Abstract
High reliance on chemical pesticides for controlling phytophagous pests in agro-ecosystems has resulted in different negative effects, and this issue dramatically changed our attitude in pest management programs. Among different safe alternatives for combating pest populations in agro-ecosystems, biological control has considerable potential by utilization of other living organisms including predators, parasitoids and entomopathogens. Pathogenic agents are diverse group of biological operators which exhibit reliable activities in different situations and hence, their application in agro-ecosystems has significantly increased. However, to maximize the benefits and increase the effectiveness of these natural enemies, “Integrated Biological Control” (IBC) could be applied as a promised strategy. This approach not only increases the effectiveness of native natural enemies, but also has confirmed impacts on exotic agents. Furthermore, IBC could reveal actual capacity of these pathogenic agents for regulating population density of target organisms, playing a critical role for successful implementation of biocontrol programs. On the other hand, simultaneous application of entomopathogens and other natural enemies may adversely affects their biological performance, especially in the case of insect predators/parasitoids, as discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Currently, agricultural systems (agro-ecosystems) only partially statisfy food requirements of increasing populations. Different factors restrict successful production of these systems in different parts of the world. Among them, arthropod pests (insects and mites) are considered as most dangerous factors (Fathipour and Sedaratian 2013). Among different management strategies for suppression of such organisms, application of chemical pesticides is still practiced by farmers and growers (Nauen et al. 2001; Ditillo and Walgenbach et al. 2016). However, high reliance on chemical pesticides and their extensive usage has resulted in many deleterious effects (e.g. negative effects on non-target organisms, hazard to human health, pest resistance, resurgence and outbreak, environmental pollutions, toxic residues in agricultural products etc.). The above-mentioned disadvantageous, and also the increasing global concerns about food safety and security, revealed a need of changes in pest management programs (Mohan et al. 2008). Accordingly, organic agriculture is urgently required as this option could minimize negative effects of chemical pesticides in agro-ecosystems (Fathipour and Maleknia 2016).
Despite conventional agriculture, to achieve sustainable management and regulation of pest populations, modern agriculture relies on more eco-friendly options. These focus on integrated pest management (IPM) programs with special emphasis on non-chemical methods on host plant resistance (HPR), interference tactics by sex pheromones and biological control (biocontrol) (Sedaratian et al. 2009, 2013; Fernandez et al. 2017). Bio control is an effective strategy for management of destructive organisms (insects, mites, weeds and plant diseases) by the utilization of other living organisms known as natural enemies or biocontrol agents (e.g. predators, parasitoids and entomopathogens). This procedure typically involves deliberate human activities and is considered as an inseparable component of any IPM program, based on three basic strategies: introduction, augmentation and conservation (Rechcigl and Rechcigl 1998). However, natural enemies play a deterministic role in the success of such programs and, as a first step before application, their efficiency, together with their possible interactions with other organisms should be accurately investigated (Jervis 2005).
In comparison with other natural enemies, entomopathogens show a huge diversity. They consist of several groups of living organisms including entomopathogenic fungi, bacteria, viruses, nematodes and protists, which cause severe and often lethal infections in target organisms. Entomopathogens provide a non-chemical alternative for sustainable management of pest populations. Although our knowledge about these natural enemies steady increased in the last century, specific gaps remained on different aspects of such microbial agents. Today, many entomopathogens are commercially produced, formulated and released in agro-ecosystems for management of arthropod pests in a process similar to synthetic pesticides.
Undoubtedly, widespread use of entomopathogenic agents in natural environments has resulted in undefined effects which need to be investigated, in particular for their simultaneous interactions with other natural enemies eventually applied (Koul and Dhaliwal 2002). In such cases, the main concern is the likelihood of detrimental interactions occurring between entomopathogens and predators/parasitoids, especially when antagonistic interactions disrupt effectiveness of pest management programs (Sedaratian et al. 2014). To develop widespread usage of these microbial agents in organic agriculture, our knowledge about such interactions should hence be extended. In fact, we need more detailed information to evaluate safety of microbial agents towards other non-target organisms in agro-ecosystems. In the current chapter, different research projects performed to evaluate possible interactions between entomopathogenic agents and insect predators/parasitoids are reviewed. Furthermore, a concise interpretation of such interactions is presented, with a discussion on future evolution of microbial pest control as well as microbial biopesticides.
9.2 Definition and Basic Principles of Biological Control
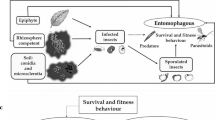
Generally, biological control could be defined as intentional practices involving the application of natural enemies (predators, parasitoids and pathogens) to reduce damage caused by phytophagous arthropods (insects and mites), weeds and plant diseases. Accordingly, the main objective of such programs is minimizing the undesirable effects of target pests and involves regulation of their populationdynamics (Crawley 1989). DeBach (1964) stated that biological control, considered as a part of natural control, could be described as the activity of natural enemies in maintaining population density of other organisms at a lower equilibrium level than would occur in the absence of these agents (Fig. 9.1). In fact, the concepts of “population regulation” and “equilibrium level” are inseparable parts of biological control. To regulate the population of any target organism, different factors should act (separately or in combination) in direct or inverse density-dependent manners (Huffaker et al. 1984).
Success biocontrol programs is achieved when a significant reduction in population density of a target pests occurs, with eventual maintenance below any economic threshold at non-pest status (DeBach and Rosen 1992; van Driesche and Bellows 1988). In such circumstances, stable interactions between population of pests and their natural enemies should occur, with a decline in pest population density, expected following the introduction of the biocontrol agents (Fig. 9.1). Success of biological programs may be affected by several factors (biotic and abiotic) and therefore, these programs have no similar outcomes. DeBach and Rosen (1992) stated that from 164 species of insect pests subjected to biological control programs, 75 cases resulted in “complete” success, 74 were “substantial” and 15 achieved a “partial” control. Keeping this in view, it is noticeable that among non-chemical strategies used in management programs of arthropod pests (e.g. biological, cultural, physical, mechanical, genetic, interference and etc.), biological control achieved greatest number of success (DeBach and Rosen 1992). However, any biocontrol program aims at increasing natural control of pest population. To achieve this goal, fundamental understanding of many aspects of population ecology, of both pests and their natural enemies, is urgently needed (Mills and Getz 1996).
Biological control is compatible with other management strategies for combating pest populations. This approach is considered as a reliable alternative to suppress pest damages and reduce deleterious effects of chemical pesticides. Accordingly, in modern agriculture, biological programs are strongly considered as the cornerstone of sustainability, and reliance on their applications is a key factor to guarantee food security. On the other hand, biological agents regulate population density of other living organisms at the field/greenhouse conditions. Hence, their impacts on population structure of both target and non-target organisms, as well as the environmental benefits derived, should be investigated with more accuracy.
9.3 Natural Enemies as Reliable Tools for Biological Programs
Biocontrol agents (natural enemies) have an impact on in designing biological programs and their performance affects their success rate. In tri-trophic systems (Fig. 9.2), natural enemies are placed at the top of the food chain (third level) but are limited by the abundance of the herbivorous populations (Hariston et al. 1960; Koul and Dhaliwal 2003). In these chains, direct and indirect interactions exist among different food levels and feeding activities of natural enemies, on the different herbivorous life stages (top-down effects). These interactions play a crucial role in regulating population fluctuations of undesirable organisms. On the other hand, different attributes of the first (host plants) and second (phytophagous pests) levels could significantly affect the biological performance of natural enemies (bottom-up effects) (Fathipour and Sedaratian 2013).
During recent years, considerable efforts were performed to evaluate practical and theoretical aspects of natural enemies. In this view, increasing demands for predators and parasitoids, simple life cycles of most natural enemies (particularly parasitoids), relative ease for mass rearing and investigations on these organisms in laboratory conditions resulted in an increased global attention, facilitating further research projects (Jervis 2005). Herein, brief information about different groups of natural enemies used in biological programs is presented.
9.3.1 Predators
In general, predation is defined as a biological interactions between two organisms where one of them (predator) kills and eats another ones (prey). Predators attack and kill many preys during their life span in both immature and adult stages. These natural enemies can be found in different agricultural and natural habitats. Several groups of animals have predatory behavior on insect and mite pests in agro-ecosystems (Koul and Dhaliwal 2003). Table 9.1 lists some of the most important groups of insect predators used in biological control programs. Feeding behavior of predators, as concerns their choice of prey, ranges from specialized to generalists (Hoffmann and Frodsham 1993). Unfortunately, although some predators are extremely useful agents, some of them have predation behaviors also on other beneficial organisms. From the view point of biology, each species presents a different life-cycle. The life history of common predators is well investigated, but our knowledge about many species is still very limited (Hokkanen 1993).
However, efficiency varies among species. Some predators have considerable impact on suppression of a prey population. For example, in the case of homopterous insects, where the insect body is covered by a waxy layer and contact chemicals have no sufficient effects, predators exhibit a reliable performance. Another success has been obtained in the case of lepidopteran pests which have borer and internal feeding behaviors (Dhaliwal and Arora 2001). It should be mentioned that some predators may have only a minor role by themselves, but contribute to overall pest mortality or provide good control at a late season.
9.3.2 Parasitoids
A parasitoid is an organism that lives, feeds and develops inside (endoparasitoid) (Fig. 9.3) or outside (ectoparasitoid) its host’s body. In fact, female individuals deposit their fertile eggs in/on the body of their hosts, and the hatched larvae consume the host tissues.
In more cases, only immature stages feed on their hosts and adult individuals have a nectar-feeding behavior. Adult females of certain parasitoids, attacking scales and whiteflies, kill their hosts and provide important sources of control, causing host mortality by their parasitism activity. In nature, most insect parasitoids belong to some groups of wasps (Order: Hymenoptera) or flies (Order: Diptera) (Table 9.2).
In contrast with true parasites (fleas and ticks), feeding activity of immature stages of parasitoids kill their hosts. Furthermore, also the adult true parasites feed on their hosts. Unlike the predators, during their life span the parasitoids often consume only one host, which is not killed immediately.
9.3.3 Pathogens
Pathogen is any microorganism (e.g., fungi, bacteria, viruses, nematodes and protista) that can infect and kill their hosts (Khetan 2001). Some of the most important entpmopathogens are shown in Table 9.3.
Deleterious impacts of chemical pesticides increased our need for safe alternatives to these compounds. This situation elicited considerable interests in entomophatogens as reliable and effective agents for suppression of insect pests in agro-ecosystems (Sedaratian et al. 2013, 2014). Under appropriate environmental conditions (e.g., extended period of high humidity or dense pest populations), entomopathogens produce an epizootic in natural populations of different arthropods, drastically decreasing their numbers (Mracek and Sturhan 2000; Udayababu et al. 2012; Haar et al. 2018). As microbial pesticides, some of these organisms such as Bacillus thuringienis Berliner (Bt), Metarhizium anisopliae (Metch.) Sorok. and Beauveria bassina (Balsamo) are commercially available. The application of entomopathogens in management programs of different pests is favorable since their action occurs without introducing any toxic and non-biodegradable compounds to the environment, and no residue is present on agricultural products (Zimmermann 2007).
9.3.3.1 Fungi
As a diverse group of microorganisms, true fungi have about 1.5 million different species (Schmit and Mueller 2007). Among them, 700 species from 90 genera are documented with insecticidal activities (Roberts and Humber 1981). They belong to two distinct phyla: Entomophthoromycota and Ascomycota (Order: Hypocreales) (Humber 2012). The most common attribute used to consider fungi as natural groups is their sexual fruiting structure. Other characters of fungi are their feeding behavior, and structure, the unicellular (yeasts) or hyphal (filamentous) development and their reproductive strategy (both sexual [Telomorph] and asexual [Anamorph]) (Fig. 9.4).
The ability of producing sexual spores does not occur (or is rare) in many entomopathogenic fungi. Member of this group have mycelial forms that produce asexual spores (conidia). However, given their visible appearance, the hosts infected by fungal entomopathogens are very typical. In comparison with other groups, these agents directly penetrate the host cuticle and have no need for ingestion (Fig. 9.4). Having this trait enables them to parasitize phloem-feeder insects (aphids and whiteflies), which have no feeding activity on sprayed leaves of host plants (Gonzalez et al. 2016).
First attempt to use entomopathogenic fungi for control of insect pests was performed by the Russian scientist Eli Metchnikoff. In fact, he found that soils contaminated with fungal conidia could infect insect larvae. Eventually, he cultured these agents on a artificial substrate (sterilized beer mash) and tested their pathogenecity against different insect pests (Steinhaus 1975). de Faria and Wraight (2007) revealed that 170 microbial products have been developed using fungal metabolites of at least 12 species of entomopathogenic fungi.
Microsporidia
Recent molecular observations transferred the Phylum Microsporidia from Protista to Fungi (Corradi and Keeling 2009) and revealed that this group is related to Zygomycetes (Corradi and Slamovits 2010). Pathogenic activity of Microsporidia was reported both on insect pests and beneficial species. However, symptoms observed in individuals infected by Microsporidia are clearly different from those due to other fungi (Microsporidia have no fruiting bodie). Nosema bombycis Nageli is one of the most important species that infects silkworms, Bombyx mori L., producing dark spots on the larval cuticle named “pebrine”. Efforts by Louis Pasteur around 1870 resulted in strategies for controlling this disease and saved this industry in France. As previously mentioned, these microorganisms have undesirable effects on populations of beneficial insects, especially in high-density colonies. For example, Nosema apis (Zander 1909) and Nosema ceranae (Fries) are considered as dangerous pathogens of honey bees (Paxton 2010). Nosema bombi (Kudo) is pathogenic on bumble bees (Camerona et al. 2011). However, some species of Microsporidia — e.g. Paranosema locustae (Canning), Vavraia culicis (Weiser), Nosema pyrausta (Paillot), N. portugal and Endoreticulatus sp. — have a documented pathogenicity and regulate the population density of several different insect pests. These issue revealed a critical need to concentrate research projects on this group of natural enemies (Lewis et al. 2009).
In most species, infection will start by ingestion of spores during feeding activity of susceptible hosts. In the next step, ingested spores are activated in the host alimentary track and for this, several factors such as gut pH and ions (or their combination) play a main activation role (Keohane and Weiss 1998). With germination of activated spores, polar filaments are extruded and extend rapidly from the swollen spores. The emerged filament penetrates into the host cell and then, all the cellular content of the microsporidian spore (nucleus, membranes, and etc.) are injected into the cytoplasm of the host cells (Williams and Keeling 2005) (Fig. 9.5). After this stage, being deprived of mitochondria, the microsporidia vegetative stage utilizes adenosine triphosphate (ATP) from its host cells as an energy source (Keeling et al. 2010). However, through an unknown mechanism, some species could infect adjacent cells and tissues by moving from infected to healthy cells.
9.3.3.2 Bacteria
Bacteria are among the first life forms that appeared on earth. They consist of a widely diversified group of prokaryotic (deprived of a nuleus) microorganisms with different shapes, mostly spherical (cocci), rod-shaped (bacilli) or spiral (spirochaetes). Advances in microscopy during late 19th and early 20th centuries significantly increased knowledge also on the entomopathogenic bacteria. The Japanese scientist Shigetane Ishiwata conducted the first investigations on silkworm, B. mori , to resolve the problem known as the “sotto-byo-kin” disease of larvae (Aizawa 2001). Finally, his researches led to the identification of a spore-forming bacterium called Bacillus sotto . In 1909, German scientist Ernest Berliner found a similar case on the Mediterranean flour moth, Anagasta kuehniella Zeller, larvae and named the bacterium as B. thuringiensis . However, performed studies by another German scientist, Mattes in 1927 eventually led to the first commercial formulation of this bacterium in 1938 (Milner 1994). Currently, biopesticides with bacterial metabolites and Bt-crops which express insecticidal toxins of B. thuringiensis in their tissues are commonly used strategies in integrated management of insect pests.
