Abstract
Heavy metals are available abundantly in nature, especially in the soil, mines, drinking water, some in vapor form in the air, and also constitute the Earth’s crust. These are widely used in pesticides, herbicides, paints, gasoline, etc., and their main route of exposure encompasses anthropogenic sources. Among several heavy metals, lead, cadmium, mercury, and arsenic cover the most part. One of the most important contributing factors are industrial pollutants that have an important role in contaminating the plant and marine life, which indirectly affect the human health. The brain is the functional unit of body which is sensitive to such heavy metals, and suffers a lot through their contamination in comparison to the other parts of the body. If the exposure of heavy metals becomes prolonged, they will have deleterious effects on the nervous system. Heavy metals toxicity is responsible for many neurodegenerative diseases particularly Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, and attention-deficit hypertensive disorders. There are number of epidemiological, experimental, in vivo and in vitro data which represent the significant association or correlation between the exposure of heavy metals and neurotoxicity. The probable reason behind this correlation is mostly due to oxidative stress, the participation of certain proteins/enzymes as well as an interruption in the normal secretion of neurotransmitters on account of heavy metal exposure. The resultant effects and intensity of diseases can be prevented by taking the adequate preventive measures with possible therapeutic interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Heavy metals can be termed as the metals that weigh more than 5 g cm−3, such as arsenic, cadmium (Cd), lead (Pb), and mercury (Hg). Almost there exist 40 elements that are included in the category of heavy metals out of which the abovementioned heavy metals play an important contribution in the toxicity and neurological disorders. Naturally, they are found in the Earth’s crust in the form of dispersed rocks and ores. The anthropogenic contribution of heavy metals in the biosphere has been associated with industrialization and urbanization; due to this fact, they are widely available in the soil and aquatic environment. These metals become the cause of adverse reactions occurring in plants, animals, and human and affect the entire ecosystem [1]. In human, they are inhaled, ingested, and got in contact with skin, resulting in the inhibition of growth during the developmental stage, mental retardation, death of either particular cells of organism or whole of it, abnormalities in the immune system, endocrine system, and overall metabolism.
There are two ways of destructing metabolic functions; firstly, in the brain, heart, liver, kidneys, and some other parts, the heavy metals get accumulated and affect the proper functioning of them. Secondly, the displacement of necessary minerals from their origin, with heavy metals, occurs, which ultimately disrupts the biological functioning [2]. Therefore, considering these types of abnormal alterations in the body of human, there should be some safety standard levels of interactions with heavy metals for the protection of health and those interactions must be within the safest limits [3].
This chapter mainly focuses on the effects of Pb, Cd, Hg, and arsenic on neurological health and their underlying mechanisms. These metals when present in the body in excess amount cause toxicity in the form that they disrupt the mitochondrial function and disable the activity of enzymes. Most importantly, they induce the oxidative stress and increase the production of reactive oxygen species (ROS). Many epidemiological and clinical studies have been conducted that show the correlation between the exposure of heavy metals and neurological disorders, such as Alzheimer’s disease, autism spectrum disorders (ASD), amyotrophic lateral sclerosis (ALS), Gulf War syndrome, Guillain–Barré disease, Huntington’s disease, multiple sclerosis, Parkinson’s disease, and Wilson’s disease [4]. For instance, considering the exposure of Hg, it is involved in the lipid peroxidation and becomes the source of cell damage that is a similar process incriminated in case of Parkinson’s disease pathogenesis [5]. This chapter also explains the ways of prevention as well as the protection against the exposure of heavy metals and possible therapeutic interventions being applied to relieve the symptoms of neurological disorders. There are also certain antidotes that are used in case of life-threatening exposure.
Sources of Exposure to Toxic Metals
Lead
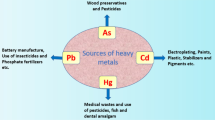
Lead (Pb) is one of the highly toxic metals that has a bright silvery, moderately bluish appearance. Industrial processes, food, drinking water, and smoking are the main sources of exposure to it. The other origins include gasoline and house paints that emerge from storage batteries, toys, lead bullets, faucets, etc. The particles of soil, sediment, and sewage sludge get strongly bind to Pb in the environment. Human beings are exposed to Pb through vehicle exhaust, industrial fumes, and contaminated food and water. Fixation of Pb to the soil particles and flow into the water generally cause the exposure to human beings. The occupational exposure of Pb gives rise to many neurological and non-neurological signs and symptoms such as headaches, encephalopathy, loss of memory, hallucination, dullness, irritability, poor attention span anemia, nausea, muscular tremor, and saturnism. The cross-sectional (descriptive and analytical) survey conducted on 40 female solderers who were working in 2 electrical parts manufacturing factories in Neyshabur city in 2017–2018. Their blood test showed increased Pb concentration, as they were highly exposed to Pb during work [6]. Another cross-sectional study held in Duhok City, Kurdistan Region, Iraq, found that the main exposure of Pb was occupational that the workers were being employed as gasoline power generators, traffic policemen, and working in petrol filling stations and batteries repairing workshops [7]. A survey-based study was performed among Australian workers to estimate the prevalence of work-related exposure of organic and inorganic Pb compounds. The conclusion described that this occupational exposure could be the leading cause of life-threatening diseases [8]. In an experimental study, the influence of occupational exposure of Pb was evaluated on hematological indices among petrol station attendants and automobile mechanics in Nnewi, South-East Nigeria. The results indicated that Pb exposure leads to the adverse effects on the hematopoietic system; as a fact, they were highly exposed to Pb and alcohol was exacerbating the hepatotoxic signs and symptoms due to this Pb subjection [9]. The possible sources of Pb pollution in the environment have been shown in Fig. 4.1.
