Abstract
Surfactant protein D (SP-D), expressed in respiratory epithelium, is important for modulating both innate and adaptive immunity against inhaled allergens. This chapter evaluates the key immunomodulatory role of SP-D in allergic airway inflammatory events. Studies have shown that SP-D ameliorated various cellular mechanisms implicated in pulmonary allergic reactions, including allergic eosinophilia through induction of apoptosis and clearance of activated eosinophils, and inhibition of basophils activation. In addition, blocking allergen-specific IgE synthesis, T cell proliferation, and hindrance to the binding of allergen-IgE complex to B lymphocytes are also brought about by SP-D. In vitro, a recombinant fragment of human SP-D, composed of homotrimeric neck and C-type lectin domains (rfhSP-D), binds to allergens derived from Aspergillus fumigatus, house dust mite and grass pollen in a calcium-dependent manner and inhibits specific IgE interaction with allergens. Murine models of hypersensitivity reactions, when treated with rfhSP-D, showed reduced levels of IgE, clearance of peripheral and pulmonary eosinophilia, and Th2 polarisation to Th1 type. SP-D knock-out mice accordingly show hypereosinophilia and predominantly exaggerated Th2 response following allergen challenge; these features can be ameliorated by rfhSP-D treatment. The current state of the filed appears to suggest that rfhSP-D is an excellent therapeutic immunomodulator in airway allergic diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Allergic airway diseases are multifactorial and influenced by genetic, immune and environmental components (Allinne et al. 2019; Polverino et al. 2018). These diseases are growing challenges to global health (Pawankar 2014). The pattern of pulmonary inflammation such as rhinorrhoea, airway hyperresponsiveness and airway obstruction are exhibited as clinical features in asthma and allergic rhinitis (Jeffery and Haahtela 2006). Allergic airway disease is largely established by the overexpression of IgE generated in response to allergens that are innocuous to non-allergic individuals (Kratzer and Pickl 2016; Voskamp et al. 2020).
Peripheral blood mononuclear cells such as Dendritic cells (DCs), T and B lymphocytes, and granulocytes like mast cells and eosinophils, are critical in allergic reactions through the secretion of an array of mediators with airway constrictive and pro-inflammatory consequences (Figs. 1 and 2) (Méndez-Enríquez and Hallgren 2019). Immune hyperresponsiveness toward specific environmental allergens can lead to airway remodelling and pulmonary tissue damage (Palm et al. 2012; Kuruvilla et al. 2019). The current novel therapeutic regimens for airway allergic diseases, apart from allergen- immunotherapy, are mainly biologics that block allergic mediators such as cytokines and cellular receptors, such as Omalizumab, Dupilumab, mepolizumab, and Benralizumab (Staubach et al. 2018; Hellings et al. 2017; Trischler et al. 2017; Fitzgerald et al. 2016). However, concerns have been raised about long-term side effects, prediction of treatment responses, the ability of these agents to promote tolerance induction, and the high financial cost (Breiteneder et al. 2019). Therefore, novel inhibitors of allergen-specific IgE and Th2 cytokines are much needed as an alternative therapeutic modality with lower costs and a broader scope of application. Studies have shown the pivotal role of surfactant protein D (SP-D) in modulating immune hyperresponsiveness in the lung; this infers its importance to be considered an alternative therapeutic candidate in hindering pulmonary allergic reactions (Schleh et al. 2012; Sorensen 2018; Carreto-Binaghi and Taylor 2016; Winkler and Hohlfeld 2013). This chapter will highlight the immunomodulatory effects of SP-D in type I mediated hypersensitivity focusing on its impact on immune cells that fuel allergic airway reactions.
