Abstract
Acute Stanford type A dissection complicated by neurologic injury, mesenteric ischemia, limb malperfusion, or aortic rupture represents an especially challenging clinical scenario. This entity represents a true surgical emergency, requiring rapid determination of operative candidacy and technical strategy. Much debate remains about optimal surgical and interventional management, in particular whether central aortic repair or fenestration/peripheral reperfusion should take precedence. This chapter highlights important clinical features of complicated aortic dissection management. Technical details encompassing aortic arch replacement and elephant trunk techniques as well as visceral, renal and lower extremity reperfusion are discussed. Finally, the requirement for multidisciplinary involvement within the “aortic team” and capacity for both open surgical and endovascular techniques within major aortic centers is discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
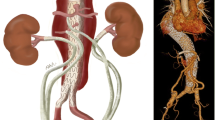
Acute aortic dissection is a rare, life-threatening condition with an incidence ranging from 5 to 10/100,000 person-years [1]. Cardiac surgeons have long recognized that this disease process is clinically challenging with a high mortality rate. Among the simplest and earliest classification systems, the Stanford paradigm proposed in 1970 by Dailey et al. established surgical repair as the standard of care for the ‘Type A’ variant involving the ascending aorta (Fig. 1) [2]. While the wealth of experience treating this entity over the subsequent five decades has reaffirmed the need for prompt surgical intervention, one central theme remains certain: not all aortic dissections are created equal. Within the cohort of patients referred for prompt surgical repair of acute type A aortic dissection (ATAAD), many potential complicating factors contribute to operative candidacy, optimal interventional strategy and morbidity/mortality risk. In particular, the presence of neurologic injury, mesenteric malperfusion, limb ischemia or shock mandate rapid decisive action. When present, these factors comprise a heterogeneous “complicated ATAAD” variant with heightened technical challenges and surgical risk. Whereas the debate around neurologic status reflects a question of ‘if’ an operation should be attempted, the presence of malperfusion or limb ischemia raises important considerations of ‘how’ it should be performed. Various institutional paradigms have been built around theories on optimal management, reflecting the lack of clear consensus across the specialty about how to optimally manage these difficult problems. The growing body of literature surrounding complicated aortic dissection management underscores the need for centralized cardiac surgery referral centers capable of interdisciplinary aortic interventions and the rapidly evolving practice of the modern aortic surgeon. This chapter presents pertinent lessons learned from institutional experience and multi-center databases to highlight branch points in the treatment algorithm for complicated ATAAD.
Pre-Operative Evaluation
Operative Candidacy
Decisions on operative candidacy for ATAAD in general are made difficult by the very poor outcomes of medical management alone. While modern mortality estimates for medical management alone are implicitly limited by selection and reporting biases, data from the International Registry of Acute Aortic Dissection (IRAD) database showed 57% mortality for patients treated medically. Surgical outcomes have steadily improved since the inception of the IRAD database with reported multi-center surgical mortality rates falling from 25% to 18% between 1995 and 2013 [3]. Findings from the IRAD database also highlight age-dependent increases in mortality risk regardless of treatment modality but consistent superiority of surgical treatment up to 80 years of age [4]. The paucity of data for patients over 80 within this cohort precludes robust determination of optimal management for octogenarians. Given these dichotomous outcomes, every ATAAD patient should be considered for operative repair. With few exceptions, our default pathway is immediate transfer directly to a hybrid operating room and preparation for central aortic repair. The presence of distal malperfusion, which may affect one or multiple organ beds, represents a central branch point in treatment algorithm for patients presenting with ATAAD.
Malperfusion
Review of computed tomography imaging to confirm the diagnosis and determine the extent of dissection is critical to operative planning. In particular, the extent of dissection into the aortic arch vessels should be assessed to determine the feasibility of axillary artery perfusion strategies. Involvement of mesenteric, renal, and iliac artery branches should also be evaluated.