Cadmium
The seventh most toxic heavy metal is Cd that is the by-product of zinc production, through which human can get exposed. It was first used during the World War I, in place of tin and as a pigment in paint industries. It is relatively water-soluble than other heavy metals; so, the accumulation occurs in the soil and ultimately in fruits and vegetables. Nowadays, it is being used in rechargeable batteries, alloys, and also in tobacco smoke. There are several epidemiological studies including cohort and cross-sectional studies that bring attention to nonoccupational and occupational exposure of Cd. Human beings are exposed to Cd by inhaling and ingesting it, via tobacco smoke and agriculture crops. After inhalation, Cd enters into the brain via olfactory bulb and even it enters into the brain via cerebrospinal fluid (CSF) barrier [10]. The sources of exposure of Cd in an adult urban population in southern Brazil and its level in the blood were investigated. It was concluded that levels of Cd in the blood were associated with smoking and alcohol drinking; these parameters were the main causative factors to the increased concentration of Cd in the blood [11]. A study carried out among Canadian adults aged from 20 to 79 years showed that smoking has a major contribution to Cd exposure; while, the diet has not contributed a lot [12]. Another study describes that occupational exposure of Cd could be associated with neurological signs and symptoms of myalgic encephalomyelitis/chronic fatigue syndrome [13]. These are the neurological diseases characterized by widespread inflammation and multisystemic neuropathology [14]. The occupational exposure of Cd encompasses those people working as technicians in jewelry industries. Such industries usually make jewelry that is not of pure precious metals rather it contains some heavy metals like Cd, to make them cheaper. The occupational exposure of a few heavy metals was studied, among the workers in jewelry manufacturing. The results manifested that workers were significantly exposed to Cd as it was released during the jewelry processing, as compared to the control group. The mean concentration of Cd in their urine samples was 12.65 (+ SD 11.12) μg/L and among the controls it was 4.66 ± 2.27 μg/L [15].
Mercury
Mercury (Hg) is found naturally, possessing shiny silver-white liquid appearance without any odor and exists in three forms, i.e., as a metallic element, inorganic salts, and organic compound. Such forms have different bioavailability and toxicity. Hg is transformed into methylmercury and dimethylmercury either biologically or chemically, where methylmercury is bioaccumulative and causes toxicity. It is inhaled and ingested by human through the vapors of Hg in elemental form and food containing methylmercury, respectively. Anthropogenic activities of human in agriculture, mining, municipal wastewater discharges, incineration, and discharges of industrial wastewater are the main sources of exposure on humans. The most direct exposure to human involved is through amalgams which are used as a tooth filler to prevent it from decaying. A study was conducted to assess the levels of aluminum, Pb, and Hg in the hair of 100 autistic Egyptian children; their ages ranged from 2.5 to 15 years so that the environmental and genetic risk factors associated with them could be estimated. The result of this study exhibited the significant levels of aluminum, Pb, and Hg in the hair of autistic children than patients which were kept as controls. There was a positive correlation between the maternal fish consumptions and level of Hg metal and in this study; it was also found that the level of Hg in children with ASD was increased as the usage of maternal dental amalgam increased, albeit not significantly increased, in terms of stats [16]. A 2014 meta-analysis of the evidence of the impact of prenatal and early infancy Hg exposures on autism risk found a significant correlation between the increased exposures of environmental Hg and an increased risk in ASD [17].
Arsenic
Arsenic is found in organic and inorganic form. Fertilizers, phosphates, paints, dyes, semiconductors, drugs are the sources of exposure. The encounter with human may occur by drinking water, contaminated with arsenical compounds; present in pesticides, disposals, and natural mineral deposits/ores. This type of exposure is particularly more associated with toxicity caused by arsenic compounds which is usually termed as arsenicosis [18]. The occupational exposure of arsenic encompasses the herbicides and pesticide production, mining, smelting, manufacturing of glass, semiconductors, and some professions like carpentry that incriminate the removal or exposure to structures/materials treated with arsenate wood preservatives [19]. According to US Agency for Toxic Substances and Disease Registry publication, inhalation and the dermal layer is contemplated as a minor way of exposure in the general population, but a major way of exposure of arsenic is the occupational worker [20]. Arsenic exposure through water and consumption of rice was investigated in epidemiological studies that manifested the association with this type of exposure and increased urine concentration of arsenic in 229 pregnant women [21]. There are many metal mines that contain arsenic in South Korea. These mines contribute to the contamination of the environment [22]. Thus, the accumulation of arsenic during the childhood in the body could lead to the neurobehavioral abnormalities during puberty [23]. The association of exposure of arsenic with neuronal development and behavioral disorder was found through meta-analysis, which showed that arsenic exposure affected memory, verbal, and performance domains in children, although to a lesser extent [24].
