Abstract
The embryo of humans and other mammals requires the presence of two subcellular structures, named centrioles. The centrioles act as nucleation points for two massive structures that help build the skeleton of the cell, the centrosome, and the cilium. Centrosomes nucleate and anchor star-shaped arrays of microtubules known as asters that assist in cell division. Cilia (known as the flagella in sperm cells) are hair-like structures that mediate cell movement and signaling. Two centrioles are maintained in each cell of the developing embryo by precisely duplicating and segregating during each cell cycle. It is accepted that the mature oocytes of mammals do not contain centrioles, and therefore do not contribute centrioles to the embryo. Similarly, it is accepted that, in humans and other non-murine mammals, the mature sperm has one centriole with a typical structure, which is contributed to the embryo during fertilization. But, how the embryo acquires its first two centrioles, or what their precise role in the early embryo is, is only now starting to be revealed. Recently, it was discovered that human and bovine sperm also have a second, atypical centriole, and this centriole is functional in the embryo. Therefore, the typical and atypical centrioles of the sperm appear to be the first two centrioles of the embryo. Here, we will focus on sperm centrioles in humans and other mammals, their formation during spermatogenesis, and their role in the embryo.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Introduction
Centrioles are the fundamental building blocks of two unique subcellular organelles, the centrosome and the cilium (aka flagellum in sperm); the sperm alone, not the oocyte, provides them to the embryo upon fertilization. Centrioles are the only known structure that is exclusively paternally inherited. While this unusual pattern of inheritance has been known for over 100 years, many mysteries remain regarding the role of the centrioles in reproduction (Scheer 2014). Here we review the recent discovery of a sperm centriole with atypical structure and composition, which is surprising considering the high conservation of centriolar structure exhibited throughout evolution (Carvalho-Santos et al. 2010). We start with a general overview of the typical centriole structure. We then describe the centrioles in the sperm and zygotes of insects, where atypical centrioles were first discovered; next, centrioles in mammalian sperm and zygotes, where atypical centrioles were subsequently found; and, then the odd exception of mice, where sperm centrioles are not detected. Finally, we speculate why atypical centrioles may have formed and discuss the clinical relevance of sperm centrioles, methods to detect them, and their potential implications in infertility.
General Overview: Typical Centriole Structure, Composition, Function, and Formation
The centriole is a complex subcellular organelle whose cellular role ranges from assisting in cell division to cilium formation for sensory functions and motility. Because of this wide range of functions, centrioles consist of many parts. The most basic, hallmark features of a centriole are its ninefold symmetric cylindrical microtubules and its strict number control; cycling cells contain exactly two centrioles at the G1 phase and have precise mechanisms to maintain this number. The two centrioles differ in age, structure, and function; one is referred to as the mother (mature) and the other is the daughter (immature). Neither centriole is immediately essential as a microtubule-organizing center for division, but they are necessary for maintaining proper centriole numbers, and they are fundamentally essential for cilia formation. Malformation or improper centriole numbers can cause developmental defects, cellular dysfunction, and cell death. Many tissues developing from the embryo need centrioles, so, the two functional centrioles are essential for the embryo. Therefore, this section will focus on the structure, components, and functions of typical centrioles in cycling cells, such as those arising from the differentiating embryo.
Typical Centrioles Are Cylindrical Cytoplasmic Structures Made of Nine Triplet Microtubules
Centrioles are cylindrical, proteinaceous structures found in the cytosol. Centrioles are heavily conserved across animal evolution; most animals studied have centrioles (with the notable exception of in planarians worms (Azimzadeh et al. 2012)). Since their structure is so iconic, electron microscopy has provided a structure-based definition that has been used nearly exclusively since centrioles were described using this technology in the mid-twentieth century (Burgos and Fawcett 1956).
The centriole’s most distinguishing component is its nine microtubule triplets (Fig. 1). These microtubules form the centriole wall and are named the A, B, and C microtubules, from the innermost to the outermost, respectively. The A microtubule is round, whereas the B and C microtubules are incomplete and attached to the adjacent microtubules (reviewed by Winey and O’Toole 2014). The lengths of the microtubules are different, with the A and B microtubules being longer than the C microtubule (Fig. 1a). The diameter of the centriole is approximately 250 nm, and the length ranges from 200 to 500 nm, depending on what phase of the cell cycle the cell is in (reviewed by Avidor-Reiss and Gopalakrishnan 2013a). The centriole is a polar structure; the minus end of the microtubules is at the base of the centriole and the plus end is at the tip.
Typical Centrioles Often Have Associated Substructures that Correspond to the Centriole’s Maturity
Centrioles often have associated substructures within the cylinder, in the lumen, and peripheral to the wall (reviewed by Winey and O’Toole 2014). Since the mother and daughter centrioles differ in maturity, they each have distinctive features that are used to identify them.
The mother centriole has nine pairs of appendages and can form a cilium and the centrosome (Fig. 1). The mother’s distal appendages are fibrous extensions of filaments present at the centriole tip that anchor the centriole to the cell membrane, allowing primary cilia formation. Since the cilium is exclusively nucleated from the mother centriole, it is logical that these appendages are absent from the daughter centriole. The subdistal appendages appear as a triangular structure attached to the microtubule triplets, which anchor the microtubules that emanate from the centriole (Bowler et al. 2019; Kodani et al. 2013; Paintrand et al. 1992) (Fig. 1a).
The daughter centriole often has a cartwheel structure inside the base of the cylinder. The cartwheel is primarily made up of an evolutionarily conserved centriole-specific protein, Sas-6, and has nine spokes that connect to the microtubular wall. While the cartwheel is essential for the normal formation of the microtubular wall, it only partially mediates the ninefold symmetry of the microtubular wall (Hilbert et al. 2016). This cartwheel helps form the new centriole (Fig. 1b) (Cavalier-Smith 1974), but upon maturity, it is no longer needed. Therefore, the mother centriole usually lacks a detectable cartwheel (Lange and Gull 1996; Paintrand et al. 1992).
In both the mother and daughter centrioles, the tip of the lumen is filled with proteins. These proteins are evolutionarily conserved and centriole-specific. However, their precise role is unclear (Azimzadeh et al. 2009; Pearson et al. 2009). Despite these proteins not being well understood, electron microscopy has identified “helical disks” in the distal lumen (Ibrahim et al. 2009; Paintrand et al. 1992), and super-resolution microscopy suggests that the lumen is highly organized (Sydor et al. 2018).
A Typical Centriole Forms the Centrosome or the Cilium/Flagellum
Centrioles have two main functions; the first function is essential: the mother centriole forms a cilium for sensory functions and motility. The second, and perhaps more well-known function is to form a centrosome that nucleates a vast cytoskeleton that is important for intracellular transport and cell division.
The most essential function of the centriole is to template the polymerization and extension of the centrioles’ A and B microtubules to create a doublet microtubule-based axoneme that acts as the skeleton of the cilium/flagellum. The cilium functions as a sensory and motility organelle (reviewed by Azimzadeh and Marshall 2010). For example, in the retina, the rod and cones are sensory cilia that recognize light (reviewed by Yildiz and Khanna 2012). In contrast, the motile cilium of the sperm cell, the flagellum, propels the sperm cell to the egg (Fawcett 1975).
The second function of the centriole, forming a centrosome, involves the recruitment and organization of pericentriolar material (aka PCM), a protein mass that surrounds the centriole (Fig. 1b) (Bornens 2002). From the PCM, the centrosome nucleates and anchors a star-shaped (aster) microtubule network, and therefore, the centriole initiates the formation of a significant component of the cytoskeleton (Bobinnec et al. 1998). The sperm centriole forming a centrosome is particularly important after fertilization because the large aster (aka the sperm aster) mediates the migration of the pronuclei (Navara et al. 1994).
While centrioles are considered the major microtubule organizing center of the cell, alternate and compensatory mechanisms exist for exceptional circumstances and specific cell type programs. For example, in oocytes, planarians, and plants, accurate division occurs in the absence of centrioles, in some circumstances, other organelles can adopt the microtubule organizing function of the centrioles. Additionally, in sick, centriole-lacking cells, inaccurate division occurs without centrioles (Vitre and Cleveland 2012). Therefore, in a cell type-specific manner, centrioles can be essential, nonessential, or any degree of importance in between, but because centrioles are essential for typical, cycling cells, we consider two centrioles to be essential unless alternate molecular pathways have been shown.
Centriole Maturation and Duplication Regulates Centriole Number and Structure
Centriole number is regulated by a precise mechanism of duplication, maturation, and segregation. When a cell prepares to divide, each centriole duplicates once; by coordinating centriole duplication with the cell cycle, a healthy cell guarantees that it forms only one new centriole near each preexisting centriole (reviewed by Avidor-Reiss and Gopalakrishnan 2013b).
In the typical centriole biogenesis pathway, a cell alternates between having two and four centrioles. During mitosis (M), each daughter cell receives two connected (engaged) centrioles as part of the centrosome at its spindle pole. After division, in Gap One (G1) phase in each daughter cell, the pair of centrioles migrates to the cell periphery, where the mother centriole docks to the cell membrane and forms a cilium. Once the cell receives a signal to move forward into Synthesis (S) phase, the DNA replicates while the cilium starts to disassemble. Furthermore, the centriole forms a centrosome, and each centriole forms a new centriole precursor (procentriole) perpendicular to its wall. Consequently, the cell ends the S phase with four centrioles organized into two pairs, each containing a new procentriole and an older centriole, either the mother or the now maturing daughter. As the cell progresses to the Gap two (G2) phase, the two pairs of centrioles separate from each other and the procentrioles begin to mature, becoming what is sometimes referred to as granddaughter centrioles. Each pair of centrioles (mother and granddaughter, or maturing daughter and granddaughter) recruits PCM and assembles an aster. Next, the cell enters mitosis and the centrosomes push each other to opposite sides of the cells. Each centrosome localizes to one spindle pole and helps determine the axis of the spindle. During mitosis, the new centriole in each pair is disengaged but is kept next to the older centriole via a protein linker (Kuriyama and Borisy 1981; Vorobjev and Chentsov Yu 1982). During mitosis, each pair of centrioles localize to opposite poles of the spindle and segregates into different daughter cells (Tsou and Stearns 2006).
Because of this strict number-regulating pathway, a centriole needs two cell cycles from its initial formation to reach functional maturity. The procentriole, formed during the S phase as an immature centriole, will gradually gain distinct abilities in G2 and M; it then becomes a daughter centriole (G1 and subsequent S, M, and G2), and finally a mother centriole (G1). Only when a centriole enters the G1 phase for the second time does it become a mature, mother centriole and it is only when it has reached maturity that it can form a cilium for the first time.
