Abstract
Intracranial hypertension (IH) is a common and significant secondary insult in patients with acute neurologic injury. Clinical signs in patients with altered level of consciousness poorly predict IH, except in advanced stages. Head computed tomography (CT) scans are the initial examination in these patients since it can detect structural causes for IH and provides surgery indication. Optic nerve sheath ultrasound has good accuracy for detecting IH and can be useful as a triage tool in some settings.
While it is possible to treat IH with noninvasive tools, invasive monitoring of intracranial pressure (ICP) is the gold standard. A threshold for treatment of IH above 22 mmHg has been established in the Brain Trauma Foundation Guidelines. The two fundamental principles to treat IH are surgical treatment if indicated as soon as possible and to correct anatomic and physiologic derangements that worsen cerebral edema. To correct these anatomic factors, the head of the bed should be elevated and attention should be paid to neck position, avoiding jugular compression. The main goals of physiological variables are normal arterial oxygen and carbon dioxide partial pressures, mean cerebral perfusion pressure 60–70 mmHg (or individualized according to IH response), avoiding hyperthermia, anemia, and hypoglycemia.
If IH persists after these fundamental principles were applicable, sequential stepwise therapies are suggested. They are generally ordered according to the magnitude of collateral effects since they can be lifesaving but none of them are innocuous: cerebrospinal fluid drainage, optimizing sedation, hyperosmolar therapy, induced hypocapnia, metabolic suppression with barbiturates, mild hypothermia, and decompressive craniectomy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intracranial hypertension
- Acute neurologic injury
- Optic nerve sheath ultrasound
- Secondary brain insult
- Cerebral perfusion pressure
- Cerebrospinal fluid drainage
- Analgesia
- Propofol
- Hyperosmolar therapy
- Induced hypocapnia
- Barbiturates
- Mild hypothermia
- Decompressive craniectomy
1 Introduction
The concept of a pressure–volume relationship inside the skull and, therefore, the understanding of how this relationship affects the intracranial pressure (ICP) was first mentioned in 1783, by Alexander Monro, and supported by Monro’s student George Kellie in 1824. The Monro-Kellie doctrine [1] states that the human skull is a rigid compartment with a fixed volume and with three main components within: brain parenchyma, blood, and cerebrospinal fluid (CSF). These three components are in a dynamic equilibrium, and an increase in any of them or the appearance of any space-occupying lesion is followed by a decrease in the other components without any significant increase in ICP [2]. However, this equilibrium mechanism is maintained until a threshold is reached where the ICP increases exponentially with any increase of volume inside the skull [1, 3].
A dynamic, non-linear relationship exists between the intracranial volume and ICP (Fig. 12.1). Initially, in this curve, in the segment I, an excellent compensatory mechanism can prevent significant increases in ICP despite rising in intracranial volume. In segment II, a poor autoregulatory mechanism is present, when buffering capabilities are being exceeded, with a near-linear ICP response to the increases in volume, until a critical threshold (segment III) is reached, when autoregulation fails [4].
Intracranial hypertension (IH), sustained or not, is harmful leading to secondary brain injury, herniation, and possible brain death if untreated [5]. Therefore, aggressive treatment should be provided as soon as possible. For many years the cutoff for diagnosing IH has been debated. The last edition of Brain Trauma Foundation guidelines established that sustained ICP > 22 mmHg diagnosis IH [4]. However, recent evidence suggests that more important than a single cutoff value is the ICP dose [5, 6].
Intracranial pressure dose represents the time spent over the threshold and its intensity (area under the curve ICP x Time), and together with cerebral perfusion pressure (CPP) should be considered when treating a neurocritical patient [7, 8].
The earliest reports of ICP monitoring are from Guillaume and Janny in 1951, and Lundberg in 1960, in which ICP estimation was done from backpressure of CSF in a tube manometry. More accurate and safer methods for continuous monitoring of the ICP are available today [3].
