Abstract
A pituitary carcinoma (PC) is a rare neoplasm, accounting for only 0.2% of pituitary tumors, and is defined by the presence of noncontiguous metastatic disease. Its management requires a multimodal approach including surgery, irradiation, and medical therapy. Stereotactic radiosurgery (SRS) by means of the Gamma Knife or CyberKnife may be considered potentially useful in such cases. It has mainly been applied for localized metastases and symptomatic lesions, but it may also be effective in control of aggressive tumor growth at the primary site after sufficient surgical debulking of the lesion. Given the infrequency of PC and their heterogeneous nature with regard to the histopathological type, local extension, and location of metastases, large clinical series have not been compiled to date. While, in such cases, SRS is certainly not curative and does not prevent disease progression, it is quite reasonable to incorporate this treatment option into a multimodal management strategy and apply it judiciously at the treating clinician’s discretion on a case-by-case basis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- CyberKnife radiosurgery
- Gamma Knife radiosurgery
- Metastatic disease
- Pituitary adenoma
- Pituitary carcinoma
- Stereotactic radiotherapy
Introduction
While the majority of pituitary adenomas (PA) are benign and slow-growing lesions, they frequently demonstrate somewhat invasive growth [1, 2]. Additionally, certain histological subtypes of these tumors (e.g., sparsely granulated somatotroph adenomas, silent corticotroph adenomas, and Crooke cell adenomas) are more prone to invasion and to difficulty in achieving persistent remission [3]. However, despite frequent locally invasive or clinically aggressive behavior, only 0.2% of PA eventually metastasize [4]. The presence of noncontiguous metastasis (but not specific histological features) defines a pituitary carcinoma (PC).
General Characteristics of Pituitary Carcinomas
PC often show acquisition of mutations in TP53 and significantly increased MIB-1 immunolabeling for Ki-67 protein [2, 5]. However, in some cases, the MIB-1 index is low and overlaps with the expected values seen in benign PA (i.e., <3%) [2, 6]. Whereas only half of PA are hormone-secreting, nearly 90% of PC have detectable hormone production [7,8,9,10], and the majority of them secrete either prolactin or adrenocorticotropic hormone (ACTH) [8].
Although there is typically a delay (mean 6.5 years) from diagnosis of a PA to development of metastatic disease, such tumors are rapidly progressive once they show noncontiguous spread. The overall survival rate of patients at 1 year after diagnosis of the first metastasis is only 33% [9]. While initial management of PA (other than prolactinomas) is usually microsurgical tumor resection, PC require a multimodal treatment strategy including surgery, irradiation, and medical therapy. Nevertheless, because of the rarity of these tumors, neither treatment outcomes nor management paradigms—specifically, in regard to fractionated radiotherapy (FRT), stereotactic radiosurgery (SRS), chemotherapy, or their combination—have been fully defined.
Radiotherapy and Stereotactic Radiosurgery for Pituitary Adenomas
Irradiation of the sellar and parasellar area has been used to treat PA since the early 1900s and constitutes an important adjunct to their management [7, 11, 12]. Nowadays, treatment may be done either as conventional FRT or as SRS, and both of these techniques have increasingly become a standard second-line therapeutic option for several types of PA, including ACTH-secreting tumors (causing Cushing’s disease), growth hormone (GH)–secreting tumors (resulting in acromegaly or gigantism), and carefully selected nonfunctioning pituitary tumors [7, 13].
