Abstract
The lectin chaperones calreticulin (CALR) and calnexin (CANX), together with their co-chaperone PDIA3, are increasingly implicated in studies of human cancers in roles that extend beyond their primary function as quality control facilitators of protein folding within the endoplasmic reticulum (ER). Led by the discovery that cell surface CALR functions as an immunogen that promotes anti-tumour immunity, studies have now expanded to include their potential uses as prognostic markers for cancers, and in regulation of oncogenic signaling that regulate such diverse processes including integrin-dependent cell adhesion and migration, proliferation, cell death and chemotherapeutic resistance. The diversity stems from the increasing recognition that these proteins have an equally diverse spectrum of subcellular and extracellular localization, and which are aberrantly expressed in tumour cells. This review describes key foundational discoveries and highlight recent findings that further our understanding of the plethora of activities mediated by CALR, CANX and PDIA3.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Calreticulin (CALR) and calnexin (CANX) are calcium-binding lectin-like molecular chaperones responsible for the proper folding of newly synthesized glycoproteins. Each can be found in a complex with PDIA3, a thiol oxidoreductase with protein disulfide isomerase activity, to form the CALR/CANX cycle that is a key part of the quality control for new proteins being made and assembled in the lumen of the endoplasmic reticulum (ER) (Adams et al. 2019; Halperin et al. 2014). An increasing revelation gained from studies spanning over two decades have highlighted additional non-classical roles for CALR, CANX and PDIA3 in multiple cellular locales that include the nucleus, the extra-ER cytosol and on the extracellular surface of the plasma membrane (Gold et al. 2010; Hettinghouse et al. 2018). However, their relative low abundance in subcellular compartments outside of the ER presents challenges in studies seeking to elucidate their specific functional significance. Here, we will review some of the implications of these findings in the cellular and pathobiology of cancers.
9.2 Expression of CALR in Human Cancers
Many ER chaperones are known to be aberrantly expressed in a variety of tumour tissues. As the expression of ER chaperones is highly regulated in normal cells, the detection and quantification of these ER chaperones in patients’ tumour cells may have potential prognostic significance (Gutierrez and Simmen 2014). Among these trio of proteins, the expression of CALR in various cancers have been the most investigated, owing largely to CALR’s enhanced significance and prominence as a pro-phagocytic signal deemed important for innate and adaptive anti-tumour immunity.
In a survey of multiple malignancies including hematologic and solid tumours, cell surface CALR levels were inherently much higher when compared to their normal cell counterparts (Chao et al. 2010). However, this did not necessarily translate to a better disease outcome, as the increased surface CALR on tumour cells was accompanied by enhanced expression of the anti-phagocytic CD47 signal receptor. In the same study, the authors found that increased expression of CALR mRNA is associated with an adverse clinical prognosis in neuroblastoma, bladder cancer and mantle cell lymphoma (Chao et al. 2010). It’s unclear how the increased CALR mRNA may account for increased CALR protein expressed in the various subcellular locales that include the cell surface.
An immunohistological survey of resected pancreatic cancers identified an association between high CALR expression and poor patient survival (Matsukuma et al. 2016). High CALR expression was also associated with a poorer prognosis for patients with nasopharyngeal carcinoma (Han et al. 2019b). CALR was also found to be highly expressed in oral squamous cell carcinoma tumour tissues in comparison to matched non-cancerous tissues (Chiang et al. 2013). This study also showed that stable knockdown of CALR expression led to reduced cell proliferation, colony forming potential and anchorage-independent growth. Immunohistological analysis of oral squamous cell carcinoma tissues also identified an association between elevated CALR expression with advanced staging and reduced term survival in patients (Harada et al. 2017). A recent analysis of gene expression datasets across a large cohort of patients that included breast, kidney and brain cancers, found a high level of consistency that high CALR mRNA expression associated with the worst survival outcomes (Han et al. 2019a). However, the authors cautioned that the enhanced transcript expression data have not been correlated with increased protein expression.
Thus, it is interesting that CALR deficiency can also be associated with poorer prognosis in other malignancies. CALR is highly expressed in the normal prostate, and seen to be downregulated in prostate cancer specimens from more advanced stages of disease (Alur et al. 2009). Accordingly, enforced CALR overexpression in prostate cancer cells inhibited cell proliferation in both monolayer and soft agar conditions, concomitant with reduced growth and metastasis in an orthotopic xenograft tumour model (Alur et al. 2009). Additionally, high expression of CALR in non-small cell lung cancer is associated with a most favorable prognosis for patients (Fucikova et al. 2016); this retrospective study found high CALR expression in tumour tissues correlated with dense intratumoral infiltration with immune cell subtypes suggestive of an enhanced adaptive immune response. Similar observations were also made in ovarian cancer carcinoma, where high CALR expression is associated with enhanced immune response and improvement in patients’ survival (Kasikova et al. 2019; Stoll et al. 2016). Importantly, this correlation was seen in treatment-naïve primary tumours, suggesting an ongoing and effective anti-tumour immune response prior to the onset of chemotherapy.
