Abstract
Coronavirus disease 2019 (COVID-19) pandemic continues devastating effects on healthcare systems. Such a crisis calls for an urgent need to develop a risk stratification tool. The present chapter aimed to identify laboratory and clinical correlates of adverse outcomes in patients with COVID-19. To this end, we conducted a systematic evaluation of studies that investigated laboratory abnormalities in patients with COVID-19 and compared i. patients with a severe form of disease and patients with a non-severe form of the disease, ii. patients who were in critical condition and patients who were not in critical condition, and iii. patients who survived and patients who died. We included 54 studies in the data synthesis. Compared to patients with a non-severe form of COVID-19, patients who had a severe form of disease revealed higher values for white blood cells (WBC), polymorphonuclear leukocytes (PMN), total bilirubin, alanine aminotransferase (ALT), creatinine, troponin, procalcitonin, lactate dehydrogenase (LDH), and D-dimer. By contrast, platelet count, lymphocyte count, and albumin levels were decreased in patients with a severe form of COVID-19. Also, patients with a severe phenotype of disease were more likely to have diabetes, chronic heart disease, chronic obstructive pulmonary disease (COPD), cerebrovascular disease, hypertension, chronic kidney disease (CKD), and malignancy. Compared to patients who survived, patients who died had higher WBC, PMN, total bilirubin, ALT, procalcitonin, IL-6, creatinine, PT, lymphocyte count, platelet count, and albumin. Also, non-survivors revealed a higher prevalence of diabetes, chronic heart disease, COPD, cerebrovascular disease, and CKD. Meta-analyses identified several laboratory parameters that might help the prediction of severe, critical, and lethal phenotypes of COVID-19. These parameters correlate with the immune system function, inflammation, coagulation, and liver and kidney function.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biomarker
- Clinical
- COVID-19
- D-dimer
- Diagnostic
- Immune system
- Inflammation
- Interleukin 6
- Laboratory
- Mortality
- Prognostic
- Severity
- Systematic review
- Meta-analysis
22.1 Introduction
In December 2019, pneumonia cases with unknown etiology emerged in Wuhan, China (Lu et al.). Lower respiratory tract sampling and sequencing process could reveal a novel coronavirus from the family of betacoronavirus, which was subsequently named 2019 novel coronavirus (2019-nCoV) (Zhu et al. 2020). This family also included severe acute respiratory syndrome (SARS) and the Middle East respiratory syndrome (MERS), which have caused outbreaks over the last two decades (Ksiazek et al. 2003; de Wit et al. 2016; Jabbari et al. 2020). Coronavirus disease 2019 (COVID-19) has rapidly spread throughout the world, posing a global emergency (Hanaei and Rezaei 2020; Rezaei 2020a).
The Chinese Centers for Disease Control and Prevention (CDC) has suggested classifying COVID-19 patients according to their clinical state into three categories. Mild disease manifestations include no pneumonia or mild pneumonia and occur in 81% of cases. Severe disease characterized by dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation (SpO2) ≤ 93%, PaO2/FiO2 ratio < 300, and/or lung infiltrates >50% within 24–48 h is present in 14% of cases. Critical conditions accompanied by respiratory failure, septic shock, and/or multiple organ dysfunction or failure occur in 5% of cases (The Novel Coronavirus Pneumonia Emergency Response Epidemiology 2020). While most patients experience mild respiratory illness, some cases, especially the old population and patients with comorbid conditions, develop a severe form of disease requiring intensive management (Guan et al. 2020b; Lotfi and Rezaei 2020). Chinese CDC has reported an overall case fatality rate of 2.3%, with the majority of deceased patients being 60 years old and above and having preexisting comorbid conditions such as hypertension, cardiovascular disease, and diabetes (The Novel Coronavirus Pneumonia Emergency Response Epidemiology 2020; Shamshirian and Rezaei 2020). Accordingly, the resistance and susceptibility to disease are governed by genetic factors and nongenetic factors such as biological age, pregnancy, and chronic conditions, which affect the expression of the receptors which mediate virus entry, i.e., angiotensin-converting enzyme 2 (ACE2), and the function of the immune system (Yousefzadegan and Rezaei 2020; Ahmadi et al. 2020; Ahanchian et al. 2020; Darbeheshti and Rezaei 2020; Babaha and Rezaei 2020; Mirbeyk and Rezaei 2020).