Therapeutic irradiation over multiple fractions offers the benefit of reduced cranial nerve toxicity, particularly for lesions adjacent to the optic apparatus [14]. Conversely, SRS allows highly selective and conformal high-dose single-session treatment—in particular, resulting in a shorter time to remission in cases of hormone-secreting PA [15,16,17]. This modality is based on precise stereotactic localization of a radiographically defined target and focusing of converging beams of irradiation on it with a steep dose falloff at the margin of the treatment volume. The most common technological devices used for SRS of PA are the Leksell Gamma Knife (Elekta AB; Stockholm, Sweden), which is typically a frame-based technique, and the CyberKnife (Accuray; Sunnyvale, CA, USA) which allows for frameless treatment, thus facilitating administration of multisession SRS and hypofractionated stereotactic radiotherapy (SRT). Usually, the prescribed marginal dose in cases of nonfunctioning PA ranges from 12 to 20 Gy, but it is greater (from 18 to >25 Gy) in hormone-secreting tumors, since the goals of SRS in such cases include both lesion growth control and endocrine remission [18].
Impact of Radiotherapy on Tumorigenesis of Pituitary Carcinomas
Many patients who eventually develop a PC initially present with a locally invasive tumor and multiple recurrences at the primary site. Because, in such cases, FRT is often part of combined treatment, it has been suggested that irradiation itself may have an impact on tumorigenesis of PC. Lall et al. [19] found that in 45 out of 46 reported cases of such tumors (98%), sellar/parasellar irradiation was given before appearance of metastases. However, a “post hoc ergo propter hoc” fallacy must be avoided; it is evident that FRT is more likely to be recommended for locally aggressive neoplasms [20]. Of note, other authors have not found the aforementioned association. In an earlier review, Mountcastle et al. [21] noted that fewer than half of the patients with PC (18 out of 38) had previously received sellar irradiation. In concordance, analysis of the Surveillance, Epidemiology, and End Results (SEER) database revealed seven cases of PC, but only in one of them was FRT given beforehand [22].
Radiotherapy for Pituitary Carcinomas
Although conventional FRT has been utilized for scattered cases of PC for several decades, few related data have been reported. For example, on the basis of a clinicopathological study, Pernicone et al. [9] presented one of the largest series of such tumors and briefly noted that 10 out of 15 patients received radiotherapy for treatment of metastases, but they did not provide either treatment details or outcome data. Given the infrequency of PC and their heterogeneous nature (in particular, with regard to the histopathological type, local extension, and location of metastases), large clinical series have not been compiled to date. Nevertheless, analysis of the several case reports that have described application of FRT for pituitary tumors with metastatic spread and the results of such treatment allows some understanding of the radiobiology of these rare neoplasms.
Efficacy of Combined Treatment
FRT has not typically been used as a stand-alone treatment for distant metastases or aggressive local extension of PC (Table 1). Two reports have described its postoperative administration after surgical tumor debulking, which resulted in local control for 3 years in one patient [6] and for 5 years in another [25]. Alternatively, FRT has been applied concurrently with systemic anticancer drugs, such as cisplatin, temozolomide (TMZ), and the mammalian target of rapamycin (mTOR) inhibitor everolimus [6, 23, 24, 26]. In one patient, chemotherapy with cisplatin combined with spinal irradiation resulted in tumor control for 2 years and a reduction in radicular pain [23]. In two other comparable cases of thoracic vertebral body and pelvis/sacrum metastases treated by a combination of everolimus and FRT, short follow-up periods precluded analysis of treatment effectiveness, but at least one patient experienced symptomatic improvement [26]. In two reports, irradiation was administered concurrently with TMZ and provided effective medium-term tumor growth control (for 1–1.5 years) [6, 24]. Of note, chemotherapy with TMZ, an alkylating agent with good penetration through the blood–brain barrier, may result in an initial response of the PC, but these neoplasms still progress eventually [27,28,29]. Finally, one patient, in addition to postoperative local-field FRT for a tumor extending into the orbit, received hypofractionated SRT (70 Gy in ten fractions) concurrently with TMZ for portacaval lymph node metastasis, which resulted in its control for 2.5 years [6]. Overall, these data suggest that while the results of FRT alone for PC are generally unknown, its combination with chemotherapy may contribute to effective local tumor control, at least during medium-term follow-up. Importantly, the treatment has usually been well tolerated, and minimal adverse radiation effects have been noted.