To start its pathogenic activity, bacteria enter their host body from different routes. Although ingestion during host feeding activity is considered as the main pathway, bacteria could also infect their host from their integument, respiratory system and eggs. After ingestion, bacteria disrupt midgut epithelial cells and spread into haemolymph. In the following stage they cause bacteremia (without producing toxins and harmful factors) or septicemia (release of toxins together with bacteria reproduction). Finally, entomopathogenic bacteria kill their hosts and external symptoms appear (tissue necrosis, color changes, soft and flaccid tissues).
Entomopathogenic bacteria are classified in the groups of true bacteria (Eubacteria). Considering the presence and structure of cell walls, they are classified into three major divisions including Firmicutes, Gracilicutes and Tenericutes (Gram-positive and Gram-negative cell walls, and without a cell wall, respectively) (Jurat-Fuentes and Jackson 2012). Binary division is the usual strategy for reproduction, in which clonal copies of mother cells are produced as daughter cells. However, these organisms are present in different habitats including soil, water, acidic hot springs, deep parts of Earth’s crust and even radioactive waste (Fredrickson et al. 2004).
9.3.3.3 Viruses
Also the development of insect virology is related to the silkworm industry. In fact, signs and symptoms of infected insects, caused by entomopathogenic viruses, were described by early researchers (Merian 1679; Nysten 1808). However, the study of “melting” disease in the caterpillars of silkworm resulted in the identification of refractive crystal-like bodies in the cells of infected individuals only in recent times. This was the first finding about what we actually know as Nucleo Polyhedral Viruses (NPVs). After this discovery, several researchers continued their studies on entomopathogenic viruses (Bergold 1947). By using an electronic microscopes, the first electron micrograph of NPVs was published by Bergold (1947).
Viruses have no free-living lifestyle and therefore, cannot be classified as true living organisms. In addition, obligate parasitism forces them to depend on the host cells for crucial physiological functions such as reproduction. Shape and size of viruses differ regarding the arrangement of their genomic and protein structures. Entomopathogenic organisms show different shapes such as rods or spheres (Rogers 2011).
Entomopathogenic viruses (Alphanodaviruses, Dicistroviruses, Flaviviruses, Iflaviruses, Tetraviruses, Cypoviruses etc.) have been reported form different insect orders such as Coleoptera, Hymenoptera, Lepidoptera, Orthoptera and Diptera (Murphy et al. 1995; Chen et al. 2012). Viral genomes (DNA or RNA) represent the most important component which conducts the infection process. Similar to entomopathogenic bacteria, infection usually began when viral occlusion bodies are ingested by susceptible hosts. After entrance, the alkaline pH of midgut environment provides a suitable conditions for the ingested bodies. Afterward, the viral genome translocates to the nucleus of midgut epithelial cells. In the next step, the basement lamina cells surrounding the tracheal system are infected and then the infection spreads into other tissues. Entomopathogenic viruses show favourable traits, such as the narrow specificity and host range, a considerable environmental safety, a reliable virulence to target organisms, and the rapid spread of epizootics in the field conditions. These group of biological agents are hence among the promised natural enemies used for biological control of insect pests and should be considered for designing new and sustainable bio-insecticides (Chen et al. 2012).
9.3.3.4 Nematoda
Another important group of natural enemies is that of entomopathogenic nematodes. For the first time, these agents were described as “worms” on grasshoppers and then reported on bumble bees, ants and other hosts (Gould 1747). Using improved microscopes, morphological attributes were described by Kirby and Spence (1822). Several years later, the first entomopathogenic nematode, Steinernema kraussei (Steiner), was extracted from infected sawflies by Steiner (1923). Glaser (1931) could successfully rear Steinernema glaseri (Steiner) under laboratory conditions. Among different species of nematodes which are associated with insects, seven families including Sphaerularidae, Neotylenchidae, Mermithidae, Allantonematidae, Rhabditidae, Steinernematidae and Heterorhabditidae, attained more considerations (Kaya and Stock 1997; Lacey et al. 2001; Grewal et al. 2005). The majority of species used in biological programs belong to the two families Steinernematidae and Heterorhabditidae (Lewis and Clarkey 2012).
After arthropoda, the members of Phylum Nematoda show a huge diversity of habitats, in comparison with other groups of animals (Tanada and Kaya 1993). Their dependency on water is noticeable and approximately all species require this vital element for reproduction. Life style ranges from free-living to facultative or obligate parasites of other animals or plants. Pathogenic activity of some nematodes (Heterorhabditidae, Steinernematidae and some Rhabditidae) on insects is associated to the occurrence of symbiotic bacteria (Lewis and Clarkey 2012). It is documented that symbiotic bacteria from two genera Xenorhabdus and Photorhabdus have close evolutionary relations, being congruent with the entomopathogenic nematode genera Steinernema and Heterorhabditis, respectively (Ulug et al. 2014). To initiate a new infection, the nematode infective juveniles (IJs) f search their hosts and penetrate into their body. After entrance, each nematode releases its symbiotic bacteria in the haemocoel, infecting its host. These bacteria multiply and kill the hosts, becoming a food resource for growth and development of the entomopathogenic nematodes, inside the insect cadaver. Most nematodes complete up to three generations in their hosts and then spread to the environment as new IJs (Lewis et al. 2006). Some species are facultative parasites of insects (Phaenopsitylenchidae), whereas others have harmless phoretic relation (e.g., Rhabditidae, Diplogasteridae, Cephalobidae and etc.) (Poinar 1975).
9.3.3.5 Protista
Unlike previously-mentioned groups, these natural enemies have an unicellular organization, being one the most diverse groups of living organisms (Adl et al. 2005; Cavalier-Smith 2010). All species occur in aquatic and semi-aquatic environments and have an endosymbiotic lifestyle (Lange and Lordy 2012). Protista have both sexual and asexual (binary/multiple division) reproduction mechanisms. The relationships between Protista and insects range from mutualism to commensalism and parasitism. In the case of a pathogenic activity, chronic diseases may occur within the host populations (Solter et al. 1997). Accordingly, pathogenic effects on the host population may be unnoticed. Generally, the detection of external symptoms of protistan infections may be difficult. In the case of severe infections, larvae has swollen and show a whitish appearance. Furthermore, infected larvae show abnormal movements. In comparison with other entomopathogens, Protista have a larger size and their detection on cadavers of dead individuals is hence less difficult. With the aid of a light microscope, protistans are visible on special cadaver tissues, especially the midgut epithelial cells and the malpighian tubules. After infection, the reproductive phase occurs, during which resistant spores are produced to originate new infection cycles.
In this group, several taxa such as Amoebozoa, Apicomplexa (Eugregarinorida, Neogregarinorida, Coccidia), Ciliophora, Euglenozoa and Helicosporidia exhibit considerable insecticidal activity. Their potential could be trusted in management programs of different insect pests.
9.4 Integrated Biological Control and Effectiveness of Biological Control Programs
In insect pests management programs, integration of compatible strategies is one of the most reliable solutions to enhance effectiveness of control efforts (Fathipour and Sedaratian 2013). Current opinions should be revised and new approaches must be designed, to achieve the highest efficiency, due to the diversity and reproductive potential of insect populations. A review of literatures showed that the success of biological programs is affected by different factors, and that the final output may be lower than the desired expectations. Gurr and Wratten (1999) stated that among the performed classical biocontrol programs, the success rate was very low (about 10%), a disappointing statement. These researchers argued that one of the most important reasons which negatively affect the final goal of such programs is caused by ignoring the requirements of natural enemies. However, to maximize the benefits and increase the effectiveness of biocontrol programs, an attitude change is needed to achieve an “integrated biological control” (IBC) that could serve as a promised tool. To date, this term has been used to describe different types of integration. Barbagallo et al. (1982) used this term for a situation in which several natural enemies were released into a specific agro-ecosystem to suppress populations of multiple pests. Sher and Parrella (1996) described under this term the intentional application of more than one natural enemy to control a target pest. In another statement, Gurr et al. (1998) used this term for combined application of different approaches of biological control (classic, conservation and augmentation). Gurr and Wratten (1999) indicated that the potential of classical biological control could be completely attained when basic requirements of biocontrol agents are supplied. These include nectar and pollen (Jervis et al. 1996; Riahi et al. 2016; Khanamani et al. 2017), moderated microclimate (Thomas et al. 1992) and alternative host/prey (Perrin 1975), supplied via habitat manipulation (conservation). Accordingly, they define IBC as a coupled usage of both classical and conservation strategies. Furthermore, they stated that this approach not only increase the effectiveness of native agents, but also has confirmed impacts on exotic natural enemies. However, to achieve the highest efficiency in biological programs, IBC is inevitable. This strategy could in fact reveal the actual capacity of natural enemies for regulating the density of target organisms, and plays a critical role for success implementation of biocontrol programs in future years.
9.5 Simultaneous Applications of Entomopathogens and Insect Predators/Parasitoids in IPM
Deleterious effects of chemical pesticides, used against phytophagous pests, changed our mind in pest management and elicited increasing demands for safe alternatives such as IPM programs (Kogan 1998). In modern agriculture, IPM is the main strategy for managing pest populations. As the most practicable and acceptable procedure, this strategy also appeared as the best solution to minimize undesirable effects of chemical pesticides and reach a sustainable agriculture (Fathipour and Sedaratian 2013).
In IPM programs different compatible strategies such as chemical, cultural, mechanical, physical and interference tactics, as well as biological methods, may be applied to regulate population density of herbivorous mites and insects (Metcalf and Luckmann 1994). Biological control is one of the most promised components and in some circumstances it may be considered as a cornerstone. However, limitations exist in natural conditions of agro-ecosystems, as biocontrol agents alone are often unable to minimize the population density of a target organism. To increase effectiveness of biological programs, integrated usage of natural enemies offer higher reliable options, as shown by numerous research works.
The effects of the entomopathogenic bacterium B. thuringiensis on biological performance of Rogas lymantriae Watanabe, during integrated biocontrol of Lymantria dispar (L.), was investigated by Wallner et al. (1983). Hilbeck et al. (1998b) found that B. thuringiensis has negative effects on survivorship and development of Chrysoperla carnea (Stephens), whose larvae are predators of aphids. Synergistic interactions between B. thuringiensis and Campoletis chlorideae Uchida was documented by Mohan et al. (2008). Carvalho et al. (2012) evaluated interactions between Podisus nigrispinus (Dallas), the predatory bug of larval and pupal stages of Plutella xylostella (L.), and B. thuringiensis . Sedaratian et al. (2014) evaluated possible effects of B. thuringiensis on biological performance of Habrobracon hebetor (Say) during integrated biological control of Helicoverpa armigera (Hubner).
Aqueel and Leather (2013) evaluated integrated biocontrol of aphids by the fungus Verticillium lecanii (Zimmerman) and Harmonia axyridis (Pallas). Labbe et al. (2009) documented the compatibility of B. bassiana with two natural enemies of Trialeurodes vaporariorum Westwood (the predatory bug Dicyphus hesperus Knight and the parasitoid wasp Encarsia formosa Gahan). Mahdavi et al. (2013) argued that the two entomopathognic fungi B. bassiana and M. anisopliae had little negative effects on biological efficacy of H. hebetor. Effects of B. bassiana and Metarhizium brunneum Petch on oviposition behavior of the parasitoid wasp Trybliographa rapae Westwood were analyzed by Rannback et al. (2015). Bayissa et al. (2016) revealed that the simultaneous application of M. anisopliae and predatory ladybird Cheilomenes lunata (F.) could enhance the biocontrol efficiency of different aphids on crucifers and okra. The combined application of Lecanicillium muscarium (Petch) and the two-spotted ladybird, Adalia bipunctata (L.), for integrated biological control of black bean aphid, Aphis fabae Scopoli, was investigated by Mohammed (2018).
In the case of other entomopathogens, Murray et al. (1995) evaluated interactions between nuclear polyhedrosis virus (NPV) and larval stages of three solitary endoparasitoids Hyposoter didymator Thunberg, Cotesia kazak (Telenga) and Microplitis demolitor Wilkinson in biocontrol program of H. armigera . Furthermore, possible effects of NPV on the parasitoid wasp H. hebetor were studied by Stoianova (2007).
For integrated biological control of Plodia interpunctella Hubner, compatibility of entomopathogenic nematode, Heterorhabditis indica Poinar, Karunakar, and David with H. hebetor for coupled application was evaluated by Mbata and Shapiro-Ilan (2010). Atwa et al. (2013) assessed interactions of the koinobiont parasitoid Microplitis rufiventris Kokujev and two pathogenic nematodes Steinernema carpocapsae (Weiser) and H. bacteriophora (Poinar) during a biocontrol program of Spodoptera littoralis (Spodli). Effects of Heterorhabditis amazonensis Andaló, Nguyen and Moino on the predatory beetle Calosoma granulatum Perty, both natural enemies of Spodoptera frugiperda (J. E. Smith), was estimated under laboratory condition (Mertz et al. 2015).
Microsporidia also affect biological traits of natural enemies. Possible interactions between Vairimorpha sp. and Trichogramma chilonis Ishii in their simultaneous application for biological control of P. xylostella was studied by Schuld et al. (1999). Other authors examined combination of microsporidian entomopathogens with parasitoid wasps Macrocentrus grandii Goidanich (Andreadis 1980) and Pediobius foveolatus (Crawford) (Own and Brooks 1986).
9.6 Effects of Entomopathogens on Predators/Parasitoids
One of the most interesting combinations in IBC is simultaneous application of entomopathogens and insect predators/parasitoids. Chandler et al. (2011) stated that in situations in which other natural enemies are unavailable or have no desirable efficiency, entomopathogens could act as a reliable alternative or back-up strategy. In such circumstances different direct and indirect interactions (synergistic, antagonistic and additive) could occurr and any unpredictable outcome may also be expected (Goettel et al. 2010). Accordingly, as first step, compatibility of entomopathogens with other natural enemies (especially predators and parasitoids) should be carefully monitored as well as their possible side effects on non-target organisms including pollinators, birds, mammals, fishes etc.. In extensive applications of microbial products or wide-spread use of broad spectrum entomopathogens, such interactions were frequently observed (Zimmermann 2007). Safety of entomopathogens is crucial for other natural enemies which persist on the host plants during the cropping cycle (from planting to harvest), to minimize negative effects on their efficiency.
9.6.1 Top-Down Effects of Entomopathogens and Biological Alternations in Predators/Parasitoids
In some circumstances entomopathogens have top-down effects on predators/parasitoids as fourth trophic level. In fact, pathogenicity of these microorganisms on predators/parasitoids has different consequences and affects some key biological traits such as mortality, developmental stages, fecundity, sex ratio etc. In the following, some of the most important top-down effects of entomopathogens on predators/parasitoids are discussed. Investigating different aspects of such effects is very important, and should be emphasized for future studies.