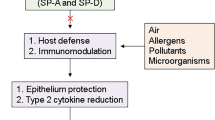
Lungs are constantly challenged with Allergens and microorganisms leading to the production of several defense mechanisms. This includes formation of anatomical epithelium barrier, mucus production and mucociliary clearance surfactant protein. In airway surfaces Clara cells stimulate SP-A and SP-D production after allergen exposure. Small particles might reach to the alveolar gas-exchange region of the lungs. Defense mechanisms get activated and peripheral airspace starts secreting surfactants, other opsonins and innate immune cells, i.e., macrophages and neutrophils
(a) hydrophilic surfactant proteins SP-A and SP-D are multimeric proteins where single subunit monomer contains lectin domain (CRD), neck region and a collagen like domain. (b) Trimeric subunits come together to form oligomeric structure form through non-covalent bond to forms octamers (18 monomers) for SP-A, and dodecamers (12 monomers) for SP-D (c)
Surfactant Protein D: Molecular Structure and Function
Initial purification and characterization of SP-D in the late 80s and early 90s paved the way for the determination of the structure and the immune functions of SP-D (Persson et al. 1989; Possmayer 1988; Malhotra et al. 1990, 1992). SP-D protein is a member of collectin family of innate carbohydrate pattern recognition molecules; it is calcium-dependent C-type lectin, and has multimeric structure (Holmskov et al. 1994). It is synthesised by airway epithelium, mainly type II pneumocytes and non- ciliated Clara cells (Crouch et al. 1992). It is hydrophilic with 43 kDa molecular weight; its monomer structure is composed of four regions: a short N-terminal region attached to a collagen-like domain, followed by an α-helical coiled-coil neck region, and C-type lectin or carbohydrate recognition domain (CRD) (Fig. 3) (Håkansson et al. 1999). The collagen region can form triple-helical structures, and by virtue of trimerizing capability of neck region, the C-terminal CRD region forms a trimeric structure. This subunit can further oligomerise due to N-terminal region cross-linking to yield dodecamers. The homotrimeric CRD region mediates binding to various ligands on pathogens/allergens that exhibiting carbohydrate and charge patterns, whereas the collagen region binds to putative receptors on effectors cells such as macrophages (Kishore et al. 2006). SP-D multimerization is required for enhancing CRD binding affinity of to its ligands (Wright 2005). Interestingly, in the late 90s, a functional recombinant fragment of human SP-D (rfhSP-D) comprised of trimeric CRD and α-helical coiled neck region was expressed in E. coli and subsequently characterised for potential therapeutic applications via in vitro, in vivo and ex vivo allergic models (Kishore et al. 1996, 2006; Wang et al. 1996).
Surfactant Protein D: Immunomodulatory Mechanisms in Allergic Airway Inflammatory Events
The idea of immunomodulation by SP-D in allergy stems from the observations by Wang et al. who showed that SP-D (purified from human lung lavage) as well as rfhSP-D can bind to house dust mite extracts (Dermatophagoides pteronyssinus; Der p) and purified native Der P1 in a calcium- and carbohydrate-dependent manner, thus, inhibiting Der p- IgE complex formation in vitro (Wang et al. 1996). Likewise, SP-D and rfhSP-D bound to Aspergillus fumigatus allergens and reduced histamine release from sensitised basophils derived from allergic bronchopulmonary aspergillosis (ABPA) patients (Madan et al. 1997). These findings suggested that SP-D binding to allergens can suppress early phase of inducing allergic airway reactions.
Protection by SP-D Against Allergic Eosinophilia
Infiltration of lung tissues with eosinophils is a sign of allergic airway inflammation ((Felton et al. 2014). This process involves eosinophilic differentiation and migration from the bone marrow to the lungs as a result of Th2 derived cytokines, particularly IL-13 and IL-5 (Esnault and Kelly 2016). Activation of eosinophils gives rise to the release of pro-inflammatory cytokines and cytotoxic proteins, including eosinophilic peroxidase (EPO), major basic protein (MBP) and reactive oxygen species (Acharya and Ackerman 2014). This leads to more destruction in the airway tissues and increases the tone of inflammatory allergic events (Yousefi et al. 2018).