Intracranial pressure monitoring offers more than a continuous pressure value. It allows continuous monitoring of the cerebral perfusion pressure (CPP), which is equal to mean arterial pressure (MAP) – ICP. Also, it will enable evaluation of the pressure-reactivity index, which estimates cerebrovascular reaction and ICP waveform. Since IH is a heterogeneous and complex disease, the more information is gathered from the ICP monitoring more tailored and assertive can the neurocritical care management be [4].
Despite the strong correlation between IH and mortality, other factors such as metabolic disarrangements might be present before any significant ICP elevation. This understanding leads to the concept of multimodal neuromonitoring strategies, considering other variables, together with ICP values, to guide the clinical decision [4, 8].
2 Clinical Presentation
Clinical symptoms of IH are diverse and depend on the underlying etiology and time of installation (acute or chronic), being more clinically evident in acute cases [4, 9]. Most of the time, clinical manifestations are related to global or hemispheric dysfunction rather than focal findings [4].
These symptoms include headache, decreased level of consciousness, nausea, vomiting, diplopia, and sixth cranial nerve palsy. The Cushing’s triad (bradycardia, hypertension, and irregular breathing or apnea) with all its three components is an uncommon feature occurring most frequently with high and acute increases in ICP and late phases of intracranial hypertension (herniation syndrome and brain dead) [3, 4, 9].
Although intracranial hypertension is a global phenomenon most of the time, in some situations, it might be a compartmentalized syndrome due to intracranial anatomical landmarks, such as falx and tentorium cerebelli. In these situations, herniation can occur from those points of higher to points of lower pressure [9]. Common herniation syndromes are subfalcine, uncal, and foraminal.
Transtentorial or uncal herniation is the prototype of these syndromes and is marked by acute loss of consciousness, ipsilateral pupillary dilatation, and contralateral hemiparesis. These clinical manifestations are related to compression of ascending arousal pathways, oculomotor nerve, and corticospinal tract, respectively. Ipsilateral cerebral infarction can be present due to occlusion of the posterior cerebral artery [9].
3 Physical Examination
Unfortunately, physical examination alone has low sensitivity for the diagnosis of IH [9]. A decreased level of consciousness has very low specificity. It may be present in many other clinical conditions, especially in the setting of a prior known neurologic disease, such as traumatic brain injury (TBI). Abnormal motor posture (Glasgow coma scale motor score ≤3) has both low sensitivity and low specificity. Pupillary dilation has low sensitivity, but it is more specific, although it is a late sign of elevated ICP, as well as are other clinical signs seen in herniation syndromes.
4 Imaging
Computed tomography (CT) is the most widely available imaging method for the evaluation of neurocritical patients and is extremely useful when IH is suspected. It allows the diagnosis of underlying pathologies and is helpful for non-invasive evaluation of the presence of IH. A recent meta-analysis showed that compression or effacement of the basal cisterns (Fig. 12.2) has good sensitivity (85.9%) but low specificity (61%) for the diagnosis of elevated ICP. The presence of any midline shift also has good sensitivity, especially if 5–10 mm or >10 mm [9].
The optic nerve sheath diameter (ONSD) measurement using ultrasound is a reproducible easy to perform non-invasive method to detect elevated ICP with a steep learning curve [10]. It evaluates the retrobulbar segment of the optic nerve, which is surrounded by subarachnoid space, which distends with increased ICP. There are different cut-offs described in the literature that provide different sensitivity and specificity. There is no consensus about the optimal threshold to detect elevated ICP, with values <5 mm probably not associated with increased ICP and values >6 mm highly associated with increased ICP [11]. Its dynamic variation, however, might be of limited interpretation given the nerve sheath might remain enlarged for a variable and undetermined period after an episode of increased ICP. Another concern is the variable response to therapeutic interventions. For this reason, it has a limited role in patients with known intracranial hypertension. However, it is useful as a fast screening method after neurological worsening [10].
Transcranial doppler indices (pulsatility index and methods derived from flow velocity) have low accuracy in estimating ICP and are not recommended in the diagnosis of intracranial hypertension [12, 13].