9.6.1.1 Mortality
In some situations, widespread use of entomopathogens for managing pest populations may affect non-target organisms present in the same agro-ecosystem (Oluwafemi et al. 2009). Sedaratian et al. (2014) showed that, during integrated management of H. armigera by B. thuringiensis and H. hebetor, this microbial antagonist negatively decreased survivorship of H. hebetor. Adverse effects of B. thuringiensis on other two bracon wasps, Bracon instabilis Marsh and Apanteles litae Nixon, was reported by Salama et al. (1996) during the integrated management of Phthorimaea operculella (Zeller). Similar deleterious effects of B. thuringiensis were mentioned on the parasitoid wasp Meteorus pulchricornis (Wesmael), a biocontrol agent of H. armigera (Walker et al. 2007). In another case, combination of this entomopathogen and H. hebetor to manage P. interpunctella seriously increased mortality of the parasitoid wasp (Oluwafemi et al. 2009). The same results were reported in the case of other organisms. For example, Ulug et al. (2014) stated that when predators consumed infective juvenile of entomopathogenic nematodes, severe infection could be detected in their populations. Similarly, Mertz et al. (2015) showed that when the larvae of the carabid beetle C. granulatum consumed infected larvae of S. frugiperda with entomopathogenic nematodes, a severe mortality occurred 6 days after feeding.
Studies on entomopathogenic fungi showed different outputs.. Ekesi et al. (1999) showed that one of the most important entomopathogenic fungi, M. anisopliae, had no adverse effects on populations of non-target organisms. is. Jacobson et al. (2001) revealed that B. bassiana , another entomopathogenic fungus applied for biological control of arthropod pests, had no significant effects on mortality of different life-stages of the predatory mite Neoseiulus cucumeris (Oedemans). Effect of this pathogenic fungus on several non-target organisms was, however, documented by Ludwig and Oetting (2001). Effects of different B. bassiana strains with considerable virulence on five phytoseiid mites (N. cucumeris, N. californicus (McGregor), N. womersleyi Xin, Liang and Ke, Phytoseiulus persimilis Athias-Henriot and Amblyseius swirskii Athias-Henriot) were evaluated by Wu et al. (2016). Results revealed that the strains tested had no pathogenecity on predatory mites and no significant mortality was recorded. Shipp et al. (2012) described that B. bassiana (GHA isolate) had serious negative effects on a population of the predatory bug Orius sp. Their results revealed that toxicity of B. bassiana is related to experimental conditions, as the tests performed showed a laboratory mortality higher than that observed in greenhouse conditions. Hajek and Goettel (2000) and Jaronski et al. (2003) stated that entomopathogenic fungi have wider host ranges under laboratory conditions. This issue was addressed as differences between physiological (under laboratory conditions) and ecological (in nature) host ranges (Hajek and Butler 2000). In fact, microorganisms with pathogenic activity on non-target organisms under laboratory conditions may have no infections on the same organisms in nature.
9.6.1.2 Duration of Different Life Stages
In IBC of H. armigera using B. thuringiensis and H. hebetor, the entomopathogenic bacterium prolonged immature development of the parasitoid wasp (Sedaratian et al. 2014). Bernal et al. (2002) observed similar findings in Parallorhogas pyralophagus (Marsh), a parasitoid wasp of Eoreuma loftini (Dyar). Similar results were reported when studying the parasitoid wasp M. rufiventris females developed on infected larvae of S. littoralis (El-Maghraby et al. 1988). Such adverse effects on growth of the parasitoid wasp Microplitis croceipes (Cresson) were also described by Blumberg et al. (1997).
When M. anisopliae (isolate M14) was applied together with H. hebetor to manage a population of H. armigera , effects on larval development prolongation were recorded and confirmed (Jarrahi and Safavi 2016). Prolonged pupal development was observed in the parasitoid wasp Aphidius matricariae Haliday when developed on aphids treated with M. anisopliae (Rashki et al. 2009). However, in contrast with these observations, Fatiha et al. (2008) stated that V. lecani had no significant effect on development of the coccinelid beetle Seranjium japonicum Chapin. Murray et al. (1995) showed that entomopathogenic viruses (NPVs) have negative effects on larval development of three parasitoids of H. armigera . These researchers suggest that a time interval of at least 3 days is required between parasitization and NPV exposure to minimize such adverse effects. A similar time interval was proposed by Brown et al. (1989) to minimize NPV effects on development of the parasitoid wasp Glabromicroplitis croceipes (Cresson) applied for IBC of Heliothis virescens (F.).
In earlier study, Huger and Neuffer (1978) found a prolonged adult longevity of the braconid wasp Ascogaster quadridentata Wesmael when its host was infected by Nosema carpocapsae . Futerman et al. (2006) showed that development of the parasitoid wasp Asobara tabida Nees within hosts infected by the microsporidian Tubulinosema kingi Kramer prolonged its development. Data reported by Simoes et al. (2012) showed that immature development of Cotesia flavipes (Cameron) increased and its adult longevity was decreased when the parasitoids developed inside hosts infected by Nosema sp. Hoch et al. (2000) reported that the duration of the larval period of the parasitoid wasp Glyptapanteles liparidis (Bouche) was prolonged on infected larvae of L. dispar , when the latter were infected by the microsporidian Vairimorpha disparis. In another study, Hoch et al. (2002) described that infection of L. dispar larvae by V. disparis changed its carbohydrate and fatty acid contents, reducing the host nutritional quality for normal development of G. liparidis. In another study, effects of the microsporidia Nosema adaliae (Steele and Bjornson) and Tubulinosema hippodamiae (syn. Hippodamia convergens Guérin-Méneville) on development of two-spotted ladybird, A. bipunctata, were described by Steele and Bjornson (2014) under laboratory conditions. Results confirmed extension of larval development on preys infected by N. adaliae, but the other pathogen had no significant effects on the duration of life stage. Furthermore, comparison of pteromalid wasp Muscidifurax raptor Girault and Sanders infected and uninfected by Nosema muscidifuracis (Becnel and Geden) confirmed that this microsporidian prolonged the parasitoid development (Geden et al. 1995). Godfray (1994) noticed that nutritional quality of the parasitoid hosts has confirmed effects on its development. Similarly, Murugan et al. (2000) and Mohan et al. (2008) stated that induced changes in parasitoid hosts after ingestion of pathogenic microorganisms may influence the development and foraging of their parasitoids.
9.6.1.3 Fecundity
In addition to developmental periods and mortality, another direct effect of entomopathogens on insect predators/parasitoids is their possible effects on fecundity (Nielsen et al. 2005). It is documented that Nosema bordati Goudegnon could significantly reduce fecundity of C. flavipes when simultaneously applied for managing Chilo partellus Swinhoe (Bordat et al. 1994). Simoes et al. (2012) evaluated possible effects of Nosema sp. extracted from the sugarcane borer, Diatraea saccharalis (Fabricius), on biological performance of theparasitoid. Their results revealed negative effects on potential of progeny parasitoid production. Geden et al. (1995) compared fecundity of the pteromalid wasp M. raptor , treated and untreated with N. muscidifuracis . Their data revealed that infection dramatically decreased the reproductive potential of this parasitoid. Jarrahi and Safavi (2016) described interactions between H. hebetor and M. anisopliae during management program of H. armigera confirming that the parasitoid wasp had significantly lower daily and total fecundity on infected hosts. Negative effects of B. thuringiensis on fecundity of H. hebetor were reported by Sedaratian et al. (2014). The same results were reported by other researchers (Baur and Boethel 2003; Sanders et al. 2007; Sharma et al. 2008).
However, a reduction of fecundity could be related to several factors. Roy and Pell (2000) described that fungal infection affects physiological functions of female parasitoids and this issue could directly affects their fertilization rate. Another possible reason for fecundity reduction in population of natural enemies is septicemia (Sedarataian et al. 2014). On the other hand, in circumstances in which microbial products are commercially used in large scale, other formulation components may have unknown effects on fecundity of predators/parasitoids (Flexner et al. 1986; Teera-Arunsiri et al. 2003).
9.6.1.4 Sex Ratio
One of the most important indirect effects of entomopathogens on predators/parasitoids populations is their possible impact on the sex ratio (ratio of male to female offspring) especially in the case of parasitoid wasps where the haplo-diploid mechanism allows female individuals to determine the offspring sex ratio. Considering the polygamic behavior of male individuals (fertilization of different females by one male), an increase in female progeny is so beneficial for biological control purposes and enhances the final efficiency of these programs. Different elements such as genetic factors, female wasp density, age of female and male parents, extreme temperature, relative humidity, photoperiod, host size, density, age and sex, as well as its nutritional quality could affect sex ratio of natural enemies (Legner and Badgley 1982; Kido et al. 1983; Morse 1994). Prior to oviposition, female individuals evaluate nutritional quality of their preys/hosts and then selectively decide to deposit female or male eggs. Undoubtedly, entomopathogenic agents have several effects on their hosts including reduction in size and nutritional quality and this issue could affects sex ratio of their natural enemies. However, when natural enemies detect favorable conditions, they alter their sex ratio to female-biased offsprings, in order to build up the future population (Kant et al. 2012).
It is documented that larvae of H. armigera infected by B. thuringiensis have no significant effects on offspring sex ratio of H. hebetor (Sedaratian et al. 2014). Similar outputs were reported by Sharma et al. (2008) when evaluating the effects of this bacterium on the sex ratio of the parasitoid wasp C. chlorideae . Mohammed and Hatcher (2017) investigated sex ratio of the parasitoid wasp Aphidius colemani Viereck on Myzus persicae (Sulzer) treated with the pathogenic fungus L. muscarium. Results obtained showed that extension of time interval between parasitoid introduction and fungus application strongly changed adverse effects on the parasitoid sex ratio. Accordingly, they revealed that offspring sex ratio was not significantly affected when a time interval of 6–7 days was considered between application of the parasitoid wasp and pathogenic fungus. The number of emerged female faced a significant reduction (40%) when this interval was lower than 5 days. In previous study, Aqueel and Leather (2013) described that V. lecani significantly affected the sex ratio of A. colemani emerged from treated aphids.
Geden et al. (2002) observed that the sex ratio of Tachinaephagus zealandicus Ashmead on hosts infected by Nosema sp. was altered favoring the male progeny. During another study, Schuld et al. (1999) showed that ingestion of the microsporidian Vairimorpha sp. had no significant effects on sex ratio of the parasitoid wasp T. chilonis. Similar to this report, Saleh et al. (1995) explained that N. pyrausta did not affect the sex ratio of the parasitoid wasp Trichogramma nubilale Ertle and Davis, when developed on infected eggs of Ostrinia nubilalis Hubner. Steele and Bjornson (2012) showed that offspring sex ratio in A. bipuctata was not affected by the microsporidian N. adaliae.
9.6.2 Entomopathogen Effects on Behavioral Characters of Predators/Parasitoids
In addition to biological attributes, entomopathogens could considerably affect behavioral attributes of insect predators/parasitoids. Accordingly, this issue was subjected to different research studies. In this section, some of the most important findings are mentioned.
9.6.2.1 Pathogen Detection Strategy and Avoidance by Insect Predators/Parasitoids
Before oviposition, a female individual (predator/parasitoid) complete a sequence of steps to select the best site for construction of next generation. In the first step, it must find the habitat of its preys/hosts. Then, the female individual locates the preys/hosts in their habitats. Finally, preys/hosts are evaluated by the females to achieve the best decision for oviposition. Vinson (1976) reviewed the process of host assessment by parasitoids and argued that different factors such as size, movement, shape, sound and chemical cues (volatiles), from host feces or injured host plant tissues, were employed for host-selection. Among these factors, the volatiles emitted from host plants or preys/hosts play a key role for detecting infected patches (Afsheen et al. 2008; Nilsson et al. 2011).
However, to minimize any undesirable effect on subsequent generations and maximize immature survivorship, growth, development as well as adult fitness, it is very important that female individuals provide the best food resources. As previously mentioned, pathogenic infections seriously decrease preys/hosts quality with deleterious effects on biological performance of their natural enemies (Mesquita and Lacey 2001). Therefore, the ability of females to discriminate uninfected preys/hosts from infected ones is crucial and is considered as the first defense mechanism of predators/parasitoids against pathogenic infections (Ormond et al. 2011).
Several researchers stated that parasitoid wasps could recognize hosts infected by pathogenic fungi form healthy ones (Fransen and van Lenteren 1993; Mesquita and Lacey 2001). The ability of the tachinid parasitoid Compsilura concinnata (Meigen) to discriminate hosts infected with B. thuringiensis from healthy larvae was noticed by Erb et al. (2001). Rannback et al. (2015) concluded that when the parasitoid wasp T. rapae was exposed to B. bassiana and Metarhizium brunneum Petch, it could discriminate M. brunneum. The predatory ladybird, C. lunata does not prefer aphids infected by M. anisopliae, and this behavior provides sustainable management on crucifers and okra (Bayissa et al. 2016). Such behavior was observed in Anthocoris nemorum (L.) which avoids depositing its eggs on leaves treated with B. bassiana to decrease the risk for its progeny (Meyling and Pell 2006). However, in such situations, when predators/parasitoids discriminate infected resources and avoid them, some undesirable effects may also occur. Although avoidance of contaminated area decreases infection risks, Pourian et al. (2011) discussed that this behavior in predatory bugs increased time required for prey searching and dramatically decreases their predation rate and biological efficiency.
On the other hand, some natural enemies could not avoid contaminated preys/hosts. It is documented that the parasitoid wasp Cephalonomia tarsalis (Ashmed) equally parasitized hosts, Oryzaephilus surinamensis (L.) infected and uninfected by B. bassiana (Lord 2001). Hoch et al. (2000) concluded that the braconid wasp G. liparidis has the same parasitism rate on hosts healthy or infected by Vairimorpha sp. Similarly, T. nubilale has no ability to detect eggs infected by N. pyrausta from uninfected ones (Saleh et al. 1995). These findings are in agreement with those reported by Geden et al. (1992). Baverstock et al. (2005) showed that Aphidius ervi Haliday has no ability to recognize aphids infected by Pandora neoaphidis Remaudiere and Hennebert. Fransen and van Lenteren (1993) indicated that E. formosa could not distinguish whiteflies infected by entomopathogenic fungi. Mesquita and Lacey (2001) stated such shortcoming in the aphid parasitoid Aphelinus asychis Walker. As noticeable point, if natural enemies consume infected preys/hosts, efficiency of entomopathogens may be also moderately decreased (Roy et al. 2008).
9.6.2.2 Possible Effects of Entomopathogens on Foraging Behaviors of Predators/Parasitoids
Different factors (temperature, host plant, pesticide, host/prey attributes, pathogens etc.) could affect biological performance of natural enemies (Wang and Ferro 1998; Moezipour et al. 2008). Such effects are reflected in biological and behavioral changes of natural enemies. Therefore, it is very important to evaluate foraging behaviors of predators/parasitoids when these agents are exposed to infected resources both directly and indirectly. In addition to host preference, entomopathogens could seriously affect other foraging behaviors of predators/parasitoids. Pourian et al. (2011) investigated possible effects of onion thrips, Thrips tabaci Lindeman infected by M. anisopliae, on some behavioral traits of the anthocorid predatory bug, Orius albidipennis Reut, reporting that the searching time on infected preys significantly increased. Furthermore, O. albidipennis had a lower feeding time on treated individuals. Negative effects of M. anisopliae on the predation rate were also detected.
Alma et al. (2010) reported that when the pathogenic fungus Isaria fumosorosea Wize infected immature whitefly stages, the predatory bug D. hesperus significantly altered its predation behavior. Similarly, Pell and Vandenberg (2002) revealed that this fungus changed the predation behavior of the predatory ladybird, H. convergens. In another case, Sewify and El-Arnaouty (1998) stated that V. lecanii dramatically suppressed searching behavior and feeding capacity of the common green lacewing, C. carnea.
