Abstract
Colorectal cancer is one of the most commonly diagnosed incurable multifactorial malignancies in the world. To date, there are no promising noninvasive therapeutic tools that have achieved CRC prognosis, survival, and recurrence in clinical settings. We are now very familiar with the most famed term “metabolic reprogramming” that cancer cells preferably employ to meet their rapid bioenergetic and ATP synthesis requirements. Glutamine is the most abundant amino acid in human blood plasma and is known for its significant pleotropic role in the metabolic network.
Here, we exposed the metabolic distortion associated with the metabolism of glutamine in the CRC. Classically, findings have shown that dysregulated glutamine metabolism is significantly associated with CRC growth, survival, metastases, and recurrence. As a result, blocking signaling pathways, enzymes, and transporters associated with glutamine metabolism could be a gold standard strategy to hijack the development of CRC. We hope that this strategy will help to systematically target, manage, and cure CRC.
Yashwant Kumar Ratre and Henu Kumar Verma contributed equally to this work as first authors.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Colorectal cancer (CRC) is ranked as the third most common form of malignancy and the fourth leading cause of cancer-related death with approximately 1.8 million new cases and 881,000 deaths in 2018 (Bray et al. 2018). Drug resistance and recurrence have a significant challenge in the treatment of cancer, including CRC (Russi et al. 2019; Zare-Bandamiri et al. 2017; Verma et al. 2020a). However, it was expected that the global burden of CRC will increase by almost 60% by 2030. Colorectal malignancy has a significant effect on the quality of life and is a major economic burden on the society and CRC patients (Sun and Zhu 2018; Ferlay et al. 2018).
It is widely accepted that CRC is a multifactorial disease developed by the accumulation of multiple abnormal events, including lifestyle, environmental factors, mutations in anti-proliferative genes (tumor suppressor), and expression of oncogenes (Nguyen and Duong 2018). Studies have shown that CRC is also associated with metabolic changes resulting in a significant number of metabolites (Kong et al. 2018). Recently, few prognostic factors are considered to be a promising survival resource for CRC patients, namely, TNM staging, histological parameter, tumor location, and carcinoembryonic antigen (CEA) rates (Arnold et al. 2017; Zhang et al. 2015).
At present, due to limited treatment choices and inadequate diagnosis, the median overall survival of CRC metastases decreased by 60% with a 5-year survival rate, and this may be more important in the advance stage of the disease due to the late detection and resistance of patients to certain combination therapy (Park et al. 2014). Consequently, due to lack of therapeutic choices and inadequate management, there is an immediate need to create a gold standard compound and therapeutic to cope with colorectal cancer.
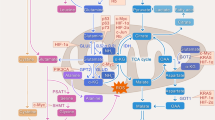
Metabolic pathways are fundamental sources of ATP and serve as a recycling plant for the generation/regeneration of macromolecules required for the life of tumor cells. Most cancer biologists have shown that cancer cells have a license to interrupt normal metabolic pathways by metabolic rewiring to replenish their nutritional requirements that are essential to their rapid growth and spread. Metabolic rewiring is caused by the alteration of the expression of various metabolic enzymes, tumor suppressor genes, and oncogenes that redefine the transport and flow of metabolites through the metabolic network to help cancer cells. Warburg effect is the most desirable and recognized hallmark of cancer accompanied by glutamine (GLU) addiction (Yang et al. 2017; Pavlova and Thompson 2016).
Glucose is the primary metabolic food for cancer cells compared to amino acids, usually glutamine, which also acts as a fuel source and contributes to tumor development and proliferation (Scopelliti et al. 2018). Over the last few decades, studies have shown that the reported requirement of metabolic precursors, carbon skeleton, and nitrogen makes cancer cells more dependent on glutamine. Avid intake of glutamine and cancer cell metabolism have also become areas of focus in the exploration of new therapeutic targets in cancer patients in recent years (Luengo et al. 2017). Studies have mainly focused on the diagnostic value of glutamine in the CRC, and minimal studies have examined the prognosis and predictive effects of glutamine (Sirniö et al. 2019; Choi and Park 2018). In the last few years, the glutamine metabolism has gained much attention due to its more consumption by cancer cells in an unusual way, distinct from normal cells. The goal of this chapter is to highlight the role of glutamine metabolism as a potential therapeutic tool for targeting CRC to reduce future burdens in the global context.
15.2 Metabolism of Cancer and Normal Cells
Cancer cell metabolism plays a key role in tumorigenesis and tumor cell proliferation, development, and metastases. In current scenario, cancer metabolism has become a major area of science research, which may provide some information to find new and effective therapeutic approaches to prevent cancer growth (Vernieri et al. 2016). In certain respects, the main metabolic pathways in cancer cells vary in comparison to normal cells, such as their unique capacity to regulate genetics and cellular dynamics, a process known as metabolic rewiring .
Cell metabolism is a biochemical transformation that occurs in all living organisms. Cells are proficient recyclers that break down the complex macromolecules into smaller building blocks leading to the generation of energy units in the form of metabolic currency (ATP) through a process known as catabolism . The anabolic pathway is an energy-consuming process that builds new macromolecules (proteins, lipids, and DNA) to form the cells’ biomass. Historically, the only pathways of biosynthesis, such as nucleotides and deoxynucleotides, are intensively studied and targeted fruitfully in cancer therapy (Wilson et al. 2014; Villa et al. 2019).
All living cells depend on metabolic pathways to obtain adequate fuel and metabolic ingredients for the maintenance, proliferation, and critical cell homeostasis. Under normal non-proliferative conditions , cells activate metabolic machines to accommodate ATP intake for reproduction and survival through two oxygen-dependent processes, namely, aerobic respiration and oxidative phosphorylation , where 36 ATP molecules are produced while in anaerobic respiration (Pasteur effect) only 2 ATPs are produced under hypoxic or stressful conditions. Both of these pathways required glucose uptake in order to gain the “energy currency” of the cell.
Classically, healthy or quiescent cells primarily follow aerobic respiration/oxidative phosphorylation to meet their bioenergetic requirements, while cancer cells use metabolic pathways quite differently from normal cells based on their rapid demand for energy production. To satisfy growth and high proliferation rates, cancer cells prudently adopt various metabolic phenotypes to increase the uptake of nutrients from extracellular environments through the deregulation of metabolic fitness, which is now an emerging hallmark of cancer. The physiological and biochemical aspects of tumor tissues and normal tissues are quite different in many ways.
The majority of these are due to the difference between cancer cell vasculatures and normal cells. Poorly formed tumor vasculature defines a hypoxic microenvironment that results in low levels of nutrients and high levels of waste products (Lugano et al. 2020). Cancer cells respond to such abnormal conditions and adjust their cell metabolism to proliferate and survive even in the worst condition. Aberrant cancer metabolism is one of the silent features of colorectal cancer, which involves the modulation of bioenergetic pathways to meet the rapid demand for bio-molecules to maintain their high rate of growth and energy consumption (DeBerardinis and Chandel 2016).
Colorectal cancer cells are widely known to upregulate in various metabolic pathways, including glycolysis, glutaminolysis, fatty acid biosynthesis, and single-carbon metabolism with oncogenic mutations and loss of tumor suppressor genes (Brown et al. 2018). Overall, cancer cells are switched to increase the rate of aerobic glycolysis despite the presence of an adequate concentration of oxygen and use glucose as a well-known source of energy to accelerate their uncontrolled growth and division. In addition, high pyruvate production by glycolysis is reduced to lactate by fermentation rather than by oxidative phosphorylation.
Primary and metastatic CRCs have been reported to have often shown increased glucose intake and increased intratumoral lactate, as confirmed by tumor imaging with 18F-deoxyglucose positron emission tomography (18FDG-PET) compared to adjacent normal intestinal tissue (Satoh et al. 2017; Maffione et al. 2015). Defective aerobic glycolysis is not effective in producing enough ATP (2 ATP/glucose vs ~36 ATP/glucose by OXPHOS) and it therefore accelerates its nutrient flow by rewiring glycolysis and associated pathways to generate ATP quickly. It can also provide a biosynthetic advantage by supplying precursors and reducing agents for macromolecule synthesis (Burns and Manda 2017).