Invasive ICP monitoring should be considered in patients at risk for intracranial hypertension [4, 14]. However, there are possible complications for this invasive procedure, and there are settings where it may not be readily available. In these scenarios, physicians should gather information from physical examination, head CT, and non-invasive methods to diagnosis increased ICP. Individually, none of these are sufficiently accurate in diagnosing IH when compared to invasive monitoring. Physicians should use a combination of these findings, along with pre-test probability, to interpret them adequately.
5 Etiology
As discussed, the clinical presentation of IH is variable and depends on its etiology and time of installation [4, 9]. The initial symptoms and clinical findings such as headache, nausea, vomiting, and diplopia are unspecific, and other diagnostics should be considered. These initial symptoms may be accompanied by clinical findings, which may suggest a specific etiology. Some clinical pictures may be archetypal. For instance, acute onset of headache, nausea, vomiting, fever, and neck stiffness is suggestive of meningitis.
Therefore, it is essential to know that the initial presentation of a patient with IH may be unspecific, and any suspicion should guide further investigation. The physician must go through two different steps during the diagnostic process. The first one is the diagnosis of IH, and the second is its etiology (Table 12.1). At initial presentation, the differential diagnosis of headache, nausea, and vomiting are numerous.
When facing a patient with likely IH in a more advanced stage, it is essential to know that there is a common pathway for all the pathologic processes leading to an impaired level of consciousness. We can divide the diagnosis of the reduced level of consciousness into two groups: those with focal neurologic signs and those without (Table 12.2).
The first group (with focal neurologic signs) of differential diagnosis comprises central nervous system structural lesions and some exogenous intoxications. In this situation, an urgent head CT scan is paramount for diagnosis. The second group (without focal neurologic signs) comprises toxic metabolic causes, including most of the exogenous intoxications, inflammatory, and infectious diseases. Patients in this group are more likely to have systemic conditions, so a thorough clinical history and physical examination are paramount. Also, in this group, a head CT scan and an electroencephalogram (EEG) are helpful, since mass effect lesions (e.g., diffuse cerebral edema) or EEG abnormalities may also be the cause or symptoms.
6 Treatment
6.1 Initial Treatment
Despite the myriad of therapeutic interventions for treating IH, success is heavily dependent on the two cornerstones [4]: early removal of surgically treatable lesions and early and continuous correction of anatomic and physiologic derangements that can worsen cerebral edema.
When IH is documented or suspected (based on clinical–radiological findings), surgically treatable space-occupying lesions should be removed as soon as possible, and any delays increase the secondary injury to the brain parenchyma.
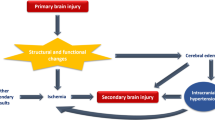
Correction of underlying anatomic and physiologic disorders that can worsen both cerebral edema and secondary injury is of pivotal importance. Secondary brain injury is any factor that occurs immediately after the primary injury and worse neurological outcomes [15], for example, hypoxia, hyperthermia, and hypoglycemia. Avoiding secondary injury is also one of the cornerstones of the care of neurocritical patients, and its prognostic impact is independent of its effect on intracranial pressure. For example, in patients with traumatic brain injury, a systolic blood pressure <90 mmHg increases unfavorable outcomes by an odds ratio of 3.5 [16].
Intracranial hypertension is often refractory when these physiologic alterations are not under close control. Additionally, specific treatments for refractory intracranial hypertension, such as hyperventilation, barbiturates, induced hypothermia, or decompressive craniectomy, have significant side effects [17], however, their benefit might not be apparent for all patients.
It is essential to make sure all these initial targets (Table 12.3) are reached as soon as possible in all patients with suspected IH before escalating to a sequential stepwise approach.
The goal in this initial approach is to avoid any factors that increase the likelihood of secondary brain injury or might affect either the ICP or the CPP [18]. Known risk factors for secondary brain injury such as hypoxia, hypoglycemia, hyperthermia, hypotension, and decreased CPP must be corrected. Therefore, one should aim to achieve the values summarized in Table 12.3 [4]. For patients with invasive ICP monitoring, the CPP is 60–70 mmHg, however for patients with suspected IH without invasive ICP monitoring, the mean arterial pressure target (MAP) target is 90–100 mmHg. For patients with invasive ICP monitoring is possible in some situations to tailor the CPP target according to the intracranial pressure response, with the use of cerebral autoregulation surrogates, such as the pressure reactivity index (for details on this topic, see Copplestone and Welbourne, 2018).