It was found that high levels of anti-CALR autoantibodies in serum is a potential biomarker for bladder cancer, although a correlation between survival and serum anti-CALR levels could not be established (Minami et al. 2014). The level of soluble CALR was found to be significantly elevated in the serum of lung cancer patients when compared to healthy subjects (Liu et al. 2012). In the same study, serum CALR was found to be higher for patients receiving chemotherapy compared to those who did not. It is worth noting that a serum CALR survey of patients with chronic lymphocytic leukemia taken at diagnosis revealed a correlation between higher CALR levels to earlier onset of disease progression that necessitated treatment (Molica et al. 2016). It’s a compelling notion that a simple blood test for soluble CALR may provide not only diagnostic and prognostic information, but may also be useful to assess treatment response.
Although it may seem contradictory that CALR can be seen to both promote and suppress oncogenesis, these findings illustrate the complexity and dynamic nature of CALR’s role in various biological processes. It seems clear that a simple relationship between CALR expression and tumour aggressiveness cannot be made at this point. It is likely the value of such a relationship will depend not only on the individual tumour types, but also on the consideration of how the CALR protein is aberrantly expressed and distinguished at the subcellular level. In a later section, we will consider how the subcellular biology of CALR may promote either a pro- or anti-tumour effect.
9.3 Expression of PDIA3 and CANX in Human Cancers
Like CALR, PDIA3 is also known to be aberrantly expressed in various types of cancer. High expression of PDIA3 in patients’ hepatocellular carcinoma tissues linked to hepatitis infection was associated with poorer prognosis, increased tumour cell proliferation and decreased apoptosis (Liu et al. 2017; Takata et al. 2016). High PDIA3 expression could have prognostic significance in ovarian cancer, being overexpressed in malignant tissues in comparison to normal, and increasing expression was correlated with tumour progression and poor survival (Samanta et al. 2017). Similarly, high expression of PDIA3 mRNA in clear cell renal carcinoma was associated with poor survival and mechanistically linked to enhanced oncogenic signaling through STAT3 (Liu et al. 2019b). On the other hand, gastric cancer tissues in patients are observed to have a low level of PDIA3 expression when compared to normal mucosa or low grade gastric tumors. However, gastric cancer tissues with the highest PDIA3 levels was associated with favourable staging and survival outcome (Leys et al. 2007; Shimoda et al. 2019). Thus, these findings demonstrate that PDIA3 expression, like CALR, may have both tumour promotion and suppression properties.
Patient cohort studies involving CANX expression lag behind those of CALR and PDIA3. Still, the available studies provide an early consensus that increased CANX expression is generally associated with a poorer prognosis. High expression of CANX mRNA was correlated with poorer survival for breast cancer patients (Alam et al. 2019). In a study that evaluated expression of several ER-stress chaperones in colorectal cancer, increased CANX protein expression, but not of CALR, GRP78 or GRP94, was predictive of poorer clinical outcome (Ryan et al. 2016). A proteomics based approach comparing individual-matched pairs of tumour and normal tissue from patients with oral squamous cell carcinoma found significantly elevated CANX expression in tumour cells (Chen et al. 2019). This study also evaluated immunohistological staining of tumour tissues distinguished at the subcellular level, revealing that high plasma membrane localized CANX was correlated with poorer patient survival, while enrichment of cytosolic CANX was not. Furthermore, surgical derived tumour tissues with increased membranous CANX expression was found to contain fewer infiltrated T-cells, suggesting an immune-suppressive role for CANX (Chen et al. 2019). This phenomenon is supported by the finding that CANX expression was inversely correlated with MHC1 presentation in breast cancers (Alam et al. 2019). Given that CANX and CALR are both PDIA3 binding proteins, it is intriguing to consider that overexpression of CANX may reduce the level of CALR expressed, thus accounting for CANX’s role in immune suppression.
9.4 Cell Surface CALR Signals Immunogenic Cell Death
The Nomenclature Committee on Cell Death defined immunogenic cell death (ICD) as a “regulated cell death that is sufficient to activate an adaptive immune response” (Galluzzi et al. 2018). In this regard, the discovery that cell surface exposed CALR functions as a damage associated molecular pattern (DAMP) able to stimulate engulfment by professional phagocytes (Gardai et al. 2005) was another landmark moment that promoted investigation of CALR’s role in an extra-ER locale. The significance for cancer studies was further exemplified when it was found that certain cytotoxic agents, including anthracyclines that are strongly favored for inclusion in most combination chemotherapies, can efficiently induce surface CALR exposure (Obeid et al. 2007). Mechanistically, drug-induced surface CALR occurs rapidly within several hours and is considered to be pre-apoptotic, since this phenomena preceded surface presentation of phosphaditylserine. It was observed also that drug treatment resulted in PDIA3 being co-presented on the cell surface (Obeid et al. 2007). Other ER-chaperones including GRP78, PDIA1, and GRP94 are also presented on the cell surface (Wiersma et al. 2015), although not all necessarily involve induction of cell stress. Of note, the membrane integral protein CANX has been characterized on the surface of many cell types at low levels (Okazaki et al. 2000), but with yet unclear physiologic roles. Still, surface CALR remains unique in being the only ER-chaperone with DAMP-functionality able to stimulate an immune-response. Hence, there has been much research interest seeking to understand the molecular mechanisms that regulate trafficking of CALR from its predominant ER localization to the cell surface in ICD, and how this phenomenon may be exploited to enhance the anti-tumour immune response.