However, glucose has certain limitations: since it can only supply carbon skeleton for macromolecule biosynthesis, it cannot transport other fuels such as amino acids and glutathione needed by cancer cells during rapid proliferation for nucleotide synthesis and maintenance of oxidative stress. Studies have shown that, after glucose, glutamine is also the most widely used nutrient by cultured cancer cells compared to other non-essential amino acids (Hosios et al. 2016), although abnormal fatty acid metabolism, nucleotides, folate, acetate, and proteins have also been reported (Pavlova and Thompson 2016). Therefore, cancer cells are easily “addicted” to the nutrients, and their withdrawal may eventually result in cell death by apoptosis. Glutamine is a prominent substrate not only for the production of macromolecule biosynthesis (lipids, proteins, and nucleotides) and nicotinamide adenine dinucleotide phosphate (NADPH), but also it serves as an important anaplerotic substrate for TCA or Krebs in proliferating cells (Nguyen and Durán 2018). The difference in metabolism between cancer and normal cells is expected to provide opportunities for the development of innovative cancer treatments. Modulated expression of enzymes and transporters involved in various metabolic pathways and nutrient transports in cancer are given in Fig. 15.1.
15.2.1 Glutamine Metabolism
Over the last few decades, oncometabolomics has emerged as a renowned field of interest in cancer biology and has played an important role in targeting various cancers, including colon cancer, and continues to impact our knowledge and understanding of oncology. The addition of new discoveries on cancer metabolism continues to surprise science and opens up additional avenues of research on cancer control. Since the Warburg observation of cancer metabolism, the main focus has been on the fundamental metabolism of carbon, including glycolysis, citric acid cycle, and pentose phosphate pathway.
Current studies have shed light on the role of amino acids in the metabolism of cancer. Amino acids are the main substrate for protein synthesis in cells and tissues. They are vital to the construction and integrity of every cell, without which cells are unable to maintain their morphology and to perform their normal metabolic functions (Vazquez et al. 2016). It can be obtained exogenously and endogenously and has a very significant presence for cell survival, maintenance, and proliferation.
Amino acids also contribute to a wide range of cellular processes, such as energetic regulation, redox homeostasis, nucleotide synthesis, lipids, glucosamines, antioxidants, and polyamines. Hence, this diverse dimension of metabolic activities has made amino acid metabolism increasingly common in cancer research, particularly glutamine metabolism.
Glutamine is the most abundant free amino acid in blood plasma and skeletal muscle (Mayers and Vander Heiden 2015). Glutamine is conventionally a non-essential amino acid, but it has recently been known as a “conditionally essential” nutrient when cells are highly proliferative or have some physiological condition, such as pathogen infection and starvation (Scalise et al. 2020). Further, it is highly versatile amino prudently transferred to its carbon, amino, and amide nitrogen for the generation of other amino acids, nucleotides, and amino sugars. In addition, multiple functions and signaling pathways are involved (Bott et al. 2019).
In cancer cells, glutamine is an alternative source of energy after the glycolytic pathway. Apart from the quiescent cell, cancer cells showed 10–100 times higher glutamine intakes than any others. Higher use of glutamine and subsequent “glutamine dependence ” are frequently seen in several cancers, including colorectal cancer, as a result of aberrantly expressed oncogenes and loss of tumor suppressor genes (Miyo et al. 2016; Balsa-Martinez and Puigserver 2015).
As described above, most cancer cells excrete carbon as lactate in the extracellular environment due to deregulated aerobic glycolysis. It makes cancer cells more addicted to glutamine, which not only supplies carbon but also acts as a source of nitrogen for nucleic acid de novo biosynthesis, nicotinamide adenine dinucleotide (NAD), hexosamine, and other non-essential amino acids (NEAAs). It also acts as a precursor to the synthesis of antioxidant enzyme glutathione (GSH) for redox balancing under stressful conditions (Altman et al. 2016).
In contrast, glutamine also induces the absorption of essential amino acids , such as leucine through the plasma membrane, from the extracellular environment via the LAT1/SLC7A5 bidirectional transporter in conjunction with the glutamine efflux. This also stimulates mTOR signaling and helps with unnecessary ammonia and glutamate (GLU) recycling (Scalise et al. 2017). Glutamine participates actively in metabolic rewiring for the replenishment of TCA cycle metabolic intermediates (Hensley et al. 2013). In addition to glutamine, other branched-chain amino acids (BCAAs) such as valine, leucine, and isoleucine can also help promote the growth of cancer cells as an opportunistic fuel for the TCA cycle (Green et al. 2016).
Rapidly proliferating cancer cells make extensive use of glutamine through rewiring associated pathways to accumulate biomass and produce ATP. A tracer finding has shown that up to 50% of NEAAs needed for protein synthesis by cancer cells originate from glutamine, exploring the significant role of glutamine during uncontrolled growth and proliferation (Alberghina and Gaglio 2014).
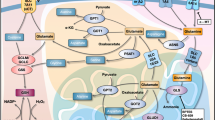
First, glutamine gives its amide nitrogen to glutamine acid (glutamate) catalyzed by cytoplasmic glutamine (GLS1). GLU is the primary nitrogen donor for other NEAA synthesis. GLU is exchanged for glutamate dehydrogenase (GLUD) with α-ketoglutarate which releases toxic ammonium and further synthesizes lipids and amino acids and gains ATP through the TCA cycle followed by OXPHOS (Li and Le 2018). These biochemical reactions also result in the production of reducing agent NADPH, which is simultaneously used to regenerate glutathione (GSH) which acts as an electron acceptor to counteract reactive oxygen species (ROS) generated in cancer cells during higher metabolic flux (Zhou et al. 2014).
Furthermore, transaminases transfer the rest of nitrogen residues to different α-ketoacids which are glucose or glutamine carbon catabolites by different aminotransferases such as glutamate-pyruvate transaminase (GPT), glutamate-oxalate transaminase (GOT), and phosphoserine aminotransferase 1 (PSAT1) which transfer amino nitrogen from GLU to alanine, aspartate, and phosphoserine, respectively. Aminotransferases also produce α-ketoglutarate without the release of ammonium (Lieu et al. 2020).
Alpha-ketoacid , such as glutamate gamma-semialdehyde, is used to synthesize ornithine that is part of the urea cycle. Serine participates in protein synthesis and also releases some extra carbon from glycolysis into the extracellular environment of cancer cells. Aspartate enters the urea cycle and participates in biosynthesis of asparagine. It also serves as a substrate for purine and pyrimidine synthesis (DeBerardinis and Cheng 2010). Phosphoserine acts as a precursor to glycine and cysteine biosynthesis as part of a single-carbon metabolism. Both amino acids are important for the synthesis of glutathione. In addition, glycine is a key source of carbon and nitrogen in the purine ring (Amelio et al. 2014). Ornithine is a precursor to arginine synthesis. Glutamic acid is donating its carbon and nitrogen to the synthesis of proline. It also produces glutamine via glutamine synthetase (GS) catalysis.
15.2.2 Glutamine Transporters and Cancer
Understanding and targeting cancer cell metabolism has become a well-known task for biochemists and researchers around the world. Transition has been found in the metabolism of glutamine in several different cancers, including colon cancer, which has been observed almost uniformly. As discussed above, glutamine is a vital nutrient known for the plethora of biological functions, including cell growth, proliferation, anaplerosis, redox balance, acid-base balance, and detoxification (NH3) in mammalian cells.
In addition, two supplementary metabolic pathways are also glutamine-dependent: the first glutamine/GABA-glutamine pathway between glutamatergic/GABAergic neurons in the brain and the second glutamine pathway in cancer cells, where glutamine enters the TCA cycle and is subsequently converted to malate and reduced to pyruvate by malic enzyme and then lactate through the action of lactate dehydrogenase. As a result, various glutamine transporters are hired to maintain the concentration of glutamine in blood plasma and cells to perform all of the abovementioned functions, including the maintenance of rapid proliferation and cell survival in cancer cells. The blocking of these transporters may also work as a therapeutic weapon in many cancers, including CRC (Cruzat et al. 2018; La Vecchia and Sebastián 2020).
Glutamine is a hydrophilic molecule that cannot simply be diffused into cells across the plasma membrane; therefore, our metabolic system obtains various transporters for transmembrane transfer of glutamine inside the cells. Until recently, 14 glutamine transporters in the plasma membrane have been identified in mammalian cells that recognize and transport glutamine as a substrate (Table 15.1). For humans, glutamine transporters have a 44–55% sequencing identity, and neutral amino acid transporters have a 57% identity. According to the Human Genome Organisation nomenclature, these 14 transporters are distributed among four major gene families, namely, SLC1, SLC6, SLC7, and SLC38. Under normal conditions, glutamine reaches the cells from the extracellular space via the four carrier groups (SLC) of transporters that import glutamine and other amino acids (Scalise et al. 2018). Remember that not all transporters are limited to glutamine and not even just the inflow of glutamine into the cells.
