Abstract
Coronavirus disease 2019 (COVID-19) is a highly contagious infectious disease that can rapidly escalate to respiratory failure and death. It has infected millions of people worldwide. The trajectory of this disease continues to progress in some areas of the United States and worldwide. The Institute for Health Metrics now predicts a resurgence of infections in the fall of 2020. The pathogenesis of COVID-19 includes an inflammatory phase with either resolution or the potential to accelerate to a cytokine storm, characterized by high interleukin (IL)-6 and other inflammatory markers. COVID-19 is a condition without a gold-standard treatment. The US Federal Drug Administration (FDA) issued an emergency use authorization for remdesivir in severe cases of COVID-19, which shortened the recovery time in hospitalized patients with lower respiratory tract infection in one study. Although several vaccine trials are underway, no vaccines are available for primary prevention of COVID-19 at this time. Dietary supplement sales have dramatically risen during the COVID-19 pandemic despite depressed economic conditions. Commonly used immune-modulating dietary supplements, including vitamin D, ascorbic acid, zinc, and melatonin, are reviewed in this manuscript highlighting biological plausibility for salutary benefit against COVID-19. Ongoing clinical trials recruiting subjects at the time of this writing are provided for each dietary supplement.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
29.1 Introduction
29.1.1 COVID-19: Epidemiology and Pathogenesis
Coronavirus disease 2019 (COVID-19) is responsible for the third major coronavirus (CoV) pandemic in recent history (Mahase 2020). There is no definitive treatment for COVID-19, which remains a highly contagious infectious disease that can escalate rapidly to respiratory failure and death (Mahase 2020). The pandemic of COVID-19 has infected over nine million people worldwide, including over two million people in the United States ((JHU); Jin et al. 2015; Urwyler et al. 2019). The trajectory of disease continues to progress in some areas of the United States and worldwide, and the University of Washington Institute for Health Metrics and Evaluation (IHME) now predicts a resurgence of infections in the fall of 2020 ((IHME) 2020). Overall, 81% of adults infected by COVID-19 self-resolve while 19% progress, and many require hospitalization ((JHU)). Older people aged 65 or over and those with comorbidities (immunocompromised, cardiopulmonary disease, cancer, diabetes, obesity, and kidney failure) are most at risk to experience rapid disease progression, respiratory deterioration requiring intensive care unit (ICU) admission, and mortality (Park et al. 2020; Zhou et al. 2020). COVID-19 is classified according to four levels of severity based on symptoms: mild, moderate, severe, and critical (Siddiqi and Mehra 2020). Critical disease has a 49% case fatality rate related to acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), septic shock, and multiorgan failure) (Wang et al. 2020c).
The pathogenesis of COVID-19 illness proceeds variably according to distinct phases of the disease:
-
Incubation with asymptomatic viral replication.
-
Symptomatic with constitutional, respiratory, and other systemic symptoms (i.e., gastrointestinal) of variable duration and severity.
-
An inflammatory phase with either resolution or acceleration to a cytokine storm, whereby severe illness is associated with increased plasma concentrations of pro-inflammatory cytokines, including interleukin (IL)-6, IL-10, granulocyte colony-stimulating factor (G-CSF), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein (MIP)-1α, and tumor necrosis factor (TNF)-α (Huang et al. 2020; Alunno et al. 2020). Most severe conditions, such as multiorgan failure, occur in those with high IL-6 levels (Han et al. 2020).
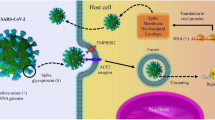
The coronavirus responsible for COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also attaches to toll-like receptors (TLRs) in pulmonary macrophages, causing IL-1-beta production and inflammasome activation. Once inflammasomes are activated, and injurious pro-inflammatory cytokines are produced, an extensive injury with loss of lung tissue and subsequent fibrosis with permanent respiratory dysfunction may result (Conti et al. 2020). Respiratory decompensation is frequent and can include the development of ARDS requiring mechanical ventilation. Angiotensin-converting enzyme 2 (ACE2) converts angiotensin I to angiotensin (1–7), which regulates blood pressure, systemic vascular resistance, and fluid-electrolyte balance. COVID-19 infects human cells as spike (S) protein enters cells via ACE2 receptors. Once inside the cell, furin facilitates the cleavage of COVID-19 spike protein by transmembrane protease serine 2. The glycoprotein cleavage byproduct of S protein facilitates the entry of viral genetic material into the cell. This step appears to be required for infection of lung tissues and occurs in other aggressive viral infections such as dengue, avian influenza, and anthrax (Shang et al. 2020; Walls et al. 2020). However, furin protease activation has not been seen with prior coronavirus infections or its ancestor viruses (i.e., SARS-CoV-1). Conditions associated with elevated furin, including diabetes, obesity, and hypertension, overlap significantly with vulnerability to the severe form of COVID-19. ACE2 is constitutively expressed in respiratory and oral epithelium, lung parenchyma, and other tissues, including gut epithelium. Increased ACE2 activity under physiologic conditions appears to protect against COVID-19 (Brojakowska et al. 2020). However, higher expression of ACE2 on the surface of cells in COVID-19 is linked to heightened disease severity (Brojakowska et al. 2020). Furthermore, it seems that furin is implicated in the pathogenesis of SARS-CoV-2 infection and potentially in the increased rates of human-to-human transmission. ACE2 expression appears to be associated with COVID-19 severity.
NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) is an intracellular sensor that detects a broad range of microbial motifs. Inflammasomes are formed by different substances, including lipopolysaccharide (LPS) from bacterial cell walls, pathogen-associated molecular patterns (PAMPs) from viruses, bacteria, and fungi. Inflammasomes can be activated by damage-associated molecular patterns (DAMPs) and pro-inflammatory cytokines (IL-1β, TNFα) (Korakas et al. 2020). Activation of the NLRP3 inflammasome appears to be another critical event in the acceleration of the inflammatory phase of the disease to cytokine storm (Fig. 29.1). Inflammasomes activate caspase-1 leading to pyroptosis, a pathway of cell death is uniquely dependent on caspase-1 (Fink and Cookson 2005), and stimulate maturation and secretion of the pro-inflammatory cytokines, interleukin-1beta (IL-1β) (Parisi and Leosco 2020) and interleukin-18 (IL-18) through nuclear factor kappa-B (NF-κB) signaling. A cytokine storm may occur in COVID-19 patients characterized by a failure of the immune system to counter regulate NLRP3 inflammasome activity (Paramo 2020). Modulation of ACE2 expression and furin proteases and prevention of the induction NLRP3 are targets of therapy in COVID-19.
