Abstract
Sleep is considered an important modulator of the immune response. Thus, a lack of sleep can weaken immunity, increasing organism susceptibility to infection. The function of sleep in altering immune responses must be determined to understand how sleep deprivation increases the susceptibility to viral, bacterial, and parasitic infections. There are several alterations in the immune system after reduced sleep, such as impaired mitogenic proliferation of lymphocytes, decreased HLA-DR expression, the upregulation of CD14+, and variations in CD4+ and CD8+ T lymphocytes, which have been observed during partial sleep deprivation. Also, steroid hormones not only regulate sexual behavior but also influence sleep. Thus, we hypothesize that sleep and the immune–endocrine system have a bidirectional relationship in governing various physiological processes, including immunity to infections. This chapter discusses the evidence on the bidirectional interaction between sleep and immune system. Because sleep is essential in the maintenance of homeostasis, immune system changes must be adapted to elicit changes in sleep patterns and other physiological parameters during the immune response to infections to which the organism is continuously exposed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sleep
- Immune system
- Immunity
- Network
- Cytokines
- Sleep deprivation
- REM sleep
- Lymphocytes
- T cytotoxic cells
- NK cells
- B lymphocytes
- Infection
- Health
- Sleeping disorders
Introduction
Sleep is a physiological process that has been proposed to have restorative and regulatory properties [1, 2]. Sleep has garnered particular interest in recent years due to its potential influence on the immune system. Many studies have demonstrated that total sleep deprivation and rapid eye movement (REM) sleep deprivation modify various components of the immune system, such as the percentage of cell subpopulations (e.g., CD4+, CD8+, and NK) and cytokine levels (e.g., IFN-g, TNF-a, and IL-1) [3, 4]. Also, conversely, sleep patterns are altered during the immune response, suggesting that sleep and the immune response are linked through bidirectional communication [5].
Sleep can be defined as a state of immobility resulting from the decreased ability to respond to external stimuli and is distinguished from coma and analgesia because it is rapidly reversible. Further, when deprived of sleep the organism tends to recover, depending on the extent and duration of sleep loss. The existence of this “rebound” after sleep deprivation suggests that sleep is not simply a period in which activity and alertness decline, it is a vital process that modulates various physiological functions [6].
Sleep has specific electroencephalographic (EEG) patterns in mammals and birds, which divide the sleep process into several stages. In addition, electromyograms (EMGs) and electrooculograms (EOGs) are used to differentiate the phases of sleep. Based on these parameters, several stages of sleep have been proposed: wakefulness, light sleep (two stages), slow-wave sleep, and rapid eye movement (REM) sleep, each of which has specific electrical patterns [7, 8]. Based on the classification of sleep stages, a hypnogram can be constructed describing the number of episodes, duration, rhythmicity, and latency of overnight sleep. Sleep patterns differ between species and during ontogeny and are altered in sleeping disorders (dyssomnia) or when a medical, psychiatric, or neurological disease develops [8, 9]. During sleep, important processes occur in endocrine function in mammals, for example, the rise in the levels of hormones such as prolactin and growth hormone [9, 10]. On the contrary, cortisol levels decline, observing an increase before wake up, which demonstrates the existence of a connection between sleep and other physiological events [9,10,11]. Studies on total sleep deprivation and REM sleep deprivation suggest that sleep has an important function in memory consolidation, learning, and neuronal plasticity [11,12,13,14]; although it has also been proposed to be a mechanism to conserve and recover energy [1].
The Function of Sleep
One of the crucial questions in the sleep study is: what is the function of sleep and particularly what is the role that REM sleep plays in the organism? Various theories have been postulated about its function that have been divided into three large groups that involve different types of sleep functions; the first group includes theories that propose sleep as a mechanism to conserve energy; a second group establishes sleep as a facilitator of learning and memory through the generation of changes in brain plasticity and synaptogenesis; and the last group proposes sleep as a process of restoration of various cellular components and biosynthesis of macromolecules [14]. Despite the various theories that explain the existence of sleep, its functions in mammals remain unclear. Some other studies suggest that duration of sleep may be related to the protection against oxidative stress, whereas previous studies have shown a phylogenetic correlation between sleep time and metabolic rate [15]. A high metabolism is linked to a greater number of biochemical changes, several of which have been related to sleep control. A high metabolic rate results in the generation of high levels of reactive oxygen species (ROS) by the mitochondria, and this generation of ROS has been linked to aging [16]. On the other hand, it has been shown that sleep deprivation in the rat produces an increase in oxidative stress; interestingly, it was found that the most noticeable changes occurred in a region of the brain with higher rate of protein synthesis and presumably of the generation of ROS. So, one of the theories is that at higher metabolic rates longer periods of sleep are required to disrupt ROS-induced damage in brain cells, and thus facilitate the synthesis and activity of molecules that protect brain cells against oxidative stress [15].