Most of them accept only neutral amino acids, while others accept both neutral and cationic amino acids. Likewise, under normal conditions, most of these transporters are influx extracellular glutamine into the cells and vice versa. Of note, there are a variety of glutamine transporters that are overexpressed in cancer cells to provide food. Nonetheless, some of the inhibitors against these transporters are currently under review or in a clinical trial. ASCT2 is a Na + upregulated and downregulated transporter of Myc and retinoblastoma protein (Rb), respectively, and belongs to the SLC1 carrier family of genes and facilitates the transport of neutral amino acids, such as glutamine (Reynolds et al. 2014; Gao et al. 2009).
ASCT2 has long been considered a primary high-affinity importer of glutamine to the plasma membrane. SLC1A5 is the only carrier in this family of genes that recognizes glutamine as a substrate (Scalise et al. 2017). In particular, there has been an increased focus on alanine-serine-cysteine transporter 2 (ASCT2) encoded by the SLC1A5 gene, also recognized as an excitatory amino acid transporter (EAAT1-5) due to their elevated expression in various cancers including CRC (Toda et al. 2017; Liu et al. 2018).
The study showed that expression of miRNA-137 altered expression of ASCT2. In addition, it was confirmed that overexpression of ASCT2 was significantly associated with decreased expression of miRNA-137 in CRC (Dong et al. 2017). Recently, a Japanese research group reported that the mutation in the KRAS gene has been significantly associated with ASCT2 expression. They also noticed that the overexpression of the ASCT2 transporter was significantly correlated with tumor depth and vascular invasiveness in KRAS-mutant CRC (Toda et al. 2017).
SLC6A14 belongs to the family of SLC6 genes known as Na+/Cl−-coupled neurotransmitter transporters and recognizes all proteinogenic amino acids except glutamate and aspartate. It is also known as ATB0,+ (amino acid transporter with neutral amino acids as a substrate). SLC6A14 is somewhat distinct from SLC1A5, SLC7A5, and SLC7A11 because it is tissue-specific and expressed in the lungs, trachea, salivary gland, pituitary, intestinal tract, and colon. It carrier can transport not only EAAs but also most non-essential amino acids such as glutamine, arginine, serine, and glycine, which are important for the supply of metabolic fuel necessary for tumor development.
SLC7A5 (LAT1) is an Na+-independent two-way transporter of glutamine, also invlove in transporting BCAAs and neutral amino acids, including EAAs (isoleucine, histidine, methionine, leucine, phenylalanine, tryptophan, tyrosine, valine) (Kandasamy et al. 2018). SLC7A5 is expressed in the colon, fetal liver, intestine, ovary, testis, placenta, spleen, blood-brain barrier, activated lymphocytes, skeletal muscle, heart, lung, thymus, and kidney (Wang and Holst 2015; Pochini et al. 2014). SLC7A5 is an amino acid exchanger that regulates the parallel extracellular efflux of glutamine and the intracellular flow of leucine.
This exchange is regulated by HIF-2α and Myc, which results in the activation of mTORC1 signaling and regulates cell proliferation, differentiation, and growth (Nakazawa et al. 2016). Due to its prominent function in the control of various metabolic enzymes, this transporter has been seen in several cancers such as breast, esophageal, non-small cell lung carcinoma, prostate, biliary, oral, gastric, pancreatic, and colon cancer. Hayase et al. conducted a clinical study and found that the upregulation of the LAT1 transporter was significantly associated with increased cell proliferation in colorectal cancer (Hayase et al. 2017).
Both SLC7A6 and SLC7A7 are neutral/cationic transporters of the SLC7 gene family encoding y + LAT2 and y + LAT1 proteins, of which SLC7A6 is expressed predominantly in the small intestine, parotides, heart, kidney, lung, testis, and brain. There are unique transport functions such as neutral amino acid inflows and Na+-dependent efflux. Y + LAT2 was discovered as an arginine/glutamine exchanger in the brain.
Increased expression of SLC7A7 is associated with a high prognosis of several cancers such as malignant glioma and multiple myeloma (Fan et al. 2013) and drug resistance to ovarian cancer and gastric cancer (Cheng et al. 2010; Verma 2020b). Similarly, Lu et al. reported that overexpression of SLC7A6 and SLC7A7 was also essential to the proliferation and development of human colorectal cancer tissue relative to normal colon tissue (Lu et al. 2013). SLC7A8 gene encodes for neutral LAT2 transporter (system L amino acid transporter) where system L is preferred for leucine.
This is identical to SLC7A5 in the selectivity and unique function of the substrate and plays a key role in the discharge of amino acids ingested in the blood from the intestinal and kidney epithelial cells. There is no information as to whether SLC7A8 plays any important role in cancer. Nevertheless, some emerging evidence indicate that many cancers are correlated with high expression of LAT2, but none of them confirm and correlate significantly (Barollo et al. 2016). SLC7A9 is also a Na+-independent neutral/cationic transporter that is strongly expressed in the stomach, kidney, and placenta. Cationic amino acid is believed to flow through the cells in conjunction with neutral amino acid efflux from the cells. Recently, it has been shown that SLC7A9 is correlated with the proliferation and survival of certain cancers such as breast cancer and papillary thyroid cancer (Jiang et al. 2017; Shen et al. 2018).
Among the 14 glutamine transporters, 6 belong to the SLC38 genes family called SNATs (sodium-coupled neutral amino acid transporters) : SNAT1, SNAT2, SNAT3, SNAT5, SNAT7, and SNAT8. Proteins in this gene family transport sodium-coupled neutral, cationic, and anionic amino acids and are considered to be the main transporter of glutamine in human cells. Such transporters are found in intestinal and renal epithelial cells, membranes of the blood vessels, and astrocytes.
Of the six SLC38 gene family, three (SNAT1, SNAT2, and SNAT8) belong to system A-type transporter (Na+-dependent transportation) common to neutral amino acids like alanine, while SNAT3, SNAT5, and SNAT7 prefer system N transporter (Na+-dependent transportation) selective for glutamine, asparagine, and histidine; all of these amino acids carry nitrogen atoms in the side chain. SLC38 transporters are commonly recognized for their various biological functions, such as cell proliferation, differentiation, and survival, as they are essential transporters of glutamine (Hägglund et al. 2011).
As a result, the expressions of these transporters are deregulated in many cancers; some studies revealed that SNAT2 could play a role in promoting tumor growth. Some evidence indicates that SNAT2 is a transcriptional target for the TP53 tumor suppressor gene (Grewal et al. 2009). SLC38A1 and SLC38A2 are essential sources of glutamine for glutaminolysis (Bröer et al. 2016). Similarly, elevated levels of SNAT1 in breast and hepatocellular carcinoma were observed (Wang et al. 2013).
Silencing of SLC38A1 also reduced the growth of colon and pancreatic cancer cell lines (Zhou et al. 2017; Xie et al. 2014). It is important to note that SLC38A5 is a transcriptional target for oncogene c-Myc. This transporter is likely to be upregulated in some cancer cells. DeBerardinis et al. reported an increase in the expression of SLC38A5 transporter in Myc-driven cancer cells (DeBerardinis et al. 2007). In addition, SLC38A3 also plays a vital role in the activation of oncogene due to high reactive oxygen generation in the tumor inflammatory environment (Rubio-Aliaga and Wagner 2016).
15.2.3 Different Enzymes Associated with Glutamine Metabolism in CRC
Glutaminolysis is one of the highly regulated metabolic pathways seen in many cancers. This pathway is widely known for the cancer metabolism due to the conditionally essential amino acid glutamine that the cancer cells are strongly addicted. Various metabolic enzymes are involved in the metabolism of glutamine, which plays many biochemical and physiological roles in normal metabolic signaling.