Central role of NLRP3 inflammasome activation in the severe symptomatic phase of COVID-19 and potential options for treatment. The DAMPs released after NLRP3 inflammasome activation have a dual function. In a normal immune reaction, they induce the necessary co-stimulatory activation of the APC, but they also play a role in resolution and tissue regeneration. Only in case of a hyperactivation of the NLRP3 inflammasome DAMPs are released in high concentrations and result in pyroptosis, high-mobility group box 1 (HMGB1) release, activation of macrophages, neutrophil infiltration and reduced apoptosis, excessive cytokine production (IL-1β, IL-2, IL-6, IL-17, TNF-α, GM-CSF, IFN-γ, CXCL10, CCL2, and CCL3, cytokine storm), and fibrosis
DAMPs damage-associated molecular patterns, NLRP3 NOD-, LRR- and pyrin domain-containing protein 3, PAMPs pathogen-associated molecular patterns, IL-1β interleukin-1 beta, IL-2 interleukin-2, IL-6 interleukin-6, IL-7 interleukin-7, TNF-a tumor necrosis factor-alpha, CXCL10 interferon gamma-induced protein 10 or chemokine 10, GM CSF granulocyte colony-stimulating factor, CCL2 C-C motif chemokine ligand 2, CCL3 C-C motif chemokine ligand 3, NF-κB nuclear factor kappa-B, Th17 T-helper 17 cells, HMGB1 high-mobility group box 1
Adapted with permission (van den Berg and te Velde 2020)
29.1.2 COVID-19: Current Treatment Paradigm
Investigational therapies against COVID-19 that are being tested include antiviral agents, immune modulators, cellular therapies, vaccines, convalescent plasma, traditional Chinese medicines, combination agents, or other medications (Bhagavathula et al. 2020; Guo et al. 2020). Hospitalization for COVID-19-related complications is primarily for supportive care, as therapies to prevent the progression of the respiratory disease remain investigational.
The treatment with the most testing thus far has been the antiviral remdesivir. A double-blind, randomized placebo-controlled trial (RCT) of 1063 patients with COVID-19 and lower respiratory tract involvement compared to remdesivir (200 mg loading on day one, 100 mg daily days 2–10) to placebo and showed benefit in shortening recovery time from 15 to 11 days (rate ratio for recovery, 1.32; 95% CI, 1.12–1.55; P < 0.001) (Beigel et al. 2020). The benefit was seen mainly in those with a severity score of 5 (required oxygen), which represents more severe disease. Remdesivir may facilitate quicker recovery for patients who are hospitalized with COVID-19 and require supplemental oxygen therapy.
Wang et al. reported a double-blind RCT of 237 hospitalized patients with COVID-19 lower respiratory tract infections and severe disease, comparing intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2–10 in single daily infusions) to placebo infusions for 10 days (Wang et al. 2020d). The primary endpoint was clinical improvement up to day 28, defined as the time from randomization to the point of a decline of two levels on a six-point ordinal scale of clinical status (from 1 = discharged to 6 = death) or discharged alive from the hospital, whichever came first. The study was terminated before reaching the anticipated sample size as stringent public health measures in Wuhan created difficulties with enrollment. The intention to treat populations did not show a difference in time to clinical improvement. However, those who had symptom onset in less than 10 days before treatment appeared to trend toward benefit with remdesivir over placebo.
Goldman et al. conducted a randomized non-placebo-controlled clinical trial of remdesivir in 397 patients who underwent 1:1 randomization with intravenous remdesivir for either 5 days or 10 days. A total of 200 subjects were treated for 5 days, and 197 were treated for 10 days. Remdesivir was administered intravenously to subjects at 200 mg on day 1 and then 100 mg once daily after that. The primary endpoint was the clinical status of subjects on day 14, assessed on a 7-point ordinal scale. There was no difference between a 5-day course and a 10-day course of remdesivir in non-ventilated subjects with severe COVID-19. Both groups showed an improvement by reduction of 2 more points in the 7-point ordinal scale [64% (5 days) vs. 54% (10 days), p = 0.14] (Goldman et al. 2020). However, a placebo was not utilized, limiting the conclusions drawn from the study.
Treatment to prevent the progression of COVID-19 to severe disease is lacking, and the public is using a variety of nutraceutical agents as a preventative measure to prevent or mitigate the progression of COVID-19 (Table 29.1) (Hemila and Chalker 2013). The nutraceutical agents with biological plausibility (Iddir et al. 2020) with the potential to address in part the pathophysiology of COVID-19 are being explored in clinical trials (clinicaltrials.gov, WHO database) and are reviewed below.
29.2 Dietary Supplements Being Studied for COVID-19
29.2.1 Ascorbic Acid
Ascorbic acid contributes to immune defense by supporting various cellular functions of both the innate and adaptive immune systems (Holmannova et al. 2012). Ascorbic acid accumulates in phagocytic cells, such as neutrophils, and can enhance chemotaxis, phagocytosis, generation of reactive oxygen species, and ultimately microbial killing (Carr and Maggini 2017). Ascorbic acid has a long history of use for viral respiratory infections, and there is in vitro evidence of its activity against coronavirus in chick embryo ciliated tracheal organ cultures (Atherton et al. 1978). Intravenous ascorbic acid has been shown to reduce the length of stay and ventilator requirements in the critical care unit setting. Vitamin C has been shown to shorten the duration of mechanical ventilation by about 20% in patients who required mechanical ventilation for over a 24 h period (95% CI 7.7% to 27%; p = 0.001) (Hemila and Chalker 2019). In combination with thiamine (Amrein et al. 2011), ascorbic acid and hydrocortisone may improve outcomes among patients with a critical illness such as sepsis and ARDS (Hager et al. 2019, 2020; Fowler 3rd et al. 2019, 2020; Kim et al. 2018, 2019; Hwang et al. 2019; Shin et al. 2019). The putative mechanisms include improvement of pulmonary capillary integrity, correction of sepsis-induced coagulopathy, attenuation of oxidative endothelial injury, and lowering of serum TNF-alpha (Chen et al. 2014).