An important distinction related to the complicated ATAAD is the concept of “dynamic” versus “static” obstruction of the affected branch vessel [5]. Dynamic obstruction, which results from collapse of the true lumen by the pressurized false lumen, is rectified by central aortic repair and true lumen pressure/flow restoration. Conversely, static obstruction arises from tear entry or intussusception into the branch vessel and subsequent thrombosis (Fig. 2). In this scenario, central aortic repair does not resolve flow obstruction and delays reperfusion to the affected vascular bed until secondary branch vessel intervention is performed. Careful assessment of the celiac and superior mesenteric arteries on CTA imaging is imperative to determine whether static or dynamic malperfusion is present because delayed mesenteric reperfusion may be lethal following open aortic repair. This upfront distinction is less relevant to myocardial or cerebral malperfusion, both of which can be addressed via reconstruction or bypass of the affected branch vessels as part of the central repair strategy. Intervention for static renal malperfusion, typically via endovascular stenting or dissection flap fenestration can typically be delayed until after primary surgery in a staged approach. Iliac malperfusion can be managed intraoperatively with secondary arterial cannulation of the affected extremity during primary aortic repair and secondary bypass when necessary. Thus, we regard the combination of clinical mesenteric malperfusion syndrome with appearance of static SMA or celiac obstruction on CT imaging as the exception to the “central repair first” algorithm and treat these patients with upfront endovascular stenting.
While CTA imaging is helpful in identifying affected aortic branch vessels and vascular territories, malperfusion is a clinical diagnosis. The presence of peritonitis, hematochezia or ileus on pre-operative imaging should alert the surgeon to the possibility of ongoing mesenteric ischemia. Similarly, oliguria is suggestive of renal hypoperfusion. New neurologic deficits or pulseless extremities imply neurologic and limb malperfusion states, respectively. Unsurprisingly, both the extent and location of malperfusion syndromes affect mortality. In our own series, patients with visceral malperfusion had higher unadjusted mortality (28.6%) than renal or limb ischemia (16.1% and 14.5%, respectively), and patients with multiple affected vascular beds were at further increased risk [6]. Multi-center data from the German Registry for Acute Aortic Dissection Type A (GERAADA) demonstrated stepwise increases in operative mortality with increased number of malperfused vascular beds (12.6% with no malperfusion up to 43.4% with three affected systems) [7]. Lawton et al. demonstrated through retrospective review of their single institution series that the constellation of malperfusion and severe metabolic acidosis (base deficit or −10 or more) was uniformly fatal [8].
In light of these challenges, the group at University of Michigan has set forth an upfront reperfusion strategy utilizing endovascular fenestration or SMA stenting followed by an observation period prior to central aortic repair for ATAAD patients with visceral malperfusion syndromes [9]. Yang et al. reported outcomes for 82 patients treated with this approach over two decades at Michigan; for the 47 patients (57%) who survived to open repair they observed equivalent operative mortality compared to patients without malperfusion, however 31 patients (37%) died from aortic rupture or organ failure following endovascular treatment [10]. Our institutional philosophy remains centered around prompt central aortic repair as the primary strategy to restore true lumen flow and resolve malperfusion states except when clinical gut malperfusion and static celiac or SMA obstruction are encountered. We recently reported outcomes for 82 patients presenting with ATAAD and visceral, renal or peripheral malperfusion syndromes (26.9% of the all patients undergoing surgery for ATAAD extending beyond the ascending aorta) [6]. We observed no significant difference for in-hospital mortality in patients presenting with ATAAD with malperfusion (13.4%) compared to ATAAD alone (8.5%). Unsurprisingly, we observed increased need for aortic branch interventions for the malperfused group (12.3% versus 5.7% at 10 years, Fig. 3).
Malperfusion did not confer increased mortality risk in ATAAD patients treated with central repair strategy (top) but did correlate with increased branch interventions (bottom). Reproduced with permission from [6]
Cumulatively, these studies highlight the difficulty in applying rigid treatment algorithms to this highly variable clinical entity and the importance of pre-operative evaluation for malperfusion states. Regardless of general philosophy about the best initial treatment approach for complicated ATAAD, these challenges underscore the importance of both open surgical and endovascular capabilities in major referral centers.