Belmain et al. (2002) and Sullivan and Berisford (2004) showed that specific cues from pathogenic fungi could act as repellents for phytophagous pests and their natural enemies. Meyling and Pell (2006) found that when A. nemorum encountered B. bassiana-infected aphids Acyrthosiphon pisum (Harris), it changed its predation behavior. These researchers stated that sporulating cadavers of infected hosts have repellent effects on A. nemorum.
Attack rates of the parasitoid wasp A. ervi was significantly reduced on aphids infected by the pathogenic fungus P. neoaphidis (Pope et al. 2002). Similar findings were reported by Baverstock et al. (2005). Another strategy is the rejection of a prey/host. Rejection behavior was observed in some natural enemies. It was observed that the parasitoid wasp E. formosa when locating microhabitats, searched its host and rejected those infected by pathogenic fungus Aschersonia aleyrodis (Webber), after probing (Fransen and van Lenteren 1993).
Effect of B. thuringiensis on functional response of Trichogramma brassicae Bezdenko was described by Vaez et al. (2013). Results obtained exhibited that exposure to infected eggs of H. armigera had no significant effects on functional response of this wasp. In both infected and uninfected eggs a type III response was recorded. Furthermore, infected eggs increased handling time and decreased searching efficiency of T. brassicae. Farrokhi et al. (2010) compared functional response of T. brassicae on Wolbachia-infected and uninfected hosts. These researchers reported that infection had no significant effects on this behavioral function. In contrast, Dong et al. (2017) studied the functional response of Trichogramma dendrolimi Matsumura on eggs of the Asian corn borer, Ostrinia furnacalis Guenée infected and uninfected by Wolbachia, at three constant temperatures (20, 25 and 30 °C). Their results revealed that Wolbachia sp. could affect functional response of T. dendrolimi and its effect was temperature-dependent.
In addition to the above-mentioned alterations, entomophatogenic agents could indirectly affect behavioral attributes of insect predators/parasitoids. Wu et al. (2016) observed that the predatory mite Neoseiulus barkeri (Hughes) displayed self-grooming behavior to remove fungal conidia from its body surface. However, although different arthropods exhibit grooming behavior to remove undesirable agents, such as pathogenic conidia and parasitic mites (Farish 1972), Wekesa et al. (2007) explained that this behavior may reduce searching ability and predation rate.
9.6.2.3 Intra-Guild Predation Between Entomopathogens and Predators/Parasitoids
As a crucial point, it is necessary for any agricultural producer to evaluate its cropping system, as concerns how the interacting components formed food/trophic levels (Fig. 9.2). In these systems, natural enemies (predators/parasitoids and pathogens) occupy the highest position (3th level) and can regulate the population of herbivorous organisms (second level) via top-down regulatory efforts, mainly known as biological control. The success rate of biological programs highly depends on intentional manipulation of possible interactions among tri-trophic levels. However, due to lower species diversity, agro-ecosystems provide suitable conditions for such manipulations (Finke and Denno 2004).
One of the most promising procedures to optimize efficiency of biological programs is introducing new beneficial organisms (Stevens and Stuart 2008). Undoubtedly, this process may result in several interferences and cause intra-guild predation (Denno et al. 2008; Ali et al. 2013) which dramatically affects adequate control of herbivores (Rosenheim et al. 1995). Straub et al. (2008) explained that intra-guild interactions could occurr during combined application of at least two natural enemies against the same pest species. Such interactions were frequently detected in biological communities and may be observed when biocontrol agents compete and exploit the same organisms in a similar manner.
Unidirectional intra-guild interactions, i.e. between entomopathogenic fungi and insect predators/parasitoids, are asymmetric, favoing pathogenic agents. In fact, because of their wide host range, these agents may infect different life stages of insect predators/parasitoids and significantly decrease their population levels and efficiency (Brodeur and Rosenheim 2000). Fransen and van Lenteren (1993) recognized that the entomopathogenic fungus A. aleyrodis drastically infected the parasitoid wasp E. formosae, after contact with parasitized whiteflies.
In addition to contact pathogenicity, ingestion of entomopathogens by predators/parasitoids could amplify such negative effects. Pell et al. (1997) reported feeding activity of coccinellid and carabid beetles on aphids heavily infected by P. neoaphidis. In another study, Askary and Brodeur (1999) observed that when larval parasitoids consumed infected aphid tissues, fungal spores were accidentally ingested. Sedaratian et al. (2014) stated that feeding activity of H. hebetor on larvae of H. armigera treated with B. thuringinesis caused ingestion of the entomopathogenic bacterium. In this scenario, the parasitoid biological performancewas seriously affected.
9.6.3 Other Effects
In addition to the above-mentioned effects, entomopathogens could also directly affect predators/parasitoids. Idris et al. (2001) revealed that when the parasitoid wasp Diadegma semiclausum Hellen consume infected larvae of the diamondback moth, Plutella xylostella infected by microsporidian Vairimorpha sp., emerged females have deformed wings. Such individuals faced several difficulties for their flying and searching activities, and were unable to compete with other individuals. Furthermore, results showed that infected parasitoids had smaller size in comparison to healthy ones, affecting the parasitoid fitness. In another study, Hoch et al. (2000) documented that individuals of the parasitoid wasp G. liparidis, emerged from host L. dispar infected by the microsporidian V. disparis, had a smaller size. Additionally, the individuals developed on infected hosts had a lower weigh. A further effect of entomopathogens concerns the egg viability of predators/parasitoids. A study by Pozzebon and Duso (2009) revealed that B. bassiana significantly reduced the egg hatching rate in P. persimilis.
9.6.3.1 Entomopathogen Effects on Immune System of Phytophagous Pests and Its Impact on Predators/Parasitoids
The insect immune system can suppress undesirable alien factors (fungi, bacteria, viruses, nematodes, protists, endoparasitoids etc.) via two different mechanisms namely humoral and cellular responses. In the humoral mechanism several antimicrobial peptides such as lectins, lysozyme, and attacin are produced and underpin insect fight vs introduced agents. Cellular function involves different mechanisms including phagocytosis of introduced materials by hemocytes, nodulation (trapping introduced agents by a net of hemocytes) and encapsulation (surrounding too large materials by thin layers of flatted hemocytes) (Jiravanichpaisal et al. 2006). In the case of nodulation and encapsulation, another reaction usually occurs, which is recognized as melanization. This process involves production of the pigment melanin to construct a hard and impenetrable envelope around alien factors (Cerenius et al. 2008). The role of some enzymes in the melanization process is documented by several researchers. For instance, Popham et al. (2004) stated that higher levels of phenoloxidase in H. virescence resulted in a higher degree of melanotic encapsulation of baculovirus-infected cells. It is documented that pathogenic infection engages immune defense of phytophagous insects and alters their vulnerability to predators/parasitoids. In such situations, they usually try to compensate this shortcoming. Cessation of feeding on contaminated resources has been described as one of the most common responses to increased immune responses in such circumstances (Adamo et al. 2007, 2010).
Insects’ immune reactions to entomopathogens affect predators/parasitoids in different manners. Appropriate immune responses could help contaminated individuals to recover from pathogenic infections. The lack of suitable responses or weak reactions will lead to the insects’ death or to chronic infections, respectively. Alive individuals with chronic symptoms often have lower quality and could not supply nutritional requirements for growth and development of predators/parasitoids. This issue could indirectly affect biological performance of these beneficial organisms. As previously mentioned, predators/parasitoids, with developed detection and avoidance behaviors, could minimize such adverse complications. Otherwise, their biological performance may severely decrease. Sedaratian et al. (2014) revealed that when the ectoparasitoid wasp H. hebetor consumed Bt-contaminated food resources, its biological performance was significantly reduced. If contaminated individuals were selected for oviposition by female endoparasitoids, a higher mortality of immature parasitoids was observed (Sanders et al. 2007). In another word, if immune functions of contaminated hosts could not destroy entomopathogens, ingestion of their tissues may negatively affect biological performance of both predators and parasitoids.
Activation of immune responses in sick individuals involves energy consumption that may decrease their defensive power against predators/parasitoids. In such situations, predators/parasitoids will gain higher number of preys/hosts with a lower energy consumption. In the case of endoparasitoids, encapsulation is the most common response of the insect immune system (Blumberg 1997). This mechanism may reduce parasitoid efficiency in biological programs, prevent successful establishment of exotic parasitoids in new regions or disrupt mass rearing efforts. However, if the host immune system is engaged in the suppression of an invasive pathogen, its performance for parasitoid encapsulation will inevitably decrease. This condition may hence increase the biological performance of biological programs. It is noticeable that some parasitoid wasps have a symbiotic mutualism relationship with different microorganisms which protect their immature stages from encapsulation. This mechanism is described in next sections.
9.6.3.2 Effects of Entomopathogens on Physiological Systems of Predators/Parasitoids
Consumption of infected preys/hosts by insect predators/parasitoids has several effects on their physiological functions, especially in the case of endoparasitoids. However, exposure to entomopathogeneic agents could also affect physiological functions of predatory insects. When predators/parasitoids feed on infected haemolymph and tissues of preys/hosts, a variety of unexpected outcomes may be expected (Futerman et al. 2005). Pathogenic effects on reproductive, digestive and immune systems of predators/parasitoids are the most important physiological involvements occurring in these natural enemies after infection.
Infection of reproductive system may result in vertical transmission of entomopathogens to subsequent generation (Mazzone 1985). It was observed that the parasitoid wasp M. grandii, when developing into O. nubilalis hosts infected by N. pyrausta , transmitted the entomopathogenic microsporidian to its offspring (Siegel et al. 1986). Brooks (1973) stated that some parasitoids were susceptible to the microsporidian pathogens attacking their hosts. In another study, Roy et al. (2006) showed that ingestion of pathogenic fungi significantly decreased fecundity of natural enemies. Consumption of infected preys with low nutrition quality caused detectable reduction in reproductive performance (Pozzebon and Duso 2009). In fact, such food resources could not provide the nutrients required for egg production and this issue disrupts the physiological functions of the reproductive system. Pozzebon and Duso (2009) showed that activity of the predatory mite, P. persimilis, on Tetranychus urticae Koch treated with B. bassiana , dramatically reduced its ability for egg production. Furthermore, the number of fertile eggs was also affected. One of the possible reasons for reduction of egg production is resources diverting from the reproductive to the immune system. In fact, to minimize mortality, also the natural enemies consume their energy resources for defense mechanisms. Seiedy et al. (2012) reported that ingestion of preys infected by B. bassiana seriously affected the fecundity of P. persimilis. These researchers assumed that the activation of the immune system and the production of secondary metabolites for suppressing aggressive agents significantly disrupted the reproductive system of the predatory mite.
In addition to reproductive and immune systems, the digestive canal, which has a vital functions in supplying required energy for growth and development of predators/parasitoids, could also affected. Moawed et al. (1997) showed that negative effects of microsporidan on endoparasitoids include the disruption of the nutritional balance in the digestive canal of parasitoid larvae, due to direct infection or aggression of undigested spores. Furthermore, this accumulation significantly decreased available space for food storage (Saleh et al. 1995). Schuld et al. (1999) showed that during feeding activity of T. chilonis larvae on larvae of P. xylostella treated with Vairimorpha sp., the microsporidian was detectable in the parasitoid intestinal lumen 3 days after parasitization, and then was dispersed to other tissues including flight muscles and the nervous system.
9.6.3.3 Catastrophic Synchronization Caused by Entomopathogens and Impact on Predators/Parasitoids
For the first time, the hypothesis of “catastrophic synchronization” was proposed by Godfray and Chan (1990) as an unusual output of extensive application of chemical pesticides. In fact, these researchers illustrated a specific scenario in which the population of a target organisms is synchronized at a particular stage after pesticide application. As a result, synchronized populations interrupt the biological performance of insect predators/parasitoids that are active on other life stages of the target pests, and require food resources for their growth and development. Catastrophic synchronization shifts the multiple structure of a pest population towards a single stage one. Thus the natural enemies (especially predators/parasitoids) encounter undesirable conditions. In such situations, pest resurgence may occur as a result of unavailability of preferred stages for biological activities of predators/parasitoids. Furthermore, predators/parasitoids may reduce their reproduction potential, migrate from such environment or tolerate starvation. Pest resurgence from catastrophic synchronization was reported for coconut (Perera et al. 1988) and coffee (Waage 1989) pests.
However, although no documented information is available regarding synchronization induced in pest populations structure by entomopathogens, more attentions should be devoted to investigate this hypothesis, especially in the case of extensive application of entomopathogens in agro-ecosystems. This is especially important for large scale application of commercial formulations of entomopathogens or genetically modified host plants. Data by Sedaratian et al. (2013) revealed that commercial formulation of B. thuringiensis had more toxicity to first instars of H. armigera , whereas last instars had a relative resistance to the bacterium. Accordingly, long time application of such formulations could induce a synchronized structure in target host populations and negatively affect natural enemies such as the green lacewing, C. carnea, which feeds on first instars of H. armigera . In another case, entomopathogenic nematodes could be candidate. As previously mentioned, this group of entomopathogens infects the insect life stages in soil. With increasing population density of pathogenic nematodes, the number of infected pests in soil will increase and this issue could synchronize other stages of the pests.
9.7 Application Management of Entomopathogens Increase Their Compatibility with Predators/Parasitoids
Simultaneous application of different natural enemies is inevitable in IBC programs. As previously mentioned, synchronized application of entomopathogenic agents and insect predators/parasitoids may also have some negative outcomes on biological performance of these natural enemies. Therefore, it is very important to fully investigate different aspects of such integrations and reduce potential negative effects. One of the most reliable strategies to increase biological safety of entomopathogenic agents is their application management in agro-ecosystems, where other beneficial agents such as insect predators/parasitoids coexist. Such efforts attempt to minimize direct contact of these microbial agents with predators/parasitoids.
9.7.1 Importance of Monitoring Population Fluctuations of Phytophagous Pests
In modern agriculture, all control efforts must be applied at their appropriate time. In fact, ancient attitudes for calendar-based application of control strategies was changed in favor of need-base application. For this, we designed a monitoring schedule to attentively check all biological activities and population fluctuations of phytophagous hosts/preys and their natural enemies. Such monitoring activities enable agricultural producers to make accurate decisions, selecting the best strategy in an appropriate time. However, economic criteria play a basic role for implementation of control strategies in IPM (Fig. 9.2). Accordingly, each strategy is only applied when the highest performance is achieved.
Data collected during monitoring activities enable pest managers to consider a reasonable time period between intentional application of entomopathogens and release programs of insect predators/parasitoids. This period of time may considerably decrease the overall adverse effects of entomopathogens on biological performance of predators/parasitoids (Fransen and van Lenteren 1993). Furthermore, sampling target organisms during monitoring activities may reveal the level of naturally occurring infections with pathogenic agents. Consequently, when naturally infections in population of target organisms are considerable, the release of insect predators/parasitoids is not a good idea. On the other hand, if monitoring efforts revealed noticeable activities of predators/parasitoids, it is better to avoid intentional application of entomopathogens which have the same ecological niche. Such findings will help in accurate decision-making, in order to minimize direct contaminations of predators/parasitoids with pathogenic agents (Jacobson et al. 2001).
9.7.2 Genetically Modified Plants and Their Effects on Predators/Parasitoids
Genetically modified plants which express B. thuringiensis toxins in their tissues (Bt-crops) offer a reliable tool for suppressing pest populations in intensive agro-ecosystems, and their applications reduce pesticide usage (Lovei and Arpaia 2005). Tobacco and tomato were the first transgenic plants which express insecticidal Bt delta-endotoxins (van Frankenhuyzen 1993). Currently, these manipulated crops (tomato, cotton, potato, maize, rice and etc.) are commercially cultivated in different countries such as United States, Canada, Japan, Mexico, Argentina and Australia (Frutos et al. 1999).