SP-D has been shown to bind to eosinophils via its CRD and CD32 and block chemotaxis release and degranulation of Eosinophil Cationic peptide (Von Bredow et al. 2006). In addition, Madan et al. have demonstrated that SP-D and rfhSP-D reduced eosinophilia, allergen-specific IgE and IL-2, IL-4, and IL-5 levels in pulmonary hypersensitivity murine models induced by A. fumigatus allergens (Madan et al. 2001). Similarly, Singh et al. found that rfhSP-D significantly reduced specific IgE, Il-4 and IL-5 levels, and peripheral eosinophilia in pulmonary mice models triggered by Der p (Singh et al. 2003). Furthermore, a study by Mahajan et al. demonstrated ex vivo that rfhSP-D increased apoptosis of activated eosinophils derived from allergic patients, without affecting eosinophils from healthy donors (Mahajan et al. 2008). Additionally, A. fumigatus allergen sensitized mice treated with SP-D (or rfhSP-D) showed a reduction in eosinophilia and eotaxin level, and consequently lower airway hyperresponsiveness (Erpenbeck et al. 2006). Thus, SP-D has a crucial role in regulating eosinophilia in allergic airway inflammation.
Allergen Uptake and Antigen Presentation Modulation
SP-D can effectively bind to allergens and enhance the uptake of allergens by alveolar macrophages, an important step in modulating the allergic airway inflammation ((Haczku 2008). Alveolar macrophages and Dendritic cells (DCs) play a crucial role in allergic pulmonary inflammation driven by T lymphocytes (Moser and Murphy 2000; Novak et al. 2004; Desch et al. 2013). DCs are accountable for inducing activation and differentiation of T lymphocyte in allergic airway reactions (Matzinger 1994). SP-D has been shown to bind to immature DCs in a carbohydrate and calcium-dependent manner (Brinker et al. 2001). Hansen et al. showed that SP-D can attenuate antigen presentation by DCs in the lung (Hansen et al. 2007). Additionally, SP-D interferes with DC maturation and TNF-α secretion in the pulmonary mice model (Hortobágyi et al. 2008). Therefore, SP-D may alter the allergen-antigen presentation by DCs to T lymphocytes which inhibits the intensification of airway allergic events (Fig. 4).
Alveolar macrophages have a crucial role in maintaining mucosal immune tolerance in the lung (Macaubas et al. 2003). Liu et al. have examined the role of SP-D on activated alveolar macrophages during allergic pulmonary inflammation in Derp-sensitised mice where it worked via blocking Nitric Oxide (NO) and TNF-α productions (Liu et al. 2005). Additionally, elevated expression of allergen-induced TLR4 in AMs of SP-D null mice has been reported (Schaub et al. 2004). These results indicated that SP-D can suppress inflammatory mediators in alveolar macrophages in allergic airway inflammation. Consistent with these observations, SP-D treatment in an allergic murine model increased levels of IL-10, IL-12, and IFN-γ in bronchoalveolar lavage fluid and reduced goblet cell hyperplasia. When alveolar macrophages were cultured in the presence of SP-D and allergen together, heightened levels of IL-10, IL-12, and IFN-γ were produced, suggesting alveolar macrophages being a target for SP-D actions against the development of airway hyperreactivity and inflammation (Takeda et al. 2003).
Modulation of Lymphocytes Mechanisms in Allergic Pulmonary Reactions
Inhalation of allergens in atopic patients induce inflammatory events involving mainly allergen-specific IgE and degranulation of mast cell and eosinophils; these events are orchestrated by B and T lymphocytes including IL-4, IL-5, and IL-13. Asthmatic patients have a high population of activated pulmonary T lymphocytes (CD4+) characterised by elevated CD25+ and CD69+ expression (Corrigan et al. 1993). Genetically manipulated mice with Th2 cytokines deficiency have shown an absence of pulmonary allergic reaction features (Hamelmann et al. 2000). Thus, SP-D null mice show persistent activation of T lymphocytes in the lung in response to exogenous antigens (Fisher et al. 2002).
SP-D inhibits T lymphocyte proliferation in response to antigenic and mitogenic activation (Borron et al. 1998; Vass et al. 2004). SP-D has been shown ex vivo to inhibit histamine release and lymphocyte proliferation in asthmatic patients induced by phytohemagglutinin (PHA) and Der p (Wang et al. 1998). Moreover, high level of CTLA4 (a negative regulator of T-lymphocytes) has been reported in the presence of SP-D in vitro and in vivo (Lin et al. 2010). These results underline the potential role SP-D in regulate T-lymphocyte activation and proliferation expression in airway allergy.