Neurologic Complications
Among complicating factors, neurologic injury (ranging from transient mild deficits to overt obtundation) is present in 10–15% of patients presenting with ATAAD in modern series and is associated with significantly higher mortality risk [11]. We do not withhold surgery for patients presenting with stroke or obtundation/coma. We recently reported our 10-year experience of 345 ATAAD repair cases, of which 50 (14.4%) presented with neurologic injury. While concerns exist about potential conversion of ischemic insults to hemorrhagic stroke following systemic heparinization, we observed intracranial hemorrhage in only 2 patients (4%) after aortic repair on cardiopulmonary bypass [12]. In our experience, time-to-operation did not predict neurologic or survival outcomes in ATAAD patients with stroke (Fig. 4). Conversely, Estrera et al. reported on 16 ATAAD patients treated surgically after presenting with stroke; post-operative neurologic improvement occurred only in patients who underwent repair within 10 h of symptoms [13]. Tsukube et al. analyzed outcomes in 27 ATAAD patients presenting with coma and found improved mortality (14% vs. 67%) and neurologic recovery (86% vs. 17%) in patients who underwent surgery within 5 h of symptoms [14]. Furthermore, subset analysis of the International Registry of Acute Aortic Dissection (IRAD) database has revealed return of brain function in 84.3% of patients with stroke and 78.8% of those with coma after aortic repair [15]. Collectively, these data support an immediate operative approach to resolve dynamic obstruction of aortic branch vessels for ATAAD patients presenting with neurologic injury. We therefore do not advocate for operative delays for cerebrovascular imaging or clinical observation.
Time-to-operation was a poor predictor for lack of neurologic recovery in ATAAD patients with neurologic insults. Reproduced with permission from [12]
Physical Exam
The majority of ATAAD patients are transferred to central referral centers from peripheral hospitals, necessarily producing a delay of several hours between diagnosis and operation [16]. In complicated cases, this time period may present dynamic changes in hemodynamic status, acid/base balance, and neurologic exam. Upon arrival to the operating room, a rapid neurologic assessment, abdominal exam and determination of peripheral pulses should be performed. Hemodynamic assessment must occur in parallel with preparation for general anesthesia. Hypotension or overt shock, which may reflect impending tamponade physiology or aortic rupture, are independent predictors of mortality in ATAAD patients [17].
Operative Technique
Anesthetic Considerations
Induction of general anesthesia represents a period of vulnerability for patients with ATAAD. Nearly one-fifth of patients with ATAAD present with some degree of cardiac tamponade [18]. The surgical team should be present and ready to commence the operation at the time of induction. Blood products should be available and central intravenous access obtained. Transesophageal echocardiography after anesthesia induction is useful to confirm the diagnosis of dissection, determine the degree of pericardial effusion and assess aortic valve regurgitation. As a period of circulatory arrest is uniformly necessary during distal graft anastomosis with the unclamped aorta, EEG and monitoring of cerebral oxygen saturation with near-infrared spectroscopy (NIRS) is advisable.
Cerebral Protection Strategy
The goal of central aortic repair for complicated ATAAD is to re-establish true lumen flow, resect the primary intimal tear and reverse distal malperfusion. A variety of distal repair strategies may be employed depending on the extent of dissection and clinical scenario. Regardless of whether the operative approach calls for partial or total arch replacement, a period of hypothermic circulatory arrest is required to complete the repair. Systemic cooling is a mainstay of cerebral protection, though the extent of cooling varies among surgeons. Deep hypothermia (18–20 °C) can be safely employed for arch repairs with circulatory arrest times up to 50 min without adjunct cerebral perfusion with good long-term outcomes in elective cases, though short-term results in dissection patients are less favorable [19]. When combined with selective antegrade cerebral perfusion (SACP), Algarni et al. reported that moderate hypothermia (22–28 °C) was superior to deep cooling during ATAAD repair (circulatory arrest time 25.9 ± 14.3 versus 28.9 ± 19.9 min) [20]. Leshnower et al. similarly showed that moderate hypothermia with unilateral SACP was safe for patients undergoing total arch replacement in both elective cases and dissections [21].