O’Callaghan et al. (2005) described that one of the main benefits of Bt-crops is their insecticidal specificity. In contrast with chemical pesticides, these crops only affect target organisms. However, although Bt-crops significantly decrease pesticide usage in agro-ecosystems, their possible effects on non-target organisms such as insect predators/parasitoids is a main, global concern. Different researchers showed that insect predators/parasitoids may receive Bt-toxins from infected preys/hosts (Obrist et al. 2006). However, researches on possible effects of Bt-crops on insect predators/parasitoids reported different outputs. Torres and Ruberson (2006) showed that Bt-cotton expressing the Cry1Ac toxin had no detectable effects on the predatory bug Podisus maculiventris (Say). In another study, the same findings were reported when O. insidiosus consumed Bt-treated preys (Al-Deeb et al. 2001). In contrast, it was observed that Bt-cotton containing the Cry1Ac toxin significantly affected survivorship of two predatory bugs Geocoris punctipes (Say) and Orius tristicolor (White) (Ponsard et al. 2002). Hilbeck et al. (1998a) found that predatory lacewing, C. carnea, had higher mortality and lower development rates when preys reared on Bt-crops were consumed. However, Lovvei et al. (2009) stated that insect parasitoids have more sensitivity to Cry toxins than predators. Candolfi et al. (2004) compared a population of the parasitoid wasp Macrocentrus cingulum Brischke in two Bt and conventional corn fields, and showed that the parasitoid had lower biological activity in the Bt-corn field. In another study, Xia et al. (1999) stated that specialist parasitoids that parasitized H. armigera had a lower population density in Bt-cotton fields. However, although some findings revealed that insect predators/parasitoids had lower biological activity on transgenic plants, Romeis et al. (2004) indicated that such results reflect adverse effects of feeding activity of predators/parasitoids on food resources with lower nutritional quality and were not directly related to Bt transgenic crop. On the other hand, it should be noticed that alternative food resources are more available in field conditions for insect predators/parasitoids, and this issue minimizes the adverse effects of Bt-toxins.
9.7.3 Microbial Biopesticides
Increased global demands for widespread application of entomopathogens has resulted in manufacturing commercial formulations of these microorganisms, commonly indicated as biopesticides. It is noticeable that the majority of these pathogen-based bioinsecticides was assigned to entomopathogenic bacterium B. thuringiensis . Koul and Dhaliwal (2002) described that commercial formulations of B. thuringiensis have lower undesirable effects on insect predators/parasitoids than chemical insecticides. Although these products may contain microorganisms, their metabolites or combination of both elements, only products with living organisms may be considered in biological control efforts. Considering several benefits, one of the main advantages of biopesticides is their ecological selectivity for non-target organisms. In fact, regarding monitoring outcomes, pest managers should use these products when populations of other natural enemies, especially insect predators/parasitoids, have low density, in order to minimize any adverse effects on their performance.
Among different microbial bioinsecticides, commercial formulations of entomopathogenic viruses have the lowest negative effects on insect predators/parasitoids. In the case of pathogenic viruses, commercial formulations only contain members from family Baculoviridae. Since this family has a narrow host range and its pathogenic activity is recorded on specific insects, extensive application as commercial biopesticides has the lowest negative effects on non-target organisms (Cory and Myers 2003).
de Faria and Wraight (2007) stated that over 120 fungal formulations were globally applied in management programs of different insect pests. However, most of these mycopesticide products are based on spores of B. bassiana , M. anisopliae, I. fumosorosea, L. longisporum, L. muscarium and Hirsutella thompsonii Fisher (Jaronski 2010). The wide host range of entomopathogenic fungi suggests caution when applying them in agro-ecosystems.
9.8 Changes in Environmental Conditions Alter Entomopathogen Effects on Predators/Parasitoids
Similar to all living organisms, biological activities of entomopathogens are completely dependent on environmental conditions (abiotic factors). Accordingly, unfavorable conditions significantly reduce pathogenicity of these agents. Consequently, in IBC programs, if intentional application of insect predators/parasitoids is performed when environmental conditions are unfavorable or sub-optimal, possible intra-guild interactions could be minimized.
As we know, entomopathogenic agents are a diverse group of natural enemies which have different environmental requirements. For example, high doses of ultra violet rays (UV) in field conditions negatively affects pathogenicity of B. thuringiensis (Sedaratian et al. 2013). Furthermore, other environmental factors, including temperature and rainfall, could affect residual life of this pathogenic agent (Frye et al. 1973; Salama et al. 1983; Pedersen et al. 1997). Soil moisture has critical impact on biological activities of entomopathogenic nematodes and dried conditions may cause significant deleterious effects on their performance. Besides, application of chemical pesticides and fertilizers in soil environments could have negative effects on biological performance of these biocontrol agents. Such conditions minimize their possible effects on target pest populations, as well as affecting other beneficial agents i.e. the predatory beetles from family Carabidae.
Among different environmental factors, relative humidity has considerable effects on biological performance of entomopathogenic fungi. Under low humidity conditions, germination of infective spores of pathogenic fungi seriously decreased, drastically suppressing fungal epizootics. In addition to relative humidity, other abiotic factors such as temperature, rain, and sunlight could also affect these fungi (Jaronski 2010). However, such limitations may hinder desirable delivery of lethal effects of entomopathogenic agents, with significant restrictions in their pathogenicity, both on target and non-target organisms. These informations could help pest managers to manipulate negative interactions between entomopathogenic agents and insect predators/parasitoids, in order to enhance biological efficiency of IBC programs.
9.9 Symbiotic Interactions Between Entomopathogens and Insect Predators/Parasitoids
As previously stated, biological performance of predators/parasitoids may be adversely affected by the defense mechanisms of the target pests. This issue is evident in the case of endoparasitoids which deposit their eggs inside the host body, where they spend their immature development. In this time, host defense strategies activate and try to eliminate invasive factors (immature stages of parasitoids such as egg, larvae and etc.). Regarding the relatively large size of invasive particles, encapsulation is the most important mechanism employed by the host to suppress alien factors (see Sect. 9.6.3.1). On the other hand, endoparasitoids also utilize defense strategies to overcome such immune responses, and could successfully facilitate their immature development into the host haemocoel.
To achieve this goal, one known mechanisms is the mutualistic relationship detected between Ichneumonoidea wasps (Ichneumonidae and Braconidae) and polydnaviruses. However, although effects of entomopathoges on predators/parasitoids are usually negative, this mutualistic relation revealed a positive effect on the biological performance of endoparasitoids. Tan et al. (2018) defined it as obligatory mutualism. Webb et al. (2006) stated that about 30,000 species of endoparasitoid wasps from both Ichneumonidae and Braconidae families have specific mutualistic viruses. Herniou et al. (2013) revealed an approximately 100 million years evolutive background for this relation.
A symbiotic virus integrates its genome into the wasp genome with replication of the viral particles in the reproductive system of female parasitoids. However the infection process and expression of viral genes only occur in the host tissues (especially salivary glands) (Herniou et al. 2013). During oviposition, female parasitoids inject the symbiotic virus in the host body. The particles injected engage the host immune system and manipulate it to allow a successful development of the deposited eggs (Beckage 1998). Rodriguez-Perez and Beckage (2008) described that polydnaviruses injected into the haemocoel of the sugarcane borer, D. saccharalis, significantly reduced immune responses of caterpillars towards the eggs deposited by the parasitoid wasp C. flavipes. In previous studies, Rodriguez-Perez and Beckage (2006) explained that polydnaviruses reduce the adhesive attributes of the host haemocytes. Thereafter, the encapsulation process is disrupted and the eggs deposited by the parasitoids successfully complete their development.
9.10 Future Research Directions
Entomopathogens need more attention to investigate different aspects including widespread application in a large scale, pest resistance or possible interactions with non-target organisms. Even though considerable efforts were conducted to evaluate different attributes of entomopathogens in recent years, our knowledge in some areas is still restricted. One of the main gaps is our knowledge about the epizootiology of these organisms. More research projects should be designed to evaluate factors affecting epizootiology of these entomopathogenic agents in natural conditions. However, because different factors are involved, multidisciplinary efforts by different specialists should be contributed, from fields such as insect pathology, entomology, ecology, agronomy etc.Comprehensive research projects may also enhance our knowledge about possible effects of climatic changes on entomopathogens. Another directions to minimize adverse effects of entomopathogens on non-target organisms, such as pollinators, predators and parasitoids, involve the development of novel delivery tactics. To achieve this goal, Vega et al. (2012) suggested application of endophytic entomopathogenic fungi.
Our knowledge about the ecology of microsporidia, as well as their possible impacts on predators/parasitoids, is still restricted, This is a main area for future studies on this group. In addition, more taxonomic studies are also needed. Similarly, there is an obvious gap in our systematic information about entomopathogenic nematodes. In this group, our current knowledge is focused on two families, Heterorhabditidae and Steinernematidae. Therefore, future studies should deserve more considerations to other families.
To challenge chemical pesticides, efforts on commercial formulations are required. However, in contrast with chemicals, entomopathogens are living organisms and this vital point causes some difficulties for their packing, storage and application. On the other hand, commercial formulations should be ecologically selective to minimize possible adverse effects on non-target organisms. This issue is so crucial for non-specific organisms such as pathogenic microsporidia. In the case of entomopathogenic nematodes, since these agents have close symbiotic relation with Photorhabdus and Xenorhabdus , understanding their nutritional contributions will facilitate mass production efforts under in-vitro conditions.
In some circumstances molecular studies are needed. For example, resistance mechanisms of target organisms to different groups of entomopathogens or their metabolites are important fields that should be comprehensively pursued. Another area is the vertical and horizontal transmission of different organisms in populations of both target and non-target species. Shapiro-Ilan et al. (2012) stated that the gene flow between population of entomopathogens and target organisms represents an open field in molecular studies. In the case of entomopathogenic viruses, insect cell cultures will provide appropriate tools to evaluate different aspects of virus biology and infection, replication and transmission mechanisms. Therefore, this is a clear direction to develop our knowledge on entomopathogenic viruses. In addition, Harrison and Hoovery (2012) highlighted our gap in understanding host responses to viral infections. These researchers suggest more studies on mass production of entomopathogenic viruses in insect cells to reduce the cost of commercial formulations. In the case of entomopathogenic bacteria, molecular screening could optimize the discovery of novel isolates as well as virulent factors. Furthermore, genetic studies could be applied to generate new toxins with higher pathogenic activity and specificity, also helpful for designing new transgenic crops.
9.11 Conclusion
Deleterious effects of chemical pesticides have changed our attitude in pest management programs, with more emphasis given to eco-friendly strategies. In recent years, entomopathogenic agents have been considered as one of the most reliable and safe alternatives. Furthermore, diversity of these biological agents allows agricultural producers to select appropriate options for controlling target organisms, in different circumstances. Considerably, our current knowledge about possible effects of these biological agents on non-target organisms, such as insect predators and parasitoids, is still limited. Therefore, before widespread application, compatibility of these microbial agents with other natural enemies (especially insect predators/parasitoids), during simultaneous applications, should be investigated. Such assessments must involve different entomopathogenic effects on predators/parasitoids, including biological, ecological, physiological, immunological and behavioral studies. Such evaluations may play a significant role in successful implementation of IBC. Although the term “success” has wide definitions, in IBC our criteria involve the intentional application of entomopathogens as a reliable tool, with the highest and lowest negative effects on target and non-target organisms, respectively. Some findings showed that entomopathogens could have adverse effects on other beneficial organisms. Therefore, comprehensive assessments are urgently needed to minimize such undesirable effects on non-target organisms, reducing the risk associated with widespread applications of these biocontrol agents.
References
Adamo, S. A., Fidler, T. L., & Forestel, C. A. (2007). Illness-induced anorexia and its possible function in the caterpillar, Manduca sexta. Brain, Behavior, and Immunity, 21, 292–300.
Adamo, S. A., Bartlett, A., Le, J., Spencer, N., & Sullivan, K. (2010). Illness-induced anorexia may reduce trade-offs between digestion and immune function. Animal Behaviour, 79, 3–10.
Adl, S. M., Simpson, A. G. B., Farmer, M. A., Andersen, R. A., Anderso, O. R., et al. (2005). The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. Journal of Eukaryotic Microbiology, 52, 399–451.
Afsheen, S., Wang, X., Li, R., Zhu, C. S., & Lou, Y. G. (2008). Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci., 15, 381–397.
Aizawa, K. (2001). Shigetane Ishiwata: His discovery of sotto-kin (Bacillus thuringiensis) in 1901 and subsequent investigations in Japan. In Proceedings of a centennial symposium commemorating ishiwata’s discovery of Bacillus thuringiensis. Japan: Kurume, November 1–3, 2001.
Al-Deeb, M., Wilde, G., & Higgins, R. (2001). No effect of Bacillus thuringiensis corn and Bacillus thuringiensis on the predator Orius insidiosus (Hemiptera: Anthocoridae). Environmental Entomology, 30, 625–629.
Ali, J. G., Campos-Herrera, R., Alborn, H. T., Duncan, L. W., & Stelinski, L. L. (2013). Sending mixed messages: A trophic cascade produced by a belowground herbivore-induced cue. Journal of Chemical Ecology, 39, 1140–1147.
Alma, C. R., Gillespie, D. R., Roitberg, B. D., & Goettel, M. S. (2010). Threat of infection and threat-avoidance behavior in the predator Dicyphus hesperus feeding on whitefly nymphs infected with an Entomopathogen. Journal of Insect Behavior, 23, 90–99.
Andreadis, T. G. (1980). Nosema pyrausta infection in Macrocentrus grandii, a braconid parasite of the European corn borer, Ostrinia nubilalis. Journal of Invertebrate Pathology, 35, 229–233.
Aqueel, M. A., & Leather, S. R. (2013). Virulence of Verticillium lecanii (Z.) against cereal aphids; does timing of infection affect the performance of parasitoids and predators? Pest Management Science, 69, 493–498.
Askary, H., & Brodeur, J. (1999). Susceptibility of larval stages of the aphid parasitoid Aphidius nigripes to the entomopathogenic fungus Verticillium lecanii. Journal of Invertebrate Pathology, 73, 129–132.
Atwa, A. A., Hegazi, E. M., Khafagi, W. E., & Abd El-Aziz, G. M. (2013). Interaction of the koinobiont parasitoid Microplitis rufiventris of the cotton leafworm, Spodoptera littoralis, with two entomopathogenic rhabditids, Heterorhabditis bacteriophora and Steinernema carpocapsae. Journal of Insect Science, 13, 84.
Barbagallo, S., Longo, S., & Patti, I. (1982). Preliminary results of integrated biological control in eastern Sicily to control the citrus mealybug and the citrus whitefly (translated from Italian). Fruits, 36, 115–121.
Baur, M. E., & Boethel, D. J. (2003). Effect of Bt-cotton expressing Cry1A(c) on the survival and fecundity of two hymenopteran parasitoids (Braconidae, Encyrtidae) in the laboratory. Biological Control, 26, 325–332.
Baverstock, J., Alderson, P. G., & Pell, J. K. (2005). Influence of the aphid pathogen Pandora neoaphidis on the foraging behavior of the aphid parasitoid Aphidius ervi. Ecological Entomology, 30, 665–672.
Bayissa, W., Ekesia, S., Mohameda, S. A., Kaayab, G. P., Wagachab, J. M., et al. (2016). Interactions among vegetable infesting aphids, the fungal pathogen Metarhizium anisopliae (Ascomycota: Hypocreales) and the predatory coccinellid Cheilomenes lunata (Coleoptera: Coccinellidae). Biocontrol Science and Technology, 26(2), 274–290.
