Unfortunately, there is no standard treatment against COVID-19 today. However, a series of clinical trials are being conducted to investigate potentially effective interventions to combat SARS-CoV-2. For this, some drugs are being evaluated, among them, chloroquine (CQ) and hydroxychloroquine (HCQ) [5].
CQ, also known as 4-aminoquinoline, has been in clinical use since 1944. HCQ is a derivative of QC, and was synthesized in 1946 with the addition of a hydroxyl group to CQ as shown in Fig. 1. These drugs have been widely used in the treatment of malaria and also in the treatment of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, among others, due to their immunomodulatory role [6].
In vitro studies have shown that both substances have an anti SARS-CoV-2 effect. CQ and HCQ are able to block viral infection by increasing the endosomal pH needed for viral/cell fusion, and also interfere with the glycosylation of cell receptors of the new virus. In addition, these drugs are widely distributed throughout the body after oral administration, including the lungs [7].
Although both drugs have shown antiviral activity against the new coronavirus, HCQ appears to have a more satisfactory action. Its clinical safety profile is better than that of CQ during prolonged use and allows higher daily doses, presenting a lower risk of drug interactions [6, 8].
Despite the large number of articles published on this issue in the last 3 months, some data are available only in open observational studies, case reports and case series. All drugs are currently used based on their in vitro activities or previous clinical experience [9].
Many clinical trials in humans are ongoing, but their results are not yet available [9]. Thus, there is currently no strong evidence from well-designed controlled studies in humans on therapies for COVID-19. In addition, the evidence on the effects of drugs on patients is extremely limited [5].
Therefore, given the current scenario, the objective of this work is to systematically review studies published in the literature that conducted clinical trials on individuals with COVID-19 to evaluate the effects and efficacy of chloroquine or hydroxychloroquine.
2 Materials and Methods
The first step in all scientific work that consists of a systematic review is the elaboration of a question that is sufficiently clear, objective and directed to the theme of the research to be developed. Thus, its elaboration is allowed using a tool designated by the acronym PICO. In this tool, the letters correspond to: “P” for population, “I” intervention, “C” comparison, and “O” outcome.
Thus, the question defined was the following: “In patients diagnosed with COVID-19 who received drugs to treat the disease, does chloroquine, compared to hydroxychloroquine, result in better clinical outcomes?”.
From that, the descriptors for the search of scientific articles were defined, which were: chloroquine, hydroxychloroquine, COVID-19, clinical trial and patients. Then, the search for scientific articles was performed in the following databases: COCHRANE, MEDLINE, IEEE XPLORE, PORTAL CAPES and BIREME.
The searches strings were made with the association of the keywords terms described. In each database, the strings were: (“chloroquine” AND “hydroxychloroquine” AND “COVID-19” AND “clinical trial”) or (“chloroquine” AND “hydroxychloroquine” AND “COVID-19” AND “patients”) or (“chloroquine” AND “hydroxychloroquine” AND “COVID-19” AND “clinical trial” AND “patients”). All articles found in each search were added to a spreadsheet, this being the inclusion criterion adopted. All descriptors were searched in Portuguese, English and Spanish.
The searches for scientific works were carried out between March and Mai 2020. No specific time interval was defined for the publication period to have access to a larger set of results.
Then, the article exclusion process started, adopting three criteria:
-
Repetition of articles;
-
Failure to adapt to the proposed theme;
-
Articles that did not contribute significantly to the research, or with incomplete texts.
A tool called PRISMA was used to report this items, by the identification, selection, eligibility, and inclusion of articles. It features preferred reporting items for systematic reviews and meta-analysis.
In addition, it is also important to consider in a systematic literature review the quality of selected studies. This measure was obtained following some criteria [10], defined by the evaluation of the following questions according to the score of 1 point for YES; 0.5 point for PARTIALLY; and 0 for NOT.
A—Is the article based on research, or is it only based on expert opinion?