Vitamin C supplementation could help the prevention and treatment of respiratory and systemic infections (Turski et al. 2020; Carr and Maggini 2017). High doses of ascorbic acid reduce the severity and duration of common cold symptoms caused by rhinovirus, a coronavirus with a typical mild self-limited course if untreated (Hemila and Chalker 2013). The treatment of critically ill patients with ascorbic acid has shown mixed results on mortality, length of intensive care unit stay, and duration of mechanical ventilation (Carr 2019a, b; Hager et al. 2020). There are some clinical trials involving ascorbic acid for COVID-19 underway (Carr 2020). A clinical trial to investigate vitamin C infusion for the treatment of severe 2019-nCoV-infected pneumonia will treat 140 patients with intravenous vitamin C at a dose of 24 g/day versus matching placebo for 7 days. Study endpoints include the need for mechanical ventilation, vasopressor drug requirements, sequential organ failure assessment scores, intensive care unit length of stay, and 28-day mortality (Identifier: NCT04264533na). Other clinical trials involving ascorbic acid for COVID-19 are registered in clinical trials.gov, and the World Health Organization Trial Registry Network (WHO ICTRP database).
29.2.2 Phytochemicals
Phytonutrients are plant-derived biochemicals, some of which impart disease-fighting health-benefits when ingested. Some phytonutrients are powerful anti-inflammatory agents modulating NLRP3 inflammasome activation, thereby mitigating degenerative diseases (Table 29.2 and Fig. 29.2) (Jahan et al. 2017b). The following phytonutrients described here are essential to consider for COVID-19, due to their actions on NLRP3 activation, viral replication, and immunity. Curcumin can exert its anti-inflammatory role mainly by preventing the activation of NLRP3 inflammasomes (Hasanzadeh et al. 2020; Olcum et al. 2020). Curcumin downregulates NF-kappa B (NF-κB) signaling, interrupts IL-1β maturation, and reduces the secretion and release of interleukins. Collectively, these actions are the most prominent mechanisms of curcumin in modulating inflammasomes (Figs. 29.3 and 29.4) (Hasanzadeh et al. 2020). A recent review of phytochemicals and their influence on intracellular signaling mechanisms of action on NLRP3 inflammasome activation focused on sulforaphane (SFN), curcumin, and resveratrol (RSV) (Olcum et al. 2020). SFN, which is present in cruciferous vegetables, was identified as a potent inhibitor of NLRP3 inflammasome activation. SFN is also known as a strong promoter of the Nrf2 transcription factor, which is the primary regulator of numerous cytoprotective, antioxidant, and anti-inflammatory genes in various tissues and cell types, and it has a role in maintaining cellular redox balance (Fig. 29.4). RSV can suppress NLRP3 inflammasome by different mechanisms and pathways, including NAD-dependent deacetylase sirtuin-1 (SIRT1) and autophagy activation. RSV appears to be a potent SIRT1 activator, which alters and inhibits the acetylation of inflammatory proteins (Sui et al. 2016). In most of the studies, RSV inhibits NLRP3 inflammasome via enhancing autophagy by activating SIRT1 (Qi et al. 2019). RSV may also upregulate ACE2 expression and may benefit the host against COVID-19 infection (Horne and Vohl 2020).
Schematic model illustrating the underlying anti-inflammatory mechanisms of curcumin through regulation of NLRP3 inflammasome activity. Curcumin could suppress the activity of NLRP3 inflammasome through different pathways, including deterrence of K+ efflux, inhibition of ER stress and decreased levels of ROS and TXINP through AMPK activation, prevention of NLRP3 components assembly via blocking the binding of ASC to NLRP3, and suppression of NF-κB signaling pathway, which leads to the prevention of NLRP3 and pro-IL-1β expression
PAMPS pathogen-associated molecular patterns, DAMPS damage-associated molecular patterns, TLR toll-like receptor, TNFR tumor necrosis factor receptor, NF-κB nuclear factor kappa-B, NLRP3 NOD-like receptor pyrin domain-containing 3, IL-1β interleukin-1β, IL-18 interleukin 18, AMPK 5′ adenosine monophosphate-activated protein kinase, ER endoplasmic reticulum, ROS reactive oxygen species, TXNIP thioredoxin-interacting protein, ATP adenosine triphosphate, P2 × 7R purinergic 2 × 7 receptor, MSU monosodium urate crystal, K+ potassium, Ca+ calcium, ASC apoptosis-associated speck-like protein containing a caspase recruitment domain
Adapted with permission (Hassanzadeh et al. 2020)
NLRP3 inflammasome suppression mechanisms of sulforaphane (SFN), curcumin, and resveratrol (RSV). All three phytochemicals use NF-kB inhibition to suppress inflammasome activation. Other than this mechanism, SFN leads to inflammasome suppression via Nrf2 activation, STAT-1 activation. RSV and curcumin lead to inflammasome suppression via TXNIP inhibition or leading to AMPK-induced autophagy
Adapted with permission (Olcum et al. 2020)
Inflammasome activation pathways concerning disease development and phytonutrients: i, some inflammasome agonists as ATP, triggers P2X7-dependent pore formation by the pannexin-1 hemichannel, allowing extracellular agonists to enter the cytosol and directly trigger inflammasome assembly; ii, crystalline or particulate inflammasome agonists that are engulfed by the cells have characteristic physical properties which lead to lysosomal rupture. The inflammasome senses lysosomal content released in the cytoplasm, for example, via cathepsin B-dependent processing of a direct NLRP3 ligand; iii, all danger-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), stressed mitochondria including ATP and particulate/crystalline activators, cause the generation of reactive oxygen species (ROS). A ROS-mediated pathway triggers inflammasome complex formation; and iv, toll-like receptor (TLR) senses lipopolysaccharides and prime the NF-κB, a transcription factor which triggers pro-IL-1 and pro-IL-18 expressions which sequentially get converted into IL-1beta and IL-18. Caspase-1 clustering induces autoactivation and caspase-1-dependent maturation and secretion of pro-inflammatory cytokines, such as interleukin-1beta (IL-1b) and IL-18. Against all possible pathways, phytochemicals (shown by green leaf) are used for the therapeutic activity to inhibit inflammasome induced diseases
Adapted with permission (Jahan et al. 2017a)
Other NLRP3 activation inhibitors include green tea phytochemicals (Zhang et al. 2019; Wang et al. 2020a). Phytochemicals can also prevent and or mitigate COVID-19 by serving as virus main protease (Mpro) inhibitors to replication. A study using molecular docking technology revealed that Phaseolus vulgaris phytochemicals had maximum binding with Mpro and ACE2, while quercetin 3-glucuronide-7-glucoside and quercetin 3-vicianoside gave even better binding energy with both the targets (Joshi et al. 2020). Quercetin has been shown to inhibit hepatitis C viral replication (Khan et al. 2013) and modulate NLRP3 inflammasome activation, is antifibrotic by stabilizing mast cell function, and promotes resolvins, which stabilize collateral damage to host tissues. A subsequent study also using molecular docking technology showed that robust one exhibited excellent binding affinity properties against Mpro of SARS-CoV-2 (Rasool et al. 2020). This phytonutrient antioxidant flavonoid compound has also been shown to be an inhibitor against the protease of the dengue virus (Mishra et al. 2016). RSV has also been shown to have in vitro activity against coronaviruses such as MERS-CoV and is effective clinically against rhinovirus (Baldassarre et al. 2020; Lin et al. 2017).