The argument that sleep has a vitally important function is compelling as lack of sleep in rodents and flies can cause death faster than food deprivation [17]. On the other hand, the amount and nature of sleep are related to age, body size, and ecological variables, such as whether animals live a terrestrial environment or aquatic environment, their diet, and the safety of the place where they sleep. Sleep can be an effective process that performs certain functions, but variations in sleep expression indicate that these functions may differ between species [18].
Diverse evidence suggests that REM sleep and non-REM sleep have distinct functions; most theories suggest that non-REM sleep plays an important role in energy conservation, while REM sleep is involved in the recovery of nervous system, learning, neuronal plasticity, and synaptogenesis [14].
It has been proposed that REM sleep is a state of periodic brain activation during sleep that can participate in recovery processes and emotional regulation [19]. Physiologically, it has also been observed that during this stage important processes occur in mammalian endocrine function, as it is during this stage that the highest levels of hormones such as prolactin and growth hormone are reached, in comparison to decreased cortisol, and it is right to wake up when it reaches its maximum levels [10], which could show the existence of sleep interactions with phenomena typical of other physiological systems.
Sleep Deprivation
Sleep deprivation consists of either a complete lack of sleep over a certain period of time or a reduction in optimal sleep time. A chronic reduction in sleep time or fragmentation of sleep leads to sleep cycle disruption and may have consequences similar to those observed by acute sleep deprivation, such as alterations in cognitive functions, attention, and operating memory. Sleep deprivation for several days is usually performed in extreme situations or under experimental conditions [20].
In humans, clinical symptoms of sleep deprivation or restriction usually include an increase in reaction time to any stimulus, distraction, disturbances in attention and concentration, as well as difficulty in memorizing the new information. Higher stress level is observed; tiredness, drowsiness and irritability increase; decreased effectiveness and motivation when working [20]. Total sleep deprivation in rats causes their death over a period of approximately 3 weeks, presenting a physical deterioration, with ulcerations on the skin, tail, and legs; alterations in motor and postural coordination (ataxia); increases in food intake accompanied by considerable weight loss and increased energy spent [21]. Acute sleep deprivation impairs the integrity of cognitive processes, such as attention, learning, and memory. This deterioration is accompanied by a change in brain metabolic activity [22]. Sleep restriction, which consists of decreased sleep time, is observed as the most common form of deprivation in humans. Restriction and sleep disorders have been associated with a wide range of health consequences, including an increased risk of hypertension, diabetes, obesity, depression, heart attack, and stroke [23].
Immune System
The primary function of the immune system is to defend the body from infections due to pathogens, to external chemical and biological agents (nonpathogens) or self-transformed cells through early innate immunity and subsequent adaptive responses [24].
Innate immunity is the first line of defense; Its two primary functions are to isolate and destroy invading pathogens through inflammatory processes and to recognize and process antigens to trigger acquired immunity [25]. Both types of immunity include cellular and biochemical mechanisms that are designed to respond quickly to infections and accurately distinguish between native and foreign materials [24, 25].
In innate immunity, for example, foreign pathogens are recognized by pattern recognition receptors (PRRs), which are encoded in the germline, have broad specificity for detecting molecular structures that are unique to such organisms, and are evolutionarily conserved. These unique molecular patterns in pathogens are known as pathogen-associated molecular patterns (PAMPs) [26]. PAMPs are generally components of the bacterial cell wall, such as lipopolysaccharide (LPS) and peptidoglycan. Other important PAMPs include β-glucan (a cell wall component of fungi) and viral nucleic acids (DNA and RNA), all of which have specific structural characteristics [26]. There are various receptors that recognize PAMPs, the most extensively studied of which are toll-like receptors (TLRs), comprising 13 types that recognize a wide range of PAMPs. TLRs bind to molecules, such as large lipopeptides in bacteria and mycoplasma [27]. NLRs form another group of PRRs that act as intracellular sensors that detect viral DNA and RNA [28]. The activation of TLRs by their bacterial ligands induces an inflammatory response that stimulates macrophages, which produce proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), interferon-gamma (IFN-γ), and interleukin-6 (IL-6), which coordinate local and systemic inflammatory immune responses [29]. TNF-α and IL-1β are triggered in the local endothelium to induce vasodilatation and increase permeability of blood vessels, promoting the recruitment of serum proteins and leukocytes to the site of infection. IL-1β and IL-6 together, interacting to hepatocytes, activate them to produce acute phase proteins that activate complement and opsonize pathogens, to be phagocytosed by neutrophils and macrophages [29]. TLRs are expressed in other effector cells of the innate immune system, such as neutrophils, monocytes, NK cells, and γδ T cells [29], which can co-express more than one type of TLR. Phagocytic leukocytes, such as eosinophils, basophils, and mast cells, are the principal effectors of innate immunity, the main function of which is to ingest and kill pathogens [30]. Other types of phagocytes participate in these processes, acting as antigen-presenting cells (APCs) and generating antigenic peptides that activate specific immune responses particularly foreign antigens that are partially degraded by T lymphocytes [31].