As with most ER-lumen resident proteins, CALR is translationally directed into the ER-lumen via an N-terminal signal peptide that is subsequently proteolytically removed. CALR’s retention and enrichment in the ER is further mediated by the C-terminal KDEL motif, which engages KDEL-receptors for retrograde transport from the Golgi back to the ER (Gold et al. 2010; Wiersma et al. 2015). Despite these targeted enrichment and retention mechanisms, it was estimated that up to 14% of total CALR resides in the extra-ER cytosol in non-stressed cells, and that the cytosolic form is without the signal peptide (Afshar et al. 2005; Shaffer et al. 2005). This highlighted that extra-ER forms of CALR likely originates from the ER-luminal pool.
The advent of CRISPR-Cas9 gene editing methods ushered new abilities to easily generate loss of function cellular models to facilitate our lab’s studies of ER to surface trafficking of CALR. In this manner, we showed that surface CALR induced by doxorubicin- or oxaliplatin-treatment of wildtype Jurkat T-lymphoblasts was completely abolished in CALR−/− cells (Liu et al. 2016). Importantly, drug-induced increases of surface CALR in CALR−/− cells could only be rescued upon re-expression of CALR containing the signal peptide, while expression of the truncated cytosol-targeted variant, which bypasses the ER-lumen, resulted in constitutively high levels of surface CALR detected, irrespective of drug-treatment. This indicated that the bulk of drug-induced surface CALR is derived from the ER-luminal pool, and involved transit through the cytosol. Interestingly, it was shown that inhibition of ER to Golgi transport using brefeldin A, as well as inhibition of actin polymerization using latrunculin B, resulted in reduced drug-induced surface CALR (Garg et al. 2012). Thus, it remains likely that drug stress induced ER to surface translocation of CALR may involve multiple mechanisms.
It is now evident that CALR cannot translocate to the cell surface on its own. It was shown that anthracyclines induced surface presentation of PDIA3 in addition to CALR (Obeid et al. 2007). It was reported that in murine cell models, knockdown of PDIA3 inhibited surface CALR presentation, and likewise, knockdown of CALR inhibited surface PDIA3 presentation (Obeid 2008; Panaretakis et al. 2008), suggesting CALR and PDIA3 are co-required in ER to surface trafficking. Our studies using the human Jurkat derivative CALR−/− and PDIA3−/− cells revealed that PDIA3 is similarly required for surface CALR presentation, while PDIA3 remains efficiently translocated to the surface in a CALR-independent manner (Liu et al. 2019a). In addition, we found that non-drug treated PDIA3−/− cells is devoid of a cytosolic pool of CALR typically observed in wildtype cells, indicating that PDIA3 is a critical mediator of possibly all extra-ER localized CALR. These findings highlight an indispensable role for PDIA3 to facilitate ER to cytosol to surface presentation of CALR that serves the immunogenic role important for ICD. In this regard, CALR-PDIA3 is a sufficient and necessary ‘immunogenic complex’ able to translocate to the cell surface.
What about CANX? Given that PDIA3 act as a co-chaperone with the ability to bind CALR or CANX to form a stable complex (Leach et al. 2002; Russell et al. 2004), it is likely the subcellular localization of the trio of proteins will overlap. It’s interesting to note that surface CANX was originally described as being enriched on thymocytes (Wiest et al. 1995), which are immature T-lymphocytes. Indeed, ultraviolet light-induced apoptosis of Jurkat cells was accompanied by surface CANX expression (Franz et al. 2007). It’s not clear if this process requires PDIA3, but we do note that Jurkat cells lacking CALR presented increased levels of surface PDIA3 upon drug treatment (Liu et al. 2019a). Unlike CALR, surface CANX is not known to be immunogenic, but an intriguing postulate would predict that tumour cells expressing higher CANX to CALR ratios may present decreased levels of surface CALR owing to competition for binding to PDIA3, and hence be less immunogenic. Likewise, increased expression of CALR and/or PDIA3 may enhance ICD, and this balance of CALR, CANX and PDIA3 may account for differences in their prognostic values for cancers as reflected in the preceding section.
9.5 Intersection of α-Integrins, CALR and ICD
Integrins are transmembrane heterodimeric cell adhesion receptor proteins consisting of α- and β-subunits. The human genome encodes 21α and 9β isoforms, which combine to form 21 known αβ-heterodimers. The integrin extracellular domains mediate attachment to other cells or to the extracellular matrix, while the cytosolic ‘tails’ mediate intracellular signaling by interaction with downstream effector proteins (Hynes 2002; Takada et al. 2007).
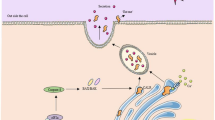
Prior to cell surface CALR being identified as a DAMP, CALR was identified as an interacting protein with the membrane proximal GFFKR motif found on the cytoplasmic tail of α-integrins (Rojiani et al. 1991). GFFKR is a highly conserved motif amongst all 21 α-integrin tails, serving a critical structural function by interacting with its β-subunit counterpart to form the ‘inner membrane clasp’ and stable αβ-heterodimer expression (Fig. 9.1). Subsequently, it was shown that physiological cell adhesion, which entails the conformational activation of integrins, inducibly enhances the interaction of α-integrins with CALR, and that Calr−/− mouse embryonic fibroblasts exhibit defects in integrin-mediated adhesion and Ca2+ flux (Coppolino et al. 1995, 1997).