However, if the ICP persists >22 mmHg after these initial measures, we suggest an orderly sequential stepwise approach to guide treatment. We choose to divide this approach in conventional treatment and options for refractory intracranial hypertension based on the scope of evidence available for each therapy.
6.2 Conventional Treatment
The interventions described below must be performed in a stepwise approach following the order: cerebrospinal fluid drainage (when applicable), optimizing sedation, hyperosmolar therapy, neuromuscular blockade, and transitory induced hypocapnia [3].
6.3 Cerebrospinal Fluid (CSF) Drainage
The CSF drainage can only be performed in patients with external ventricular drainage (EVD) placed, which is most of the time inserted together with an ICP monitor [4]. One should not drain CSF through a lumbar puncture (except in specific situations), which might lead to downward herniation. The EVD can be used as a therapeutic and monitoring tool. In situations of increased ICP, the drainage of small volumes of CSF can lead to a significant decrease in ICP since most of these patients are in the segment III of the intracranial pressure–volume curve (Fig. 12.1).
There are some concerns that continuous CSF drainage trough the EVD might lead to ventricular collapse and EVD obstruction (which might lead to an increase in ICP), however, a continuous drainage strategy is recommended by the Brain Trauma Foundation since it is an efficient way to reduce ICP in trauma patients. Therefore, proper functioning of the EVD must be guaranteed, and additional drainage might be necessary in situations of persistent increase in ICP.
6.4 Sedation
Appropriate analgesia should be a priority in acute neurologic ill patients. Once pain is resolved, a higher dose of analgesics usually does not confer an additional benefit on controlling the ICP [4, 19].
The use of sedatives with the goal of deep sedation (burst suppression) does not confer any benefits in the long-term prognosis of the acute neurologic ill patient without IH. Furthermore, heavy sedation lengthens the duration of mechanical ventilation, which increases the risk of adverse events such as nosocomial infections. Also, it might reduce cerebral perfusion pressure and decrease the sensitivity of clinical neurologic monitoring.
However, if IH persists after the initial treatment (Table 12.3) and cerebrospinal fluid drainage if there is a ventricular drain in place, sedation and mechanical ventilation are recommended to decrease the ICP. Propofol or midazolam are the most used drugs; both act on the gamma-amino-butyric acid (GABA) receptor and decreases brain metabolism. Sedation should be titrated to avoid agitation and consequently increase in cerebral oxygen consumption and to control the IH, but not necessarily to induce coma in all acute neurologic ill patients. We suggest using the least amount of sedation to achieve two practical goals in patients with IH: Richmond Agitation-Sedation Scale (RASS) <1 and intracranial pressure <22 mmHg.
In some cases, higher sedatives doses are needed to accomplish both targets, especially to control the ICP, leading in these patients to more profound sedation (sometimes RASS – 5).
We suggest using propofol (maximum dose 5 mg/kg/h) as first-line sedative due to its short half live, allowing constant neurologic reevaluation and titration. Midazolam is an option in patients at risk for the propofol-infusion syndrome (young male patients and/or use of propofol for >72 h), patients with severe systolic dysfunction, or needing high-dose vasopressors.
6.5 Hyperosmolar Therapy
Mannitol and hypertonic saline are the next steps if IH persists despite optimized sedation (RASS – 5) and pain control [20, 21]. While both are effective in reducing the ICP, hypertonic saline has less frequent serious adverse events. Mannitol-induced polyuria can reduce cerebral perfusion pressure and titrate the mannitol dosing is challenging since the osmolar gap is not routinely measured in the majority of intensive care units, also using mannitol in oliguric patients can cause acute pulmonary edema. We recommend infusions of hypertonic saline if ICP persists >22 mmHg with maximal dosing titrated by serum sodium targeting serum sodium 140–155 mEq/L. There is no consensus on which concentration is better. Therefore, different concentrations (3%, 7.5%, 20%) can be used. We suggest 0.5 mL/kg sodium chloride 20% in 10 minutes in cases of cerebral herniation.