Recognition of antigens by the adaptive immune system is mediated by specific receptors. These receptors are also encoded in the germline, and through somatic recombination, random combinations of segments of these genes can generate a large and diverse repertoire of receptors with high specificity [32]. The resulting products are clonally distributed in antigen-specific T and B lymphocytes , which express receptors that are specific for one antigen, and specific populations are selected to expand in response to the pathogen [32]. T cells recognize peptides through the T-cell receptor (TCR), which triggers different mechanisms that will determine the fate of the T cell. There are two chief groups of conventional T cells: T helper (Th) cells that express the CD4 co-receptor and cytotoxic T lymphocytes that bear CD8 [31, 32]. Both cell types recognize an antigenic peptide that must be complexed to the major histocompatibility complex class II (MHC II) molecules, whereas B cells recognize the antigen by binding to a 3D molecular determinant (epitope) [31, 32]. In turn, certain Th cells interact with B cells, through which the latter produce large amounts of immunoglobulin or antibody. Every B cell produces antibodies, with a unique specificity, that neutralize and destroy the antigen [32]. Innate and acquired immune responses require a network of molecules that signal and orchestrate them [31]. These molecules (cytokines) are synthesized by all classes of immune cells and many other cell types. Generally, cytokines act as proinflammatory, regulatory, or anti-inflammatory molecules and can be classified depending on the subtype of lymphocytes that produced them, as Th0, Th1, Th2, Th3, or Th17, although the actual classification is broader and more complex [33, 34]. Cytokines participate in innate and adaptive immune responses [34]. Th1 cells and activated macrophages primarily secrete IFN-γ and other cytokines that mediate the response against intracellular pathogens and induce B cells to synthesize IgG2 antibodies. Th2 cells preferentially respond to multicellular parasites and produce IL-4, IL-5, and IL-13 [35], which modulate the function of eosinophils, basophils, and mucosal epithelial lymphocytes. IL-5 specifically instructs lymphocytes to produce IgE antibodies. Th17 cells induce cell types, such as epithelial cells, to produce IL-17 and chemokines that recruit neutrophils to the site of infection and are involved in the response against extracellular bacteria and fungi [33]. The differentiation of Th cells into various lineages is controlled by master transcription factors, the expression of which is regulated by cytokines that are produced and governed by APCs in response to activation by PAMPs. Thus, the adaptive immune response results in antigen -specific activation that is orchestrated by the innate immune response.
Sleep, Brain, and Immune System
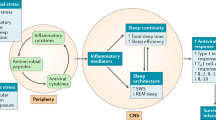
The brain is linked to the immune system, and similar interactions occur during sleep, wherein brain activity changes, resulting in the putative “awake brain” and “sleeping brain.” There is evidence that the expression of molecules, such as neurotransmitters, hormones, and cytokines, is modulated while the subject sleeps, and human studies have described changes in the serum levels of some of these components during sleep; specifically, the secretion of IL-1β, IL-10, IL-12, and TNF-α by monocytes and dendritic cells peaks during sleep, independently of circadian rhythms (Fig. 7.1). This behavior may be directly related to sleep, because when the animal is deprived of the rhythmicity of these cytokines [36,37,38], the changes in expression wane. Also, blood levels of monocytes, T cells, and NK cells follow a clear circadian rhythm regarding the sleep–wake cycle [36]. Notably, other neuroendocrine mediators, such as prolactin, cortisol, and norepinephrine, also exhibit circadian rhythms, but their secretion pattern is more related to the sleep–wake cycle, and all of these compounds modulate the immune response [39]. Conversely, certain cytokines affect sleep, such as IL-1β, which, when administered intracerebroventricularly to rabbits and rats, increases the duration of non-REM sleep (Fig. 7.1). This effect is abolished when IL-1β antagonists are given [40]. Administration of the cytokines TNF-α and IFN-α has the same effect as IL-1β [40, 41].