Integrins are αβ-heterodimeric cell adhesion receptors. (a) Integrin activation switches the conformation from low- to high-affinity ligand binding state, to include exposure of the substrate binding site and physical separation of the αβ transmembrane and cytosolic domains. (b) Cytoplasmic domain sequence alignment of all 21 human α-integrin isoforms. The juxta-membrane GFFKR motif is highly conserved in 21, and fully conserved in 16
Admittedly, it was rather by accident that work conducted in our lab led to a ‘rediscovery’ of CALR-integrin interaction relevant to cancer studies. We rationalized that a phenomenon known as cell adhesion mediated drug resistance (CAM-DR) involved α-integrin tail interactions with downstream effector proteins, and proceeded to express a tail-truncated version of integrin α4, called α4δ, with only the minimal KxGFFKR cytosolic tail to maintain αβ-heterodimer pairing. Unexpectedly, rather than abrogating CAM-DR, α4δ-expression enhanced chemoresistance in a manner that bypassed the requirement for cell adhesion (Liu et al. 2013). This phenomenon was reproduced by expression of Tacδ, essentially a non-integrin dummy transmembrane receptor with the same KxGFFKR cytosolic tail, while a scrambled version KxRFGFK that does not bind CALR failed to do so. Furthermore, whereas cells expressing wildtype α4 required cell adhesion to stimulate Akt phosphorylation, Ca2+-flux and integrin-CALR interaction, α4δ and Tacδ cells activated these pathways constitutively in an adhesion-independent manner (Liu et al. 2013). We extended these findings to show that drug induced surface CALR expression was attenuated for cells plated to engage integrins, while α4δ and Tacδ expression decreased surface CALR expression in an adhesion-independent manner (Liu et al. 2016). Further to this, treatment of cells with either chemical- or antibody-based agonists of integrin activation resulted in complete suppression of drug-induced surface CALR exposure, while cells with overall reduced α-integrin expression enhanced it (Liu et al. 2019a). The integrin modulatory effect on cell surface CALR is functionally relevant, as cells with activated integrins, stimulated via adhesion or molecular agonists, exhibited reduced phagocytosis by macrophages concomitant with reduced surface CALR levels. In sum, our findings add a new layer to the pro-tumour survival effects mediated by integrins to include suppression of a surface immunogen important in anti-tumour immunity.
Importantly, GFFKR as a highly conserved motif found in the cytosolic tail of α-integrins provision a widely applicable universal mechanism in multiple cancer cell types where various integrin-substrate interactions can potentiate reduced therapy-induced ICD. Mechanistically, it is interesting to speculate how CALR-integrin binding can occur in a manner stimulated by cell adhesion and how this binding reduces surface CALR presented. Structural studies of the integrin αβ-transmembrane helices provide evidence that the GFFKR moiety transitions from a partial membrane-embedded state in inactive integrins, to full exposure within the cytosol when activated (Kim et al. 2011; Lau et al. 2009). In addition, the transmembrane and cytosolic domains of αβ-integrins is physically separated, facilitating access for binding by cytosolic effector proteins. More recently, proximity ligation assays (PLA) was used to provide in situ evidence that CALR-integrin interaction occurs at the membrane proximal cytosolic compartment, and the interaction was enhanced upon treatment with agonists that activate integrins (Ohkuro et al. 2018). The same study also showed that a small molecule inhibitor able to disrupt CALR-GFFKR binding in vitro also disrupted CALR-integrin binding in situ, and blockaded integrin-mediated cell-substrate adhesion. It remains to be shown if this small molecule inhibitor may be used as a therapeutic to enhance both the chemosensitivity of tumour cells and immunogenic cell death via increased surface CALR presentation.
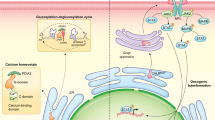
Collectively, the combined studies support a model whereby conformational change accompanying integrin activation exposes the GFFKR moiety of α-integrin within the membrane proximal cytosol available to bind CALR (Fig. 9.2). This pool of CALR is dramatically increased for cells treated with ICD-inducers, which in the absence of activated integrins, translocate to the cell surface to act as the immunogen. When integrins are activated, GFFKR-mediated integrin-CALR binding act to sequester CALR within the cytosol, thereby limiting the levels of surface CALR presented.