6.6 Neuromuscular Blockade
In selected cases of IH, especially in patients with uncontrolled shivering despite the usual treatment (extremities warming, opioids, magnesium, and others), a trial of neuromuscular blockade might help to decrease the ICP. Also, the use of neuromuscular blockade might lower the intraabdominal pressure and facilitate ventilation and CO2 control. We suggest a trial of cisatracurium 0.2 μg/kg IV push. If a sustained decrease in ICP is observed, continuous infusion until control of other factors associated with increased ICP might be beneficial [22].
6.7 Induced Hypocapnia
In situations of acute elevation of ICP or herniation syndrome, controlled hypocapnia targeting an arterial CO2 between 30 and 35 mmHg decreases the ICP allowing a safe time to adjust other therapies and image evaluation. However, this hypocapnia must be controlled in both its intensity and duration, since hypocapnia leads to cerebral vasoconstriction and consequently decreases cerebral blood flow [23].
6.8 Imaging
In situations of acute unexplained neurologic deterioration (decrease in GCS by two or more points, new anisocoria, herniation syndrome, or any other significant new neurologic alteration) or unexplained increase in ICP, it is advisable to consider obtaining new image of the brain, to exclude surgical treated causes alongside with acute treatment to control ICP.
The series of interventions mentioned are called first and second tiers therapy and are described in Algorithms 1 and 2 for patients with and without ICP monitors, respectively.
6.9 Treatment Options – Refractory Measures
For patients with refractory ICP elevation despite first and second tiers describe above, rescue therapy strategies such as decompressive craniectomy, barbiturates, or mild hypothermia might be useful.
6.10 Barbiturates
Barbiturates (thiopental and pentobarbital) have potent effects on cerebral metabolic demand and cerebral blood flow, inducing a state of cerebral metabolic suppression [24]. These drugs have serious side effects, like hypotension, long half-life (jeopardizing neurological examination in the meanwhile, eventually even delaying brain death diagnosis), paralytic ileus, potassium shift disturbances, and increased risk of pneumonia. Loading doses of three are followed by 1–4 mg/kg/h. We suggest administration in 10 minutes and close hemodynamic during loading dose. Patients should be monitored with continuous electroencephalography to achieve a burst-suppression pattern, also an indication of its maximal effect. Other sedatives are then ceased. Hypokalemia may occur with induction and hyperkalemia as a rebound when the infusion is suddenly stopped. Despite the impact on ICP reduction, there is no convincing evidence that barbiturates improve outcomes in patients with severe TBI, possible due to harmful side effects (hypotension adversely affecting cerebral perfusion and increased risk of infection). Its use should be considered when ICP is measured invasively, and intracranial hypertension is refractory to first and second-tier treatments. In that scenario, one must individualize its use either as a therapeutic bridge to surgical decompression or, knowledgeable of its severe side effects, when surgery is not the best option.
6.11 Hypothermia
Therapeutic hypothermia decreases cerebral metabolism, lowering oxygen consumption, and CO2 production, which may lead to ICP reduction. It has severe side effects, such as metabolic acidosis, electrolyte disturbances (mainly hypokalemia), arrhythmias and bradycardia, coagulopathy, and increased risk of infections. There are various methods to induce hypothermia. Most commonly, cold intravenous infusion, gastric lavage, and surface cooling blankets are used, but other automated devices are also available. The induction phase should be fast, followed by a sustained maintenance phase for 24–48 hours; then, the rewarming phase must be slow and controlled (maximum of 0.5°C per hour) due to the risk of rebound intracranial hypertension and sudden metabolic disturbances (hyperkalemia). During induction, patients may present shivering, which increases CO2 production. It can be managed through warming of the extremities and boluses of opiates and sedatives; eventually, neuromuscular blockers are necessary. Continuous core temperature monitoring is essential to avoid sudden and frequent temperature changes and adequate induction and rewarming speed. Despite its theoretical protective effects, clinical trials have failed to show that hypothermia improves outcomes in patients with intracranial hypertension.