This canonical centriole biogenesis pathway is used in most animal cell types studied, with a few notable exceptions. The first major exception is multiciliated cells; during differentiation, cells acquire supernumerary centrioles through alternate molecular pathways that are not tied to the cell cycle but are part of the cell’s specific differentiation program (reviewed by Tang 2013). For example, ciliated epithelial cells have hundreds of centrioles (aka basal bodies) (Vladar and Stearns 2007). The other major exception to canonical biogenesis is de novo centriole formation. De novo centriole formation is well-established in two circumstances, mouse embryos, which we will address in the section titled “The Confusing Status of Murine Sperm Centrioles,” and manipulated cell culture systems. When centrioles are ablated or excised, structurally normal centrioles are formed de novo; however, they form in variable numbers (Uetake et al. 2007). Likewise, de novo formation can be induced using overexpression of certain centriolar proteins, but it also results in supernumerary centrioles (Rodrigues-Martins et al. 2007). Supernumerary centrioles are known to cause aneuploidy (Ganem et al. 2009). However, over time some tissue culture cells with supernumerary centrioles can regain the proper number of centrioles (Wong et al. 2015).
Insect’s Sperm and Zygote Have a Typical Centriole and an Atypical Centriole
Like sperm cells in most animal species, insect sperm consists of two major parts, the head and a tail (Fig. 2a). The sperm tail contains the axoneme, which is the cytoskeletal basis for sperm movement, and a long mitochondrial derivative that acts as a second structural element in the tail (Chen et al. 2017; Fabian and Brill 2012; Noguchi et al. 2012). The neck connects the head and tail and contains two centrioles in many animals. These centrioles are traditionally named the distal centriole and proximal centriole (Ritter 1919; Thomas Harrison Montgomery 1912). The distal centriole is farther away from the nucleus, closer to the cell membrane, and nucleates the axoneme. Alternatively, the proximal centriole is near the nucleus, and farther away from the cell membrane, and does not nucleate an axoneme. For a long time, it was thought that insects had only one centriole, the distal centriole (reviewed by Callaini et al. 1999; Fuller 1993; Phillips 1970; Riparbelli et al. 2010). However, more recently, it became evident that some insects have an additional second centriole that has an atypical structure and composition. The second, atypical centriole does not form the axoneme, and is, therefore, analogous to the proximal centriole; it is known as the Proximal Centriole-Like structure (PCL) (reviewed byAvidor-Reiss et al. 2015). Likely, a second centriole is also present in the sperm of other insect species, but it has not been observed because the techniques utilized were not sensitive enough to detect the PCL, due to its very small size, easily disrupted structure, and irregular features.
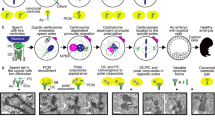
Drosophila sperm centrioles. (a) An overview of sperm morphology. (b–d) Spermiogenesis begins as a round spermatid with a single centriole and a centriole like structure (PCL) (b). Then during spermiogenesis, the axoneme extends, nuclear morphology changes and the centrioles are remodeled to produce a (c) spermatozoa with two atypical centrioles. Both centrioles are contributed to the zygote (d) where they each recruit PCM, organize microtubules, act as platforms for the formation of a new, typical, daughter centriole, and form a bipolar spindle. Atypical centrioles are highlighted in green. DC distal centriole, PCM pericentriolar material, PCL proximal centriole-like, Ax axoneme, NK Nebenkern derivative (mitochondria), ZdC zygotic daughter centriole
Insect Sperm Often Has Two Atypical Centrioles
Extensive literature has been published on the fine ultrastructure of the sperm of various insects (reviewed by Jamieson et al. 1999). Because insects are one of the most diverse classes of animals, it is not surprising that these publications describe a wealth of different findings in the neck. Adding to this complexity, the varied centriole structure in sperm means that the literature is extremely varied in their identification. Recently though, as techniques have become more sensitive, it seems likely that in male insects, meiosis culminates with the sperm containing precisely two centrioles, although their structure and composition are varied (reviewed by Avidor-Reiss et al. 2015) (Fig. 2b).
During spermiogenesis, the spermatids undergo a series of morphological changes that produce an elongated spermatozoon with two centrioles. At the same time, both centrioles undergo a process termed centriole remodeling, which in some insects, leaves one detectable centriole, and one that is too small and unusual to be detected using standard techniques (Fig. 2c). Remodeling is presumably related to the function of the centriole in the mature sperm; however, the exact relationship of remodeling, sperm function, and embryonic development has not been fully evaluated yet.
The most easily identifiable centriole in insect haploid sperm cells is the distal centriole, which forms the axoneme. Centriole remodeling culminates in several atypical features in the distal centriole that vary heavily from species to species. In Drosophila, the centriole’s diameter is reduced, causing the transition from a round lumen, to an elliptical shape (Khire et al. 2016). Some insects’ (e.g., Drosophila and honey bees) distal centrioles have nine triplet microtubules (Hoage and Kessel 1968; Tates 1971), while others have doublets (e.g., Tribolium (Dias et al. 2015; Fishman et al. 2017). Sometimes the two central microtubules from the axoneme are observed permeating the centriole lumen (reviewed in Avidor-Reiss 2018). Despite the distal centriole’s wealth of atypical features in various insects, it can be recognized using electron microscopy.
Where the distal centriole is recognizable, the structure of the second centriole in insects is less regular, both in spermatids and spermatozoon. In some insects, such as Adalia, the proximal centriole has singlet microtubules in spermatids but lacks microtubules in the spermatozoon (Dallai et al. 2017). In Drosophila and Tribolium, the second centriole is extremely difficult to recognize because it lacks microtubules at all stages (spermatids and spermatozoa) (Gottardo et al. 2015; Khire et al. 2016). However, because it is not connected to the axoneme, but still appears to perform centriolar functions, it is presumed to be the analog of the Proximal Centriole.
In Drosophila spermatids, the PCL is composed of an electron-dense material that forms a ring around a central tubule, which is not a microtubule (Fig. 2b) (Gottardo et al. 2015; Khire et al. 2016). The PCL in Tribolium spermatids also lacks microtubules; it is composed of an electron-dense structure surrounded by translucent material (Fishman et al. 2017). In Drosophila, during spermiogenesis, centriole remodeling modifies the structure of the PCL, the electron-dense material is wholly reduced, leaving it with only a central tubule (Khire et al. 2016) (Fig. 2c).
The PCLs in Drosophila and Tribolum went undetected for many years because they are minuscule and difficult to distinguish from cytosolic structures; they additionally lack centriolar hallmarks, such as microtubules. And yet, despite their atypical features, they were determined to be centrioles because they appear to follow the typical centriole-formation pathway. This conclusion is supported in Drosophila, where after fertilization, the PCL performs centriolar functions, namely recruiting PCM and acting as a platform for the formation of a procentriole (Fig. 2d) (Blachon et al. 2014).
The PCL appears to resemble the early procentriole, and therefore is thought to be a form of centriolar neoteny (reviewed by Avidor-Reiss and Turner 2019; Jo et al. 2019), where juvenile characteristics are retained during maturation. It is not well understood why a neotenic PCL benefits the sperm, but we speculate that it plays a role in motility, perhaps related to the small size of the sperm neck.
Zygote Centrioles
Upon fertilization, the spermatozoon provides the distal centriole and the proximal centriole (or PCL) to the zygote. Both centrioles, despite their atypical features, are essential for development (Fig. 2d) (Khire et al. 2015, 2016). After fertilization, they recruit the maternal PCM and form a centrosome and aster, which allows for male and female pronuclei congregation (Blachon et al. 2014; Riparbelli et al. 2000). Then, the distal centriole and the PCL duplicate and each acts as a platform for the formation of a new, typical procentriole. Thus, the resulting zygote forms two centrosomes, one with the distal centriole and its zygotic daughter centriole, and the other with the PCL and its zygotic daughter centriole. Despite the atypical structure of both sperm centrioles, they direct the formation of typical centrioles, suggesting that centriolar microtubules do not employ a template mechanism to form new centrioles (Avidor-Reiss et al. 2012).
In the zygote, the apparent role of the centriole is to form a centrosome and regulate centriole number. It is unlikely that the PCL, with its microtubule-lacking structure, can form a cilium without regaining its typical structure, and there is no evidence to suggest that it is ever regenerated into a typical centriole. Likewise, the distal centriole remains attached to the axoneme for several cell divisions which would logically impede its ability to form a cilium (Riparbelli and Callaini 2010). Thus, both the PCL and the distal centriole cannot form a cilium, but this is not an issue as the first cilia in the embryo forms much later in development.
Typical and Atypical Sperm Centrioles Function in the Zygote of Non-murine Mammals
Much like insects, mammalian sperm consists of two major parts, the head and the tail, which are connected by a neck that contains the two centrioles (Fawcett and Phillips 1969) (Fig. 3a). Unlike the PCL in insects, the proximal centriole is easily identifiable in most mammals studied (with the exception of murines, see section “The Confusing Status of Murine Sperm Centrioles”) due to its prominent and characteristic centriolar microtubules; the distal centriole in mammalian sperm is the source of much confusion. The distal centriole can easily be found in early spermatids but was not identified for many years in the spermatozoon due to its modification via centriole remodeling during spermatogenesis.
Humans and bovine sperm centrioles. (a) Overall sperm morphology. Spermiogenesis begins with a round spermatid (b), with a typical proximal and distal centriole. The Annulus is the structure homologous to the insect transition zone (Avidor-Reiss and Leroux 2015; Basiri et al. 2014). Throughout spermiogenesis, centrioles are modified while the axoneme extends and the nucleus is reshaped to produce a spermatozoa (c). Upon fertilization, the spermatozoa contribute both centrioles to the oocyte to produce a zygote. After fertilization, these sperm centrioles recruit PCM, organize microtubules, act as a platform for the formation of two new, typical centrioles, and form a bipolar spindle (d). Atypical centrioles are highlighted in green. PCM pericentriolar material, ODF outer dense fibers, PC proximal centriole, DC distal centriole, ZdC zygotic daughter centriole, Ax axoneme
Mammalian spermatogenesis occurs within the seminiferous tubules of the testes (reviewed by Hess and De Franca 2009; Wistuba et al. 2007). Spermatogonial stem cells are the stem cell niche from which sperm cells differentiate. They reside in the outer layer of cells along the periphery of the seminiferous tubule, adjacent to the epithelium (basal lamina). As they develop and differentiate, they move inwards, toward the lumen. Due to this development pattern, a single cross section of a seminiferous tubule contains several different stages of sperm, which allows for a parsimonious determination of developmental processes (reviewed by Roosen-Runge 1977). After several mitotic divisions, the spermatogonia enter meiosis as primary spermatocytes, and then when they enter meiosis II, they are termed secondary spermatocytes. Finally, after meiosis, they are termed spermatids. While no longer in a state of division, round spermatids begin to undergo dramatic morphology change in a process referred to as spermiogenesis. After the elongated spermatid releases excess cytoplasm, the spermatozoon is released into the seminiferous tubule’s lumen. The spermatozoa continue to mature in the epididymis (reviewed by Neto et al. 2016).
During spermiogenesis, the distal centriole nucleates an axoneme and densely packed, specialized structural features form in the sperm’s neck, including the striated columns, capitulum, and fibrous sheath. Interestingly, at the same time, the proximal centriole nucleates an axoneme-like structure known as the centriolar adjunct (not to be confused with insect sperm’s PCM-like structure of the same name). The centriolar adjunct is a mysterious feature because its function and mechanism of formation are unknown. Furthermore, the proximal centriole has long been accepted by some researchers as the less mature daughter centriole, which should not be capable of nucleating an axoneme. This apparent disagreement with the known functions of the daughter centriole has prompted an investigation into the origin of the proximal centriole, and because the distal centriole abuts the side of the proximal centriole, which resembles the engaged orientation where the immature daughter centriole abuts the side of the mature mother, it has been proposed that the proximal centriole’s origin is actually the mother centriole, and the distal centriole originates from the daughter (Alieva et al. 2018). Intriguingly, the adjunct is eliminated in the spermatozoa of most animals, including rhesus monkeys, but not in humans, indicating that it is a sign of relative immaturity (neoteny) of human sperm or that it has a unique function in humans (Manandhar et al. 2000b; Zamboni 1971).