Stereotactic Radiosurgery for Pituitary Carcinomas
In our own experience, SRS is used most commonly to treat focal metastases of PC defined on magnetic resonance imaging (MRI), and is applied less often for sellar/parasellar tumors themselves, since prior surgery and proximity to the optic nerves and chiasm impose more ambiguous radiographic margins of the target lesion and limit radiation dosimetry. Moreover, as a result of previous FRT, adjacent critical neurovascular structures’ tolerance of additional high-dose irradiation may be significantly decreased.
We were able to identify only two reports (Table 2) on SRS for metastasizing pituitary tumors—that is, for true PC. In both cases, the neoplasm initially presented as a prolactinoma. Phillips et al. [30] described a patient who had aggressive local growth of the tumor at the primary site and presented with a dural-based metastasis in the right temporal area 22 months after the initial manifestation of the disease. Gamma Knife surgery (GKS) targeted the sellar/parasellar lesion and resulted in its shrinkage, but the untreated temporal mass continued to grow. Despite salvage chemotherapy with TMZ, the patient died 15 months after irradiation [30]. Park et al. [31] reported a PC metastasizing into the fourth ventricle 7 years after its initial presentation and management. The tumor was subtotally resected, and the residual mass was treated with GKS (with a marginal dose of 16 Gy at the 50% isodose line), which led to its control during 3 years of follow-up [31]. The treatment results described in these reports corroborate our own experience in similar cases well (Figs. 1 and 2).
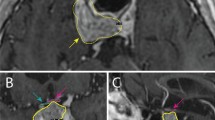
An 81-year-old woman with a dural-based metastasis noted 7 years after presentation with a prolactin-secreting pituitary macroadenoma. Therapy with a dopamine agonist at the time of initial diagnosis proved ineffective in controlling tumor growth; thus, the intra- and suprasellar portions of the lesion were removed via the transsphenoidal approach. Subsequently, the patient underwent two left-sided craniotomies for resection of the relapsing neoplasm and received adjuvant fractionated radiotherapy for a residual mass in the left cavernous sinus. Three months after her second craniotomy, tumor metastasis was disclosed. Magnetic resonance imaging (MRI) demonstrated a nodular contrast-enhancing lesion adjacent to the left frontobasal dura (a, arrow), which had not been visible during the prior imaging examination. At that time, the patient’s serum prolactin level was 727 ng/mL, and MRI showed residual fibrosis and an invasive tumor in the left side of the sella and in the superior portion of the adjacent cavernous sinus (b). Growth of the metastatic tumor was well controlled by Gamma Knife radiosurgery, with a marginal dose of 20 Gy delivered at the 50% isodose line. However, 7 months later, multiple new dural-based metastases appeared, shortly before the death of the patient from pneumonia
A 23-year-old woman with metastasis to the lower clivus noted 8 years after presentation with clinical hypercortisolism caused by an adrenocorticotropic hormone (ACTH)–secreting pituitary macroadenoma with suprasellar extension and invasion of the right cavernous sinus. The patient underwent (in succession) transsphenoidal tumor debulking, craniotomy for further tumor debulking, fractionated radiotherapy for the residual lesion (total dose 48.8 Gy), a second craniotomy, and chemotherapy, first with capecitabine and temozolomide, and then with carboplatin and 5-fluorouracil (which yielded tumor shrinkage and lowered, but did not normalize, her plasma ACTH level). As the clival metastasis (a) demonstrated resistance to chemotherapy and continued to grow, at 2 years after its initial discovery, it was treated with Gamma Knife radiosurgery, with a marginal dose of 16 Gy delivered at the 50% isodose line (b; the isodose lines corresponding to 24, 16, and 8 Gy are shown; note the minimal irradiation of the adjacent brainstem). After treatment, the lesion demonstrated a prominent volume reduction and a sustained and durable response. Four years later, the patient remained alive with radiographically stable disease and partially controlled hypercortisolism
