Abstract
Fremanezumab, a human monoclonal antibody against CGRP ligand, was approved by FDA for the treatment of episodic and chronic migraine in September 2018. This chapter reviews and discusses data on the pharmacokinetics, metabolism, clinical efficacy, and safety of fremanezumab as reported in the scientific literature.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Introduction to the Compound
Fremanezumab (AJOVY®) has been the second FDA-approved humanized monoclonal antibody for the treatment of migraine, in September 2018. Moreover, it received the EU market authorization in March 2019. In 2020, the National Institute for Health and Care Excellence (NICE) has issued a positive opinion regarding the use of AJOVY (Fremanezumab) as preventative chronic migraine drug in the Final Appraisal Determination (FAD). NICE recommends the administration of AJOVY in patients with chronic headaches who have not responded to at least three previous drug prophylactic treatments.
10.2 Chemistry
The chemical name of Fremanezumab (synonyms TEV-48125, LBR-101, PF-04427429, RN307) is Immunoglobulin G2, antihuman alpha calcitonin gene-related peptide/beta calcitonin gene-related peptide (CGRP) [1]. Fremanezumab is a humanized immunoglobulin G2 (IgG2) Δa monoclonal antibody (mAb) derived from a murine precursor with a molecular weight of approximately 148 kDa. It has been mutated in the Fab variable region of the heavy chain to increase affinity and to limit antibody Fc effector function. Moreover, two mutations (A330S and P331S) were introduced into the constant region of the heavy chain to limit antibody Fc effector function (ADCC and CDC). It is administered subcutaneously with a quarterly dosing of 675 mg as three 225 mg SC injections every 3 months, while the monthly dosing is 225 mg SC injection each month [1].
10.3 Fremanezumab Pharmacodynamics and Pharmacokinetics
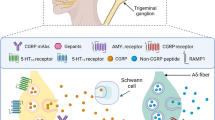
Fremanezumab shows typical pharmacokinetic features of other therapeutic antibodies. Fremanezumab targets both α and β-CGRP isoforms, preventing their binding to CGRP and AMY1 receptors. Fremanezumab can target specifically CGRP ligand and it is unclear if fremanezumab is also able to bind AM or other human calcitonin receptors family compounds [2]. A phase I clinical trial conducted in order to assess the pharmacokinetics of fremanezumab different subcutaneous doses on Japanese and Caucasian healthy subjects showed that CMAX were 0.91, 1.04, and 1.14 for 225 mg, 675 mg and 900 mg doses, respectively [3]. Across doses, mean TMAX was the same for both ethnicities for 225 and 675 mg doses (7 and 5 days) and similar for Japanese and Caucasian subjects at the 900 mg dose level (11 and 7 days, respectively). Plasma concentrations of fremanezumab reach the maximum peak (CMAX) and overall (all AUCs) within 5–7 days for all three doses administered. As expected, plasma concentrations increased with increasing doses, with gradual decline thereafter. In both ethnic study subject groups, subcutaneous fremanezumab, binding to protective receptors as the other IgG2 molecules, has a long mean half-life ranging from 31 to 39 days in both Japanese and Caucasian groups [3]. However, a further Phase I program [4] assessed that half-life of fremanezumab was 45 days in healthy volunteers. Across all the three administered doses, both CL/F (0.08–0.09 mL/min) and Vz/F (5.71–6.43 L) were similar, suggesting minimal distribution to the extravascular tissues. However, data regarding the protein binding of fremanezumab were not available. As all other monoclonal antibody agents, hepatic or renal impairment is not expected to affect fremanezumab pharmacokinetics, even if no study has included patients affected by hepatic or renal disorders. Nevertheless, monoclonal antibody agents like fremanezumab are not eliminated via hepatic, renal, or biliary routes, but they are known to be mainly eliminated via intracellular enzymatic proteolysis, producing small peptides and amino acids [2]. For these reasons fremanezumab, being an anti-CGRP mAb, exhibits PK/PD advantages of target specificity, prolonged half-lives, reduced potential for hepatotoxicity and limited drug–drug interactions [5, 6].