Similarly, to centriole remodeling in insects, centriole remodeling occurs during spermiogenesis. During centriole remodeling, while structural changes occur, some proteins are reduced, while others are enriched. Centriole remodeling results in a spermatozoon with a mildly affected proximal centriole and a dramatically remodeled distal centriole, that is unrecognizable using standard criteria. Remodeling continues as the spermatozoa mature in the epididymis, and may even continue beyond that (Simerly et al. 2016). However, the mechanism that controls centriole remodeling, and the purpose of remodeling process, is unknown.
Non-murine Mammalian Sperm Have Two Centrioles, One Typical and One Atypical in Structure
During centriole remodeling, the round spermatid’s two typical centrioles (Fig. 3b) undergo a series of changes that disguise the centrioles. At this time, the PCM transforms into the striated column and capitulum (Fig. 3) (Fawcett and Phillips 1969) and typical PCM proteins, such as γ-tubulin, PCNT, CEP152, and CEP192, are reduced from the striated columns and capitulum (Fawcett and Phillips 1969). However, centriole proteins that are not part of the PCM of most cell types, RTTN and CEP295, are found in the capitulum and striated columns. Sperm specific proteins, SPAG4 (Shao et al. 1999), Speriolin (Goto and Eddy 2004), and SPATA6 (Yuan et al. 2015) also localize to these structures.
Remodeling changes occur in the proximal centriole, although it keeps its typical microtubule structure (Manandhar et al. 2000a; Schatten 1994) (Fig. 3c). The proximal centriole is marked by classical centriolar proteins such as CETN1, CEP135, CEP120, and CEP76, but it lacks centriole wall proteins CNTROB and RTTN (Fishman et al. 2017; Manandhar et al. 2000a). This unusual protein composition suggests that, even with a typical shape, the proximal centriole is partially remodeled.
The greatest enigma of the mammalian sperm neck has been the distal centriole. While the nine triplets of microtubules of the distal centriole are easily identified in spermatids, the identity or even the existence of the distal centriole has been debated for nearly half a century (Fawcett and Phillips 1969).
The Discovery of the Atypical Centriole in Non-murine Mammals
The historic inability to recognize the distal centriole lead to the degeneration hypothesis, which speculated that the distal centriole was reduced to a nonfunctional remnant during spermatogenesis, leaving behind the electron light region called the vault, and occasionally some disorganized microtubules (Manandhar and Schatten 2000; Manandhar et al. 2000b). The distal centriole lacks RTTN, CEP295, CEP135, and CEP120 (centriole wall proteins); CEP76 and CP110 (centriolar tip proteins); and CEP164 and CEP89 (appendage proteins) (Fishman et al. 2017). While the degeneration hypothesis was accepted for many years, it was recently disproved when it was discovered that the distal centriole is present and functional, albeit with an atypical structure that prevented detection in the past.
Unlike the typical cylinder shape with triplet microtubules, the distal centriole has splayed microtubule doublets, and a restricted protein profile (Fishman et al. 2018) (Fig. 3c). These identification obstructions were overcome by three technological improvements: (1) improved electron microscopy and fixation/preparation, (2) highly specific antibodies against recently discovered centriolar components, and (3) super-resolution microscopy.
-
1.
In the past, sperm centrioles were studied in chemically fixed samples. Part of the difficulty in identifying the distal centriole using the standard, chemical fixation electron microscopy is that the distal centriole’s microtubules are masked by closely associated dark electron-dense structures. With new fixation techniques, namely high-pressure freeze-substitution preparation, the resolution is improved enough that eight to nine doublet microtubules are visible in a unique configuration; these microtubules are splayed out and flattened around the vault.
-
2.
The distal centriole also went unnoticed because classic centriolar proteins were either undetectable or inconsistent. For example, CETN1 inconsistently showed unequal staining in the proximal and distal centriole, which was interpreted as a sign that the distal centriole degenerates partially or completely (Manandhar et al. 2000b). However, previously unstudied centriole lumen proteins such as POC1B and POC5 can be easily and consistently identified in both centrioles using immunofluorescence, as can CETN1, with improved fixation conditions. Furthermore, POC1B labels the distal centriole more prominently than the proximal, suggesting that the distal centriole is not degendered but rather remodeled into an atypical, almost unrecognizable structure.
-
3.
With the recent advancement of super-resolution microscopy, the fine structure of the distal centriole was reexamined. The splayed doublets noted in the electro-micrographs are flanked by rod structures labeled with POC5 and POC1B. With further improved resolution, Stochastic Optical Reconstruction Microscopy shows rods of POC5. The exact interactions between POC5, POC1B, CETN1, and microtubules are unknown. It is also possible that other interacting partners have yet to be identified.
To date, the atypical distal centriole has been meticulously described in human and bovine sperm. Although the remodeled distal centriole is expected to be present in most non-murine mammals, this remains to be tested. Interestingly, the atypical distal centriole appears to have a distinct size in different animals (smaller in humans as compared to bovine). It would be important to see size variation between non-murine mammals and to gain insight into the reason for size variability. The exact role of the remodeled distal centriole is unknown, but speculations exist (see section “The Evolution of the Atypical Centriole” below).
The Two Sperm Centrioles Function in the Zygote of Non-murine Mammals
Because centrioles are so important for accurate cell division and forming cilia (Bornens 2012) and the embryo is a cycling cell, it makes sense that centrioles are similarly important for the developing embryo. Abnormal centriole numbers can result in aneuploidy and, during development, can lead to serious birth defects or spontaneous abortion (Sathananthan et al. 1996; Vitre and Cleveland 2012). This logic has been used as the primary support for the idea that centrioles are essential for the embryo; experimental evidence of this in mammals, is limited, but the idea is universally accepted.
Since centrioles are essential for the embryo, it is interesting that the oocyte, which provides most of the organelles an embryo needs to develop, has no centrioles (Manandhar et al. 2005; Namgoong and Kim 2018; Szollosi et al. 1972). During oogenesis, the centrioles are eliminated through a centrosome reduction program that varies between animals. In mammals, centrioles disappear during the Pachytene stage (during prophase of meiosis I), meaning that the oocyte lacks centrioles during both the first and the second meiotic divisions, and likely does not contribute any centrioles to the offspring (reviewed in Manandhar et al. 2005). Therefore, if the embryo is to inherit centrioles to use as a platform for the formation of new centrioles or for centriole number control, it must inherit the centrioles from the sperm or form them de novo (Fig. 4).
The elimination of centrioles in the oocyte appears to be an active, dominant process, not simply the result of protein degradation over time. This is exemplified when centrioles were transferred during somatic cell nuclear transfer in pigs; CETN1/2 labeling showed degradation of the somatic centrioles (Manandhar et al. 2006). One interpretation of this observation is that the developmental program in the oocyte can affect the sperm centriole once introduced to the egg. If the oocyte has a dominant centriole reduction program that acts on sperm centrioles, it is possible that the sperm coevolved with postfertilization centrosome reduction to minimize the centriolar contribution (the passive hypothesis described in section “Atypical Sperm Centrioles May Have Passively Evolved Due to Lack of Necessity”).
Because of the ethical considerations surrounding this work, and because mice are not a suitable model system for investigating the centriole’s role in human reproduction, direct observations of the centrioles behavior in normal zygote is limited to small observational studies, mostly in nonviable embryos (Kai et al. 2015; Sathananthan et al. 1996). Although some of these studies recognize embryonic centrioles using electron microscopy or PCM-specific antibodies, it is difficult to determine whether or not their centrioles resemble the centrioles of healthy embryos. Furthermore, it is impossible to know if the centrioles were the cause of the embryo’s defect, or if they were simply affected by defects in another process that also plagued the embryo. As a result, most studies on viable non-murine embryos, especially in recent years, use embryos from livestock species, such as cattle; more research on these species will benefit the understanding of reproduction (Madeja et al. 2019; Polejaeva et al. 2016).
After fertilization, the sperm tail remains associated with the male pronucleus during decondensation, and recruitment of maternal PCM proteins suggests that the centrosome remains affiliated with the sperm tail (Wu et al. 1996). After the completion of meiosis II and the extrusion of the second polar body, the centrosome continues to recruit PCM proteins and forms the sperm aster, which congregates the male and female pronuclei (Fig. 4a). At this point in cattle zygotes, CEP152 can be detected in two foci, one of which is attached to the sperm axoneme, suggesting that both the proximal and remodeled distal centriole recruit PCM. Next, centriole duplication takes place. Prior to entry into mitosis, foci can be seen using anti-CEP152, two of which are co-labeled with anti-Sas6, indicating that they are newly formed centrioles (Fishman et al. 2018). This suggests that the sperm’s centrioles, despite being atypical, are still able to perform key functions of centrioles, forming an aster and acting as a platform for centriole duplication.
After the formation of the new centrioles, the zygote enters mitosis, forms a bipolar spindle, and divides, providing each of the two resulting blastomeres with exactly two centrioles. Interestingly, throughout this first division, the distal centriole remains attached to the sperm’s axoneme. This was observed originally because the axoneme appeared attached to one of the spindle poles during the first division, and later the centriole itself was identified in this pole. While the axoneme can be observed for several cell cycles, it is not clear if the centriole remains attached to it, or how long the centriole remains attached and thereby incapable of nucleating a cilium. The asymmetry of the zygote’s centrosomes, due in part to the presence of the axoneme, means that the contents of the two blastomeres is inherently unequal. The implications, if any, of this unequal inheritance on the blastomeres or their eventual fates are unknown.
The Confusing Status of Murine Sperm Centrioles
For many reasons, including generation time, ease of breeding, and maintenance costs, mice have been the predominant model system for scientific inquiry across biology, including the fields of reproduction and development. However, it has become evident that mice do not adequately model human development, so interrogating these differences provides insight into the evolution of alternative mechanisms in mice and humans.
While human and murine sperm have the same major parts, murine sperm have three obvious differences (Fig. 5a), namely, (1) the head is elongated and has a hook shape of unknown function (Tourmente et al. 2016); (2) the tail is longer than most other mammals (Gomendio and Roldan 1991); and (3) the neck is attached to the side of the nucleus (Fawcett 1970). These differences could potentially be linked to the centrioles, as the centrioles of mouse sperm are also notably different when compared to those of humans and other mammals. Unlike in other mammals, the current dogma is that centrioles are absent in the spermatozoa of mice, rats, and hamsters (reviewed in Schatten 1981) (Fig. 5b), and therefore findings in mouse sperm and embryos have limited application to humans. However, the dogma needs additional validation in context of the discovery of an unexpected atypical centriole in humans. Reevaluating whether mice have or do not have sperm centrioles has major implications. If mice truly lack centrioles completely during early development, research would provide insight into how cells can function without centrioles, and if mice have extremely atypical sperm centrioles, it would open the door to experiments on how and why atypical centrioles form—experiments that are difficult in humans or other larger model systems.