The Eurotherm3235 [25] trial assigned patients with TBI and ICP >20 mmHg despite first-tier treatment to receive either hypothermia between 32 and 35 °C or standard therapy. Trial recruitment was suspended for safety reasons, indicating worse outcomes (GOS-E at 6 months) in the hypothermia group. Recently, the POLAR [26] trial evaluated the role of early prophylactic hypothermia in severe TBI. Patients were enrolled both out-of-hospital and at emergency departments and assigned to hypothermia between 33 and 35 °C for at least 72 hours or normothermia (target of 37 ± 5 °C). There was no difference in neurologic outcomes (GOS-E) in 6 months.
Given these recent results with even a signal of harm from a trial, therapeutic hypothermia is not currently recommended as a standard treatment in intracranial hypertension. Its use should be limited to selected cases of ICP refractory to first and second-tier therapies. In our practice, a target core temperature of 36–37 °C is the usual goal of treatment.
6.12 Decompressive Craniectomy
Craniectomy is an effective therapy in reducing and even normalizing ICP by removing a large segment of the skull, thus increasing cranial vault volume for allowing brain tissue swelling (according to Monro–Kellie Doctrine) without or with lower impact on the ICP. When performed, it should be of sufficient size (at least 10–12 cm in diameter), involving temporal and parietal bones (unilateral or hemicraniectomy), including middle cranial fossa. A large proportion of ICP reduction is achieved through durotomy.
Randomized clinical trials showed that decompressive craniectomy is effective in reducing ICP and possibly increases patient’s survival, but it does not improve long term functional outcomes. In the 2011 DECRA trial [27], patients with severe TBI and early refractory ICP (ICP >20 mmHg for 15 minutes within 1 hour) despite optimized first tier therapies (sedation, normocapnia, hyperosmolar therapy, and external ventricular drainage) were randomized within 72 hours of injury to undergo bifrontotemporoparietal decompressive craniectomy or continuing standard care. Early surgical intervention decreased ICP and length of stay in the ICU but was associated with unfavorable neurological outcomes (worst Extended Glasgow Outcome Scale GOS-E), with no difference in 6 months mortality.
Later, the RESCUEicp trial [28] studied craniectomy as a rescue maneuver. In this study, patients with TBI and refractory ICP elevation (>25 mmHg for 1–12 hours), despite first and second-tier therapies (sedation, mild hypocapnia, hyperosmolar therapy, blood pressure augmentation, external ventricular drainage, and mild hypothermia) were randomly assigned to surgery (either hemicraniectomy or bifrontal craniectomy, at the discretion of neurosurgeons) or continuing medical therapy, with the option to add barbiturates. Again, the surgical group achieved lower ICP values, but this time with lower 6 months mortality (26.9% × 48.9%) but higher rates of vegetative state, lower severe disability, and upper severe disability. Rates of favorable outcomes (moderate disability and good recovery) were similar between groups.
Nowadays, craniectomy is considered a salvage therapy in the context of large middle cerebral artery infarction and severe traumatic brain injury. It could be a therapeutic alternative to barbiturates and mild hypothermia in the management of intracranial hypertension refractory to first and second-tier measures, but no late enough that harmful effects of prolonged elevated ICP can no longer be avoided. The decision to perform this procedure should be individualized and consider the patient’s values and preferences through family members’ perspective, the timing of the procedure and patient’s age, considering it may increase survival, but it does not improve long term neurologic outcomes.
7 Complications
Intracranial hypertension and its effects on intracranial physiology, if left untreated, may lead to brain death, permanent neurological dysfunction, or devastating neurological outcomes. On the other hand, the treatment for IH itself poses substantial side effects.