Beckage, N. E. (1998). Modulation of immune responses to parasitoids by polydnaviruses. Parasitology, 116, 57–64.
Belmain, S. R., Simmonds, M. S. J., & Blaney, W. M. (2002). Influence of odor from wood-decaying fungi on host selection behavior of deathwatch beetle, Xestobium rufovillosum. Journal of Chemical Ecology, 28, 741–754.
Bergold, G. H. (1947). Die Isolierung des Polyeder-Virus und die Natur der Polyeder. Zeitschrift für Naturforschung B, 2, 122–143.
Bernal, J. S., Griset, J. G., & Gillogly, P. O. (2002). Impacts of developing on Bt maize-intoxicated hosts on fitness parameter of a stem borer parasitoid. Journal of Entomological Science, 37, 27–40.
Blumberg, D. (1997). Parasitoid encapsulation as a defense mechanism in the Coccoidea (Homoptera) and its importance in biological control. Biological Control, 8, 225–236.
Blumberg, D., Navon, A., Keren, S., Goldenberg, S., & Ferkovich, S. M. (1997). Interactions among Helicoverpa armigera (Lepidoptera: Noctuidae), its larval endoparasitoid Microplitis croceipes (Hymenoptera: Braconidae), and Bacillus thuringiensis. Journal of Economic Entomology, 90, 1181–1186.
Bordat, D., Goudegnon, A. E., & Bouix, G. (1994). Relationships between Apanteles flavipes (Hym.: Braconidae) and Nosema bordati (Microspora, Nosematidae) parasites of Chilo partellus (Lep.: Pyralidae). Entomophaga, 39, 21–32.
Brodeur, J., & Rosenheim, J. A. (2000). Intraguild interactions in aphid parasitoids. Entomologia Experimentalis et Applicata, 97, 93–108.
Brooks, W. M. (1973). Protozoa: Host-parasite-pathogen interrelationships. Miscellaneous Publications of the Entomological Society of America, 9, 105–111.
Brown, J. R., Phillips, J. R., & Yearian, W. C. (1989). Transmission of Heliothis NPV by Microplitis croceipes (Cresson) in Heliothis virescens (F.). Southwestern Entomology, 14, 139–146.
Camerona, S. A., Lozier, J. D., Strange, J. P., Koch, J. B., Cordes, N., Solterd L. F., & Griswold, T. L. (2011). Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences, 108(2), 662–667.
Candolfi, M., Brown, K., Reber, B., & Schmidli, H. (2004). A faunistic approach to assess potential side-effects of genetically modified Bt-corn on non-target arthropods under field conditions. Biocontrol Science and Technology, 14, 129–170.
Carvalho, V. F. P., Vacari, A. M., Pomari, A. F., De Bortoli, C. P., Ramalho, D. G., & De Bortoli, S. A. (2012). Interaction between the predator Podisus nigrispinus (Hemiptera: Pentatomidae) and entomopathogenic bacteria Bacillus thuringiensis. Environmental Entomology, 41(6), 1454–1461.
Cavalier-Smith, T. (2010). Kingdoms Protozoa and Chromista and the eozoan root of the eukaryotic tree. Biology Letters, 6, 342–345.
Cerenius, L., Lee, B. L., & Soderhall, K. (2008). The proPO-system: Pros and cons for its role in invertebrate immunity. Trends in Immunology, 29, 263–271.
Chandler, J. A., Lang, J. M., Bhatnagar, S., Eisen, J. A., & Kopp, A. (2011). Bacterial communities of diverse Drosophila species: Ecological context of a host-microbe model system. PLoS Genetics, 7, 474.
Chen, Y. P., Becnely, J. J., & Vallesy, S. M. (2012). RNA viruses infecting pest insects. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 133–170). London: Academic.
Corradi, N., & Keeling, P. J. (2009). Microsporidia: A journey through radical taxonomical revisions. Fungal Biology Reviews, 23, 1–8.
Corradi, N., & Slamovits, C. H. (2010). The intriguing nature of microsporidian genomes. Briefing in Functional Genomics, 10, 115–124.
Cory, J. S., & Myers, J. H. (2003). The ecology and evolution of insect baculoviruses. Annual Review of Ecology, Evolution, and Systematics, 34, 239–272.
Crawley, M. J. (1989). Chance and timing in biological invasions. In J. A. Drake (Ed.), Biological invasions: A global perspective (pp. 407–423). Chichester: Wiley.
de Faria, M. R., & Wraight, S. P. (2007). Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43, 237–256.
DeBach, P. (1964). Biological control of insect pests and weeds. London: Chapman and Hall.
DeBach, P., & Rosen, D. (1992). Biological control of by natural enemies (2nd ed.). Cambridge: Cambridge University Press.
Denno, R. F., Gruner, D. S., & Kaplan, I. (2008). Potential for entomolopathogenic nematodes in biological control: A meta-analytical synthesis and insights from trophic cascade theory. Journal of Nematology, 40, 61–72.
Dhaliwal G.S., Arora R. (2001). Integrated pest management: Concepts and approaches. Kalyani Publishers, New Delhi.Kennedy G.G.
Ditillo J.L., , Walgenbach J.F. (2016). Effects of insecticides and fungicides commonly used in tomato production on Phytoseiulus persimilis (Acari: Phtyoseiidae). Journal of Economic Entomology 109 (6), 2298–2308.
Dong, H., Liu, Q., Xie, L., Cong, B., & Wang, H. (2017). Functional response of Wolbachia-infected and uninfected Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) to Asian corn borer, Ostrinia furnacalis Guenée (Lepidoptera: Pyralidae) eggs. Journal of Asia-Pacific Entomology, 20, 787–793.
Ekesi, S., Maniania, N. K., Ampong-Nyarko, K., & Onu, I. (1999). Effect of intercropping cowpea with maize on the performance of Metarhizium anisopliae against Megalurothrips sjostedti (Thysanoptera: Thipidae) and predators. Environmental Entomology, 28, 1154–1161.
El-Maghraby, M. M. A., Hegab, A., & Yousif-Khalil, S. I. (1988). Interactions between Bacillus thuringiensis Berl., Beauveria bassiana (Basl.) Vuill. and host/parasitoid system Spodoptera littoralis (Boisd.)/Microplitis rufiventris Kok. Journal of Applied Entomology, 106, 417–421.
Erb, S. L., Bourchier, R. S., van Frankenhuyzen, K., & Smith, S. M. (2001). Sublethal effects of Bacillus thuringiensis Berliner subsp. kurstaki on Lymantria dispar (Lepidoptera: Lymantriidae) and the tachinid parasitoid Compsilura concinnata (Diptera: Tachinidae). Environmental Entomology, 30, 1174–1181.
Farish, D. J. (1972). The evolutionary implications of qualitative variation in the grooming behaviour of the Hymenoptera (Insecta). Animal Behaviour, 20, 662–676.
Farrokhi, S., Ashouri, A., Shirazi, J., Allahyari, H., & Huigens, M. E. (2010). A comparative study on the functional response of Wolbachia-infected and uninfected forms of the parasitoid wasp Trichogramma brassicae. Journal of Insect Science, 10, 1–11.
Fathipour, Y., & Maleknia, B. (2016). Mite predators. In Omkar (Ed.), Ecofriendly pest management for food security (pp. 329–366). San Diego: Elsevier.
Fathipour, Y., & Sedaratian, A. (2013). Integrated management of Helicoverpa armigera in soybean cropping systems. In H. El-Shemy (Ed.), Soybean-pest resistance (pp. 231–280). Rijeka: InTech.
Fatiha, L., Huang, Z., Ren, S. X., & Ali, S. (2008). Effect of Verticillium lecanii on biological characteristics and life table of Serangium japonicum (Coleoptera: Coccinellidae), a predator of whiteflies under laboratory conditions. Insect Sci., 15, 327–333.
Fernandez, M. M., Medina, P., Wanumen, A., Del Estal, P., Smagghe, G., & Vinuela, E. (2017). Compatibility of sulfoxaflor and other modern pesticides with adults of the predatory mite Amblyseius swirskii. Residual contact and persistence studies. BioControl, 62, 197–208.
Finke, D. L., & Denno, R. F. (2004). Predator diversity dampens trophic cascades. Nature, 429(6990), 407–410.
Flexner, J. L., Lighthart, B., & Croft, B. A. (1986). The effects of microbial insecticides on non-target beneficial arthropods. Agriculture, Ecosystems & Environment, 16, 203–254.
Fransen, J. J., & van Lenteren, J. C. (1993). Host selection and survival of the parasitoid Encarsia formosa on greenhouse whitefly, Trialeurodes vaporariorum in the presence of hosts infected with the fungus Aschersonia aleyrodis. Entomologia Experimentalis et Applicata, 69, 239–249.
Fredrickson, J. K., Zachara, J. M., Balkwill, D. L., Kennedy, D., Li, S. M., Kostandarithes, H. M., Daly, M. J., Romine, M. F., & Brockman, F. J. (2004). Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford site, Washington state. Applied and Environmental Microbiology, 70(7), 4230–4241.
Frutos, R., Rang, C., & Royer, M. (1999). Managing insect resistance to plants producing Bacillus thuringiensis toxins. Critical Reviews in Biotechnology, 19, 227–276.
Frye, R. D., Scholl, C. G., Scholz, E. W., & Funke, B. R. (1973). Effect of weather on a microbial insecticide. Journal of Invertebrate Pathology, 22, 50–54.
Futerman, P. H., Layen, S. J., Kotzen, M. L., Franzen, C., & Kraaijeveld, A. R. (2005). Fitness effects and transmission routes of a microsporidian parasite infecting Drosophila and its parasitoids. Parasitology, 132, 479–492.
Futerman, P., Layen, S. J., Kotzen, M. L., Franzen, C., Kraaijeveld, A. R., & Godfray, H. C. (2006). Fitness effects and transmission routes of a microsporidian parasite infecting Drosophila and its parasitoids. Parasitology, 132, 479–492.
Geden, C. J., Smith, L., Long, S. J., & Rutz, D. A. (1992). Rapid deterioration of searching behavior, host destruction, and fecundity of the parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae) in culture. Annals of the Entomological Society of America, 85, 179–187.
Geden, C. J., Long, S. J., Rutz, D. A., & Becnel, J. J. (1995). Nosema disease of the parasitoid Muscidifurax raptor (Hymenoptera: Pteromalidae): Prevalence, patterns of transmission, management, and impact. Biological Control, 5, 607–614.
Geden, C. J., Ferreira, M., de Almeida, A., & do Prado A.P. (2002). Effects of Nosema disease on fitness of the parasitoid Tachinaephagus zealandicus (Hymenoptera: Encyrtidae). Environmental Entomology, 32(5), 1139–1145.
Glaser, R. W. (1931). The cultivation of a nematode parasite of an insect. Science, 73, 614–615.
Godfray, H. C. J. (1994). Parasitoids. Princeton: Princeton University Press.
Godfray, H. C. J., & Chan, M. S. (1990). How insect trigger single-stage outbreaks in tropical pests. Functional Ecology, 4, 329–338.
Goettel, M. S., Eilenberg, J., & Glare, T. (2010). Entomopathogenic fungi and their role in regulation of insect populations. In L. I. Gilbert & S. S. Gill (Eds.), Insect control: Biological and synthetic agents (pp. 387–432). Amsterdam: Academic.
Gonzalez, F., Tkaczuk, C., Dinu, M. M., Fiedler, Z., Vidal, S., Zchori-Fein, E., & Messelink, G. J. (2016). New opportunities for the integration of microorganisms into biological pest control systems in greenhouse crops. Journal of Pest Science, 89, 295–311.
Gould, W. (1747). An account of English ants. London: A. Millar.
Grewal, P. S., Ehlers, R. U., & Shapiro-Ilan, D. I. (2005). Nematodes as biocontrol agents. Wallingford: CABI.
Gurr, G. M., & Wratten, G. M. (1999). Integrated biological control: A proposal for enhancing success in biological control. International Journal of Pest Management, 45(2), 81–84.
Gurr, G. M., Wratten, S. D., Irvin, N. A., Hossain, Z., Baggen, L. R., Mensah, R. K., Walker, P. W. (1998). Habitat manipulation in Australasia: recent biocontrol progress and prospects for adoption. In M. P. Zalucki, R. A. I. Drew, and G. G. White (Eds.), Pest management-future challenges. Proceedings of the Sixth Australasian applied entomological research conference, Brisbane, Australia, pp. 225–235.
Haar, P. J., Bowling, R., Gardner, W. A., & Buntin, G. D. (2018). Epizootics of the entomopathogenic fungus Lecanicillium lecani (Hypocreales: Clavicipitaceae) in sugarcane aphid (Hemiptera: Aphididae) populations infesting grain sorghum in Georgia and Texas. Journal of Entomological Science, 53(1), 104–106.
Hajek, A. E., & Butler, L. (2000). Predicting the host range of entomopathogenic fungi. In P. A. Follett & J. J. Duan (Eds.), Nontarget effects of biological control (pp. 263–276). Dordrecht: Kluwer Academic Publishers.
Hajek, A. E., & Goettel, M. S. (2000). Guidelines for evaluating effects of entomopathogens on nontarget organisms. In L. A. Lacey & H. K. Kaya (Eds.), Manual of field techniques in insect pathology (pp. 847–868). Dordrecht: Kluwer Academic Publishers.
Hariston, N. G., Smith, F. E., & Slobodkin, L. B. (1960). Community structure, population control and competition. The American Naturalist, 94, 421–425.
Harrison, R., & Hoovery, K. (2012). Baculoviruses and other occluded insect viruses. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 73–131). London: Academic.
Herniou, E. A., Huguet, E., Theze, J., Bezier, A., Periquet, G., & Drezen, J. M. (2013). When parasitic wasps hijacked viruses: Genomic and functional evolution of polydnaviruses. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences, 368(1626), 20130051.
Hilbeck, A., Baumgartner, M., Fried, P. M., & Bigler, F. (1998a). Effects of transgenic Bacillus thuringiensis corn-fed prey on mortality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Environmental Entomology, 27, 480–487.
Hilbeck, A., Moar, W., Pusztain-Carey, M., Filippini, A., & Bigler, F. (1998b). Toxicity of Bacillus thuringiensis Cry1Ab toxin to the predator Chrysoperla carnea (Neuroptera: Chrysopidae). Environmental Entomology, 27, 1255–1263.
Hoch, G., Schopf, A., & Maddox, J. V. (2000). Interactions between an entomopathogenic microsporidium and the endoparasitoid Glyptapanteles liparidiswithin their host, the gypsy moth larva. Journal of Invertebrate Pathology, 75, 59–60.
Hoch, G., Schafellner, C., Henn, M. W., & Schopf, A. (2002). Alterations in carbohydrate and fatty acid levels of Lymantria dispar larvae caused by a microsporidian infection and potential adverse effects on a co-occurring endoparasitoid Glyptapanteles liparidis. Archive of Insect Biochemistry and Physiology, 50, 109–120.
Hoffmann, M. P., & Frodsham, A. C. (1993). Natural enemies of vegetable insect pests. Ithaca: Cooperative Extension, Cornell University.
Hokkanen, H. M. T. (1993). New approaches in biological control. In D. Pimentel (Ed.), Handbook of pest management (pp. 185–198). New Delhi: CBS Publishers and Distributors.
Huffaker, C. B., Dahlsten, D. L., Janzen, D. H., & Kennedy, G. G. (1984). Insect influences in the regulation of plant populations and communities. In C. B. Huffaker & R. L. Rabb (Eds.), Ecological entomology (pp. 659–691). New York: Wiley.
