Abstract
Early breast cancer detection decreases mortality. As a result, it is imperative that primary care physicians are able to identify those individuals who are at an increased risk for breast cancer and understand how to help the patients manage that risk. Hereditary cancer syndromes in particular are dangerous and under-recognized. Various risk assessment tools are available for patients who are not at hereditary risk to help determine who may be eligible for enhanced screening or those who may benefit from preventive medication, and many imaging centers have now incorporated a form of risk assessment with associated recommendations into their reporting structure. Some patients even wish to consider risk-reducing surgery, which requires special counseling and support. All women should be risk stratified as early as possible so that those at a high risk can benefit from risk-stratified care. The management of patients at increased risk for the development of breast cancer has become increasingly challenging and nuanced with the exploding field of germline genetic testing and its implications for personalized care, and an increasing number of available options for high-quality supplemental breast imaging, particularly for the patient with dense breast tissue. This chapter aims to outline the identification and management of the high-risk patient, focusing on salient genetic and nongenetic risk factors, available options for risk management, and recommendations for risk modification for all women.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Breast cancer is now the leading cause of cancer in women worldwide and has surpassed lung cancer in mortality [1]. It is a profound public health issue. In the United States in 2019, 268,600 cases of invasive breast cancer were diagnosed and 41,760 women died from the disease [2]. Rates are expected to increase over the upcoming decades, due not only to a combination of aging and improved detection but to an increase in sedentary lifestyle patterns and poor diet. There are several approaches available to increase the chances of diagnosing breast cancer at an early, curable stage, or reducing the chance of getting breast cancer at all. Some examples include enhanced surveillance with periodic enhanced breast magnetic resonance imaging (MRI), preventive medication, or even risk-reducing surgery or mastectomy (RRM). These interventions cannot be employed widely, as each has a cost of its own – financial, physical, or psychological (or all three). The risks and benefits of risk management must be considered on an individual basis, and this requires knowledge of the assessment of risk and the proposed risks and benefits of the interventions offered. Being at “high risk” for breast cancer can be defined in different ways. Patients at the highest risk have germline genetic variants conferring a fivefold or greater increased risk for the development of breast cancer; patients are also considered high risk with benign atypical lesions, a history of therapeutic chest irradiation, moderate risk germline genetic variants conferring a two- to fivefold risk for the development of breast cancer, extreme breast density, an estimated lifetime risk of breast cancer of 20% or greater (based on models using family history), or a personal or family history of breast cancer. The remainder of women are considered to be at “average” risk as currently we do not have tools to accurately identify low risk women. All women need to remain vigilant about screening as the majority of breast cancers occur sporadically. Most breast cancers are not attributable to risk factors other than female gender and increasing age. There are, however, risk factors that allow certain women to be more proactive.
Identification of the High-Risk Patient
Traditional risk factors for the development of breast cancer include family history, early menarche, late menopause, nulliparity or late age at first birth (over the age of 30), benign atypical breast lesions such as atypical ductal or lobular hyperplasia or lobular carcinoma in situ, or prior therapeutic chest irradiation, particularly under the age of 30. More recently, alcohol consumption, obesity, and combined hormone replacement therapy have been the focus of increased attention and in the mid 1990s, BRCA1 and BRCA2 were identified in causal association with the study of very-high-risk families. Over time, many other genes have been identified that are linked with breast cancer, and in fact, ~12% of breast cancers are associated with germline mutations [3, 4] and up to 25% of ovarian cancers [5]. In late 2013, multigene cancer panels were introduced clinically and now represent the vast majority of tests ordered for identification of those at hereditary risk, making testing more efficient, available, and affordable. Breast density is emerging as another important risk factor currently being incorporated into risk modeling, and the cumulative effects of common allelic variants will play an increasingly important role in risk stratification going forward, both for average and for high-risk women. Of these risk factors, pathogenic germline genetic variants confer the highest levels of risk, with benign atypical lesions, extreme breast density, and prior thoracic irradiation being also important points of focus.
Risk assessment and identification of women at high risk allow for referral to healthcare providers with expertise in cancer genetics counseling and testing for breast cancer-related germline mutations (e.g., BRCA), patient counseling about risk-reduction options, and cascade testing to identify family members who may be at increased risk. It will also identify those at increased risk for those at increased risk for other than genetic predisposition and will encourage conversation around modifiable risk factors for all, which we will also discuss.
When identifying patients at risk for hereditary cancer, families will often exhibit many more cancers than would be expected, cancers occur at earlier ages than would be expected, and rare cancers such as pancreatic cancer and ovarian cancer are seen more frequently (See Table 4.1).
Hereditary Cancer
Review
Hereditary breast and ovarian cancer syndrome (HBOC) is due to either a BRCA 1 or 2 gene mutation on chromosomes 17q and 13q, respectively [6]. Breast cancer in BRCA 1-positive women is diagnosed by age 50 up to 40% of the time and in BRCA2-positive women up to 30% of the time [7]. In families with both male and female breast cancer, BRCA 2 mutations (or PALB2 mutations) are suspected [7, 8]. Patients with BRCA 1 or 2 mutations have a lifetime risk of developing breast cancer of up to 70% [7]. Patients with BRCA 1 gene mutations are more likely than BRCA 2 mutations to develop estrogen receptor negative, progesterone receptor negative, and human epidermal growth factor receptor 2 (HER2) non-amplified (known as “triple negative”) breast cancers. With BRCA1 there is a lifetime risk of ovarian cancer up to 44%, felt to start at the age of 40, a less than 5% risk of pancreatic cancer, and a prostate cancer risk in males for which screening is offered at the age of 40 [7, 9]. BRCA2 confers up to a 17–18% lifetime risk of ovarian cancer beginning at the age of 50, a 5–10% risk of pancreatic cancer, a small risk of melanoma, and a higher risk of prostate cancer in males for which screening is recommended at the age of 40 [7, 9]. A comprehensive assessment for determining who may qualify for genetic testing for HBOC can be found in Table 4.1.
Hereditary diffuse gastric cancer syndrome (HDGC) is caused by truncating or missense germline E- cadherin mutations (CDH1) on chromosome 16q22.1 [10]. HDGC has been associated with an increased risk of the lobular subtype of breast cancer, with a lifetime estimate of ~55% and diffuse gastric cancer, or linitis plastica [11]. Women with CDH1 mutations from highly penetrant families have been reported to have lifetime risks for the development of diffuse gastric cancer of 56–83% with an average age of onset of 37 years; however, an estimate of the CDH1 penetrance without ascertainment bias for families rich in gastric cancer was 33% [11]. Total gastrectomy is recommended between age 18 and 40 [12].
PTEN hamartoma tumor syndrome (PHTS) is caused by an autosomal dominant germline mutation of the PTEN tumor suppressor gene located on chromosome 10q23 [13]. These alterations have been found to occur irregularly across exons with varying types of mutations (frameshift, missense, etc.) [14]. The primary clinical concern in these patients is the increased lifetime risks of breast, endometrial, thyroid (follicular or papillary), colon, melanoma, and renal cell cancers. Autism is seen in young children, and thyroid cancer can be seen very early; thyroid ultrasound initiation is recommended at the age of 7 [9]. It has been shown that PTEN-positive women have twice the risk of developing any type of cancer than PTEN-positive men [14]. The cumulative lifetime risk for female invasive breast cancer is 70–85% [13, 14]. Other features of the syndrome commonly include macrocephaly (head circumference 58 cm or greater in women or 60 cm or greater in men), biopsy-proven trichilemmomas, thyroid nodules, or goiter or uterine fibroids. Features that are also associated are gastrointestinal hamartomas including ganglioneuromas and esophageal glycogenic acanthoses, Lhermitte-Duclos disease, macular pigmentation of the glans penis, acral keratosis (palmoplantar keratotic pits and/or acral hyperkeratotic papules), mucocutaneous neuromas, oral papillomas (particularly on the tongue and gingiva), lipomas, and vascular malformations.
Peutz-Jeghers syndrome (PJS) is an autosomal dominant polyposis disorder characterized by a germline mutation in the serine/threonine kinase tumor suppressor gene (STK11) on chromosome 19p13 in most patients [15]. Patients with Peutz-Jeghers syndrome have an increased risk of gastrointestinal, breast, gynecologic (uterine, ovarian, and cervical), pancreatic, and lung cancers. The lifetime risk of female breast cancer is 44–50% by the age 70, regardless of the type of mutation [15]. The lifetime risk for pancreatic cancer is also very significant, at least >15%, but the precise estimates are not clear given the paucity of data. Patients will commonly have freckling of the mouth, lips , nose, eyes, genitalia, or fingers.
Li-Fraumeni syndrome (LFS) is an autosomal dominant disorder characterized by a germline mutation in the TP53 gene, which codes for a transcription factor associated with cell proliferation and apoptosis [16]. This mutation confers a lifetime cancer risk of 93% in women (mainly breast cancer) and 68% in males [16]. Breast cancers can occur very early. It is also prudent to avoid therapeutic radiation therapy in Li-Fraumeni patients who develop cancer when possible. There is felt to be a 5–10% absolute risk of pancreatic cancer, but the syndrome is characterized by a wide variety of cancers throughout the life span including soft tissue sarcomas, osteosarcomas, colon cancer, gastric cancer, adrenocortical tumors, and brain tumors.
PALB2 has emerged as an important highly penetrant breast cancer-associated gene. It is a partner and localizer of BRCA2 with a lifetime risk of breast cancer of 53%, a lifetime risk for ovarian cancer of 5%, 5–10% for pancreatic cancer, and 1% for male breast cancer [8].
ATM
Pathogenic or likely pathogenic variants in the ATM (ataxia-telangiectasia mutated) gene increase the risk for breast cancer with a lifetime risk between 15% and 40% (moderate risk), a <3% risk of ovarian cancer, ~5–10% risk for pancreatic cancer, and an elevated risk for prostate cancer [9].
CHEK2 is another moderate risk gene with an estimated lifetime risk of breast cancer of 15–40% and an elevated risk for colorectal cancer. Screening colonoscopies are recommended at the age of 40 and then every 5 years [9]. Of note is that patients with CHEK2 mutations are strongly predisposed to developing estrogen-receptor positive breast cancers; this has important implications for chemoprevention.
Under-recognition: The Scope of the Problem
A 2017 study analyzing National Health Interview Survey data estimated that 1.2–1.3 million US women with a history of breast and/or ovarian cancer have not undergone testing. Less than one in five women with a history of breast or ovarian cancer who meet National Comprehensive Cancer Network (NCCN) criteria have undergone the recommended genetic testing. The majority have never even discussed the option of testing with their providers [9, 17]. It is estimated that more than 90% of unaffected BRCA1 and BRCA2 carriers have not been offered testing [18].
Evaluation for the presence of a hereditary cancer syndrome includes careful assessment of personal and family history and tumor characteristics. For example, BRCA1, BRCA2, BARD1, and PALB2 mutations are enriched in estrogen receptor (ER)-negative and human epidermal growth factor receptor 2 (HER2)-negative tumors. TP53 mutations are enriched in HER2 positive tumors and ATM and CHEK2 mutations are enriched in ER+ tumors [19]. Identifying women at increased risk for hereditary cancer is a vital component of patient care. Diagnosis of pathogenic germline mutations in both cancer patients and their families can prevent future cancers in both risk stratifying the patient for heightened surveillance for other cancers and implementation of risk-reducing measures, but also for testing other family members at risk to identify other mutation carriers. These interventions have the potential to significantly decrease the hereditary cancer burden.
We commonly see these patients or their unaffected relatives in our clinics. In a large recent survey of women visiting two busy gynecology practices, 23.8% met criteria for genetic testing [20]. Even in patients with a personal history of breast or ovarian cancer meeting criteria for testing, only 15.3% have undergone testing [17]. This represents an enormous opportunity for prevention and early detection for the attentive clinician. Early identification of families at risk may inform recommendations for more comprehensive screening and risk-reducing strategies and may even have important treatment implications for patients diagnosed with breast or ovarian cancer.
The USPSTF recommends that primary care clinicians assess a woman’s personal and family history and ancestry and refer to genetic counseling as indicated [21]. The American College of Obstetricians and Gynecologists (ACOG) recommends that OB/GYNs perform a risk assessment updated regularly. The assessment includes information on personal and family history, including pathology, imaging, and evaluation of other risk factors for cancer. If a hereditary cancer risk assessment is revealing, referral to a genetics specialist is indicated [22].
The genetic counseling process is very important for patients. Ensuring informed consent and patient comprehension of potential results is critical in the genetic testing process. Prior to proceeding with genetic testing, a patient is encouraged to pursue pretest genetic counseling. Family history is expanded, and the potential is discussed to reveal impact beyond breast cancer risks, impact to family members, incidental findings, and findings of uncertain clinical significance. Cost, insurance coverage, and laws protecting individuals from job or healthcare discrimination based on genetic information are discussed. If there is a known pathogenic variant in the family, patients understand that they will have a true positive or true negative test. With highly penetrant genes, “true negatives” return to population risk, whereas with moderate risk genes, “true negatives” are still presumed to be at increased risk due to the potential of shared environmental exposures and the possible contribution of other factors not related to the identified moderate risk gene. Patients must understand the meaning of uninformative negative results and variants of uncertain significance (VUS).
Counseling is vital to ensure patient comprehension and ease in the delivery of results. Specific examples underscore the value of pretest counseling. Broad pan-cancer gene panels can reveal mutations in common genes such as monoallelic MUYTH or APCI1307K in the Ashkenazi community, which are felt to be unrelated to breast cancer; conversely, finding a true germline mutation in TP53 revealing significant cancer risks has profound impact on the patient and also on their children. Mutations in CDH1 revealing potentially very high gastric cancer risks (particularly in a family with no prior gastric cancer history) lead to very challenging discussions around the possibility of risk-reducing gastrectomy. Finally, with panel testing, variants of uncertain significance are common, occurring approximately 25% of the time [3, 4, 23, 24]. A VUS indicates that a gene mutation has been identified that has an unknown effect on protein function and an uncertain association with cancer risk. A VUS can also be a source of uncertainty for providers and may lead to overtreatment, excessive surveillance, and unnecessary preventive measures [25].
The results disclosure conversation includes the impact of the findings on the patient and their family, risk management options, and provision of available resources. Discussion of management should outline recommendations related to multidisciplinary care if other organs are at risk. As more is learned about the different hereditary syndromes conferring risk, strategies for risk management continue to evolve, often necessitating a multidisciplinary team of subspecialists to care for these patients. (See Table 4.2). The mutations associated with hereditary breast cancer are inherited in an autosomal dominant fashion. This means that a single copy of the disease-associated mutation is enough to cause the disease. Parents, siblings, and children of the carrying a mutation have a 50% chance to also have that mutation. Extended family members may also be at increased risk. Identifying at-risk family members and discussing strategies for the patient to notify them is essential. Caution should be given to interpretation of variants of uncertain significance (VUS) and uninformative negative results. In both scenarios, a patient’s personal and family history, not their genetic test result, should be used to determine medical management recommendations.