Mice sperm centrioles. (a) Overall sperm morphology. Spermiogenesis begins with a round spermatid (b), with a typical proximal and distal centriole. Throughout spermiogenesis, these centrioles are extensively modified while the axoneme extends and the nucleus is reshaped to produce a spermatozoa that seems to lack centrioles completely (c). After fertilization, two bipolar spindles form that each has PCM, but no centrioles have been observed (d). It is accepted that the sperm, oocyte, and zygote lack centrioles altogether. Centrioles are not detected until the blastocyst stage. PCM pericentriolar material, ODF outer dense fibers, Ax axoneme
Mouse Sperm Centriole Degeneration Starts in the Testis and Continues in the Epididymis
Murine spermatogenesis appears similar to that of humans, with development taking place in the seminiferous tubules in the same general pattern. And similarly, murine sperm also go through a centrosome reduction program. In early spermatogenesis, CETN1/2 localizes to the proximal and distal centrioles—both of which have visible centriolar microtubules and PCM components such as γ-tubulin (Manandhar et al. 1998) (Fig. 5b). But in the subsequent elongated spermatid stage, the γ-tubulin disappears from both centrioles, indicating that the PCM undergoes reduction. Next, CETN1/2 disappears first from the distal centriole, and later from the proximal centriole in epididymal sperm (Manandhar et al. 1998; Schatten 2016) (Fig. 5c). The Centrosome Remodeling Program in humans and cattle has a Reduction and Enrichment subprogram (section “Typical and Atypical Sperm Centrioles Function in the Zygote of Non-murine Mammals”), but in mice, we speculate that the either the enrichment subprogram is attenuated or absent altogether, or the reduction subprogram is far more dominant. Regardless of the mechanism, the final product is an ejaculated spermatozoon, which seemingly lacks centrioles.
Mice Are Thought to Have No Functional Sperm Centriole
Several observations support the idea that mouse sperm lack centrioles and that centrioles form de novo from zygotic proteins. While this hypothesis is well accepted, the observations supporting it have some alternate explanations.
Studies that look directly for centrioles and conclude that they are absent due to several observations show that (1) triplet microtubules are not detected in the neck region of mouse spermatozoa by electron microscopy (Manandhar et al. 1998), nor are centrioles with typical ultrastructure detected after fertilization (Szollosi et al. 1972). (2) centriolar proteins (e.g., CETN1/2, and γ-tubulin) are not detected in the neck of spermatozoa (Schatten et al. 1985, 1986), or the embryo (Schatten et al. 1985); centrioles do not appear until the 32/64-cell stage (Simerly et al. 1993; Gueth-Hallonet et al. 1993; Coelho et al. 2013), (Bangs et al. 2015). (3) after fertilization, instead of forming a dominant sperm aster like other mammalian embryos that have paternal centriole inheritance, mouse embryos form many mini asters that appear random; there is no immediate dominant microtubule organizing center (Calarco 2000; Clift and Schuh 2015; Coelho et al. 2013; Schatten et al. 1985). These mini asters are organized by small electron dense aggregates and can be detected by anti-PCM antibodies (Calarco-Gillam et al. 1983; Hiraoka et al. 1989; Houliston et al. 1987; Maro et al. 1985; Szollosi et al. 1972). Several antibodies against PCM proteins have been used to study the distribution of PCM including the human auto-antiserum 5051 (Calarco-Gillam et al. 1983), the monoclonal antibodies MPM-1 and MPM-2 recognizing mitotic phosphoprotein and γ-tubulin antibodies (Gueth-Hallonet et al. 1993). All these antibodies label the spindle poles in embryo, suggesting that these proteins participate in the function of the spindle poles (Calarco-Gillam et al. 1983; Hiraoka et al. 1989; Maro et al. 1985), but it should be noted that acentriolar spindle poles have PCM in the absence of centrioles (Debec et al. 2010), and therefore PCM alone is not inherently evidence of centrioles.
Alternatively, the inability of these studies to detect the centrioles can be explained by the use of older and insufficiently sensitive technologies (namely fixation methods and antibodies); inability to detect or visualize a centriole should not be confused with total absence. With the advent of new tools, such as High-Pressure Freezing–Freeze Substitution and Cryo Electron Microscopy, and Super-resolution microscopy (i.e., STORM), the sperm neck needs to be reexamined. Furthermore, with the discovery of the atypical centrioles in Drosophila and human sperm, future studies need to consider the possibility of functionally competent centrioles that do not resemble typical centrioles.
Two studies type that looks for centrioles indirectly by examining their microtubule organizing function and suggest that centrioles are absent. (1) The zygote spindle appears anastral and barrel-shaped (Schatten et al. 1985) (Fig. 5d). This barrel shape is different from the spindles of other mammalian embryos, but similar to the shape of the meiotic spindle, which lacks centrioles (Sakai et al. 2011; Simerly et al. 2019; Wu et al. 1996). (2) The sperm tail is not associated with the spindle pole in mice (Simerly et al. 1993) like it is in humans and other mammals (Navara et al. 1994; Wu et al. 1996). These two types of observations indeed suggest mechanistic differences between mice and non-murine mammals, but it is worth noting that after the formation of the mini asters, the embryo eventually does form two bipolar spindles (Reichmann et al. 2018). How the poles are selected from the mini asters is not known, but extremely atypical centrioles are one potential explanation.
Two studies type that looks at the requirement of the sperm or sperm tail suggests centrioles are absent. (1) Embryos made from injection of a sperm head with no tail, and thereby no centriole, result in viable offspring (Yan et al. 2008). (2) Parthenogenic activation of mouse oocytes, which guarantees no paternal centriole contribution, can result in healthy pups that develop into healthy, fertile adults (Kono et al. 2004). These two types of observations suggest that centrioles are not essential for early development. However, both experiments are inefficient in producing live pups. Regardless, it is known that cell culture cells can form centrioles de novo (Uetake et al. 2007) but most of the time, these cells will form the wrong number of centrioles and will develop aneuploidy (Vitre and Cleveland 2012). However, some of the time (Wong et al. 2015) they recover the correct centriole number and proliferate. It is unclear if the success of nucleus-only injection or parthenogenesis is due to a chance formation of exactly two centrioles or a compensatory mechanism, but it is expected that the embryo cells end up with the correct number of centrioles for the pups to be healthy.
In addition to these alternate explanations, there are experiments that can be interpreted to suggest that mouse sperm could have extremely atypical centrioles. (1) Some centriolar microtubules are observed using electron microscopy (Iwashita and Oura 1980; Manandhar et al. 1998); and (2) an experiment using injection of mouse sperm into a cat egg results in the formation of a sperm aster (Comizzoli et al. 2006; Jin et al. 2012). The latter experiment was conducted to determine whether the aster formation program was maternally or paternally derived; they assumed that when injecting a mouse sperm into a cat egg the resulting embryo would be acentriolar, and therefore the sperm aster that resulted must be a feature of a maternally derived program. However, there is an alternate explanation: the sperm contains a highly atypical, possibly inactive, but not incompetent centriole. This centriole, in the presence of the correct maternally derived program, would be able to facilitate, or at least act as a landmark for the formation of a dominant microtubule organizing center. This ability of atypical centriole is also supported by the discovery that overexpressing CETN2 in mice results in the retention of CETN2-enriched foci, which reside at the spindle poles during metaphase. It suggests that these CETN2-enriched foci are at least competent to act as platforms for the recruitment of microtubules (Simerly et al. 2018). It is unclear if the CETN2 foci simply stay out of the way, or actively orchestrate recruitment.
One way to reconcile all these observations is that very atypical centrioles are present in the mouse sperm, but they are not functional postfertilization because of the presence of mouse egg-derived inhibitory factors, but in the context of a different developmental program, they may be competent to orchestrate aster formation, or at least not interfere.
In conclusion, the dogma is that the sperm centrioles do not organize a functional centrosome in the mice zygote, and instead, centrioles are formed de novo from a maternally derived program, and there is plenty of evidence to support this idea. But having a maternally derived dominant developmental program is not mutually exclusive with the existence of a sperm centriole. The idea that mouse sperm lack centrioles altogether is widely accepted, but the discovery of a highly atypical centriole in non-murine spermatozoa, and the possible alternate explanations for these experiments necessitate a reevaluation of the dogma.
The Evolution of the Atypical Centriole
Across evolution, sperm centrioles execute essential centriolar functions in the zygote, regardless of atypical structure in some species. The ancestral “primitive” sperm had two typical centrioles in its neck (reviewed in Avidor-Reiss 2018), and the evolution of atypical centrioles in insect and non-murine mammalian sperm, as well as the loss of centrioles in murine sperm presumably occurred more recently. Furthermore, since many of the animals between insects and humans have two typical centrioles, it appears that atypical centrioles evolved independently in insects and mammals. But if atypical centrioles truly are the product of two evolutionary events, this begs the question as to why atypical centrioles evolved. We propose two hypotheses: a passive and an active hypothesis.
Sperm Competition Drives Sperm Evolution, and May Have Actively Selected for Atypical Centrioles
The active hypothesis is based on the premise atypical centriole have advantages. Because sperm undergo such extreme competition, especially in polyandrous species, there is significant evolutionary pressure that drives sperm evolution. Because of this evolutionary pressure, sperm are some of the body’s most evolved cells, with even closely related species having vastly different sperm morphologies (Firman and Simmons 2009). Postmating sexual selection causes spermatozoa to undergo a direct selection process and they undergo rapid evolution (reviewed in Birkhead and Pizzari 2002; Parker 1984).
Sperm competition exhibited when sperm from different males competes to fertilize the egg—often favors speed or progressive motility, so for insects, and maybe mice, having a very small atypical centriole could allow for a smaller sperm neck region, and thereby a faster, more hydrodynamic sperm.
Sperm selection also takes place due to female cryptic choice, when physical and chemical properties of the female tract provide an advantage to sperm from one male over others. Having a splayed distal centriole could give mammalian sperm more efficient planar movement to navigate the complex physical environment of the female reproductive tract. This idea is supported by the observation that structural symmetry in the flagellum correlates with fertilization types. In animals that use external fertilization (reviewed in Avidor-Reiss 2018), the flagellum has pseudo radial symmetry (ninefold symmetry). However, in many animals with internal fertilization, including the atypical non-murine mammalian distal centriole, the flagellum, and the centriole, have increased pseudo bilateral symmetry (twofold symmetry).
Additionally, it is possible that some zygotic processes or sperm/egg complementation, that we do not yet understand, benefit from an atypical centriole. The active hypothesis would speculate that there is some fundamental difference between sperm or zygote function of animals with atypical sperm centrioles and animals with typical sperm centrioles.