Huger, A. M., & Neuffer, G. (1978). Infection of the braconid parasite Ascogaster quadridentata (Hymenoptera: Braconidae) by a microsporidan of its host Laspeyresia pommonella. Mitt Biol Bundesanst Land- Forstwirtsch Berl-Dahl, 180, 105–106.
Humber, R. A. (2012). Entomophthoromycota: A new phylum and reclassification for entomophthoroid fungi. Mycotaxon, 120, 477–492.
Idris, A. B., Zainal-Abidin, B. A., Noraini, I., & Hussan, A. K. (2001). Diadegma semiclausum as a possible factor for the horizontal transmission of microsporidial disease of diamondback moth, Plutella xylostella L. Pakistan Journal of Biological Science, 4, 1353–1356.
Jacobson, R. J., Chandler, D., Fenlon, J., & Russell, K. M. (2001). Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Science and Technology, 11, 391–400.
Jaronski, S. T. (2010). Ecological factors in the inundative use of fungal entomopathogens. BioControl, 55, 159–185.
Jaronski, S. T., Goettel, M. S., & Lomer, C. J. (2003). Regulatory requirements for ecotoxicological assessments of microbial insecticides – How relevant are they? In H. M. T. Hokkanen & A. E. Hajek (Eds.), Environmental impacts of microbial insecticides (pp. 237–260). Dordrecht: Kluwer Academic Publishers.
Jarrahi, A., & Safavi, S. A. (2016). Sublethal effects of Metarhizium anisopliae on life table parameters of Habrobracon hebetor parasitizing Helicoverpa armigera larvae at different time intervals. BioControl, 61(2), 167–175.
Jervis, M. A. (2005). Insect as natural enemies, A practical perspective (755 pp). Dordrecht: Springer.
Jervis, M. A., Kidd, N. A. C., & Heimpel, G. E. (1996). Parasitoid adult feeding behaviour and biocontrol. Biocontrol News and Information, 17, 11–26.
Jiravanichpaisal, P., Lee, B. L., & Soderhall, K. (2006). Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology, 211, 213–236.
Jurat-Fuentes, J. L., & Jackson, T. A. (2012). Bacterial entomopathogens. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 265–351). London: Academic.
Kant, R., Minor, M. A., Trewick, S. A., & Sandanayaka, W. R. M. (2012). Body size and fitness relation in male and female Diaeretiella rapae. BioControl, 57, 759–766.
Kaya, H. K., & Stock, S. P. (1997). Techniques in insect nematology. In L. A. Lacey (Ed.), Manual of techniques in insect pathology (pp. 281–324). New York: Academic.
Keeling, P. J., Corradi, N., Morrison, H. G., Haag, K. L., Ebert, D., Weiss, L. M., Akiyoshi, D. E., & Tzipori, S. (2010). The reduced genome of the parasitic microsporidian Enterocytozoon bieneusi lacks genes for core carbon metabolism. Genome Biology and Evolution, 2, 304–309.
Keohane, E. M., & Weiss, L. M. (1998). Characterization and function of the microsporidian polar tube: A review. Folia Parasitologica, 45, 117–127.
Khanamani, M., Fathipour, Y., Talebi, A. A., & Mehrabadi, M. (2017). How pollen supplementary diet affect life table and predation capacity of Neoseiulus californicus on two-spotted spider mite. Systematic & Applied Acarology, 22(1), 135–147.
Khetan, S. K. (2001). Microbial pest control. New York: Marcel Dekker.
Kido, H., Flaherty, D. L., Bosch, D. F., & Valero, K. A. (1983). Biological control of grape leafhopper. In California Agriculture (pp. 4–6). Berkeley: California Agricultural Experiment Station.
Kirby, W., & Spence, W. (1822). Diseases of insects. In Hurst, Rees, Orme, & Brown (Eds.), An introduction to entomology: Or elements of the natural history of nematodes (pp. 197–232). London: Longman.
Kogan, M. (1998). Integrated pest managements: Historical perspectives and contemporary developments. Annual Review of Entomology, 43, 243–270.
Koul, O., & Dhaliwal, G. S. (2002). Microbial biopesticides. London: Taylor and Francis.
Koul, O., & Dhaliwal, G. S. (2003). Predators and parasitoids: An introduction. London: Taylor and Francis.
Labbe, R. M., Gillespie, D. R., Cloutier, C., & Brodeur, J. (2009). Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Science and Technology, 19(4), 429–446.
Lacey, L. A., Frutos, R., Kaya, H. K., & Vail, P. (2001). Insect pathogens as biological control agents: Do they have a future? Biological Control, 21, 230–248.
Lange, C. E., & Lordy, J. C. (2012). Protistan entomopathogens. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 367–394). London: Academic.
Legner, E. F., & Badgley, M. E. (1982). Improved parasites for filth fly control. California Agriculture, 36, 27.
Lewis, E. E., & Clarkey, D. J. (2012). Nematode parasites and entomopathogens. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 395–424). London: Academic.
Lewis, L. C., Sumerford, D. V., Bing, L. A., & Gunnarson, R. D. (2006). Dynamics of Nosema pyrausta in natural populations of the European corn borer, Ostrinia nubilalis. BioControl, 51, 627–642.
Lewis, L. C., Bruck, D. J., Prasifka, J. R., & Raun, E. S. (2009). Nosema pyrausta: Its biology, history, and potential role in a landscape of transgenic insecticidal crops. Biological Control, 48, 223–231.
Lord, J. C. (2001). Response of the wasp Cephalonomia tarsalis (Hymenoptera: Bethylidae) to Beauveria bassiana (Hyphomycetes: Moniliales) as free conidia or infection in its host, the Sawtoothed Grain Beetle, Oryzaephilus surinamensis (Coleoptera: Silvanidae). Biological Control, 21, 300–304.
Lovei, G. L., & Arpaia, S. (2005). The impact of transgenic plants on natural enemies: A critical review of laboratory studies. Entomologia Experimentalis et Applicata, 114, 1–14.
Lovvei, G. L., Andow, D. A., & Arpaiaa, S. (2009). Transgenic insecticidal crops and natural enemies: A detailed review of laboratory studies. Environmental Entomology, 38(2), 293–306.
Ludwig, S. W., & Oetting, R. D. (2001). Susceptibility of natural enemies to infection by Beauveria bassiana and impact of insecticides on Ipheseius degenerans (Acari: Phytoseiidae). Journal of Agricultural and Urban Entomology, 18, 169–178.
Mahdavi, V., Saberm, M., Rafiee-Dastjerdi, H., & Mehrvar, A. (2013). Susceptibility of the hymenopteran parasitoid, Habrobracon hebetor (Say) (Braconidae) to the entomopathogenic fungi Beauveria bassiana Vuillemin and Metarhizium anisopliae Sorokin. Jordan Journal of Biological Science, 6, 17–20.
Mazzone, H. M. (1985). Pathology associated with baculovirus infection. In K. Maramorosh & K. E. Sherman (Eds.), Viral insecticides for biological control (pp. 81–120). Orlando: Academic.
Mbata, G. N., & Shapiro-Ilan, D. I. (2010). Compatibility of Heterorhabditis indica (Rhabditida: Heterorhabditidae) and Habrobracon hebetor (Hymenoptera: Braconidae) for biological control of Plodia interpunctella (Lepidoptera: Pyralidae). Biological Control, 54, 75–82.
Merian, M. S. (1679). Der Raupen wunderbare Verwandelung und sonderbare Blumen-nahrung. Nurnberg: J. A. Graff.
Mertz, N. R., Agudelo, E. J. G., Sales, F. S., & Junior, A. M. (2015). Effects of entomopathogenic nematodes on the predator Calosoma granulatum in the laboratory. Journal of Insect Behavior, 28(3), 312–327.
Mesquita, A. L. M., & Lacey, L. A. (2001). Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biological Control, 22, 51–59.
Metcalf, R. L., & Luckmann, W. H. (1994). Introduction to insect pest management. New York: Wiley.
Meyling, N. V., & Pell, J. K. (2006). Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecological Entomology, 31, 162–171.
Mills, N. J., & Getz, W. (1996). Modelling the biological control of insect pests: A review of host-parasitoid models. Ecological Modelling, 92(2–3), 121–143.
Milner, R. J. (1994). History of Bacillus thuringiensis. Agriculture, Ecosystems & Environment, 49, 9–13.
Moawed, S. M., Marei, S. S., Saleh, M. R., & Matter, M. M. (1997). Impact of Vairimorpha ephestiae (Microspora: Nosematidae) on Bracon hebetor (Hymenoptera: Braconidae), an external parasite of the American bollworm, Heliothis armigera (Lepidoptera: Noctuidae). European Journal of Entomology, 94, 561–565.
Moezipour, M., Kafil, M., & Allahyari, H. (2008). Functional response of Trichogramma brassicae at different temperatures and relative humidities. Bulletin of Insectology, 62(2), 245–250.
Mohammed, A. A. (2018). Lecanicillium muscarium and Adalia bipunctata combination for the control of black bean aphid, Aphis fabae. BioControl, 63, 277–287.
Mohammed, A. A., & Hatcher, P. E. (2017). Combining entomopathogenic fungi and parasitoids to control the green peach aphid Myzus persicae. Biological Control, 110, 44–55.
Mohan, M., Sushil, S. N., Bhatt, J. C., Gujar, G. T., & Gupta, H. S. (2008). Synergistic interaction between sublethal doses of Bacillus thuringiensis and Campoletis chlorideae in managing Helicoverpa armigera. BioControl, 53, 375–386.
Morse, D. H. (1994). The effect of host size on sex ratio in the Ichneumonid wasp, Trychosis cyperia, a spider parasitoid. American Midland Naturalist, 131, 281–287.
Mracek, Z., & Sturhan, D. (2000). Epizootic of the entomopathogenic nematode Steinernema intermedium (Poinar) in an aggregation of the bibionid fly, Bibio marci L. Journal of Invertebrate Pathology, 76(2), 149–150.
Murphy, F. A., Fauquet, C. M., Bishop, D. H. L., Ghabrial, S. A., Jarvis, A. W., Martalli, G. P., Mayo, M. A., & Summers, M. D. (1995). Virus taxonomy: Classification and nomenclature of viruses. Dordrecht: Springer.
Murray, D. A. H., Monsour, C. J., Teakle, R. E., Rynne, K. P., & Bean, J. A. (1995). Interactions between Nuclear Polyhedrosis Virus and three larval parasitoids of Helicoverpa armigera (Hiibner) (Lepidoptera: Noctuidae). Journal of the Australian Entomological Society, 34, 319–322.
Murugan, K., Senthil, K. N., Jeyabalan, D., Senthil, N. S., Sivaramakrishnan, S., & Swamiappan, M. (2000). Influence of Helicoverpa armigera (Hubner) diet on its parasitoid Campoletis chlorideae Uchida. Insect Science and its Application, 20, 23–31.
Nauen, R., Stumpf, N., Elbert, A., Zebitz, C. P. W., & Kraus, W. (2001). Acaricide toxicity and resistance in larvae of different strains of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pest Management Science, 57, 253–261.
Nielsen, C., Skovgard, H., & Steenberg, T. (2005). Effect of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) on survival and reproduction of the filth fly parasitoid, Spalangia cameroni (Hymenoptera: Pteromalidae). Environmental Entomology, 34(1), 133–139.
Nilsson, U., Rannback, L. M., Anderson, P., Eriksson, A., & Ramert, B. (2011). Comparison of nectar use and preference in the parasitoid Trybliographa rapae (Hymenoptera: Figitidae) and its host, the cabbage root fly, Delia radicum (Diptera: Anthomyiidae). Biocontrol Science and Technology, 21, 1117–1132.
Nysten, P. H. (1808). Recherches sur les maladies des vers a soie et les moyens de les prevenir. Paris: De L’Imprimerie Imperiale.
O’Callaghan, M., Glare, T. R., Burgess, E. P. J., & Malone, L. A. (2005). Effects of plants genetically modified for insect resistance on non-target organisms. Annual Review of Entomology, 50, 271–292.
Obrist, L. B., Klein, H., Dutton, A., & Bigler, F. (2006). Assessing the effects of Bt maize on the predatory mite Neoseiulus cucumeris. Experimental and Applied Acarology, 38, 125–139.
Oluwafemi, A. R., Raol, Q., Wang, X. Q., & Zhang, H. Y. (2009). Effect of Bacillus thuringiensis on Habrobracon hebetor during combined biological control of Plodia interpunctella. Insect Science, 16, 409–416.
Ormond, E. L., Thomas, A. P. M., Pell, J. K., Freeman, S. N., & Roy, H. E. (2011). Avoidance of a generalist entomopathogenic fungus by the ladybird, Coccinella septempunctata. Microbial Ecology, 77, 229–237.
Own, S. O., & Brooks, W. M. (1986). Interactions of the parasite Pediobius foveolatus (Hymenoptera: Eulophidae) with two Nosema spp. (Microsporida: Nosematidae) of the Mexican bean beetle (Coleoptera: Coccinellidae). Environmental Entomology, 15, 32–39.
Paxton, R. J. (2010). Does infection by Nosema ceranae cause colony collapse disorder in honey bees (Apis mellifera)? Journal of Apiculture Research, 49, 80–84.
Pedersen, A., Dedes, J., Gauthier, D., & van Frankenhuyzen, K. (1997). Sublethal effects of Bacillus thuringiensis on the spruce budworm, Choristoneura fumiferana. Entomologia Experimentalis et Applicata, 83, 253–262.
Pell, J. K., & Vandenberg, J. D. (2002). Interaction among aphid Diuraphis noxia, the entomopatogenic fungus Paecilomyces fumosoroseus and the coccinellid Hippodamia convergens. Biocontrol Science and Technology, 12, 217–224.
Pell, J. K., Pluke, R., Clark, S. J., Kenward, M. J., & Alederson, P. G. (1997). Interactions between two aphid natural enemies, the entomopathogenic fungus Pandora neoaphidis Remau-diere & Hennebert (Zygomycetes: Entomophthorales) and the predatory beetle Coccinella septempunctata L. (Coleoptera: Coccinellidae). Journal of Invertebrate Pathology, 69, 261–268.
Perera, P. A. C., Hassell, M. P., & Godfray, H. C. J. (1988). Population dynamics of the coconut caterpillar, Opisina aeronsella Walker (Lepidoptera: Xylorctidae) in Sri Lanka. Bulletin of Entomological Research, 78, 479–497.
Perrin, R. M. (1975). The role of the perennial stinging nettle Urtica dioica as a reservoir of beneficial natural enemies. Annals of Applied Biology, 81, 289–297.
Poinar, G. O. (1975). Description and biology of a new insect parasitic rhabditoid, Heterorhabditis bacteriophora n.gen. n.sp. (Rhabditida: Heterorhabditidae n.fam.). Nematologica, 21, 463–470.
Ponsard, S., Gutierrez, A. P., & Mills, N. J. (2002). Effect of Bt-toxin (Cry1Ac) in transgenic cotton on the adult longevity of four heteropteran predators. Environmental Entomology, 31, 1197–1205.
Pope, T., Croxson, E., Pell, J. K., Godfray, H. C. J., & Muller, C. B. (2002). Apparent competition between two species of aphid via the fungal pathogen Erynia neoaphidis and its interaction with the aphid parasitoid Aphidius ervi. Ecological Entomology, 27, 196–203.
