Abstract
The Region of Central and South America represents an eclectic scenery with regards to its economic and social indicators and its ecological and geographical range. This is a Region with a vast wildlife diversity, which also holds the largest livestock producing and exporting countries. In this context, many opportunities for interaction at the wildlife–livestock interface arise, enabling the occurrence of important infectious diseases.
This chapter describes the regional insights of diseases such as rabies, avian influenza, leishmaniasis, brucellosis, bovine tuberculosis, and a group of arboviruses; together with the strategies for their surveillance and control at the wildlife–livestock interface. Besides, foot-and-mouth disease (FMD) is presented here, not because wild animal species have been relevant to FMD control in the Region (as it happens in other regions), but because of the opposite—representing an alternative epidemiological scenario for this disease.
The chapter identifies several knowledge gaps, particularly those in relation to disease occurrence, to the identification of hosts/reservoirs and their role, and to disease dynamics. In response, strategies for enhanced surveillance and new research objectives are proposed; particularly those aiming at detecting the presence of different pathogens (e.g., avian influenza, tuberculosis, brucellosis, West Nile virus, etc.), either in the livestock population or among wildlife, and their interactions at this interface. On the horizon, despite the efforts made and some progress achieved, bat-transmitted rabies will remain as the disease transmitted from wildlife to livestock with a major impact in the Region.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The Region of Central and South America, which extends from northern Guatemala to Tierra del Fuego, is full of sharp contrasts in many of its descriptive parameters (e.g., economic, social, geographic, agriculture and livestock production, wildlife, etc.). While it is in fact one of the Regions with the largest biodiversity, it also encloses the largest livestock producing and exporting countries. Against this complex regional backdrop, many opportunities for interaction arise at the wildlife-livestock interface, for which it is necessary to assess their reciprocal impact. On one hand, to explore whether livestock diseases are posing a threat for wildlife conservation; but at the same time, determining how the interaction with wildlife reflects on livestock diseases.
This scenario at the wildlife-livestock interface enables the occurrence of important infectious diseases, including rabies, avian influenza, leishmaniasis, brucellosis, bovine tuberculosis, and a group of arboviruses (Cunningham et al. 2017). These diseases imply different impacts (on the economy, on public health, on conservation); have different epidemiology, with uneven prevalence distribution across the Region and several ways of transmission (with or without the role of vectors); and present different threats and opportunities. Thus, a detailed focus on the regional outlook of these diseases would allow getting a comprehensive view of potential situations that can be encountered within the topic.
Beyond the issue of disease sharing at the wildlife-livestock interface, it is important to identify those strategies for surveillance and control that are contributing to resolve the spillovers; particularly comparing what it is being done with what should be done. Likewise, in this heterogeneous context, there is an opportunity to describe the main knowledge gaps about those interactions not well understood yet and to add to the wishlist those potential key topics that need more and better research. Additionally, since the increase in deforestation has been one of the major problems due to the development of the livestock sector in the Region, it is necessary to recognize the direct impact on wildlife and on the occurrence of vector-borne diseases.
Socioeconomical and Biogeographical Circumstances
The Region represents a complex socioeconomic area where the wide income inequality is still a major problem (Tsounta and Osueke 2014; World Bank 2014). In the past, its natural resources, such as precious metals, sugar, rubber, coffee, and cacao, made it a strategic Region; and likewise, it was also favored by the recent pattern of global recovery (Sinnott et al. 2010). In fact, the national policies implemented across the countries have contributed to support the domestic demands in the regional larger economies, as well as external demand from fast-growing emerging markets, boosting export (Sinnott et al. 2010).
A large part of the Region is often affected by natural hazards such as earthquakes, volcanic eruptions, tsunamis, or hurricanes, together with the alternation of serious floods and droughts linked to El Niño-Southern Oscillation (Tollefson 2014). These natural disasters disturb the basic welfare state (e.g., food security, access to drinking water, housing, etc.) and have an impact on the occurrence of infectious diseases in human being, livestock, and wildlife (Watson et al. 2007; Cohen and Thompson 2012; Kouadio et al. 2012). Central and South America includes a variety of geographical features such as the Amazonian rainforest, the biome of Pampas, the tropical wetlands of the Pantanal, the Andean mountain range, tropical forest, large rivers, and a combination of natural and human-made grasslands and shrublands across the whole Region.
The livestock regional map has drastically changed over the last five centuries, along with the pastures-deforestation landscape. Among the Pre-Columbian civilizations, the only domesticated species were the dog, turkey, guinea pig, and Andean camelids (Martínez et al. 2012). Livestock species (including cattle, horses, donkeys, pigs, sheep and goats) were brought since the late fifteenth century, in the first trips to the Americas from Europe during the colonization. The cattle expanded throughout the Americas, adapting to a wide range of environmental conditions and giving origin to what is currently known as Creole cattle (Martínez et al. 2012; Anderson et al. 2015). After nearly 300 years of expansion, together with the development of more intensive production systems, several other European breeds were brought (Martínez et al. 2012). Likewise, at end of the nineteenth century, Bos indicus cattle breeds were also introduced and quickly disseminated throughout the Americas, especially in tropical regions (Martínez et al. 2012).
Among the major livestock diseases occurrence in the Region, Foot-and-Mouth Disease (FMD) would be on the top. It was introduced at the end of the nineteenth century in South America, and it has never been detected in Central America (Naranjo and Cosivi 2013). FMD is still one of the major livestock diseases worldwide as a result of the highly infectious nature of the virus and the significant direct impact of the virus on animal production and trade (Garland and de Clercq 2011; Clavijo et al. 2017)—and hence on food security. This disease, now almost eradicated, has contributed to the framing of the veterinary services of the Region on strategies such as surveillance, control programs, prevention, and emergency preparedness (Clavijo et al. 2015). In South America, the total annual costs of the countries involved in FMD control, surveillance, and prevention are estimated to be over one thousand million USD per year (Centro Panamericano de Fiebre Aftosa – OPS/OMS 2017).
The livestock expansion did not take place without an impact on the wildlife landscape. It is estimated that between 1990 and 2010, deforestation reached 88 million hectares in Central and South America, where large-scale forest conversion for livestock-based and commercial agriculture is a primary cause of deforestation (Lambin et al. 2003; FAO 2017a). Hence, the areas for livestock grazing have expanded in the past and will continue to increase, although at a moderate pace, in this Region (Alkemade et al. 2013). The largest deforestation front took place in the Brazilian Amazon, which extended more recently outside Brazil, east of the Andes, and even to Venezuela (Lambin et al. 2003). Likewise, the Chaco (that includes areas of Argentina, Bolivia, Brazil, and Paraguay) and parts of Atlantic forest in South America have been identified as areas of forest loss. Central America has significant deforestation areas in the Yucatan and at the Nicaraguan border with Honduras and Costa Rica (Lambin et al. 2003).
The Prevalent Livestock, Farm Typologies in Every Region, and Opportunities for Interface
Its geography, and its associated climatic conditions, draws an eclectic map of livestock production, with a broad variety of species and ways of production, which ranges from farming of llamas in the Andes to the vast territories of cattle grazing in grasslands, together with key areas for pig and poultry intensive production. In fact, the Region has the elements to be a major livestock producer, to meet local food needs, and contributing to world food security (FAO 2017b).
(a–b) Camera trappings of cattle and ocelot (Leopardus pardalis) in the same spot in Ecuador. Tropical forests are increasingly destroyed to clear land that is ultimately used for agriculture, the largest driver of tropical deforestation globally. (c) There are vast forested areas at risk of conversion for pastureland expansion in South America. The picture shows a cleared area where black vultures (Coragyps atratus) wait for cows to defecate and/or give birth. (d) The capybara (Hydrochoerus hydrochaeris) is the largest living rodent in the world (wild or raised under free-ranging or semifree-ranging conditions), and increasingly present in urban areas, living near bodies of water. The capybara is one of the wildlife species in the Region susceptible to FMDV (image: “Wild Llanos—Agro Tours” Colombian Llanos, Yopal, Casanare). (e) Vicuña (Vicugna vicugna) in Chimborazo (Ecuador). The vicuña is one of the two wild South American camelids which live in the high alpine areas of the Andes, the other being the guanaco (Lama guanicoe). Vicuñas are the wild ancestors of domesticated alpacas. Sheep (f) occupy the same habitat (except extremely high altitudes) as camelids including vicuña and guanaco, see also next figure) (image: C. Gortazar)
(a) The greater rhea (Rhea americana) is a South American ratite endemic to Argentina, Bolivia, Brazil, Paraguay, and Uruguay. This bird has been greatly affected by habitat alteration (farming) and hunting. The image shows a close interaction with cattle (image: M. M. Guerisoli-Grupo de Ecología Comportamental de Mamíferos). (b) These images illustrate the human face of the triple human-wildlife-livestock interface; a peccary piglet is fed by local villagers in proximity of domestic pets and poultry in Peru (images: C. Gortazar). (c–e) Livestock, such as horses, and guanacos, and huemul (Hippocamelus bisulcus) share habitat in the Magallanes Region (Torres del Paine National Park, Chile) (images: Ezequiel Hidalgo). The guanaco is the wild ancestor of the lama and inhabits the Andean and Patagonian steppes from sea level to high altitudes. It is considered a key engineer species for the Patagonian steppe. According to historical data, guanaco populations in pre-Columbian times reached 30–40 million specimens from the north of Peru to the extreme south of Chile, at present (IUCN) the total world population would not exceed 600,000 individuals. (f) Pudu (Puda puda), a species considered as high probability of population decline, and cattle sharing habitat Nahuelbuta National Park of the Bio Bio Region in Chile (images: Dario Moreira Arce). (g) Wild–domestic carnivore interaction by a dog approaching an American sea lion (Otaria flavescens) carcass in the Chilean coast (image: C. Gortazar)
The Region combines subsistence livestock production and smallholders with a strong intensive animal farming industry, which positions some countries of the Region among the largest cattle, pig, and poultry producers in the world. In fact, the livestock sector has boomed in recent decades, particularly in the Southern Cone, due to the growth in world food demand (FAO 2017b). Four countries of the Region (Brazil, Uruguay, Paraguay, and Argentina) appear among the top ten exporters of beef and veal in the world (USDA 2018). Likewise, Brazil together with Chile are among the top ten pork exporters in the world, and Brazil and Argentina among the top ten broiler meat exporters (USDA 2018).