Atypical Sperm Centrioles May Have Passively Evolved Due to Lack of Necessity
The passive hypothesis is based on the premise that atypical centriole evolved because of relaxed centriolar requirement. This could be due to the relative independence of the oocyte, or because sperm centrioles do not form cilia in early embryogenesis. Experiments showing centriolar reduction of somatic centrioles in a porcine embryo (Manandhar et al. 2006) suggest that the oocyte actively silences centrioles, and therefore there is no embryonic necessity for typical centrioles. Likewise, when human sperm was introduced to a hamster egg (whose developmental program resembles that of the mouse), no sperm aster was formed (Hewitson et al. 1997), supporting the idea that the hamster zygote does not use a centriole-based mechanism. Perhaps the oocyte contains most of the developmental program needed to direct divisions, and therefore the sperm does not need to provide typical centrioles but only a rudimentary centriole that recruits maternal proteins to execute other beneficial functions. Therefore, evolutionary drift has changed the centrioles with no major effect on fertility.
Centrioles appear not essential for division in early mouse embryos and since cilia appear only much later in the blastocyst, the main canonical roles of centrioles are not employed here (Simerly et al. 1993). They do appear to assist in bringing the two pronuclei in many species, and they may be essential only as a mechanism to control centriole numbers so that the tissues differentiating from the embryo have precisely two centrioles. Furthermore, this hypothesis would explain why centrioles vary so much even between species with atypical centrioles (Fig. 6).
Phylogenetic comparison of spermatozoa centrioles. The majority of animals studied have two centrioles that vary in structure, from PCLs to typical centrioles, to more recognizable atypical centrioles. Neither the type of modification nor the specific centriole that is modified, is completely conserved, suggesting that insects and mammals evolved atypical centrioles independently. Furthermore, the unusual lack of centrioles in murine sperm suggests that they extended their centriole degeneration program, using a similar, but more extreme, mechanism to that of other mammals. PCL proximal centriole-like, SDC sperm distal centriole
Sperm Centrioles Contribute to Infertility and Reduced Male Fecundity
After one year of attempting conception, 10–14% of couples in the United States are unable to conceive (review in Chandra et al. 2013; Kelley et al. 2019; Kumar and Singh 2015) (Fig. 7). Out of these couples, 40–50% is due to male factor infertility (reviewed in Kumar and Singh 2015; Chandra et al. 2013; Kelley et al. 2019) and 15–30% of couples suffer from unexplained infertility (reviewed in Isidori et al. 2006; Quaas and Dokras 2008; Thonneau et al. 1991). These statistics show that both male factor infertility and unexplained infertility are prevalent.
Origins of infertility. About 1/10 couples experience infertility, and of those, about 1/3 experience male infertility. Male infertility is diagnosed because of low count, motility defects, or morphology defects—centrioles play a potential role in motility and morphology; specifically, we expect that they are a candidate cause of head/neck severing, bulging midpieces, multiple tails, and other MMAF disorders. The frequency of these conditions is not known. It is also possible that centriolar defects affect neither morphology nor motility, but affect the developmental outcome, meaning that individuals with centriolar defects could be mistakenly labeled as maternal infertility. Centrioles are a candidate cause for any disorder listed in red
The standard of care for infertility starts with timed intercourse, lifestyle modification (such as; weight loss), medication, intrauterine insemination (IUI), and ends with assisted reproductive technology (ART), usually in the form of Intracytoplasmic Sperm Injection (ICSI) (reviewed in Quaas and Dokras 2008). These latter treatments are expensive, with an average cost of $5894 for medication, $10,696 for IUI, and $61,377 for ART (Katz et al. 2011); depending on location, these treatments may not be covered by insurance. Also, ART involves multiple surgical procedures, which increase the risk of complications for the female partner compared to the other less invasive treatments (Wang and Sauer 2006). These treatments can overcome defects related to motility and sperm fusion with the egg; however, if the centrioles are defective after fertilization, current treatment options are unable to address defects (reviewed in Quaas and Dokras 2008; Van Blerkom 1996a). To improve patient care, it would be essential to identify men with centriole-based infertility. With a diagnosis, couples would likely save money on healthcare and reduce the number of invasive procedures.
A Link Between Centriole Defects and Male Infertility
A few studies indirectly associated a suspected sperm centriole defect with a zygote developmental defect (Chemes and Rawe 2003; Moretti et al. 2017; Rawe et al. 2008; Van Blerkom 1996b). However, these studies have a small sample size or are case studies (reviewed in Avidor-Reiss et al. 2019). It is important to note that these papers are not true experiments, because they are observational studies; as such, their findings are strictly limited to correlations. And yet, despite the lack of causative, direct evidence, it is accepted that centrioles are essential for, and heavily implicated in human fertility (reviewed in Avidor-Reiss et al. 2019; Palermo et al. 1997; Schatten 1994).
Studies that conclude that there is a connection between centriole defects and infertility can be separated into two categories, studies that describe a direct centriolar defect, and those that describe defects that indirectly could be associated with the centrioles, or centriole-associated structures.
There are two case studies that directly attribute a case of infertility to malfunctional centrioles. The first describes two infertile patients who exhibit centriolar adjunct defects (Garanina et al. 2019). This study found that the sperm of infertile men had a longer centriolar adjunct when compared to five fertile patients, suggesting that the centriole adjunct defect is correlated to infertility. Another study found a missense mutation (D455V) in the centriole protein, CEP135, in an infertile man (Sha et al. 2017). The mutated protein was mislocalized and formed ectopic aggregates, and the sperm had multiple morphological abnormalities of flagella (MMAF), resulting in immotility. When his sperm was used to fertilize using intracytplasmic sperm injection (ICSI), the embryo developed for several days, but the pregnancy failed, suggesting that centrioles play a role in embryo development after the zygote stage. This mutation in CEP135 is a candidate cause for a common clinical phenotype: most IVF embryos do cleave, but one of the major failure points during development occurs between the eight-cell stage and blastocyst formation. Because these embryos were implanted prior to blastocyst, it is not clear exactly when they died, but CEP135, and centrioles as a whole, could be candidates for the nearly 50% of embryos that fail to reach the blastocyst stage or the nearly 75% that fail to implant. Understanding even a fraction of this large group of embryonic failures could dramatically improve IVF efficiency.
The second category of studies that suggest an association between sperm centrioles and infertility focuses on broader neck defects. Because the centrioles are in the neck, which connects the head and tail, it is possible that the infertile men from these studies have centriolar defects too (Rawe et al. 2002). This category includes head–neck defects (sometimes called the Head Tail Coupling Apparatus, HTCA), where the head is severed from the tail, in varying degrees of severity (reviewed in Chemes 2012). This defect can be rescued by injection of the separated sperm head and tail in close proximity. Contrastingly, when a sperm head without the tail was injected into an oocyte, embryo development failed (Palermo et al. 1997). This suggests that, unlike in mice, the tail in human sperm is essential for fertilization possibly because the centrioles are attached to the tail (Emery et al. 2004; Gambera et al. 2010).
Overall, only a few studies have observed defects in centrosome proteins, location, number, or structure in infertile men. These few studies have introduced the idea that centrioles may be important for fertility in humans, but due to their observational nature, any conclusions are limited to correlation and association. More comprehensive studies on the subject are needed to determine the degree of association between centrosomal defects and infertility, and experiments need to be conducted in nonhuman, non-murine models, such as livestock species (cattle, sheep, and pigs) to delineate the centrosomal role and mechanism in the embryo to ultimately determine causal candidates for centriole-based infertility. Conducting this work in livestock species will have major implications for humans, but will also help the agricultural industry breed more efficiently.
A Link Between Centriole Defect and Human Embryo Development
Because of the ethical and legal considerations surrounding human embryos, one of the most prevalent methods of examining the centrioles in human embryos is to look at arrested embryos. Most of these embryos have been tripolar, presumably due to polyspermy (Kai et al. 2015; Sathananthan et al. 1991). Inherently, polyspermic embryos have supernumerary centrioles, but it is impossible to tell whether the centrioles are the reason for their arrest or artifacts of the failed development. For this reason, currently, we do not know how to diagnose malformed centrioles by looking at the sperm, nor can we identify centriolar defects by looking at embryonic outcomes alone. Therefore, an important first step in the clinical application of reproductive centriole research is identifying centriolar defects that cause infertility or failed development, and fully characterizing the developmental phenotype.
More Research Is Needed for Effective Treatment of Centriole-Based Infertility
Currently, there is no treatment for centriole-based infertility (Pandruvada et al. 2021; Royfman et al. 2020; Turner et al. 2020). However, as we learn more about centriole defects and their effect on motility and the embryo, potential treatment avenues can be investigated. One potential treatment for centriole-based infertility would be to select sperm for their centriole quality. Since it is possible that the centrioles affect the sperm’s motility or morphology, developing light-microscopy observable criteria that are associated with functional centrioles would allow clinicians to select for sperm with good centrioles. Identifying sperm with functional centrioles would be especially helpful in patients with especially heterogenous sperm (Hinduja et al. 2010).
If the patient’s sperm centrioles are homogenous and defective to the point that embryonic development fails, another option may be to replace the defective centrioles with centrioles from a donor. A few studies have demonstrated that a fertile embryo develops when the tail with the centrioles is injected near the sperm head (Emery et al. 2004; Gambera et al. 2010), using a donor’s sperm tail and the patient’s sperm head could potentially overcome the centriole defect while still producing a genetically related child. This idea is similar to mitochondrial replacement therapy (MRT), where the parental DNA is transferred from a diseased oocyte to an enucleated donor (Craven et al. 2010; Tachibana et al. 2018).
Methods of Studying Sperm Centrioles Past, Present, and Future
Centrioles are challenging to study because they are so small that their internal structure cannot be resolved by classic light microscopy, and all techniques currently used have limitations. Therefore, the study of centrioles is reliant on multi-approach techniques that are expensive, time-consuming, and not accessible for clinical laboratories. Ultimately, there is a need to develop biomarkers and techniques with higher throughput and specificity, such as super-resolution microscopy and image-based flow cytometry. Here we review and evaluate the many techniques that exist for studying centrioles.
Transmission Electron Microscopy (TEM)
Transmission electron microscopy (TEM) is commonly used to study centrioles as it provides the highest level of structural detail. Sperm used in TEM are fixed, either through chemical fixation (Oliveira et al. 2011) or through cryofixation (Ounjai et al. 2012). The sample is then mounted, sectioned, stained, and imaged. Although useful in viewing cellular structures, TEM does not provide information on specific protein distribution (Chemes et al. 1987; Moretti et al. 2017; Oliveira et al. 2011). TEM is extremely expensive and laborious, so it is inaccessible in most clinical settings, and unsuitable for large-scale studies (Chemes et al. 1987; Moretti et al. 2017).
Despite its incompatibility in clinical settings, TEM has been used in several studies researching sperm centrioles. Sperm centrioles in gametes, fertilized embryos, and in preimplantation embryos examined using TEM suggested that human centrosome inheritance is paternal and that inheritance of abnormal centrosomes can result in infertility (Sathananthan 1998). TEM was also used to evaluate the sperm of a patient with severe asthenoteratozoospermia, and it was determined that the observed alterations in the sperm head–tail junction and attachment were due to an abnormal centriole resulting in improper aster formation and defective embryos (Rawe et al. 2002). Additionally, TEM was primarily used to discover that subjects with unexplained infertility have a significantly longer centriolar adjunct compared to healthy subjects (Garanina et al. 2019). Each of the studies described earlier examined no more than a few patients, demonstrating the limited capacity of this technique.