Certain features suggestive of a possible hereditary cancer syndrome are well known: breast cancer diagnosed at a young age (50 years or younger), multiple primary tumors, several close blood relatives with the same type of cancer, and male breast cancer, for example. The Society of Gynecologic Oncology recommends that timely and universal genetic testing is recommended for women with ovarian, fallopian tube, and peritoneal cancers [26]. Other features perhaps less commonly appreciated are triple-negative breast cancer diagnosed at age 60 or younger, pancreatic cancer at any age, and prostate cancer (particularly metastatic or of intraductal histology) [27]. Couch et al. reported on a large series of patients with triple-negative breast cancer (TNBC) who underwent multigene panel testing finding a high frequency of gene mutations (14.6%) and suggested that all patients with TNBC regardless of age or family history, be offered genetic testing, at least for BRCA1 and BRCA2 [27]. Prevalence of actionable germline mutations in prostate cancer patients (aggressive and non-aggressive) may be as high as 10–15% [28, 29] and up to 14.1% with unselected pancreatic cancer patients [30].
A group that deserves special mention are those of Ashkenazi ancestry who have a 2–2.5% incidence of carrying a BRCA mutation. Historically, the vast majority of those with mutations harbored one of the three Ashkenazi “founder mutations,” two in BRCA1 and one in BRCA2, and thus testing was modified to address only these variants, the multi-site 3 test. It has recently been shown that pathogenic variants in other non-founder BRCA genes and mutations in other genes are not uncommonly found in Ashkenazi patients and multigene panel testing should be likely be offered to this population [31, 32]. In fact, given the prevalence of mutations seen in this population, population testing is being considered, with National Comprehensive Cancer Center (NCCN) guidelines suggesting “consideration of testing” in unaffected Ashkenazi individuals [9].
There are several arguments in favor of more liberal testing guidelines. Pathogenic variants are relatively common and are actionable. Guidelines that restrict genetic testing by personal and family history have gaps. A recent study looked at more than 1000 patients with breast cancer and demonstrated that there was essentially no difference in the finding of pathogenic variants between patients who met testing criteria compared to those who did not [33]. Patients are missed by current testing guidelines. Genetic testing and its results are acceptable to patients, and genetic testing is increasingly affordable.
In February of 2019, the American Society of Breast Surgeons released a Consensus Guideline that genetic testing should be made available to all patients with a history of breast cancer [34]. This has been hotly debated with concerns over available genetic counseling resources and possible gaps in interpretation of results by both patients and providers leading to possible overtreatment [25]. Clinicians have a great deal of influence over patient decisions and need to clearly understand and communicate testing and treatment implications, if they are to test independently.
That being said, patients want to know. The public’s interest in genetics and genomics continues to increase, and there has been corresponding and unprecedented growth in direct-to-consumer genetic testing. Now available are multigene cancer panels using next-generation sequencing with associated genetic counseling which may help bridge the gap in some instances where up-front genetic counseling resources are limited, when at-risk patients would prefer to test privately and when patients have concerns about insurability and do not understand laws in place (such as GINA in 2008) protecting Americans from discrimination based on their genetic information in both health insurance and employment [35]. Providers need to understand the differences between these actionable clinical grade tests and the single-nucleotide polymorphism (SNP)-based recreational tests available.
Pathogenic variants in breast cancer susceptibility genes result in a higher risk for development of disease, earlier age at onset, and an increased risk for a second or phenotypically related cancer. Early identification may inform strategies for enhanced surveillance, preventive medication, or risk-reducing surgery. The American College of Radiology stated “All women, especially black women and those of Ashkenazi Jewish descent, should be evaluated for breast cancer risk no later than age 30, so that those at higher risk can be identified and can benefit from supplemental screening” (African Americans have a disproportionate burden of aggressive early-onset breast cancer) [36]. Providers should have a consistent method to evaluate and update personal and family history on a regular basis, identifying those at risk, and reassessing survivors for the need for updated testing.
Screening and management guidelines for individuals with hereditary breast cancer syndromes continue to evolve. While subspecialists may be involved in enhanced surveillance and preventive care options, the primary care physician (PCP) is uniquely poised to centralize the patient’s care, with both a broader perspective and knowledge of the patient’s competing medical issues, risks, and preferences, and the imaging center has the unique capability of capturing a large number of women who may not be receiving information from their healthcare providers about breast cancer risk.
Benign Atypical Lesions
ADH/ALH/LCIS and FEA
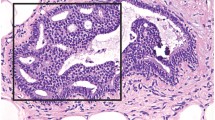
The normal life cycle of the breast is characterized by hormones and growth factors acting on stromal and epithelial cells to regulate development, maturation, and differentiation of breast tissue. At puberty, estradiol and progesterone levels increase to initiate breast development. Ten to 100 subsegmental ducts end in glandular units called terminal-duct lobular units. These subsegmental ducts lead to 20–40 segmental ducts that lead to 5–10 primary milk ducts at the nipple. Over time, in response to hormonal stimuli in an exaggerated fashion, there is enhancement of lobular tissue and stroma in some individuals, leading to hypertrophy and “fibrocystic change,” but also in some, to an increase in cellular proliferation [37, 38]. Although there does appear to be some degree of increased risk associated with benign proliferative lesions without atypia such as usual duct hyperplasia, papillary lesions, radial scar, and sclerosing adenosis (on the order of a relative risk of ~1.5–2.0) [38, 39], proliferative lesions with atypia (atypical ductal hyperplasia and atypical lobular hyperplasia) confer more significant risk (RR ~fourfold) [38, 40]. Atypical hyperplasia is a pathologic diagnosis, usually found incidentally on a biopsy of a mammographic abnormality or breast mass. ADH is characterized by a proliferation of uniform epithelial cells filling part of the involved duct (see Fig. 4.1). ALH is characterized by monomorphic dyscohesive cells filling part of the involved lobule (see Fig. 4.2). Frequently used risk models, such as the Gail model (BCRAT) or the IBIS model (Tyrer-Cuzick), do not provide accurate risk estimates for women with atypical hyperplasia. In absolute terms, it can be more useful to think about risk in terms of a cumulative risk over time. Atypical hyperplasia confers a risk of breast cancer of ~30% at 25 years [40, 41]. Atypical hyperplasia is found in approximately 10% of biopsies with benign findings [42]. Only a small minority of these women are offered enhanced surveillance or preventive medication, despite their very high risk, and of those who are offered the medication, few accept [43].
Breast lesions are believed by many to progress in a linear fashion from usual duct hyperplasia – UDH (ductal hyperplasia without atypia) – to atypical ductal hyperplasia and then to ductal carcinoma in situ and invasive ductal cancer, but true, causal relationships have not been well established. ADH differs from DCIS only with regard to the extent of proliferation of the abnormal cell proliferation. The atypical cell population in ADH shows high levels of estrogen receptor expression and shares molecular alterations with DCIS providing strong evidence that ADH is an early lesion in the development pathway of breast cancers. Flat epithelial atypia may be an intermediary step between UDH and ADH. FEA shares molecular and genetic alterations with the cells of ADH, low-grade DCIS, and low-grade invasive cancers, providing evidence that it may be a precursor. In the case of lobular histology, atypical lobular hyperplasia is felt to progress to lobular carcinomas in situ to invasive lobular cancer, as one possible mechanism [44]. ALH differs from LCIS with regard to the extent of involvement of the lobular units. In ALH , the atypical cell population distends less than 50% of the acinar spaces in the involved lobules; any greater involvement is categorized as LCIS. Observations that ALH and LCIS are clonal and contain the same genetic alterations found in adjacent invasive lobular carcinomas have generated interest in the theory that LCIS is a precursor lesion in addition to being a marker of increased risk [45]. Other mechanisms for cancer development and progression are actively being investigated, but interruption of the progression of atypical hyperplasia with preventive medication is an extremely important clinical intervention, and in fact preventive tamoxifen was shown in the Breast Cancer Prevention Trial P-1 to reduce the risk of estrogen-sensitive breast cancers in women with atypical hyperplasia by 86% [46]. These lesions are considered risk markers, because the cancers that subsequently develop are not necessarily in the area of the atypia and may even occur in the contralateral breast.
The younger a woman is when she is diagnosed with atypical hyperplasia, the higher her risk of developing breast cancer [40, 47]. Though risk models tend to be additive regarding family history and atypical hyperplasia, the two risk factors often go together, and it has been showed that the associated risks should not be added; but likely the same pathologic process.
It is also consistently observed that among women who develop atypical hyperplasia (either ductal or lobular) in whom cancer develops, the vast majority develop estrogen-receptor-positive invasive ductal disease [48]. The cumulative incidence of breast cancer increases linearly over time [49]. Kerlikowske looked at another large cohort of women participating in the Breast Cancer Surveillance Consortium evaluating 1.2 million women age 35–74 looking at factors specific for estrogen receptor-positive and estrogen receptor-negative breast cancer and found that for women age 40 years, compared with no prior biopsy, for ER+ disease, hazard ratios were 1.53 vs. 1.26 for non-proliferative disease, 1.63 vs. 1.41 for proliferative disease without atypia (UDH, radial scar, papilloma, or sclerosing adenosis), and 4.47 vs. 0.20 for proliferative disease with atypia. Women at the highest risk had lobular carcinoma in situ (LCIS) that were primarily at risk for ER+ cancers [50]. This has important implications for efforts at risk reduction through endocrine manipulation.
Lobular carcinoma in situ is diagnosed microscopically when more than half of the acinar spaces in a lobule are distended and distorted by a dyscohesive proliferation of small uniform cells that are strongly estrogen receptor positive and have a low proliferation rate and loss of the CDH1 gene encoding E-cadherin (See Fig. 4.3). The pleomorphic variant is characterized by cells that show marked nuclear pleomorphism, often with central necrosis. It can be estrogen receptor positive or negative, high or low grade, and have high or low proliferation rates, but it also has loss of the CDH1 gene encoding E-cadherin. It often occurs in the setting of concurrent invasive lobular cancer.
LCIS is often multifocal and is bilateral in one-third of patients [51]. A common misconception, as with ALH, is that invasive cancers developing after LCIS will be invasive lobular cancers; in fact, the majority that develop are invasive ductal cancers [52, 53]. Currently, LCIS is thought of as likely an indicator of increased breast cancer risk and a non-obligate precursor lesion. King published a series on 1060 patients with LCIS participating prospectively in a surveillance program over a 29-year period and reported a 2% annual incidence of breast cancer [54]. In a population based study of 19,462 women diagnosed with LCIS from the SEER database between 1983 and 2014, the cumulative incidences of subsequent breast malignancy were 11.3% (95% CI, 10.7–11.9%) and 19.8% (95% CI, 18.8–20.9%) at 10 and 20 years, respectively [55]. At a median follow-up of 8.1 years (range 0–30.9 years), primary breast cancer was diagnosed in 9.4% of the cohort [55].
Flat epithelial atypia (FEA) is a relatively new term established in 2003 meaning replacement of the luminal epithelial cells with one to several layers of a single epithelial cell type showing low-grade cytologic atypia. The lesions have a tendency to calcify and are seen in 3.8–10% of breast biopsies performed for mammographic calcifications [56, 57]. A study from the Mayo cohort showed that FEA did not further increase breast cancer risk among women with atypical hyperplasia and the risk associated with FEA was similar to that of patients with proliferative lesions without atypia. FEA should not be considered equivalent to ADH and ALH with regard to cancer risk assessment, risk modeling, or patient management [58]. It should not be entered into the Gail or Tyrer-Cuzick risk model as an equivalent to atypical hyperplasia.
The National Comprehensive Cancer Network (NCCN) has recognized the risk associated with both atypical hyperplasia (ADH/ALH) and LCIS and recommends that annual contrast-enhanced screening breast MRI be recommended for these patients, when their calculated estimated lifetime risk is 20% or greater [12].
Breast Density
Dense breast tissue as measured by mammography has long been recognized as an important independent risk factor for the development of breast cancer but has only been received recent focus, likely as a result of the opportunity for supplemental imaging on the basis of breast density knowledge. Women with the highest degree of breast density or “extremely dense breasts” are felt to be at four- to fivefold increased risk as compared with those at the lowest density “fatty replaced” [59,60,61]. In fact, it is felt to be one of the strongest risk factors for the development of breast cancer [62]. Dense breast tissue contains a higher proportion of stromal and glandular tissue. According to classic studies in twins, heritability accounts for approximately 60 percent of the variation in breast density [61].
A large study of pooled data in the modern era looking at mammography density and the risk of breast cancer by age and tumor characteristics in 3414 women with breast cancer and 7199 without who underwent screening mammography showed that density was associated with all breast cancer subtypes, but particularly large tumors and node-positive tumors across all age groups, and estrogen-receptor-negative status among women under age 55, suggesting high mammographic density plays an important role in tumor aggressiveness, especially in younger women [63].
Risk of combined postmenopausal hormone therapy is also related to mammographic density. Postmenopausal hormone therapy, in particular estrogen plus progestin, increases breast density [59, 64,65,66] and breast cancer risk. A large study published by Kerlikowske collected data on 587,369 women who underwent 1,349,027 screening mammograms collecting data on BIRDAS breast density, age, menopausal status, and current HT use, assuming a normal BMI. They found the use of postmenopausal hormone therapy, in particular estrogen plus progestin therapy, is associated with higher breast cancer risk among with higher breast density compared to postmenopausal women with high breast density that do not take hormone therapy. Studies have shown that postmenopausal estrogen use alone does not result in an increase in breast cancer incidence [67]. In this study, estrogen alone was associated with higher breast cancer risk among women with high breast density compared with postmenopausal women with high breast density that did not take HT but to a lesser extent than estrogen plus progestin therapy, and there was no increase among postmenopausal women with average breast density. For example, in women age 55–59 years with extremely dense tissue, the 5-year risk was 2.4% for non-users, 3% for estrogen-only users, and 4.2% for combined HT users. Low breast density was associated with a low risk of breast cancer for women of all ages regardless of HT use [68]. Postmenopausal women with high breast density may want to consider the added risk of breast cancer when deciding on initiating postmenopausal therapy, or on duration of therapy. Proposed mechanisms include that perhaps the hormone therapy slows the normal process of breast involution that occurs with aging. Additionally, it is postulated that combined hormone therapy may stimulate proliferation of greater numbers of epithelial and stromal cells in the breast associated with high breast density to promote tumorigenesis and increase breast cancer risk [68].
As mentioned, high breast density appears to be a heritable risk factor and is associated with the development of particular estrogen-receptor subtypes. The strength of association of breast density decreases with older age for estrogen-receptor-positive breast cancer. In comparison, the incidence of estrogen-receptor negative receptor cancer is stable with increasing age and the association with breast density remains elevated across all ages, suggesting that there could be continued genetic influence of breast density for the development of estrogen-receptor-negative breast cancer as women age. In support of this, at least some single-nucleotide variants associated with breast density are also preferentially associated with estrogen-receptor-negative breast cancers [50].
A provacative case control study looked at the population attributable risk of clinical risk factors for breast cancer of patients in the Breast Cancer Surveillance Consortium. Many established breast cancer risk factors are used in clinical risk prediction models, although the proportion of breast cancers explained by these factors is unknown. The study design was a case control study with 1:10 matching, and both pre- and postmenopausal women were included. A total of 18,437 women with invasive breast cancer or ductal carcinoma in situ were enrolled as cases and matched to 184,309 women without breast cancer, with a total of 58,146 premenopausal and 144,600 postmenopausal women enrolled in the study. Breast density was the most prevalent risk factor for both the premenopausal and postmenopausal women and had the largest effect on the population attributable risk proportion: 39.3% (95% CI, 36.6–42.0%) of premenopausal women and 26.2% (95% CI, 24.4–28.0%) of cancers in postmenopausal women were attributable to increased density (of note, 22.8% (95% CI, 18.3–27.3%) of breast cancers could potentially be averted if all overweight and obese women attained a body mass index of less than 25 [69].