The forecast indicates that the Region will maintain a position among the leaders in meat production. Brazil’s growth will benefit from an abundant supply of natural resources, and will also count on noteworthy potential contributions to additional meat production from Argentina (OECD/FAO 2017). These two countries from the Region, together with China, India, Mexico, and Pakistan, will account for 75% of the additional beef produced by 2026 (OECD/FAO 2017).
These promising regional scenarios on livestock production are hampered by concerns on: the limited availability of quality animal feed and by an inefficient use of available resources; the risk of introduction of transboundary animal diseases and costs related to livestock disease prevention, surveillance, and control; and damages associated with the conversion of land use and threats due to the negative impact of climate change on the livestock sector (Sinnott et al. 2010; PANAFTOSA 2015; FAO 2017b).
Livestock expansion into the Americas has also interacted with the wildlife populations. Thus, the Desmodus rotundus (common vampire bat) has a preference for the blood of large mammals, such as horses, cattle, and pigs, and since the introduction of these livestock species into the New World (Turner 1975), the D. rotundus populations adapted to use this source of feeding which allowed a population burst. It has been demonstrated that high livestock densities are an important factor to understand the pattern of bat expansion (Reis et al. 2007). Likewise, deforestation has been one of the straightforward and determinant causes for climate and environmental changes, favoring the expansion of leishmaniases to areas with no previous transmission (Cardenas et al. 2006, 2008; Gottwalt 2013). Indeed, several studies have demonstrated the effect of the actions generated by agriculture and livestock in the occurrence of vector-borne diseases, such as leishmaniases (Mayen 2003; Molyneux 2003; FAO 2012; Gottwalt 2013).
The Wildlife
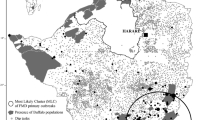
The wildlife outlook is also heterogeneous, considering that six of the nine countries with the greatest diversity of terrestrial fauna on the planet are within the Central and South American regions (Butler 2016, Figs. 1 and 2). A large part of this diversity is composed of species of three orders (i.e., Chiroptera, rodents, and primates), recognized as hosts or reservoirs for major animal diseases and zoonoses (Jones et al. 2008; Olival et al. 2017). In addition, the Region has an eclectic variety of species of ungulates (South American camelids, cervids, tapirids, tayasuids), carnivores (canids, didelphids, felids, procyonids, mephitids, mustelids), and the greatest diversity of birds on the planet. In short, this scenario represents a wide range of potential hosts for relevant infectious diseases at the livestock/wildlife interface. All this enables an epidemiological scenario for the occurrence of important infectious diseases, having the wildlife as a modulator, such as rabies, avian influenza, West Nile virus (WNV), brucellosis, or tuberculosis.
While a number of studies to investigate the exposure (or infection) of livestock pathogens to wildlife species have been carried out (De Sousa et al. 2017; Maciel et al. 2017), only a few have shown disease or pathological implications (Deem et al. 2002; Filoni et al. 2009; Morales et al. 2017). There is a series of parameters (e.g., clinical signs assessment, anatomo-pathology/histopathology, or laboratory diagnostic such as molecular characterization of the agent) allowing the evaluation of the consequences in the conservation of a species that could be used to help understand the direct impact of livestock diseases on wildlife health (Hidalgo-Martínez et al. 2008; Preece et al. 2017; Grogan et al. 2018). Evidence of this impact has been identified in other regions of the world. For Central and South America, however, the episodes of mortality in wildlife are associated with other factors than the transmission of the pathogen at the interface of the wildlife-livestock. Indeed, there is no evidence in this Region, either through molecular epidemiology or pathological studies, to confirm any substantial impact of livestock on wildlife health.
In the Region, rabies, leishmaniasis, Venezuelan equine encephalomyelitis (VEE), leptospirosis, and avian influenza appear among the diseases with implications on wildlife-livestock interface that are most often addressed in scientific publications (Wiethoelter et al. 2015) (Table 1). In the rabies aerial cycle, in which bats are the reservoir of the virus, the presence of rabies-infected bats in urban and rural areas represents risks to livestock and public health, due to feeding from large herbivores and to synanthropic habit (Kotait et al. 2009). Although other species of hematophagous bats are present in the Region, i.e., Diaemus youngi, Diphylla ecaudata (Reis et al. 2007), the D. rotundus (common vampire bat) is the main responsible for the incursions of rabies (associated with attacks) from the aerial cycle into the rural cycle, that is characterized by the transmission of rabies to livestock (and human) populations (Reis et al. 2007; Kotait et al. 2009; Albas et al. 2011). D. rotundus has a wide distribution, being the most common and abundant species of vampire bat, found from the north of Mexico to the north of Argentina. It feeds from mammal’s blood, both wild and domestic, including man and occasionally on birds (Reis et al. 2007). In its natural habitat, its roosts are caves, stone burrows, hollow trees, fallen trees, and even termite nests (Gomes and Uieda 2004). Deforestation and occupation of wilderness by livestock and other man-made activities (Kunz and Fenton 2003), forced them to adapt to the new conditions in urban areas (Reis et al. 2002). Besides D. rotundus, other bats have shown to play a role in the disease maintenance, contributing in that way to the aerial cycle (Calisher et al. 2006), but without participating in the transmission of rabies to livestock.
Venezuelan equine encephalitis (VEE), eastern equine encephalitis (EEE), west equine encephalitis (WEE), and West Nile encephalitis (WNE) are emerging arboviruses that can affect humans, equines, other mammalian species, and birds. These pathogens represent another example where livestock affecting diseases are influenced by wildlife. As in other diseases transmitted by arthropods, they have cycles involving wild vertebrates (reservoirs/amplifiers), mosquitos (vectors) equines, and humans (final hosts) (Mesa et al. 2005). These are characterized by different epidemiological attributes; all of them, however, are involved in a complex interaction between equine livestock and a wide range of wildlife reservoirs that include both mammals and birds, with the additional potential impact on public health. The cycle for leptospirosis also involved wildlife, and the most frequent reservoirs in the Region are members of the order Carnivora (e.g., N. vison, C. thous, and N. nasua), that may get exposed to these bacteria by consuming infected prey, such as rodents (Vieira et al. 2017). Avian influenza is another key disease having wildlife reservoirs. Among the reported events of avian influenza in the Region, most of them has been associated with migratory birds, particularly of the orders Anseriformes and Charadriiformes (Hurtado et al. 2016; Afanador-Villamizar et al. 2017), which play a major role in the disease spread and its transmission to domestic poultry.
Leishmaniasis, conversely, is an example that contributes to illustrate the complex interface between wildlife and livestock that has an impact on human health. The parasite does not have direct implications for livestock; however, the deforestation, which in turn, is partially associated with livestock expansion has contributed to change the dynamics of leishmaniasis and favored human contact with Leishmania wild vectors. Its complex and dynamic transmission cycle is characterized by a variety of vectors, reservoirs, and Leishmania. Twenty-two Leishmania species—15 in the Americas—have been identified as being pathogenic to humans (Alvar et al. 2012; Desjeux 2004; World Health Organization 2010). In Central and South America members of the family Canidae, of the order Rodentia and of the infraclass Marsupialia have been identified as the main reservoirs (Roque and Jansen 2014). The maintenance of the Leishmania in nature depends on the transmission cycle, which can be zoonotic (as in this Region) or anthroponotic. Likewise, the different species of phlebotomine, involved in the transmission, present a unique and complex biology that affects the distribution, cycle, and control of the disease (Gramiccia and Gradoni 2005; WHO 2010).
The Disease at the Interface: One Health Perspective
The concept of One Health comes to recognize the inextricable relation between its three main components: animal, human, and environment health (Gibbs 2005; Zinsstag et al. 2011). There are indeed interesting contributions brought with the application of this concept, and it also helps to think broadly about problems and solutions beyond the boundaries of just one component. Thus, it is used in this chapter to explore different scenarios of health interaction between wildlife (a term that, here, intentionally merges together wild animals and their habitats) and livestock; which additionally, when relevant, is tangentially presented with the human interplay.
Within the One Health triad, the human component cannot be ignored, even when it is not the priority of this chapter, since humans interfere, module, and somehow control the wildlife-livestock interface. The human population in the Region is mainly distributed in urban areas. Since it is the world’s most urbanized Region (UN-HABITAT 2012; Atlantic Council 2014), the rural population needs to be protected and more valued—as it holds a socioeconomic and environmental relevance. Among developing regions, Latin America has possibly the longest experience in the application of the territorial development approach. National programs for rural territorial development, aimed at alleviating poverty, improving education levels, and reducing regional disparities, are underway in Argentina, Brazil, Colombia, Costa Rica, Honduras, Mexico, and Nicaragua (FAO 2017a). Besides, rural incomes respond to agricultural development, even though many rural households do not necessarily rely on agriculture as their direct or main income source (de Ferranti et al. 2005). Thus, policy reforms that began in the 1980s led to an overall growth and trade which contributed to increase the rural income, the reduction of poverty, and improvements in welfare indicators (de Ferranti et al. 2005; FAO 2017a).
Apart from some of the abovementioned diseases (rabies, avian influenza, leishmaniasis, and arboviruses), other three major diseases with an important economic impact on livestock production are also explored here: FMD, brucellosis, and bovine tuberculosis. Hence, these diseases are used as a model to present the main features of One Health interaction together with the problems and opportunities. FMD is presented in this chapter not because wild animal species are relevant to FMD control in the Region (as it happens in other regions such as Africa), but because of the opposite—it represents an exception. Brucellosis and bovine tuberculosis (both zoonoses) are briefly presented as a complex interaction between livestock species and wildlife, which is not yet understood and hence presents opportunities to throw some light on the aspects of the epidemiology.