Some risks are inherent to the treatment. Since all patients with IH are under invasive mechanical ventilation, there is a risk for ventilation associated pneumonia (VAP) and ventilator-induced lung injury (VILI). The same for central line-associated bloodstream infections. The sedation used for reducing brain oxygen consumption might lead to hemodynamic instability in different degrees, depending on the dose and agent used. Osmolar therapy to minimize brain edema leads to fluid depletion and may cause dehydration and hydroelectrolytic disturbances in the case of mannitol or hypernatremia and fluid overload when using hypertonic saline. Induced hypocapnia causes cerebral vasoconstriction and may lead to brain ischemia.
In advanced stages, if left untreated, independent of the cause of the IH, a common pathway is seen. The increase in the ICP leads to compression of brain stem structures and brain herniation. Breathing pattern alterations may then occur depending on the herniation, leading to a gasping pattern and possible cardiovascular collapse, culminating in cardiorespiratory arrest.
Metabolic suppression with barbiturates causes hemodynamic instability, hypokalemia, and might increase the risk of infection. Hypothermia causes severe electrolytic disturbances and may increase the risk of infection. Surgical treatments such as the use of EVD or decompressive craniectomy also may increase the risk of infection. Vasopressors are frequently used to achieve MAP targets and might cause arrhythmias and increase the risk of vascular ischemia.
The occurrence of seizures, convulsive, or nonconvulsive status is not uncommon in patients with acute neurological insults and are associated with worse neurological outcomes. Therefore, seizure prophylaxis is indicated for selected patients. Some patients might develop central diabetes insipidus and other sodium disturbances (cerebral salt wasting syndrome and syndrome of inappropriate antidiuretic hormone secretion) may also be present and should be treated accordingly. Patients with IH have increased risk for upper gastrointestinal bleeding, and stress ulcer prophylaxis is recommended in the acute phase.
Therefore, when managing a patient with IH, it is paramount to be aware of the most common complications. Due to its severity and frequency of poor neurological outcomes, patients with IH require an aggressive management strategy. A comprehensive plan, weighting the pros and cons of each conduct, with precisely timing interventions, is the best tool for managing patients with IH.
Pearls/Tips
-
When managing IH without direct ICP monitoring, a well-structured clinical/imaging protocol is necessary. The criteria for acquiring a new head CT must be low. Optic nerve sheath diameter (ONSD) measurement is a useful triage tool is this setting.
-
When IH is suspected or documented, aggressive treatment must be provided even before acquiring a new CT image or installing invasive ICP monitoring.
-
If available, we recommend invasive intraventricular ICP monitoring with EVD in patients with suspected IH.
-
Despite the myriad of therapeutic interventions for treating IH, success is heavily dependent on two cornerstones: early removal of surgically treatable lesions and early and continuous correction of anatomic and physiologic derangements that can worsen cerebral edema (Table 12.3).
-
If IH is refractory to these initial maneuvers, a stepwise approach is suggested: cerebrospinal fluid drainage (when applicable), optimizing analgesia and sedation, hyperosmolar therapy, neuromuscular blockade (when indicated), and mild induced hypocapnia. We suggest propofol as first line sedation agent and hypertonic saline for hyperosmolar therapy.
-
If IH is refractory to initial maneuvers and to conventional treatment, rescue therapy strategies include metabolic suppression with barbiturates, mild hypothermia, and/or decompressive craniectomy.
References
Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–8.
Posner JB, Plum F, Oxford university press. Plum and Posner's diagnosis of stupor and coma. New York: Oxford University Press; 2007. Available from: https://doi.org/10.1093/med/9780195321319.001.0001.
Stocchetti N, Maas AI. Traumatic intracranial hypertension. N Engl J Med. 2014;370(22):2121–30.
Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GW, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15.
Sheth KN, Stein DM, Aarabi B, Hu P, Kufera JA, Scalea TM, et al. Intracranial pressure dose and outcome in traumatic brain injury. Neurocrit Care. 2013;18(1):26–32.
Vik A, Nag T, Fredriksli OA, Skandsen T, Moen KG, Schirmer-Mikalsen K, et al. Relationship of "dose" of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109(4):678–84.