Breast density is an important and increasingly recognized independent risk factor for the development of breast cancer that is likely largely heritable and may be associated with more aggressive disease, particularly in younger women, and higher risk in postmenopausal women on hormone replacement therapy. It seems prudent that a woman embarking on a shared decision-making discussion with her healthcare provider about screening mammography from the age of 40–49 consider a baseline mammogram to have knowledge about her mammographic density to aid in informing that decision and that breast density enter into the shared decision-making process around hormone replacement in the postmenopausal setting.
Therapeutic Irradiation
Breast cancer is the most common secondary solid tumor following pediatric Hodgkin lymphoma and is associated with the therapy for the primary malignancy. Women receiving therapeutic thoracic irradiation prior to the age of 30 (e.g., for treatment of Hodgkin lymphoma) is a significant risk factor for the development of breast cancer. Results from a case control study of women treated at a young age (<30) for Hodgkin lymphoma with thoracic radiation indicated that the estimated cumulative absolute risk for breast cancer at 55 years of age was 29% (95% CI, 20.2–40.1%) for a women treated at 25 years of age with 40 Gy of radiation and no alkylating agents [70]. A more recent paper reported on female childhood cancer survivors treated with chest irradiation who were participants in the CCSS (Childhood Cancer Survivor Study) showed the cumulative incidence of breast cancer by age 50 years was 30% (95% CI, 25–34), with a 35% incidence among Hodgkin lymphoma survivors overall (95% CI, 13–25), respectively [71].
Mantle field radiation historically represented the standard of care for patients with supradiaphragmatic Hodgkin lymphoma. Currently, coupled with effective multi-agent chemotherapy, radiotherapy fields can be reduced and smaller fields have been shown to be associated with fewer radiation-associated malignancies. A more recent study looking at a modern smaller field radiotherapy showed that it was not associated with a greater risk of secondary breast cancer than chemotherapy alone [72].
Intriguingly, a breast cancer polygenic risk score developed for risk stratification in the general population was also found to be useful in substratifying risk in survivors of Hodgkin lymphoma that would be more prone to developing breast cancer [73].
Current NCCN guidelines for Screening and Diagnosis for a woman who has received thoracic radiation therapy between the ages of 10 and 30 are breast awareness (women should be familiar with their breasts and promptly report changes to their healthcare provider), annual clinical encounter 8 years after RT is complete and until the age of 25 (clinical encounter meaning at minimum medical and family history should be obtained and the encounter should encompass ongoing risk assessment, risk reduction counseling, as well as a clinical breast exam by a licensed provider), and, beginning at the age of 25, clinical encounters every 6–12 months with the addition of annual contrast-enhanced breast MRI. An annual screening mammogram (with consideration of tomosynthesis) is added 8 years after RT but not prior to the age of 30. Consideration is given to whole breast ultrasound or contrast-enhanced mammography for those who qualify for but cannot undergo MRI, and patients should also be offered risk reduction strategies, though chemoprevention has not been specifically studied in this patient population [12]. Consideration can be given to risk-reducing mastectomy per NCCN guidelines should that be the preference of the patient after careful informed shared decision-making and consent [27]. Counseling regarding the degree of protection offered by such surgery and the degree of cancer risk should be provided. It is important that the potential psychosocial effects of risk-reducing mastectomy are addressed. Nipple-sparing mastectomy has been suggested as a safe and effective risk reduction strategy [74].
Risk Modeling
It is well known that breast cancer can run in families, and it is not uncommon for a woman who has a family history to suffer from anxiety around fear of the disease. Individuals with a family history of breast cancer frequently overestimate their risk and are relieved when presented with quantitative information suggesting that their risk is lower than they would have predicted [75, 76]. Conversely, a healthy woman with a benign atypical biopsy may be convinced to embark on preventive medication when she sees a mathematical estimate of her risk over time. Modeling can be used for prediction of an individual’s risk of carrying a genetic mutation, calculation of risk for inclusion in clinical trials, and calculation of a lifetime risk for purposes of enhanced clinical surveillance. Third-party payors certainly consider quantitative risk assessment data in their determinations of medical necessity for contrast-enhanced breast MRI, an expensive medical test.
Risks are often expressed as either relative risks or absolute risks. Relative risk expresses the strength of association between exposure to a risk factor and the presence of breast cancer. For example, the relative risk of breast cancer conferred by atypical hyperplasia is about four. This means that women with atypical hyperplasia develop breast cancer about 4 times more frequently than similar women without atypical hyperplasia. Absolute risk is the percent chance that some event will happen over some specified time. The same woman might be informed that her absolute risk of developing breast cancer is ~30% over the next 25 years.
Cancer risk models use personal and family history information to calculate the probability that an individual carries a pathogenic variant in a cancer predisposition gene or to estimate the probability that the woman will develop cancer over time. In the United States, guidelines using personal and family history for genetic testing largely govern referrals and reimbursement. Risk modeling is seldom employed for estimation of the likelihood of carrying a pathogenic variant (such as BRCAPRO or the UPENNII model) presently as testing has become more affordable and available. Risk prediction models are more commonly used to identify women without genetic mutations who may be at an elevated risk for breast cancer and those who may benefit from additional counseling, supplemental screening, or chemoprevention [77, 78]. Currently, there are several risk assessment tools available, each with their own values and flaws. While some models include hormonal factors and body mass index, others focus only on family history and hereditary risk. As our understanding of breast cancer risk has evolved, many of the risk models have also been updated to incorporate additional risk factors including breast density and racial background [79]. As a result, recognizing the differences between each risk model is critical in determining its proper utilization in decision-making for prevention and screening.
Gail Model
The Gail model is probably the most commonly used model. It can be accessed at http://cancer.gov/bcrisktool/default.aspx. It asks five questions, takes about a minute to complete, estimates the 5-year and lifetime risk of breast cancer in women >35 years of age, and is the preferred model of the United States Preventive Services Task Force for estimation of 5-year risk; it is felt that the benefits generally outweight the risks of preventative medication if the 5-year risk per Gail model is 3% or greater (in the absence of medication contraindications) [80]. The National Comprehensive Cancer Network (NCCN) uses the Gail model 5-year risk of >1.67% to recommend discussion around chemoprevention with all patients [27].
The model was initially developed based on of data from the Breast Cancer Detection Demonstration Project (BCDDP), a large screening study that included over 250,000 women age 35–74 years. Developed by Dr. Mitchell Gail and colleagues at the National Cancer Institute (NCI), it was one of the earliest tools created to determine a woman’s risk for developing invasive breast cancer, carcinoma in situ, or LCIS. It uses a logistic regression model to estimate a woman’s lifetime risk and 5-year risk of breast cancer, accounting for a woman’s age, ethnicity, age at menarche, parity, immediate family history, previous biopsies, and their histologies [81]. This was later updated and validated to create the modified Gail model, which has been implemented in a variety of formats. The modified version can predict both the 5-year risk and the lifetime risk of invasive breast cancer in women up to age 90 [79, 82].
While the Gail model is one of the most studied and validated tools, it does have limitations. The model does not accurately calculate risk in high-risk populations, those with strong family histories, a history of mantle radiation, or underlying LCIS or a history of breast cancer [79, 83]. It cannot be used for women under age 35 and considers only a fraction of family history data. It only includes female first-degree relatives, does not include age at diagnosis of affected relatives, and does not include paternal family history or family history of other cancers. Among ethnic backgrounds, it is well validated in White populations; however, it may underestimate the risk of breast cancer in African American, Asian, and Hispanic women. As a result, several extensions of the Gail model have been developed in order to provide more estimations of risk in these populations [79, 81]. The Gail model can also overestimate the risk in women with a history of benign breast biopsies. It can help predict women who may be candidates for risk-reducing medications when the 5-year risk is >1.67%; however, it is not appropriate for determining women who may benefit from supplemental screening. Overall, the use of the Gail model is appropriate for women over the age of 35 who are not at hereditary risk and are undergoing regular mammographic screening [79].
Tyrer-Cuzick Model
A more comprehensive model, the Tyrer-Cuzick model , available at http://www.ems-trials.org/riskevaluator/, takes a bit more time to complete, but is more comprehensive, taking into consideration biometrics, reproductive factors, and multigenerational family history as well as breast density to provide a 5-year, 10-year, and lifetime risk for the development of breast cancer. A pedigreed version can be printed for the patient and imported into the clinical note.
The Tyrer-Cuzick (T-C) model was originally designed in 2004 and developed from data from the International Breast Cancer Intervention Study (IBIS). Later versions have incorporated data from the United Kingdom Thames Cancer Registry 2005–2009. The model includes hereditary, hormonal, and pathologic risk factors to determine a woman’s short term and lifetime risk. There are multiple versions available; however, the newest version, v8, also incorporates breast density, which is known to be an independent risk factor for breast cancer. In the T-C model, lifetime risk can be estimated up to age 85, and it is also able to calculate the probability of BRCA1 and BRCA2 mutations [83]. The T-C model includes multiple risk factors including height, weight, age of menarche, parity, breast biopsy history and pathology, menopausal status, use of hormone therapy, family history of breast, and ovarian cancer including first-, second-, and third-degree relatives, as well as Ashkenazi Jewish ancestry and BRCA status [83]. Given its comprehensive nature, T-C demonstrates better calibration and predictive accuracy when compared to other models such as Gail [79].
The T-C model has been shown to overestimate cancer risk in women with atypical hyperplasia and LCIS or in women with a less strong family history. In contrast, it may underestimate the risk in women with very strong family histories [79, 83]. It can be used in women under the age of 35. A 10-year risk of 5% or greater per the T-C model is accepted by the American Society of Clinical Oncology as a equivalent to a 5-year risk per Gail of 3% or greater in terms of the benefits of chemoprevention likely outweighing the risks (assuming no medication contraindications) [84].
Barlow/Breast Cancer Surveillance Consortium
The Breast Cancer Surveillance Consortium (BCSC) model or Barlow model was first described in 2006 using data collected from the BCSC which included women ages 35–84 at the time of their screening mammograms. The model itself is designed to predict the risk of invasive breast cancer or DCIS within 1 year of a woman’s screening mammogram and does not apply to women with a prior history of cancer, prior mastectomy, or prior breast augmentation [79]. It is divided into two parts: premenopausal and postmenopausal. The premenopausal model incorporates a woman’s age, breast density, prior breast biopsies, as well as family history. The postmenopausal model considers the same factors but also includes demographic risk factors, BMI, and hormonal factors. Because this model incorporates breast density, it is best suited to be used at mammographic facilities where breast density is readily available. It is currently not available as a web-based tool but is widely used in research [79].
Claus
The Claus model was originally developed from a population-based, case study (the Cancer and Steroid Hormone Study) involving 4730 women ages 20–54 years old with known breast cancer and matching it to 4688 controls in the same geographic region and 5-year categories of age [83]. The model itself was intended to be used in women with a family history of breast cancer and uses only hereditary variables (family history and age at diagnosis) to predict the lifetime risk of breast cancer. Segregation analysis revealed the presence of an autosomal dominant genotype carried by 1 in 300 people, which lead to an elevated risk [85]. Output information from the Claus model includes a lifetime risk of breast cancer up to age 79 and includes both invasive breast cancer and DCIS [83]. The Claus model should not be used in women with a known genetic mutation, as it will underestimate overall risk. It has not been validated outside the original cohort and does not incorporate any other risk factors.
BRCAPRO
BRCAPRO was originally developed in 1997 as a model used to determine the risk of carrying BRCA1 mutation and later extended to include BRCA2 [79, 85]. The model is also designed to determine lifetime risk but is highly sensitive at predicting the probability of carrying a deleterious mutation in these two genes. BRCAPRO can be used in both men and women with and without a family history to determine the probability of having BRCA1 or BRCA2 mutation, developing ovarian cancer or invasive breast cancer and, more recently added, the risk for developing contralateral breast cancer in those women with a known breast cancer history. BRCAPRO also incorporates pathologic markers for breast cancer including ER, CK14, CK5/6, and HER2 status [79]. Currently, DCIS is not specially accounted for in the BRCAPRO model [86].
BOADICEA/CanRisk
CanRisk is now available as the updated version of BOADICEA at www.canrisk.org.
The Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA) was first introduced in 2002 and later refined in 2004 [79]. Similar to BRCAPRO, it also calculates the likelihood of carrying BRCA1 or BRCA2 mutations, overall lifetime risk, in addition to including the effects of an added polygenic risk score, even for moderately penetrant gene carriers [85]. Risk is calculated using the Bayes’ theorem and takes into account family history of ovarian and breast cancers, tumor pathology, and demographic influences [87]. Short-term and lifetime risks for both breast and ovarian cancer are predicted.
Future directions will employ incorporation of risk-reducing strategies such as weight loss and chemoprevention into modeling, ease of communication to patients to facilitate understanding and decision-making, and further validation in non-White populations, particularly for the single-nucleotide variants. More information must also be incorporated with regard to competing risks, particularly in light of the obesity epidemic and its associated morbidity.
Discussion and Clinical Implementation
Determining breast cancer risk requires a comprehensive assessment that is often a multifaceted approach. First and foremost, one needs to ask whether the patient has a pedigree suggestive of genetic predisposition. If a woman meets criteria for genetic evaluation, then that is the most critical element of risk assessment.
It is then important to understand the clinical implications of various models in order to determine which model will have the best predictive accuracy for your patients. The Gail and Claus models primarily estimate the risk of breast cancer over a span of time and continue to be widely accepted and well validated among physicians and researchers [79]. The Gail model has undergone a variety of modifications to account for a more refined risk assessment for particular populations. An important limitation of these models is that while they remain strongly calibrated, they lack “discriminatory accuracy,” suggesting their stronger value within a given population, but limited efficacy in predicting an individual woman’s risk for breast cancer [79]. Tyrer-Cuzick, BRCAPRO, and BOADICEA/CanRisk not only estimate lifetime risk but can also assess the probability of carrying a pathogenic mutation. T-C also incorporates body mass index, extended family history and structure, and hormonal factors that allow it to be best applied to the general population vs. solely those with an elevated familial risk and at this point in time is the most comprehensive with the greatest ease of use [79].
In a large study by McCarthy et al. [88], the performance, validity, and accuracy of various models (Gail, Claus, BCSC, TC, and BRCAPRO) were studied with regard to both DCIS and invasive breast cancer risk. A total of 35,921 women aged 40–84 who underwent routine mammographic screening were included and followed for 6 years. Among all models, there was comparable moderate discrimination; however, the Gail model had a marginally higher accuracy than BRCAPRO and T-C in the general population, and BCSC had the highest overall accuracy in women who had a readily available breast density [88]. When determining calibration, the Gail model and BCSC were superior when compared to T-C; however, this was thought to be due to the fact that the data was pulled from a general mammography clinic vs. a high-risk clinic. The ability to assess a full family pedigree in an alternative clinic and input it into either the T-C or the BRCAPRO model could have led to better predictability. Overall the results of this study are reassuring that among all models there is comparable calibration and predictive accuracy among women in the general population [88]. More data is needed to evaluate their roles in predicting individual risk among both general and high-risk populations.
Polygenic Risk Score
The likelihood that a woman will develop breast cancer during her lifetime is influenced by her hereditary makeup. Recall that there are essentially three types of genetic variation felt to contribute to risk:
-
1.
Pathogenic variants in rare highly penetrant genes such as BRCA1, BRCA2, PTEN, TP53, CDH1, and PALB2 which are associated with ~10% of all breast cancers.
-
2.