Box 1 Rabies
The rabies terrestrial cycle in the Region, mostly transmitted by dogs—with an apparent insignificant role of native terrestrial wild mammals—is under efficient control measures in most of the countries. The aerial cycle, however, remains a serious issue (Albas et al. 2011). It is estimated that D. rotundus rabies seroprevalence is more than 40% (da Costa and Fernandes 2016), particularly associated with variant 3 in this species (Schneider et al. 2009). So far, Rabies is the disease transmitted from wildlife to livestock that has the major impact in the Region. Today, in geographic areas with no control measures this disease might cause the death of around 100–500,000 cattle per year, and forecasts for the coming decades report that conditions in the Region may become increasingly favorable to predation of herds by D. rotundus (Swanepoel 1994; Lee et al. 2012). In Brazil, during the years 2002 to 2015, more than 665 million bovines were vaccinated against rabies throughout the national territory, which reveals an annual mean vaccination of 51 million animals (MAPA 2015). In addition to vaccination costs, Belotto et al. (2005) estimated that losses to livestock production in Latin America amounted to US$50 million per year. Although predation of humans is not the first choice for hematophagous bats, when there is a reduction or absence of local herds, leading to a decrease in the feeding sources, the attack on humans might increase, as it has been reported particularly in the Amazon (Schneider et al. 2009). Furthermore, herbivores that were bitten and infected by vampire bats, sometimes, accidentally transmit rabies to people; therefore, beyond economic losses, herbivorous rabies also represents an impact on public health (Kotait et al. 2009).
Surveillance of rabies in wild animals involves veterinarians, forest rangers, local people, and animal rights groups, as it is important to monitor the behavior of wild animals, especially in endemic areas for rabies. Rabies must be a notifiable disease (both in humans and animals) in all countries (WHO 2013; OIE 2018), which requires the active involvement of the local health services and private veterinarians and owners of domestic herbivores—that should inform the official veterinary services. Also, suspected bites with consequent nervous symptoms in people or animals, and the presence of bat roost should be reported. The official veterinary service, in turn, must attend to the notifications by collecting samples and investigating epizootics. Upon deciding on the implantation of any strategy, it is necessary to distinguish rabies reservoirs from animals that are just susceptible to rabies infection, so that their epidemiological importance can be established, and suitable surveillance and control measures can be implemented. D. rotundus is thus the target of surveillance and control strategies in the countries. Latin America has an extensive network of more than 100 laboratories to diagnose cases of rabies in humans and animals, as well as the typification of the viral variant (REDIPRA 2003). Annually, the official veterinary services notify in Latin America, more than 1500 herbivore rabies outbreaks with laboratory confirmation (SIRVERA 2018). However, it is believed that official notifications represent only 10% of total outbreaks (Kotait 1998). Among the factors for the underreporting are the difficulty to collect and send samples to the official laboratory services in a country, an insufficient number of professionals to implement surveillance, unspecific clinical signs that can be mistaken with other diseases, difficulty to reach some territories, and local population ignorance about rabies (Mayen 2003; Belotto et al. 2005; Mallewa et al. 2007).
Box 2 Avian Influenza
Avian influenza is classified in two forms according to its pathogenicity level, i.e., high and low (OIE 2017). While highly pathogenic avian influenza (HPAI) is a key disease at the wildlife-livestock interface in other regions, that is not the case for Central and South America, where this form has only been reported in Chile (Mathieu et al. 2015). In poultry, low pathogenic avian influenza (LPAI) has been only reported in Belize and Chile (WAHIS-OIE 2018) for the H5 and H7 OIE notifiable subtypes (OIE 2017); however, other low pathogen strains might be present in the poultry population of the Region (Bravo-Vasquez et al. 2016; Jiménez-Bluhm et al. 2016). The scenario for LPAI is different in wildlife, with several reports informing of the presence of this form in Argentina, Bolivia, Brazil, Chile, Colombia, and Peru (Spackman et al. 2006; Pereda et al. 2008; Ghersi et al. 2009; Karlsson et al. 2013; De Araujo et al. 2014; Mathieu et al. 2015). In general, the number of isolates has been small in the Region, in correlation with the small sampling size of the surveys. This said, it is necessary to consider that some evidence suggests that the prevalence of avian influenza might be actually lower in this Region than in other parts of the world (Hurtado et al. 2016). Avian influenza is only briefly presented in this chapter due to its low frequency of occurrence in poultry across Central and South America, and to the limited knowledge on its presence in wild birds.
Box 3 Equine Encephalitis and West Nile Encephalitis
Among the group of diseases caused by arboviruses presented here, three of them (i.e., EEE, WEE, and VEE) only occur in the American continent; whereas WNE is also found in Asia, Africa, and Europe. They are associated with specific environmental characteristics together with economic, political, and social factors (Mesa et al. 2005). VEE is of great importance because of its severity, high morbidity, and lethality in equines, and also due to its periodical presentations in epizootics and epidemics in a great part of the American continent (Acha and Szyfres 2003). One of the major outbreaks of VEE in Central and South America occurred in 1969, involving 31,000 humans and the death of approximately 20,000 horses only in Ecuador, resulting in losses of around US$1,200,000 (Acha and Szyfres 2003). The high morbidity and mortality in equine livestock, as occurred in 1969, affects the rural economy of the Region, since these animals are used in agricultural tasks and individual transport. In addition, VEE impacts on public health, due to the incapacitation of workers, treatment costs, and hospitalization of individuals, cause a decrease in community well-being and might contribute to a collapse in health services (Acha and Szyfres 2003; Alder et al. 2005; Mesa et al. 2005).
The EEE virus has been isolated in most of the countries of the American continent, including Brazil, Argentina, Colombia, Peru, Panama, Haiti, Guatemala, Mexico, USA, and Canada (OIRSA and PAHO 2014). The EEE virus variant found in North America is more pathogenic to horses and humans than the variant present in South and Central America (Smith et al. 2009). In recent years, the EEE and VEE have been showing unusual behavior in the Region and outbreaks of both diseases were reported in humans and horses in Panama in 2010. These occurrences in humans may be the result of increased human contact with enzootic transmission cycles, genetic changes in EEE viral strains that resulted in increased human virulence, or an alteration of host range (Carrera et al. 2013). The Table 1 provides a summary of the major diseases at the wildlife-livestock interface presented in this chapter.).
Like EEE and VEE, WEE virus circulates in regions of North, Central, and South America. Thus, WEE has been identified from Argentina to western Canada, usually presenting sporadic cases in horses in a widespread manner (OIRSA and PAHO 2014). It is worth mentioning that in the enzootic cycle of equine encephalitis, the transmission involves rodents (VEE) and wild birds (EEE and WEE) to a variety of mosquitoes. Equines and humans may accidentally engage in this cycle when they enter the enzootic ecosystem and get affected when susceptible (e.g., born after an epizootic, unvaccinated, or that came from free areas) (Mesa et al. 2005).
The occurrence of VEE, EEE, and WEE epidemics poses a serious risk to Central and South American countries since most countries have favorable conditions for the development and distribution of these arboviruses (Acha and Szyfres 2003). Between 2000 and 2015, several outbreaks of equine encephalitis were reported in different countries of Latin America (Table 2). The main factors associated with the emergence of epidemics in the region are: the presence of reservoirs and vectors, the population of susceptible equines, migrations, human displacement and their congregation in areas surrounded by amplifying animals, expansion of agricultural frontiers, climatic changes, water accumulation, and the poor hygienic conditions (PAHO 2011).
The WNE is relatively new in the Americas—first reported in 1999 (USA) (Lanciotti et al. 1999)—but has managed to spread rapidly, particularly in North America, by finding ecological conditions and habitats that favor the migration of many species of birds—virus amplifiers in this region. The most pressing concern regarding the reports of WNE in the Region is the absence of robust data on the disease burden in people, horses, or birds (Komar and Clark 2006). Serological evidence indicates that the virus has spread southwards in different countries of Central and South America (Table 2). In Argentina, the viral particles identified as WNV have already been isolated in horses with neurological symptomatology (Morales et al. 2006; Chancey et al. 2015). Although the disease has not yet posed a serious threat to the Region, the high equine population density and ecological conditions favorable to the proliferation of arthropods are factors that may result in WNV epidemics in this region (Ward 2005; Komar and Clark 2006). The role of migratory birds in the spread of WNV is still unknown, but the rapid diffusion of the agent in the USA and later in Central and South America points to a probable participation of these birds (LaDEAU et al. 2007). In this respect, the zoological parks can be used as sentinels for WNV in natural or urban environments (Ludwig et al. 2002; Pultorak et al. 2011).
Box 4 Leishmaniasis
In the past, in Central and South America, leishmaniases had two well-established transmission patterns: visceral leishmaniasis was considered a primarily rural disease, and cutaneous leishmaniasis had a predominantly sylvatic pattern. Since the 1980s, other transmission patterns became epidemiologically important, determining different scenarios (Maia-Elkhoury et al. 2008; Salomón et al. 2015). These scenarios were established when cutaneous and visceral leishmaniasis began to occur in peri-urban/urban areas, since new risk factors and changes in the biology of the wild vector favored their adaptation to domestic environments and to a greater contact with man (Desjeux 2004; Rangel and Vilela 2008; Salomón et al. 2006, 2008, 2015). This process was consolidated with the increase of cases, a 30% increase in total cases of cutaneous leishmaniasis during the period of 2001–2010, and widening the geographic of the disease, reaching large cities and other countries of the region (Maia-Elkhoury et al. 2008, 2016; Salomón et al. 2015; PAHO 2016).
Risk factors such as deforestation, modification of the land usage, climate change, and migration have contributed to this growth due to greater humans–vectors–reservoirs contact (Maia-Elkhoury et al. 2016). Different reports from Argentina, Bolivia, Brazil, Colombia, Mexico, Costa Rica, and Peru have shown a relationship between the occurrence of cutaneous leishmaniasis and environmental alterations due to climate change, deforestation, and economic activities, including agriculture (Davies et al. 2000; Cardenas et al. 2006, 2008; Chaves and Pascual 2006; Valderrama-Ardila et al. 2010; Gottwalt 2013).