Mechanism of Induction of Neurotoxicity
The general mechanism of heavy metal–induced neurotoxicity is almost the same. Nearly, all heavy metals particularly Pb, Cd, mercury, and arsenic induce the cellular damage by the production of free radicals or ROS like O2•, OH, NO•, RO•, and ONOO•, H2O2. This production is accelerated when the availability of antioxidants is reduced or the overall balance of activity of antioxidant enzymes (SOD, GSH, GST, catalase) is disturbed as shown in Fig. 4.2. Few of them also produce reactive nitrogen species (RNS). In addition to this, the release of synaptic neurotransmitters is also drastically affected, resulting in neurotoxicity mainly, impairment in the body balance, tremors, hearing and vision problems, loss in memory, low IQ level, learning disabilities, and several others.
Lead-Induced Neurotoxicity
There are some mechanisms involved through which Pb induces the neurotoxicity, and Fig. 4.3 shows the possible mechanisms involved in the induction of neurotoxicity that can be associated with the morphological and pharmacological effects.
Some in vitro studies reveal that Pb can inhibit the Na+/K+-ATPase in the cell membrane and activate the protein tyrosine kinase in the capillary, which hinders the energy metabolism [25]. Two mechanisms are associated with the production of ROS by which deterioration in living systems occurred. The first one is the direct generation of free radicals like O2 and H2O2 due to the overload of heavy metals. The second one is the indirect mechanism that is executed by the depletion of antioxidants and inhibition of enzymes. The enzymes with antioxidant activity that Pb inhibits are delta-aminolevulinic acid dehydratase (ALAD) and glutathione reductase (GSR). The inhibition of ALAD and GSR causes the imbalance, and the resultant effect will be oxidative damage to DNA and lipids. The inhibition of ALAD gives rise to the circulating ALA, which is also termed as a weak gamma-aminobutyric acid (GABA) agonist, this causes in the reduction of releasing of GABA through presynaptic inhibition leading to the onset of excitatory activity as seen in some neurodegenerative diseases. Pb not only participates in the production of ROS but also in the production of RNS, which has a detrimental effect on vascular endothelial [3, 26]. A meta-analysis following epidemiological studies illustrated that occupational exposure of Pb attributes in endangering the development of motor neuron disease, amyotrophic lateral sclerosis. Pb affects the normal functioning of the body and causes high blood pressure, renal system damage, reduced fertility, anorexia, damage to neurons, chronic nephropathy, hyperactivity, insomnia, and learning deficits, and it is also a risk factor for Alzheimer’s disease [27]. Multiple sclerosis, an inflammatory demyelinating disease in which the protective myelin sheath covering the nerve fibers gets degenerated, in consequence of immune system attack either by environmental or pathological cause. The study conducted in Taiwan detailed the association between the concentrations of heavy metals and multiple sclerosis incidence, using spatial regression. This epidemiological study ended up by finding that soil containing Pb had a positive relation with multiple sclerosis incidence in Taiwan. Among the affected people, the ratio of males was higher as compared to the females.
Cadmium-Induced Neurotoxicity
Some evidences illustrate morphological changes and biochemical changes due to Cd toxicity , and it might be the contributing factor in neurodegenerative diseases, mainly Alzheimer’s disease and Parkinson’s disease. Figure 4.4 displays the general mechanism of action of Cd that disrupts the synaptic transmission. The neurons in the cerebral cortex are considered to be the targets that are involved in Cd-induced toxicity and apoptosis as well. The biochemical changes mediated by Cd are associated with an imbalance between the excitatory and inhibitory neurotransmitters and levels of antioxidants in the brain. Studies have shown that the release of acetylcholine is inhibited by the interference in the metabolism of calcium, whereas the sensibility of serotonin increases on account of raised levels of Cd [28].
Mechanism of action of cadmium in disruption of synaptic transmission. The blockage of Ca++ channels leads to decrease in synaptic vesicle proton gradient and exocytotic release of glutamate. The binding of Cd to thiol groups of Glu transporters induces reduction in synaptic vesicles proton gradient and also decreases in transporter-mediated Glu uptake. These alterations ultimately cause synaptic impairment
In animal studies, increase in exposure to Cd causes a significant increase in Cd concentration in the brain that ultimately results in the biochemical changes which are related to the changes in the synthesis and release of neurotransmitters, disturbances in memory/behavior also the alteration in the function of receptors and ion channels occurs [29]. These neuronal and CNS disturbances happen because of the morphological damage in choroid plexus (a plexus of cells responsible for the production of cerebrospinal fluid), induced by a high concentration of Cd. The mechanism behind the biochemical changes related to Cd exposure entails the interference in the cholinergic system. Acetylcholine being a vital neurotransmitter in this system controls various cognitive functions, the levels of acetylcholine are maintained by the balance of enzymatic activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Cd hinders this balance of synaptic neurotransmitters. Experimental studies detected the alterations in AChE and Na+/K+-ATPase enzymes in the cerebellum, cerebral cortex, hypothalamus, and hippocampus of adult Wistar rats that were exposed with Cd for several days [28]. Cd induces the neurotoxicity by altering the permeability of BBB, resulting in amyloid-β (Aβ) aggregation; a protein linked with Alzheimer’s disease and by the production of tau neurofibrillary tangles. Several human aging studies are associating Alzheimer’s disease with Cd exposure, as it is thought that Cd impairs the cognitive function [10]. In vitro study was conducted to analyze the neurotoxic effect of cadmium selenide (CdSe) and its potential uptake in neural cell lines by using PC-12 cells of rats, and the results showed that neurodegeneration occurs at higher level of CdSe exposure [30].