Interaction of IL-1 with serotonin in NREM sleep regulation. IL-1 and serotonin (5-HT) interact at different sites in the brain to regulate NREM sleep. Interactions between IL-1, serotonin, and GABA are shown that are important during NREM sleep regulation. In the core of dorsal raphe nuclei (DRN), IL-1 microinjections promote NREM sleep; IL-1 reduces the rate of firing of active serotonin neurons in wakefulness through increased inhibitory effects of GABA. (a) In the preoptic hypothalamic area and the basal brain stem region (POA/BF), IL-1 stimulates the secretion of 5-HT; (b) 5-HT inhibits cholinergic neurons involved in cortical activation; and (c) stimulates the synthesis of IL-1; (d) while inhibiting wakefulness-promoting neurons and active sleep-promoting neural populations in POA/BF. In POA/BF, IL-1 is subjected to a potent inhibitory homeostatic control of corticosteroids secreted by the adrenal cortex (f). Corticosteroids in turn depend on the activity of the hypothalamus–pituitary–adrenal axis, which is activated by the serotonergic system (g). IPSP inhibitory postsynaptic potential, Ach Acetylcholine, ACTH adrenocorticotropic hormone, CRH corticotropin-stimulating hormone, IPSP inhibitory post-synaptic potential, PVN paraventricular hypothalamus nucleus. (From Imeri L, Opp MR. [51]. Reprinted with permission from Springer Nature)
The Role of Sleep in Regulating the Immune System
In recent decades, various works have shown that sleep seems to be associated with the regulation of the immune system and immune response, and that lack of sleep induces vulnerability to develop certain disorders [42].
In this respect, some authors propose that sleep participates in the phase of the formation of immune memory, making the comparison with the consolidation of neurobehavioral memory [12,13,14], in which the information is transferred from a short to long term in the acquisition, in a similar way sleep can participate in the acquisition of immune memory, as well as the recovery phase and in the immunological synapse by recruiting cells to the antigen presenters to be presented to the T helper lymphocytes [43].
Other work provides evidence that the concentration of circulating immune cells and cytokines are subject to sleep regulation, as higher or lower systemic levels are reached during sleep, depending on the cell population and the interleukins [42,43,44]. Cell populations, such as neutrophils, monocytes, and NK cells, present their lowest blood levels during sleep, an opposite behavior is observed in B lymphocytes, T cytotoxic, and T helper cells, which reach their highest levels during sleep [45]. Similarly, IL1-O is known to reach its minimum blood levels during sleep, while TNF has a contrary behavior, reaching its maximum concentrations during the night. It should be noted that sleep regulation on cell concentration at the systemic level may differ from the regulatory effect it exerts on their cytokine production or secretion [46].
About the regulatory effect that sleep can have on the immune system has emerged an interesting but unproven idea, which addresses that the dream has evolved to play an important role in protecting animals against parasitic infections [47]. This theory is derived from the clinical observation of close physiological relationship between sleep and the immune system. This relationship suggests that species that have evolved to longer sleep duration seem to be able to increase investment in their immune systems and be better protected against parasites [47].
To prove this possibility, the authors made a comparative analysis among 26 species of mammals, of their sleep characteristics, confronting them with different parameters of their immune system [47]. According to the authors, there is a strong correlation between the increases in sleep duration in different mammals with the increase in immune defenses measured through the number of circulating immune cells [47]. It observed a positive correlation between the amount of both non-REM sleep and REM sleep with the number of cells in the white formula, while cells of the red formula had no significant variation [47]. Neutrophils that account for about 47% of white blood cells that rank as the first line of defense against pathogen attacks, themselves increased in relation to the increase in sleep [48]. Similarly, lymphocytes that account for about 44% of white blood cells, and which are related to acquired immunity, also increased at the same time in the 26 species of mammals studied [47, 48]. In addition, both eosinophils and basophils that together add up to about 6% of white blood cells also have this positive correlation. On the other hand, the number of pathogens that can infect each species studied was determined, it was found that the relative infection state had a negative correlation respect to total sleep time, that is, as sleep time increased the relative infection state decreased [47]. Just as this study provides new information about sleep, it also opens up questions. For example, only monocytes, which are about 5% of white blood cells, did not present a positive correlation with the amount of sleep. So, we would have to try to explain both the positive relationship of the amount of sleep with most of immune cells and the lack of this correlation with the monocytes. Why don’t these cells behave like the rest of their sleep peers?