29.2.3 Melatonin
Melatonin is receiving increasing attention in the press as a natural product with a multitude of biological effects that may be relevant to COVID-19 (Reiter et al. 2020). Melatonin attenuates several actions that protect the host against COVID-19, including pro-inflammatory cytokine production, inducible nitric oxide synthases, neuronal nitric oxide synthase, cyclooxygenase-2, high-mobility group box 1 signaling, TLR4 activation, inflammasome NLRP3 activation, and NF-κB activation (El-Missiry et al. 2020). Melatonin induces anti-inflammatory cytokines while having a high antioxidant capacity, which buffers the injurious inflammatory injury during the resolution phase of COVID-19 (El-Missiry et al. 2020). In COVID-19, melatonin may increase resistance to infection by upregulating ACE2 expression while inhibiting NLRP3 inflammasome activation (Zhang et al. 2019). Multiple actions of melatonin as an anti-inflammatory, antioxidant, and antiviral (against other viruses) make it a reasonable choice for use (Reiter et al. 2020). Mitochondrial intracellular heme oxygenase (HO-1) is low in the elderly, hypertensives, and diabetics, which may relate to COVID-19 susceptibility, and melatonin raises HO-1 (Hooper 2020). Two active COVID-19 clinical trials include melatonin. One clinical trial (NCT04409522) is evaluating the therapeutic effects of high-dose melatonin (9 mg) vs. usual care for 7–10 days in 55 patients with COVID-19 while measuring inflammatory cytokines and pathways to include NLRP3 inflammasome activation. The other study (NCT04353128) involves 450 Spanish healthcare workers who will be randomized to either melatonin 2 mg or placebo before bed for 12 weeks as primary prevention of COVID-19. The World Health Organization (WHO) database lists two melatonin intervention COVID-19 studies: evaluation of the efficacy of melatonin tablets as auxiliary medication in accelerating the improvement of the COVID-19 symptoms and clinical findings (IRCT20200408046988N1) and the effect of melatonin on the quality of sleep in COVID-19 patients (IRCT20200411047030N1).
29.2.4 Vitamin D
Vitamin D, once converted to the active 1,25hydroxyvitamin D (1,25VitD) form, has endocrine and paracrine properties. The paracrine action of 1,25VitD may be most important for immunity. Activated vitamin D augments innate cellular immunity against microbes, protects against bacterial and viral acute respiratory tract infections (including influenza), and regulates adaptive immune responses such as those seen in the COVID-19-associated cytokine storm (Zdrenghea et al. 2017; Martineau et al. 2017). Vitamin D influences T-helper cell (Th) cell differentiation by its effect on antigen-presenting cells (APCs). Vitamin D is involved in APC activity that modulates T-cell differentiation into an effector cell with pro-inflammatory (Th1) or anti-inflammatory (Th2) properties; thus, modulation of APCs is crucial in initiating and maintaining adaptive immune response and self-tolerance. Vitamin D modulates adaptive immunity by suppressing Th1 and Th17 responses that are overactivated in a COVID-19 cytokine storm (Wu and Yang 2020; Miraglia Del Giudice et al. 2018). Vitamin D induces Th2 cytokines such as IL-10, which counterbalance Th1 pro-inflammatory cytokines while increasing T-regulatory cells (Tregs) and their activity (Fawaz et al. 2016). By inducing Tregs and increasing their numbers, vitamin D harmonizes inflammatory responses. In COVID-19, there are reports that Th1/Th17 cytokines are high, Th2 cytokines such as IL-10 are low, and Tregs are low in number and dysfunctional in the setting of a cytokine storm (Wang et al. 2020b; Chen et al. 2020).
Vitamin D enhances innate cellular immunity to prevent and mitigate viral respiratory infections. There are two main mechanisms by which vitamin D has been shown to prevent viral respiratory tract infections. One is the promotion of respiratory epithelial and alveolar barrier function junctions to prevent the infiltration of immune cells in the lungs and other respiratory tissues. The other is an increased immune-enhanced viral killing while avoiding injurious inflammatory response (Grant et al. 2020). Vitamin D enhances the production of β-defensin-2 and LL-37 cathelicidin. Pulmonary epithelial cells have a high expression of 1α-hydroxylase, which produces calcitriol, the active form of vitamin D. Calcitriol inhibits bronchial smooth muscle cell proliferation and elaboration of pro-inflammatory cytokines, chemokines, and matrix metalloproteinases, preventing lung injury (Sandhu and Casale 2010). Vitamin D upregulates cAMP, not only by monocytes and macrophages but also in cells participating in the innate immune system, including the respiratory tract, by increasing their antimicrobial activity and epithelial barrier function (Dhawan et al. 2015).