Pathogenic variants in still rare but slightly more common moderately penetrant genes such as CHEK2 and ATM which explain an additional 4–8% of family history. Mutation risk is lower, but the single mutation is still impactful enough to significantly influence breast cancer risk.
-
3.
Genome-wide association studies have identified common single-nucleotide polymorphisms that, by themselves, confer very little risk (usually between 1.05-fold and 1.50-fold); however, acting in concert, these common changes may explain up to 18% of additional associated familial cancer risk [89]. There are currently over 300 known common genetic variants (SNPs) or SNVs associated with increased risk, some specific for estrogen-receptor-positive disease and some for estrogen-receptor-negative disease.
Improved stratification of breast cancer risk is essential for optimizing clinical benefit from screening and risk-reducing procedures. Polygenic risk scores can be expected to add an additional layer of stratification, although precisely how best to combine the scores with traditional tools remains unclear.
These single-nucleotide polymorphisms (SNPs) occur approximately every 300 nucleotides in the human genome and are present in >1% of a population [90, 91]. Polygenic risk scores incorporate SNPs in an appropriately weighted fashion, adding an element to risk stratification that is independent of other risk factors.
There has been a lot of investigation looking at the polygenic risk score as an independent risk factor which can now be incorporated into both the Tyrer-Cuzick and the BOADICEA/CanRisk models for risk estimation, but also research looking at SNVs as modifiers of risk in gene mutation carriers. Couch et al. looked at breast and ovarian cancer penetrance in BRCA carriers as related to their polygenic risk score, and there was marked variability. For breast cancer, lifetime risk estimates ranged from 28% at the minimum PRS to nearly 100% at the highest. For some patients this may ultimately be important in clinical decision-making. For ovarian cancer, risk was substratified between 9% and 96%, but without effective screening, risk-reducing BSO would still be recommended with a 9% risk [92].
A recent study by Gallagher et al. (2020) looked at stratification of breast cancer risk by an 86-SNV score in non-carriers, carriers of pathogenic variants in moderate-risk breast cancer genes, and carriers of highly penetrant gene mutations. They observed significant stratification of risk, particularly in CHEK2 carriers. The median lifetime risk estimate seen in CHEK2 carriers in the study was 23%, but at the minimum polygenic risk score, the adjusted lifetime risk was 6.6%, and at the maximum, it was 70.6% [93]. This may ultimately be clinically important for carriers of moderate risk genes in risk management decision-making.
Polygenic risk scores are likely to be an important future direction for individualized risk assessment for both women who carry pathogenic mutations in breast cancer predisposing genes [93,94,95] and in those who do not. In the future, the polygenic risk score may be helpful in substratifying women at high risk for breast cancer, despite negative genetic testing results, average risk women making decisions about screening, women with family history making decisions about preventive medication, or even those at hereditary cancer risk faced with decisions about risk reducing surgeries. Together with pathogenic mutations in highly and moderately penetrant genes, SNPs are expected to evolve into an important component of genetic breast cancer risk assessment. At the present time, however, there are no validated studies to support the use of polygenic risk scores in clinical settings, and data is lacking in non-White populations. The effects of many of these SNVs are population specific.
Risk Management
The three pillars of risk management are enhanced surveillance, risk-reducing agents, and risk-reducing surgery. The fourth, which applies to all women, is lifestyle modification. The utility and benefit of each are, to a large degree, dependent on condition-specific empiric cancer risks, family history, comorbidities, and patient preference. We will address each in turn. Management guidelines derived through expert review and consensus are available for the hereditary cancer syndromes via the National Comprehensive Cancer Network (NCCN) [27]. These frequently updated recommendations are available at www.nccn.org. Consultation with a genetic specialist is critical for patients identified at increased risk for hereditary cancer. Evaluation will provide patients with a detailed explanation of the cancer risks and current management guidelines for their particular condition. Each of these conditions also confers increased risk for cancer in at least one additional site. The management of these additional risks is beyond the scope of this chapter; however, comprehensive care for these patients requires a familiarity with other cancer risks so that adequate referrals may be provided.
The starting point for screening of all women is the clinical encounter which includes a personal and family history followed by a breast cancer risk assessment and a clinical breast examination. The age at onset and frequency of the encounter depends on the age and risk assessment of the patient. In a systematic review of several case-controlled studies that included clinical breast examination as part of the screening modality, the sensitivity of clinical breast examination was found to be 54% and specificty 94% [96]. The clinical exam is important in order to detect early stage palpable cancers, especially those that are mammographically occult (e.g., lobular carcinomas). Breast self-awareness is also recommended; that is, women should be familiar with their breasts and promptly report any changes to their healthcare provider.
Imaging in High-Risk Patients
Screening Mammography: Special Challenges
The presence of dense breast tissue decreases the sensitivity of screening mammography to detect small lesions and may obscure visualization of an underlying cancer. About half of all women of screening age have “dense” breast tissue referred to as “heterogeneously dense” or “extremely dense” by American College of Radiology (ACR) Breast Imaging Reporting and Data System (BI-RADS) nomenclature. The sensitivity of screening mammography for women with almost entirely fatty breasts is 88% as compared with 82% for women with scattered fibroglandular densities, 69% for women with heterogeneously dense breasts, and 62% for women with extremely dense breasts [97, 98]. Women at higher than average risk for breast cancer typically begin screening at an early age, when density is typically even higher, and sensitivity has been reported as low as 31% [99]. In women actually screened, mortality reductions are even greater. One analysis looked at deaths in screened vs. unscreened women. Seventy-one percent of the deaths observed in the study occurred in unscreened women with a median age at diagnosis of fatal cancer of 49 years [100]. Mammography screening fulfills all requirements for an effective screening test. It detects cancers earlier, at a more curable size, reducing cancer deaths in randomized controlled trials and when introduced into the population, is associated with a decrease in deaths from the disease.
Younger patients typically have higher breast density and tend to present with more aggressive cancers [97, 101,102,103]. They also present with higher interval cancers [104]. Thus, density definitely presents a challenge in the high-risk patient, particularly the young patient, or any woman with dense tissue.
Choosing the Modality: Digital Mammography Versus Digital Breast Tomosynthesis
Current NCCN guidelines recommend consideration of tomosynthesis for high-risk women who are undergoing screening mammography [105]. Tomosynthesis allows acquisition of three-dimensional (3D) data using a moving x-ray and digital detector. These data are reconstructed using computer algorithms to generate thin sections of images. It is associated with a supplemental yield of ~1.5 cancers/1000 women screened and reduces the callback rate for noncancerous findings significantly in women with dense breasts when performed in conjunction with a standard two-dimensional digital mammogram. It is more sensitive for cancer detection in all categories of breast density except for fatty replaced breasts [106,107,108,109]. A current limitation to digital breast tomosynthesis is the increased radiation dosage, a potential concern in young nulliparous women. The dosage however, still falls below the limit set by the FDA for standard mammography. Of note is that BRCA2 carriers demonstrate higher benefit of cancer detection on mammography prior to age 40, in comparison to BRCA1 carriers [110].
Interpreting dense mammograms is challenging. Radiologists should minimize interruptions, develop standardized search patterns, and use specific hanging protocols for interpretation of screening mammograms. The radiologist must look at the entire mammogram for large findings and global differences. Next, the borders of dense fibroglandular tissue with fatty tissue are evaluated for changes such as retraction, protrusions, or spicules extending from the underlying tissue. Special attention is paid to retro-glandular fat, anterior mammary fat, the axillary tail, and the axilla. Lastly, the dense breast tissue is evaluated in detail looking for subtle masses, distortion, calcifications, or asymmetries.
NCCN Criteria for Screening MRI
The sensitivity of contrast-enhanced breast MRI at detecting breast cancer is higher than the sensitivity of mammography, although the specificity of MRI is often lower, resulting in a higher rate of false-positive findings [111]. Microcalcifications, often the earliest sign of breast cancer, are also not detected with MRI [112]. The high sensitivity is based on the fact that tumors attract new blood vessels to provide nutrients for them to grow (angiogenesis). These vessels can be visualized as “enhancement” on contrast-enhanced MRI when the tumor is as small as 2 mm in size [113]. These vessels are leaky and the large gadolinium molecules extravasate into the breast cancer stroma. Sensitivity of MRI alone ranges between 75.2% and 100% and is generally over 80% and specificity climbs from 90% to 97%, implying that the lower specificity is mainly a first-round effect [114].
In women with a history of thoracic radiation between ages 10 and 30 years, a known genetic predisposition to breast cancer, or a lifetime risk of 20% or greater based on models such as Claus or Tyrer-Cuzick, the National Comprehensive Cancer Network recommends annual MRI as in addition to annual mammography [105]. Women with lobular carcinoma in situ or atypical hyperplasia with a calculated lifetime risk of 20% or greater should also be considered for breast MRI. The age at which to begin MRI screening in those at hereditary risk is outlined in the NCCN guidelines for High Risk/Familial Breast/Ovarian/Pancreatic care [9]. In those for whom MRI is being ordered due to family history of the disease, MRI begins 10 years prior to when the youngest family member was diagnosed with breast cancer, but not prior to the age of 25 years, or age 40 years (whichever comes first.) For those receiving MRI due to prior therapeutic chest irradiation, MRI begins 8 years after RT but not prior to age 25 years. An important group to remember is untested first-degree relatives of highly penetrant gene mutation carriers (BRCA1, BRCA2, PTEN, TP53, PALB2, and CDH1). This group of patients is eligible for MRI screening even if they are not ready or willing to undergo genetic testing at the time that MRI screening would be recommended. Whole breast ultrasound can be considered or contrast-enhanced mammography for those who qualify for but cannot undergo MRI [9].
Although there is no direct evidence that MRI reduces mortality, supplementing annual screening with MRI facilitates early disease detection in high-risk patients [9]. There are (nor will there be) no randomized studies, so the effects of screening on breast cancer specific survival is precluded. The studies use the evidence that early detection improves outcomes (from mammography screening trials) as an argument for MRI screening. The tumors that are detected tend to be smaller (the fraction <1 cm is much higher than in women screened only with mammography) and the fraction of women with positive axillary lymph nodes is much lower [115]. Evans et al. reported a significantly higher overall survival of 95.3% in women at very high risk participating in an MRI-based screening program compared with 73.7% for equally high-risk women who did not [115]. Unfortunately, there are a number of other factors that limit the uptake of MRI screening by eligible patients and compliance with recommended surveillance schedules. The key factors center around the trade-offs between the accuracy of the test versus the anxiety associated with both the experience of the test and the fear of the results, the out-of-pocket costs (many patients pay a significant portion of the cost themselves), discomfort, and time (away from work, away from home). Finally, they may fear false-positive results, particularly if they have experienced an imaging callback in the past.
ACR Criteria for Screening MRI in Breast Cancer Survivors
Breast cancer survivors commonly inquire about eligibility for screening breast MRI. It has been shown that earlier detection of second cancers, both in the ipsilateral and contralateral breast, improves overall survival, particularly in younger patients [91]. NCCN recommends annual mammograms for patients who have had breast-conserving surgery and does not comment specifically on MRI screening [105]. The American College of Radiology recommends that MRI be offered to survivors with remaining dense breast tissue (heterogeneously dense or extremely dense) or those with remaining tissue who were diagnosed under the age of 50 [116]. Diagnostically, breast MRI may be useful in women with prior history of breast cancer and suspected recurrence when clinical, mammographic, and/or sonographic findings are inconclusive. It may also be useful in patients presenting with axillary or distant metastatic disease and no mammographic or physical findings of primary breast cancer. It may also be useful in evaluating suspected cancer recurrence in patients with tissue flap reconstruction. MRI is an important tool helpful in differentiating between recurrence and fat necrosis in patients with history of breast cancer who have undergone autologous tissue flap reconstruction or those who have had fat grafting [117].
FAST/Abbreviated MRI
Recent studies have reported shortened or abbreviated MRI protocols to have similar sensitivities and specificities compared to complete MRI protocol. These studies are being used increasingly as a screening tool and could help make breast MRI a more cost-effective screening tool [118, 119].
Patients with dense breast tissue are seeking supplemental screening because of the limited sensitivity of screening mammography. Abbreviated or FAST MRI has a shorter scan time and much lower cost than full-protocol breast MRI. In 2014, Kuhl studied patients with slightly increased risk of breast cancer, dense breast tissue, and normal digital mammogram findings with abbreviated MRI. Of note, 96% of the patients in their study also had a negative screening ultrasound. The study found the same supplemental cancer detection rate (18.2/1000), sensitivity (100%), and specificity (94%) as full-protocol MRI [120]. Another study by Kuhl in 2017 evaluated FAST MRI in women with all densities who had an average risk and a negative digital mammogram result. In this group, 65% also had a negative result on screening breast ultrasound. The authors reported a supplemental cancer detection rate of 15.5 per 1000 women screened and an increased detection of poorly differentiated high-grade cancers at an early stage. The median size was 8 mm and 93.4% were node negative [121]. Conant recently reported on a single institution series at the University of Pennsylvania in asymptomatic women with dense tissue after negative digital breast tomosynthesis who were offered abbreviated breast magnetic resonance imaging. Screening with AB-MI yielded an added cancer detection rate of 27.4 per 1000 women (95% CI, 16.1–46.3) [122]. A large cross-sectional study was published by Kuhl in 2020 reporting on longitudinal follow-up at 48 sites in the United States and Germany in women with dense tissue undergoing routine screening comparing abbreviated breast magnetic resonance imaging (MRI) to digital breast tomosynthesis (DBT). AB-MRI detected significantly more invasive cancers (11.8 per 1000) than DBT (4.8 per 1000). No invasive cancer was identified by DBT alone [123]. A recent meta-analysis published in 2020 cited overall sensitivity for abbreviated MRI of 94.8% (95% CI, 85.5–98.2) and specificity of 94.6% (95% CI, 91.5–96.6) which did not differ significantly from full-protocol MRI [124]. To enable wider use of MRI and improve cost-effectiveness, the use of shorter and less costly MRI protocols is necessary. The concept of abbreviated breast MRI was introduced to enable this, reducing acquisition time to 3 minutes and making reading time much faster. Unfortunately, administration of IV contrast is still required which takes time and is costly and painful, despite the fact that the currently used macrocyclic agents are very stable and safe [125].
Other Modalities: Ultrasound, Contrast Enhanced Mammography, BSGI, and MBI
Handheld and automated ultrasound can increase cancer detection in high-risk patient with dense breasts but may increase recall and benign breast biopsies and, in the setting of contrast-enhanced MRI, only decreases specificity and should generally be avoided. It could be offered if the patient is not eligible for MRI screening or is intolerant of the test. Current NCCN guidelines do not support routine use of molecular imaging (e.g., breast specific gamma imaging, sestamibi scan, or positron emission mammography) as screening modalities. There is emerging evidence though that these tests may improve early breast cancer detection in patients with dense breast tissue; however, whole body radiation effective dose with these tests is 20–30 times higher than mammography [126].
Future Directions in Breast Imaging
Screening for breast cancer aims to reduce morbidity and mortality from the disease. Current screening modalities lack sensitivity or specificity or are expensive or not widely available. Novel techniques are being explored including contrast-enhanced spectral mammography, automated three-dimensional breast ultrasound, transmission tomography, elastography, optoacoustic imaging, abbreviated/ultrafast and diffusion-weighted MRI, and molecular breast imaging. Artificial intelligence and radiomics have the capability to refine care. Liquid biopsies and breath tests may also be available to add to the armamentarium of available choices [127].