Amyotrophic lateral sclerosis (ALS) is a disease in which the motor neurons are affected that cause the disablement of movement and control of muscles. It involves the gene mutation such as superoxide dismutase 1 (SOD1) [31]. Cd , through the induction of metallothionein (MT) expression, alters SOD1 which might influence the zinc (Zn) homeostasis. The activity of SOD1 enzyme could be decreased as the availability of Zn decreased because there might be a binding between the Zn and increased levels of MT. Contradictorily, the overload of Cd and deficiency of Zn may hinder the functions of SOD1 by the impedance of the secondary structure which induces misfolding and part aggregation of SOD1. This could ultimately bring about the risk of developing ALS [32]. Cd has the capacity of interaction with micronutrients such as Zn, copper, iron, and calcium. It replaces the Zn at MTs and replaces iron and calcium from prosthetic groups. These replacements contribute to alter the enzymatic reactions, by depleting the thiol groups present in enzymes and antioxidants. There are some physiological functions of micronutrients such as copper or Zn while Cd has no any participation in physiological functions rather it leads to the cellular damage [33].
Mercury-Induced Neurotoxicity
As aforementioned, Hg exists in three forms that are inorganic (divalent and monovalent cationic forms; Hg2+ and Hg+), organic methylmercury (MeHg), and elemental Hg vapor. These different forms induce the toxicity, distribution, and metabolism of Hg. Majorly, studies explained the neurotoxic effects of MeHg, an organic form of mercury, as this form has a relatively greater ability to enter into the CNS. The resultant effects are the hearing and speech impairment, visual disturbance, paresthesia, cerebellar ataxia, and psychiatric effects [34]. The elemental form of Hg oxidizes into an inorganic form of Hg and gets excreted through urine while the former is well distributed in the kidney and also has the capacity to accumulate in the brain [35]. The distribution of MeHg to the different regions of the brain takes place by crossing the BBB. Previous studies illustrated that the BBB is relatively more sensitive to the organic Hg rather than inorganic form, but the inorganic Hg has a direct toxic effect on BBB [36]. It is suspected that neutral amino acid carrier systems are responsible for the transportation of MeHg–cysteine complexes. In the brain, demethylation of MeHg appears to occur and the resultant inorganic form has long half-life in the thalamus and pituitary. A family of cysteine-sufficient protein, i.e., MT, has high affinity towards metals, so these proteins bind within organic form of Hg or its demethylated form [37].
One study described the effect of Hg on human neuronal-glial (HNG) cells, by the utilization of pro-inflammatory transcription factor NF-kB (p50/p65) complex as an indicator for the onset of inflammatory neurodegeneration. The results showed that mercuric sulfate significantly induced the NF-kB (p65) activator complex in HNG cells. Such activation depicts that there is a possibility of the onset of Alzheimer’s disease by heavy metals like Hg. Along with this, the mechanism involved in the production of ROS is also suspected to be activated [38]. Hg is an etiological factor for Alzheimer’s disease because of the involvement of neurofibrillary tangles consisting of hyperphosphorylated tau protein, as this phosphorylation is induced by mercury; also Hg stimulates the secretion of Aβ protein. These functional and structural changes are the major attributes in Alzheimer’s disease. Furthermore, the disturbances in neurotransmitters seen in Alzheimer’s disease are the same as seen in Hg-induced Alzheimer’s disease, particularly the reduction of acetylcholine, inhibition of serotonin binding with its receptors, and glutamate uptake [39]. Experimental studies have revealed that at sub-cytotoxic concentrations of Hg, there is no direct breakdown of DNA strands, but ROS like H2O2 caused the breakage of DNA strands. Astrocytes and microglia are affected due to the Hg exposure. Both of these cells are responsible to protect the brain activity [40].
Figure 4.5 represents the neurotoxicity induced by MeHg mediated by ROS. In step 1, MeHg inhibits the astrocytic glutamate (GLU) uptake, which results in an increased level of GLU. In step 2, release of GLU from presynaptic is stimulated. In step 3, the uptake of vesicular GLU also inhibits. In step 4, N-methyl d-aspartate (NMDA)-type GLU receptors are hyperactivated due to increased extracellular GLU levels, also the influx of calcium into neurons is raised. In step 5, the raised intracellular level of calcium causes the mitochondrial collapse. In step 6, neuronal nitric oxide synthase (nNOS) is activated. In step 7, due to nNOS activation, nitric oxide (NO) formation is increased. MeHg affects the mitochondrial electron transfer chain (mainly at the level of complexes II–III). In step 8, the formation of H2O2 and superoxide anions (O2•−) is increased. H2O2 can produce hydroxyl radical anion (•OH) through Fenton’s reaction. In step 9, MeHg-induced H2O2 levels can be a consequence of a reduction in glutathione peroxidase (GPx) activity. Lastly, the glutathione (GSH) is depleted [41, 42]. MeHg is also known to induce neurotoxicity by depositing in astrocytes and microglia, where it generates ROS. The rapid increase in ROS reduces the GSH which is supposed to detoxify the MeHg at an earlier stage [43].Epidemiological studies explain the correlation between the brain biomarkers with Hg levels in children with autism spectrum disorder (ASD), and the increase in blood mercury levels is shown to associate with the worsening of symptoms of ASD [44].