Vitamin D is well-known to participate in the defense against some respiratory pathogens, including intracellular pathogens and bacterial and viral pathogens (Anderson et al. 2020). Lower-serum vitamin D levels are associated with adult new-onset severe sepsis, including septic shock, and high doses of enteral vitamin D3 (400,000 IU) have been shown to increase circulating cAMP and reduce inflammatory cytokines IL-6 and IL-1 when compared to placebo (Quraishi et al. 2015). In viral respiratory infections, vitamin D metabolites modulate several chemokines and pro-inflammatory cytokines (CXCL8, CXCL10, TNF-α, and IL-6) (Greiller and Martineau 2015). Observational and interventional studies demonstrate that Vitamin D can impart primary and secondary protection against viral respiratory tract illness (Teymoori-Rad et al. 2019; Beard et al. 2011). A meta-analysis of 25 eligible randomized controlled trials (total 11, 321 participants) concluded that Vitamin D supplementation was safe and protected against acute respiratory tract infection (adjusted odds ratio 0.88, 95% confidence interval 0.81–0.96) (Martineau et al. 2017). Patients who were very vitamin D-deficient as defined by baseline 25-hydroxyvitamin D levels <25 nmol/L (adjusted odds ratio 0.30, 0.17–0.53) derived more protection against respiratory tract infection compared to patients who were not so identified with baseline 25-hydroxyvitamin D levels ≥25 nmol/L (adjusted odds ratio 0.75, 0.60–0.95). Those not receiving bolus doses experienced the most benefit (adjusted odds ratio 0.81, 0.72–0.91).
There is growing speculation that vitamin D deficiency may render hosts vulnerable to COVID-19 infection and that vitamin D may serve a primary and secondary preventative role (Weir et al. 2020; Grant et al. 2020; Wei and Christakos 2015; Dhawan et al. 2015). Countries related to high mortality rates early in the COVID-19 pandemic (Italy, Spain, and the United Kingdom) are more likely to suffer from lower vitamin D levels than countries that were not as severely affected (Grant et al. 2020). An analysis of a world database by investigators at Northwestern University examined severe SARS-CoV-2 illness and vitamin D deficiency prevalence; data revealed that the risk of severe SARS-CoV-2 cases among patients with severe vitamin D deficiency was 17.3%, compared with a risk of 14.6% for patients with normal vitamin D levels (a relative reduction of 15.6%) (Daneshkhah et al. 2020). The authors suggested that the correction of vitamin D deficiency may reduce SARS-CoV-2 severity by suppressing the cytokine storm. The WHO database lists one study that is actively recruiting; IRCT2020040146909N1 9 (https://en.irct.ir/trial/46875), an RCT to evaluate the efficacy of 1000 IU of vitamin D3 or placebo daily for 8 weeks. The duration of COVID-19 infection is the primary endpoint and the WHO severity scale as the secondary endpoint.
29.2.5 Zinc
Zinc is a micronutrient with an established role in robust and effective immune responses, including antiviral immunity and adaptive immune responses, including antibody formation (Gammoh and Rink 2017). Older adults (65 years and older) are at increased risk of zinc insufficiency or deficiency. Further, because of the high incidence of diarrhea with SARS-CoV2 infection (seen in approximately 20% of patients), it was possible to assume these patients were zinc insufficient (Lee et al. 2020). Zinc is particularly attractive to consider in SARS-CoV2 infection. In vitro studies show zinc to inhibit coronavirus RNA replication (Fig. 29.5) (te Velthuis et al. 2010; Read et al. 2019). Zinc lozenges at symptom onset reduce the duration of symptoms from illness attributed to more innocuous coronavirus infections (i.e., the common cold) (Mossad et al. 1996; Hemila 2017; Hemila et al. 2020). Also, hydroxychloroquine (HCQ) mobilizes zinc into lysosomes suggesting there may be synergy between HCQ and zinc to amplify efficacy (in vitro data support this synergy in cell culture assays of HCQ-induced cytotoxicity and HCQ-induced inhibition of autophagic flux) (Xue et al. 2014). The WHO database lists one study using zinc sulfate (IRCT20180425039414N2; https://en.irct.ir/trial/47516). It investigates the effect of 220 mg of zinc sulfate on the clinical course of 80 inpatients with COVID-19. Forty patients will receive a combination of zinc sulfate 220 mg with hydroxychloroquine 200 mg twice daily and then once for 5 days, and the other 40 patients will receive only hydroxychloroquine in the same manner in a parallel clinical trial at the Esfahan University of Medical Sciences in Iran (https://en.irct.ir/trial/47516).
The diverse stages of viral replication cycles that are inhibited by zinc. In vitro studies have demonstrated some mechanisms by which zinc interferes with the viral replication cycle. These include free virus inactivation: i, inhibition of viral uncoating; ii, viral genome transcription; iii and iv, viral protein translation and polyprotein processing. No studies to date, however, have demonstrated zinc-mediated inhibition of virus assembly and/or particle release
CV coronavirus, DdDp DNA-dependent DNA polymerase, EMCV encephalomyocarditis virus, FMDV foot and mouth disease virus, HCV hepatitis C virus, HIV human immunodeficiency virus, HPV human papilloma virus, HRV human rhinovirus, HSV herpes simplex virus, PV polio virus, RdRp RNA-dependent RNA polymerase, RT reverse transcriptase, SARS severe acute respiratory syndrome coronavirus, SFV Semliki Forest virus, SV sindbis virus, VZV varicella-zoster virus, Zn zinc
Adapted with permission (Read et al. 2019)
29.3 Conclusion
There is a dearth of surveys to precisely indicate the usage of dietary supplements by the public for COVID-19. However, the dietary supplement industry has reported a global boost in sales during the COVID-19 pandemic as people sought the aid of natural medicines in an attempt to prevent or mitigate COVID-19. There is some evidence to indicate that immune-modulating dietary supplements may play a role in benefiting the public from COVID-19. However, dietary supplements are not without cost or potential harm, albeit low risk in the majority of cases. Any benefit of dietary supplements against COVID-19 depends on biological plausibility, the peer-reviewed literature, not direct studies in humans, preferably RCT. The approach was heuristic and served its place at a time when morbidity and mortality spread across the world in an uncontrolled manner. Ultimately, the many clinical trials underway now that will soon illuminate whether the millions of dollars spent by the public and their actions during the COVID-19 pandemic had any merit. Only time will tell.