Because of the paucity of reports on PC treated with SRS after the appearance of metastatic spread, analysis of the results of such treatment in locally aggressive pituitary tumors just prior to development of metastases may give some insight into the radiobiological characteristics of these lesions. Sufficient surgical debulking of the neoplasm via the transsphenoidal or transcranial approach before irradiation may be an important prerequisite for attainment of an optimal outcome, since it allows reduction of the target volume and decompression of adjacent neurovascular structures—in particular, the anterior visual pathways. Ono et al. [32] described a patient complaining of double vision caused by an ACTH-secreting PA, who underwent transsphenoidal removal followed by GKS (with a marginal dose of 25 Gy) for a residual mass in the right cavernous sinus. The treatment resulted in resolution of diplopia at 3 months after irradiation, but 8 months later, the tumor demonstrated extensive regrowth into the temporal bone, resulting in lower cranial nerve palsy. The patient underwent repeat GKS (with a marginal dose of 15 Gy), which, again, resulted in resolution of symptoms. However, 3 months later, a new tumor recurrence was confirmed in the right cavernous sinus; thus, GKS (with a marginal dose of 15 Gy) was performed for the third time and led to resolution of the presenting diplopia within 1 month. Unfortunately, 9 months later, the patient was diagnosed with multiple liver metastases [32]. A very similar case from our own practice has been reported in part previously (Fig. 3) [33].
Fractionated stereotactic radiotherapy (SRT) of a recurrent pituitary carcinoma, resulting in local tumor control. A 37-year-old man presented with diplopia caused by a nonfunctioning pituitary macroadenoma and underwent subtotal lesion resection via the transsphenoidal approach and subsequent Gamma Knife radiosurgery for a residual mass at a different institution. The tumor responded to treatment but demonstrated regrowth 3 years later. Four years after the initial presentation, repeat transsphenoidal surgery was performed at our hospital, but the neoplasm relapsed within 1 year, which required its additional removal via right-sided craniotomy. With histopathology revealing an MIB-1 index of 7.1% and 3 mitoses per 10 high-power fields, further rapid progression within and adjacent to the right cavernous sinus was noted 6 months later (a). Because of the proximity of the optic apparatus and previous radiosurgery, fractionated SRT using the CyberKnife, with a total dose of 45 Gy delivered in 25 fractions, was performed (b). Although the right cavernous sinus lesion eventually regressed, out-of-field tumor progression in the sella and left cavernous sinus was noted (c). This recurrence coincided with the onset of multiple metastases in the interpeduncular fossa, the posterior fossa dura, the cerebellum, and the intradural extramedullary space at the level of C2. The patient underwent repeat craniotomy for decompression of the anterior visual pathways and was started on temozolomide; however, it had limited efficacy. He died from slow but unrelenting disease progression with extensive metastatic spread of the tumor both within the neuroaxis and to systemic sites. In total, he survived for 13 years after the initial diagnosis and 5 years after the first appearance of metastases. (Reproduced in part from McCutcheon [33])
Taken together, the findings from these cases of PC treated with SRS after and before metastatic spread suggest that this treatment modality may be effective for controlling both noncontiguous tumors and aggressive growth of the neoplasm at the primary site. Although stereotactic irradiation is certainly not curative and does not prevent remote disease progression, it may be useful for management of localized disease and symptomatic lesions. Moreover, as with PA in general, this treatment modality is likely to be specifically effective in a particular subset of patients, but, obviously, it cannot be defined without analysis of larger series, which are currently not available. Therefore, further studies performed on a multi-institutional basis, and preferably in a prospective fashion, are required for clarification of the indications for SRS, its efficacy, and the durability of the tumor response in patients with PC.
Conclusion
Few systematic studies exist on the use of SRS in cases of PC. As the histology and location of tumors, the functional status of patients, and the total disease burden have varied widely across the reported cases, meaningful clinical conclusions cannot be reached. Nevertheless, the occasionally reported results suggest that this modality may have a role in initial and salvage treatment of patients with such neoplasms. While no class I–III evidence on the use of SRS for PC currently exists, it is quite reasonable to incorporate this treatment option into a multimodal management strategy and apply it judiciously at the treating clinician’s discretion on a case-by-case basis.