Glutamine is catabolized by various enzymes, including GLS, carbamoyl-phosphate synthetase 2-aspartate transcarbamylase-dihydroorotase (CAD), or glutamine fructose-6-phosphate amidotransferase (GFAT). Traditionally, glutamine is transported to the cell by two membrane transporters, namely, SLC1A5 and SLC7A5/LAT1, and hydrolyzed to glutamate by GLS/GLS1. In addition, GDH/GLUD1 and aminotransferases convert glutamate to α-KG. Alpha-KG enters the TCA cycle to facilitate the generation of energy in the form of ATP through the production of NADH and FADH2. Glutamine also provides nitrogen and carbon backbone for macromolecules for rapidly proliferating cancer cells.
Mammalian cells encode two genes for glutaminase enzymes: the GLS1 gene is located in chromosome 2 and encodes the kidney-type glutaminase protein (KGA/GLS1) initially characterized in the kidney (Katt et al. 2017; Matés et al. 2013), while the GLS2 gene is found in chromosome 12 and encodes the liver-type protein isoform (LGA/GLS2) originally characterized in the liver. Glutaminase is critically involved in cancer cell proliferation, autophagy, signal transduction, and radioresistance (Sever and Brugge 2015; Xiang et al. 2019).
Glutaminase expression has long been found to be significantly associated with the altered expression of the c-Myc transcription factor and the mTORC1 signaling. The transcription factor c-Myc is a key regulator of the consumption of glutamine and is frequently observed in many proliferating cells, including CRC (La Vecchia and Sebastián 2020). c-Myc also induces the expression of SLC1A5 by promoting the expression of GLS1 and CAD to promote the transport and uptake of glutamine (Bott et al. 2015; Dong et al. 2020).
In addition, retinoblastoma (Rb), a tumor suppressor protein, also negatively regulates the intake of glutamine via the SLC1A5 and GLS1-dependent E2F transcription factor (Csibi et al. 2013). Correspondingly, it has also been reported that the mTOR pathway regulates the expression of GLS by facilitating the translation of c-Myc protein. Similarly, signaling pathways to Rho GTPase can also result in overexpression of NF-kB-dependent GLS1 in cancer cells (Wilson et al. 2013). Huang et al. conducted a case-control study between colorectal tumor and normal tissue and found an increased level of GLS in CRC patients compared to control (Huang et al. 2014).
Li et al. recently reported that the activation of heat shock factor 1 (HSF1) increases the expression of GLS to promote glutamine metabolism and mTOR signaling in colorectal cancer (Li et al. 2018). Unlike GLS/GLS1, the role of GLS2 in cancer metabolism is still controversial and not yet fully understood. Some findings have shown that GLS2 controls the ROS level by targeting the p53 gene by regulating the production of antioxidants (GSH) (Hu et al. 2010; Suzuki et al. 2010). Upregulated expression of GLS2 functions as a tumor suppressor and inhibits the growth and spread of cancer cells. GLS or GDH also plays a pivotal role in the glutamine metabolism by reducing glutamate to α-ketoglutarate through the action of a number of enzymes, including GDH1, GOT2, and GPT2, particularly when the first line of nutrient glucose is insufficient or under hypoxia. In addition, α-ketoglutarate enters the TCA cycle for the production of ATP synthesis and anabolic carbon for other NEAAs, lipids, and nucleotides. Csibi et al. reported that mTORC1 significantly promotes glutaminolysis by activating GDH to promote cell growth, proliferation, and metastases by inhibiting SIRT4 (Csibi et al. 2013).
Similarly, Lorin and coworkers have shown that mTORC1 activation promotes GDH-dependent autophagy by limiting the formation of ROS in cancer cells (Lorin et al. 2013). Some authors have reported overexpression of GDH enzyme in certain types of cancer such as triple-negative breast cancer, lung cancer, glioblastoma, and colorectal cancer (Jin et al. 2016). Wang and his team have recently found that upregulation of SIRT4 directly increases the expression of GLUD1, thus enhancing glutaminolysis in a deglutarylation-dependent manner. In addition, overexpression of SIRT4 has been reported to be significantly associated with poor prognosis of colorectal cancer (Wang et al. 2018).
AST is also known as glutamine oxaloacetate transaminase (GOT1 and GOT2) which is critical for redox homeostasis and growth in some cancer cells that are proliferating. Phosphoserine aminotransferase 1 (PSAT1) catalyzes the transfer of glutamate nitrogen to 3-phosphohydroxypyruvate to make phosphoserine and α-ketoglutarate as part of serine biosynthesis. High expression of PSAT1 has recently been reported to be significantly associated with poor prognosis of colorectal carcinoma (De Marchi et al. 2017).
Similarly, some authors have shown that ALT and AST are particularly associated with the prognosis of certain cancers, such as hepatocellular cancer, renal cell carcinoma, colonic cancer, pancreatic cancer, and breast cancer (Shen et al. 2014; Bezan et al. 2015; Son et al. 2013).
15.3 Targeting Glutamine Metabolism in CRC
Several accumulated data have shown the diverse and significant role of glutamine metabolism in cancer. Since the discovery of “glutamine dependence” in cancer cells, the field has continued to explore and attract many cancer biologists. It is important to develop an effective and gold standard therapeutic agent to target glutamine transporters and enzymes associated with catabolic/anabolic biosynthetic pathways. Until now, few promising compounds target the metabolism of glutamine in certain types of cancer, including colorectal cancer.
Some drugs have successfully entered the human clinical trial and/or are approved for use in cancer treatment by the FDA (Table 15.2). The discovery of versatile anti-glutamine compounds is advantageous in cancer therapy. Some small molecule analog glutamine inhibitors, such as acivicin, azaserine, and L-DON, have been tested for their anti-cancer effect in vitro/in vivo, followed by preclinical and clinical studies against multiple types of cancer, including colorectal cancer.
Huang et al. conducted a proliferative study in the colon cancer cell line (HT29, SW480) and found that L-DON significantly reduces growth and proliferation of colon cancer, leading to DNA fragmentation and apoptosis (Huang et al. 2014). In addition to their anti-proliferative role, blocking the metabolism of glutamine in cancer growth has shown significant toxicity which results in adverse effects on the gastrointestinal tract, immune cells, and central nervous system due to non-selective inhibition of the metabolism of glutamine, thus limiting the toxicity of these compounds (Lukey et al. 2013).
GLS inhibition has become much more attractive since the last few years due to the dysregulated expression of GLS in a variety of cancers. In recent years, various GLS inhibitors (BC-839, 968, BPTES) target the metabolism of glutamine by demonstrating tumor suppression in various preclinical tumor models. Of these, CB-839 is considered to be the most advanced and phase I clinical trial compound for many cancers, including colorectal cancer alone and adjuvant therapy (Gross et al. 2014).
Song et al. found that BPTES significantly decreased the growth of colon cancer by inhibiting GLS (Song et al. 2017). Similarly, compound 968 is a GLS1 allosteric regulator that inhibits the growth of Rho GTPase-induced cancer transformation in many cancers (Xie et al. 2016; Verma et al. 2019). Oxaliplatin is an antineoplastic small molecule that is the most commonly used drug for the treatment of colorectal cancer. Some drugs, such as metformin and ribavirin, have the potential to accelerate the synergetic effect of oxaliplatin when combined with compound 968 in colorectal cancer therapy (Richard and Martinez 2015).
Moreover, some studies have revealed the anti-cancer role of EGCG and R162 in colorectal cancer. Furthermore, EGCG and R162 have been shown to significantly inhibit the expression of GDH resulting in reduced tumor aggressiveness and poor prognosis of colorectal cancer (Larsen and Dashwood 2010). Glutamate dehydrogenase is recognized as a novel CRC prognosis marker (Liu et al. 2015), but the clinical significance and importance of GDH expression in colorectal carcinoma has not yet been studied. L-Asparaginase is an antineoplastic enzyme that inhibits asparagine biosynthesis in particular and acts as a depleting agent for plasma asparagine and glutamine in KRAS-mutated colorectal cancer (Altman et al. 2016).
L-Asparaginase is currently the first approved drug choice for the treatment of acute lymphoblastic anemia (ALL) patients (Egler et al. 2016). Benzylserine and GPNA also bind to the SLC1A5 transporter of glutamine and suppress cancer aggressiveness (Hassanein et al. 2015). Ma et al. reported that GPNA-induced inhibition of SLC1A5 sensitizes patients with colorectal cancer resistance to cetuximab (Erbitux) resulting in better therapeutic outcomes in CRC (Ma et al. 2018). In addition, recently published data have shown the role of the novel potent inhibitor V-9302, which inhibits SLC1A5 transporter and colorectal cancer both in vitro and in mice (Schulte et al. 2018).