Activated integrins suppress immunogenic cell death by limiting presentation of cell surface CALR. At rest, CALR (red) and PDIA3 (blue) is highly enriched in the ER-lumen. Treatment with ICD-inducers promote ER-stress and release of CALR-PDIA3 complex into the extra-ER cytosolic space. In the absence of activated integrins, the CALR-PDIA3 ‘immunogenic complex’ readily translocates to the cell surface, where CALR acts as a DAMP to promote phagocytosis. When activated, integrins undergo conformational change that exposes the membrane proximal GFFKR motif of α-integrin to bind and sequester CALR-PDIA3 complex in the cytosolic compartment and reducing the levels of surface CALR and PDIA3 presented
9.6 Regulation of Oncogenic Signaling by Lectin Chaperones
In this section, we consider the evidence that support a role for lectin chaperones in oncogenic signaling operating primarily in the extra-ER cytosol. As mentioned, α4δ or Tacδ expressing cells exhibit enhanced drug resistance concomitant with increased Akt phosphorylation and Ca2+ signaling (Liu et al. 2013). We hypothesized that increased sequestration of cytosolic CALR by GFFKR may promote increased Ca2+ influx coupled to drug efflux; alternatively, increased intracellular Ca2+ may promote enhanced Akt pro-survival signaling. Subsequently, we were able to show that CALR−/− cells exhibit reduced intracellular Ca2+-flux and increased susceptibility to chemotherapy (unpublished results); however we have not been able to resolve if the phenomena is mediated by cytosolic or total CALR that includes the ER-luminal form. Of interest, studies of myeloproliferative neoplasms associated mutant CALR (MPN-CALR) suggested MPN-CALR enhanced proliferative Akt signaling (Fu et al. 2019). However, it’s not clear if the effects are mediated via increased presence of cytosolic CALR, since MPN-CALR primarily acts as a pseudo-cytokine to specifically activate the MPL receptor and transduce JAK/STAT signaling. Resolution to this question must involve expressing MPN-CALR in a cell model that does not express MPL receptor to negate the role of cytokine signaling and to demonstrate enhanced extra-ER cytosolic accumulation of the mutant protein. This may be predicted, since MPN-CALR lacks the KDEL ER-retention sequence. Interestingly, small molecule based disruption of CALR-integrin binding inhibited inflammatory cytokine induced phosphorylation of STAT3 (Ohkuro et al. 2018), implicating a role for cytosolic CALR in oncogenic STAT3 signaling.
The involvement of a chaperone with STAT3 signaling was initially described for PDIA3. In melanoma cells, chromatin immunoprecipitation assays identified PDIA3 as a necessary STAT3 co-factor for binding DNA transcriptional elements regulated by STAT3 (Chichiarelli et al. 2010). Moreover, increased PDIA3-STAT3 complexes was detected in radioresistant laryngeal cancer cells, while PDIA3 depletion results in decreased STAT3 phosphorylation induced by irradiation (Choe et al. 2015). In contrast, Pdia3−/− mouse embryonic fibroblasts exhibit increased levels of phosphorylated STAT3 and increased transcription of a STAT3 regulated promoter (Coe et al. 2010). In this report, Calr−/− fibroblasts exhibited no differences in phosphorylated STAT3, while STAT3 regulated transcription was enhanced in a similar fashion as Pdia3−/− cells. Furthermore, PDIA3 inhibition of STAT3 transcriptional activity was partially reconstituted in Pdia3−/− fibroblasts expressing an ER-targeted form of PDIA3, but not with cytosolic PDIA3, suggesting that luminal PDIA3 modulates STAT3. STAT3 transcription activity was not affected in Canx−/− cells, but requires a functional CALR-PDIA3 complex, since re-expression of a PDIA3 binding mutant of CALR does not rescue Calr−/− cells (Coe et al. 2010). Recently, it was shown that CALR knockdown in human nasopharyngeal carcinoma cell lines inhibited cell migration, invasion and proliferation, as well as decreased total and phosphorylated STAT3 levels (Han et al. 2019b). It’s unclear what accounts for the reported differences in activities for CALR and PDIA3; different species background may play a role (mouse vs human), as so is the cell type specific context (different tissues, and normal vs tumour cells). Further studies are required to establish a clearer consensus for the role/s of PDIA3 and CALR in oncogenic STAT3 signaling.
Other prominent pathways potentially regulated by ER-chaperones include MAPK and p53. Knockdown of CALR led to decreased migration and invasion in breast cancer cells that was correlated with significantly reduced expression of genes in MAPK signaling, including MAP2K6, MAPK8, ERK2, and MAPK14, and upregulation of several target genes of p53 (Zamanian et al. 2016). This is supported by studies in pancreatic cancer cells, where CALR silencing inhibited EGF-induced pathways involved in epidermal to mesenchymal transition, including E-cadherin, β-catenin, EGFR and ERK (Sheng et al. 2017). Coincidentally, the inhibition of PDIA3 with siRNA in human acute myeloid leukemia cells resulted in decreased proliferation with increased apoptosis, concomitant with significant decreases in phosphorylated p38, JNK and ERK (Ye et al. 2018). Interestingly, CALR silencing in several solid tumours led to significant increases in caspase-dependent apoptotic proteins, even though an increase in cell death was observed (Han et al. 2019a). This was attributed to increased expression of total and activated p53, since CALR silencing induced cell death was effectively negated upon p53 activity blockade with a dominant negative peptide-based inhibitor.
The accumulated evidence clearly highlight the multitude of oncogenic pathways that are regulated by lectin-ER chaperones stemming from their varied localizations including the cell surface, extra-ER cytosol, nucleus, and of course, the ER-lumen. An interesting hypothesis for their roles in oncogenesis and tumour progression stems from their enrichment within these subcellular compartments leading to dysregulation of pathways not commonly observed in normal, non-tumour cells. A key aspect that remains to be exploited is detailed multiparameter studies that compares the relative enrichment of all three proteins to better understand their relationship as prognostic biomarkers in cancer, and their relative contribution in oncogenic signaling.
9.7 Summary Points
-
High expression of CALR and/or PDIA3 in tumour tissues may have positive or negative prognostic significance that vary by tumour type. More limited studies suggest high CANX expression is associated with a poorer prognosis.