Effect of Sleep Deprivation on the Immune System
As mentioned above, humanity has repeatedly observed how sleep deprivation makes us more vulnerable to infectious agents. However, few research groups have addressed this issue and the lack of information available is surprising. In addition, man is the only species that can voluntarily suppress his sleep, which would have some experimental advantages compared to studies in animals, to which he is forced to stay awake. In this context, the alterations that can occur in the immune system are of great importance when there is a modification in sleep or when it is deprived. In this respect, the first works were reported in humans by Palmblad and collaborators in 1976 [49]. In this first study, eight women were completely deprived of sleep for 77 hours in circumstances that simulated a battlefield. A blood sample was taken before, during, and after deprivation. The authors found no changes in leukocytes, monocytes, or circulating lymphocytes, but in interferon production and phagocytic activity [49].
Subsequently, other work showed that sleep deprivation resulted in decreased lymphocyte blastogenesis, and an increase in IL-1 and IL-2 levels, while a decrease in NK cell activity was observed during sleep deprivation [50]. In a study published by the Dinges group in 1995 conducted on private youth for 64 h, a significant increase in the percentage of NK cells, granulocytes, and monocytes was observed, while observing changes in IL-1 and IFN levels [42]. Other studies in which rats were selectively deprived of REM sleep reported an increase in total leukocytes and IgM systemic at 96 h of deprivation [39].
Furthermore, another group reported an increase in plasma levels of IL-1, IL-6, IL-10, TNF, and IL-17 when subjects were deprived of sleep in a period of 72 h [4]. These findings suggest that REM sleep deprivation involves changes in immune system modulation, perhaps increasing inflammatory processes or favoring the type of cellular response. However, little is known about the effect that sleep deprivation can have when an immune response to an infection develops, although empirically it is known that in infectious processes the sleep pattern is modified, indicating that it may be regulating the generated response [51]. Little has been studied about the relationship between sleep and immune response modulation, particularly in parasitic type infections, as well as possible mechanisms that are mediating this phenomenon [51]. REM selective sleep deprivation studies conducted in rats, as detailed in previous paragraphs, are regularly contaminated with a stress component that is inherent in the deprivation technique. Although changes have been made to reduce this component, there is still controversy in this regard. Moreover, when it comes to assessing the immune response, this controversy is even more relevant, given the marked effect of stress on the immune system. Therefore, the strategy of comparing the effects of REM sleep deprivation with some stressor that causes the usual response has occasionally been used [52].
Immune System Effect on Sleep
In general, infectious diseases, mental disorders, and physical conditions are associated with drowsiness and fatigue. After being stimulated, innate immune system cells secrete inflammatory cytokines that induce the synthesis of a different cytokine profile characteristic of each disease, including sleep disorders [39]. Proinflammatory cytokines synthesized in the periphery by the immune system reach the brain through nerves or blood and regulate sleep. The details of the interaction between sleep and an immune process have not been so widely studied, much less the effects of various types of infections on it have been clarified; however, there is evidence of a close direct and two-way relationship between them [39].
The list of cytokines and chemokines that have been studied in laboratory animals or humans that suggest an alteration or that affect sleep include: IL-1, IL-2, IL-4, IL-6, IL-8, IL -10, IL-13, IL-15, IL-18, TNF, TNF-α, IFN-1, INF-α, and macrophage protein (also known as CCL4) [1,2,3,4,5, 51]. Of these, only two substances, IL-1 and TNF, have been studied enough to claim that they are involved in the physiological (i.e., spontaneous) regulation of sleep. Evidence of a role for IL-1 and TNF in physiological sleep regulation has been derived from electrophysiological, biochemical, and molecular genetic studies [53, 54]. For example, when IL-1 was administered intravenously or intracerebroventricularly in rabbits, it resulted in an increase in NREM sleep time of approximately 60–70% [55]. The same effect has been observed with the administration of two other interleukins, such as TNF and IFN, although the effect of these two may be mediated by IL-1, as receptors for IL-1 have been found in several structures of the brain, in addition to the existence of immunoreactive hypothalamic neurons to this cytokine. Interestingly, IL-1 exerts effect on the serotonin system, involved in regulating sleep at different levels [55, 56].
The above mentioned evidence supports the existence of an interaction between components of the immune system and sleep. In this context, the alterations that can occur in sleep when there is an immune response are of great relevance. In this line of research, it is known that systemic levels of TNF-α present a circadian rhythm that coincides with sleep–wake rhythm [57]. In addition, secretion of IL-6 is negatively related to the amount of nighttime sleep. Consequently, decreased secretion of IL-6 is associated with good nighttime sleep and a feeling of well-being the next day. Since treatment with IL-6 causes drowsiness and fatigue, it is proposed that this cytokine has direct action on the central mechanisms of sleep [58].