References
Alunno A, Carubbi F, Rodriguez-Carrio J (2020) Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open 6(1):e001295. https://doi.org/10.1136/rmdopen-2020-001295
Amrein K, Ribitsch W, Otto R, Worm HC, Stauber RE (2011) Severe lactic acidosis reversed by thiamine within 24 hours. Crit Care 15(6):457. https://doi.org/10.1186/cc10495
Anderson J, Do LAH, Toh ZQ, Hoe E, Reitsma A, Mulholland K, Licciardi PV (2020) Vitamin D induces differential effects on inflammatory responses during bacterial and/or viral stimulation of human peripheral blood mononuclear cells. Front Immunol 11:602. https://doi.org/10.3389/fimmu.2020.00602
Atherton JG, Kratzing CC, Fisher A (1978) The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch Virol 56(3):195–199. https://doi.org/10.1007/bf01317848
Baldassarre ME, Di Mauro A, Labellarte G, Pignatelli M, Fanelli M, Schiavi E, Mastromarino P, Capozza M, Panza R, Laforgia N (2020) Resveratrol plus carboxymethyl-beta-glucan in infants with common cold: a randomized double-blind trial. Heliyon 6:e03814. https://doi.org/10.1016/j.heliyon.2020.e03814
Beard JA, Bearden A, Striker R (2011) Vitamin D and the antiviral state. J Clin Virol 50(3):194–200. https://doi.org/10.1016/j.jcv.2010.12.006
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fatkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, Members A-SG (2020) Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med 383:993. https://doi.org/10.1056/NEJMoa2007764
Bhagavathula AS, Aldhaleei WA, Rovetta A, Rahmani J (2020) Vaccines and drug therapeutics to lock down novel coronavirus disease 2019 (COVID-19): a systematic review of clinical trials. Cureus 12(5):e8342. https://doi.org/10.7759/cureus.8342
Brojakowska A, Narula J, Shimony R, Bander J (2020) Clinical implications of SARS-CoV-2 interaction with renin angiotensin system: JACC review topic of the week. J Am Coll Cardiol 75(24):3085–3095. https://doi.org/10.1016/j.jacc.2020.04.028
Carr AC (2019a) Duration of intravenous vitamin C therapy is a critical consideration. Crit Care Resusc 21(3):220–221
Carr AC (2019b) Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit Care 23(1):265. https://doi.org/10.1186/s13054-019-2538-y
Carr AC (2020) A new clinical trial to test high-dose vitamin C in patients with COVID-19. Crit Care 24(1):133. https://doi.org/10.1186/s13054-020-02851-4
Carr AC, Maggini S (2017) Vitamin C and immune function. Nutrients 9(11):1211. https://doi.org/10.3390/nu9111211
Chen Y, Luo G, Yuan J, Wang Y, Yang X, Wang X, Li G, Liu Z, Zhong N (2014) Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediat Inflamm 2014:426740. https://doi.org/10.1155/2014/426740
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130(5):2620–2629. https://doi.org/10.1172/JCI137244
Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents 34(2):327. https://doi.org/10.23812/CONTI-E
Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V (2020) The possible role of vitamin D in suppressing cytokine storm and associated mortality in COVID-19 patients. BMJ Yale. https://doi.org/10.1101/2020.04.08.20058578
Dhawan P, Wei R, Sun C, Gombart AF, Koeffler HP, Diamond G, Christakos S (2015) C/EBPalpha and the vitamin D receptor cooperate in the regulation of cathelicidin in lung epithelial cells. J Cell Physiol 230(2):464–472. https://doi.org/10.1002/jcp.24729
El-Missiry MA, El-Missiry ZM, Othman AI (2020) Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19. Eur J Pharmacol 882:173329. https://doi.org/10.1016/j.ejphar.2020.173329
Evans JM, Luby R, Lukaczer D, Rountree R, Stone PM, Guilliams TG, Yanuck S, Messier H, Ramsdell K, Hanaway PJ (2020) The functional medicine approach to COVID-19: virus-specific nutraceutical and botanical agents. Integr Med: Clinician’s J 19:34
Fawaz L, Mrad MF, Kazan JM, Sayegh S, Akika R, Khoury SJ (2016) Comparative effect of 25(OH)D3 and 1,25(OH)2D3 on Th17 cell differentiation. Clin Immunol 166–167:59–71. https://doi.org/10.1016/j.clim.2016.02.011
Fink SL, Cookson BT (2005) Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73(4):1907–1916. https://doi.org/10.1128/IAI.73.4.1907-1916.2005
Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2nd, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M (2019) Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 322(13):1261–1270. https://doi.org/10.1001/jama.2019.11825
Fowler AA 3rd, Fisher BJ, Kashiouris MG (2020) Vitamin C for sepsis and acute respiratory failure-reply. JAMA 323(8):792–793. https://doi.org/10.1001/jama.2019.21987
Gammoh NZ, Rink L (2017) Zinc in infection and inflammation. Nutrients 9(6). https://doi.org/10.3390/nu9060624
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Munoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A, Investigators G-U-540-5773 (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 383:1827. https://doi.org/10.1056/NEJMoa2015301
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP (2020) Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 12(4). https://doi.org/10.3390/nu12040988
Greiller CL, Martineau AR (2015) Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 7(6):4240–4270. https://doi.org/10.3390/nu7064240
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res 7(1):1. ARTN 11. https://doi.org/10.1186/s40779-020-00240-0
Hager DN, Hooper MH, Bernard GR, Busse LW, Ely EW, Fowler AA, Gaieski DF, Hall A, Hinson JS, Jackson JC, Kelen GD, Levine M, Lindsell CJ, Malone RE, McGlothlin A, Rothman RE, Viele K, Wright DW, Sevransky JE, Martin GS (2019) The vitamin C, thiamine and steroids in sepsis (VICTAS) protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials 20(1):197. https://doi.org/10.1186/s13063-019-3254-2
Hager DN, Hinson JS, Rothman RE (2020) Vitamin C for sepsis and acute respiratory failure. JAMA 323(8):791–792. https://doi.org/10.1001/jama.2019.21984
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, Jiang Y, Cheng X, Zhu C, Xia Y (2020) Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 9(1):1123–1130. https://doi.org/10.1080/22221751.2020.1770129
Hasanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921. https://doi.org/10.1016/j.phrs.2020.104921