B cells appear to be convenient target for SP-D in allergic inflammation. B lymphocytes play a crucial role in allergic airway inflammation (Ghosh et al. 2012), via production of allergen-specific IgE and secretion of IL-4, which induces Th2 differentiation (De Vooght et al. 2013; Harris et al. 2000). B lymphocyte null mice treated with cockroach allergens show low levels of Th2 cytokines (Lindell et al. 2008). In addition, allergen presentation by B lymphocytes results in T cell expansion, Th2 polarization and more allergen-specific IgE synthesis (Linton et al. 2003; Crawford et al. 2006). B lymphocytes are also involved in eosinophilic pulmonary inflammation (Drake et al. 2015).
Recently, an ex vivo study by Qaseem et al. using allergic rhinitis patients’ samples revealed that rfhSP-D suppressed basophil and B, and T lymphocytes activations. rfhSP-D bound to B lymphocytes in a calcium- and carbohydrate-dependent manner through the CRD region. Furthermore, rfhSP-D hindered allergen-IgE complexes from binding to CD23 (FcεRII). rfhSP-D also blocked IgE synthesis by B cells despite the presence of IL-4, IL-21, and CD40L (Qaseem et al. 2017). These results put together the immune functions of SP-D in modulating granulocytes and lymphocytes in airway allergic reactions. CD23 is low affinity receptor for IgE mainly on B lymphocytes which is involved in allergen-specific IgE upregulation though the interaction with CD21 (complement receptor 2) (Conrad et al. 2007). The study of Qaseem et al. revealed SP-D reduced CD23 expression on B cells.
Surfactant Protein D Expression in Allergic Airway Diseases
SP-D levels are elevated in nasal tissue of patients with chronic rhinosinusitis (Ooi et al. 2007). High serum levels of SP-D have reported in allergic patients following allergen challenge (Koopmans et al. 2004). In addition, asthmatic patients showed high SP-D levels in the bronchial alveolar lavage fluid as compared with non-asthmatic controls (Cheng et al. 2000). Murine models with acute lung allergic reactions show high levels of SP-D in the pulmonary tract (Wang and Reid 2007). Thus, SP-D may serve as a biomarker for the severity of allergic immune response (Hartl and Griese 2006).
Significance and Future Direction
Research in unravelling various mechanisms of protective effects of SP-D against airway allergic diseases has highlighted the therapeutic potential of rfhSP-D. This small fragment of human SP-D seems to bind allergens, inhibit IgE-allergen interaction, suppress basophil activation, modify allergen presentation, suppress proliferation of allergen-stimulated B and T lymphocytes, induce Th2 to Th1 polarisation, and suppress IgE synthesis by primed B cells. The data so far in the field clearly point towards logical clinical trials.
References
Acharya KR, Ackerman SJ. Eosinophil granule proteins: form and function. J Biol Chem. 2014;289:17406–15.
Allinne J, Scott G, Lim WK, Birchard D, Erjefält JS, Sandén C, Ben L-H, Agrawal A, Kaur N, Kim JH. IL-33 blockade affects mediators of persistence and exacerbation in a model of chronic airway inflammation. J Allergy Clin Immunol. 2019;144:1624–37. e10
Borron PJ, Crouch EC, Lewis JF, Wright JR, Possmayer F, Fraher LJ. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161:4599–603.
Breiteneder H, Diamant Z, Eiwegger T, Fokkens WJ, Traidl-Hoffmann C, Nadeau K, O’hehir RE, O’mahony L, Pfaar O, Torres MJ. Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. Allergy. 2019;74:2293–311.
Brinker KG, Martin E, Borron P, Mostaghel E, Doyle C, Harding CV, Wright JR. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Phys Lung Cell Mol Phys. 2001;281:L1453–63.
Carreto-Binaghi LE, Taylor ML. Surfactant proteins, SP-A and SP-D, in respiratory fungal infections: their role in the inflammatory response. Respir Res. 2016;17:1–7.
Cheng G, Ueda T, Numao T, Kuroki Y, Nakajima H, Fukushima Y, Motojima S, Fukuda T. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16:831–5.
Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr Allergy Asthma Rep. 2007;7:331–7.
Corrigan C, Haczku A, Gemou-Engesaeth V, Doi S, Kikuchi Y, Takatsu K, Durham S, Kay A. CD4 T-lymphocyte activation in asthma is accompanied by increased serum concentrations of interleukin-5: effect of glucocorticoid therapy. Am Rev Respir Dis. 1993;147:540–7.
Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–506.
Crouch E, Parghi D, Kuan S, Persson A. Surfactant protein D: subcellular localization in nonciliated bronchiolar epithelial cells. Am J Phys Lung Cell Mol Phys. 1992;263:L60–6.
De Vooght V, Carlier V, Devos FC, Haenen S, Verbeken E, Nemery B, Hoet PH, Vanoirbeek JA. B-lymphocytes as key players in chemical-induced asthma. PLoS One. 2013;8:e83228.
Desch AN, Henson PM, Jakubzick CV. Pulmonary dendritic cell development and antigen acquisition. Immunol Res. 2013;55:178–86.
Drake LY, Iijima K, Hara K, Kobayashi T, Kephart GM, Kita H. B cells play key roles in th2-type airway immune responses in mice exposed to natural airborne allergens. PLoS One. 2015;10:e0121660.
Erpenbeck V, Ziegert M, Cavalet-Blanco D, Martin C, Baelder R, Glaab T, Braun A, Steinhilber W, Luettig B, Uhlig S. Surfactant protein D inhibits early airway response in Aspergillus fumigatus-sensitized mice. Clin Exp Allergy. 2006;36:930–40.
Esnault S, Kelly EA. Essential mechanisms of differential activation of eosinophils by IL-3 compared to GM-CSF and IL-5. Crit Rev Immunol. 2016;36(5):429–44.
Felton JM, Lucas CD, Rossi AG, Dransfield I. Eosinophils in the lung–modulating apoptosis and efferocytosis in airway inflammation. Front Immunol. 2014;5:302.
Fisher JH, Larson J, Cool C, Dow SW. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol. 2002;27:24–33.
Fitzgerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–41.
Ghosh S, Hoselton SA, Schuh JM. μ-chain–deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. J Immunol. 2012;189:1322–9.
Haczku A. Protective role of the lung collectins surfactant protein A and surfactant protein D in airway inflammation. J Allergy Clin Immunol. 2008;122:861–79.
Håkansson K, Lim NK, Hoppe H-J, Reid KB. Crystal structure of the trimeric α-helical coiled-coil and the three lectin domains of human lung surfactant protein D. Structure. 1999;7:255–64.
Hamelmann E, Takeda K, Haczku A, Cieslewicz G, Shultz L, Hamid Q, Xing Z, Gauldie J, Gelfand EW. Interleukin (IL)-5 but Not Immunoglobulin E Reconstitutes Airway Inflammation and Airway Hyperresponsiveness in IL-4–Deficient Mice. Am J Respir Cell Mol Biol. 2000;23:327–34.
Hansen S, Lo B, Evans K, Neophytou P, Holmskov U, Wright JR. Surfactant protein D augments bacterial association but attenuates major histocompatibility complex class II presentation of bacterial antigens. Am J Respir Cell Mol Biol. 2007;36:94–102.
Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–82.
Hartl D, Griese M. Surfactant protein D in human lung diseases. Eur J Clin Investig. 2006;36:423–35.
Hellings PW, Fokkens WJ, Bachert C, Akdis CA, Bieber T, Agache I, Bernal-Sprekelsen M, Canonica GW, Gevaert P, Joos G. Positioning the principles of precision medicine in care pathways for allergic rhinitis and chronic rhinosinusitis–A EUFOREA-ARIA-EPOS-AIRWAYS ICP statement. Allergy. 2017;72:1297–305.
Holmskov U, Malhotra R, Sim RB, Jensenius JC. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994;15:67–74.
Hortobágyi L, Kierstein S, Krytska K, Zhu X, Das AM, Poulain F, Haczku A. Surfactant protein D inhibits TNF-α production by macrophages and dendritic cells in mice. J Allergy Clin Immunol. 2008;122:521–8.