References
Meij BP, Lopes MBS, Ellegala DB, Alden TD, Laws ER Jr. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J Neurosurg. 2002;96:195–208.
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery. 1996;38:99–107.
Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134:521–35.
Scheithauer BW, Kurtkaya-Yapicier O, Kovacs KT, Young WF Jr, Lloyd RV. Pituitary carcinoma: a clinicopathological review. Neurosurgery. 2005;56:1066–74.
Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER Jr. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996;38:765–71.
Kamiya-Matsuoka C, Cachia D, Waguespack SG, Crane CH, Mahajan A, Brown PD, Nam JY, McCutcheon IE, Penas-Prado M. Radiotherapy with concurrent temozolomide for the management of extraneural metastases in pituitary carcinoma. Pituitary. 2016;19:415–21.
Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017;19:762–73.
Mete O, Lopes MB. Overview of the 2017 WHO classification of pituitary tumors. Endocr Pathol. 2017;28:228–43.
Pernicone PJ, Scheithauer BW, Sebo TJ, Kovacs KT, Horvath E, Young WF Jr, Lloyd RV, Davis DH, Guthrie BL, Schoene WC. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79:804–12.
Ragel BT, Couldwell WT. Pituitary carcinoma: a review of the literature. Neurosurg Focus. 2004;16(4):E7.
Cushing H. Further notes on pituitary basophilism. JAMA. 1932;99:281–4.
Mehta GU, Lonser RR, Oldfield EH. The history of pituitary surgery for Cushing disease. J Neurosurg. 2012;116:261–8.
Castinetti F, Brue T. Pituitary gland: Gamma Knife for Cushing disease—time for a reappraisal? Nat Rev Endocrinol. 2017;13:628–9.
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–9.
Lee CC, Vance ML, Xu Z, Yen CP, Schlesinger D, Dodson B, Sheehan J. Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab. 2014;99:1273–81.
Mehta GU, Ding D, Patibandla MR, Kano H, Sisterson N, Su YH, Krsek M, Nabeel AM, El-Shehaby A, Kareem KA, Martinez-Moreno N, Mathieu D, McShane B, Blas K, Kondziolka D, Grills I, Lee JY, Martinez-Alvarez R, Reda WA, Liscak R, Lee CC, Lunsford LD, Vance ML, Sheehan JP. Stereotactic radiosurgery for Cushing disease: results of an international, multicenter study. J Clin Endocrinol Metab. 2017;102:4284–91.
Mitsumori M, Shrieve DC, Alexander E 3rd, Kaiser UB, Richardson GE, Black PM, Loeffler JS. Initial clinical results of LINAC-based stereotactic radiosurgery and stereotactic radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys. 1998;42:573–80.
Loeffler JS, Shih HA. Radiation therapy in the management of pituitary adenomas. J Clin Endocrinol Metab. 2011;96:1992–2003.
Lall RR, Shafizadeh SF, Lee KH, Mao Q, Mehta M, Raizer J, Bendok BR, Chandler JP. Orbital metastasis of pituitary growth hormone secreting carcinoma causing lateral gaze palsy. Surg Neurol Int. 2013;4:59.
Verma J, McCutcheon IE, Waguespack SG, Mahajan A. Feasibility and outcome of re-irradiation in the treatment of multiply recurrent pituitary adenomas. Pituitary. 2014;17:539–45.
Mountcastle RB, Roof BS, Mayfield RK, Mordes DB, Sagel J, Biggs PJ, Rawe SE. Pituitary adenocarcinoma in an acromegalic patient: response to bromocriptine and pituitary testing: a review of the literature on 36 cases of pituitary carcinoma. Am J Med Sci. 1989;298:109–18.