Popham, H. J., Shelby, K. S., Brandt, S. L., & Coudron, T. A. (2004). Potent virucidal activity in larval Heliothis virescens plasma against Helicoverpa zea single capsid nucleopolyhedrovirus. Journal of General Virology, 85, 2255–2261.
Pourian, H. R., Talaei-Hassanloui, R., Kosari, A. A., & Ashouri, A. (2011). Effects of Metarhizium anisopliae on searching, feeding and predation by Orius albidipennis (Hem., Anthocoridae) on Thrips tabaci (Thy., Thripidae) larvae. Biocontrol Science and Technology, 21, 15–21.
Pozzebon, A., & Duso, C. (2009). Pesticide side-effects on predatory mites: The role of trophic interaction. In M. W. Sabelis & J. Bruin (Eds.), Trends in acarology (pp. 465–469). Dordrecht: Springer.
Rannback, L. M., Cotes, B., Anderson, P., Ramert, B., & Meyling, N. V. (2015). Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. Journal of Invertebrate Pathology, 124, 78–86.
Rashki, M., Kharazi-pakdel, A., Allahyari, H., & van Alphen, J. J. M. (2009). Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biological Control, 50(3), 324–328.
Rechcigl, J. E., & Rechcigl, N. A. (1998). Biological and biotechnological control of insect pests. Boca Raton: CRC Press.
Riahi, E., Fathipour, Y., Talebi, A. A., & Mehrabadi, M. (2016). Pollen quality and predator viability: Life table of Typhlodromus bagdasarjani on seven different plant pollens and two-spotted spider mite. Systematic & Applied Acarology, 21(10), 1399–1412.
Roberts, D. W., & Humber, R. A. (1981). Entomogenous fungi. In G. T. Cole & B. Kendrick (Eds.), Biology of conidial fungi (Vol. 2, pp. 201–236). New York: Academic.
Rodriguez-Perez, M. A., & Beckage, N. E. (2006). Co-evolutionary strategies of interaction between parasitoids and polydnaviruses. Revista Latinoamericana de Microbiología, 48(1), 31–43.
Rodriguez-Perez, M. A., & Beckage, N. E. (2008). Comparison of three methods of parasitoid polydnavirus genomic DNA isolation to facilitate polydnavirus genomic sequencing. Archives of Insect Biochemistry and Physiology, 67(4), 202–209.
Rogers, K. (2011). Bacteria and viruses. New York: Britannica Educational Publishing.
Romeis, J., Dutton, A., & Bigler, F. (2004). Bacillus thuringiensis toxin has no direct effect on larvae of the green lacewing Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae). Journal of Insect Physiology, 50, 175–183.
Rosenheim, J. A., Kaya, H. K., Ehler, L. E., Marois, J. J., & Jaffee, B. A. (1995). Intraguild predation among biological-control agents: Theory and evidence. Biological Control, 5, 303–335.
Roy, H. E., & Pell, J. K. (2000). Interactions between entomopathogenic fungi and other natural enemies: Implications for biological control. Biocontrol Science and Technology, 10, 737–752.
Roy, H. E., Brown, P. M. J., & Majerus, M. E. N. (2006). Harmonia axyridis: A successful biocontrol agent or an invasive threat? In J. Eilenberg & H. Hokkanen (Eds.), An ecological and societal approach to biological control (pp. 295–309). Dordrecht: Kluwer Academic Publishers.
Roy, H. E., Brown, P. M. J., Rothery, P., Ware, R. L., & Majerus, M. E. N. (2008). Interactions between the fungal pathogen Beauveria bassiana and three species of coccinellid: Harmonia axyridis, Coccinella septempunctata and Adalia bipunctata. BioControl, 53, 265–276.
Salama, H. S., Foda, M. S., Zaki, F. N., & Khalafallah, A. (1983). Persistence of Bacillus thuringiensis Berliner spores in cotton cultivations. Journal of Applied Entomology, 95, 321–326.
Salama, H. S., Zaki, F. N., & Sabbour, M. M. (1996). Effect of Bacillus thuringiensis endotoxin on Apanteles litae Nixon and Bracon instabilis Marsh (Hym., Braconidae), two parasitoids of the potato tuber moth Phthorimia operculella Zeller (Lep., Gelishiidae). Journal of Applied Entomology, 120, 565–568.
Saleh, M. M., Lewis, L. C., & Obrycki, J. J. (1995). Selection of Nosema pyrausta (microsporidia: Nosematidae)-infected Ostrinia nubilalis (Lepidoptera: Pyralidae) eggs for parasitization by Trichogramma nubilale (Hymenoptera: Trichogrammatidae). Crop Protection, 14(4), 327–330.
Sanders, C. J., Pell, J. K., Poppy, G. M., Raybould, A., Garcia-Alonso, M., & Schuler, T. H. (2007). Host-plant mediated effects of transgenic maize on the insect parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Biological Control, 40, 362–369.
Schmit, J. P., & Mueller, G. M. (2007). An estimate of the lower limit of global fungal diversity. Biodiversity and Conservation, 16(1), 99–111.
Schuld, M., Madel, G., & Schmuck, R. (1999). Impact of Vairimorpha sp. (Microsporidia: Burnellidae) on Trichogramma chilonis (Hymenoptera: Trichogrammatidae), a hymenopteran parasitoid of the cabbage moth, Plutella xylostella (Lepidoptera, Yponomeutidae). Journal of Invertebrate Pathology, 74, 120–126.
Sedaratian, A., Fathipour, Y., & Moharramipour, S. (2009). Evaluation of resistance in 14 soybean genotypes to Tetranychus urticae (Acari: Tetranychidae). Journal of Pest Science, 82, 163–170.
Sedaratian, A., Fathipour, Y., Talaei-Hassanloui, R., & Jurat-Fuentes, J. L. (2013). Fitness costs of sublethal exposure to Bacillus thuringiensis in Helicoverpa armigera: A carryover study on offspring. Journal of Applied Entomology, 137, 540–549.
Sedaratian, A., Fathipour, Y., & Talaei-Hassanloui, R. (2014). Deleterious effects of Bacillus thuringiensis on biological parameters of Habrobracon hebetor parasitizing Helicoverpa armigera. BioControl, 59, 89–98.
Seiedy, M., Saboori, A., & Allahyari, H. (2012). Interactions of two natural enemies of Tetranychus urticae, the fungal entomopathogen Beauveria bassiana and the predatory mite, Phytoseiulus persimilis. Biocontrol Science and Technology, 22(8), 873–882.
Sewify, G. H., & El-Arnaouty, S. A. (1998). The effect of the entomopathogenic fungus Verticillium lecanii (Zimm.) Viegas on mature larvae of Chrysoperla carnea Stephans (Neuroptera, Chrysoperla) in the laboratory. Acta Zoologica Fennica, 209, 233–237.
Shapiro-Ilan, D. I., Brucky, D., & Lacey, L. A. (2012). Principles of epizootiology and microbial control. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 29–72). London: Academic.
Sharma, H. C., Dhillon, M. K., & Arora, R. (2008). Effects of Bacillus thuringiensis d-endotoxin-fed Helicoverpa armigera on the survival and development of the parasitoid Campoletis chlorideae. Entomologia Experimentalis et Applicata, 126, 1–8.
Sher, R. B., & Parrella, M. P. (1996). Integrated biological control of leafminers, Liriomyza trifolii, on greenhouse chrysanthemums. IOBC WPRS Bulletin, 19, 147–150.
Shipp, L., Kapongo, J. P., Park, H. H., & Kevan, P. (2012). Effect of beevectored Beauveria bassiana on greenhouse beneficials under greenhouse cage conditions. Biological Control, 63, 135–114.
Siegel, J. P., Maddox, J. V., & Ruesink, W. G. (1986). Impact of Nosema pyrausta on a braconid, Macrocentrus grandii, in Central Illinois. Journal of Invertebrate Pathology, 47, 271–276.
Simoes, R. A., Reis, L. G., Bento, J. M., Solter, L. F., & Delalibera, I., Jr. (2012). Biological and behavioral parameters of the parasitoid Cotesia flavipes (Hymenoptera: Braconidae) are altered by the pathogen Nosema sp. (Microsporidia: Nosematidae). Biological Control, 63, 164–171.
Solter, L. F., Maddox, J. V., & McManus, M. L. (1997). Host specificity of microsporidia (Protista: Microspora) from European populations of Lymantria dispar (Lepidoptera: Lymantriidae) to indigenous North American Lepidoptera. Journal of Invertebrate Pathology, 69, 135–150.
Steele, T., & Bjornson, S. (2012). The effects of two microsporidian pathogens on the two-spotted lady beetle, Adalia bipunctata L. (Coleoptera: Coccinellidae). Journal of Invertebrate Pathology, 109, 223–228.
Steele, T., & Bjornson, S. (2014). Nosema adaliae sp. nov., a new microsporidian pathogen from the two-spotted lady beetle, Adalia bipunctata L. (Coleoptera: Coccinelldiae) and its relationship to microsporidia that infect other coccinellids. Journal of Invertebrate Pathology, 115, 108–115.
Steiner, G. (1923). Aplectana kraussei n.sp., eine in der blattwespe Lyda sp. parasitierende Nematoden form, nebst Bemerkungen uber das Seitenorgan der parasitischen Nematoden. Zbl. Bakt. Parasitenk. Infetionskrank Hyg Abt, 1, 14–18.
Steinhaus, E. A. (1975). Disease in a minor chord. Columbus: Ohio State University Press.
Stevens, G. N., & Stuart, R. J. (2008). The ecological complexities of biological control: Trophic cascades, spatial heterogeneity, and behavioral ecology. Journal of Nematology, 40, 59–60.
Stoianova, E. E. (2007). The effect of the Nuclear Polyhedrosis Viruses (NPVs) of some noctuidae species on the longevity of Bracon hebetor Say (Hymenoptera: Braconidae). Bulgarian Journal of Agricultural Science, 13, 197–203.
Straub, C. S., Finke, D. L., & Snyder, W. E. (2008). Are the conservation of natural enemy biodiversity and biological control compatible goals? Biological Control, 45, 225–237.
Sullivan, B. T., & Berisford, C. W. (2004). Semiochemicals from fungal associates of bark beetles may mediate host location behavior of parasitoids. Journal of Chemical Ecology, 30, 703–717.
Tan, C. W., Peiffer, M., Hoover, K., Rosa, C., Acevedo, F. E., & Felton, G. W. (2018). Symbiotic polydnavirus of a parasite manipulates caterpillar and plant immunity. PNAS, 115(20), 5199–5204.
Tanada, Y., & Kaya, H. K. (1993). Insect pathology. San Diego: Academic.
Teera-Arunsiri, A., Suphantharika, M., & Ketunuti, U. (2003). Preparation of spray-dried wettable powder formulations of Bacillus thuringiensis-based biopesticides. Journal of Economic Entomology, 96, 292–299.
Thomas, M. B., Wratte, N., & Sotherton, N. W. (1992). Creation of ‘island’ habitats in farmland to manipulate populations of beneficial arthropods: Predator densities and species composition. Journal of Applied Ecology, 29, 524–531.
Torres, J. B., & Ruberson, J. R. (2006). Interactions of Bt-cotton and the omnivorous big-eyed bug Geocoris punctipes (Say), a key predator in cotton fields. Biological Control, 39, 47–57.
Udayababu, P., Goud, S. Z., & Raja, C. (2012). Evaluation of entomopathogenic fungi for the management of tobacco caterpillar Spodoptera litura (Fabricius). Indian Journal of Plant Protection, 40(3), 214–220.
Ulug, D., Hazir, S., Kaya, H. K., & Lewis, E. (2014). Natural enemies of natural enemies: The potential top-down impact of predators on entomopathogenic nematode populations. Ecological Entomology, 39, 462–469.
Vaez, N., Iranipour, S., & Hejazi, M. J. (2013). Effect of treating eggs of cotton bollworm with Bacillus thuringiensis Berliner on functional response of Trichogramma brassicae Bezdenko. Archives of Phytopathology and Plant Protection, 46, 2501–2511.
Van Driesche, R. G., & Bellows, T. S. (1988). Host and parasitoid recruitment for quantifying losses from parasitism, with reference to Pieris rapae and Cotesia glomerata. Ecological Entomology, 13, 215–222.
Van Frankenhuyzen, K. (1993). The challenge of Bacillus thuringiensis. In P. E. Entwistle, J. S. Cory, M. J. Bailey, & S. Higgs (Eds.), Bacillus thuringiensis, an environmental biopesticide: Theory and practice (pp. 1–35). Chichester: Wiley.
Vega, F. E., Meylingy, N. V., Luangsaard, J. J., & Blackwellz, M. (2012). Fungal entomopathogens. In F. E. Vega & H. K. Kaya (Eds.), Insect pathology (pp. 171–220). London: Academic.
Vinson, B. S. (1976). Host selection by insect parasitoids. Annual Review of Entomology, 21, 109–133.
Waage, J. (1989). The population ecology of pest-insecticide-natural enemy interactions. In P. Jepson (Ed.), Pesticides and non-target invertebrates (pp. 81–83). Wimborne: Dorest: Intercept.
Walker, G. P., Cameron, P. J., MacDonald, F. M., Madhusudhan, V. V., & Wallace, A. R. (2007). Impacts of Bacillus thuringiensis toxins on parasitoids (Hymenoptera: Braconidae) of Spodoptera litura and Helicoverpa armigera (Lepidoptera: Noctuidae). Biological Control, 40, 142–151.
Wallner, W. E., Dubois, N. R., & Grinberg, P. S. (1983). Alteration of parasitism by Rogas lymantriae (Hymenoptera: Braconidae) in Bacillus thuringiensis-stressed gypsy moth (Lepidoptera: Lymantriidae) hosts. Journal of Economic Entomology, 76, 275–277.
Wang, B., & Ferro, D. N. (1998). Functional response of Trichogramma ostriniae (Hymenoptera: Trichogrammatidae) to Ostrinia nubilalis (Lepidoptera: Pyralidae) under laboratory and field conditions. Environmental Entomology, 27, 752–758.
Webb, B. A., Strand, M. R., Dickey, S. E., Beck, M. H., Hilgarth, R. S., et al. (2006). Polydnavirus genomes reflect their dual roles as mutualists and pathogens. Virology, 347, 160–174.
Wekesa, V. W., Moraes, G. J., Knapp, M., & Delalibera, I. (2007). Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulus longipes (Acari: Phytoseiidae). Biological Control, 41, 408–414.
Williams, B. A. P., & Keeling, P. J. (2005). Microsporidian mitochondrial proteins: Expression in Antonospora locustae spores and identification of genes coding for two further proteins. Journal of Eukaryotic Microbiology, 52, 271–276.
Wu, S., Xie, H., Li, M., Xu, X., & Lei, Z. (2016). Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Experimental and Applied Acarology, 70, 421–435.
Xia, J. Y., Cui-Jin, J., Ma, L. H., Dong, S. L., & Cui, X. F. (1999). The role of transgenic Bt cotton in integrated insect pest management. Acta Gossypii Sinica, 11, 57–64.
Zander, E. (1909). Tierische Parasiten als Krankheitserreger bei der Biene. Leipziger Bienenzeitung, 24, 147–150.
Zimmermann, G. (2007). Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Science and Technology, 17(9), 879–920.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sedaratian-Jahromi, A. (2021). Effects of Entomopathogens on Insect Predators and Parasitoids. In: Khan, M.A., Ahmad, W. (eds) Microbes for Sustainable lnsect Pest Management. Sustainability in Plant and Crop Protection, vol 17. Springer, Cham. https://doi.org/10.1007/978-3-030-67231-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-67231-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67230-0
Online ISBN: 978-3-030-67231-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)