Western Blot
Western blots are commonly performed to detect specific proteins from a cell lysate. However, it only informs us indirectly about centrioles since it characterizes total protein in the cell. Because centriolar proteins are found largely in the cytoplasm, more so than in the centriole itself, it is difficult to evaluate the centriole-specific population of a given protein (Gavini and Parameshwaran 2019). This is especially true for embryos, which contain high levels of centrosomal proteins in the cytoplasm. One way to overcome this limitation is to separate the sperm centrioles biochemically and then analyze them via Western blot. However, these techniques are time-consuming and rely on purity that is difficult to achieve (Firat-Karalar et al. 2014).
Despite its limitations, Western blots have been used in several studies: higher levels of centrin were found in normozoospermic samples when compared to oligoasthenozoospermic (Hinduja et al. 2010). Additionally, expression of centriolar protein Tektin-2 (TEKT2) is decreased in cryopreserved human sperm when compared to fresh sperm, which may contribute to the loss of motility observed in cryopreserved sperm (Alshawa et al. 2019). An antisperm antibody that targets TEKT2 was also found in samples from infertile men (Zangbar et al. 2016).
Immunohistochemistry
Immunohistochemistry (IHC) is a staining technique that allows for the visualization of specific proteins within a tissue. IHC uses antibodies, similar to Western Blots; however, rather than labeling proteins within a lysate, samples are sectioned and mounted on a microscope slide (Erdogan et al. 2005). IHC allows the proteins to remain in their original distribution within cells, which enables the precise localization. However, IHC is a subjective technique that relies on high signal-to-noise ratio and quantitative methods are relative, not absolute, which makes these experiments difficult to replicate (Walker 2006). Furthermore, its use for analyzing sperm centrioles is very limited because of their size and IHCs diffuse staining, but the complete absence of centrioles and the axoneme was visible in spermatids with spermatogenetic arrest (Aumuller et al. 1987).
Immunofluorescence with Classic or Super-Resolution Microscopy
Similar to IHC, Immunofluorescence is a staining technique that allows for the precise localization of a protein in a cell and can determine if the protein is present in the centrioles, but it exhibits more focused staining and yields higher resolution images. Immunofluorescence also allows for quantification, although normalization is needed to account for experiment-to-experiment variation (Petrunkina and Harrison 2013). While immunofluorescence is technically and logistically feasible in most clinics, it is rarely used in diagnostic tests and standards have not been developed to diagnose sperm centriole defects. The lack of use in clinics is partially due to the reliance on highly specific, expensive antibodies that require optimization, especially in centrioles, due to the dense proximity of proteins and epitope masking. Using classic microscopy, immunofluorescence can be used to localize proteins to a centriole, but because of the small size of centrioles (~200 nm wide), it is difficult to determine the localization of proteins to specific substructures inside a centriole because the resolution of light microscopy is limited by the wavelength of the light (~400–700 nm, depending on the fluorophore used, and the size of the antibody). Super-resolution can circumvent this limit, and provide a higher resolution (up to 10 nm), but super-resolution microscopes are not yet widely available, they require extensive optimization, and they are much more time consuming than other light microscopy techniques.
Flow Cytometry
Flow cytometry is similar to immunofluorescence in that cells are fixed and stained with a fluorescently labeled antibody against a specific protein, but flow cytometry provides the ability to quickly quantify many cells. Stained cells are placed into a flow cytometer that passes each individual cell through a laser. The level of fluorescence within the cell is automatically measured and photos can be taken to allow for morphological analysis. Flow cytometry’s main advantage is its speed and precision: millions of cells can be analyzed quickly and protein quantification and cell sorting can be automated, thus reducing error and subjectivity.
Flow cytometry has not been performed on sperm centrioles yet, but it has been performed in a post acrosomal bovine sperm protein (Kennedy et al. 2014). Sperm were categorized based on levels of fluorescence and then subcategorized based on morphology. This study was able to correlate protein levels with certain sperm morphology and conception rate, thus exemplifying how flow cytometry could be used for sperm diagnostics in the future.
Altogether, the techniques available for the fine localization and quantification of centriolar proteins are improving, but each technique has its own benefits and limitations. The combinatory approaches currently used are not feasible for regular clinical applications, so there is a significant need to develop these techniques to make them feasible for clinical applications. Finally, the described techniques involve treatment (fixation or lysis) of the sperm that renders it unusable for fertilization, so these techniques are strictly able to determine correlation, which could improve diagnostics. The development of techniques that do not interfere with the viability of the sperm would allow for sperm selection and thus will become important as we learn more about what characteristics make one sperm better than another for IVF.
Final Remarks
Despite that sperm centrioles have been studied for more than two centuries, many aspects of their structure, function, and precise role during reproduction remain unclear. With recent technological advancements, centrioles are emerging as a hotbed of novel biology during reproduction and development that will have major implications for human reproductive health and agricultural production. As a result, there is a need for direct studies comparing the biology of sperm centrioles in various animal groups, especially with humans. It is essential to develop new and improved techniques to uncover the role of the centriole during sperm movement, fertilization, and embryo development.
References
Alieva I, Staub C, Uzbekova S, Uzbekov R (2018) A question of flagella origin for spermatids; mother or daughter centriole? In: Uzbekov RE (ed) Flagella and cilia. Nova Science Publishers, Inc., New York
Alshawa E, Laqqan M, Montenarh M, Hammadeh ME (2019) Influence of cryopreservation on the CATSPER2 and TEKT2 expression levels and protein levels in human spermatozoa. Toxicol Rep 6:819–824
Aumuller G, Fuhrmann W, Krause W (1987) Spermatogenetic arrest with inhibition of acrosome and sperm tail development. Andrologia 19:9–17
Avidor-Reiss T (2018) Rapid evolution of sperm produces diverse centriole structures that reveal the most rudimentary structure needed for function. Cell 7:67
Avidor-Reiss T, Gopalakrishnan J (2013a) Building a centriole. Curr Opin Cell Biol 25:72–77
Avidor-Reiss T, Gopalakrishnan J (2013b) Cell cycle regulation of the centrosome and cilium. Drug Discov Today Dis Mech 10:e119–e124
Avidor-Reiss T, Leroux MR (2015) Shared and distinct mechanisms of compartmentalized and cytosolic ciliogenesis. Curr Biol 25:R1143–R1150
Avidor-Reiss T, Turner K (2019) The evolution of centriole structure: heterochrony, neoteny, and hypermorphosis. Results Probl Cell Differ 67:3–15
Avidor-Reiss T, Gopalakrishnan J, Blachon S, Polyanovsky A (2012) Centriole duplication and inheritance in Drosophila melanogaster. In: Schatten H (ed) The centrosome: cell and molecular mechanisms of functions and dysfunctions in disease. Humana, New York, pp 3–31
Avidor-Reiss T, Khire A, Fishman EL, Jo KH (2015) Atypical centrioles during sexual reproduction. Front Cell Dev Biol 3:21
Avidor-Reiss T, Mazur M, Fishman EL, Sindhwani P (2019) The role of sperm centrioles in human reproduction – the known and the unknown. Front Cell Dev Biol 7. https://doi.org/10.3389/fcell.2019.00188
Azimzadeh J, Marshall WF (2010) Building the centriole. Curr Biol 20:R816–R825
Azimzadeh J, Hergert P, Delouvee A, Euteneuer U, Formstecher E, Khodjakov A, Bornens M (2009) hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J Cell Biol 185:101–114
Azimzadeh J, Wong ML, Downhour DM, Sánchez Alvarado A, Marshall WF (2012) Centrosome loss in the evolution of planarians. Science 335:461–463
Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV (2015) Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol 17:113–122
Basiri ML, Ha A, Chadha A, Clark NM, Polyanovsky A, Cook B, Avidor-Reiss T (2014) A migrating ciliary gate compartmentalizes the site of axoneme assembly in Drosophila spermatids. Curr Biol 24:2622–2631
Birkhead TR, Pizzari T (2002) Postcopulatory sexual selection. Nat Rev Genet 3:262–273
Blachon S, Khire A, Avidor-Reiss T (2014) The origin of the second centriole in the zygote of Drosophila melanogaster. Genetics 197:199–205
Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Edde B, Bornens M (1998) Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol 143:1575–1589
Bornens M (2002) Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol 14:25–34
Bornens M (2012) The centrosome in cells and organisms. Science 335:422–426
Bowler M, Kong D, Sun S, Nanjundappa R, Evans L, Farmer V, Holland A, Mahjoub MR, Sui H, Loncarek J (2019) High-resolution characterization of centriole distal appendage morphology and dynamics by correlative STORM and electron microscopy. Nat Commun 10:993
Burgos MH, Fawcett DW (1956) An electron microscope study of spermatid differentiation in the toad, Bufo arenarum Hensel. J Biophys Biochem Cytol 2:223–240
Calarco PG (2000) Centrosome precursors in the acentriolar mouse oocyte. Microsc Res Tech 49:428–434
Calarco-Gillam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M (1983) Centrosome development in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell 35:621–629
Callaini G, Riparbelli MG, Dallai R (1999) Centrosome inheritance in insects: fertilization and parthenogenesis. Biol Cell 91:355–366
Carvalho-Santos Z, Machado P, Branco P, Tavares-Cadete F, Rodrigues-Martins A, Pereira-Leal JB, Bettencourt-Dias M (2010) Stepwise evolution of the centriole-assembly pathway. J Cell Sci 123:1414–1426
Cavalier-Smith T (1974) Basal body and flagellar development during the vegetative cell cycle and the sexual cycle of Chlamydomonas reinhardii. J Cell Sci 16:529–556
Chandra A, Copen CE, Stephen EH (2013) Infertility and impaired fecundity in the United States, 1982–2010: data from the National Survey of family growth. Natl Health Stat Rep 67:1–18, 11 p following 19