10.4 Fremanezumab Clinical Efficacy
The clinical efficacy of fremanezumab as a preventative treatment for episodic and chronic migraine conditions was assessed in two multicenter, randomized placebo-controlled, 3-month studies. In the study conducted for the evaluation of fremanezumab clinical efficacy in episodic migraineurs, 875 patients were enrolled and divided (1:1:1) into three groups: 290 patients were assigned to the AJOVY 225 mg monthly group, 291 patients were administered with AJOVY 675 mg quarterly (an initial dose of 675 mg and then two placebo doses) and 294 patients received the placebo dose monthly. The study has included patients using additional prophylactic drugs (21% of patients), while excluded patients affected from several pathological conditions, such as cardiovascular disease, deep vein thrombosis, transient ischemic attack, or other vascular or thrombotic events. The primary endpoint was the reduction of the monthly migraine days at 9–12 weeks from baseline [7]. During the 12 weeks after the first dose, the least-square mean (LSM) change value in migraine days per month was significantly reached for both dose regimens, resulting in 4.9 days for the monthly dosing group (LSM change from baseline of −3.7 days), 5.3 days for higher dosing (LSM change from baseline of −3.4 days), and 6.5 days for the placebo group (LSM change from baseline of −2.2 days). Thus, compared to placebo, both fremanezumab 225 mg and 625 mg were effective in reducing the mean number of migraine days (1.5 and 1.3 days respectively, p < 0.001), over the 3-month period. Secondary endpoints included the following: (i) ≥50% reduction in mean migraine days per month; (ii) the reduction of any acute migraine drugs from baseline to 12 weeks; (iii) mean migraine days per month; (iv) mean monthly migraine days in patients not receiving concomitant prophylactic treatments for migraine; (v) mean change in Migraine Disability Assessment (MIDAS) score. The study showed a 50% decrease of the monthly migraine days (at week 12) in 47% of subjects administered with fremanezumab 225 mg monthly, in 44.4% of subjects administered with a single 675 mg injection, and in 27.9% of subjects receiving placebo. The migraine days per month requiring an additional acute treatment were 4.4 for the monthly dosing group (LSM change from baseline, −3.0 days), 4.6 for 675 mg dose (LSM change from baseline, −2.9 days), and 5.8 in placebo group (LSM change from baseline, −1.6 days). Moreover, the MIDAS score reduced significantly in fremanezumab-treated groups. In particular, after 4 weeks from the administration, MIDAS decreased from 38 to 12.6 in the monthly dosing group, from 47.1 to 14.6 in the quarterly dosing group, and from 37.3 to 19.4 in the placebo group. A total of 791 patients completed the 3-month double-blind phase [7].
Another study [8], aimed to evaluate the efficacy of Fremanezumab in patients with chronic migraine, randomized 1130 patients (headache occurring on ≥15 days, with characteristics of migraine headache on ≥8 days) in two different groups: 375 patients received a 675 mg starting dose followed by 225 mg monthly, 375 patients received a single dose of 675 mg every 3 months (quarterly) and 371 patients were administered with placebo. The primary endpoint of this study was the reduction of the mean number of headaches per month from baseline. The results showed a decrease of 4.3 days for the quarterly dosing group, 4.6 for monthly group, and 2.5 in the placebo dosing. The secondary endpoints of the study consisted of: (1) a decrease of the mean number of monthly migraine days, significantly improved in all three groups; (2) a reduction of at least 50% of the mean headache days and this was achieved in 38, 41, and 18% of patients of the quarterly, monthly and the placebo groups respectively (p < 0.001 for both comparisons with placebo); (3) a change from baseline in monthly average number of days of additional acute headache treatment (−4.2, −3.7, −1,9 for 225 mg, 675 mg, placebo respectively); (4) the reduction in the 6-item Headache Impact Test (HIT-6) score, that resulted significantly decreased in fremanezumab quarterly (6.4 ± 0.5 points) and fremanezumab monthly (6.8 ± 0.4 points) groups with respect to placebo (4.5 ± 0.5 points; p < 0.001 for both dose regimens). A total of 1034 patients completed the 3-month double-blind phase [8].
10.5 Fremanezumab Safety and Tolerability
Preclinical and clinical studies demonstrated that fremanezumab does not determine changes in vital signs or laboratory tests (including liver enzymes); also, cardiac electrical conduction or repolarisation and other important changes in the ECG parameters were not observed [9]. However, AJOVY may cause allergic reactions in the injection site, including itching, rash, and hives that can happen within hours and up to 1 month after receiving AJOVY. In fact, it is contraindicated in subjects with severe hypersensitivity to fremanezumab-vfrm or to any of the other prefilled syringe excipients. Adverse reactions were reported by ≥2% of patients on AJOVY and greater than placebo. Only less than 2% of the subjects discontinued AJOVY because of side effects [7, 8].
Belonging to the therapeutic protein drug family, AJOVY has a potential for immunogenicity. In order to assess this clinical parameter, AJOVY was monitored by analyzing antidrug antibodies (ADA) and also the neutralizing antibodies in the treated patients. The results on the first 3 months showed that treatment-emergent ADA responses were observed in 0.4% of the sample. Only one patient developed neutralizing antibodies at day 84. ADA responses increased up to 1.6% of the sample in the long-term treatment. Of these 1.6% of patients developing ADA, 17 also had a neutralizing activity in their post-dose samples. Further studies are needed to assess if this fremanezumab-vfrm antibody development affects efficacy and safety of AJOVY [2].
Regarding pregnancy and lactation, there are no adequate data on the risks related to the use of AJOVY in pregnant women and on the presence of fremanezumab-vfrm in human milk. Also, for paediatric and geriatric use data on safety and efficacy are not available.
10.6 Ongoing Trials
Up to date, several clinical trials are ongoing to assess the efficacy and safety of fremanezumab in different pathological conditions.
One study is investigating the efficacy of fremanezumab in reducing pain in patients with interstitial cystitis–bladder pain syndrome (IC-BPS). A secondary objective of the study is to evaluate the effect of fremanezumab on other efficacy measures, including pain, voiding frequency, urinary symptoms, and quality of life. Another secondary objective of the study is to evaluate the safety and tolerability of fremanezumab administered subcutaneously in adult patients with IC-BPS [10].