B—Is there a clear statement of the research objectives?
C—Was the methodology used adequate to meet the objectives of the survey?
D—Was the participant recruitment strategy adequate to meet the research objectives?
E—Was there a control group with which the results could be compared?
F—Was the data analysis rigorous enough?
G—Was there a clear statement of results?
3 Results
This section will be divided into three subsections: the first, presents the processes and quantity of articles in each stage after applying the exclusion criteria; the second subsection describes the results found in the studies; and finally, the third subsection presents the qualitative evaluation table, based on the criteria described above.
3.1 Selection Process of Studies Included
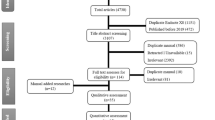
Initially, the search presented 402 works. After excluding those that were repeated, 381 remained, and these, 342 were selected primarily. Then, with the removal of those that did not fit the theme, the number of studies reduced to 118. Then, 113 with full text were excluded for not evaluating the drugs chloroquine and hydroxychloroquine for through clinical trials in humans. Thus, 5 articles constituted this work. The step-by-step of deleting studies in each stage is represented by Fig. 2.
3.2 Description of Study Results
Will be presented below: 1 study that evaluated the efficacy of two different dosages of CQ, and 4 studies that evaluated the efficacy of HCQ, all characterized by clinical trials in humans that presented COVID-19. It is worth mentioning that the conventional treatment applied to patients in the control group of some studies basically refers to the use of oxygen therapy, antivirals, antibacterials and immunoglobulin.
Borba et al. [11] conducted a randomized, double-blind clinical trial, with the objective of comprehensively evaluating the safety and efficacy of two different dosages of CQ as adjunctive therapy for hospitalized patients with severe COVID-19 in Manaus, Brazil. The 81 eligible participants were allocated to receive in the proportion of 1:1 by nasogastric tube high oral dose of CQ (600 mg of CQ twice daily for 10 days or total dose of 12 g); or low dose of CQ (450 mg for 5 days, twice a day only on the first day or total dose of 2.7 g). It was observed that the high dose of CQ generated a prolongation of the QT interval, observed on the electrocardiogram, of approximately 500 ms greater (25%) than the lowest dose. This prolongation means that the heart muscle takes longer than normal to recharge between beats, which can trigger tachycardias. In addition, the highest dose also tended to be more lethal (17%) than the lowest dose. The mortality rate was 13.5% (95% Confidence Interval [CI] 6.9–23.0), overlapping the CI of historical data from similar patients who did not use CQ (95% CI 14.5–19.2). In 14 patients with paired samples, respiratory secretion on day 4 was negative in only one patient. Thus, the authors concluded that: the highest dose of CQ (total dose of 12 g in 10 days) should not be recommended due to safety concerns regarding the prolongation of the QT interval and increased lethality in the population; and among patients randomized to the lowest dose group (5 days of treatment, 2.7 g total dose), given the limited number of enrolled patients, it was not possible to estimate a clear benefit of CQ in patients with severe COVID-19.
One of the first published studies that evaluated the use of HCQ in humans was developed in France by Gautret and collaborators [12]. The authors analyzed the role of HCQ in respiratory viral loads, including 20 patients in the experimental group and 16 in the control group. The protocol proposed by this study considered the administration of 600 mg of HCQ per day, divided into 3 doses, in the medication treatment group; in the control group, patients received conventional therapy. Depending on the patient's clinical presentation, azithromycin was added to the treatment. The outcome considered was the absence of viruses on day 6 after the inclusion of the drugs. The study showed a significant reduction in the viral transport of COVID-19 in patients in the experimental group compared to the control group, and revealed that azithromycin added to HCQ was significantly associated with elimination of the virus. However, it is important to note that this study has serious methodological complications such as bias due to non-randomization of patients, small sample size and short follow-up.