Evidence collected over the past few years also suggests that these cytokines are synthesized directly by brain cells that also express specific receptors for them. In this respect, cytokines are also known to be de novo synthesized and secreted by neurons and glia, and that there are immunoreactive neurons for IL-1 and TNF located in regions of the brain that are involved in the regulation of sleep–wake, such as the hypothalamus, hippocampus, and brainstem [51]. Even more, IL-1 and TNF receptors are also present in various areas of the brain, such as the choroid plexus, hippocampus, hypothalamus, brainstem, and cortex, and are expressed in neurons and astrocytes [59].
In this context, IL-1 and TNF increase the non-REM (NREM) sleep in several species (rat, mouse, monkey, cat, rabbit, and sheep), regardless of the administration route. NREM sleep that initiates as a result of IL-1 or TNF administration has some physiological sleep characteristics in the sense that it remains episodic and is easily reversible when the animal is stimulated. Although IL-1 usually causes NREM sleep fragmentation, the magnitude and duration of its effects depend on dosage and time of administration: very high doses suppress NREM sleep in rodents but, if IL-1 is administered before the dark phase of the light–dark cycle, NREM sleep increases [60]. In addition, the antagonists of these cytokine systems also attenuate the increase in NREM sleep caused by excessive food intake or acute temperature elevation, which are associated with increased production of IL-1 or TNF. Consistently, knockout mice that lack the receptor to IL-1 and the receptor for TNF, both type 1, spend less time in NREM sleep than control mice [60, 61].
Taken together, evidence suggests that IL-1 and 5-HT systems participate in reciprocal interactions that contribute to NREM sleep regulation. IL-1 improves 5-HT axonal release and stimulates IL-1 synthesis, while inhibiting wakefulness promoting neurons. IL-1 also inhibits serotonin-wake-active cell bodies in the dorsal raphe nuclei. Therefore, IL-1 exerts opposite effects on the serotonin cell bodies and axon terminals. These effects complement each other, and both contribute to the same functional result, NREM sleep enhancement [51].
Sleep During Infection
The above mentioned evidence supports the existence of an interaction between components of the immune system and sleep. In this context, the alterations that can occur on sleep when there is an immune response are of great relevance. In this respect, a wide variety of infectious diseases have been linked to sleep disorders, particularly it has been documented that infectious agents such as viruses or parasites are capable of infecting the CNS thus generating such disorders, either by the effect of the immune response generated against the infection or by direct effect of the pathogen [62]. One of the first diseases in which alterations in the sleep pattern was described was lethargic encephalitis. Lethargic encephalitis is a CNS disorder characterized by pharyngitis, followed by the presence of sleep disorders including drowsiness, sleep inversion, or insomnia [62]. Some recent studies have associated this disease with an autoimmune pathology, although its etiology is not known for certain [62].
Most of the pathogens that cause this type of sleep disorders are viruses. In particular, patients infected with the virus such as human immunodeficiency (HIV) (which also affect the CNS) suffer alterations such as fatigue and sleep disorders from the asymptomatic stage [39]. Reports show disruption of the physiological organization of sleep, which can appear from the early stage and progress throughout infection in both adult and child individuals [39]. These reports described the decrease in REM sleep, delta wave sleep or SUN, and can progress as the disease progresses to subsequently have shortened total sleep time and reduced sleep stage two, while increasing wakefulness [39].
Since sleep disturbances appear from an early stage in infection, it has been proposed that these alterations are caused by direct infection in the CNS; some peptides of the virus may be involved [39]. Studies with cats infected with the feline variety of the virus show alterations similar to those found in HIV-infected or AIDS patients [39], so these models are a good experimental strategy to elucidate the mechanisms by which this infection causes sleep pattern alterations; these alterations may be performed by the direct action of virus components on the CNS or by the action of immune system in response to the virus infection.
Patients infected with other types of viruses such as rabies, hepatitis C, or chickenpox have similar symptoms, such as reduced REMS and total sleep time. However, it has not been possible to differentiate whether these disorders are caused by the virus itself or as a consequence of the immune response generated to counteract it [39]. In this respect, some studies propose that sleep disturbances may be caused by the continued exposure to cytokines of innate immune system, such as the case of IFN-α, proposing that these cytokines reduce sleep continuity and induce a consistent pattern with insomnia and alertness [5]. Other infectious agents can cause sleep disturbances indirectly by affecting other systems such as respiratory or endocrine, but not the centers involved in sleep regulation; however, most infectious processes, particularly during the acute phase, coincide in altering the sleep pattern, usually causing an increase in the duration of SUN and decreased wakefulness and/or REM sleep [39]. This alteration that can be observed in a generalized way during an infectious process could be a mechanism of the organism to adapt to these circumstances; forcing the greatest energy supply to the immune system, so it can be able to eliminate the infection (Fig. 7.2).