Chemes HE (2012) Sperm centrioles and their dual role in flagellogenesis and cell cycle of the zygote. In: Schatten H (ed) The centrosome: cell and molecular mechanisms of functions and dysfunctions in disease. Humana, Totowa, NJ, pp 33–48
Chemes EH, Rawe YV (2003) Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update 9:405–428
Chemes HE, Carizza C, Scarinci F, Brugo S, Neuspiller N, Schwarsztein L (1987) Lack of a head in human spermatozoa from sterile patients: a syndrome associated with impaired fertilization. Fertil Steril 47:310–316
Chen JV, Buchwalter RA, Kao L-R, Megraw TL (2017) A splice variant of centrosomin converts mitochondria to microtubule-organizing centers. Curr Biol 27:1928–1940.e1926
Clift D, Schuh M (2015) A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat Commun 6:7217
Coelho PA, Bury L, Sharif B, Riparbelli MG, Fu J, Callaini G, Glover DM, Zernicka-Goetz M (2013) Spindle formation in the mouse embryo requires Plk4 in the absence of centrioles. Dev Cell 27:586–597
Comizzoli P, Wildt DE, Pukazhenthi BS (2006) In vitro development of domestic cat embryos following intra-cytoplasmic sperm injection with testicular spermatozoa. Theriogenology 66:1659–1663
Craven L, Tuppen HA, Greggains GD, Harbottle SJ, Murphy JL, Cree LM, Murdoch AP, Chinnery PF, Taylor RW, Lightowlers RN, Herbert M, Turnbull DM (2010) Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease. Nature 465:82–85
Dallai R, Mercati D, Lino-Neto J, Dias G, Lupetti P (2017) Evidence of a procentriole during spermiogenesis in the coccinellid insect Adalia decempunctata (L): an ultrastructural study. Arthropod Struct Dev 46:815–823
Debec A, Sullivan W, Bettencourt-Dias M (2010) Centrioles: active players or passengers during mitosis? Cell Mol Life Sci 67:2173–2194
Dias G, Lino-Neto J, Dallai R (2015) The sperm ultrastructure of Stictoleptura cordigera (Fussli, 1775) (Insecta, Coleoptera, Cerambycidae). Tissue Cell 47:73–77
Emery BR, Thorp C, Malo JW, Carrell DT (2004) Pregnancy from intracytoplasmic sperm injection of a sperm head and detached tail. Fertil Steril 81:686–688
Erdogan D, Elmas C, Ilgaz C (2005) Visualization of sperm by immunohistochemistry: application of alternative counterstains. Arch Androl 51:233–238
Fabian L, Brill JA (2012) Drosophila spermiogenesis: big things come from little packages. Spermatogenesis 2:197–212
Fawcett DW (1970) A comparative view of sperm ultrastructure. Biol Reprod 2(Suppl 2):90–127
Fawcett DW (1975) The mammalian spermatozoon. Dev Biol 44:394–436
Fawcett DW, Phillips DM (1969) The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec 165:153–164
Firat-Karalar EN, Sante J, Elliott S, Stearns T (2014) Proteomic analysis of mammalian sperm cells identifies new components of the centrosome. J Cell Sci 127:4128–4133
Firman R, Simmons L (2009) Sperm competition and the evolution of the sperm hook in house mice. J Evol Biol 22:2505–2511
Fishman EL, Jo K, Ha A, Royfman R, Zinn A, Krishnamurthy M, Avidor-Reiss T (2017) Atypical centrioles are present in Tribolium sperm. Open Biol 7:160334
Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, Khanal S, Miller AL, Simerly C, Schatten G, Loncarek J, Mennella V, Avidor-Reiss T (2018) A novel atypical sperm centriole is functional during human fertilization. Nat Commun 9:2210
Fuller MT (1993) Spermatogenesis. In: Bate M, Martinez-Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 71–174
Gambera L, Falcone P, Mencaglia L, Collodel G, Serafini F, De Leo V, Piomboni P (2010) Intracytoplasmic sperm injection and pregnancy with decapitated sperm. Fertil Steril 93:1347.e1347–1347.e1312
Ganem NJ, Godinho SA, Pellman D (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460:278–282
Garanina AS, Alieva IB, Bragina EE, Blanchard E, Arbeille B, Guerif F, Uzbekova S, Uzbekov RE (2019) The centriolar adjunct-appearance and disassembly in spermiogenesis and the potential impact on fertility. Cell 8:180
Gavini K, Parameshwaran K (2019) Western blot (protein immunoblot). StatPearls, Treasure Island, FL
Gomendio M, Roldan ER (1991) Sperm competition influences sperm size in mammals. Proc Biol Sci 243:181–185
Goto M, Eddy EM (2004) Speriolin is a novel spermatogenic cell-specific centrosomal protein associated with the seventh WD motif of Cdc20. J Biol Chem 279:42128–42138
Gottardo M, Callaini G, Riparbelli MG (2015) Structural characterization of procentrioles in Drosophila spermatids. Cytoskeleton (Hoboken) 72:576–584
Gueth-Hallonet C, Antony C, Aghion J, Santa-Maria A, Lajoie-Mazenc I, Wright M, Maro B (1993) Gamma-tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci 105(Pt 1):157–166
Hess RA, De Franca LR (2009) Spermatogenesis and cycle of the seminiferous epithelium. In: Molecular mechanisms in spermatogenesis. Springer, New York, pp 1–15
Hewitson L, Haavisto A, Simerly C, Jones J, Schatten G (1997) Microtubule organization and chromatin configurations in hamster oocytes during fertilization and parthenogenetic activation, and after insemination with human sperm. Biol Reprod 57:967–975
Hilbert M, Noga A, Frey D, Hamel V, Guichard P, Kraatz SHW, Pfreundschuh M, Hosner S, Flückiger I, Jaussi R, Wieser MM, Thieltges KM, Deupi X, Müller DJ, Kammerer RA, Gönczy P, Hirono M, Steinmetz MO (2016) SAS-6 engineering reveals interdependence between cartwheel and microtubules in determining centriole architecture. Nat Cell Biol 18:393–403
Hinduja I, Baliga NB, Zaveri K (2010) Correlation of human sperm centrosomal proteins with fertility. J Hum Reprod Sci 3:95–101
Hiraoka L, Golden W, Magnuson T (1989) Spindle-pole organization during early mouse development. Dev Biol 133:24–36
Hoage TR, Kessel RG (1968) An electron microscope study of the process of differentiation during spermatogenesis in the drone honey bee (Apis mellifera L.) with special reference to centriole replication and elimination. J Ultrastruct Res 24:6–32
Houliston E, Pickering SJ, Maro B (1987) Redistribution of microtubules and pericentriolar material during the development of polarity in mouse blastomeres. J Cell Biol 104:1299–1308
Ibrahim R, Messaoudi C, Chichon FJ, Celati C, Marco S (2009) Electron tomography study of isolated human centrioles. Microsc Res Tech 72:42–48
Isidori AM, Pozza C, Gianfrilli D, Isidori A (2006) Medical treatment to improve sperm quality. Reprod Biomed Online 12:704–714
Iwashita T, Oura C (1980) A three dimensional analysis of the capitellum and striated columns in the sperm neck region of the mouse. Okajimas Folia Anat Jpn 56:361–382
Jamieson BGM, Dallai R, Afzelius BA (1999) Insects: their spermatozoa and phylogeny. Science Publishers, Inc., New York
Jin YX, Cui XS, Yu XF, Lee SH, Wang QL, Gao WW, Xu YN, Sun SC, Kong IK, Kim NH (2012) Cat fertilization by mouse sperm injection. Zygote 20:371–378
Jo KH, Jaiswal A, Khanal S, Fishman EL, Curry AN, Avidor-Reiss T (2019) Poc1B and Sas-6 function together during the atypical centriole formation in Drosophila melanogaster. Cell 8:841
Kai Y, Iwata K, Iba Y, Mio Y (2015) Diagnosis of abnormal human fertilization status based on pronuclear origin and/or centrosome number. J Assist Reprod Genet 32:1589–1595
Katz P, Showstack J, Smith JF, Nachtigall RD, Millstein SG, Wing H, Eisenberg ML, Pasch LA, Croughan MS, Adler N (2011) Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril 95:915–921
Kelley AS, Qin Y, Marsh EE, Dupree JM (2019) Disparities in accessing infertility care in the United States: results from the National Health and Nutrition Examination Survey, 2013–16. Fertil Steril 112:562–568
Kennedy CE, Krieger KB, Sutovsky M, Xu W, Vargovic P, Didion BA, Ellersieck MR, Hennessy ME, Verstegen J, Oko R, Sutovsky P (2014) Protein expression pattern of PAWP in bull spermatozoa is associated with sperm quality and fertility following artificial insemination. Mol Reprod Dev 81:436–449
Khire A, Vizuet AA, Davila E, Avidor-Reiss T (2015) Asterless reduction during spermiogenesis is regulated by Plk4 and is essential for zygote development in Drosophila. Curr Biol 25:2956–2963
Khire A, Jo KH, Kong D, Akhshi T, Blachon S, Cekic AR, Hynek S, Ha A, Loncarek J, Mennella V, Avidor-Reiss T (2016) Centriole remodeling during spermiogenesis in Drosophila. Curr Biol 26:3183–3189
Kodani A, Salomé Sirerol-Piquer M, Seol A, Garcia-Verdugo JM, Reiter JF (2013) Kif3a interacts with Dynactin subunit p150 Glued to organize centriole subdistal appendages. EMBO J 32:597–607
Kono T, Obata Y, Wu Q, Niwa K, Ono Y, Yamamoto Y, Park ES, Seo J-S, Ogawa H (2004) Birth of parthenogenetic mice that can develop to adulthood. Nature 428:860–864
Kumar N, Singh AK (2015) Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci 8:191–196
Kuriyama R, Borisy GG (1981) Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol 91:814–821
Lange BM, Gull K (1996) Structure and function of the centriole in animal cells: progress and questions. Trends Cell Biol 6:348–352
Madeja ZE, Pawlak P, Piliszek A (2019) Beyond the mouse: non-rodent animal models for study of early mammalian development and biomedical research. Int J Dev Biol 63:187–201
Manandhar G, Schatten G (2000) Centrosome reduction during Rhesus spermiogenesis: gamma-tubulin, centrin, and centriole degeneration. Mol Reprod Dev 56:502–511
Manandhar G, Sutovsky P, Joshi HC, Stearns T, Schatten G (1998) Centrosome reduction during mouse spermiogenesis. Dev Biol 203:424–434
Manandhar G, Simerly C, Schatten G (2000a) Centrosome reduction during mammalian spermiogenesis. Curr Top Dev Biol 49:343–363
Manandhar G, Simerly C, Schatten G (2000b) Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum Reprod 15:256–263
Manandhar G, Schatten H, Sutovsky P (2005) Centrosome reduction during gametogenesis and its significance. Biol Reprod 72:2–13
Manandhar G, Feng D, Yi YJ, Lai L, Letko J, Laurincik J, Sutovsky M, Salisbury JL, Prather RS, Schatten H, Sutovsky P (2006) Centrosomal protein centrin is not detectable during early pre-implantation development but reappears during late blastocyst stage in porcine embryos. Reproduction 132:423–434
Maro B, Howlett SK, Webb M (1985) Non-spindle microtubule organizing centers in metaphase II-arrested mouse oocytes. J Cell Biol 101:1665–1672
Montgomery TH (1912) Human spermatogenesis spermatocyte and spermiogenesis a study in inheritance. J Acad Nat Sci Phil 15:1
Moretti E, Pascarelli NA, Belmonte G, Renieri T, Collodel G (2017) Sperm with fibrous sheath dysplasia and anomalies in head-neck junction: focus on centriole and centrin 1. Andrologia 49. https://doi.org/10.1111/and.12701
Namgoong S, Kim N-H (2018) Meiotic spindle formation in mammalian oocytes: implications for human infertility. Biol Reprod 98:153–161
Navara CS, First NL, Schatten G (1994) Microtubule organization in the cow during fertilization, polyspermy, parthenogenesis, and nuclear transfer: the role of the sperm aster. Dev Biol 162:29–40
Neto FTL, Bach PV, Najari BB, Li PS, Goldstein M (2016) Spermatogenesis in humans and its affecting factors. Semin Cell Dev Biol 59:10–26