Role of methyl mercury in the alteration of synaptic signaling and production of ROS [42]
Arsenic-Induced Neurotoxicity
The two forms of organic and inorganic arsenic metabolites exist in trivalent or pentavalent oxidation states. These different states have various biological effects. The metabolic pathway of arsenic is shown in the Fig. 4.6. The primary pathways of arsenic metabolism are oxidative methylation and GSH conjugation. Inorganic arsenic (V) is reduced to arsenic (III), which is important for methylation in mammals. Inorganic arsenic (III) is methylated to methylarsonic acid (MMA) and dimethylarsinic acid (DMA) by alternating the reduction of pentavalent arsenic to trivalent arsenic [45] as shown in Fig. 4.6.
Metabolic pathway of arsenic. (Adopted from [45])
Arsenic can cross BBB , which comprises three cellular components that are endothelial cells, astrocytes, and pericytes (PCs). The diffusion of gases, water, and nonpolar molecules occurs via the diffusion barrier or tight junctions (TJs) that are present between the endothelial cells. The destruction in this barrier due to increased arsenic exposure could lead to the development of neurodegenerative disease [46]. In controlled experimental study in Swiss albino mice, there was a significant reduction in GSH level in the brain of arsenic-treated mice. This explains that arsenic also causes a reduction in antioxidative enzymes, due to oxidative stress. It was found that neurobehavioral changes along with the reduction in cholinesterase enzymes also occurred [47]. Another study described that postnatal low concentration of arsenic exposure in rats induces autism-like behavior which includes problems linked with learning abilities and social skills. Moreover, abnormal frontal cortex neurogenesis was seen and this effect was produced by arsenic exposure [48]. The possibility of this unusual neurological behavior could be because of increased oxidative stress and decreased ATP production with the disturbances and mutations in structural and functional maturity of nerve cells, owing arsenic exposure [49]. There are high levels of inorganic arsenic in drinking water due to industrial pollution, the major source of exposure to arsenic. For the investigation of inorganic arsenic-induced apoptosis in the cerebral cortex and in vivo analysis was carried out in mice. There were some underlying mechanisms connected to this apoptosis. Figure 4.7 indicates the reduced GSH levels in cerebral cortex in inorganic arsenic-exposed mice. These mice were exposed to 0, 0.5, and 5 ppm inorganic arsenic via the drinking water for almost 6 consecutive weeks in the presence or absence of N-acetylcysteine (150 mg/kg/day). This apoptotic effect could be the cause of Alzheimer’s disease [50].
Effect of different doses of arsenic exposure on glutathione (GSH) level in cerebral cortex in inorganic arsenic-exposed mice. (Adopted from [50] after some modifications)
In Japan, people who had survived with the probable exposure of arsenic , neurological and electrophysiological sign and symptoms showed that these residential people complained about the hearing problem. On examination, it was discovered that sensory dysfunction worsened gradually [51]. Figure 4.8 shows the pathways by which arsenic induces neurotoxicity. The first pathway illustrates that exposure of arsenic accelerates the activity of ROS and lipid peroxidase enzymes but decreases the activity of SOD, this gives rise in oxidative stress. The second one explains the apoptosis in cerebral neurons by the upregulation and activation of p38 MAPK, JUNK3. The third pathway depicts the effect of arsenic exposure which contributes to the destabilization and disruption of the cytoskeletal framework by the alteration of protein composition or protein hyperphosphorylation [52].
The mechanism in Fig. 4.9 shows how neurodegenerative prototypic proteinopathy diseases like Alzheimer’s disease are occurred due to the involvement of inorganic arsenic exposure. The in vivo study using transgenic animals described that the presence of amyloid plaques and neurofibrillary tangles containing hyperphosphorylated tau protein along with oxidative stress can be the contributing factors in the development of Alzheimer’s disease [53].
Schematic representation of mechanism of arsenic-induced neurodegeneration. (Adopted from [53] after some modifications)
Preventive Interventions
The overall preventive measures for heavy metals include cessation of smoking, more intake of iron-containing diet and filtered drinking water, maintenance of adequate occupational hygiene, and avoid further exposure if once affected. Some specific preventive and therapeutic interventions have been discussed ahead. Environmental poisoning of Pb cannot always be handled by the chelation using dimercaptopropane sulfonate (DMPS), dimercaptosuccinic acid (DMSA), dimercaprol (British Anti-Lewisite, BAL), and CaNa2EDTA, as there are the chances of redistribution of Pb even after the chelation therapy. So, the levels of Pb in the blood must be monitored and screened at appropriate intervals to avoid and prevent the neurotoxic effects in case of presence of toxic level of Pb in the blood. Most importantly, Pb exposure can be reduced and prevented through the awareness of its possible hazards [54]. Likewise, in Pb chelators, clinical studies have shown that usage of EDTA, DMPS, DMSA, and British Anti-Lewisite (BAL) could help out in Cd-induced toxicity. Selenium (Se) is thought to act as a protective agent in Hg-induced neurotoxicity. Experimental studies indicate the association between Hg and Se, generally in nervous tissue and particularly in the whole brain [55]. The in vivo study indicated the shielding effect of Vitamin C in metal-induced toxicity [56]. In rodent studies, loss of neurogenesis in adults can be reduced by almost complete eradication of exposure to arsenic in water or drinking water containing arsenic [57]. The use of arsenic chelators, Se, and Zn can lessen the damage that occurred due to arsenic exposure. The antioxidant and antidotal property of Se is highly investigated through experimental studies. Se induces antioxidant activity by the expression of selenoproteins which include thioredoxin reductases and glutathione peroxidases. These proteins help in the reduction of ROS production. Moreover, the Se accelerates the capacity of conjugation reaction of arsenic, i.e., methylation of arsenic aids in the excretion of methylarsinous acid (MMA) into the bile. The methylation process takes place in the liver as it has relatively higher concentration of GSH. So, overall Se promotes the detoxification process [58, 59]. Studies have also found that the arsenic-induced deficiencies in mice can be recovered by the treatment of Zn as it elevates GSH level and ameliorates lipid peroxidation, consequently assisting in the reduction of oxidative stress [60].