Hara et al. reported that monoclonal antibody Ab3-8 functions as an antagonist to SLC1A5 and significantly inhibits the growth of KRAS-mutated colon cancer cell line HT29 followed by inhibition of glutamine uptake (Hara et al. 2020). Similarly, anti-human SLC7A5 monoclonal antibody Ab1 is also associated with poor growth and survival of colon cancer (Ueda et al. 2019). Compound JHP20 dramatically blocks the transport of amino acids by SLC7A5 transporter, leading to inhibition of mTOR signaling. It has been shown that JPH203 potentially induced inhibition of leucine uptake by targeting SLC7A5 transporters, which ultimately results in reduced proliferation and cell death in colorectal cancer (Oda et al. 2010).
To date, JPH203 is currently the only SLC7A5 inhibitor to be evaluated in clinical trials for colorectal cancer. A transaminase inhibitor AOA is also associated with poor prognosis and inhibition of colorectal cancer cell growth in vitro and in vivo (Hao et al. 2016). Sulfasalazine (SSZ) is an anti-inflammatory compound that preferably binds to the SLC7A11 cystine transporter and inhibits the growth of colon cancer followed by apoptosis and cell death (Arensman et al. 2019). However, due to its off-target effects in clinical settings, this compound has been limited.
15.4 Future Prospective
Colorectal cancer is one of the most common diseases with high morbidity and mortality affecting millions of lives worldwide. Till now, surgery and chemotherapy are the only treatment options for metastatic CRC that contribute to higher overall survival. However, due to organ-specific toxicity and poor specificity, chemotherapy as a medical procedure is hampered. Over the past few decades, glutamine has been the most regularly tested and studied nutrient in the area of cancer metabolism. Cancer cells have the ability to adopt the metabolic process required for rapid growth and proliferation in order to accommodate the use of nutrients. Glutamine metabolism is a central metabolic pathway that plays a key role in cancer growth and survival regulation by modulating the bioenergetic signal and redox balance by acting as a precursor to biomass accumulation and ATP synthesis.
The key factors that control metabolic reprogramming and make cancer cells more addicted to glutamine are alteration in intrinsic tumor suppressors and oncogenes along with tumor microenvironment. Therefore, some key therapeutic solution may be inserted to target specific cancer metabolism pathways, and a new option may be developed to improve the therapeutic efficiency and specificity. In addition, targeting glutamine metabolism can provide a new roadmap and promising strategy for the pharmacological design of gold standard compounds for the treatment of advanced colorectal cancer and/or chemo/drug resistance. Moreover, anti-glutamine compounds and chemosensitizing drugs as a direct or adjuvant therapy may also work toward improving the quality of life in patients with CRC.
Abbreviations
- 968:
-
5-[3-Bromo-4-(dimethylamino)phenyl]-2,3,5,6-tetrahydro-2,2-dimethyl-benzo[a]phenanthridin-4(1H)-one
- ABCA1:
-
ATP-binding cassette transporter A1
- ACSL1:
-
Adipose acyl-CoA synthetase-1
- AGPAT1:
-
1-Acylglycerol-3-phosphate o-acyltransferase 1
- ALL:
-
Acute lymphoblastic leukemia
- ALT:
-
Alanine aminotransferase
- AOA:
-
Aminooxyacetate
- AST:
-
Aspartate aminotransferase
- ATP:
-
Adenosine triphosphate
- BCH:
-
2-Aminobicyclo-(2,2,1)-heptane-2-carboxylic acid
- BPTES:
-
Bis-2-(5-phenylacetamido-1,3,4-thiadiazol2-yl)ethyl sulfide
- CAD:
-
Carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, dihydroorotase
- CB-839:
-
Telaglenastat
- COX2:
-
Cyclooxygenase-2
- CRC:
-
Colorectal cancer
- DNA:
-
Deoxyribonucleic acid
- E2F:
-
E2 promoter binding factor
- EAA:
-
Essential amino acid
- EAAT:
-
Excitatory amino acid transporter
- EGCG:
-
Epigallocatechin gallate
- FADH2:
-
Flavin adenine dinucleotide
- FASN:
-
Fatty acid synthase
- FBP1:
-
Fructose-1,6-bisphosphatase
- FDA:
-
US Food and Drug Administration
- GABA:
-
Gamma aminobutyric acid
- GFAT:
-
Glutamine fructose-6-phosphate amidotransferase
- GLN:
-
Glutamine
- GLS:
-
Glutaminase
- GLU:
-
Glutamate
- GLUD:
-
Glutamate dehydrogenase
- GLUT1:
-
Glucose transporter 1
- GOT2:
-
Glutamate-oxalate transaminase
- GPNA:
-
L-γ-Glutamyl-p-nitroanilide
- GPT2:
-
Glutamate-pyruvate transaminase
- GSH:
-
Glutathione
- HIF-2α:
-
Hypoxia-inducible factor-2
- HK:
-
Hexokinase
- HND:
-
Hartnup disorder
- HSF1:
-
Heat shock factor 1
- JPH203:
-
(S)-2-amino-3-(4-((5-amino-2-phenylbenzo[d]oxazol-7-yl)methoxy)-3,5-dichlorophenyl
- KRAS:
-
Kirsten rat sarcoma viral oncogene
- LDH:
-
Lactate dehydrogenase
- L-DON:
-
6-Diazo-5-oxo-L-norleucine
- MCT4:
-
Monocarboxylate transporter 4
- miRNA:
-
MicroRNA
- MPC1:
-
Mitochondrial pyruvate carrier 1
- mTOR:
-
Mammalian target of rapamycin
- NAD:
-
Nicotinamide adenine dinucleotide
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- NEAA:
-
Non-essential amino acid
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NH3:
-
Ammonia
- OXPHOS:
-
Oxidative phosphorylation
- p53:
-
Tumor suppressor 53
- PET:
-
Positron emission tomography
- PHGDH:
-
Phosphoglycerate dehydrogenase
- PKCζ:
-
Protein kinase C zeta
- PKM2:
-
Pyruvate kinase muscle isozyme M2
- PPARG:
-
Peroxisome proliferator-activated receptor gamma
- PSAT1:
-
Phosphoserine aminotransferase 1
- R162:
-
2-Allyl-1-hydroxy-9,10-anthraquinone
- Rb:
-
Retinoblastoma
- ROS:
-
Reactive oxygen species
- SAM:
-
S-Adenosylmethionine
- SCD1:
-
Stearoyl-coenzyme A desaturase 1
- SIRT4:
-
Sirtuin-4
- SLC1:
-
Solute carrier 1
- SLC1A5:
-
Solute carrier family 1 member 5
- SLC38:
-
Solute carrier 38
- SLC38A1:
-
Solute carrier family 38 member 1
- SLC38A2:
-
Solute carrier family 38 member 2
- SLC38A3:
-
Solute carrier family 38 member 3
- SLC38A5:
-
Solute carrier family 38 member 5
- SLC38A7:
-
Solute carrier family 38 member 7
- SLC6:
-
Solute carrier 6
- SLC6A14:
-
Solute carrier family 6 member 14
- SLC6A19:
-
Solute carrier family 6 member 19
- SLC7:
-
Solute carrier 7
- SLC7A5:
-
Solute carrier family 7 member 5
- SLC7A6:
-
Solute carrier family 7 member 6
- SLC7A7:
-
Solute carrier family 7 member 7
- SLC7A8:
-
Solute carrier family 7 member 8
- SLC7A9:
-
Solute carrier family 7 member 9
- SSZ:
-
Sulfasalazine
- TCA:
-
Tricarboxylic acid
- TNM:
-
Tumor node metastasis
- V-9302:
-
2-Amino-4-bis(aryloxybenzyl)aminobutanoic acids
- α-KG:
-
Alpha-ketoglutarate
References
Ahluwalia, G., Grem, J., Hao, Z., & Cooney, D. (1990). Metabolism and action of amino acid analog anti-cancer agents. Pharmacology & Therapeutics, 46(2), 243–271.
Alberghina, L., & Gaglio, D. (2014). Redox control of glutamine utilization in cancer. Cell Death & Disease, 5(12), e1561.
Altman, B. J., Stine, Z. E., & Dang, C. V. (2016). From Krebs to clinic: Glutamine metabolism to cancer therapy. Nature Reviews. Cancer, 16(10), 619–634.