Box 5 Foot-and-Mouth Disease
Central America is free from the disease—in fact, it is considered historically free—while in the South American countries the disease is heading to its eradication (Clavijo et al. 2017). In this subregion, FMD virus infection has been caused by viral types O, A, and C, which reached a wide distribution during the first half of the twentieth century. While it was recognized that bovines are responsible for the presentation of the main patterns of the diseases, it was suspected that wild animals could have also played a role in the persistence of the virus in the field during the interepidemic periods (Rosenberg and Gomes 1977). An FMD outbreak in Colombia in 1976, where type A virus was isolated from a capybara (Hydrochoerus hydrochoeris) presenting clinical lesions and living with pigs and cattle (Brasileira and Agropecuária) brought the attention to this species due to its abundance in endemic areas and its high degree of ecological competition with the bovine species (Rosenberg and Gomes 1977). The susceptibility of the species to FMDV infection has been confirmed both by intramuscular route and by direct contact. It has been also proved that capybaras inoculated with the virus could transmit the infection to cattle and pigs by direct contact, which was evidenced by both the appearance of clinical signs together with virus isolation and antibody production. But even though it has been postulated that this rodent could contribute to the spread of the infection due to their migratory habits, it would not act as natural reservoirs (Gomes and Rosenberg 1984).
There is little information on the epidemiology of FMD in South American camelids from field research. In only one report, mild clinical signs were observed in alpacas (Vicugna pacos) in connection with an outbreak occurring in cattle (Wernery and Kaaden 2004). Experimental studies in llamas (Lama glama) and alpacas indicate that they can be infected by direct contact, but in general, they are not susceptible animals and do not represent a risk to transmit the infection. Likewise, their carrier status has not been verified in South American camelids (Wernery and Kaaden 2004). Serological studies have been carried out on Mexican deer (Blastocerus dichotomus), free-ranging vicunas (Vicugnas vicugnas) in Argentina and Bolivia (Marcoppido et al. 2010; Beltrán-Saavedra et al. 2011), gray brocket deer (Mazama gouazoubira) in Bolivia (Deem et al. 2004), in pampas deer (Ozotoceros bezoarticu sceler) in Argentina (Uhart et al. 2003), and guanacos (Lama guanicoe) in Argentina (Karesh et al. 1998) with no evidence of FMDV infection.
Beyond the valuable experimental work that has been done to evidence FMD infection in wild species, the literature does not clearly distinguish between evidence of infection and the ability to maintain infection at the population level, i.e., it might persist and be transmitted to other species (Weaver et al. 2013). Despite many speculations about the possible role of wild animals as reservoirs of FMD in South America, to date, there is no robust evidence to support such a hypothesis. Moreover, the fact that no outbreaks have been linked to transmission of the infection by wild animals also stands against that theory. In addition, in South America, systematic vaccination of the bovine species, leaving goats, sheep, and pigs as sentinels, has succeeded in eradicating the disease in most of the subcontinent (Sutmoller et al. 2003; Clavijo et al. 2017). This situation contributes to confirm that in those regions where the reservoir of FMD virus has been domestic animals, the disease eradication from them has usually led also to the disappearance of the infection of wild animals (Thomson et al. 2003).
Box 6 Brucellosis and Bovine tuberculosis
Brucellosis and bovine tuberculosis are present in the livestock population of most of the countries in South and Central America (WAHIS-OIE 2018); and the implementation of successful control/eradication strategies for these diseases are of the utmost importance to this Region, following the examples from others such as North America and Europe. The presence of these two infectious diseases is closely linked to underprivileged socioeconomic factors, being among the diseases with the greatest impact in low-income populations (Perry et al. 2002), and both are regarded as neglected human diseases according to the World Health Organization. Besides the impact on public health, they cause major economic losses in the livestock sector. It was estimated that yearly losses related to bovine brucellosis in Central America could reach up to US$25 million; while in South America, only in Brazil, the losses could be in the range of US$250 million (Moreno 2002; Santos et al. 2013). Although there is a lack of published data about specific losses due to bovine tuberculosis in this Region, the reported global annual losses of US 3 billion can help provide an idea of the current economic importance of this disease in the Region (Garnier et al. 2003).
In other regions, wild mammal species are known to act as reservoirs of Brucella and M. bovis, playing an important role in their dissemination and maintenance (Muñoz et al. 2010; Palmer 2013; Van Campen and Rhyan 2010). In Central and South America, although some studies reported several terrestrial wild mammal species naturally exposed or infected by Brucella spp. and M. bovis (Tables 3 and 4), there is a lack of information regarding their epidemiological role. Thus, considering that Brucella spp. and M. bovis are found in livestock and wild animals in Central and South America and that the expansion of cattle ranching over wildlife natural habitats increases the chances of contact and mutual disease transmission (Bengis et al. 2002), there is a crucial need to understand the wildlife-livestock interface for these diseases in this Region. On one hand, there is a need to clarify the impact of these diseases on native wildlife species. While on the other hand, the fact that wild species are known as reservoirs of these etiological agents in other regions reflects the need to clarify the epidemiological link between wildlife and livestock in this Region, particularly to determine if wild animals constitute reservoirs able to maintain and transmit Brucella spp. and M. bovis back to adjacent domestic animal populations (Van Campen and Rhyan 2010).
Management Practices at the Interface
Depending on the scenario, different approaches are used in the management and control of the diseases at the interface. Some of the strategies are focused on the management of the wildlife population, as it is done for rabies in the bat roosts; for which it is recommended to have a georeferenced registry. Among the interventions implemented in the roosts, the application of anticoagulant paste to decrease the population of D. rotundus is a widely used option provided for in the national strategies (Reis et al. 2007; MAPA 2009; Lee et al. 2012). Captured bats are coated with the paste, that when are released and get back to the roosts, will be transferred to others during the social grooming (when bats engage in cleaning one another). There are researches, however, arguing that these strategies aiming at decreasing bats population are not effective (Streicker et al. 2012), besides being regarded as a poorly humane practice. There exists a potential for ingestion of anticoagulant poisons by other bat species or through contamination of shared roosts, included endangered ones, although D. rotundus occupy specific sites within roosts and this may reduce impacts on other bat species (Wohlgenant 1994). As an alternative, other strategies to reduce the damage caused by rabies in livestock are suggested, such as the use of an oral vaccine, which is applied on captured bats and released to other roosts cohabitants in a similar manner that the anticoagulant paste (Lee et al. 2012). This alternative, however, still requires further investigations due to its unknown effectiveness as a control measure in the Region.
Other strategies for control should be based on areas at risk. Thus, in the control of rabies in herbivores, once the risk areas are defined, it is necessary to optimize the use of resources and reduce costs to ensure complete vaccination coverage. Another fundamental action for a control program is the educational campaigns to establish activities of health education to the population, using materials with images, such as posters and educational books for children, bringing information about the specific area in native languages that allow efficient sensitization of the population at risk (da Costa and Fernandes 2016). In addition, it is important to take on board a multidisciplinary strategy, involving all relevant stakeholders, in the prevention and control of disease at the interface. In fact, official programs start to recognize that an engagement with animal rights organizations by bringing them to the discussion table at the planning stages against rabies can deliver niche-specific approaches to local problems (Del Rio Vilas et al. 2017). The prevention and control of bat-transmitted rabies should involve health and agriculture with environment, education, housing, and infrastructure sectors from each country (Schneider et al. 2009).
Another essential component in the management of the diseases at the interface involves strengthening laboratory capacity for diagnosis to support the epidemiological surveillance. This is particularly important for arboviruses, and countries should prioritize the implementation of new diagnostic techniques for EEE, VEE, WEE, and WNO, ensure a continuous supply of essential inputs for the diagnosis, and provide the necessary laboratory equipment to ensure that both diagnosis and communication of results are timely (ICA-INS-MAVDT 2004; OIRSA and PAHO 2014). The measures for the prevention and control of these arboviruses should include training plans for health professionals, health promotion and education programs, quality control of vaccines, followup and evaluation of vaccination activities, timely attention to outbreaks, movement control of susceptible domestic animals, research, vector control, and community participation (Mesa et al. 2005).
Systems for enhancing surveillance such as target or risk-based strategies should be utilized to increase the sensitivity and timely detection of disease at the wildlife-livestock interface. These enhanced strategies should indeed be applied to diseases such as avian influenza, for which the regional epidemiological surveillance targeting wildlife is weak; and it would require extensive and constant efforts focused on local and migratory wild birds (Hurtado et al. 2016; Afanador-Villamizar et al. 2017). Furthermore, surveillance strategies for avian influenza need to be adapted to target the wildlife–poultry interface, focused on subpopulations at greater risk, such as backyard poultry units or farms with poor biosecurity near wetlands with the presence of aquatic wild birds and/or on the route of migratory birds. For the arboviruses, it is important, as well, to conduct risk characterization and monitoring (through entomological studies, detection of wild bird mortality, detection of viral circulation in wild reservoirs and sentinels, ecological and environmental characterization). These elements provide information to alert agriculture and health authorities in order to timely establish methods to prevent and control equine encephalitis in the region (OIRSA and PAHO 2014).
Research of Diseases at the Wildlife-Livestock Interface
For a region that comprises a high percentage of the wildlife diversity of the Earth, it actually produces substantially less scientific knowledge on wildlife diseases than others, and therefore only a little—occasionally nothing—is known of endemic wild animal species (Wiethoelter et al. 2015). The lack of systematic research on baseline wildlife diseases in this Region hampers the comparisons with other regions of the world like North America, Europe, or Africa. As presented here, there are some descriptive data of diseases of importance in the livestock-wildlife interface, with some public health implications.
The greatest need comes from the lack of systematic studies that allow responding to basic occurrence, prevalence studies, identification of hosts and determination of their role, disease dynamics and spread, short-term and long-term studies, address of entire host communities, disease monitoring in natural populations, need for new and validated diagnostic techniques.
Likewise, knowledge on socioeconomic aspects contributes to inform the decision-making process about control strategies. Some data are already available for rabies in herbivores; indeed it has become apparent that, since the initiation of bat control methods and vaccines for cattle in the Region, the number of reported cattle deaths due to rabies declined from 500,000 in 1968 (Arellano-Sota and Arellano-Sota 1988) to 9904 in 1983 and 1580 in 2006 (Lee et al. 2012). However, there is still a need to add more knowledge about the socioeconomic factors of rabies aerial cycle occurrence, maintenance, and evolution; in order to establish more effective measures for its control in the endemic regions (Kotait et al. 2009). For other important livestock diseases, such as brucellosis and bovine tuberculosis, it is still essential to elucidate the epidemiological role of wildlife to define efficient control strategies or even to move into their eradication.