Conclusion
The vulnerability of heavy metals primarily Pb, Cd, Hg, and arsenic is strongly linked with the sufferings of neurotoxicity or neurodegenerative diseases. The clinical signs and symptoms in Alzheimer’s disease, multiple sclerosis, and Parkinson’s disease are almost the same as that of indications or manifestations observed with the subjection of these heavy metals. A firm need of awareness regarding the risk of hazardous metals, yet useful in certain means and along with this the preventive measures during the handling of these metals must be practiced as a means for sound and safe health.
References
Sharma RK, Agrawal M. Biological effects of heavy metals: an overview. J Environ Biol. 2005;26(2 Suppl):301–13. PubMed PMID: 16334259. Epub 2005/12/13. eng.
Singh R, Gautam N, Mishra A, Gupta R. Heavy metals and living systems: an overview. Indian J Pharmacol. 2011;43(3):246–53. PubMed PMID: 21713085. eng.
Wu X, Cobbina SJ, Mao G, Xu H, Zhang Z, Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23(9):8244–59. PubMed PMID: 26965280. Epub 2016/03/12. eng.
Chen P, Miah MR, Aschner M. Metals and neurodegeneration. F1000Res. 2016;5:F1000. Faculty Rev-366. PubMed PMID: 27006759. eng.
Rybicki BA, Johnson CC, Uman J, Gorell JM. Parkinson’s disease mortality and the industrial use of heavy metals in Michigan. Mov Disord. 1993;8(1):87–92. PubMed PMID: 8419812. Epub 1993/01/01. eng.
Mohammadyan M, Moosazadeh M, Borji A, Khanjani N, Rahimi MS. Investigation of occupational exposure to lead and its relation with blood lead levels in electrical solderers. Environ Monitor Assess. 2019;191(3):126. 2019/02/04.
Al-Dabbagh SA, Al-Timimi DJ, Al-Dosky AH. Occupational exposure to lead in Duhok city, Kurdistan region, Iraq. Duhok Med J. 2011;5(2):76–85.
Driscoll TR, Carey RN, Peters S, Glass DC, Benke G, Reid A, et al. The Australian work exposures study: occupational exposure to lead and lead compounds. Ann Occup Hyg. 2015;60(1):113–23.
Ibeh N, Aneke J, Okocha C, Okeke C, Nwachukwuma J. The influence of occupational lead exposure on haematological indices among petrol station attendants and automobile mechanics in Nnewi, South-East Nigeria. J Environ Occup Sci. 2016;5(1):1.
Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, et al. Heavy metals exposure and Alzheimer’s disease and related dementias. J Alzheimers Dis. 2020;1:1–28.
Martins AC, Urbano MR, Almeida Lopes ACB, Carvalho MFH, Buzzo ML, Docea AO, et al. Blood cadmium levels and sources of exposure in an adult urban population in southern Brazil. Environ Res. 2020;187:109618. 2020/08/01/.
Garner R, Levallois P. Cadmium levels and sources of exposure among Canadian adults. Health Rep. 2016;27(2):10–8. PubMed PMID: 26885840. Epub 2016/02/18. eng.
Pacini S, Fiore MG, Magherini S, Morucci G, Branca JJV, Gulisano M, et al. Could cadmium be responsible for some of the neurological signs and symptoms of myalgic encephalomyelitis/chronic fatigue syndrome. Med Hypotheses. 2012;79(3):403–7. 2012/09/01/.
Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–38. PubMed PMID: 21777306. Pubmed Central PMCID: Pmc3427890. Epub 2011/07/23. eng.
Ahed JA, Ahmad MB, Zeid A-H, Abdalrahman A-B, Murtala M, Abubakar Abdulsalam B, et al. Occupational exposure to nickel, cadmium and copper among workers in jewelry manufacturingoccupational exposure to nickel, cadmium and copper among workers in jewelry manufacturing. Eur Sci J. 2014;10(15):159–169.
Mohamed Fel B, Zaky EA, El-Sayed AB, Elhossieny RM, Zahra SS, Salah Eldin W, et al. Assessment of hair aluminum, lead, and mercury in a sample of autistic Egyptian children: environmental risk factors of heavy metals in autism. Behav Neurol. 2015;2015:545674. PubMed PMID: 26508811. Pubmed Central PMCID: Pmc4609793. Epub 2015/10/29. eng.
Yoshimasu K, Kiyohara C, Takemura S, Nakai K. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neuro Toxicol. 2014;44:121–31. 2014/09/01/.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7(2):60–72. PubMed PMID: 26109881. Epub 11/15. eng.