Amelio, I., Cutruzzolá, F., Antonov, A., Agostini, M., & Melino, G. (2014). Serine and glycine metabolism in cancer. Trends in Biochemical Sciences, 39(4), 191–198.
Arensman, M. D., Yang, X. S., Leahy, D. M., et al. (2019). Cystine–glutamate antiporter xCT deficiency suppresses tumor growth while preserving antitumor immunity. Proceedings of the National Academy of Sciences, 116(19), 9533–9542.
Arnold, D., Lueza, B., Douillard, J. Y., et al. (2017). Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Annals of Oncology, 28(8), 1713–1729.
Balsa-Martinez, E., & Puigserver, P. (2015). Cancer cells hijack gluconeogenic enzymes to fuel cell growth. Molecular Cell, 60(4), 509–511.
Barollo, S., Bertazza, L., Watutantrige-Fernando, S., et al. (2016). Overexpression of L-type amino acid transporter 1 (LAT1) and 2 (LAT2): Novel markers of neuroendocrine tumors. PLoS One, 11(5), e0156044.
Bezan, A., Mrsic, E., Krieger, D., et al. (2015). The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. The Journal of Urology., 194(1), 30–35.
Bott, A. J., Peng, I. C., Fan, Y., et al. (2015). Oncogenic Myc induces expression of glutamine synthetase through promoter demethylation. Cell Metabolism, 22(6), 1068–1077.
Bott, A. J., Maimouni, S., & Zong, W.-X. (2019). The pleiotropic effects of glutamine metabolism in cancer. Cancers (Basel)., 11(6), 770.
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., & Jemal, A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424.
Bröer, A., Rahimi, F., & Bröer, S. (2016). Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. The Journal of Biological Chemistry., 291(25), 13194–13205.
Brown, R. E., Short, S. P., & Williams, C. S. (2018). Colorectal cancer and metabolism. Current Colorectal Cancer Reports., 14(6), 226–241.
Burns, J. S., & Manda, G. (2017). Metabolic pathways of the Warburg effect in health and disease: Perspectives of choice, chain or chance. International Journal of Molecular Sciences, 18(12), 2755.
Cheng, L., Lu, W., Kulkarni, B., et al. (2010). Analysis of chemotherapy response programs in ovarian cancers by the next-generation sequencing technologies. Gynecologic Oncology, 117(2), 159–169.
Choi, Y., & Park, K. (2018). Targeting glutamine metabolism for cancer treatment. Biomolecules & Therapeutics, 26(1), 19–28.
Cormerais, Y., Giuliano, S., LeFloch, R., Front, B., Durivault, J., Tambutté, E., et al. (2016). Genetic disruption of the multifunctional CD98/LAT1 complex demonstrates the key role of essential amino acid transport in the control of mTORC1 and tumor growth. Cancer Research, 76(15), 4481–4492.
Cruzat, V., Macedo Rogero, M., Noel Keane, K., Curi, R., & Newsholme, P. (2018). Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients, 10(11), 1564.
Csibi, A., Fendt, S.-M., Li, C., et al. (2013). The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell, 153(4), 840–854.
De Marchi, T., Timmermans, M. A., Sieuwerts, A. M., et al. (2017). Phosphoserine aminotransferase 1 is associated to poor outcome on tamoxifen therapy in recurrent breast cancer. Scientific Reports, 7(1), 2099–2099.
DeBerardinis, R. J., & Chandel, N. S. (2016). Fundamentals of cancer metabolism. Science Advances, 2(5), e1600200.
DeBerardinis, R. J., & Cheng, T. (2010). Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene, 29(3), 313–324.
DeBerardinis, R. J., Mancuso, A., Daikhin, E., et al. (2007). Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proceedings of the National Academy of Sciences, 104(49), 19345–19350.
Dixon, S. J., Patel, D. N., Welsch, M., Skouta, R., Lee, E. D., Hayano, M., et al. (2014). Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife, 3, e02523.
Dong, J., Xiao, D., Zhao, Z., et al. (2017). Epigenetic silencing of microRNA-137 enhances ASCT2 expression and tumor glutamine metabolism. Oncogenesis, 6(7), e356.
Dong, Y., Tu, R., Liu, H., & Qing, G. (2020). Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduction and Targeted Therapy, 5(1), 124–124.
Eads, J., Krishnamurthi, S., Saltzman, J., Bajor, D., Vinayak, S., Barnholtz-Sloan, J., et al. (2018). Phase I clinical trial of the glutaminase inhibitor CB-839 plus capecitabine in patients with advanced solid tumors. Journal of Clinical Oncology, 36(15_suppl), 2562–2562.
Egler, R. A., Ahuja, S. P., & Matloub, Y. (2016). L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. Journal of Pharmacology and Pharmacotherapeutics, 7(2), 62–71.
Fan, S., Zhao, Y., Li, X., et al. (2013). Genetic variants in SLC7A7 are associated with risk of glioma in a Chinese population. Experimental Biology and Medicine (Maywood, N.J.), 238(9), 1075–1081.
Ferlay, J., Colombet, M., Soerjomataram, I., et al. (2018). Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. European Journal of Cancer (Oxford, England : 1990), 103, 356–387.
Gao, P., Tchernyshyov, I., Chang, T.-C., et al. (2009). c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature, 458(7239), 762–765.
Green, C. R., Wallace, M., Divakaruni, A. S., et al. (2016). Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nature Chemical Biology, 12(1), 15–21.
Grewal, S., Defamie, N., Zhang, X., et al. (2009). SNAT2 amino acid transporter is regulated by amino acids of the SLC6 gamma-aminobutyric acid transporter subfamily in neocortical neurons and may play no role in delivering glutamine for glutamatergic transmission. The Journal of Biological Chemistry., 284(17), 11224–11236.
Gross, M. I., Demo, S. D., Dennison, J. B., et al. (2014). Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Molecular Cancer Therapeutics, 13(4), 890–901.
Häfliger, P., Graff, J., Rubin, M., Stooss, A., Dettmer, M., Altmann, K., et al. (2018). The LAT1 inhibitor JPH203 reduces growth of thyroid carcinoma in a fully immunocompetent mouse model. Journal of Experimental & Clinical Cancer Research, 37(1).
Hägglund, M. G., Sreedharan, S., Nilsson, V. C., et al. (2011). Identification of SLC38A7 (SNAT7) protein as a glutamine transporter expressed in neurons. The Journal of Biological Chemistry, 286(23), 20500–20511.
Hao, Y., Samuels, Y., Li, Q., et al. (2016). Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nature Communications, 7(1), 11971.
Hara, Y., Minami, Y., Yoshimoto, S., et al. (2020). Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Medicine, 9(1), 302–312.
Hassanein, M., Qian, J., Hoeksema, M. D., et al. (2015). Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. International Journal of Cancer, 137(7), 1587–1597.
Hayase, S., Kumamoto, K., Saito, K., et al. (2017). L-type amino acid transporter 1 expression is upregulated and associated with cellular proliferation in colorectal cancer. Oncology Letters, 14(6), 7410–7416.
Hensley, C. T., Wasti, A. T., & DeBerardinis, R. J. (2013). Glutamine and cancer: Cell biology, physiology, and clinical opportunities. The Journal of Clinical Investigation., 123(9), 3678–3684.
Hosios, A. M., Hecht, V. C., Danai, L. V., et al. (2016). Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Developmental Cell, 36(5), 540–549.
Hu, W., Zhang, C., Wu, R., Sun, Y., Levine, A., & Feng, Z. (2010). Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proceedings of the National Academy of Sciences, 107(16), 7455–7460.
Huang, F., Zhang, Q., Ma, H., Lv, Q., & Zhang, T. (2014). Expression of glutaminase is upregulated in colorectal cancer and of clinical significance. International Journal of Clinical and Experimental Pathology, 7(3), 1093–1100.
Jiang, Y., Cao, Y., Wang, Y., et al. (2017). Cysteine transporter SLC3A1 promotes breast cancer tumorigenesis. Theranostics., 7(4), 1036–1046.
Jin, L., Alesi, G. N., & Kang, S. (2016). Glutaminolysis as a target for cancer therapy. Oncogene, 35(28), 3619–3625.
Kandasamy, P., Gyimesi, G., Kanai, Y., & Hediger, M. A. (2018). Amino acid transporters revisited: New views in health and disease. Trends in Biochemical Sciences, 43(10), 752–789.