Jeffery PK, Haahtela T. Allergic rhinitis and asthma: inflammation in a one-airway condition. BMC Pulmon Med. 2006;6:S5.
Kishore U, Wang J-Y, Hoppe H-J, Reid KB. The α-helical neck region of human lung surfactant protein D is essential for the binding of the carbohydrate recognition domains to lipopolysaccharides and phospholipids. Biochem J. 1996;318:505–11.
Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–315.
Koopmans J, Van Der Zee J, Krop E, Lopuhaä C, Jansen H, Batenburg J. Serum surfactant protein D is elevated in allergic patients. Clin Exp Allergy. 2004;34:1827–33.
Kratzer B, Pickl WF. Years in review: recent progress in cellular allergology. Int Arch Allergy Immunol. 2016;169:1–12.
Kuruvilla ME, Lee FE-H, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56:219–33.
Lin K-W, Jen KY, Suarez CJ, Crouch EC, Perkins DL, Finn PW. Surfactant protein D-mediated decrease of allergen-induced inflammation is dependent upon CTLA4. J Immunol. 2010;184:6343–9.
Lindell DM, Berlin AA, Schaller MA, Lukacs NW. B cell antigen presentation promotes Th2 responses and immunopathology during chronic allergic lung disease. PLoS One. 2008;3:e3129.
Linton P-J, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–83.
Liu CF, Chen YL, Shieh CC, Yu CK, Reid K, Wang JY. Therapeutic effect of surfactant protein D in allergic inflammation of mite-sensitized mice. Clin Exp Allergy. 2005;35:515–21.
Macaubas C, Dekruyff RH, Umetsu DT. Respiratory tolerance in the protection against asthma. Curr Drug Target Inflammat Allergy. 2003;2:175–86.
Madan T, Kishore U, Shah A, Eggleton P, Strong P, Wang J-Y, Aggrawal S, Sarma P, Reid K. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–9.
Madan T, Kishore U, Singh M, Strong P, Clark H, Hussain EM, Reid KB, Sarma PU. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001;107:467–75.
Mahajan L, Madan T, Kamal N, Singh VK, Sim RB, Telang SD, Ramchand CN, Waters P, Kishore U, Sarma PU. Recombinant surfactant protein-D selectively increases apoptosis in eosinophils of allergic asthmatics and enhances uptake of apoptotic eosinophils by macrophages. Int Immunol. 2008;20:993–1007.
Malhotra R, Thiel S, Reid K, Sim R. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990;172:955–9.
Malhotra R, Haurum J, Thiel S, Sim RB. Interaction of C1q receptor with lung surfactant protein A. Eur J Immunol. 1992;22:1437–45.
Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045.
Méndez-Enríquez E, Hallgren J. Mast cells and their progenitors in allergic asthma. Front Immunol. 2019;10:821.
Moser M, Murphy KM. Dendritic cell regulation of Th 1-Th 2 development. Nat Immunol. 2000;1:199–205.
Novak N, Allam JP, Betten H, Haberstok J, Bieber T. The role of antigen presenting cells at distinct anatomic sites: they accelerate and they slow down allergies. Allergy. 2004;59:5–14.
Ooi EH, Wormald PJ, Carney AS, James CL, Tan LW. Surfactant protein d expression in chronic rhinosinusitis patients and immune responses in vitro to Aspergillus and alternaria in a nasal explant model. Laryngoscope. 2007;117:51–7.
Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72.
Pawankar R. Allergic diseases and asthma: a global public health concern and a call to action. World Allergy Organ J. 2014;7(1):12.
Persson A, Chang D, Rust K, Moxley M, Longmore W, Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989;28:6361–7.
Polverino E, Dimakou K, Hurst J, Martinez-Garcia M-A, Miravitlles M, Paggiaro P, Shteinberg M, Aliberti S, Chalmers JD. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;2018:52.
Possmayer F. Pulmonary perspective. Am Rev Respir Dis. 1988;138:990.