Interactions during infectious processes. It is shown the immune response resulting from the invasion of a pathogen with the consequent secretion of immune mediators, such as interleukins and cytokines, which is accompanied by the response at the endocrine level and the nervous system. Secreted substances can find their targets at the systemic level or they can cross the blood–brain barrier to reach their receptors in different neural structures, or may have a modulation via vagal, in order to modulate the response mechanisms aimed at maintaining homeostasis. This modulation can also be used by pathogens to ensure the establishment of the infection, completing its life cycle and ensuring its offspring. (From Ibarra-Coronado EG, et al. [39]. Creative Commons Attribution 3.0 Unported (CC BY 3.0). Available at https://www.hindawi.com/journals/jir/2015/678164/)
It has also observed that substances associated with bacterial infections are able to induce sleep [63]. Among these substances are the components of the cell wall of bacteria. In this respect, it is known that muramyl peptide is capable of inducing an excess of SUN when administered in rabbits, rats, and cats [63], while LPS and antigen A produce an increase in both the amount of SUN and its amplitude while suppressing REM sleep when administered in rabbits via intracerebral ventricular and intravenous [64]. In humans, Salmonella abortus endotoxin produces a noticeable diminution of both wakefulness and REM sleep, accompanied by an increase in NREM sleep, in addition to causing disturbances during the day, mainly daytime sleepiness [50].
References
Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–60.
Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31(3):441–57.
Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30(4):401–11.
Yehuda S, Sredni B, Carasso RL, Kenigsbuch-Sredni D. REM sleep deprivation in rats results in inflammation and interleukin-17 elevation. J Interf Cytokine Res. 2009;29(7):393–8.
Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–80.
Dement WC. History of sleep medicine. Neurol Clin. 2005;23(4):945–65.
Fuller PM, Gooley JJ, Saper CB. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythm. 2006;21(6):482–93.
Miyazaki S, Liu CY, Hayashi Y. Sleep in vertebrate and invertebrate animals, and insights into the function and evolution of sleep. Neurosci Res. 2017;118:3–12.
Dent RR, Guilleminault C, Albert LH, Posner BI, Cox BM, Goldstein A. Diurnal rhythm of plasma immunoreactive beta-endorphin and its relationship to sleep stages and plasma rhythms of cortisol and prolactin. J Clin Endocrinol Metab. 1981;52(5):942–7.
Cauter EV, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. J Am Med Assoc. 2000;284(7):861–8.
Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265(5172):679–82.
Stickgold R, Walker MP. Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 2005;28(8):408–15.
Sara SJ. Sleep to remember. J Neurosci Off J Soc Neurosci. 2017;37(3):457–63.
Frank MG, Heller HC. The function(s) of sleep. Handb Exp Pharmacol. 2019;253:3–34.
Hill VM, O'Connor RM, Sissoko GB, Irobunda IS, Leong S, Canman JC, et al. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018;16(7):e2005206.
Deepashree S, Niveditha S, Shivanandappa T, Ramesh SR. Oxidative stress resistance as a factor in aging: evidence from an extended longevity phenotype of Drosophila Melanogaster. Biogerontology. 2020;20(4):497–513.
Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990;48(5):749–53.
Rattenborg NC, de la Iglesia HO, Kempenaers B, Lesku JA, Meerlo P, Scriba MF. Sleep Research Goes Wild: New Methods and Approaches to Investigate the Ecology, Evolution and Functions of Sleep. Philosophical transactions of the Royal Society of London. Series B Biol Sci. 2017;372(1734):20160251.
Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264–71.
Malik SW, Kaplan J. Sleep deprivation. Prim Care. 2005;32(2):475–90.
Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12(1):13–21.
Gou XJ, Cen F, Fan ZQ, Xu Y, Shen HY, Zhou MM. Serum and brain metabolomic variations reveal perturbation of sleep deprivation on rats and ameliorate effect of total ginsenoside treatment. Int J Genom. 2017;2017:5179271.
Grandner MA, Jackson NJ, Pak VM, Gehrman PR. Sleep disturbance is associated with cardiovascular and metabolic disorders. J Sleep Res. 2012;21(4):427–33.
Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(1):1–13.
Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–26.
Kawai T, Akira S. Pathogen recognition with toll-like receptors. Curr Opin Immunol. 2005;17(4):338–44.
Takeda K, Akira S. Toll-like Receptors. Curr Protoc Immunol. 2015;109:14.12.1–14.12.10.
Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–50.
Kumar H, Kawai T, Akira S. Toll-like receptors and innate immunity. Biochem Biophys Res Commun. 2009;388(4):621–5.
Unanue ER. The regulatory role of macrophages in antigenic stimulation. Part two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136.