-
ICD-inducer mediated presentation of surface CALR on tumour cells is dependent on PDIA3 to facilitate translocation of the CALR-PDIA3 as a complex from the ER-lumen to the extracellular cell surface.
-
Activated integrins mediate the suppression of surface CALR presentation in ICD. The mechanism involves a conserved cytosolic GFFKR motif on α-integrin that facilitate binding to and predicted sequestration of CALR in the cytosol.
-
CALR and PDIA3 can modulate activities of oncogenic pathways including STAT3, Akt, MAPK, p53, and Ca2+ flux manifested in alterations of cell adhesion, migration, proliferation, and cell death.
9.8 Future Issues
-
It is not known if surface CALR presented on the surface of ICD-inducer treated tumour cells requires physical interaction with a receptor-like protein. LRP1 (LDL receptor-related protein 1) is a known trans-acting CALR receptor expressed on macrophages that may facilitate this role as a cis-acting receptor on tumour cells.
-
Thus far, functional integrin suppression of surface CALR have been studied with leukemic blasts that grow viably in suspension. It remains to be shown if cells of solid tumour origin, which are anchorage-dependent to remain viable, are inherently more resistant to ICD-inducing therapies.
-
A number of existing biologics target integrin-substrate interactions that may be evaluated as ICD-enhancing therapeutics. Consideration must be given to ensure that these agents act in a manner to blockade integrin function without their activation, which would achieve the opposite desired effect whereby immunogenicity of the tumour is reduced.
-
Due to their multi-locale nature, there remains a continuing challenge to resolve the compartment specific functions of CALR, CANX and PDIA3 in a manner distinct from their roles as ER-chaperones. This limitation can be partly addressed by cell models that achieve compartment specific expression of the respective proteins that allows distinction between activities mediated by their extra-ER localization and those originating from the ER-lumen.
-
Fundamental reported differences exist for signaling attributed to CALR and PDIA3 expression. Further work will be required to resolve if the differences could be species dependent, tissue cell type dependent, or even differences arising from oncogenic transformation vs normal cells.
-
Is surface CANX on tumour cells immunosuppressive? Or is this role facilitated by competitive inhibition of surface CALR involving limited PDIA3?
Abbreviations
- CAM-DR:
-
Cell adhesion mediated drug resistance
- CALR:
-
Calreticulin, also known as calregulin, CRT, CRP55, CaBP3 and ERp60
- CANX:
-
Calnexin, also known as CNX, IP90 and P90
- DAMP:
-
Damage associated molecular patterns
- ER:
-
Endoplasmic reticulum
- PDIA3:
-
Protein disulfide isomerase A3, also known as ERp57, ER60, GRP57, GRP58
- ERK:
-
Extracellular signal regulated kinase
- ICD:
-
Immunogenic cell death
- MAPK:
-
Mitogen activated protein kinase
- STAT3:
-
Signal transducer and activator of transcription 3
References
Adams BM, Oster ME, Hebert DN (2019) Protein quality control in the endoplasmic reticulum. Protein J 38:317–329. https://doi.org/10.1007/s10930-019-09831-w
Afshar N, Black BE, Paschal BM (2005) Retrotranslocation of the chaperone calreticulin from the endoplasmic reticulum lumen to the cytosol. Mol Cell Biol 25:8844–8853. https://doi.org/10.1128/MCB.25.20.8844-8853.2005
Alam A et al (2019) SMAR1 favors immunosurveillance of cancer cells by modulating calnexin and MHC I expression. Neoplasia 21:945–962. https://doi.org/10.1016/j.neo.2019.07.002
Alur M et al (2009) Suppressive roles of calreticulin in prostate cancer growth and metastasis. Am J Pathol 175:882–890. https://doi.org/10.2353/ajpath.2009.080417
Chao MP et al (2010) Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2:63ra94. https://doi.org/10.1126/scitranslmed.3001375
Chen Y et al (2019) Calnexin impairs the antitumor immunity of CD4(+) and CD8(+) T cells. Cancer Immunol Res 7:123–135. https://doi.org/10.1158/2326-6066.CIR-18-0124
Chiang WF et al (2013) Calreticulin, an endoplasmic reticulum-resident protein, is highly expressed and essential for cell proliferation and migration in oral squamous cell carcinoma. Oral Oncol 49:534–541. https://doi.org/10.1016/j.oraloncology.2013.01.003
Chichiarelli S et al (2010) Role of ERp57 in the signaling and transcriptional activity of STAT3 in a melanoma cell line. Arch Biochem Biophys 494:178–183. https://doi.org/10.1016/j.abb.2009.12.004
Choe MH et al (2015) ERp57 modulates STAT3 activity in radioresistant laryngeal cancer cells and serves as a prognostic marker for laryngeal cancer. Oncotarget 6:2654–2666. https://doi.org/10.18632/oncotarget.3042
Coe H, Jung J, Groenendyk J, Prins D, Michalak M (2010) ERp57 modulates STAT3 signaling from the lumen of the endoplasmic reticulum. J Biol Chem 285:6725–6738. https://doi.org/10.1074/jbc.M109.054015