Interesting Cases
Case 1
A 50-year-old Korean-American medical professional underwent screening mammogram showing bilateral breast calcifications (BIRADS 0). She returned for diagnostic imaging and was found to have probably benign calcifications in the right upper outer breast for which 6-month follow-up was recommended (BIRADS 3) and more suspicious calcifications in the left upper outer breast for which biopsy was recommended (BIRADS 4). Her mother had breast cancer at age 76. Figure 4.4 shows initial screening mammogram of the left breast.
She underwent needle localization biopsy on the left (rather than stereotactic biopsy due to body habitus and breast size) showing ALH and fibrosis. Her data were entered into the Gail risk assessment model with a 5-year risk of developing breast cancer of 5.9% and a lifetime risk of 31.6%.
The patient had a follow-up bilateral diagnostic imaging showing stable benign calcifications on the right (BIRADS 2) and new probably benign calcifications in the left upper outer breast for which six-month follow-up was recommended (BIRADS 3). A screening breast MRI (Fig. 4.5) showed a suspicious mass in the right upper outer breast (BIRADS 5) and non-mass-like enhancement at 12 pm (BIRADS 5). She went on to right ultrasound-guided biopsy, which showed ALH/LCIS in both locations. Surgical excision revealed multifocal invasive lobular carcinoma, Bloom-Richardson grade I on the right. She had unilateral mastectomy on the right, continues with high-risk screening, and is doing well 6 years later.
Case 2
A 32-year-old patient presented for risk assessment and to establish breast care. Her age of menarche was 12. Her maternal aunt developed breast cancer at 50 and had negative BRCA testing and her mother recently developed breast cancer at 53 and had negative multigene panel testing. A maternal uncle had renal cell cancer and her paternal grandfather had esophageal cancer. There was no history of Ashkenazi Jewish ancestry. Her physical examination was normal. Her lifetime risk for breast cancer was estimated at 27% using the T-C model and she was recommended to undergo annual clinical breast examinations until the age of 40, baseline mammogram at the age of 35 with annual mammograms and MRI to begin at 40. There was also a discussion about tamoxifen chemoprevention to be deferred until she is done childbearing. A screening mammogram, which had been ordered in conjunction with the visit, was performed (Fig. 4.6). Calcifications were seen in the right breast for which stereotactic biopsy was recommended (Fig. 4.7). It showed poorly differentiated triple-negative invasive ductal carcinoma. She went on to mastectomy which showed a small focus of poorly differentiated invasive ductal cancer arising in a background of extensive high-grade comedo-type DCIS. One of three sentinel lymph nodes showed macrometastatic disease and seven additional nodes were removed. This young woman with Stage II (T1aN1M0) TNBC began chemotherapy with ACT. She received goserelin for ovarian function protection. MyRisk panel testing revealed a deleterious BRCA1 mutation, subsequently demonstrated in the patient’s father.
Case 3
A 40-year-old patient presented for risk assessment and to establish breast care. Her mother developed breast cancer at 61 and was BRCA negative. Her maternal grandmother developed breast cancer at 49. Her exam was normal. Her mammograms were normal and demonstrated heterogeneously dense breast tissue. She was offered preventive tamoxifen but declined. Her lifetime risk as estimated by the T-C model was 23%. Six months later, a screening MRI was ordered which showed a 6.3 cm area of ductal and segmental enhancement on the right side (Fig. 4.8). Second-look ultrasound showed no abnormality and MRI-guided biopsy showed carcinoma in situ of mixed type, which was strongly ER+/PR+. She had bilateral mastectomies that showed 10 mm of carcinoma in situ on the right with negative margins (20 mm), negative sentinel lymph nodes, and negative pathology of the left breast. She underwent DIEP flap reconstruction. She saw medical oncology and it was felt tamoxifen was not necessary. She did decide to have genetic panel testing which was negative.
Chemoprevention
Breast cancer chemoprevention refers to the use of preventive medication to decrease the risk of the development of breast cancer in high-risk women. Specifically, tamoxifen and raloxifene, which are two selective estrogen receptor modulators (SERMs), have been shown in randomized, controlled trials to reduce the risk of estrogen-receptor-positive breast cancer and are FDA approved for breast cancer risk reduction. Additionally, exemestane and anastrozole, which are aromatase inhibitors (AIs), have also been shown to reduce the risk of breast cancer specifically in postmenopausal women. Yet despite the efficacy of these medications, they continue to not be widely adopted in everyday practice [128]. It is estimated that over 2,000,000 women in the United States would benefit from preventative medication, but very few of them take it. In a study of 22,235 Medicare eligible women, raloxifene was used in only 2.5% and 4% of women with an elevated 5-year estimated risk of 1.66–3% and >3%, respectively. Data from review of Part D claims revealed that use of raloxifene was 6.6% in women in the highest risk category (5-year risk >3%) [129]. The limited use of these medications is likely due to lack of physician comfort and knowledge in prescribing the medications and fear of adverse effects on the part of the patient. The following will highlight the medications commonly used for breast cancer chemoprevention, those individuals who are most likely to benefit, and common side effects associated with use [130]. A comprehensive table demonstrating all of the risk reducing agents can be seen in Table 4.3.
Tamoxifen and Raloxifene
Tamoxifen and raloxifene are SERM medications that have agonist effects on estrogen receptors in the uterus, vagina, liver, and bone but have antagonistic effects on the estrogen receptors in the breast. Tamoxifen has long been used in the treatment of hormone-positive breast cancers. The interest in tamoxifen as a preventive agent came from the finding that while it reduced the risk of recurrence of primary breast cancers, substantial reductions in contralateral breast cancers were also noted [131]. Several large randomized controlled trials have studied the risks and benefits of tamoxifen and raloxifene for use as preventative agents and will be discussed here. It should be noted that no trial has shown a survival advantage with risk-reducing medication.
NSABP P-1 Study: The Breast Cancer Prevention Trial
The largest trial evaluating the use of tamoxifen as a preventive agent was the Breast Cancer Prevention Trial (NSABP P-1) . Results were published in 1997. This study looked at 13,388 women (age 35 years or older) who were estimated to be at increased risk for the development of breast cancer (5-year Gail model risk score >1.67%, age >60, or history of LCIS) and randomly assigned them to either tamoxifen (20 mg/day) or placebo for 5 years [130, 132]. After a median follow-up of 4.6 years, the use of tamoxifen was shown to reduce the risk of invasive and noninvasive cancers by 50% among all age groups [4]. Additionally, it was noted that women who had a history of atypical hyperplasia had an 86% reduction in breast cancer risk with tamoxifen [132, 133]. Women of all ages were noted to have benefit with tamoxifen use.
While tamoxifen is promising with regard to effects on breast cancer risk reduction, it also carries a variety of adverse effects that should be considered prior to its use. In young, healthy premenopausal women, there was no increased risk of serious side effects. However, in postmenopausal women, tamoxifen use increased the risk of endometrial cancer, venous thromboembolism, and cataracts [132, 134]. In the P-1 study, the annual incidence of endometrial cancer in those women taking tamoxifen was 2 per 1000 women (all Stage 1 localized tumors) [132]. These findings were consistent with those seen in other trials evaluating the use of tamoxifen in the treatment setting. Women who are known to be at risk for the development of venous thromboembolism (e.g., those with a prior history of blood clot or stroke or those with risk factors such as a known coagulation disorder, active smoking history, or obesity) should not be offered tamoxifen for chemoprevention. Additionally, those women who report abnormal bleeding during their course of treatment with tamoxifen should notify their physicians immediately and undergo an appropriate workup. Finally, tamoxifen is teratogenic and should not be used in pregnant women or in those planning on becoming pregnant.
Most of the historical data regarding raloxifene comes from osteoporosis studies in women at average risk for the development of breast cancer. The Multiple Outcomes Raloxifene Evaluation (MORE) trial was a large double-blind placebo-controlled study that was designed to evaluate the risk of vertebral facture on 60 mg or 120 mg of raloxifene, or placebo [135]. While the study showed raloxifene to be an effective medication for reducing the risk for vertebral fractures, in a secondary analysis, it also was shown to decrease the incidence of invasive breast cancers. The MORE trial concluded that during 4 years of treatment, raloxifene reduced the risk of estrogen receptor positive tumors by 70% in postmenopausal women with known osteoporosis [135, 136]. The Continuing Outcomes Relevant to Evista (CORE) trial examined the same women from the MORE trial who opted to continue raloxifene therapy for an additional 4 years. In women who took raloxifene for an additional 4 years, the risk of invasive breast cancer was reduced by 59%. The combined data from both the MORE and CORE trials concluded that the incidence of estrogen receptor-positive invasive breast cancer was reduced by 66% in those postmenopausal women who had taken raloxifene for 8 years [136, 137]. Raloxifene was not found to increase the risk of endometrial cancer in these trials, and these data prompted a head-to-head comparison of tamoxifen and raloxifene in women at increased risk.
STAR Trial
The Study of Tamoxifen and Raloxifene (STAR) trial compared the use of tamoxifen 20 mg to raloxifene 60 mg over a duration of 5 years in 19,749 postmenopausal women (age 35 years or older) with a median age of 58.5 years who had an average 5-year risk for the development of breast cancer of 4.03%. The initial results of the study showed that raloxifene was as effective as tamoxifen in reducing the risk of invasive breast cancer after a median follow-up of 47 months [136]. At 81 months, however, raloxifene was slightly less effective than tamoxifen yielding a 38% reduction in invasive breast cancer [138]. Additionally, in women with atypical hyperplasia, raloxifene was 78% as effective as tamoxifen, and there was no statistically significant difference between the two for LCIS risk reduction [137]. Given the improved safety profile in postmenopausal women with a uterus, it remains an important option.
Ultimately the use of tamoxifen and raloxifene should be individualized based on a patient’s risk for the development of breast cancer, personal and family medical history, and patient preference. It is imperative for physicians to be able to appropriately identify patients who may benefit from risk-reducing medications . While tamoxifen is FDA approved for the prevention of breast cancer in premenopausal women, increased side effects of endometrial hyperplasia and cancer, blood clots, and cataracts may pose a potential threat to many postmenopausal women. Raloxifene may be a safer alternative in these women, especially those who have their uterus. The most common side effects seen with both tamoxifen and raloxifene are hot flashes and night sweats. Tamoxifen is often associated with vaginal discharge and raloxifene may result in vaginal dryness, though both medications are generally well tolerated [139]. The United States Preventive Services Task Force has suggested that women with an estimated 5-year breast cancer risk of 3% or greater are likely to have more benefit than harm from using tamoxifen or raloxifene (assuming that there are no contraindications to the use of the medications) [140].
Low-Dose Tamoxifen
An attractive alternative regimen for preventive therapy is supported by a recent trial published in the Journal of Clinical Oncology from Italy in 2019. A multicenter trial of 500 women with intraepithelial neoplasia including atypical hyperplasia and lobular carcinoma in situ were followed for a median of 5.1 years with a primary end point of invasive breast cancer or ductal carcinoma in situ. Patients were given 5 mg daily for 3 years (as opposed to the traditional 20 mg daily for 5 years) and the same 50% reduction in breast cancer events was seen with limited toxicity [141].
Exemestane
Exemestane was first studied in 2004 as a potential alternative to SERMs for breast cancer chemoprevention. The NCIC Clinical Trials Group Mammary Prevention Trial (NCIC CTG MAP.3) was a randomized, double-blind, placebo-controlled study that compared 25 mg exemestane +/− celecoxib vs. placebo [142]. A total 4560 postmenopausal women who were estimated to be at increased risk for breast cancer were randomized to receive either 25 mg exemestane plus placebo, 25 mg exemestane plus celecoxib, or placebo plus placebo to be administered once daily for a duration of 3 years. Exemestane was found to reduce the incidence of invasive breast cancer by 65% as compared to placebo [142]. The majority of these cancers were estrogen-receptor positive; however, there were also reductions seen in HER-2-positive tumors, which often carry a poorer prognosis. Additionally, there were notable reductions in noninvasive breast cancers in addition to precursor lesions including atypical hyperplasia and LCIS. The most common associated side effects included menopausal symptoms such as hot flashes, night sweats, insomnia, and arthralgias; however, the medication is generally well tolerated. Unlike tamoxifen, there was no endometrial hyperplasia or venous thromboembolism [139, 143]. A slight decrease in bone mineral density was seen in MAP3 (2–7%); however, in several other trials, upon discontinuation of exemestane, improvement toward baseline bone density was seen. As a result, exemestane serves as a feasible alternative for chemoprevention in postmenopausal women who have contraindications to tamoxifen or raloxifene. Despite the 3-year duration of the MAP3 trial, NCCN recommends 5 years of use in the preventive setting, with a baseline bone density prior to initiation of therapy and monitoring as indicated [81].
Anastrozole
Anastrozole is an aromatase inhibitor that has also been studied as a potential risk-reducing agent in postmenopausal women. The International Breast Cancer Intervention Study II (IBIS-II) was a large, double-blind, placebo-controlled study that measured the safety and efficacy of anastrozole vs. placebo in women age 40–70 years with an elevated risk for breast cancer [144]. A total of 3864 women were enrolled and randomized to receive either 1 mg of anastrozole daily or placebo for a total of 5 years. At the end of 5 years, there was a 50% reduction in invasive breast cancers seen in women on anastrozole vs. placebo (32 cases vs. 64 cases, respectively; HR 0.50; 95% CI, 0.32–0.76; p = 0.0001) [131, 144]. There were also reductions in ER-positive disease and DCIS, but no reductions were noted in ER-negative disease. While the use of anastrozole decreased the risk of breast cancer in all groups, notable reductions were seen in women with a history of atypical hyperplasia or LCIS [144]. Similar to the MAP3 trial, there were no major adverse events seen in women on anastrozole. Joint pain and vasomotor symptoms were commonly seen in both the use of anastrozole and exemestane; however, vasomotor symptoms were more prevalent in IBIS II in women on anastrozole. Additionally, an increased report in dry eyes, dry mouth, and hypertension were noted. Though there was no increased risk of fractures in women on anastrozole in IBIS-II, clinicians should still monitor bone status, as there have been some reductions in bone density seen with the use of aromatase inhibitors in general [144]. Anastrozole, like exemestane, can be considered as an alternative to raloxifene in postmenopausal women at an elevated risk for breast cancer.
Risk-Reducing Surgery
Risk-reducing mastectomy (RRM) is associated with a decreased risk of breast cancer of 90–95% [145] and decreased breast cancer specific mortality in BRCA carriers with breast cancer who choose contralateral risk-reducing mastectomy [146]. NCCN guidelines support a discussion around RRM for women with pathogenic variants in BRCA1, BRCA2, PTEN, TP53, and PALB2 given their very high levels of risk. Consideration can also be given to those who received therapeutic chest irradiation between the ages of 10 and 30 or those with a compelling family history without hereditary explanation [27]. Risk-reducing mastectomy is never a recommendation as it is a highly personal choice and screening is highly sensitive at detecting breast cancer early.
Surgical options for RRM include total or simple mastectomy (removal of the both breasts, nipple-areola complex, and the overlying skin), skin-sparing mastectomy (removal of the both breasts, nipple-areola complex with preservation of the overlying skin), or nipple-sparing mastectomy (removal of the both breasts with preservation of the nipple-areola complex and the overlying skin). The timing of risk-reducing mastectomy is highly dependent on personal and family medical history and personal choice.
Nipple-sparing mastectomy (NSM) has emerged over recent decades as an option for treatment and prevention of breast cancer with optimal cosmesis. In addition, the procedure also facilitates the process of single-stage breast reconstruction, and to date evidence is growing in terms of oncologic safety [74].