Qaseem AS, Singh I, Pathan AA, Layhadi JA, Parkin R, Alexandra F, Durham SR, Kishore U, Shamji MH. A recombinant fragment of human surfactant protein D suppresses basophil activation and T-helper type 2 and B-cell responses in grass pollen–induced allergic inflammation. Am J Respir Crit Care Med. 2017;196:1526–34.
Schaub B, Westlake R, He H, Arestides R, Haley K, Campo M, Velasco G, Bellou A, Hawgood S, Poulain FR. Surfactant protein D deficiency influences allergic immune responses. Clin Exp Allergy. 2004;34:1819–26.
Schleh C, Rothen-Rutishauser BM, Blank F, Lauenstein HD, Nassimi M, Krug N, Braun A, Erpenbeck VJ, Gehr P, Hohlfeld JM. Surfactant Protein D modulates allergen particle uptake and inflammatory response in a human epithelial airway model. Respir Res. 2012;13:1–10.
Singh M, Madan T, Waters P, Parida SK, Sarma PU, Kishore U. Protective effects of a recombinant fragment of human surfactant protein D in a murine model of pulmonary hypersensitivity induced by dust mite allergens. Immunol Lett. 2003;86:299–307.
Sorensen GL. Surfactant protein D in respiratory and non-respiratory diseases. Front Med. 2018;5:18.
Staubach P, Metz M, Chapman-Rothe N, Sieder C, Bräutigam M, Maurer M, Weller K. Omalizumab rapidly improves angioedema-related quality of life in adult patients with chronic spontaneous urticaria: X-ACT study data. Allergy. 2018;73:576–84.
Takeda K, Miyahara N, Rha Y-H, Taube C, Yang E-S, Joetham A, Kodama T, Balhorn AM, Dakhama A, Duez C. Surfactant protein D regulates airway function and allergic inflammation through modulation of macrophage function. Am J Respir Crit Care Med. 2003;168:783–9.
Trischler J, Lieb A, Arnold M, Schulze J, Rosewich M, Schubert R, Bottoli I, Zielen S. Omalizumab effectively protects against early and late allergic responses in asthma after 4 weeks. Allergy. 2017;72:1912–5.
Vass G, Scanlon S, Beers M, Haczku A. Surfactant protein (SP)-D suppresses antigenic and mitogenic T cell activation in vitro. J Allergy Clin Immunol. 2004;113:S252.
Von Bredow C, Hartl D, Schmid K, Schabaz F, Brack E, Reinhardt D, Griese M. Surfactant protein D regulates chemotaxis and degranulation of human eosinophils. Clin Exp Allergy. 2006;36:1566–74.
Voskamp A, Kormelink TG, Van Wijk RG, Hiemstra P, Taube C, De Jong E, Smits HH. Modulating local airway immune responses to treat allergic asthma: lessons from experimental models and human studies. Semin Immunopathol. 2020;2020:1–16.
Wang J-Y, Reid KB. The immunoregulatory roles of lung surfactant collectins SP-A, and SP-D, in allergen-induced airway inflammation. Immunobiology. 2007;212:417–25.
Wang J-Y, Kishore U, Lim B-L, Strong P, Reid KB. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106:367–73.
Wang J-Y, Shieh C-C, You P-F, Lei H-Y, Reid KB. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–8.
Winkler C, Hohlfeld JM. Surfactant and allergic airway inflammation. Swiss Med Wkly. 2013;143:w13818.
Wright JR. Immunoregulatory functions of surfactant proteins. Nat Rev Immunol. 2005;5:58–68.
Yousefi S, Sharma SK, Stojkov D, Germic N, Aeschlimann S, Ge MQ, Flayer CH, Larson ED, Redai IG, Zhang S. Oxidative damage of SP-D abolishes control of eosinophil extracellular DNA trap formation. J Leukoc Biol. 2018;104:205–14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Singh, I., Beirag, N., Kishore, U., Shamji, M.H. (2021). Surfactant Protein D: A Therapeutic Target for Allergic Airway Diseases. In: Kishore, U., Madan, T., Sim, R.B. (eds) The Collectin Protein Family and Its Multiple Biological Activities. Springer, Cham. https://doi.org/10.1007/978-3-030-67048-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-67048-1_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67047-4
Online ISBN: 978-3-030-67048-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)