Hassanzadeh S, Read MI, Bland AR, Majeed M, Jamialahmadi T, Sahebkar A (2020) Curcumin: an inflammasome silencer. Pharmacol Res 159:104921
Hemila H (2017) Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open 8(5):2054270417694291. https://doi.org/10.1177/2054270417694291
Hemila H, Chalker E (2013) Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev (1):CD000980. https://doi.org/10.1002/14651858.CD000980.pub4
Hemila H, Chalker E (2019) Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients 11(4). https://doi.org/10.3390/nu11040708
Hemila H, Haukka J, Alho M, Vahtera J, Kivimaki M (2020) Zinc acetate lozenges for the treatment of the common cold: a randomised controlled trial. BMJ Open 10(1):e031662. https://doi.org/10.1136/bmjopen-2019-031662
Holmannova D, Kolackova M, Krejsek J (2012) Vitamin C and its physiological role with respect to the components of the immune system. Vnitr Lek 58(10):743–749
Hooper PL (2020) COVID-19 and heme oxygenase: novel insight into the disease and potential therapies. Cell Stress Chaperones 25:707. https://doi.org/10.1007/s12192-020-01126-9
Horne JR, Vohl MC (2020) Biological plausibility for interactions between dietary fat, resveratrol, ACE2, and SARS-CoV illness severity. Am J Physiol Endocrinol Metab 318(5):E830–E833. https://doi.org/10.1152/ajpendo.00150.2020
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Hwang SY, Park JE, Jo IJ, Kim S, Chung SP, Kong T, Shin J, Lee HJ, You KM, Jo YH, Kim D, Suh GJ, Kim T, Kim WY, Kim YJ, Ryoo SM, Choi SH, Shin TG, Korean Shock Society I (2019) Combination therapy of vitamin C and thiamine for septic shock in a multicentre, double-blind, randomized, controlled study (ATESS): study protocol for a randomized controlled trial. Trials 20(1):420. https://doi.org/10.1186/s13063-019-3542-x
Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, Bohn T (2020) Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients 12(6):1562. https://doi.org/10.3390/nu12061562
Jahan S, Kumar D, Chaturvedi S, Rashid M, Wahajuddin M, A Khan Y, N Goyal S, R Patil C, Mohanraj R, Subramanya S (2017a) Therapeutic targeting of NLRP3 inflammasomes by natural products and pharmaceuticals: a novel mechanistic approach for inflammatory diseases. Curr Med Chem 24(16):1645–1670
Jahan S, Kumar D, Chaturvedi S, Rashid M, Wahajuddin M, Khan YA, Goyal SN, Patil CR, Mohanraj R, Subramanya S, Ojha S (2017b) Therapeutic targeting of NLRP3 inflammasomes by natural products and pharmaceuticals: a novel mechanistic approach for inflammatory diseases. Curr Med Chem 24(16):1645–1670. https://doi.org/10.2174/0929867324666170227121619
Jin D, Wu S, Zhang YG, Lu R, Xia Y, Dong H, Sun J (2015) Lack of vitamin D receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin Ther 37(5):996–1009 e1007. https://doi.org/10.1016/j.clinthera.2015.04.004
Joshi T, Joshi T, Sharma P, Mathpal S, Pundir H, Bhatt V, Chandra S (2020) In silico screening of natural compounds against COVID-19 by targeting Mpro and ACE2 using molecular docking. Eur Rev Med Pharmacol Sci 24(8):4529–4536. https://doi.org/10.26355/eurrev_202004_21036
Khan M, Masoud MS, Qasim M, Khan MA, Zubair M, Idrees S, Ashraf A, Ashfaq UA (2013) Molecular screening of phytochemicals from Amelanchier Alnifolia against HCV NS3 protease/helicase using computational docking techniques. Bioinformation 9(19):978–982. https://doi.org/10.6026/97320630009978
Kim WY, Jo EJ, Eom JS, Mok J, Kim MH, Kim KU, Park HK, Lee MK, Lee K (2018) Combined vitamin C, hydrocortisone, and thiamine therapy for patients with severe pneumonia who were admitted to the intensive care unit: propensity score-based analysis of a before-after cohort study. J Crit Care 47:211–218. https://doi.org/10.1016/j.jcrc.2018.07.004
Kim WY, Jung JW, Choi JC, Shin JW, Kim JY (2019) Subphenotypes in patients with septic shock receiving vitamin C, hydrocortisone, and thiamine: a retrospective cohort analysis. Nutrients 11(12):2976. https://doi.org/10.3390/nu11122976
Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, Palaiodimou L, Kokkinos A, Lambadiari V (2020) Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab 319(1):E105–E109. https://doi.org/10.1152/ajpendo.00198.2020
Lee IC, Huo TI, Huang YH (2020) Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc 83:521. https://doi.org/10.1097/JCMA.0000000000000319
Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC (2017) Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis 17(1):144. https://doi.org/10.1186/s12879-017-2253-8
Mahase E (2020) Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ-Brit Med J 368:m1036. ARTN m1036. https://doi.org/10.1136/bmj.m1036
Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA Jr (2017) Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356:i6583. https://doi.org/10.1136/bmj.i6583
Miraglia Del Giudice M, Indolfi C, Strisciuglio C (2018) Vitamin D: immunomodulatory aspects. J Clin Gastroenterol 52 Suppl 1, Proceedings from the 9th Probiotics, Prebiotics and New Foods, Nutraceuticals and Botanicals for Nutrition & Human and Microbiota Health Meeting, held in Rome, Italy from September 10–12, 2017:S86–S88. https://doi.org/10.1097/MCG.0000000000001112
Mishra AK, Upadhyay R, Chaurasia JK, Tiwari KN (2016) Comparative antioxidant study in different flower extracts of Nyctanthes arbor-tristis (L.) (Oleaceae): an important medicinal plant. Braz J Bot 39(3):813–820. https://doi.org/10.1007/s40415-016-0283-x
Mossad SB, Macknin ML, Medendorp SV, Mason P (1996) Zinc gluconate lozenges for treating the common cold. A randomized, double-blind, placebo-controlled study. Ann Intern Med 125(2):81–88. https://doi.org/10.7326/0003-4819-125-2-199607150-00001
Olcum M, Tastan B, Ercan I, Eltutan IB, Genc S (2020) Inhibitory effects of phytochemicals on NLRP3 inflammasome activation: a review. Phytomedicine 75:153238. https://doi.org/10.1016/j.phymed.2020.153238
Paramo JA (2020) Inflammatory response in relation to COVID-19 and other prothrombotic phenotypes. Reumatol Clin. https://doi.org/10.1016/j.reuma.2020.06.004
Parisi V, Leosco D (2020) Precision medicine in COVID-19: IL-1beta a potential target. JACC Basic Transl Sci 5:543. https://doi.org/10.1016/j.jacbts.2020.04.006