Coppolino M, Leung-Hagesteijn C, Dedhar S, Wilkins J (1995) Inducible interaction of integrin alpha 2 beta 1 with calreticulin. Dependence on the activation state of the integrin. J Biol Chem 270:23132–23138
Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S (1997) Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature 386:843–847
Franz S et al (2007) After shrinkage apoptotic cells expose internal membrane-derived epitopes on their plasma membranes. Cell Death Differ 14:733–742. https://doi.org/10.1038/sj.cdd.4402066
Fu C et al (2019) AKT activation is a feature of CALR mutant myeloproliferative neoplasms. Leukemia 33:271–274. https://doi.org/10.1038/s41375-018-0224-8
Fucikova J et al (2016) Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res 76:1746–1756. https://doi.org/10.1158/0008-5472.CAN-15-1142
Galluzzi L et al (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25:486–541. https://doi.org/10.1038/s41418-017-0012-4
Gardai SJ et al (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123:321–334. https://doi.org/10.1016/j.cell.2005.08.032
Garg AD et al (2012) A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 31:1062–1079. https://doi.org/10.1038/emboj.2011.497
Gold LI et al (2010) Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 24:665–683
Gutierrez T, Simmen T (2014) Endoplasmic reticulum chaperones and oxidoreductases: critical regulators of tumor cell survival and immunorecognition. Front Oncol 4:291. https://doi.org/10.3389/fonc.2014.00291
Halperin L, Jung J, Michalak M (2014) The many functions of the endoplasmic reticulum chaperones and folding enzymes. IUBMB Life 66:318–326. https://doi.org/10.1002/iub.1272
Han A et al (2019a) Calreticulin is a Critical Cell Survival Factor in Malignant Neoplasms. PLoS Biol 17:e3000402. https://doi.org/10.1371/journal.pbio.3000402
Han Y et al (2019b) High expression of calreticulin indicates poor prognosis and modulates cell migration and invasion via activating Stat3 in nasopharyngeal carcinoma. J Cancer 10:5460–5468. https://doi.org/10.7150/jca.35362
Harada K, Takenawa T, Ferdous T, Kuramitsu Y, Ueyama Y (2017) Calreticulin is a novel independent prognostic factor for oral squamous cell carcinoma. Oncol Lett 13:4857–4862. https://doi.org/10.3892/ol.2017.6062
Hettinghouse A, Liu R, Liu CJ (2018) Multifunctional molecule ERp57: From cancer to neurodegenerative diseases. Pharmacol Ther 181:34–48. https://doi.org/10.1016/j.pharmthera.2017.07.011
Hynes RO (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687
Kasikova L et al (2019) Calreticulin exposure correlates with robust adaptive antitumor immunity and favorable prognosis in ovarian carcinoma patients. J Immunother Cancer 7:312. https://doi.org/10.1186/s40425-019-0781-z
Kim C, Schmidt T, Cho EG, Ye F, Ulmer TS, Ginsberg MH (2011) Basic amino-acid side chains regulate transmembrane integrin signalling. Nature 481:209–213. https://doi.org/10.1038/nature10697
Lau TL, Kim C, Ginsberg MH, Ulmer TS (2009) The structure of the integrin alphaIIbbeta3 transmembrane complex explains integrin transmembrane signalling. EMBO J 28:1351–1361. https://doi.org/10.1038/emboj.2009.63
Leach MR, Cohen-Doyle MF, Thomas DY, Williams DB (2002) Localization of the lectin, ERp57 binding, and polypeptide binding sites of calnexin and calreticulin. J Biol Chem 277:29686–29697. https://doi.org/10.1074/jbc.M202405200
Leys CM, Nomura S, LaFleur BJ, Ferrone S, Kaminishi M, Montgomery E, Goldenring JR (2007) Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery 141:41–50. https://doi.org/10.1016/j.surg.2006.05.009
Liu R et al (2012) Calreticulin as a potential diagnostic biomarker for lung cancer. Cancer Immunol Immunother 61:855–864. https://doi.org/10.1007/s00262-011-1146-8
Liu CC, Leclair P, Yap SQ, Lim CJ (2013) The membrane-proximal KXGFFKR motif of alpha-integrin mediates chemoresistance. Mol Cell Biol 33:4334–4345. https://doi.org/10.1128/MCB.00580-13
Liu CC, Leclair P, Monajemi M, Sly LM, Reid GS, Lim CJ (2016) alpha-Integrin expression and function modulates presentation of cell surface calreticulin. Cell Death Disease 7:e2268. https://doi.org/10.1038/cddis.2016.176
Liu M, Du L, He Z, Yan L, Shi Y, Shang J, Tang H (2017) Increased ERp57 expression in HBV-related hepatocellular carcinoma: possible correlation and prognosis. Biomed Res Int 2017:1252647. https://doi.org/10.1155/2017/1252647
Liu CC et al (2019a) Integrins and ERp57 coordinate to regulate cell surface calreticulin in immunogenic cell death. Front Oncol 9:411. https://doi.org/10.3389/fonc.2019.00411
Liu Y et al (2019b) Upregulation of ERp57 promotes clear cell renal cell carcinoma progression by initiating a STAT3/ILF3 feedback loop. J Exp Clin Cancer Res 38:439. https://doi.org/10.1186/s13046-019-1453-z
Matsukuma S et al (2016) Calreticulin is highly expressed in pancreatic cancer stem-like cells. Cancer Sci 107:1599–1609. https://doi.org/10.1111/cas.13061