Katt, W. P., Lukey, M. J., & Cerione, R. A. (2017). A tale of two glutaminases: Homologous enzymes with distinct roles in tumorigenesis. Future Medicinal Chemistry, 9(2), 223–243.
Kong, C., Gao, R., Yan, X., & Qin, H. (2018). Research progression of blood and fecal metabolites in colorectal cancer. IJS Oncology, 3(1), e51.
Korangath, P., Teo, W., Sadik, H., Han, L., Mori, N., Huijts, C., et al. (2015). Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clinical Cancer Research, 21(14), 3263–3273.
La Vecchia, S., & Sebastián, C. (2020). Metabolic pathways regulating colorectal cancer initiation and progression. Seminars in Cell & Developmental Biology, 98, 63–70.
Larsen, C., & Dashwood, R. (2010). (−)-Epigallocatechin-3-gallate inhibits met signaling, proliferation, and invasiveness in human colon cancer cells. Archives of Biochemistry and Biophysics, 501(1), 52–57.
Li, T., & Le, A. (2018). Glutamine metabolism in cancer. Adv. Exp. Med. Biol., 1063, 13–32.
Li, J., Song, P., Jiang, T., Dai, D., Wang, H., Sun, J., et al. (2018). Heat shock factor 1 epigenetically stimulates glutaminase-1-dependent mTOR activation to promote colorectal carcinogenesis. Molecular Therapy, 26(7), 1828–1839.
Lieu, E. L., Nguyen, T., Rhyne, S., & Kim, J. (2020). Amino acids in cancer. Experimental & Molecular Medicine, 52(1), 15–30.
Liu, G., Zhu, J., Yu, M., et al. (2015). Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients. Journal of Translational Medicine, 13(1), 144.
Liu, Y., Zhao, T., Li, Z., Wang, L., Yuan, S., & Sun, L. (2018). The role of ASCT2 in cancer: A review. European Journal of Pharmacology, 837, 81–87.
Lorin, S., Tol, M. J., Bauvy, C., et al. (2013). Glutamate dehydrogenase contributes to leucine sensing in the regulation of autophagy. Autophagy, 9(6), 850–860.
Lu, Y., Wang, W., Wang, J., et al. (2013). Overexpression of arginine transporter CAT-1 is associated with accumulation of L-arginine and cell growth in human colorectal cancer tissue. PLoS One, 8(9), e73866.
Luengo, A., Gui, D. Y., & Vander Heiden, M. G. (2017). Targeting metabolism for cancer therapy. Cell Chemical Biology, 24(9), 1161–1180.
Lugano, R., Ramachandran, M., & Dimberg, A. (2020). Tumor angiogenesis: causes, consequences, challenges and opportunities. Cellular and Molecular Life Sciences, 77(9), 1745–1770.
Lukey, M. J., Wilson, K. F., & Cerione, R. A. (2013). Therapeutic strategies impacting cancer cell glutamine metabolism. Future Medicinal Chemistry, 5(14), 1685–1700.
Lynch, G., Kemeny, N., & Casper, E. (1982). Phase II evaluation of DON (6-Diazo-5-Oxo-L-Norleucine) in patients with advanced colorectal carcinoma. American Journal of Clinical Oncology, 5(5), 541–543.
Ma, H., Wu, Z., Peng, J., et al. (2018). Inhibition of SLC1A5 sensitizes colorectal cancer to cetuximab. International Journal of Cancer, 142(12), 2578–2588.
Ma, M., Chen, G., Wang, P., Lu, W., Zhu, C., Song, M., et al. (2015). Xc− inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism. Cancer Letters, 368(1), 88–96.
Maffione, A. M., Lopci, E., Bluemel, C., Giammarile, F., Herrmann, K., & Rubello, D. (2015). Diagnostic accuracy and impact on management of (18)F-FDG PET and PET/CT in colorectal liver metastasis: A meta-analysis and systematic review. European Journal of Nuclear Medicine and Molecular Imaging, 42(1), 152–163.
Matés, J. M., Segura, J. A., Martín-Rufián, M., Campos-Sandoval, J. A., Alonso, F. J., & Márquez, J. (2013). Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Current Molecular Medicine, 13(4), 514–534.
Mayers, J. R., & Vander Heiden, M. G. (2015). Famine versus feast: Understanding the metabolism of tumors in vivo. Trends in Biochemical Sciences, 40(3), 130–140.
Miyo, M., Konno, M., Nishida, N., et al. (2016). Metabolic adaptation to nutritional stress in human colorectal cancer. Scientific Reports, 6, 38415.
Mueller, C., Al-Batran, S., Jaeger, E., Schmidt, B., Bausch, M., Unger, C., & Sethuraman, N. (2008). A phase IIa study of PEGylated glutaminase (PEG-PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors. Journal of Clinical Oncology, 26(15_suppl), 2533–2533.
Muto, Y., Furihata, T., Kaneko, M., Higuchi, K., Okunushi, K., Morio, H., et al. (2018). Different response profiles of gastrointestinal cancer cells to an L-type amino acid transporter inhibitor, JPH203. Anticancer Research, 39(1), 159–165.
Nakazawa, M. S., Eisinger-Mathason, T. S. K., Sadri, N., et al. (2016). Epigenetic re-expression of HIF-2α suppresses soft tissue sarcoma growth. Nature Communications, 7(1), 10539.
Nguyen, H. T., & Duong, H. Q. (2018). The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncology Letters, 16(1), 9–18.
Nguyen, T.-L., & Durán, R. V. (2018). Glutamine metabolism in cancer therapy. Cancer Drug Resistance., 1(3), 126–138.
Nicklin, P., Bergman, P., Zhang, B., Triantafellow, E., Wang, H., Nyfeler, B., et al. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell, 136(3), 521–534.
Oda, K., Hosoda, N., Endo, H., et al. (2010). L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Science, 101(1), 173–179.
Park, J. H., Richards, C. H., McMillan, D. C., Horgan, P. G., & Roxburgh, C. S. D. (2014). The relationship between tumour stroma percentage, the tumour microenvironment and survival in patients with primary operable colorectal cancer. Annals of Oncology, 25(3), 644–651.
Pavlova, N. N., & Thompson, C. B. (2016). The emerging hallmarks of cancer metabolism. Cell Metabolism, 23(1), 27–47.
Pochini, L., Scalise, M., Galluccio, M., & Indiveri, C. (2014). Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Frontiers in Chemistry, 2, 61–61.
Reynolds, M. R., Lane, A. N., Robertson, B., et al. (2014). Control of glutamine metabolism by the tumor suppressor Rb. Oncogene, 33(5), 556–566.
Richard, S., & Martinez, M. V. (2015). Sensitization to oxaliplatin in HCT116 and HT29 cell lines by metformin and ribavirin and differences in response to mitochondrial glutaminase inhibition. Journal of Cancer Research and Therapeutics, 11(2), 336–340.
Rubio-Aliaga, I., & Wagner, C. A. (2016). Regulation and function of the SLC38A3/SNAT3 glutamine transporter. Channels (Austin, Tex.), 10(6), 440–452.
Russi, S., Verma, H. K., Laurino, S., et al. (2019). Adapting and surviving: Intra and extra-cellular remodeling in drug-resistant gastric cancer cells. International Journal of Molecular Sciences, 20(15), 3736.
Satoh, K., Yachida, S., Sugimoto, M., et al. (2017). Global metabolic reprogramming of colorectal cancer occurs at adenoma stage and is induced by MYC. Proceedings of the National Academy of Sciences, 114(37), E7697–E7706.
Scalise, M., Pochini, L., Galluccio, M., Console, L., & Indiveri, C. (2017). Glutamine transport and mitochondrial metabolism in cancer cell growth. Frontiers in Oncology, 7, 306.
Scalise, M., Pochini, L., Console, L., Losso, M. A., & Indiveri, C. (2018). The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Frontiers in Cell and Development Biology, 6, 96–96.
Scalise, M., Pochini, L., Galluccio, M., Console, L., & Indiveri, C. (2020). Glutamine transporters as pharmacological targets: From function to drug design. Asian Journal of Pharmaceutical Sciences, 15(2), 207–219.
Schulte, M., Fu, A., Zhao, P., Li, J., Geng, L., Smith, S., et al. (2018). Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nature Medicine, 24(2), 194–202.