Apart from those diseases mentioned here, there are still other livestock diseases to be added to the “wish list” for more structured research on the interface livestock-wildlife: e.g., bluetongue, classical swine fever, Tick-borne diseases (such as bovine anaplasmosis and babesiosis), Aujeszky’s disease, Q fever, Pasteurella spp., Newcastle disease, or disease such as canine distemper virus, feline leukemia virus, feline parvovirus, toxoplasmosis. Besides, more studies will be needed to confirm these implications for species with a high probability of population decline and extinction by infectious diseases at the interface as a cause such as Darwin’s fox (Pseudolapex fulvipes), huemul (Hippocamelus bisulcus), maned wolf (Chrysocyon brachyurus), chacoan peccary (Catagonus wagneri), pudu (Pudu puda), and marsh deer (Blastocerus dichotomus).
Other regional outstanding topics comprise the possible role of wild exotic species introduced in the dynamics of pathogens (e.g., red deer, wild boar, fallow deer), and the impact of wild species released from rescue centers (or those seizures of wildlife trafficked illegally or in private hands) in the spreading of pathogens or in the environmental contamination with antibiotics. Finally, another line of applied research should be focused on evaluating the detection of zoonotic infectious agents in bushmeat.
Conclusion and Perspectives
Unfortunately, there is a relevant knowledge gap on the importance of infectious diseases at the wildlife-livestock interface for Central/South American countries, where on balance there are more questions than answers. This increasing recognition of the importance of wildlife in the dynamic of emerging infectious diseases is becoming a big challenge for the countries in the Central and South American regions. There is a lack of adequate scientific information that responds to the regional scenario (Wiethoelter et al. 2015; Olival et al. 2017), which urges the need for more epidemiological studies in wildlife (e.g., reservoirs, occurrence, risk factors) to clarify its role in the transmission dynamics of diseases of economic importance. Probably, many pathogens and hosts have not been listed here because there is not data/research about them. Without this information, a further understanding and management of the system under a One Health perspective is not possible.
A hard topic addressed in this chapter is the impact of deforestation on the wildlife-livestock interface, since it creates favorable conditions for some vectors and allows the displacement and adaptation of others to new environments (Molyneux 2003; FAO 2012; Gottwalt 2013). This land transformation problem and its consequences need to be monitored, and structural national and Regional solutions, based on regulatory policies, should be implemented. In addition, over the last few years, there has been a global awareness of the need to discuss and define strategies to minimize the impacts of social, environmental, and economic changes on the health of the population. Nevertheless, this scenario is still limited by the uncertainties and scarcity of sustainable projects, as well as specific tools to support decision-makers in defining effective interventions to reduce health risks and control vector-borne diseases.
Undoubtedly, bat-transmitted rabies will remain for a while on the table as a top health problem at the wildlife-livestock interface. Certainly, the eradication of the disease is not an achievable target due to its epidemiology; however, it urges consensus among the different stakeholders on the control strategies to be implemented. Another relevant disease at the interface to be monitored in the near future is avian influenza. HPAI is present worldwide and has been reported several times recently in North America, so the Region needs to remain vigilant as the disease could be spread southward via continental bird migrations. Likewise, it is important to monitor the occurrence and distribution of LPAI, in both poultry and wildlife populations, as the virus can be transboundary spread through wild birds and has the capacity to evolve to highly pathogenic forms (Suarez et al. 2004; Spackman et al. 2006; Hurtado et al. 2016).
It is highly recommended to implement comprehensive programs involving integrated surveillance and other strategies for enhancing surveillance, that could contribute to detect the presence of different pathogens either of the livestock population or among wildlife. The revision of the surveillance strategies needs to be accompanied by the strengthening of the laboratory diagnosis capacities, particularly on arboviruses. Likewise, biosecurity policies in farming need to be revised and strengthened. Both, official services and the private sector must take a more proactive role to keep away, insofar as possible, some of the diseases mentioned in this chapter (e.g., avian influenza, bovine TB, and brucellosis).
References
Abdala AA, Garbaccio S, Zumárraga M, Tarabla HD (2015) Mycobacterium bovis en fauna silvestre de la cuenca lechera de Santa Fe, Argentina. Rev Argent Microbiol 47:174–182. https://doi.org/10.1016/j.ram.2015.04.005
Acha PN, Szyfres B (2003) Zoonoses and communicable diseases common to man and animals: volume II: Chlamydioses, rickettsioses, and viruses. PAHO Sci Tech Publ 2:408. https://doi.org/10.1016/0167-5877(89)90014-7
Afanador-Villamizar A, Gomez-Romero C, Diaz A, Ruiz-Saenz J (2017) Avian influenza in Latin America: a systematic review of serological and molecular studies from 2000-2015. PLoS One 1–21. https://doi.org/10.1371/journal.pone.0179573
Aguilar PV, Greene IP, Coffey LL, Medina G, Moncayo AC, Anishchenko M, Ludwig GV, Turell MJ, O’Guinn ML, Lee J, Tesh RB, Watts DM, Russell KL, Hice C, Yanoviak S, Morrison AC, Klein TA, Dohm DJ, Guzman H, Travassos Da Rosa APA, Guevara C, Kochel T, Olson J, Cabezas C, Weaver SC (2004) Endemic Venezuelan equine encephalitis in northern Peru. Emerg Infect Dis 10:880–888. https://doi.org/10.3201/eid1005.030634
Albas A, Campos AC d A, Araujo DB et al (2011) Molecular characterization of rabies virus isolated from non-haematophagous bats in Brazil. Rev Soc Bras Med Trop 44:678–683. https://doi.org/10.1590/S0037-86822011000600006
Alder M, Clark K, Jarvis S et al (2005) Comment One medicine? Vet Rec 157:669. https://doi.org/10.1136/vr.157.22.669
Alkemade R, Reid RS, van den Berg M et al (2013) Assessing the impacts of livestock production on biodiversity in rangeland ecosystems. Proc Natl Acad Sci 110:20900–20905. https://doi.org/10.1073/pnas.1011013108
Alvar J, Vélez ID, Bern C et al (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7. https://doi.org/10.1371/journal.pone.0035671
Alves L, Albertti G (2014) Detecção De Micobactérias Em Animais Silvestres Em Sub-Regiões Do Pantanal Sul-Mato-Grossense Sub-Regiões Do Pantanal Sul-Mato-Grossense
Anderson DM, Estell RE, Gonzalez AL et al (2015) Criollo cattle: heritage genetics for arid landscapes. Rangelands 37:62–67. https://doi.org/10.1016/j.rala.2015.01.006
Antunes J, Machado GP, Costa LF, Fornazari F, Cipriano JRB, Appolinario CM, Allendorf SD, Bagagli E, Teixeira CR, Megid J (2010) Comparison of infection by Brucella spp. in free-ranging and captive wild animals from Sao Paulo state, Brazil. J Venom Anim Toxins Incl Trop Dis 16:654–658
Arellano-Sota C, Arellano-Sota C (1988) Vampire bat-transmitted rabies in cattle. Rev Infect Dis 10:S707–S709. https://doi.org/10.1093/clinids/10.Supplement_4.S707
Atlantic Council (2014) Urbanization in Latin America
Belotto A, Leanes LF, Schneider MC et al (2005) Overview of rabies in the Americas. Virus Res 111:5–12. https://doi.org/10.1016/j.virusres.2005.03.006
Beltrán-Saavedra L, Nallar-Gutiérrez R, Ayala G (2011) Estudio sanitario de vicuñas en silvestría del Área Natural de Manejo Integrado Nacional Apolobamba, Bolivia. Ecol en Boliv 46:14–27
Bengis RG, Kock RA, Fischer J (2002) Infectious animal diseases: the wildlife/livestock interface. Rev Sci Tech 21:53–65. https://doi.org/10.20506/rst.21.1.1322
Bosch I, Herrera F, Navarro J-C, Lentino M, Dupuis A, Maffei J, Jones M, Fernández E, Pérez N, Pérez-Emán J, Guimarães AE, Barrera R, Valero N, Ruiz J, Velásquez G, Martinez J, Comach G, Komar N, Spielman A, Kramer L (2007) West Nile virus, Venezuela. Emerg Infect Dis 13:651–653. https://doi.org/10.3201/eid1304.061383
Bravo-Vasquez N, Di Pillo F, Lazo A et al (2016) Presence of influenza viruses in backyard poultry and swine in El Yali wetland, Chile. Prev Vet Med 134:211–215. https://doi.org/10.1016/j.prevetmed.2016.10.004
Butler RA (2016) The top most biodiverse countries. Mongabay
Calisher CH, Childs JE, Field HE et al (2006) Bats: important reservoir hosts of emerging viruses. Clin Microbiol Rev 19:531–545
Campos KF, De Oliveira CHS, Reis AB, Yamasaki EM, de Farias Brito M, Andrade SJT, Duarte MD, Barbosa JD (2013) Surto de encefalomielite equina Leste na Ilha de Marajó, Pará. Pesqui Vet Bras 33:443–448. https://doi.org/10.1590/S0100-736X2013000400005