Similarly, individual surgeons and institutions utilize multiple variations of cerebral perfusion strategies. While SACP comprises strategies to perfuse the cerebral vessels directly via ostial cannulation of the innominate and/or carotid artery or indirectly via the axillary artery, retrograde cerebral perfusion (RCP) utilizes reversed cardiopulmonary bypass flow through the superior vena cava. Some groups advocate for RCP, which is technically simpler and faster [22], but SACP is utilized more frequently worldwide and has been associated with better long-term outcomes in some studies [23, 24]. SACP may be performed using unilateral or bilateral approaches; advocates for bilateral cannulation argue that only a minority of patients have a functionally complete Circle of Willis (as few as 28% among aortic surgery patients as assessed by transcranial doppler) [25]. Nevertheless unilateral SACP was equivalent to bilateral cannulation in a German study of over 1000 patients undergoing aortic arch repair using mild hypothermia [26]. For ATAAD cases, we use moderate hypothermia and SACP via the right axillary artery with few exceptions (extensive dissection into axillary artery or hemodynamic instability). We employ cerebral oximetry intraoperatively to monitor left-sided perfusion and use bilateral cerebral perfusion only when concern for inadequate cerebral protection arises.
Arterial Cannulation Site
The choice of cannulation sites for cardiopulmonary bypass varies among surgeons and clinical scenarios. Our primary goal is to establish antegrade perfusion for CPB, which can be done via axillary, innominate, or carotid artery graft, direct aortic true lumen cannulation over a wire with TEE guidance [27], or transapical placement of an aortic cannula across the aortic valve [28]. Reestablishing true lumen pressure, which may reduce dynamic malperfusion while on cardiopulmonary bypass, is a central benefit of these antegrade strategies. Retrograde arterial perfusion via femoral cannulation is our last resort, given uncertainty about the relative pressurization of true and false lumens and increased stroke rate compared to central cannulation [29, 30]. Nevertheless, in an unstable patient, emergency percutaneous or open femoral cannulation may be required prior to sternotomy.
Our preferred arterial cannulation method is the creation of a right axillary artery chimney graft, which can be employed in most cases. This technique requires a separate infraclavicular incision ideally prior to sternotomy and is therefore best suited for hemodynamically stable patients. Direct cannulation of the axillary artery is not advisable. The vessel lumen should be inspected for evidence of dissection prior to end-to-side anastomosis using a Dacron graft.
An adjunct arterial graft may be added into the arterial circuit to address malperfusion states. This technique is particularly useful to perfuse an ischemic limb due to proximal iliac occlusion or provide unilateral cerebral perfusion distal to a proximally obstructed carotid takeoff [31]. Antegrade placement of a superficial femoral artery cannula may also be considered for distal perfusion of malperfused lower extremities [32].
Exposure and Dissection
Standard median sternotomy and pericardiotomy are performed, frequently releasing a bloody pericardial effusion which can improve hemodynamics in unstable patients. Following systemic heparinization, central venous cannulation is achieved via the right atrium and a retrograde cardioplegia catheter is directed into the coronary sinus. Dissection of the aorta can be performed prior to commencing cardiopulmonary bypass to minimize time on pump. The arch branches are dissected to achieve circumferential control. The axillary chimney graft is then connected to the bypass circuit with standard connectors and cardiopulmonary bypass commenced. Left ventricular vent placement via the right superior pulmonary vein is advisable given the likelihood of significant aortic regurgitation. Systemic cooling is then undertaken; we cool to a core temperature of 28 °C for limited arch operations and 24 °C if the need for total arch replacement is anticipated. Retrograde cardioplegia is administered via the coronary sinus and the distal ascending aorta is cross-clamped. Direct handheld cardioplegia administration should be used cautiously if the coronary ostia are involved with the proximal extent of dissection.
Limited Root Repair or Aortic Root Replacement
Following transection, the aorta is then resected down to one centimeter above the aortic valve commissures. Stay sutures above the commissures assist with exposure and evaluation of the aortic root and valve leaflets. Aortic valve resuspension and primary re-approximation of dissected aortic layers represents the standard proximal repair strategy in uncomplicated dissection. Frequently this can be completed while cooling prior to distal repair. Evaluation of the coronary ostia for involvement by the proximal extent of dissection requires close attention.