Patients need to understand that chest wall sensation will be markedly reduced or absent and that their tissue will not be completely removed but as much will be removed as possible. It will still be recommended that they come in annually for a clinical evaluation. Furthermore, they must anticipate issues with body image changes and sexuality. If it is possible to involve a health psychologist familiar with this field, it will help the patient to meet with him or her before the procedure, and afterward.
Breast Reconstruction
The primary goals of breast reconstruction following mastectomy include reestablishing the breast shape, optimizing symmetry, and recreating the nipple and areolar complex if desired by the patient (and it has been surgically removed). In general, patients prefer the approach of immediate reconstruction, but sometimes it is deferred if a patient is smoking or obese due to the increased risk of complications in these settings. Managing expectations and working with the multidisciplinary team is an essential component of the shared decision-making process.
Implant-based breast reconstruction begins either at the time of mastectomy (referred to as immediate breast reconstruction) or at some time following mastectomy (delayed breast reconstruction). Immediate breast reconstruction has historically been performed with placement of a tissue expander behind the pectoralis muscle at the time of mastectomy, followed by later placement of a permanent breast implant. In some women, direct-to-implant reconstruction is possible without the placement of a tissue expander. Recently, many plastic surgeons are opting for a prepectoral placement of the implant. Oncologic surveillance, particularly for chest wall recurrence, poses unique challenges in women with prepectoral implant-based breast reconstruction and a standard of care remains to be established. Mammography is not useful following mastectomy with implant reconstruction, and full-protocol MRI is expensive and not currently indicated in this low-risk setting.
Autologous tissue flap reconstruction may also be performed in either an immediate or a delayed fashion. Pedicled flaps may be offered as an option if resources to perform free tissue transfer are not available. Tissue-based breast reconstruction has the potential to achieve a more natural consistency. The key distinctions between implant and autologous reconstruction, which should be communicated as an essential component of the preoperative counseling, include a much lengthier operative time and a longer time for recovery. Donor sites may include the abdomen, thighs, back, or gluteal region, with the abdomen overwhelmingly being the most common. When compared, patient-reported outcomes following both implant-based and autologous breast reconstruction have revealed that long-term satisfaction is greater among patients who opted for an autologous approach [147].
Psychological Assessment and Counseling
Women undergoing elective RRM should be ideally referred for psychological assessment and counseling preoperatively. A mental health and substance use and abuse history is often not explored by the breast or plastic surgical staff and may be relevant in preparation for this type of procedure both in terms of pain control and adjustment to the change in body image. There is also typically a lot of loss and trauma that these patients have experienced in living with a hereditary cancer syndrome (e.g., death of close loved ones) and this may influence decision-making and coping styles. It is important to explore a patient’s motivation for surgery, assess their support structure and mental health stability, and, further, assess their capacity for decision-making, optimizing preparation for surgery, and ability to manage changes postoperatively. The patient needs to weigh the risks and benefits of their different options including enhanced surveillance and preventive medication and also envision best and worse-case scenarios for surgical outcomes, particularly if they are unsure about their choice.
Women who do experience regret often have been forced into the decision by the doctor or family or had significant complications. Patients may also benefit from relaxation training and other coping skills. Including partners or other social supports in counseling may help to strengthen social support for the surgery. Patients with significant anxiety or depression may benefit from further psychologic interventions, both preoperatively and postoperatively.
Family planning, sexuality, self-image, and the anxiety associated with both cancer risks and surveillance are all factors women consider when deciding whether and when to undergo RRM. A survey of 12 high-risk women who elected RRM elicited feelings of some regret in a minority (25%) of patients, while all of those surveyed expressed a sense of relief and reduced anxiety related to both cancer risk and screenings [148]. Another cohort of 14 women surveyed post-RRM reported initial distress related to physical appearance, self-image, and intimacy but also reported a significant decrease in anxiety related to breast cancer risk and were largely satisfied with their decision [149].
Lifestyle Modifications
Modifiable Risk Factors
BMI/Exercise
It has long been accepted that an elevated body mass index (BMI) can increase one’s risk for several chronic diseases [150]. However, the relationship between BMI and breast cancer risk is more complex and largely modified by both age and menopausal status. Specifically, premenopausal women with a higher BMI are at lower risk for the development of breast cancer, whereas postmenopausal women with a higher BMI are at an increased risk [151]. The mechanisms linking obesity with cancer risk are an area of active investigation. In postmenopausal women, excess adipose tissue serves as key source for the production of estrogen [151]. One possible theory for risk in obese women is the higher levels of circulating estrogens [81]. There are other mechanisms occurring simultaneously that create an environment favorable for tumor formation, however. In fact, the American Society of Clinical Oncology (ASCO) estimates that at least ~30% of breast cancers in the United States are attributable to obesity [152].
The World Cancer Research Fund’s (WCRF) Continuous Update Project (CUP) is an ongoing analysis which looks at how various dietary and lifestyle habits can impact cancer risk [153]. Data from multiple studies are compiled to generate recommendations for the public on general cancer prevention. For premenopausal breast cancer, CUP identified 12 newer studies that evaluated the relationship between BMI in young adulthood and premenopausal breast cancer risk. A meta-analysis of this data showed an 18% decreased risk of breast cancer per 5 kg/m2 increase in BMI [153, 154]. This inverse relationship was still seen even after adjusting for age, alcohol intake, and reproductive factors [20]. Conversely, the Pooling Project of Prospective Studies on Diet and Cancer (Pooling Project) studied postmenopausal breast cancer risk and found that women with a BMI >28 kg/m2 were 26% more likely to develop cancer compared to lean women [155]. Moreover, the Nurses’ Health Study (NHS) also found that gaining weight in adulthood can also impact breast cancer risk [154]. In this large prospective cohort study, weight changes among women 30–55 and women >55 were followed for 24–26 years. The data suggested that weight gain of 28.0 kg/m2 or more, particularly after menopause, is associated with an increased breast cancer risk, whereas weight loss after menopause is associated with a decreased risk.
Increased levels of exercise have also been shown to influence breast cancer risk. A large population-based study of over 90,000 women aged 40–65 years showed that those who had reported more than 5 hours of vigorous exercise per week compared to those who did not had overall reductions in breast cancer risk [81]. Another large prospective cohort by Eliasson et al. also demonstrated that postmenopausal women with both higher levels of both recent and long-term total physical activity had lower breast cancer risk [156, 157]. Interestingly, the main activity in this cohort was “brisk walking” suggesting that only 5 hours per week of this could lead to a reduced risk while also falling in line with the recommendations by the American Heart Association for general physical activity [156,157,158]. Studies from CUP and WCRF also looked at several studies assessing physical activity and breast cancer risk and concluded that “vigorous physical activity probably protects against both premenopausal and postmenopausal breast cancers” [153, 154]. Ultimately, women should be encouraged to adopt an exercise regimen early and to maintain both a healthy weight and BMI through adulthood and beyond menopause [81].
Alcohol
Alcohol is a known modifiable risk factor for the development of breast cancer. There are many potential mechanisms that are postulated for this risk, and its effect is likely related to genetic and genomic differences between individuals as well.
Multiple studies have demonstrated a modest increase in risk associated with increased alcohol consumption. In a pooled-analysis by Smith-Warner et al., seven prospective cohort studies examined the association between alcohol consumption and breast cancer risk, accounting for several additional modifiable risk factors [159]. A total of 322,647 women were studied and followed for an average of 11 years. Those who consumed greater than 60 g/day had a 31% higher chance for developing invasive cancer [159]. These results were consistent even when attributing for additional factors including menopausal status. Additional data from the Cancer Prevention Study II showed that alcohol consumption also has an impact on breast cancer prognosis. Data showed that women who consumed 2–3 drinks per day had a 50% higher risk of breast cancer mortality compared to nondrinkers [159, 160] suggesting that not only does alcohol increase one’s risk, but is also associated with increased mortality from breast cancer.
The CUP performed a pooled analysis of nearly 15 cohort studies on premenopausal breast cancer risk and alcohol intake. Ten of those studies reported a statistically significant increase of 5% per 10 g of alcohol consumed per day [153]. Conversely, 35 studies were reviewed evaluating the association between alcohol and breast cancer risk in postmenopausal women and nearly all studies showed a positive association. A large meta-analysis revealed that among postmenopausal women, breast cancer increased 9% per 10 mg of ethanol consumed per day. The CUP’s panel conclusions assert that consumption of alcohol is “probably” a cause of premenopausal breast cancer and a “convincing” cause of postmenopausal breast cancer [153]. As a result, the NCCN recommends women to consume less than 1 glass of alcohol per day, defined as either 1 ounce of liquor, 8 ounces of beer, or 6 ounces of wine [81].
Breastfeeding
There is convincing evidence that breastfeeding is a protective modifiable risk factor for breast cancer [161]. The mechanism whereby breastfeeding influences breast cancer is likely multifactorial. First lactation itself produces a distinctive hormonal state in the body and is often associated with a time of amenorrhea and infertility. As a result, there is a reduction in circulating estrogen levels, which can potentially impact cancer risk. Additionally, changes within the breast tissue itself during lactation can also affect tumorogenesis [153]. Breastfeeding not only reduces breast cancer risk but also has other long-term health benefits for both the mother and the baby. Consequently, most organizations recommend breastfeeding exclusively for the first 6 months with continuance for 1 year or longer, depending on the desire of the mother and baby [162].
Screening mammography can be performed in a lactating woman, ideally after she empties her breasts by either pumping or feeding her baby. Diagnostic imaging is performed anytime during pregnancy or lactation if a woman has a concerning breast sign or symptom; if mammography is necessary in a pregnant woman, the uterus is shielded. MRI is contraindicated during pregnancy due to the necessary administration of gadolinium and is generally not performed during lactation as the hormonal effects on the breast tissue limit the sensitivity of the exam. In extremely high-risk women (e.g., highly penetrant gene mutation carriers), it is not unreasonable to perform MRI screening day 7–15 after their first menstrual cycle returns when they have stopped breastfeeding. Otherwise, the test is more sensitive if one waits until the woman is 6 months out from breastfeeding to order the breast MRI.
A large meta-analysis in 2002 pooled data from 47 different studies across 30 countries and found that the relative risk for breast cancer is reduced by 4.3% for every 1 year a woman breastfeeds [162, 163]. Additionally, a relative risk was also reduced by 7% with each individual birth [163]. Ten years later, a large systematic review revealed that women who breastfed had a 14% lower risk than women who did not breastfeed. The reduced risk was sustained regardless of the number of births and for those women who had breastfed for longer than 1 year; an even more substantial reduction in breast cancer risk was noted. Convincing evidence from additional literature also suggest that breastfeeding has an impact on the cancer subtype. A meta-analysis revealed that breastfeeding was associated with reductions in both luminal and triple-negative cancers; however, no difference was noted on the development hormone-positive breast cancers [163].
The current guidelines from NCCN and WCRF state that breastfeeding should be encouraged among reproductive-aged women and that the evidence is convincing that lactation protects against breast cancer [27, 154]. This often presents challenges for women working outside of the home.
Menopausal Hormone Therapy
The use of hormone therapy has remained a controversial topic for many decades, with many studies showing mixed results. The Women’s Health Initiative (WHI) was one of the first and largest studies that looked at the relationship between hormone therapy use and primary disease prevention. Over 150,000 postmenopausal women were enrolled and into a set of clinical trials involving either combined hormone therapy (estrogen plus progestin) for women with an intact uterus, and estrogen alone therapy for women without a uterus. The former study ended early due to finding an increase rate of breast cancer and stroke in women on combined hormonal thearpy. [27]. An attributable risk for breast cancer for a woman on combined hormone therapy in the WHI was found to be less than 1 additional case per 1000 women annually [164]. In addition to a slight increased risk, changes in breast density were seen on mammography in those on combined hormones. A statistically significant increase of 6% was noted in baseline breast density after 1 year of use [164]. At year 2, the degree of increase was decreased to 4.9% suggesting that the mammographic effects of hormone therapy are maintained but are not progressive [165]. While the WHI showed an increased risk in breast cancer in the combined therapy group, an 18-year follow-up study showed no increased risk of breast cancer related or all-cause mortality [166].
Conversely, hysterectomized women in the estrogen-only arm had lower risks of breast cancer when compared to placebo (20% at 7.1 years which did not reach statistical significance) [167]. Secondary analysis revealed that estrogen use is associated with an increase in breast density (not as significant as that which is seen with combined hormone therapy) in addition to an increase in benign proliferative breast disease. In a separate randomized controlled trial by Rohan et al., the daily use of 0.625 mg of conjugated equine estrogen was associated with a twofold increase in benign proliferative disease during a follow up period of 6.9 years [168].
While the WHI was one of the largest studies to date studying the effects hormone therapy on chronic disease and cancer risk, numerous smaller studies have also been done as a means to better understand this relationship. For those taking estrogen alone, the risk of breast cancer has been mixed. Some smaller trials have shown similar nonsignificant reductions in risk as seen in the WHI, while other observational studies show an increased risk [164]. Long-term data on prolonged use of estrogen have also been mixed – some observational data suggest an increased risk if on estrogen alone for greater than 5 years, but others have not, suggesting that duration of use may play a role in overall risk [164]. The age at which one begins hormone therapy may also be important. The average age of entry into the WHI study was 62, well past when most women would be prescribed hormone therapy, and coincidentally at the average age of diagnosis of sporadic breast cancer in the United States.
The North American Menopause Society recommends that both breast cancer risk and cardiovascular risk be discussed with all women who are considering hormone therapy [164]. Those who are at an elevated risk should carefully weigh the risks and benefits of use, and if prescribed, it should be given within 10 years of the onset of menopause. Alternatively, the use of vaginal estrogen alone can be given safely to those at average or high risk and, in certain settings, be given to breast cancer survivors in collaboration with a woman’s oncologist.
What About Contraception?
A study published in the New England Journal of Medicine [169] looked at the outcomes of 1.8 million Danish women aged 15–49 years old, who were followed for an average of over 10 years. They found that among these women, those who were currently or recently using hormonal birth control had a 20% higher relative risk for the development of breast cancer during the study period. The absolute risk associated with contraception, however, was very low; for every 7690 women using hormonal contraception for 1 year, there may be one extra breast cancer case diagnosis. Further there is no evidence that women at increased risk for the development of breast cancer should be advised against hormonal contraception. Oral contraceptives reduce the risk of ovarian cancer and endometrial cancer by 50% and have been studied in BRCA carriers who have exhibited no increased risk for breast cancer [163, 170].
Conclusion
The past several years have brought substantial developments in the field of risk assessment, germline genetic testing, and risk management. Exciting technology such as next-generation sequencing for high-throughput testing of multiple genes simultaneously at a lower cost has made genetic testing more widely available, and patients are fascinated by the information, as evidenced by the booming direct-to-consumer market. Patients and primary care providers are becoming more aware, involved, and invested. Our understanding of cancer risks associated with both genetic and nongenetic risk factors continues to improve, and the plethora of information now available leads to new challenges and nuances in clinical practice. Focus on identification of those at hereditary risk, patients with a history of therapeutic chest irradiation, and women with benign atypical biopsies such as ADH/ALH and LCIS is critical in meeting the challenge of helping those at the highest risk. Assessment of breast density is important for all women, in making choices about screening, about supplemental screening, and about risk related to the density itself. Refinement and validation of the polygenic risk score and integration into traditional risk models may lead to further personalization and risk refinement.