Cardenas R, Sandoval C, Rodriguez-Morales A (2006) P530 impact of climate variability in the occurrence of leishmaniasis in southern departments of Colombia. Int J Antimicrob Agents 29:S117–S118. https://doi.org/10.1016/S0924-8579(07)70373-4
Cardenas R, Sandoval CM, Rodriguez-Morales AJ, Vivas P (2008) Zoonoses and climate variability: the example of leishmaniasis in southern departments of Colombia. Ann N Y Acad Sci 1149:326–330. https://doi.org/10.1196/annals.1428.094
Carrera J, Forrester N, Wang E (2013) Eastern equine encephalitis in Latin America. N Engl J Med 369:732–744. https://doi.org/10.1056/NEJMoa1212628.Eastern
Centro Panamericano de Fiebre Aftosa – OPS/OMS (2017) Informe de Situación de los Programas de Erradicación de la Fiebre Aftosa. Sudamerica y Panamá en 2016
Chancey C, Grinev A, Volkova E, Rios M (2015) The global ecology and epidemiology of west Nile virus. Biomed Res Int. https://doi.org/10.1155/2015/376230
Chaves LF, Pascual M (2006) Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med 3:1320–1328. https://doi.org/10.1371/journal.pmed.0030295
Clavijo A, Sanchez-Vazquez MJ, Buzanovsky LP et al (2015) Current status and future prospects to achieve foot-and- mouth disease eradication in South America. Transbound Emerg Dis 64:31. https://doi.org/10.1111/tbed.12345
Clavijo A, Sanchez-Vazquez MJ, Buzanovsky LP et al (2017) Current status and future prospects to achieve foot-and-mouth disease eradication in South America. Transbound Emerg Dis 64:31–36. https://doi.org/10.1111/tbed.12345
Cohen MJ, Thompson B (2012) The impact of climate change and bioenergy on nutrition: pathways. Risks and Strategies for Adaptation and Mitigation
Cunha EMS, Villalobos EMC, Nassar AFC, Lara MCCSH, Peres NF, Palazzo JPC, Silva A, De Stefano E, Pino FA (2009) Prevalência de anticorpos contra agentes virais em equídeos no sul do estado de são paulo. Arq Inst Biol (Sao Paulo) 76:165–171
Cunningham AA, Daszak P, Wood JLN (2017) One health, emerging infectious diseases and wildlife: two decades of progress?. Philos Trans R Soc Lond B Biol Sci 372(1725):20160167
da Costa LJC, Fernandes MEB (2016) Rabies: knowledge and practices regarding rabies in rural communities of the Brazilian Amazon Basin. PLoS Negl Trop Dis 10:1–15. https://doi.org/10.1371/journal.pntd.0004474
Datem RED, Marañon DEL, Del LDEM (2016) No title. 1–16
Davies CR, Reithinger R, Campbell-Lendrum D et al (2000) The epidemiology and control of leishmaniasis in Andean countries. Cad Saude Publica 16:925–950. https://doi.org/10.1590/S0102-311X2000000400013
De Araujo J, De Azevedo SM, Gaidet N et al (2014) Avian influenza virus (H11N9) in migratory shorebirds wintering in the Amazon region, Brazil. PLoS One 9:1–10. https://doi.org/10.1371/journal.pone.0110141
de Azevedo SS, Silva MLCR, de Batista CS, de Gomes AAB, Vasconcellos SA, Alves CJ (2010) Detection of anti Brucella abortus, anti Brucella canis and anti Leptospira spp. antibodies in hoary foxes (Pseudalopex vetulus) from semi-arid of Paraiba state, Northeastern region of Brazil. Ciência Rural 40:190–192
de Ferranti D, Perry GE, Lederman D et al (2005) Beyond the City: the rural contribution to development in Latin America and the Caribbean
de Novaes Oliveira R, Iamamoto K, Silva MLCR, Achkar SM, Castilho JG, Ono ED, Lobo RSV, Brandão PE, Carnieli P, Carrieri ML, Kotait I, Macedo CI (2014) Eastern equine encephalitis cases among horses in Brazil between 2005 and 2009. Arch Virol 159:2615–2620. https://doi.org/10.1007/s00705-014-2121-4
De Sousa KCM, Calchi AC, Herrera HM et al (2017) Anaplasmataceae agents among wild mammals and ectoparasites in Brazil. Epidemiol Infect 145:3424–3437. https://doi.org/10.1017/S095026881700245X
Deem SL, Noss AJ, Cuéllar RL et al (2002) Sarcoptic mange in free-ranging pampas foxes in the Gran Chaco, Bolivia. J Wildl Dis 38:625–628. https://doi.org/10.7589/0090-3558-38.3.625
Deem SL, Noss AJ, Villarroel R et al (2004) Disease survey of free-ranging Grey brocket deer (Mazama gouazoubira) in the Gran Chaco, Bolivia. J Wildl Dis 40:92–98. https://doi.org/10.7589/0090-3558-40.1.92
Del Rio Vilas VJ, Freire de Carvalho MJ, Vigilato MAN et al (2017) Tribulations of the last mile: sides from a regional program. Front Vet Sci 4:2–6. https://doi.org/10.3389/fvets.2017.00004
Desjeux P (2004) Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27:305–318. https://doi.org/10.1016/j.cimid.2004.03.004
Diaz LA, Komar N, Visintin A, Juri MJD, Stein M, Allende RL, Spinsanti L, Konigheim B, Aguilar J, Laurito M, Almirón W, Contigiani M (2008) West Nile virus in birds, Argentina. Emerg Infect Dis 14:689–691. https://doi.org/10.3201/eid1404.071257
Dorneles EMS, Pellegrin AO, Péres IAHFS, Mathias LA, Mourão G, Bianchi R de C, Olifiers N, Rocha FL, Lage AP (2014) Serology for brucellosis in free-ranging crab-eating foxes (Cerdocyon thous) and brown-nosed coatis (Nasua nasua) from Brazilian Pantanal. Ciência Rural 44:2193–2196. https://doi.org/10.1590/0103-8478cr20131167
dos Reis NR, de Lima IP, Peracchi AL (2002) Morcegos (Chiroptera) da Área urbana de Londrina, Paraná, Brasil. Rev Bras Zool 19:739–746
Dupuis AP, Marra PP, Kramer LD (2003) Serologic evidence of West Nile virus transmission, Jamaica, West Indies. Emerg Infect Dis 9:860–863. https://doi.org/10.3201/eid0907.030249
Elisei C, Pellegrin A, Tomas WM, Soares CO, Araújo FR, Funes-Huacca ME, Rosinha GMS (2010) Evidência molecular de Brucella sp. em Ozotoceros bezoarticus (veado campeiro) do Pantanal Sul-Mato-Grossense. Pesqui Vet Bras 30:503–509. https://doi.org/10.1590/S0100-736X2010000600006
FAO (2012) State of the World’s forests
FAO (2017a) The state of food and agriculture. Leveraging Food Systems for Inclusive Rural Transformation
FAO (2017b) Livestock production in Latin America and the Caribbean. Reg Off Lat Am Caribb. http://www.fao.org/americas/perspectivas/produccion-pecuaria/en/. Accessed 16 June 2017
Filoni C, Pena HFDJ, Gennari SM et al (2009) Heartworm (Dirofilaria immitis) disease in a Brazilian oncilla (Leopardus tigrinus). Pesqui Veterinária Bras 29:474–478. https://doi.org/10.1590/S0100-736X2009000600006
Fuchs L, Baldone V, Fort M, Rojas C, Samartino L, Giménez H (2009) Brucelosis en el zorro gris pampeano R esumen. TEST 43:227–231
Garland AJM, de Clercq K (2011) Cattle, sheep and pigs vaccinated against foot and mouth disease: does trade in these animals and their products present a risk of transmitting the disease? OIE Rev Sci Tech 30:189–206
Garnier T, Eiglmeier K, Camus J-C et al (2003) The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci USA 100:7877–7882. https://doi.org/10.1073/pnas.1130426100
Ghersi BM, Blazes DL, Icochea E et al (2009) Avian influenza in wild birds, central coast of Peru. Emerg Infect Dis 15:935–938. https://doi.org/10.3201/eid1506.080981
Gibbs EPJ (2005) Animal and human health emerging zoonotic epidemics in the interconnected global community of infectious disease increased numbers. Vet Rec 157:673–679
Gomes I, Rosenberg FJ (1984) A possible role of capybaras (Hydrochoerus hydrochoeris hydrochoeris) in foot-and-mouth disease (FMD) endemicity. Prev Vet Med 3:197–205. https://doi.org/10.1016/0167-5877(84)90008-4
Gomes MN, Uieda W (2004) Abrigos diurnos, composição de colônias, dimorfismo sexual e reprodução do morcego hematófago Desmodus rotundus (E. Geoffroy) (Chiroptera, Phyllostomidae) no Estado de São Paulo, Brasil. Rev Bras Zool 21:629–638. https://doi.org/10.1590/S0101-81752004000300025
Gottwalt A (2013) Impacts of deforestation on vector-borne disease incidence. J Glob Health 3:16–19
Gramiccia M, Gradoni L (2005) The current status of zoonotic leishmaniases and approaches to disease control. Int J Parasitol 35:1169–1180. https://doi.org/10.1016/j.ijpara.2005.07.001
Grogan LF, Peel AJ, Kerlin D et al (2018) Is disease a major causal factor in declines? An evidence framework and case study on koala chlamydiosis. Biol Conserv 221:334–344. https://doi.org/10.1016/j.biocon.2018.03.030
Heinemann MB, Souza MDCC, Cortez A, Ferreira F, Homem VSF, Ferreira-Neto JS, Soares RM, Cunha EMS, Richtzenhain LJ (2006) Soroprevalência da encefalomielite eqüina do leste e do oeste no Município de Uruará, PA, Brasil. Brazilian J Vet Res Anim Sci 43:137. https://doi.org/10.11606/issn.1678-4456.bjvras.2006.26546
Hidalgo-Martínez A, Puerto FI, Farfán-Ale JA et al (2008) Prevalencia de infección por el virus del Nilo occidental en dos zoológicos del estado de Tabasco. Salud Publica Mex 50:76–85. https://doi.org/10.1590/S0036-36342008000100014
Hurtado R, Eric R, Vanstreels T, Paulo S (2016) Avian influenza in wild birds from South America: review, implications and perspectives. Explor Res Hypothesis Med 1:62–74. https://doi.org/10.14218/ERHM.2016.00014
ICA-INS-MAVDT (2004) Sistema de informacion y vigilancia epidemiologica de las encefalitis equinas en Colombia. Rev ICA 1–56
Jiménez-Bluhm P, Karlsson EA, Ciuoderis KA et al (2016) Avian H11 influenza virus isolated from domestic poultry in a Colombian live animal market. Emerg Microbes Infect 5:1–9. https://doi.org/10.1038/emi.2016.121