Hansen TM, Batra S, Lim M, Gallia GL, Burger PC, Salvatori R, Wand G, Quinones-Hinojosa A, Kleinberg L, Redmond KJ. Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev. 2014;37:279–86.
Beauchesne P, Trouillas J, Barral F, Brunon J. Gonadotropic pituitary carcinoma: case report. Neurosurgery. 1995;37:810–6.
Morokuma H, Ando T, Hayashida T, Horie I, Inoshita N, Murata F, Ueki I, Nakamura K, Imaizumi M, Usa T, Kawakami A. A case of nonfunctioning pituitary carcinoma that responded to temozolomide treatment. Case Rep Endocrinol. 2012;2012:Article 645914.
Arnold PM, Ratnasingam D, O'Neil MF, Johnson PL. Pituitary carcinoma recurrent to the lumbar intradural extramedullary space: case report. J Spinal Cord Med. 2012;35:118–21.
Donovan LE, Arnal AV, Wang SH, Odia Y. Widely metastatic atypical pituitary adenoma with mTOR pathway STK11(F298L) mutation treated with everolimus therapy. CNS Oncol. 2016;5:203–9.
Fadul CE, Kominsky AL, Meyer LP, Kingman LS, Kinlaw WB, Rhodes CH, Eskey CJ, Simmons NE. Long-term response of pituitary carcinoma to temozolomide: report of two cases. J Neurosurg. 2006;105:621–6.
Losa M, Bogazzi F, Cannavo S, Ceccato F, Curtò L, De Marinis L, Iacovazzo D, Lombardi G, Mantovani G, Mazza E, Minniti G, Nizzoli M, Reni M, Scaroni C. Temozolomide therapy in patients with aggressive pituitary adenomas or carcinomas. J Neurooncol. 2016;126:519–25.
Raverot G, Sturm N, de Fraipont F, Muller M, Salenave S, Caron P, Chabre O, Chanson P, Cortet-Rudelli C, Assaker R, Dufour H, Gaillard S, François P, Jouanneau E, Passagia JG, Bernier M, Cornélius A, Figarella-Branger D, Trouillas J, Borson-Chazot F, Brue T. Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J Clin Endocrinol Metab. 2010;95:4592–9.
Phillips J, East HE, French SE, Melcescu E, Hamilton RD, Nicholas WC, Fratkin JF, Parent AD, Luzardo G, Koch CA. What causes a prolactinoma to be aggressive or to become a pituitary carcinoma? Hormones (Athens). 2012;11:477–82.
Park KS, Hwang JH, Hwang SK, Kim S, Park SH. Pituitary carcinoma with fourth ventricle metastasis: treatment by excision and Gamma-Knife radiosurgery. Pituitary. 2014;17:514–8.
Ono M, Miki N, Amano K, Hayashi M, Kawamata T, Seki T, Takano K, Katagiri S, Yamamoto M, Nishikawa T, Kubo O, Sano T, Hori T, Okada Y. A case of corticotroph carcinoma that caused multiple cranial nerve palsies, destructive petrosal bone invasion, and liver metastasis. Endocr Pathol. 2011;22:10–7.
McCutcheon IE. Stereotactic radiosurgery for malignant extracerebral intracranial tumors: patient selection, efficacy, and technical nuances. Acta Neurochir Suppl. 2013;116:71–83.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors have no conflict of interest concerning the reported materials or methods.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mehta, G.U., McCutcheon, I.E. (2021). Stereotactic Radiosurgery for Pituitary Carcinoma. In: Chernov, M.F., Hayashi, M., Chen, C.C., McCutcheon, I.E. (eds) Gamma Knife Neurosurgery in the Management of Intracranial Disorders II. Acta Neurochirurgica Supplement, vol 128. Springer, Cham. https://doi.org/10.1007/978-3-030-69217-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-69217-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69216-2
Online ISBN: 978-3-030-69217-9
eBook Packages: MedicineMedicine (R0)