Huang JH, Hu KN, Ilgen J, Ilgen G. Occurrence and stability of inorganic and organic arsenic species in wines, rice wines and beers from Central European market. Food Addit Contam. 2012;29(1):85–93. PubMed PMID: 22026389. Epub 2011/10/27. eng.
Chung J-Y, Yu S-D, Hong Y-S. Environmental source of arsenic exposure. J Prev Med Public Health. 2014;47(5):253–7. PubMed PMID: 25284196. Epub 09/11. eng.
Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, et al. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108(51):20656–60. PubMed PMID: 22143778. Epub 12/05. eng.
Cho Y, Seo S, Choi SH, Lee S, Kim K, Kim HJ, et al. Association of arsenic levels in soil and water with urinary arsenic concentration of residents in the vicinity of closed metal mines. Int J Hyg Environ Health. 2013;216(3):255–62. PubMed PMID: 22704486. Epub 2012/06/19. eng.
Hong Y-S, Song K-H, Chung J-Y. Health effects of chronic arsenic exposure. J Prev Med Public Health. 2014;47(5):245–52. PubMed PMID: 25284195. Epub 09/11. eng.
Rodríguez-Barranco M, Lacasaña M, Aguilar-Garduño C, Alguacil J, Gil F, González-Alzaga B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454–455:562–77. 2013/06/01/.
Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. Biomed Res Int. 2014;2014:840547. PubMed PMID: 24516855. Pubmed Central PMCID: Pmc3909981. Epub 2014/02/12. eng.
Assi MA, Hezmee MNM, Haron AW, Sabri MYM, Rajion MA. The detrimental effects of lead on human and animal health. Vet World. 2016;9(6):660–71. PubMed PMID: 27397992. Epub 06/27. eng.
Ayangbenro AS, Babalola OO. A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health. 2017;14(1):94. PubMed PMID: 28106848. Pubmed Central PMCID: Pmc5295344. Epub 2017/01/21. eng.
Wang B, Du Y. Cadmium and its neurotoxic effects. Oxid Med Cell Longevity. 2013;2013:898034. PubMed PMID: 23997854. Pubmed Central PMCID: Pmc3753751. Epub 2013/09/03. eng.
Borisova T, Krisanova N, Sivko R, Kasatkina L, Borysov A, Griffin S, et al. Presynaptic malfunction: the neurotoxic effects of cadmium and lead on the proton gradient of synaptic vesicles and glutamate transport. Neurochem Int. 2011;59(2):272–9. PubMed PMID: 21672571. Epub 2011/06/16. eng.
Larner SF, Wang J, Goodman J, O’Donoghue Altman MB, Xin M, Wang KKW. In vitro neurotoxicity resulting from exposure of cultured neural cells to several types of nanoparticles. J Cell Death. 2017;10:1179670717694523. 2017/01/01.
Pfaender S, Grabrucker AM. Characterization of biometal profiles in neurological disorders. Metallomics. 2014;6(5):960–77. PubMed PMID: 24643462. Epub 2014/03/20. eng.
Vinceti M, Bottecchi I, Fan A, Finkelstein Y, Mandrioli J. Are environmental exposures to selenium, heavy metals, and pesticides risk factors for amyotrophic lateral sclerosis? Rev Environ Health. 2012;27(1):19–41. PubMed PMID: 22755265. Epub 2012/07/05. eng.
Espart A, Artime S, Tort-Nasarre G. Cadmium exposure during pregnancy and lactation: materno-fetal and newborn repercussions of Cd(ii), and Cd-metallothionein complexes. Metallomics. 2018;10(10):1359–67. PubMed PMID: 30221266.
Farina M, Rocha JBT, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89(15):555–63. 2011/10/10/.
Al-Saleh I, Nester M, Abduljabbar M, Al-Rouqi R, Eltabache C, Al-Rajudi T, et al. Mercury (Hg) exposure and its effects on Saudi breastfed infant’s neurodevelopment. Int J Hyg Environ Health. 2016;219(1):129–41. PubMed PMID: 26541552. Epub 2015/11/07. eng.
Lohren H, Pieper I, Blagojevic L, Bornhorst J, Galla H-J, Schwerdtle T. Neurotoxicity of organic and inorganic mercury species–effects on and transfer across the blood-cerebrospinal fluid barrier, cytotoxic effects in target cells. Perspect Sci. 2015;3:21–2.
Ceccatelli S, Daré E, Moors M. Methylmercury-induced neurotoxicity and apoptosis. Chem Biol Interact. 2010;188(2):301–8. PubMed PMID: 20399200. Epub 2010/04/20. eng.
Alexandrov PN, Pogue AI, Lukiw WJ. Synergism in aluminum and mercury neurotoxicity. Integr Food Nutr Metab. 2018;5(3):1–7. https://doi.org/10.15761/IFNM.1000214. PubMed PMID: 29938114. Epub 04/13. eng.
Siblerud R, Mutter J, Moore E, Naumann J, Walach H. A hypothesis and evidence that mercury may be an etiological factor in Alzheimer’s disease. Int J Environ Res Public Health. 2019;16(24):5152.
Pieper I, Wehe CA, Bornhorst J, Ebert F, Leffers L, Holtkamp M, et al. Mechanisms of Hg species induced toxicity in cultured human astrocytes: genotoxicity and DNA-damage response. Metallomics. 2014;6(3):662–71. PubMed PMID: 24549367. Epub 2014/02/20. eng.