Jones KE, Patel NG, Levy MA et al (2008) Global trends in emerging infectious diseases. Nature 451:990
Karesh WB, Uhart MM, Dierenfeld ES et al (1998) Health evaluation of free-ranging guanaco (Lama guanicoe). J Zoo Wildl Med 29:134–141
Karlsson EA, Ciuoderis K, Freiden PJ et al (2013) Prevalence and characterization of influenza viruses in diverse species in Los llanos, Colombia. Emerg Microbes Infect 2:e20. https://doi.org/10.1038/emi.2013.20
Kin MS, Fort M, de Echaide ST, Casanave EB (2014) Brucella suis in armadillos (Chaetophractus villosus) from La Pampa, Argentina. Vet Microbiol 170:442–445. https://doi.org/10.1016/j.vetmic.2014.01.039
Komar N, Clark GG (2006) West Nile virus activity in Latin America and the Caribbean. Rev Panam Salud Pública 19:112–117. https://doi.org/10.1590/S1020-49892006000200006
Kotait I (1998) Manejo de quirópteros em áreas urbanas. Oecologia 164:45
Kotait I, Carrieri M, Takaoka NY (2009) Raiva – Aspectos gerais e clínica. Man Técnico do Inst Pasteur 8:48
Kouadio IK, Aljunid S, Kamigaki T et al (2012) Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti-Infect Ther 10:95–104. https://doi.org/10.1586/ERI.11.155
Kunz TH, Fenton MB (2003) Bat ecology. The University of Chicago Press, Chicago
LaDEAU LS, Kilpatrick AM, Marra PP (2007) West Nile virus emergence and large-scale declines of North American bird populations. Nature 447:710–713
Lambin EF, Geist HJ, Lepers E (2003) Dynamics of land-use and land-cover change in tropical regions. Annu Rev Environ Resour 28:205–241. https://doi.org/10.1146/annurev.energy.28.050302.105459
Lanciotti RS, Roehrig JT, Deubel V et al (1999) Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. https://doi.org/10.1126/science.286.5448.2333
Lee DN, Papeş M, van Den Bussche RA (2012) Present and potential future distribution of common vampire bats in the Americas and the associated risk to cattle. PLoS One 7:1–9. https://doi.org/10.1371/journal.pone.0042466
Lombardi R, Geymonat G, Berrini R (2015) El jabalí en el Uruguay: problema, desafío y oportunidad. Florestal Atlántico Sur
Lord VR, Lord RD (1991) Brucella suis infections in collared peccaries in Venezuela. J Wildl Dis 27:477–481. https://doi.org/10.7589/0090-3558-27.3.477
Lord VR, Ricardo FC (1983) Brucella spp. from the capybara (hydrochaeris hydrochaeris) in Venezuela: serologic studies and metabolic characterization of isolates. J Wildl Dis 19:308–314. https://doi.org/10.7589/0090-3558-19.4.308
Lucero NE, Ayala SM, Escobar GJ, Jacob NR (2008) Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol Infect 136:496–503. https://doi.org/10.1017/S0950268807008795
Ludwig G V, Calle PA, Mangiafico J et al (2002) An outbreak of West Nile virus in New York City captive wildlife population
Maciel ALG, Loiko MR, Bueno TS et al (2017) Tuberculosis in southern Brazilian wild boars (Sus scrofa): first epidemiological findings. Transbound Emerg Dis 65:518–526. https://doi.org/10.1111/tbed.12734
Maia-Elkhoury AN, Carmo EH, Sousa-Gomes ML, et al (2008) Visceral leishmaniasis in Brazil: trends and challenges. Cad SaúdePública Dez 24(12):2941–2947
Maia-Elkhoury A, Yadon Z, Idali Saboya Diaz M et al (2016) Exploring spatial and temporal distribution of cutaneous leishmaniasis in the Americas, 2001–2011. PLoS Negl Trop Dis 10:2001–2011. https://doi.org/10.1371/journal.pntd.0005086
Mallewa M, Fooks AR, Banda D et al (2007) Rabies encephalitis in malaria-endemic area, Malawi, Africa. Emerg Infect Dis 13:136–139. https://doi.org/10.3201/eid1301.060810
MAPA (2009) Controle da Raiva dos Herbìvoros
MAPA (2015) Programa Nacional de Controle da Raiva dos Herbívoros. Ações de controle da raiva dos herbívoros no Brasil. Departamento de Saúde Animal-DSA/SDA/MAPA.
Marcoppido G, Parreño V, Vilá B (2010) Antibodies to pathogenic livestock viruses in a wild Vicuña (Vicugna vicugna) population in the Argentinean Andean Altiplano. J Wildl Dis 46:608–614. https://doi.org/10.7589/0090-3558-46.2.608
Martínez AM, Gama LT, Cañón J et al (2012) Genetic footprints of Iberian cattle in America 500 years after the arrival of Columbus. PLoS One 7:1–13. https://doi.org/10.1371/journal.pone.0049066
Martino PE, Montenegro JL, Preziosi JA, Venturini C, Bacigalupe D, Stanchi NO, Bautista EL (2004) Serological survey of selected pathogens of free-ranging foxes in southern Argentina, 1998–2001. Rev Sci Tech 23:801–806. https://doi.org/10.20506/rst.23.3.1521
Mathieu C, Moreno V, Pedersen J et al (2015) Avian influenza in wild birds from Chile, 2007-2009. Virus Res 199:42–45. https://doi.org/10.1016/j.virusres.2015.01.008
Mayen F (2003) Haematophagous bats in Brazil, their role in rabies transmission, impact on public health, livestock industry and alternatives to an indiscriminate reduction of bat population. J Vet Med Ser B Infect Dis Vet Public Heal 50:469–472. https://doi.org/10.1046/j.1439-0450.2003.00713.x
Medlin S, Deardorff ER, Hanley CS, Vergneau-grosset C, Siudak-campfield A, Dallwig R, Travassos A, Tesh RB, Martin MP, Weaver SC, Vaughan C, Ramirez O, Kurt K, Rica C, Biológicas EDC, Nacional U, Rica C (2016) HHS Public Access 52:883–892. https://doi.org/10.7589/2015-02-040.SEROSURVEY
Melo RM, Cavalcanti RC, Villalobos EMC, Cunha EMS, Lara MCCSH, Aguiar DM (2012) Ocorrência De Equídeos Soropositivos Para Os Vírus Das. Arq Inst Biol 79:169–175
Mesa FA, Cárdenas JA, Villamil LC (2005) Las encefalitis equinas en la salud pública
Miranda FR, Superina M, Vinci F, Hashimoto V, Freitas JC, Matushima ER (2015) Serosurvey of Leptospira interrogans, Brucella abortus and Chlamydophila abortus infection in free-ranging giant anteaters (Myrmecophaga tridactyla) from Brazil. Pesqui Vet Bras 35:462–465. https://doi.org/10.1590/S0100-736X2015000500013
Molyneux DH (2003) Royal Society of Tropical Medicine and Hygiene Meeting at Manson House, London, 20 June 2002 Climate change and tropical disease. Common themes in changing vector-borne disease scenarios. Trans R Soc Trop Med Hyg 97:129–132. https://doi.org/10.1016/S0035-9203(03)90097-6
Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, Gutierrez G, Pigretti S, Menchaca H, Garrido N, Taylor N, Fernandez F, Levis S, Enría D (2006) West Nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis 12:1559–1561. https://doi.org/10.3201/eid1210.060852
Morales N, Aldridge D, Bahamonde A et al (2017) Corynebacterium pseudotuberculosis infection in Patagonian Huemul (Hippocamelus bisulcus). J Wildl Dis 53:621–624. https://doi.org/10.7589/2016-09-213
Moreno E (2002) Brucellosis in Central America. Vet Microbiol 90:31–38. https://doi.org/10.1016/S0378-1135(02)00242-0
Muñoz PM, Boadella M, Arnal M et al (2010) Spatial distribution and risk factors of Brucellosis in Iberian wild ungulates. BMC Infect Dis 10. https://doi.org/10.1186/1471-2334-10-46
Murakami PS, Monego F, Ho JL, Gibson A, de Castro Vilani RGD, Soresini GCG, Brockelt SR, Biesdorf SM, Fuverki RBN, Nakatani SM, Riediger IN, Grazziotin AL, do Santos AP, de Barros Filho IR, Biondo AW (2012) An outbreak of tuberculosis by Mycobacterium bovis in coatis (nasua nasua). J Zoo Wildl Med 43:338–341. https://doi.org/10.1638/2010-0043.1
Nachon Cicciarella HN, Bosisio CR (2005) Enfermedades Infecciosas de los Equinos
Naranjo J, Cosivi O (2013) Elimination of foot-and-mouth disease in South America: lessons and challenges. Philos Trans R Soc Lond Ser B Biol Sci 368:20120381. https://doi.org/10.1098/rstb.2012.0381
OECD/FAO (2017) OECD-FAO Agricultural Outlook 2017–2026
OIE (2017) Infection with avian influenza viruses. In: Terrestrial animal health code, pp 1–18
OIE (2018) Diseases, infections and infestations listed by the OIE. Terr Anim Heal Code 8–11
OIE WAHIS (2012) Detailed country disease incidence. [WWW document]. OIE World Animal Health Information System. http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/statusdetail. Accessed 15 Aug 2018
OIRSA, PAHO (2014) Guía para la Vigilancia, Detección y Respuesta para las Encefalitis Equinas
Olival KJ, Hosseini PR, Zambrana-Torrelio C et al (2017) Host and viral traits predict zoonotic spillover from mammals. Nature 546:646–650. https://doi.org/10.1038/nature22975
Oliveira-Filho EF, Júnior JWP, Souza MMA, Santana VLA, Silva JCR, Mota RA, Sá FB (2012) Serologic survey of brucellosis in captive neotropical wild carnivores in Northeast Brazil. J Zoo Wildl Med 43:384–387. https://doi.org/10.1638/2009-0260.1
PAHO (2011) Encefalitis Equinas en Casos de Desastres
PAHO (2016) Leishmaniases: epidemiological report in the Americas
Palmer MV (2013) Mycobacterium bovis: characteristics of wildlife reservoir hosts. Transbound Emerg Dis 60:1–13. https://doi.org/10.1111/tbed.12115
PANAFTOSA (2015) Informe de Situación de los Programas de Erradicación de la Fiebre Aftosa. Sudamerica y Panamá en 2014
Pereda AJ, Uhart M, Perez AA et al (2008) Avian influenza virus isolated in wild waterfowl in Argentina: evidence of a potentially unique phylogenetic lineage in South America. Virology 378:363–370. https://doi.org/10.1016/j.virol.2008.06.010