In another study, described by Chen et al. [13], the efficacy and safety of HCQ in treating patients with COVID-19 were evaluated. Thirty patients participated in this study, randomized 1:1 to the experimental group and the control group. The patients in the experimental group received 400 mg of HCQ per day for five days, plus conventional treatments. The patients in the control group received only conventional treatments. The negative conversion rate of SARS-CoV-2 nucleic acid in the patients’ pharyngeal respiratory swab after drug administration was considered the primary outcome. The nucleic acid of the swabs was negative in 13 (86.7%) cases in the experimental group and in 14 (93.3%) cases in the control group (p > 0.05). Radiological progression was demonstrated in computed tomography images in five cases (33.3%) in the experimental group and seven cases (46.7%) in the control group, and all patients showed improvement in the follow-up exams. Four cases (26.7%) in the experimental group and three cases (20%) in the control group had transient diarrhea and abnormal liver function (p > 0.05). Based on the unfavorable results regarding the use of HCQ, the authors concluded that studies with a larger sample size are needed to investigate the effects of the drug in the treatment of COVID-19.
The work of Chen and collaborators [14], also aimed to assess the efficacy of HCQ in the treatment of patients with COVID-19. Thus, 62 patients diagnosed with the disease participated in the study, all of which were randomized 1:1 in two groups: the experimental group, in which the patients received, in addition to conventional therapy, a treatment of 5 days with HCQ (400 mg per day); and the control group, which received only conventional therapy. The time to clinical recovery, clinical characteristics and radiological results were considered to assess the effect of HCQ. The recovery time of body temperature and the time of remission of cough were significantly reduced in the HCQ treatment group. In addition, a greater proportion of patients with improved pneumonia in the experimental group (80.6%, 25 out of 31) compared to the control group (54.8%, 17 out of 31) was found. Therefore, the authors concluded that there was an improvement in the outcomes evaluated in a favorable way to the use of HCQ.
Finally, Mahevas et al. [15] used data collected from routine care of all adults with documented SARS-CoV-2 pneumonia who required oxygen ≥2 L/min in 4 French hospitals to assess the efficacy of HCQ. The primary outcome was the transfer of the patient to the intensive care unit (ICU) within 7 days after the inclusion of the medication and / or death from any cause. This study included 181 patients, 84 of whom received 600 mg of HCQ per day and 97 did not receive the drug, only standard supportive therapy. The initial severity was well balanced between the groups. It was observed that 20.2% of the patients in the experimental group were transferred to the ICU or died within 7 days versus 22.1% in the control group (16 vs. 21 events, relative risk [RR] 0.91, 95% CI 0, 47–1.80). In the experimental group 2.8% of the patients died within 7 days versus 4.6% in the control group (3 vs. 4 events, RR 0.61, 95% CI 0.13–2.89). In addition, 27.4% and 24.1%, respectively, developed the acute respiratory distress syndrome within 7 days (24 vs. 23 events, RR 1.14, 95% CI 0.65–2.00). Eight patients who received HCQ (9.5%) suffered changes in the electrocardiogram that required discontinuation of drug treatment. The authors concluded that the results do not support the use of HCQ in patients hospitalized with COVID-19 who require oxygen.
3.3 Qualitative Evaluation
Regarding the qualitative analysis of studies, Table 1 presents the scores for each study for each item evaluated, as well as the total scores for studies and items. The “Total” column refers to the sum of scores of evaluated items in each study, and the “Total” row refers to the sum of scores of all studies for each evaluated item.
4 Discussion
From the analysis of works included in this review, it is possible to observe that, in relation to CQ, no favorable outcomes were found in relation to its use. High doses of this drug may increase lethality due to the prolongation of the QT interval, and lower doses have not been able to estimate evident benefits in infected patients. Assessing the results of HCQ, it can be concluded that further studies are needed to effectively use this drug in the treatment of individuals with COVID-19.
Several studies have stated that the low number of participants and the relatively short time of monitoring the effects of the drugs have compromised the quality of the studies.