Noguchi T, Koizumi M, Hayashi S (2012) Mitochondria-driven cell elongation mechanism for competing sperms. Fly 6:113–116
Oliveira LZ, Hossepian de Lima VF, Levenhagen MA, Santos RM, Assumpcao TI, Jacomini JO, Andrade AF, Arruda RP, Beletti ME (2011) Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa. J Vet Sci 12:267–272
Ounjai P, Kim KD, Lishko PV, Downing KH (2012) Three-dimensional structure of the bovine sperm connecting piece revealed by electron cryotomography. Biol Reprod 87:73
Paintrand M, Moudjou M, Delacroix H, Bornens M (1992) Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struct Biol 108:107–128
Palermo GD, Colombero LT, Rosenwaks Z (1997) The human sperm centrosome is responsible for normal syngamy and early embryonic development. Rev Reprod 2:19–27
Pandruvada S, Royfman R, Shah TA, Sindhwani P, Dupree JM, Schon S, Avidor-Reiss T (2021) Lack of trusted diagnostic tools for undetermined male infertility. J Assist Reprod Genet
Parker G (1984) Sperm competition and the evolution of animal mating strategies. Academic, San Diego, CA, pp 1–60
Pearson CG, Osborn DP, Giddings TH Jr, Beales PL, Winey M (2009) Basal body stability and ciliogenesis requires the conserved component Poc1. J Cell Biol 187:905–920
Petrunkina AM, Harrison RA (2013) Fluorescence technologies for evaluating male gamete (dys)function. Reprod Domest Anim 48(Suppl 1):11–24
Phillips DM (1970) Insect sperm: their structure and morphogenesis. J Cell Biol 44:243–277
Polejaeva IA, Rutigliano HM, Wells KD (2016) Livestock in biomedical research: history, current status and future prospective. Reprod Fertil Dev 28:112–124
Quaas A, Dokras A (2008) Diagnosis and treatment of unexplained infertility. Rev Obstet Gynecol 1:69
Rawe VY, Terada Y, Nakamura S, Chillik CF, Olmedo SB, Chemes HE (2002) A pathology of the sperm centriole responsible for defective sperm aster formation, syngamy and cleavage. Hum Reprod 17:2344–2349
Rawe VY, Diaz ES, Abdelmassih R, Wojcik C, Morales P, Sutovsky P, Chemes HE (2008) The role of sperm proteasomes during sperm aster formation and early zygote development: implications for fertilization failure in humans. Hum Reprod 23:573–580
Reichmann J, Nijmeijer B, Hossain MJ, Eguren M, Schneider I, Politi AZ, Roberti MJ, Hufnagel L, Hiiragi T, Ellenberg J (2018) Dual-spindle formation in zygotes keeps parental genomes apart in early mammalian embryos. Science 361:189–193
Riparbelli MG, Callaini G (2010) Detachment of the basal body from the sperm tail is not required to organize functional centrosomes during Drosophila embryogenesis. Cytoskeleton (Hoboken) 67:251–258
Riparbelli MG, Callaini G, Glover DM (2000) Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J Cell Sci 113(Pt 18):3341–3350
Riparbelli MG, Dallai R, Callaini G (2010) The insect centriole: a land of discovery. Tissue Cell 42:69–80
Ritter WE (1919) The unity of the organism; or, the organismal conception of life. RG Badger, Boston
Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M (2007) Revisiting the role of the mother centriole in centriole biogenesis. Science 316:1046–1050
Roosen-Runge EC (1977) The process of spermatogenesis in animals. CUP Archive, London
Royfman R, Shah TA, Sindhwani P, Nadiminty N, Avidor-Reiss T (2020) Sterility, an overlooked health condition. Women 1:29–45
Sakai C, Hoshino Y, Sato Y, Sato E (2011) Evaluation of maturation competence of metaphase II oocytes in mice based on the distance between pericentriolar materials of meiotic spindle: distance of PCM during oocyte maturation. J Assist Reprod Genet 28:157–166
Sathananthan AH (1998) Paternal centrosomal dynamics in early human development and infertility. J Assist Reprod Genet 15:129–139
Sathananthan AH, Kola I, Osborne J, Trounson A, Ng SC, Bongso A, Ratnam SS (1991) Centrioles in the beginning of human development. Proc Natl Acad Sci U S A 88:4806–4810
Sathananthan AH, Ratnam SS, Ng SC, Tarin JJ, Gianaroli L, Trounson A (1996) The sperm centriole: its inheritance, replication and perpetuation in early human embryos. Hum Reprod 11:345–356
Schatten G (1981) The movements and fusion of the pronuclei at fertilization of the sea urchin Lytechinus variegatus: Time-lapse video microscopy. J Morphol 167:231–247
Schatten G (1994) The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol 165:299–335
Schatten G, Simerly C, Schatten H (1985) Microtubule configurations during fertilization, mitosis, and early development in the mouse and the requirement for egg microtubule-mediated motility during mammalian fertilization. Proc Natl Acad Sci U S A 82:4152–4156
Schatten H, Schatten G, Mazia D, Balczon R, Simerly C (1986) Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc Natl Acad Sci U S A 83:105–109
Scheer U (2014) Historical roots of centrosome research: discovery of Boveri’s microscope slides in Wurzburg. Philos Trans R Soc Lond B Biol Sci 369:20130469
Sha YW, Xu X, Mei LB, Li P, Su ZY, He XQ, Li L (2017) A homozygous CEP135 mutation is associated with multiple morphological abnormalities of the sperm flagella (MMAF). Gene 633:48–53
Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA (1999) Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol 211:109–123
Simerly CR, Hecht NB, Goldberg E, Schatten G (1993) Tracing the incorporation of the sperm tail in the mouse zygote and early embryo using an anti-testicular alpha-tubulin antibody. Dev Biol 158:536–548
Simerly C, Castro C, Hartnett C, Lin CC, Sukhwani M, Orwig K, Schatten G (2016) Post-testicular sperm maturation: centriole pairs, found in upper epididymis, are destroyed prior to sperm’s release at ejaculation. Sci Rep 6:31816
Simerly C, Manil-Ségalen M, Castro C, Hartnett C, Kong D, Verlhac M-H, Loncarek J, Schatten G (2018) Separation and loss of centrioles from primordidal germ cells to mature oocytes in the mouse. Sci Rep 8:12791
Simerly CR, Takahashi D, Jacoby E, Castro C, Hartnett C, Hewitson L, Navara C, Schatten G (2019) Fertilization and cleavage axes differ in primates conceived by conventional (IVF) versus intracytoplasmic sperm injection (ICSI). Sci Rep 9:15282
Sydor AM, Coyaud E, Rovelli C, Laurent E, Liu H, Raught B, Mennella V (2018) PPP1R35 is a novel centrosomal protein that regulates centriole length in concert with the microcephaly protein RTTN. elife 7:e37846
Szollosi D, Calarco P, Donahue RP (1972) Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci 11:521–541
Tachibana M, Kuno T, Yaegashi N (2018) Mitochondrial replacement therapy and assisted reproductive technology: a paradigm shift toward treatment of genetic diseases in gametes or in early embryos. Reprod Med Biol 17:421–433
Tang TK (2013) Centriole biogenesis in multiciliated cells. Nat Cell Biol 15:1400–1402
Tates AD (1971) Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscope study. Rijksuniversiteit de Leiden, Leiden, Netherlands
Thonneau P, Marchand S, Tallec A, Ferial M-L, Ducot B, Lansac J, Lopes P, Tabaste J-M, Spira A (1991) Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989)*. Hum Reprod 6:811–816
Tourmente M, Zarka-Trigo D, Roldan ER (2016) Is the hook of muroid rodent’s sperm related to sperm train formation? J Evol Biol 29:1168–1177
Turner KA, Rambhatla A, Schon S, Agarwal A, Krawetz SA, Dupree JM, Avidor-Reiss T (2020) Male infertility is a women’s health issue-research and clinical evaluation of male infertility is neded. Cells 9
Tsou MF, Stearns T (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442:947–951
Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G (2007) Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J Cell Biol 176:173–182
Van Blerkom J (1996a) Preimplantation embryology: sperm centrosome dysfunction: a possible new class of male factor infertility in the human. Mol Hum Reprod 2:349–354
Van Blerkom J (1996b) Sperm centrosome dysfunction: a possible new class of male factor infertility in the human. Mol Hum Reprod 2:349–354
Vitre BD, Cleveland DW (2012) Centrosomes, chromosome instability (CIN) and aneuploidy. Curr Opin Cell Biol 24:809–815
Vladar EK, Stearns T (2007) Molecular characterization of centriole assembly in ciliated epithelial cells. J Cell Biol 178:31–42
Vorobjev IA, Chentsov Yu S (1982) Centrioles in the cell cycle. I. Epithelial cells. J Cell Biol 93:938–949
Walker RA (2006) Quantification of immunohistochemistry—issues concerning methods, utility and semiquantitative assessment I. Histopathology 49:406–410
Wang J, Sauer MV (2006) In vitro fertilization (IVF): a review of 3 decades of clinical innovation and technological advancement. Ther Clin Risk Manag 2:355–364
Winey M, O’Toole E (2014) Centriole structure. Philos Trans R Soc Lond B Biol Sci 369:20130457
Wistuba J, Stukenborg J-B, Luetjens CM (2007) Mammalian spermatogenesis. Funct Dev Embryol 1:99–117
Wong YL, Anzola JV, Davis RL, Yoon M, Motamedi A, Kroll A, Seo CP, Hsia JE, Kim SK, Mitchell JW, Mitchell BJ, Desai A, Gahman TC, Shiau AK, Oegema K (2015) Cell biology. Reversible centriole depletion with an inhibitor of Polo-like kinase 4. Science 348:1155–1160
Wu GJ, Simerly C, Zoran SS, Funte LR, Schatten G (1996) Microtubule and chromatin dynamics during fertilization and early development in rhesus monkeys, and regulation by intracellular calcium ions. Biol Reprod 55:260–270
Yan W, Morozumi K, Zhang J, Ro S, Park C, Yanagimachi R (2008) Birth of mice after intracytoplasmic injection of single purified sperm nuclei and detection of messenger RNAs and MicroRNAs in the sperm nuclei. Biol Reprod 78:896–902
Yildiz O, Khanna H (2012) Ciliary signaling cascades in photoreceptors. Vis Res 75:112–116
Yuan S, Stratton CJ, Bao J, Zheng H, Bhetwal BP, Yanagimachi R, Yan W (2015) Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction. Proc Natl Acad Sci U S A 112:E430–E439
Zangbar MS, Keshtgar S, Zolghadri J, Gharesi-Fard B (2016) Antisperm protein targets in azoospermia men. J Hum Reprod Sci 9:47–52
Zamboni L, Stefanini M (1971) The fine structure of the neck of mammalian spermatozoa. Anat Rec 169:155–172
Acknowledgment
This work was supported by grant R03 HD098314 and R21 HD092700 from Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD).
Author Contributions: TAR conceived the paper and wrote it with ELF. KT, AJ, SK, BO, and PD contributed sections to the writing.
Declaration of Interests: All authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this paper
Cite this paper
Fishman, E.L. et al. (2021). The Typical and Atypical Centrioles and Their Potential Roles in the Sperm and Embryo. In: Björndahl, L., Flanagan, J., Holmberg, R., Kvist, U. (eds) XIIIth International Symposium on Spermatology. Springer, Cham. https://doi.org/10.1007/978-3-030-66292-9_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-66292-9_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-66291-2
Online ISBN: 978-3-030-66292-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)