Minami S et al (2014) Detection of tumor-associated antigens in culture supernatants using autoantibodies in sera from patients with bladder cancer. Biomed Res 35:25–35. https://doi.org/10.2220/biomedres.35.25
Molica S et al (2016) Serum levels of soluble calreticulin predict for time to first treatment in early chronic lymphocytic leukaemia. Br J Haematol 175:983–985. https://doi.org/10.1111/bjh.13907
Obeid M (2008) ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. J Immunol 181:2533–2543
Obeid M et al (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13:54–61. https://doi.org/10.1038/nm1523
Ohkuro M et al (2018) Calreticulin and integrin alpha dissociation induces anti-inflammatory programming in animal models of inflammatory bowel disease. Nat Commun 9:1982. https://doi.org/10.1038/s41467-018-04420-4
Okazaki Y, Ohno H, Takase K, Ochiai T, Saito T (2000) Cell surface expression of calnexin, a molecular chaperone in the endoplasmic reticulum. J Biol Chem 275:35751–35758. https://doi.org/10.1074/jbc.M007476200
Panaretakis T et al (2008) The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 15:1499–1509. https://doi.org/10.1038/cdd.2008.67
Rojiani MV, Finlay BB, Gray V, Dedhar S (1991) In vitro interaction of a polypeptide homologous to human Ro/SS-A antigen (calreticulin) with a highly conserved amino acid sequence in the cytoplasmic domain of integrin alpha subunits. Biochemistry 30:9859–9866
Russell SJ et al (2004) The primary substrate binding site in the b' domain of ERp57 is adapted for endoplasmic reticulum lectin association. J Biol Chem 279:18861–18869. https://doi.org/10.1074/jbc.M400575200
Ryan D et al (2016) Calnexin, an ER stress-induced protein, is a prognostic marker and potential therapeutic target in colorectal cancer. J Transl Med 14:196. https://doi.org/10.1186/s12967-016-0948-z
Samanta S et al (2017) Expression of protein disulfide isomerase family members correlates with tumor progression and patient survival in ovarian cancer. Oncotarget 8:103543–103556. https://doi.org/10.18632/oncotarget.21569
Shaffer KL, Sharma A, Snapp EL, Hegde RS (2005) Regulation of protein compartmentalization expands the diversity of protein function. Dev Cell 9:545–554. https://doi.org/10.1016/j.devcel.2005.09.001
Sheng W et al (2017) Calreticulin promotes EGF-induced EMT in pancreatic cancer cells via Integrin/EGFR-ERK/MAPK signaling pathway. Cell Death Dis 8:e3147. https://doi.org/10.1038/cddis.2017.547
Shimoda T et al (2019) Expression of protein disulfide isomerase A3 and its clinicopathological association in gastric cancer. Oncol Rep 41:2265–2272. https://doi.org/10.3892/or.2019.6999
Stoll G, Iribarren K, Michels J, Leary A, Zitvogel L, Cremer I, Kroemer G (2016) Calreticulin expression: Interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Onco Targets Ther 5:e1177692. https://doi.org/10.1080/2162402X.2016.1177692
Takada Y, Ye X, Simon S (2007) The integrins. Genome Biol 8:215. https://doi.org/10.1186/gb-2007-8-5-215
Takata H et al (2016) Increased expression of PDIA3 and its association with cancer cell proliferation and poor prognosis in hepatocellular carcinoma. Oncol Lett 12:4896–4904. https://doi.org/10.3892/ol.2016.5304
Wiersma VR, Michalak M, Abdullah TM, Bremer E, Eggleton P (2015) Mechanisms of translocation of ER chaperones to the cell surface and immunomodulatory roles in cancer and autoimmunity. Front Oncol 5:7. https://doi.org/10.3389/fonc.2015.00007
Wiest DL, Burgess WH, McKean D, Kearse KP, Singer A (1995) The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. EMBO J 14:3425–3433
Ye Q, Fu P, Dou J, Wang N (2018) Downregulation of PDIA3 inhibits proliferation and invasion of human acute myeloid leukemia cells. Onco Targets Ther 11:2925–2935. https://doi.org/10.2147/OTT.S162407
Zamanian M, Qader Hamadneh LA, Veerakumarasivam A, Abdul Rahman S, Shohaimi S, Rosli R (2016) Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell Int 16:56. https://doi.org/10.1186/s12935-016-0329-y
Acknowledgments
Research conducted in CJL’s laboratory was supported by grants from the Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Canadian Cancer Society Research Institute and the Leukemia and Lymphoma Society of Canada. We also acknowledge additional funding received from the Michael Cuccione Foundation for Childhood Cancer Research and the BC Children’s Hospital Foundation.
Conflict-of-Interest Disclosure Statement
The authors declare no existing conflict-of-interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lam, S.T.T., Lim, C.J. (2021). Cancer Biology of the Endoplasmic Reticulum Lectin Chaperones Calreticulin, Calnexin and PDIA3/ERp57. In: Agellon, L.B., Michalak, M. (eds) Cellular Biology of the Endoplasmic Reticulum . Progress in Molecular and Subcellular Biology, vol 59. Springer, Cham. https://doi.org/10.1007/978-3-030-67696-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-67696-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67695-7
Online ISBN: 978-3-030-67696-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)