Although many studies presented a control group to compare the results with an experimental group, the recruitment strategy of these patients was inadequate, as shown in Table 1 (the item with the lowest score among the others evaluated was D, with only 1 point). Moreover, it is also important to note that, in most studies, the participants were not randomly allocated to groups, which may contribute to the lack of experimental methodological rigor in the developed research. All of these aspects contribute to the fact that the study does not provide a clear statement of the results obtained.
5 Conclusion
The objective of this work was to evaluate the efficacy of two drugs used in the treatment of patients diagnosed with COVID-19: chloroquine (CQ) and hydroxychloroquine (HCQ).
We concluded that, in general, neither CQ nor HCQ presented favorable clinical outcomes in relation to incorporating these drugs in the treatment of COVID-19.
In addition, it can be observed that the studies included in this review presented two main deficiencies: a failed recruitment strategy of individuals to perform the tests and a lack of experimental methodological rigor.
References
Xu Z, Shi L, Wang Y et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 4:420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Sohrabi C, Alsafi Z, O’Neill N et al (2020) World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg 76:71–76. https://doi.org/10.1016/j.ijsu.2020.02.034
COVID, CDC; TEAM, Response (2020) Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020 MMWR Morb Mortal Wkly Rep 12:343–346 https://doi.org/10.15585/mmwr.mm6912e2
Rodriguez-Morales A, Cardona-Ospina J, Gutiérrez-Ocampo G et al (2020) Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis Travel Med Inf Dis 101623:45–50. https://doi.org/10.1016/j.tmaid.2020.101623
Jean S, Hsueh P (2020) Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther (in press). https://doi.org/10.1080/14787210.2020.1771181
Kapoor K, Kapoor A (2020) Role of Chloroquine and Hydroxychloroquine in the treatment of COVID-19 infection-a systematic literature review Medrxiv 1–17 https://doi.org/10.1101/2020.03.24.20042366
Wang M, Cao R, Zhang L et al (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 3:269–271. https://doi.org/10.1038/s41422-020-0282-0
Yao X, Ye F, Zhang M et al (2020) In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 10–282 https://doi.org/10.1093/cid/ciaa237
Esposito S, Noviello S, Pagliano P (2020) Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med 2:198–211
Dybå T, Dingsøyr T (2008) Empirical studies of agile software development: a systematic review. Inf Softw Technol 50:833–859. https://doi.org/10.1016/j.infsof.2008.01.006
Borba M, Val F, Sampaio V et al (2020) Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, phase IIb clinical trial (CloroCovid-19 study). MedRxiv. https://doi.org/10.1101/2020.04.07.20056424
Gautret P, Lagier J, Parola P et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 105949 https://doi.org/10.1016/j.ijantimicag.2020.105949
Chen J, Liu D, Liu L et al (2020) A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 2:215–219
Chen Z, Hu J, Zhang Z et al (2020) Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. MedRxiv. https://doi.org/10.1101/2020.03.22.20040758
Mahévas M, Tran V, Roumier M et al (2020) Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. https://doi.org/10.1136/bmj.m1844
Acknowledgements
To the research support agencies: FAPEMIG, CAPES and CNPq. A. A. Pereira is Fellow of CNPq, Brazil (310911/2017-6).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Mendes, L.C., Ávila, J., Pereira, A.A. (2022). Evaluation of the Efficacy of Chloroquine and Hydroxychloroquine in the Treatment of Individuals with COVID-19: A Systematic Review. In: Bastos-Filho, T.F., de Oliveira Caldeira, E.M., Frizera-Neto, A. (eds) XXVII Brazilian Congress on Biomedical Engineering. CBEB 2020. IFMBE Proceedings, vol 83. Springer, Cham. https://doi.org/10.1007/978-3-030-70601-2_308
Download citation
DOI: https://doi.org/10.1007/978-3-030-70601-2_308
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-70600-5
Online ISBN: 978-3-030-70601-2
eBook Packages: EngineeringEngineering (R0)