Decision-making about the extent of proximal repair must be predicated on maximizing each patient’s chance of survival. While young patients with uncomplicated ATAAD may tolerate longer bypass runs for root replacement, a limited root operation to minimize bypass and operative times may be more appropriate in elderly patients or those with malperfusion syndromes. We performed retrospective review of 293 patients who underwent limited root repair or full root replacement for ATAAD [33]. While there was difference in mortality between groups (Fig. 5), patients who had limited root operations were more likely to require aortic root or aortic valve reoperation (11.8% vs 0%). A limited repair strategy may therefore be most appropriate for surgeons with limited experience performing aortic root replacements or in the setting of malperfusion syndromes with the understanding that reoperation may be required.
Patients who underwent full aortic root replacement had equivalent mortality compared to those undergoing limited root repair. Reproduced with permission from [33]
In some cases, performing a full aortic root replacement is appropriate or even necessary. Aortic rupture, valve degeneration, commissural destruction, root aneurysm, poor tissue integrity or known/suspected connective tissue disorder are indications for aortic root replacement during the index operation. We generally utilize a composite valve graft (CVG) prosthesis with a patient-appropriate selection of mechanical or biologic valve. Valve-sparing aortic root replacement using the reimplantation (David V) technique may be appropriate for young patients, particularly those with connective tissue disorders, but should be used only by surgeons with substantial experience in an elective setting [34]. When full aortic root replacement (Bentall technique) is undertaken, buttons of coronary ostial tissue are fashioned for eventual reimplantation. The aortic valve leaflets are resected, annular mattress sutures are placed circumferentially, passed through the CVG prosthesis and tied down. The graft is then incised at the appropriate level for left coronary button reimplantation with end-to-side technique. We complete the graft-to-graft anastomosis prior to implanting the right coronary button to ensure appropriate height with the aortic graft in final position.
Aortic Arch Operations
Distal aortic repair commences once the desired systemic cooling threshold is reached. Adjunct cerebral protection measures such as cranial topical cooling with ice and administration of mannitol and furosemide may be used. Cardiopulmonary bypass flow is reduced to 10 mL/kg/min, the innominate and left common carotid arteries are clamped and the aortic cross-clamp is removed. Indicators of inadequate left-sided protection during SACP include discordant tympanic membrane temperatures or cerebral oxygen saturation reduction greater than 15%, which should prompt maneuvers to improve perfusion such as increasing SACP flow or transfusing to increase hematocrit. If necessary and deemed safe, a small retrograde cannula can be placed directly into the carotid artery orifice to provide bilateral cerebral perfusion.
Once adequate cerebral protection is ensured the primary intimal tear can be resected entirely. Frequently the tear can be entirely resected via excision the lesser curvature of the aortic arch and graft replacement using an extended “peninsula-style” repair (Fig. 6). The dissected layers of the distal aorta must be reapproximated to obliterate flow into the false lumen. Total arch replacement is indicated if the primary intimal tear is located within the greater curvature, significant arch aneurysm is encountered, distal arch rupture, and for patients with connective tissue disorders. In some cases, the intimal tear may extend into or originate in the descending thoracic aorta (the “retrograde type A” variant). To completely treat the primary intimal tear in this scenario, especially in the setting of malperfusion, total arch replacement with frozen elephant trunk (FET) distal extension is indicated. An invaginated graft is placed into the descending thoracic aortic true lumen and end-to-end anastomosis is completed in running fashion. The proximal, branched graft portion is then withdrawn, leaving a 5 cm cuff of graft distally. A covered 10 cm thoracic endoprosthesis can then be deployed in an antegrade fashion distally to “freeze” the surgical graft in place. We deploy these devices antegrade over a wire introduced from the femoral artery using intravascular ultrasound (IVUS) to confirm true lumen landing distally. Newer generation off-the-shelf devices with combined multi-branch arch graft and endoprostheses may also be employed for this indication. Minimizing the distal length of the endoprosthesis is critical to prevent ischemic injury to the spinal cord during FET reconstruction. This technique has good aortic outcomes with acceptable neurologic complications in experienced hands and with spinal cord protective measures [35, 36].