Murphy K, Travers P, Walport M. Janeway’s immunobiology. 7th ed. Garlan Science: New York, NY; 2008.
Schatz DG, Oettinger MA, Schlissel MS. V(D)J recombination: molecular biology and regulation. Annu Rev Immunol. 1992;10:359–83.
Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36(11):1357–66.
Reinhardt RL, Kang SJ, Liang HE, Locksley RM. T helper cell effector fates—who, how and where? Curr Opin Immunol. 2006;18(3):271–7.
Stetson DB, Voehringer D, Grogan JL, Xu M, Reinhardt L, Scheu S, et al. Th2 cells: orchestrating barrier immunity. Adv Immunol. 2004;83:163–89.
Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59.
Lange T, Dimitrov S, Fehm HL, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166(16):1695–700.
Bollinger T, Bollinger A, Naujoks J, Lange T, Solbach W. The influence of regulatory T cells and diurnal hormone rhythms on T helper cell activity. Immunology. 2010;131(4):488–500.
Ibarra-Coronado EG, Pantaleón-Martínez AM, Velazquéz-Moctezuma J, Prospéro-García O, Méndez-Díaz M, Pérez-Tapia M. The bidirectional relationship between sleep and immunity against infections. J Immunol Res. 2015;2015:678164.
Krueger JM, Walter J, Dinarello CA, Wolff SM, Chedid L. Sleep-promoting effects of endogenous pyrogen (interleukin-1). Am J Phys. 1984;246(6):R994–9.
Kimura M, Majde JA, Toth LA, Opp MR, Krueger JM. Somnogenic effects of rabbit and recombinant human interferons in rabbits. Am J Phys. 1994;267(1 Pt 2):R53–61.
Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S. Sleep deprivation and human immune function. Adv Neuroimmunol. 1995;5(2):97–110.
Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Archiv. 2012;463:121–37.
Moldofsky H. Sleep and the immune system. Int J Immunopharmacol. 1995;17:649–54.
Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immunol. 2004;18(4):341–8.
Jewett KA, Krueger JM. Humoral sleep regulation; interleukin-1 and tumor necrosis factor. Vitam Horm. 2012;89:241–57.
Preston BT, Capellini I, McNamara P, Barton RA, Nunn CL. Parasite resistance and the adaptive significance of sleep. BMC Evol Biol. 2009;9:7.
Opp MR. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evol Biol. 2009;9:8.
Palmblad J, Cantell K, Strander H, Froberg J, Karlsson CG, Levi L, et al. Stressor exposure and immunological response in man: interferon-producing capacity and phagocytosis. J Psychosom Res. 1976;20:193–9.
Asif N, Iqbal R, Nazir CF. Human immune system during sleep. Am J Clin Exp Immunol. 2017;6(6):92–6.
Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10(3):199–210.
Suchecki D, Tiba PA, Machado RB. REM sleep rebound as an adaptive response to stressful situations. Front Neurol. 2012;3:41.
Opp MR, Krueger JM. Interleukin 1-receptor antagonist blocks interleukin 1-induced sleep and fever. Am J Physiol. 1991;260(2):R453–7.
Deloria LB, Mannering GJ. Interferon induces sleep and other CNS responses in mice recovering from hexobarbital anesthesia. Neuropharmacology. 1993;32(12):1433–6.
Takahashi S, Fang J, Kapás L, Wang Y, Krueger JM. Inhibition of brain interleukin-1 attenuates sleep rebound after sleep deprivation in rabbits. Am J Phys. 1997;273(2 Pt 2):R677–82.
Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol Regul Integr Comp Physiol. 1987;253(1):R142–9.
Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, et al. Disregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2011;185(10):5796–805.
Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab. 1999;84(8):2603–7.
McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216(Pt 1):84–98.
Krueger JM. The role of cytokines in sleep regulation. Curr Pharm Des. 2008;14(32):3408–16.
Krueger JM, Majde JA, Rector DM. Cytokines in immune function and sleep regulation. Handb Clin Neurol. 2011;98:229–40.
Klein RS, Garber C, Howard N. Infectious immunity in the central nervous system and brain function. Nat Immunol. 2017;18(2):132–41.
Krueger JM, Opp MR. Sleep and microbes. Int Rev Neurobiol. 2016;131:207–25.
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–187.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gómez de León, C.T., Morales-Montor, J. (2021). Sleep and Immunity. In: Gozal, D., Kheirandish-Gozal, L. (eds) Pediatric Sleep Medicine. Springer, Cham. https://doi.org/10.1007/978-3-030-65574-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-65574-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65573-0
Online ISBN: 978-3-030-65574-7
eBook Packages: MedicineMedicine (R0)