Park M, Cook AR, Lim JT, Sun Y, Dickens BL (2020) A systematic review of COVID-19 epidemiology based on current evidence. J Clin Med 9(4):967. https://doi.org/10.3390/jcm9040967
Qi Y, Shang L, Liao Z, Su H, Jing H, Wu B, Bi K, Jia Y (2019) Intracerebroventricular injection of resveratrol ameliorated Abeta-induced learning and cognitive decline in mice. Metab Brain Dis 34(1):257–266. https://doi.org/10.1007/s11011-018-0348-6
Quraishi SA, De Pascale G, Needleman JS, Nakazawa H, Kaneki M, Bajwa EK, Camargo CA Jr, Bhan I (2015) Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: a randomized, placebo-controlled trial. Crit Care Med 43(9):1928–1937. https://doi.org/10.1097/CCM.0000000000001148
Rasool N, Akhtar A, Hussain W (2020) Insights into the inhibitory potential of selective phytochemicals against Mpro of 2019-nCoV: a computer-aided study. Struct Chem:1–7. https://doi.org/10.1007/s11224-020-01536-6
Read SA, Obeid S, Ahlenstiel C, Ahlenstiel G (2019) The role of zinc in antiviral immunity. Adv Nutr 10(4):696–710. https://doi.org/10.1093/advances/nmz013
Reiter RJ, Abreu-Gonzalez P, Marik PE, Dominguez-Rodriguez A (2020) Therapeutic algorithm for use of melatonin in patients with COVID-19. Front Med (Lausanne) 7:226. https://doi.org/10.3389/fmed.2020.00226
Sandhu MS, Casale TB (2010) The role of vitamin D in asthma. Ann Allergy Asthma Immunol 105(3):191–199.; quiz 200-192, 217. https://doi.org/10.1016/j.anai.2010.01.013
Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F (2020) Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 117(21):11727–11734. https://doi.org/10.1073/pnas.2003138117
Shin TG, Kim YJ, Ryoo SM, Hwang SY, Jo IJ, Chung SP, Choi SH, Suh GJ, Kim WY (2019) Early vitamin C and thiamine administration to patients with septic shock in emergency departments: propensity score-based analysis of a before-and-after cohort study. J Clin Med 8(1):102. https://doi.org/10.3390/jcm8010102
Siddiqi HK, Mehra M (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39:405. https://doi.org/10.1016/j.healun.2020.03.012
Sui DM, Xie Q, Yi WJ, Gupta S, Yu XY, Li JB, Wang J, Wang JF, Deng XM (2016) Resveratrol protects against sepsis-associated encephalopathy and inhibits the NLRP3/IL-1beta axis in microglia. Mediat Inflamm 2016:1045657. https://doi.org/10.1155/2016/1045657
te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ (2010) Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 6(11):e1001176. https://doi.org/10.1371/journal.ppat.1001176
Teymoori-Rad M, Shokri F, Salimi V, Marashi SM (2019) The interplay between vitamin D and viral infections. Rev Med Virol 29(2):e2032. https://doi.org/10.1002/rmv.2032
Turski WA, Wnorowski A, Turski GN, Turski CA, Turski L (2020) AhR and IDO1 in pathogenesis of Covid-19 and the “systemic AhR activation syndrome” translational review and therapeutic perspectives. Restor Neurol Neurosci 38:343. https://doi.org/10.3233/RNN-201042
Urwyler P, Boesing M, Abig K, Cattaneo M, Dieterle T, Zeller A, Bachler H, Markun S, Senn O, Merlo C, Essig S, Ullmer E, Rutishauser J, Schuurmans MM, Leuppi JD (2019) Reduction of corticosteroid use in outpatient treatment of exacerbated COPD – study protocol for a randomized, double-blind, non-inferiority study, (the RECUT-trial). Trials 20(1):727. https://doi.org/10.1186/s13063-019-3856-8
van den Berg DF, te Velde AA (2020) Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol 11:1580
Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181(2):281–292.e286. https://doi.org/10.1016/j.cell.2020.02.058
Wang D, Gao Q, Wang T, Kan Z, Li X, Hu L, Peng CY, Qian F, Wang Y, Granato D (2020a) Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res Int 127:108628. https://doi.org/10.1016/j.foodres.2019.108628
Wang F, Hou H, Luo Y, Tang G, Wu S, Huang M, Liu W, Zhu Y, Lin Q, Mao L, Fang M, Zhang H, Sun Z (2020b) The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight 5(10):e137799. https://doi.org/10.1172/jci.insight.137799
Wang Y, Wang Y, Chen Y, Qin Q (2020c) Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 92:568. https://doi.org/10.1002/jmv.25748
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C (2020d) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 395(10236):1569–1578. https://doi.org/10.1016/S0140-6736(20)31022-9
Wei R, Christakos S (2015) Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 7(10):8251–8260. https://doi.org/10.3390/nu7105392
Weir EK, Thenappan T, Bhargava M, Chen Y (2020) Does vitamin D deficiency increase the severity of COVID-19? Clin Med (Lond) 20:E107. https://doi.org/10.7861/clinmed.2020-0301
Wu D, Yang XO (2020) TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect 53(3):368–370. https://doi.org/10.1016/j.jmii.2020.03.005
Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ (2014) Chloroquine is a zinc ionophore. PLoS One 9(10):e109180. https://doi.org/10.1371/journal.pone.0109180
Zdrenghea MT, Makrinioti H, Bagacean C, Bush A, Johnston SL, Stanciu LA (2017) Vitamin D modulation of innate immune responses to respiratory viral infections. Rev Med Virol 27(1):e1909. https://doi.org/10.1002/rmv.1909
Zhang J, Lu X, Liu M, Fan H, Zheng H, Zhang S, Rahman N, Wolczynski S, Kretowski A, Li X (2019) Melatonin inhibits inflammasome-associated activation of endothelium and macrophages attenuating pulmonary arterial hypertension. Cardiovasc Res 116:2156. https://doi.org/10.1093/cvr/cvz312
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Acknowledgments
The authors would like to thank the members of the Institute for Functional Medicine Task Force (Drs. Joel Evans, Dan Lukaczer, Robert Luby, and Patrick Hanaway) and Dr. Keith Berkowitz for inspiring this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mullin, G.E., Limektkai, B., Wang, L., Hanaway, P., Marks, L., Giovannucci, E. (2021). Dietary Supplements for COVID-19. In: Rezaei, N. (eds) Coronavirus Disease - COVID-19. Advances in Experimental Medicine and Biology, vol 1318. Springer, Cham. https://doi.org/10.1007/978-3-030-63761-3_29
Download citation
DOI: https://doi.org/10.1007/978-3-030-63761-3_29
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63760-6
Online ISBN: 978-3-030-63761-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)