Aortic arch reconstruction in ATAAD. (a) Extended ‘peninsula’ style hemi-arch repair includes resection of the lesser curvature to the level of the left subclavian. (b) Total arch with frozen elephant trunk (FET, left) comprises complete arch replacement with Dacron graft and antegrade stent-graft placement into the proximal descending aorta
After distal anastomosis, a variety of strategies for arch branch anastomosis may be employed. Typically, a multi-branch graft is used to anastomose each branch individually. While an “island” of arch tissue may be fashioned and reimplanted as a single anastomosis, we do not recommend this technique as it can be difficult to obtain hemostasis of bleeding from the posterior portion. After de-airing the graft, full cardiopulmonary bypass flow is resumed, ending hypothermic circulatory arrest. Systemic re-warming, proximal repair and graft-to-graft anastomosis are then completed.
Addressing Malperfusion
Coronary malperfusion due to involvement of the coronary ostia must be recognized early to prevent acute heart failure associated with high mortality. Coronary vessels may be affected by static or dynamic malperfusion or in severe cases completely avulsed or “sheared off”. If the extent of dissection prohibits administration of handheld antegrade cardioplegia, we perform upfront coronary bypass prior to aortic repair to ensure adequate myocardial protection can be maintained.
Following central repair, attention is redirected to vascular beds with preoperative malperfusion. If abdominal distention is encountered, exploratory laparotomy should be considered to assess bowel viability. Similarly, peripheral pulses should be re-examined. Malperfused lower extremities should be closely monitored for swelling and compartment syndrome which may manifest following reperfusion.
Completion aortography may be considered to confirm mesenteric perfusion post-repair. Endovascular intervention (branch stenting, thoracic endograft distal extension, or flap fenestration) may then be performed as necessary. We do not routinely perform aortography following repair unless a specific concern persists.
Post-Operative Care
Aggressive resuscitation during and following central aortic repair is critical to reverse metabolic derangement resulting from malperfusion and cardiopulmonary bypass. Platelets and fresh frozen plasma are frequently required to address coagulopathy. Active warming may be required to maintain normothermia. Metabolic acidosis and elevated serum lactate are frequently present on arrival to the ICU and should be monitored for correction with ongoing volume resuscitation. Persistent metabolic acidosis should prompt re-evaluation for ongoing malperfusion or unrecognized bowel ischemia. A baseline neurologic status should be obtained within the first few hours in ICU; persistent obtundation or change in neurologic exam should prompt immediate head CT.
Renal malperfusion due to static obstruction may persist following central repair. Oliguria and rising serum creatinine from this entity is difficult to distinguish from more typical acute kidney injury after cardiopulmonary bypass and transient low-flow states. Devoted renal doppler ultrasound should be obtained in this setting. Delayed renal artery stenting can be undertaken following initial resuscitation in an attempt to salvage renal function.
Final Remarks
Complicated ATAAD represents a unique clinical challenge for aortic surgeons. The heterogeneous spectrum of presentation precludes the application of a “one size fits all” strategy. Despite specialized care at tertiary referral hospitals, surgical mortality remains frustratingly high. Effective management requires a broad range of skills, sound decision-making and institutional capability to perform both traditional open surgery and hybrid endovascular interventions. Care of the complicated ATAAD patient is frequently multidisciplinary, encompassing multiple consulting specialties to manage complications of malperfusion. Meticulous clinical decision-making is a central theme in the determination of operative candidacy, strategy, and extent of aortic repair in these patients, decisions which may mean the difference between life and death. Finally, ATAAD patients require lifelong surveillance with cross-sectional imaging for progressive aneurysmal dilation of the distal dissected aorta. We strongly advocate for institution-based aortic teams to manage surveillance and secondary interventions for the residual aorta. The “aortic team” consisting of cardiac and vascular surgeons and devoted cardiovascular Radiology specialists is a critical asset in the longitudinal management of this complex patient subset.
References
Mody PS, Wang Y, Geirsson A, Kim N, Desai MM, Gupta A, et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among medicare beneficiaries in the United States, 2000–2011. Circ Cardiovasc Qual Outcomes. 2014 Nov;7(6):920–8.
Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970 Sep;10(3):237–47.
Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015 Jul 28;66(4):350–8.
Trimarchi S, Eagle KA, Nienaber CA, Rampoldi V, Jonker FHW, Vincentiis CD, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010 Oct 1;140(4):784–9.
Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazanjian SN, et al. The dissected aorta: percutaneous treatment of ischemic complications – principles and results. J Vasc Interv Radiol. 1997 Aug;8(4):605–25.
Chiu P, Tsou S, Goldstone AB, Louie M, Woo YJ, Fischbein MP. Immediate operation for acute type A aortic dissection complicated by visceral or peripheral malperfusion. J Thorac Cardiovasc Surg. 2018;156(1):18–24.e3.
Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, et al. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol. 2015 Jun 23;65(24):2628–35.
Lawton JS, Moon MR, Liu J, Koerner DJ, Kulshrestha K, Damiano RJ, et al. The profound impact of combined severe acidosis and malperfusion on operative mortality in the surgical treatment of type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155(3):897–904.
Patel HJ, Williams DM, Dasika NL, Suzuki Y, Deeb GM. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg 2008 Jun;135(6):1288–1295; discussion 1295–1296.
Yang B, Norton EL, Rosati CM, Wu X, Kim KM, Khaja MS, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: a 20-year experience. J Thorac Cardiovasc Surg. 2019;158(3):675–687.e4.
Bossone E, Corteville DC, Harris KM, Suzuki T, Fattori R, Hutchison S, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013 Sep 10;128(11_suppl_1):S175–9.
Chiu P, Rotto TJ, Goldstone AB, Whisenant JB, Woo YJ, Fischbein MP. Time-to-operation does not predict outcome in acute type A aortic dissection complicated by neurologic injury at presentation. J Thorac Cardiovasc Surg. 2019;158(3):665–72.
Estrera AL, Garami Z, Miller CC, Porat EE, Achouh PE, Dhareshwar J, et al. Acute type A aortic dissection complicated by stroke: Can immediate repair be performed safely? J Thorac Cardiovasc Surg. 2006 Dec 1;132(6):1404–8.
Tsukube T, Haraguchi T, Okada Y, Matsukawa R, Kozawa S, Ogawa K, et al. Long-term outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. J Thorac Cardiovasc Surg 2014 Sep;148(3):1013–1018; discussion 1018–1019.
Di Eusanio M, Patel HJ, Nienaber CA, Montgomery DM, Korach A, Sundt TM, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013 Mar;145(3 Suppl):S213–221.e1.
Harris KM, Strauss CE, Eagle KA, Hirsch AT, Isselbacher EM, Tsai TT, et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011 Nov 1;124(18):1911–8.
Bossone E, Pyeritz RE, Braverman AC, Peterson MD, Ehrlich M, O’Gara P, et al. Shock complicating type A acute aortic dissection: Clinical correlates, management, and outcomes. Am Heart J. 2016;176:93–9.
Gilon D, Mehta RH, Oh JK, Januzzi JL, Bossone E, Cooper JV, et al. Characteristics and in-hospital outcomes of patients with cardiac tamponade complicating type A acute aortic dissection. Am J Cardiol. 2009 Apr 1;103(7):1029–31.
Damberg A, Carino D, Charilaou P, Peterss S, Tranquilli M, Ziganshin BA, et al. Favorable late survival after aortic surgery under straight deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2017;154(6):1831–1839.e1.
Algarni KD, Yanagawa B, Rao V, Yau TM. Profound hypothermia compared with moderate hypothermia in repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2014 Dec;148(6):2888–94.
Leshnower BG, Kilgo PD, Chen EP. Total arch replacement using moderate hypothermic circulatory arrest and unilateral selective antegrade cerebral perfusion. J Thorac Cardiovasc Surg. 2014 May;147(5):1488–92.
Rylski B, Bavaria JE, Milewski RK, Vallabhajosyula P, Moser W, Kremens E, et al. Long-term results of neomedia sinus valsalva repair in 489 patients with type A aortic dissection. Ann Thorac Surg 2014 Aug;98(2):582–588; discussion 588–589.
Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M, et al. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. 2016 Feb;49(2):e44–52.
El-Hamamsy I, Ouzounian M, Demers P, McClure S, Hassan A, Dagenais F, et al. State-of-the-art surgical management of acute type A aortic dissection. Can J Cardiol. 2016 Jan;32(1):100–9.
Smith T, Jafrancesco G, Surace G, Morshuis WJ, Tromp SC, Heijmen RH. A functional assessment of the circle of Willis before aortic arch surgery using transcranial Doppler. J Thorac Cardiovasc Surg. 2019;158(5):1298–304.
Zierer A, El-Sayed Ahmad A, Papadopoulos N, Moritz A, Diegeler A, Urbanski PP. Selective antegrade cerebral perfusion and mild (28°C–30°C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J Thorac Cardiovasc Surg. 2012 Nov;144(5):1042–9.
Frederick JR, Yang E, Trubelja A, Desai ND, Szeto WY, Pochettino A, et al. Ascending aortic cannulation in acute type a dissection repair. Ann Thorac Surg. 2013 May;95(5):1808–11.
Wada S, Yamamoto S, Honda J, Hiramoto A, Wada H, Hosoda Y. Transapical aortic cannulation for cardiopulmonary bypass in type A aortic dissection operations. J Thorac Cardiovasc Surg. 2006 Aug;132(2):369–72.
Benedetto U, Raja SG, Amrani M, Pepper JR, Zeinah M, Tonelli E, et al. The impact of arterial cannulation strategy on operative outcomes in aortic surgery: evidence from a comprehensive meta-analysis of comparative studies on 4476 patients. J Thorac Cardiovasc Surg. 2014 Dec;148(6):2936–2943.e1–4.
Etz CD, von Aspern K, da Rocha E Silva J, Girrbach FF, Leontyev S, Luehr M, et al. Impact of perfusion strategy on outcome after repair for acute type a aortic dissection. Ann Thorac Surg. 2014 Jan;97(1):78–85.
Rylski B, Urbanski PP, Siepe M, Beyersdorf F, Bachet J, Gleason TG, et al. Operative techniques in patients with type A dissection complicated by cerebral malperfusion. Eur J Cardiothorac Surg. 2014 Aug;46(2):156–66.
Howe KL, Harlock J, Parry D. Management of lower extremity ischaemia during type A dissection repair. EJVES Short Rep. 2018;39:44–6.
Chiu P, Trojan J, Tsou S, Goldstone AB, Woo YJ, Fischbein M. Limited root repair in acute type A aortic dissection is safe but results in increased risk of reoperation. J Thorac Cardiovasc Surg. 2018 Jan;155(1):1–7.e1.
Rosenblum JM, Leshnower BG, Moon RC, Lasanajak Y, Binongo J, McPherson L, et al. Durability and safety of David V valve-sparing root replacement in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2019;157(1):14–23.e1.
Poon SS, Tian DH, Yan T, Harrington D, Nawaytou O, Kuduvalli M, et al. Frozen elephant trunk does not increase incidence of paraplegia in patients with acute type A aortic dissection. J Thorac Cardiovasc Surg. 2020;159(4):1189–1196.e1.
Hohri Y, Yamasaki T, Matsuzaki Y, Hiramatsu T. Early and mid-term outcome of frozen elephant trunk using spinal cord protective perfusion strategy for acute type A aortic dissection. Gen Thorac Cardiovasc Surg. 2020 Mar 9;68(10):1119–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pedroza, A.J., Fischbein, M.P. (2021). Management of Complicated Acute Type A Aortic Dissection: The Stanford Approach. In: Sellke, F.W., Coselli, J.S., Sundt, T.M., Bavaria, J.E., Sodha, N.R. (eds) Aortic Dissection and Acute Aortic Syndromes. Springer, Cham. https://doi.org/10.1007/978-3-030-66668-2_26
Download citation
DOI: https://doi.org/10.1007/978-3-030-66668-2_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66667-5
Online ISBN: 978-3-030-66668-2
eBook Packages: MedicineMedicine (R0)