A second study is investigating side effects of fremanezumab when treating patients with Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) for migraine headaches. Primary outcomes are changes in migraine-related disability, headache intensity, and the evaluation of adverse event risks [11].
A third study is testing if Fremanezumab is effective in preventing chronic and episodic migraine in patients 6–17 years of age. The primary objective of this study is to evaluate long-term safety and tolerability, while the secondary objectives are to assess the efficacy of subcutaneous fremanezumab in pediatric migraineurs and to evaluate the immunogenicity of the drug and the impact of ADAs on clinical outcomes [12].
Another study is evaluating safety and efficacy of fremanezumab for the preventative treatment of migraine patients suffering from major depressive disorder. The primary outcome is the mean change in monthly average number of migraine days, while the main secondary outcomes are mean changes in depression symptoms, quality of life, and the evaluation of the occurrence of adverse events in patients taking concomitant medications [13].
A study is investigating the effectiveness of fremanezumab administered subcutaneously in reducing pain in adult patients with fibromyalgia. A secondary objective of this study is to assess the effect of fremanezumab on efficacy measures such as pain, quality of life, sleep, fatigue, improvement in health, physical functioning, and mood. Another secondary objective is to evaluate the safety and tolerability of fremanezumab administered subcutaneously in adult patients with fibromyalgia [14].
10.7 Conclusion
Fremanezumab, a humanized monoclonal antibody, inhibits the interaction of CGRP with its receptor. FDA and EMA approved its clinical use for migraine prevention in adults. The available results from two Phase II and two Phase III randomized clinical trials showed a good efficacy of treatment with subcutaneous fremanezumab for episodic and chronic migraine with respect to placebo. Adverse events were mild or moderate and related to the injection site reactions (erythema, pain, or induration), but they occurred relatively frequently. Vital signs, laboratory findings, and other clinical parameters did not show relevant changes. Several other clinical trials are actually ongoing with the aims of evaluating fremanezumab efficacy for prevention of episodic and chronic cluster headache and post-traumatic headache. Although long-term safety is still being evaluated in an ongoing trial, fremanezumab represents a potentially useful option for the management of migraine disease.
References
Lionetto L, Curto M, Cisale GY, et al. Fremanezumab for the preventive treatment of migraine in adults. Expert Rev Clin Pharmacol. 2019;12(8):741–8.
Lionetto L, Cipolla F, Guglielmetti M, Martelletti P. Fremanezumab for the prevention of chronic and episodic migraine. Drugs Today (Barc). 2019;55(4):265–76.
Cohen-Barak O, Weiss S, Rasamoelisolo M, et al. A phase 1 study to assess the pharmacokinetics, safety, and tolerability of fremanezumab doses (225 mg, 675 mg and 900 mg) in Japanese and Caucasian healthy subjects. Cephalalgia. 2018;38(13):1960–71.
Bigal ME, Escandon R, Bronson M, et al. Safety and tolerability of LBR-101, a humanized monoclonal antibody that blocks the binding of CGRP to its receptor: results of the phase 1 program. Cephalalgia. 2014;34(7):483–92.
Bigal ME, Walter S. Monoclonal antibodies for migraine: preventing calcitonin gene-related peptide activity. CNS Drugs. 2014;28(5):389–99.
Taylor FR. CGRP, amylin, immunology, and headache medicine. Headache. 2019;59(1):131–50.
Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999–2008.
Silberstein SD, Dodick DW, Bigal ME, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med. 2017;377(22):2113–22.
Walter S, Alibhoy A, Escandon R, Bigal ME. Evaluation of cardiovascular parameters in cynomolgus monkeys following IV administration of LBR-101, a monoclonal antibody against calcitonin gene-related peptide. MAbs. 2014;6(4):871–8.
https://clinicaltrials.gov/ct2/show/NCT04447729?term=fremanezumab&draw=2&rank=4. Accessed 4 Sep 2020.
https://clinicaltrials.gov/ct2/show/NCT04334408?term=fremanezumab&draw=2&rank=5. Accessed on 4 Sep 2020.
https://clinicaltrials.gov/ct2/show/NCT04530110?term=fremanezumab&draw=2&rank=3. Accessed 4 Sep 2020.
https://clinicaltrials.gov/ct2/show/NCT04041284?term=fremanezumab&draw=2&rank=10. Accessed 4 Sep 2020.
https://clinicaltrials.gov/ct2/show/NCT03965091?term=fremanezumab&draw=3&rank=11. Accessed 4 Sep 2020.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lionetto, L., Capi, M., De Angelis, V., Martelletti, P. (2021). Fremanezumab. In: Maassen van den Brink, A., Martelletti, P. (eds) Monoclonal Antibodies in Headache . Headache. Springer, Cham. https://doi.org/10.1007/978-3-030-69032-8_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-69032-8_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-69031-1
Online ISBN: 978-3-030-69032-8
eBook Packages: MedicineMedicine (R0)