Stein DM, Hu PF, Brenner M, Sheth KN, Liu KH, Xiong W, et al. Brief episodes of intracranial hypertension and cerebral hypoperfusion are associated with poor functional outcome after severe traumatic brain injury. J Trauma. 2011;71(2):364–73; discussion 73–4
Marcolini E, Stretz C, DeWitt KM. Intracranial Hemorrhage and intracranial hypertension. Emerg Med Clin North Am. 2019;37(3):529–44.
Fernando SM, Tran A, Cheng W, Rochwerg B, Taljaard M, Kyeremanteng K, et al. Diagnosis of elevated intracranial pressure in critically ill adults: systematic review and meta-analysis. BMJ. 2019;366:l4225.
Maissan IM, Dirven PJ, Haitsma IK, Hoeks SE, Gommers D, Stolker RJ. Ultrasonographic measured optic nerve sheath diameter as an accurate and quick monitor for changes in intracranial pressure. J Neurosurg. 2015;123(3):743–7.
Kishk NA, Ebraheim AM, Ashour AS, Badr NM, Eshra MA. Optic nerve sonographic examination to predict raised intracranial pressure in idiopathic intracranial hypertension: the cut-off points. Neuroradiol J. 2018;31(5):490–5.
Cardim D, Robba C, Bohdanowicz M, Donnelly J, Cabella B, Liu X, et al. Non-invasive monitoring of intracranial pressure using transcranial doppler ultrasonography: is it possible? Neurocrit Care. 2016;25(3):473–91.
Rasulo FA, Bertuetti R, Robba C, Lusenti F, Cantoni A, Bernini M, et al. The accuracy of transcranial Doppler in excluding intracranial hypertension following acute brain injury: a multicenter prospective pilot study. Crit Care. 2017;21(1):44.
Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471–81.
Hawthorne C, Piper I. Monitoring of intracranial pressure in patients with traumatic brain injury. Front Neurol. 2014;5:121.
McHugh GS, Engel DC, Butcher I, Steyerberg EW, Lu J, Mushkudiani N, et al. Prognostic value of secondary insults in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):287–93.
Stevens RD, Shoykhet M, Cadena R. Emergency neurological life support: intracranial hypertension and herniation. Neurocrit Care. 2015;23(Suppl 2):S76–82.
Rose JC, Mayer SA. Optimizing blood pressure in neurological emergencies. Neurocrit Care. 2004;1(3):287–99.
Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. 2016;20(1):128.
Gu J, Huang H, Huang Y, Sun H, Xu H. Hypertonic saline or mannitol for treating elevated intracranial pressure in traumatic brain injury: a meta-analysis of randomized controlled trials. Neurosurg Rev. 2019;42(2):499–509.
Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med. 2011;39(3):554–9.
Sanfilippo F, Santonocito C, Veenith T, Astuto M, Maybauer MO. The role of neuromuscular blockade in patients with traumatic brain injury: a systematic review. Neurocrit Care. 2015;22(2):325–34.
Coles JP, Fryer TD, Coleman MR, Smielewski P, Gupta AK, Minhas PS, et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit Care Med. 2007;35(2):568–78.
Roberts I, Sydenham E. Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev. 2012;(12):CD000033.
Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK, et al. Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med. 2015;373(25):2403–12.
Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR randomized clinical trial. JAMA. 2018;320(21):2211–20.
Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502.
Hutchinson PJ, Kolias AG, Timofeev IS, Corteen EA, Czosnyka M, Timothy J, et al. Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. 2016;375(12):1119–30.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Appendices
Algorithm 1 – For Patients Without ICP Monitoring

Algorithm 2 – For Patients with ICP Monitoring

Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bassi, E., Tomazini, B.M., Cadamuro, F.M., Roepke, R.M.L., Carneiro, B.V., Malbouisson, L.M.S. (2021). Management of Intracranial Hypertension. In: Figueiredo, E.G., Welling, L.C., Rabelo, N.N. (eds) Neurocritical Care for Neurosurgeons. Springer, Cham. https://doi.org/10.1007/978-3-030-66572-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-66572-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66571-5
Online ISBN: 978-3-030-66572-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)