Scopelliti, A. J., Font, J., Vandenberg, R. J., Boudker, O., & Ryan, R. M. (2018). Structural characterisation reveals insights into substrate recognition by the glutamine transporter ASCT2/SLC1A5. Nature Communications, 9(1), 38.
Sever, R., & Brugge, J. S. (2015). Signal transduction in cancer. Cold Spring Harbor Perspectives in Medicine, 5(4), a006098.
Sheikh, T., Patwardhan, P., Cremers, S., & Schwartz, G. (2017). Targeted inhibition of glutaminase as a potential new approach for the treatment of NF1 associated soft tissue malignancies. Oncotarget, 8(55), 94054–94068.
Shen, S. L., Fu, S. J., Chen, B., et al. (2014). Preoperative aspartate aminotransferase to platelet ratio is an independent prognostic factor for hepatitis B-induced hepatocellular carcinoma after hepatic resection. Annals of Surgical Oncology, 21(12), 3802–3809.
Shen, L., Qian, C., Cao, H., Wang, Z., Luo, T., & Liang, C. (2018). Upregulation of the solute carrier family 7 genes is indicative of poor prognosis in papillary thyroid carcinoma. World Journal of Surgical Oncology, 16(1), 235–235.
Sirniö, P., Väyrynen, J. P., Klintrup, K., et al. (2019). Alterations in serum amino-acid profile in the progression of colorectal cancer: Associations with systemic inflammation, tumour stage and patient survival. British Journal of Cancer, 120(2), 238–246.
Son, J., Lyssiotis, C. A., Ying, H., et al. (2013). Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature, 496(7443), 101–105.
Song, Z., Wei, B., Lu, C., Li, P., & Chen, L. (2017). Glutaminase sustains cell survival via the regulation of glycolysis and glutaminolysis in colorectal cancer. Oncology Letters, 14(3), 3117–3123.
Song, M., Kim, S., Im, C., & Hwang, H. (2018). Recent development of small molecule glutaminase inhibitors. Current Topics in Medicinal Chemistry, 18(6), 432–443.
Sun, X., & Zhu, M. J. (2018). Butyrate inhibits indices of colorectal carcinogenesis via enhancing α-ketoglutarate-dependent DNA demethylation of mismatch repair genes. Molecular Nutrition & Food Research, 62(10), e1700932.
Suzuki, S., Tanaka, T., Poyurovsky, M. V., et al. (2010). Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proceedings of the National Academy of Sciences of the United States of America, 107(16), 7461–7466.
Tanaka, K., Sasayama, T., Irino, Y., Takata, K., Nagashima, H., Satoh, N., et al. (2015). Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. Journal of Clinical Investigation, 125(4), 1591–1602.
Toda, K., Kawada, K., Iwamoto, M., Inamoto, S., Sasazuki, T., Shirasawa, S., et al. (2016). Metabolic alterations caused by KRAS mutations in colorectal cancer contribute to cell adaptation to glutamine depletion by upregulation of asparagine synthetase. Neoplasia, 18(11), 654–665.
Toda, K., Nishikawa, G., Iwamoto, M., et al. (2017). Clinical role of ASCT2 (SLC1A5) in KRAS-mutated colorectal cancer. International Journal of Molecular Sciences, 18(8), 1632.
Ueda, S., Hayashi, H., Miyamoto, T., et al. (2019). Anti-tumor effects of mAb against L-type amino acid transporter 1 (LAT1) bound to human and monkey LAT1 with dual avidity modes. Cancer Science, 110(2), 674–685.
Vazquez, A., Kamphorst, J. J., Markert, E. K., Schug, Z. T., Tardito, S., & Gottlieb, E. (2016). Cancer metabolism at a glance. Journal of Cell Science, 129(18), 3367–3373.
Verma, H. K., Kampalli, P. K., Lakkakula, S., Chalikonda, G., Bhaskar, L., & Pattnaik, S. (2019). A retrospective look at anti-EGFR agents in pancreatic cancer therapy. Current Drug Metabolism, 20(12), 958–966.
Verma, H. K., Ratre, Y. K., Mazzone, P., Laurino, S., & LVKS, B. (2020a). Micro RNA facilitated chemoresistance in gastric cancer: A novel biomarkers and potential therapeutics. Alexandria Journal of Medicine, 56(1), 81–92.
Verma, H. K., Falco, G., & Bhaskar, L. V. K. S. (2020b). Molecular signaling pathways involved in gastric cancer chemoresistance. In Theranostics approaches to gastric and colon cancer. Singapore: Springer.
Vernieri, C., Casola, S., Foiani, M., Pietrantonio, F., de Braud, F., & Longo, V. (2016). Targeting cancer metabolism: Dietary and pharmacologic interventions. Cancer Discovery, 6(12), 1315–1333.
Villa, E., Ali, E. S., Sahu, U., & Ben-Sahra, I. (2019). Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers (Basel)., 11(5), 688.
Wang, Q., Beaumont, K., Otte, N., Font, J., Bailey, C., van Geldermalsen, M., et al. (2014). Targeting glutamine transport to suppress melanoma cell growth. International Journal of Cancer, 135(5), 1060–1071.
Wang, Q., & Holst, J. (2015). L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. American Journal of Cancer Research, 5(4), 1281–1294.
Wang, K., Cao, F., Fang, W., et al. (2013). Activation of SNAT1/SLC38A1 in human breast cancer: Correlation with p-Akt overexpression. BMC Cancer, 13(1), 343.
Wang, Y.-Q., Wang, H.-L., Xu, J., et al. (2018). Sirtuin5 contributes to colorectal carcinogenesis by enhancing glutaminolysis in a deglutarylation-dependent manner. Nature Communications, 9(1), 545.
Wilson, K. F., Erickson, J. W., Antonyak, M. A., & Cerione, R. A. (2013). Rho GTPases and their roles in cancer metabolism. Trends in Molecular Medicine, 19(2), 74–82.
Wilson, P. M., Danenberg, P. V., Johnston, P. G., Lenz, H. J., & Ladner, R. D. (2014). Standing the test of time: Targeting thymidylate biosynthesis in cancer therapy. Nature Reviews. Clinical Oncology, 11(5), 282–298.
Xiang, L., Mou, J., Shao, B., et al. (2019). Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death & Disease, 10(2), 40–40.
Xie, J., Li, P., Gao, H.-F., Qian, J.-X., Yuan, L.-Y., & Wang, J.-J. (2014). Overexpression of SLC38A1 is associated with poorer prognosis in Chinese patients with gastric cancer. BMC Gastroenterology, 14, 70–70.
Xie, C., Jin, J., Bao, X., et al. (2016). Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget, 7(1), 610–621.
Yang, L., Venneti, S., & Nagrath, D. (2017). Glutaminolysis: A hallmark of cancer metabolism. Annual Review of Biomedical Engineering, 19, 163–194.
Zare-Bandamiri, M., Fararouei, M., Zohourinia, S., Daneshi, N., & Dianatinasab, M. (2017). Risk factors predicting colorectal cancer recurrence following initial treatment: A 5-year cohort study. Asian Pacific Journal of Cancer Prevention, 18(9), 2465–2470.
Zhang, Y., Ma, J., Zhang, S., et al. (2015). A prognostic analysis of 895 cases of stage III colon cancer in different colon subsites. International Journal of Colorectal Disease, 30(9), 1173–1183.
Zhao, J., Zhou, R., Hui, K., Yang, Y., Zhang, Q., Ci, Y., et al. (2016). Selenite inhibits glutamine metabolism and induces apoptosis by regulating GLS1 protein degradation via APC/C-CDH1 pathway in colorectal cancer cells. Oncotarget, 8(12), 18832–18847.
Zhou, D., Shao, L., & Spitz, D. R. (2014). Reactive oxygen species in normal and tumor stem cells. Advances in Cancer Research, 122, 1–67.
Zhou, F.-F., Xie, W., Chen, S.-Q., et al. (2017). SLC38A1 promotes proliferation and migration of human colorectal cancer cells. Journal of Huazhong University of Science and Technology [Medical Sciences], 37(1), 30–36.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ratre, Y.K. et al. (2021). Therapeutic Targeting of Glutamine Metabolism in Colorectal Cancer. In: Vishvakarma, N.K., Nagaraju, G.P., Shukla, D. (eds) Colon Cancer Diagnosis and Therapy. Springer, Cham. https://doi.org/10.1007/978-3-030-64668-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-64668-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-64667-7
Online ISBN: 978-3-030-64668-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)