Ongoing management of the high-risk woman involves special considerations and understanding, particularly with regard to the psychological aspects associated with high-risk care and risk reduction. Women, particularly with a hereditary predisposition to breast cancer, are faced with complex and emotional decisions about the best ways to manage and reduce their risks. Referral to multiple subspecialties is an important component of these patients’ preventive care and may include cancer genetics, a high-risk breast clinic, gynecologic oncology, and counseling services. Options for risk management include enhanced surveillance, preventive medications, and, in some cases, risk-reducing surgery. Healthy lifestyle modification should be recommended for all.
It is imperative to identify those women who are at an elevated risk for breast cancer and identify them early, before cancer develops. Physicians should be systematic when obtaining family history and make sure it is updated annually. Primary care providers need the time and comfort level to effectively counsel regarding risk reduction strategies including chemopreventive medications. It is important to remember that risk assessment and management is not a one-time conversation, but an ongoing process – personal and family history changes, and guidelines change, and with time, the relationship that develops between the patient and the provider encourages compliance, open communication, and personalized patient care.
References
Mattiuzzi C, Lippi G. Current cancer epidemiology glossary. J Epidemiol Glob Health. 2019;9(4):217–22.
Eyre H, Blount L. American Cancer Society. J Oncol Pract. 2006;2(2):99.
Tung N, Battelli C, Allen B, Kaldate R, Bhatnagar S, Bowles K, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121(1):25–33.
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;32(19):2001–9.
Weiss AS, Swisher E, Pennington KP, Radke M, Khasnavis N, Garcia RL, et al. Inherited mutations in fallopian tube, ovarian and primary peritoneal carcinoma: changes in diagnoses and mutational frequency over 20 years. Gynecol Oncol [Internet]. 2020;159(1):214–20. Available from: https://doi.org/10.1016/j.ygyno.2020.06.509.
Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995;57:1457–62.
Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402.
Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, et al. Cancer risks associated with germline PALB2 pathogenic variants: an international study of 524 families. J Clin Oncol. 2020;38(7):674–85.
Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Cancer Netw. 2020;18(4):380–91.
Hansford S, Kaurah P, Li-Chang H, Woo M, Senz J, Pinheiro H, et al. Hereditary diffuse gastric cancer syndrome CDH1 mutations and beyond. JAMA Oncol. 2015;1(1):23–32.
Roberts ME, Ranola JMO, Marshall ML, Susswein LR, Graceffo S, et al. Comparison of CDH1 penetrance estimates in clinically ascertained families vs families ascertained for multiple gastric cancers supplemental content. JAMA Oncol. 2019;5(9):1325–31.
Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2018;16(11):1362–89.
Tan M-H, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18(2):400–7.
Bubien V, Bonnet F, Brouste V, Hoppe S, Barouk-Simonet E, David A, et al. High cumulative risks of cancer in patients with PTEN hamartoma tumour syndrome. J Med Genet. 2013;50:255–63.
Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12(10):3209–15.
Gonzalez KD, Noltner KA, Buzin CH, Gu D, Wen-Fong CY, Nguyen VQ, et al. Beyond Li Fraumeni syndrome: clinical characteristics of families with p53 germline mutations. J Clin Oncol. 2009;27(8):1250–6.
Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35(34):3800–6.
Hughes KS. Genetic testing: what problem are we trying to solve? J Clin Oncol [Internet]. 2017;35(34):3789–91. Available from: https://doi.org/10.1200/JCO.2017.74.7899.
Hu C, Polley EC, Yadav S, Lilyquist J, Shimelis H, Na J, et al. The contribution of germline predisposition gene mutations to clinical subtypes of invasive breast cancer from a clinical genetic testing cohort. J Natl Cancer Inst. 2020;112(12):1231–41.
DeFrancesco MS, Waldman RN, Pearlstone MM, Karanik D, Bernhisel R, Logan J, et al. Hereditary cancer risk assessment and genetic testing in the community-practice setting. Obstet Gynecol. 2018;132(5):1121–9.
Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Risk assessment, genetic counseling, and genetic testing for BRCA -related cancer: US preventive services task force recommendation statement. JAMA – J Am Med Assoc. 2019;322(7):652–65.
Syndromes HC, Assessment R. Hereditary cancer syndromes and risk assessment. Obstet Gynecol. 2019;134(6):1366–7.
Buys SS, Sandbach JF, Gammon A, Patel G, Kidd J, Brown KL, et al. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer. 2017;123(10):1721–30.
Tung N, Lin NU, Kidd J, Allen BA, Singh N, Wenstrup RJ, et al. Frequency of germline mutations in 25 cancer susceptibility genes in a sequential series of patients with breast cancer. J Clin Oncol. 2016;34(13):1460–8.
Kurian AW, Li Y, Hamilton AS, Ward KC, Hawley ST, Morrow M, et al. Gaps in incorporating germline genetic testing into treatment decision-making for early-stage breast cancer. J Clin Oncol. 2017;35(20):2232–9.
Randall LM, Pothuri B, Swisher EM, Diaz JP, Buchanan A, Witkop CT, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: a society of gynecologic oncology white paper. Gynecol Oncol [Internet]. 2017;146(2):217–24. Available from: https://doi.org/10.1016/j.ygyno.2017.06.002.
Bevers TB, Ward JH, Arun BK, Colditz GA, Cowan KH, Daly MB, et al. Breast cancer risk reduction, version 2.2015 clinical practice guidelines in oncology clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2015;13(7):880–915.
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53.
Darst BF, Dadaev T, Saunders E, Sheng X, Wan P, Pooler L, et al. Germline sequencing DNA repair genes in 5,545 men with aggressive and non-aggressive prostate cancer. J Natl Cancer Inst [Internet]. 2020. Available from: https://doi.org/10.1093/jnci/djaa132.
Cremin C, Lee MKC, Hong Q, Hoeschen C, Mackenzie A, Dixon K, et al. Burden of hereditary cancer susceptibility in unselected patients with pancreatic ductal adenocarcinoma referred for germline screening. Cancer Med. 2020;9(11):4004–13.
Walsh T, Mandell JB, Norquist BM, Casadei S, Gulsuner S, Lee MK, et al. Genetic predisposition to breast cancer due to mutations other than BRCA1 and BRCA2 founder alleles among Ashkenazi Jewish women. JAMA Oncol. 2017;3(12):1647–53.
Frey MK, Kopparam RV, Ni Zhou Z, Fields JC, Buskwofie A, Carlson AD, et al. Prevalence of nonfounder BRCA1/2 mutations in Ashkenazi Jewish patients presenting for genetic testing at a hereditary breast and ovarian cancer center. Cancer. 2019;125(5):690–7.
Beitsch PD, Whitworth PW, Hughes K, Patel R, Rosen B, Compagnoni G, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37(6):453–60.
Manahan ER, Kuerer HM, Sebastian M, Hughes KS, Boughey JC, Euhus DM, et al. Consensus guidelines on genetic’ testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol [Internet]. 2019;26(10):3025–31. Available from: https://doi.org/10.1245/s10434-019-07549-8.
Donald CG, Sanders AK. The genetic information nondiscrimination act of 2008. J Divers Manag. 2008;3(4):33–46.
Churpek JE, Walsh T, Zheng Y, Moton Z, Thornton AM, Lee MK, et al. Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015;149(1):31–9.
Shaaban AM, Sloane JP, West CR, Moore FR, Jarvis C, Williams EMI, Foster CS. Histopathologic types of benign breast lesions and the risk of breast cancer: case-control study. Am J Surg Pathol. 2002;26(4):421–30.
Hartmann L, Sellers T, Frost M. Benign breast disease and the risk of breast cancer. N Engl J Med [Internet]. 2005 [cited 2020 Oct 31];353(3):229–37. Available from: www.nejm.org.
Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N. Lower-category benign breast disease and the risk of invasive breast cancer. Available from: https://academic.oup.com/jnci/article/96/8/616/2521209.
Hartmann LC, Radisky DC, Frost MH, Santen RJ, Vierkant RA, Benetti LL, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. 2014. Available from: www.aacrjournals.org.
Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast — risk assessment and management options. N Engl J Med. 2015;372(1):78–89.
Simpson JF. Update on atypical epithelial hyperplasia and ductal carcinoma in situ. Pathology. 2009;41(1):36–9.
Waters E, Mcneel T, Mccaskill W. Use of tamoxifen and raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134:875–80.
Library WO, Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. Invit Rev J Pathol J Pathol [Internet]. 2011;223:307–17. Available from: www.pathsoc.org.uk.
Lopez-Garcia MA, Geyer FC, Lacroix-Triki M, Marchió C, Reis-Filho JS. Breast cancer precursors revisited: molecular features and progression pathways. Histopathology. 2010;57:171–92.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–88.
Hartmann L, Sellers T, Frost M. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353(3):229–37.
Hartmann LC, Radisky DC, Frost MH, Santen RJ, Vierkant RA, Benetti LL, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila). 2014;7(2):211–7.
Hartmann LC, Degnim AC, Dupont WD. Atypical hyperplasia of the breast. N Engl J Med. 2015;372(13):1271–2.
Kerlikowske K, Gard CC, Tice JA, Ziv E, Cummings SR, Miglioretti DL, et al. Risk factors that increase risk of estrogen receptor-positive and-negative breast cancer. J Natl Cancer Inst. 2016;109(5):djw276.
Urban JA. Bilaterality of cancer of the breast. Biopsy of the opposite breast. Cancer. 1967;20(11):1867–70.
Fisher ER, Land SR, Fisher B, Mamounas E, Gilarski L, Wolmark N. Pathologic findings from the national surgical adjuvant breast and bowel project: twelve-year observations concerning lobular carcinoma in situ. Cancer. 2004;100(2):238–44.
Chuba PJ, Hamre MR, Yap J, Severson RK, Lucas D, Shamsa F, et al. Bilateral risk for subsequent breast cancer after lobular carcinoma-in-situ: analysis of surveillance, epidemiology, and end results data. J Clin Oncol. 2005;23:5534–41.
King TA, Pilewskie M, Muhsen S, Patil S, Mautner SK, Park A, et al. Lobular carcinoma in situ: a 29-year longitudinal experience evaluating clinicopathologic features and breast cancer risk. J Clin Oncol. 2015;33(33):3945–52.
Wong SM, King T, Boileau J-F, Barry WT, Golshan M, Golshan M, et al. Population-based analysis of breast cancer incidence and survival outcomes in women diagnosed with lobular carcinoma in situ. Soc Surg Oncol 70th Annu Cancer Symp. 2017;24:15–8.
Chivukula M, Bhargava R, Tseng G, Dabbs DJ. Clinicopathologic implications of “flat epithelial atypia” in core needle biopsy specimens of the breast. Am J Clin Pathol [Internet]. 2009;131:802–8. Available from: https://academic.oup.com/ajcp/article-abstract/131/6/802/1760543.
Khoumais NA, Scaranelo AM, Moshonov H, Kulkarni SR, Miller N, Mccready DR, et al. Incidence of breast cancer in patients with pure flat epithelial atypia diagnosed at core-needle biopsy of the breast. Ann Surg Oncol. 2013;20(1):133–8.
Said SM, Visscher DW, Nassar A, Frank RD, Vierkant RA, Frost MH, et al. Flat epithelial atypia and risk of breast cancer: a Mayo cohort study. Cancer. 2015;121(10):1548–55.
Byrne C, Schairer C, Brinton LA, Wolfe J, Parekh N, Salane M, et al. Effects of mammographic density and benign breast disease on breast cancer risk (United States). Cancer Causes Control. 2001;12(2):103–10.
Wolfe JN, Saftlas AF, Salan M. Mammographic parenchymal patterns and quantitative evaluation of mammographic densities: a case-control study. AJR Am J Roentgenol. 1987;148(6):1087–92.
Orman B, Oyd NF, Illian D, Ite GS, Ennifer Tone JS, Noma Unasekara AG, Allas E, Nglish DR, Argaret MCC, Redie MR, et al. Heritability of mammographic density, a risk factor for breast cancer. N Engl J Med. 2002;347:886–94.
Mccormack VA, Dos I, Silva S. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev. 2006;15(6):1159–69.
Bertrand KA, Tamimi RM, Scott CG, Jensen MR, Shane Pankratz V, Visscher D, et al. Mammographic density and risk of breast cancer by age and tumor characteristics. Breast Cancer Res. 2013;15(6):R104.
Greendale GA, Reboussin BA, Sie A, Singh HR, Olson LK, Gatewood O, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Ann Intern Med. 1999;130(4 Pt 1):262–9.
Greendale GA, Reboussin BA, Slone S, Wasilauskas C, Pike MC, Ursin G. Postmenopausal hormone therapy and change in mammographic density [Internet]. Available from: https://academic.oup.com/jnci/article/95/1/30/2520191.
Vachon CM, Sellers TA, Vierkant RA, Wu F-F, Brandt KR. Case-control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomark Prev. 2002;11(11):1382–8.
Stefanick ML, Anderson GL, Margolis KL, Hendrix SL, Rodabough RJ, Paskett ED, et al. Effects of conjugated equine estrogens on breast cancer and mammography screening in postmenopausal women with hysterectomy [Internet]. Available from: https://jamanetwork.com/.
Kerlikowske K, Cook AJ, Buist DSM, Cummings SR, Vachon C, Vacek P, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–7.
Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K. Population-attributable risk proportion of clinical risk factors for breast cancer supplemental content. JAMA Oncol. 2017;3(9):1228–37.
Travis LB, Hill D, Dores GM, Gospodarowicz M, Van Leeuwen FE, Holowaty E, et al. Cumulative absolute breast cancer risk for young women treated for Hodgkin lymphoma. J Natl Cancer Inst. 2005;97(19):1428–37.
Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Friedman DN, et al. Republic of China. J Clin Oncol. 2014;32:2217–23.
Conway JL, Connors JM, Tyldesley S, Savage KJ, Campbell BA, Zheng YY, et al. Secondary breast cancer risk by radiation volume in women with Hodgkin lymphoma. Int J Radiat Oncol Biol Phys. 2017;97(1):35–41.
Opstal-Van Winden AWJ, De Haan HG, Hauptmann M, Schmidt MK, Broeks A, Russell NS, et al. Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood. 2019;133:1130–9.
Grobmyer SR, Pederson HJ, Valente SA, Al-Hilli Z, Radford D, Djohan R, et al. Evolving indications and long-term oncological outcomes of risk-reducing bilateral nipple-sparing mastectomy. BJS Open. 2019;3(2):169–73.
Haas JS, Kaplan CP, Des Jarlais G, Gildengoin V, Pérez-Stable EJ, Kerlikowske K. Perceived risk of breast cancer among women at average and increased risk. J Women’s Heal. 2005;14(9):845–51.
Metcalfe KA, Quan M-L, Eisen A, Cil T, Sun P, Narod SA. The impact of having a sister diagnosed with breast cancer on cancer-related distress and breast cancer risk perception. Cancer. 2013;119:1722–30.
Shieh Y, Hu D, Ma L, Huntsman S, Gard CC, Leung JWT, et al. Breast cancer risk prediction using a clinical risk model and polygenic risk score. Breast Cancer Res Treat. 2016;159(3):513–25.
Gail MH, Pfeiffer RM. Breast cancer risk model requirements for counseling, prevention, and screening. J Natl Cancer Inst. 2018;110(9):994–1002.
Cintolo-Gonzalez JA, Braun D, Blackford AL, Mazzola E, Acar A, Plichta JK, et al. Breast cancer risk models: a comprehensive overview of existing models, validation, and clinical applications. Breast Cancer Res Treat. 2017;164(2):263–84.
Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Medication use to reduce risk of breast cancer: US preventive services task force recommendation statement. JAMA – J Am Med Assoc. 2019;322(9):857–67.
Bevers TB, Ward JH, Ahrendt GM, Colditz GA, Daly MB, Gandhi S, et al. National comprehensive cancer network clinical practice guidelines in oncology; breast cancer risk reduction version 1.2020 [Internet]. 2020 [cited 2020 May 7]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast_risk.pdf.
Pankratz VS, Hartmann LC, Degnim AC, Vierkant RA, Ghosh K, Vachon CM, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26(33):5374–9.
Barke LD, Freivogel ME. Breast cancer risk assessment models and high-risk screening. Radiol Clin N Am. 2017;55(3):457–74.
Visvanathan K, Fabian CJ, Bantug E, Brewster AM, Davidson NE, Decensi A, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152–65.
Evans DGR, Howell A. Breast cancer risk-assessment models. Breast Cancer Res. 2007;9(5):1–8.
Berry DA, Iversen ESJ, Gudbjartsson DF, Hiller EH, Garber JE, Peshkin BN, et al. BRCAPRO validation, sensitivity of genetic testing of BRCA1/BRCA2, and prevalence of other breast cancer susceptibility genes. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(11):2701–12.
Lee A, Mavaddat N, Wilcox AN, Cunningham AP, Carver T, Hartley S, et al. BOADICEA: a comprehensive breast cancer risk prediction model incorporating genetic and nongenetic risk factors. Genet Med. 2019;21(8):1708–18.
McCarthy AM, Guan Z, Welch M, Griffin ME, Sippo DA, Deng Z, et al. Performance of breast cancer risk-assessment models in a large mammography cohort. J Natl Cancer Inst. 2020;112(5):489–97.
Lilyquist J, Ruddy KJ, Vachon CM, Couch FJ. Common genetic variation and breast cancer risk-past, present, and future. Cancer Epidemiol Biomark Prev. 2018;27(4):380–94.
Michailidou K, Beesley J, Lindstrom S, Canisius S, Dennis J, Lush MJ, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Publ Gr. 2015;47:373–80.
Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–61.
Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, Kuchenbaecker KB, et al. Fabienne Prieur 111, Francesca Damiola 9 [Internet]. Ontario Cancer Genetics Network. [cited 2020 Nov 1]:13. Available from: www.plosgenetics.org.
Gallagher S, Hughes E, Wagner S, Tshiaba P, Rosenthal E, Roa BB, et al. Association of a polygenic risk score with breast cancer among women carriers of high- and moderate-risk breast cancer genes. JAMA Netw Open. 2020;3(7):e208501.
Muranen TA, Greco D, Blomqvist C, Aittomäki K, Khan S, Hogervorst F, et al. Genetic modifiers of CHEK2*1100delC-associated breast cancer risk. Genet Med. 2017;19(5):599–603.
Kuchenbaecker KB, Mcguffog L, Barrowdale D, Lee A, Soucy P, Dennis J, et al. Evaluation of polygenic risk scores for breast and ovarian cancer risk prediction in BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2017;109(7):djw302.
Barton M, Harris R, Sw F, Nusbaum NJ. Role of the Clinical Breast Examination in Breast Cancer Screening. J Am Geriatr Soc. 2001;49(7):991–2.
Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138(3):168–75.
Kolb TM, Lichy J, Newhouse JH. Abbreviations: BI-RADS Breast Imaging Reporting and Data System HRT hormonal replacement therapy PE physical examination comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence T. Radiology. 2002;225:165–75.
Lo G, Scaranelo AM, Aboras H, Ghai S, Kulkarni S, Fleming R, et al. Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology. 2017;285:36–43.
Webb ML, Cady B, Michaelson JS, Bush DM, Calvillo KZ, et al. A failure analysis of invasive breast cancer most deaths from disease occur in women not regularly screened. Cancer. 2014;120:2839–85.
Armes JE, Egan AJM, Southey MC, Dite GS, McCredie MRE, Giles GG, et al. The histologic phenotypes of breast carcinoma occurring before age 40 years in women with and without BRCA1 or BRCA2 germline mutations: a population-based study. Cancer. 1998;83(11):2335–45.
Dent R, Warner E. Screening for hereditary breast cancer. Semin Oncol. 2007;34(5):392–400.
Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22(12):2328–35.
Komenaka IK, Ditkoff B-A, Joseph K-A, Russo D, Gorroochurn P, Ward M, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer. 2004;100(10):2079–83.
Bevers TB, Helvie M, Bonaccio E, Calhoun KE, Daly MB, Farrar WB, et al. Breast cancer screening and diagnosis, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(11):1362–89.
Bernardi D, Li T, Pellegrini M, Macaskill P, Valentini M, Fantò C, et al. Effect of integrating digital breast tomosynthesis (3D-mammography) with acquired or synthetic 2D-mammography on radiologists’ true-positive and false-positive detection in a population screening trial: a descriptive study. Eur J Radiol [Internet]. 2018;106(May 2018):26–31. Available from: https://doi.org/10.1016/j.ejrad.2018.07.008.
Friedewald SM, Rafferty EA, Rose SL, Durand MA, Plecha DM, Greenberg JS, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA – J Am Med Assoc. 2014;311(24):2499–507.
Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol [Internet]. 2013;14(7):583–9. Available from: https://doi.org/10.1016/S1470-2045(13)70134-7.
Haas BM, Kalra V, Geisel J, Raghu M, Durand M, Philpotts LE. Comparison of tomosynthesis plus digital mammography and digital mammography alone for breast cancer screening. Radiology. 2013;269(3):694–700.
Heijnsdijk EAM, Warner E, Gilbert FJ, Tilanus-Linthorst MMA, Evans G, Causer PA, et al. Differences in natural history between breast cancers in BRCA1 and BRCA2 mutation carriers and effects of MRI Screening-MRISC, MARIBS, and Canadian studies combined. Cancer Epidemiol Biomark Prev. 2012;21(9):1458–68.
Lord SJ, Lei W, Craft P, Cawson JN, Morris I, Walleser S, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43(13):1905–17.
Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–18.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57.
Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening screening for breast cancer mammography. J Magn Reson Imaging. 2019;50:377–90.
Evans DG, Kesavan N, Lim Y. MRI Breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat. 2014;145(3):663–72.
Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol [Internet]. 2018;15(3):408–14. Available from: https://doi.org/10.1016/j.jacr.2017.11.034.
American College of Radiology. Practice parameter for the performance of contrast-enhanced magnetic resonance imaging (CE-MRI) of the breast. 2018;1076:1–11. Available from: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/MR-Contrast-Breast.pdf?la=en.
Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI. A feasibility study. Acad Radiol [Internet]. 2015;22(9):1157–62. Available from: https://doi.org/10.1016/j.acra.2015.06.004.
Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol [Internet]. 2016;13(11):R74–80. Available from: https://doi.org/10.1016/j.jacr.2016.09.031.
Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast Magnetic Resonance Imaging (MRI): first postcontrast subtracted images and maximum-intensity projection – a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32(22):2304–10.
Kuhl CK, Strobel K, Bieling H, Leutner C, Schild HH, Schrading S. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361–70.
Weinstein SP, Korhonen K, Cirelli C, Schnall MD, McDonald ES, Pantel AR, et al. Abbreviated breast magnetic resonance imaging for supplemental screening of women with dense breasts and average risk. J Clin Oncol. 2020;38(33):3874–82. https://doi.org/10.1200/JCO.19.02198.
Comstock CE, Gatsonis C, Newstead GM, Snyder BS, Gareen IF, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323(8):746–56.
Geach R, Jones LI, Harding SA, Marshall A, Taylor-Phillips S, McKeown-Keegan S, et al. The potential utility of abbreviated breast MRI (FAST MRI) as a tool for breast cancer screening: a systematic review and meta-analysis. Clin Radiol. 2021;76(2):154.e11–22.
Runge VM. Dechelation (Transmetalation). Investig Radiol. 2018;53(10):571–8.
Gradishar W, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. National comprehensive cancer network clinical practice guidelines in oncology: breast cancer version 5.2020 [Internet]. 2020 [cited 2020 Jan 9]. Available from: https://www2.tri-kobe.org/nccn/guideline/breast/english/breast.pdf.
Mann RM, Hooley R, Barr RG, Moy L. Novel approaches to screening for breast cancer. Radiology. 2020;297(2):200172.
Nichols HB, Stürmer T, Lee VS, Anderson C, Lee JS, Roh JM, et al. Breast cancer chemoprevention in an integrated health care setting. JCO Clin Cancer Inform. 2017;1:1–12.
Pinsky PF, Miller E, Heckman-Stoddard B, Minasian L. Use of raloxifene and tamoxifen by breast cancer risk level in a Medicare-eligible cohort. Am J Obstet Gynecol. 2018;218(6):606.e1–9.
Khaliq W, Visvanathan K. Breast cancer chemoprevention: current approaches and future directions. Curr Obstet Gynecol Rep. 2012;1(1):33–41.
Chlebowski RT. Current concepts in breast cancer chemoprevention. Pol Arch Med Wewn. 2014;124(4):191–9.
Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62.
Sauter ER. Breast cancer prevention: current approaches and future directions. Eur J Breast Health. 2018;4:64–71.
Pruthi S, Heisey RE, Bevers TB. Chemoprevention for breast cancer. Ann Surg Oncol. 2015;22(10):3230–5.
Cuzik J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300.
Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. JAMA [Internet]. 1999;281(23):2189–97. Available from: https://doi.org/10.1001/jama.281.23.2189.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. J Am Med Assoc. 2006;295(23):2727–41.
Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, et al. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96(23):1751–61.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, et al. Update of the national surgical adjuvant breast and bowel project Study of Tamoxifen and Raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev Res [Internet]. 2010;3(6):696 LP–706. Available from: http://cancerpreventionresearch.aacrjournals.org/content/3/6/696.abstract.
Moyer VA, on behalf of the USPSTF. Using medications to decrease the risk for breast cancer in women: recommendations from the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(10):698–708.
DeCensi A, Puntoni M, Guerrieri-Gonzaga A, Caviglia S, Avino F, Cortesi L, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol [Internet]. 2019;37(19):1629–37. Available from: https://doi.org/10.1200/JCO.18.01779.
Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–91.
Perez EA, Josse RG, Pritchard KIIJ. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen therapy: a companion study to NCIC CTG MA.17. J Clin Oncol. 2016;24(22):3629–35.
Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet [Internet]. 2014;383(9922):1041–8. Available from: https://doi.org/10.1016/S0140-6736(13)62292-8.
Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93(21):1633–7.
Metcalfe K, Gershman S, Ghadirian P, Lynch HT, Snyder C, Tung N, et al. Contralateral mastectomy and survival after breast cancer in carriers of BRCA1 and BRCA2 mutations: retrospective analysis. BMJ. 2014;348:g226.
Duraes EFR, Schwarz GS, de Sousa JB, Duraes LC, Morisada M, Baker T, et al. Factors influencing the aesthetic outcome and quality of life after breast reconstruction: a cross-sectional study. Ann Plast Surg. 2020;84(5):494–506.
Kwong A, Chu ATW. What made her give up her breasts: a qualitative study on decisional considerations for contralateral prophylactic mastectomy among breast cancer survivors undergoing BRCA1/2 genetic testing. Asian Pac J Cancer Prev. 2012;13(5):2241–7.
Lodder LN, Frets PG, Trijsburg RW, Meijers-Heijboer EJ, Klijn JGM, Seynaeve C, et al. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery). Breast Cancer Res Treat. 2002;73(2):97–112.
Harvie M, Hooper L, Howell AH. Central obesity and breast cancer risk: a systematic review. Obes Rev [Internet]. 2003;4(3):157–73. Available from: https://doi.org/10.1046/j.1467-789x.2003.00108.x
Linos E, Willett WC. Diet and breast cancer risk reduction. J Natl Compr Cancer Netw. 2007;5(8):711–8.
Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74.
World cancer research fund: about continuous update project [Internet]. [cited 2020 May 7]. Available from: https://www.wcrf.org/int/continuous-update-project.
World cancer research fund: breast cancer report [Internet]. [cited 2020 May 7]. Available from: https://www.wcrf.org/sites/default/files/Breast-cancer-report.pdf.
Liu K, Zhang W, Dai Z, Wang M, Tian T, Liu X, et al. Association between body mass index and breast cancer risk: evidence based on a dose–response meta-analysis. Cancer Manag Res. 2018;10:143–51.
Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA [Internet]. 2006;296(2):193–201. Available from: https://doi.org/10.1001/jama.296.2.193.
Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med [Internet]. 2010;170(19):1758–64. Available from: https://doi.org/10.1001/archinternmed.2010.363.
Association AH. American Heart Association Guidelines for physical activity [Internet]. 2011 [cited 2020 May 7];2012. Available from: https://www.heart.org/en/get-involved/advocate/federal-priorities/physical-activity.
Smith-Warner SA, Spiegelman D, Yaun SS, Van Den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. J Am Med Assoc. 1998;279(7):535–40.
Thun M, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW Jr, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N Engl J Med. 1997;337(24):1705–14.
Enger SM, Ross RK, Paganini-Hill A, Bernstein L. Breastfeeding experience and breast cancer risk among postmenopausal women. Cancer Epidemiol Biomark Prev. 1998;7(5):365–9.
Antsey EH, Shoemaker ML, Barrera CM, O’Neil ME, Verma ABHD. Breastfeeding and breast cancer risk reduction: implications for black mothers. Am J Prev Med. 2017;53(3):S40–6.
Beral V, Bull D, Doll R, Peto R, Reeves G. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50 302 women with breast cancer and 96 973 women without the disease. Lancet. 2002;360(9328):187–95.
Pinkerton JAV, Aguirre FS, Blake J, Cosman F, Hodis H, Hoffstetter S, et al. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24(7):728–53.
McTiernan A, Martin CF, Peck JD, Aragaki AK, Chlebowski RT, Pisano ED, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women’s health initiative randomized trial. J Natl Cancer Inst. 2005;97(18):1366–76.
Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trials. JAMA [Internet]. 2017;318(10):927–38. Available from: https://doi.org/10.1001/jama.2017.11217.
Investigators WG for the WHI. Risks and benefits of estrogen plus progestin in healthy postmenopausal women principal results from the women’s health initiative randomized controlled trial. JAMA [Internet]. 2002;288(3):321–33. Available from: https://doi.org/10.1001/jama.288.3.321.
Rohan TE, Negassa A, Chlebowski RT, Habel L, McTiernan A, Ginsberg M, et al. Conjugated equine estrogen and risk of benign proliferative breast disease: a randomized controlled trial. J Natl Cancer Inst. 2008;100(8):563–71.
Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–39.
Iodice S, Barile M, Rotmensz N, Feroce I, Bonanni B, Radice P, et al. Oral contraceptive use and breast or ovarian cancer risk in BRCA1/2 carriers: a meta-analysis. Eur J Cancer. 2010;46(12):2275–84.
Care GP. ACOG Committee opinion: counseling about genetic testing and communication of genetic test results. Obstet Gynecol. 2017;130(4):e210.
Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sahni, S.K., Sharma, N., Pederson, H.J. (2021). Management of a Woman at Elevated Risk for Breast Cancer. In: Shetty, M.K. (eds) Breast & Gynecological Diseases. Springer, Cham. https://doi.org/10.1007/978-3-030-69476-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-69476-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69475-3
Online ISBN: 978-3-030-69476-0
eBook Packages: MedicineMedicine (R0)