Perry BD, Randolph TF, McDermott JJ et al (2002) Investing in animal health research to alleviate poverty, Nairobi: I
Pisano MB, Oria G, Beskow G, Aguilar J, Konigheim B, Cacace ML, Aguirre L, Stein M, Contigiani MS (2013) Venezuelan equine encephalitis viruses (VEEV) in Argentina: serological evidence of human infection. PLoS Negl Trop Dis 7:1–7. https://doi.org/10.1371/journal.pntd.0002551
Preece ND, Abell SE, Grogan L et al (2017) A guide for ecologists: detecting the role of disease in faunal declines and managing population recovery. Biol Conserv 214:136–146. https://doi.org/10.1016/j.biocon.2017.08.014
Pultorak E, Nadler Y, Travis D et al (2011) Zoological institution participation in a West Nile virus surveillance system: implications for public health. Public Health 125:592–599. https://doi.org/10.1016/j.puhe.2011.03.013
Rangel EF, Vilela ML (2008) Lutzomyia longipalpis (Diptera, Psychodidae, Phlebotominae) and urbanization of visceral leishmaniasis in Brazil. Cad Saude Publica 24:2948–2952. https://doi.org/10.1590/S0102-311X2008001200025
REDIPRA (2003) Informe final de la IX REDIPRA
Reis NRD, Peracchi AL, Pedro WA, De Lima IP (2007) Morcegos do Brasil
Romano-lieber NS, Goldbaum M (2000) Revista de Saúde Pública. J Public Health 34:395–6, 393–4. https://doi.org/10.1590/S0034-89102000000300005
Roque ALR, Jansen AM (2014) Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl 3:251–262. https://doi.org/10.1016/j.ijppaw.2014.08.004
Rosenberg FJ, Gomes I (1977) Susceptibilidad del carpincho o capibara (Hydrochoerus hydrochoeris hydrochoeris) al virus de la fiebre aftosa. Bol Centr Panam Fiebre Aft 27–28:35–34
Salomón OD, Sosa-Estani S, Ramos K et al (2006) Tegumentary leishmaniasis outbreak in Bella Vista City, Corrientes, Argentina during 2003. Mem Inst Oswaldo Cruz 101:767–774. https://doi.org/10.1590/S0074-02762006000700010
Salomón OD, Quintana MG, Zaidenberg M (2008) Urban distribution of Phlebotominae in a cutaneous leishmaniasis focus, Argentina. Mem Inst Oswaldo Cruz 103:282–287. https://doi.org/10.1590/S0074-02762008005000016
Salomón OD, Feliciangeli MD, Quintana MG et al (2015) Lutzomyia longipalpis urbanisation and control. Mem Inst Oswaldo Cruz 110:831–846. https://doi.org/10.1590/0074-02760150207
Santos RL, Martins TM, Borges ÁM, Paixão TA (2013) Economic losses due to bovine brucellosis in Brazil. Pesqui Vet Bras 33:759–764. https://doi.org/10.1590/S0100-736X2013000600012
Schneider MC, Romijn PC, Uieda W et al (2009) Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev Panam Salud Pública 25:260–269. https://doi.org/10.1590/S1020-49892009000300010
Silva JR (2010) Pesquisa de infecções por Flavivírus da encefalite de Saint Louis, Rocio e Oeste do Nilo em cavalos, por inquérito sorológico e isolamento viral Pesquisa de infecções por Flavivírus da encefalite de Saint Louis, Rocio e Oeste do Nilo em cavalos, por i 133
Silva MLCR, Galiza GJN, Dantas AFM, Oliveira RN, Iamamoto K, Achkar SM, Riet-Correa F (2011) Outbreaks of eastern equine encephalitis in northeastern Brazil. J Vet Diagnostic Investig 23:570–575. https://doi.org/10.1177/1040638711403414
Sinnott E, Nash J, Torre A De (2010) Natural resources in Latin America and the Caribbean – beyond booms and busts? The World Bank
SIRVERA (2018) Regional information system for epidemiological surveillance of rabies. In: PANAFTOSA/OPS. http://sirvera.panaftosa.org.br. Accessed 15 Aug 2018
Smith DW, Mackenzie JS, Weaver SC (2009) Alphaviruses. In: Richman DD, Whitley RJ, Hayden FG (eds) Clinical virology. ASM, Washington, pp 1241–1274
Spackman E, McCracken KG, Winker K, Swayne DE (2006) H7N3 avian influenza virus found in a south American wild duck is related to the Chilean 2002 poultry outbreak, contains genes from equine and north American wild bird lineages, and is adapted to domestic turkeys. J Virol 80:7760–7764. https://doi.org/10.1128/JVI.00445-06
Streicker DG, Recuenco S, Valderrama W et al (2012) Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc R Soc B Biol Sci 279:3384–3392. https://doi.org/10.1098/rspb.2012.0538
Suarez DL, Senne DA, Banks J et al (2004) Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg Infect Dis 10:693–699. https://doi.org/10.3201/eid1004.030396
Sutmoller P, Barteling SS, Olascoaga RC, Sumption KJ (2003) Control and eradication of foot-and-mouth disease. Virus Res 91:101–144. Pii S0168-1702(02)00262-9
Swanepoel R (1994) Rabies. In: Coetzer JAW, Thomson FR, Tustin RC (eds) Infections diseases of livestock, with special reference to southern Africa, vol 1. Oxford University Press, Oxford, pp 392–400
Szyfres B, González Tomé J (1966) Natural Brucella infection in argentine wild foxes. Bull World Health Organ 34:919–923
Thomson GR, Vosloo W, Bastos ADS (2003) Foot and mouth disease in wildlife. Virus Res 91:145–161. https://doi.org/10.1016/S0168-1702(02)00263-0
Tollefson J (2014) El Niño tests forecasters. Nature 508:20–21. https://doi.org/10.1038/508020a
Tsounta E, Osueke AI (2014) What is behind Latin America’ s declining income inequality? IMF Work Pap 14:4–25. https://doi.org/10.1111/rode.12260
Turner DC (1975) The vampire bat: a field study in behavior and ecology. Johns Hopkins University Press, Baltimore
Uhart MM, Vila AR, Beade MS et al (2003) Health evaluation of pampas deer (Ozotoceros bezoarticus celer) at Campos del Tuyú wildlife reserve, Argentina. J Wildl Dis 39:887–893. https://doi.org/10.7589/0090-3558-39.4.887
UN-HABITAT (2012) State of Latin American and Caribbean cities 2012. Towards a new urban transition
USDA (2018) Livestock and poultry: World Markets and Trade 25
Valderrama-Ardila C, Alexander N, Ferro C et al (2010) Environmental risk factors for the incidence of American cutaneous leishmaniasis in a sub-Andean zone of Colombia (Chaparral, Tolima). Am J Trop Med Hyg 82:243–250. https://doi.org/10.4269/ajtmh.2010.09-0218
Valero N, Larreal Y, Arias J, Espina LM, Maldonado MB, Melean E, Estévez J, Pérez M, Ávila J, Luzardo M, Morell P, Urdaneta N (2004) Seroprevalence of the Venezuelan equine encephalitis of Equidae population of Zulia state, Venezuela, 1999–2001 XIV, 324–330
Van Campen H, Rhyan J (2010) The role of wildlife in diseases of cattle. Vet Clin North Am – Food Anim Pract 26:147–161. https://doi.org/10.1016/j.cvfa.2009.10.008
Vieira AS, Pinto PS, Lilenbaum W (2017) A systematic review of leptospirosis on wild animals in Latin America. Trop Anim Health Prod 50:229–238. https://doi.org/10.1007/s11250-017-1429-y
Vilcarromero S, Aguilar PV, Halsey ES, Laguna-Torres VA, Razuri H, Perez J, Valderrama Y, Gotuzzo E, Suárez L, Céspedes M, Kochel TJ (2010) Venezuelan equine encephalitis and 2 human deaths, Peru. Emerg Infect Dis 16:553–556. https://doi.org/10.3201/eid1603.090970
WAHIS-OIE (2018) WAHIS. In: World Animal Health Information Database Interface
Ward MP (2005) Epidemic West Nile virus encephalomyelitis: a temperature-dependent, spatial model of disease dynamics. Prev Vet Med 71:253–264. https://doi.org/10.1016/j.prevetmed.2005.07.008
Watson JT, Gayer M, Connolly MA (2007) Epidemics_after_Natural_Disasters_Yeeyan.Pdf. 13:1–5. https://doi.org/10.3201/eid1301.060779
Weaver GV, Domenech J, Thiermann AR, Karesh WB (2013) Foot and mouth disease: a look from the wild side. J Wildl Dis 49:759–785. https://doi.org/10.7589/2012-11-276
Wernery U, Kaaden OR (2004) Foot-and-mouth disease in camelids: a review. Vet J 168:134–142. https://doi.org/10.1016/j.tvjl.2003.10.005
WHO (2010) Control of the leishmaniases. WHO Tech Rep Ser 5:22–26. https://doi.org/10.1038/nrmicro1766
WHO (2013) WHO Expert Consultation on rabies. Second report. WHO Tech Rep Ser 982:1–139. 92-4-120931-3
Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM (2015) Global trends in infectious diseases at the wildlife–livestock interface. Proc Natl Acad Sci 112:9662–9667. https://doi.org/10.1073/pnas.1422741112
Wohlgenant TJ (1994) Roost interactions between the common vampire bat (Desmodus rotundus) and two frugivorous bats (Phyllostomus discolor and Sturnira lilium) in Guanacaste. Costa Rica Biotropica 26:344–348
World Bank (2014) GINI index (World Bank estimate)
Zinsstag J, Schelling E, Waltner-Toews D et al (2011) From “one medicine” to “one health” and systemic approaches to health and well-being. Prev Vet Med 101:148–156. https://doi.org/10.1016/j.prevetmed.2010.07.003
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sanchez-Vazquez, M.J. et al. (2021). Characteristics and Perspectives of Disease at the Wildlife-Livestock Interface in Central and South America. In: Vicente, J., Vercauteren, K.C., Gortázar, C. (eds) Diseases at the Wildlife - Livestock Interface. Wildlife Research Monographs, vol 3. Springer, Cham. https://doi.org/10.1007/978-3-030-65365-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-65365-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-65364-4
Online ISBN: 978-3-030-65365-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)