Farina M, Avila DS, da Rocha JBT, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62(5):575–94. 2013/04/01/.
Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011;256(3):405–17. 2011/11/01/.
Ni M, Li X, Rocha JBT, Farina M, Aschner M. Glia and methylmercury neurotoxicity. J Toxicol Environ Health Part A. 2012;75(16–17):1091–101. 2012/08/15.
Kern JK, Geier DA, Sykes LK, Haley BE, Geier MR. The relationship between mercury and autism: a comprehensive review and discussion. J Trace Elements Med Biol. 2016;37:8–24. 2016/09/01/.
Mochizuki H. Arsenic neurotoxicity in humans. Int J Mol Sci. 2019;20(14):3418. PubMed PMID: 31336801. Pubmed Central PMCID: Pmc6678206. Epub 2019/07/25. eng.
Medda N, Patra R, Ghosh TK, Maiti S. Neurotoxic mechanism of arsenic: synergistic effect of mitochondrial instability, oxidative stress, and hormonal-neurotransmitter impairment. Biol Trace Elem Res. 2020;198(1):8–15. PubMed PMID: 31939057.
Sharma A, Kshetrimayum C, Sadhu HG, Kumar S. Arsenic-induced oxidative stress, cholinesterase activity in the brain of Swiss albino mice, and its amelioration by antioxidants vitamin E and coenzyme Q10. Environ Sci Pollut Res. 2018;25(24):23946–53. PubMed PMID: 29948670.
Zhou H, Zhao W, Ye L, Chen Z, Cui Y. Postnatal low-concentration arsenic exposure induces autism-like behavior and affects frontal cortex neurogenesis in rats. Environ Toxicol Pharmacol. 2018;62:188–98. 2018/09/01/.
Gandhi D, Kumar R. Arsenic toxicity and neurobehaviors: a review. Innov Pharm Pharmacother. 2013;1(1):1–15.
Yen CC, Ho TJ, Wu CC, Chang CF, Su CC, Chen YW, et al. Inorganic arsenic causes cell apoptosis in mouse cerebrum through an oxidative stress-regulated signaling pathway. Arch Toxicol. 2011;85(6):565–75. 2011/06/01.
Ishii N, Mochizuki H, Ebihara Y, Shiomi K, Nakazato M. Clinical symptoms, neurological signs, and electrophysiological findings in surviving residents with probable arsenic exposure in Toroku, Japan. Arch Environ Contam Toxicol. 2018;75(4):521–9. 2018/11/01.
Mohammed Abdul KS, Jayasinghe SS, Chandana EPS, Jayasumana C, De Silva PMCS. Arsenic and human health effects: a review. Environ Toxicol Pharmacol. 2015;40(3):828–46. 2015/11/01/.
Niño SA, Morales-Martínez A, Chi-Ahumada E, Carrizales L, Salgado-Delgado R, Pérez-Severiano F, et al. Arsenic exposure contributes to the bioenergetic damage in an Alzheimer’s disease model. ACS Chem Neurosci. 2019;10(1):323–36. 2019/01/16.
Kim H-C, Jang T-W, Chae H-J, Choi W-J, Ha M-N, Ye B-J, et al. Evaluation and management of lead exposure. Ann Occup Environ Med. 2015;27:30. PubMed PMID: 26677413. eng.
Bjørklund G, Aaseth J, Ajsuvakova OP, Nikonorov AA, Skalny AV, Skalnaya MG, et al. Molecular interaction between mercury and selenium in neurotoxicity. Coord Chem Rev. 2017;332:30–7. 2017/02/01/.
Khan R, Ali S, Mumtaz S, Andleeb S, Ulhaq M, Tahir HM, et al. Toxicological effects of toxic metals (cadmium and mercury) on blood and the thyroid gland and pharmacological intervention by vitamin C in rabbits. Environ Sci Pollut Res. 2019;26(16):16727–41. 2019/06/01.
Liu S, Piao F, Sun X, Bai L, Peng Y, Zhong Y, et al. Arsenic-induced inhibition of hippocampal neurogenesis and its reversibility. Neurotoxicology. 2012;33(5):1033–9. PubMed PMID: 22561869. Epub 2012/05/09. eng.
Zwolak I. The role of selenium in arsenic and cadmium toxicity: an updated review of scientific literature. Biol Trace Element Res. 2020;193(1):44–63. PubMed PMID: 30877523. Epub 03/15. eng.
Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 2014;1(2):132–47. PubMed PMID: 24860722. eng.
Ahmad M, Wadaa MA, Farooq M, Daghestani MH, Sami AS. Effectiveness of zinc in modulating perinatal effects of arsenic on the teratological effects in mice offspring. Biol Res. 2013;46(2):131–8. PubMed PMID: 23959010. Epub 2013/08/21. eng.
Conflict of Interest
Nothing to declare.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rehman, Q., Rehman, K., Akash, M.S.H. (2021). Heavy Metals and Neurological Disorders: From Exposure to Preventive Interventions. In: Akash, M.S.H., Rehman, K. (eds) Environmental Contaminants and Neurological Disorders. Emerging Contaminants and Associated Treatment Technologies. Springer, Cham. https://doi.org/10.1007/978-3-030-66376-6_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-66376-6_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66375-9
Online ISBN: 978-3-030-66376-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)