With the increasing number of people infected with the virus, the burden of COVID-19 on healthcare systems has been overwhelming. Thus, early and efficient detection of high-risk patients is a priority for maximizing the utilization of limited resources, while current diagnostics are not as rapid and as reliable as the current pandemic condition needs (Basiri et al. 2020a). Furthermore, no clinically proven specific antiviral agent has been confirmed for COVID-19. However, approaches targeting the immune system and virus entry, computational methods of drug discovery, regenerative medicine, medical biotechnology, and picotechnology offer hope for the future amid the pandemic (Mohamed et al. 2020b; Rezaei 2020b; Rabiee et al. 2020; Basiri et al. 2020b; Sharifkashani et al. 2020; Lotfi et al. 2020; Mansourabadi et al. 2020; Pashaei and Rezaei 2020; Fathi and Rezaei 2020; Jahanshahlu and Rezaei 2020b). Thus supportive management, including oxygen therapy and conservative fluid management, along with anti-inflammatory and antiviral agents, remains to be the most important management strategy (Cascella et al. 2020; Saghazadeh and Rezaei 2020b). Dynamic changes of laboratory parameters, as well as clinical and imaging findings of patients infected with COVID-19, have been depicted in the literature (Zhou et al. 2020a; Liu et al. 2020a). Results are, however, not consistent. The present chapter aims to identify laboratory and clinical correlates of adverse outcomes in patients with COVID-19. For this purpose, a systematic evaluation of literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
22.2 Methods
22.2.1 Search Strategy
The published literature was searched from December 2019 to April 20, 2020. Databases including EMBASE, MEDLINE via PubMed, and Scopus were searched for observational studies of COVID-19. The keywords for search strategy included “Wuhan seafood market pneumonia virus,” “COVID19,” “2019-nCoV,” “novel coronavirus,” “coronavirus disease-19,” “severe acute respiratory syndrome coronavirus 2,” “SARS-CoV-2,” “characteristic,” “finding,” “lab,” “imaging,” “comorbidit*,” “sever*,” “critical,” “hospital*,” “ICU,” “non-ICU,” “death,” “mortality,” and “surviv*.” Detailed search strategies are presented in Table 22.1.
22.2.2 Study Selection
The studies identified were imported into an EndNote library. After duplicate removal, the two groups (three reviewers in each group) performed the screening titles and abstracts for inclusion and then reviewing the full text of potentially relevant articles. Any questions or conflicts were resolved through discussion with the members of the other group. Concerning studies with overlapping samples, the study that had the largest sample size was included in the final analysis. Studies that i. included subjects with confirmed COVID-19 based on laboratory testing; ii. evaluated individual laboratory characteristics, symptoms, and comorbidities in predicting severe COVID-19 infection or mortality caused by COVID-19 infection; and iii. were written in English were eligible to be included in the systematic review. If the criteria for disease severity were not determined, then the study was excluded (Table 22.2). Also, types of articles other than original observational studies, e.g., opinion, editorial, case report, book chapter, review, and meta-analysis, studies confined to the pediatric population or pregnant women, and studies rated as low-quality evidence were not included.
22.2.3 Data Extraction and Quality Assessment
We used the Newcastle-Ottawa scale (NOS) and its modified version for the assessment of the quality of retrospective cohort studies and cross-sectional studies. This scale gives the maximum score of 9 points for the least risk of bias in four domains: the selection of study groups (4 points), comparability of groups (2 points), and ascertainment of exposure and outcomes (3 points). Age and sex were defined as the most relevant covariate for comparability. For the cross-sectional studies, we used the modified NOS, which gives a maximum of 10 scores for the least risk of bias in three domains: the selection of study groups (5 points), comparability of groups (2 points), and ascertainment of exposure and outcomes (3 points).
Three authors independently extracted the data by a predesigned spreadsheet in Excel, including the author’s name, publication year, setting (country/hospital name), study design, sample size, clinical and laboratory and comorbidities, and severity criteria.
22.2.4 Data Synthesis
All between-group meta-analyses were performed using Comprehensive Meta-Analysis. For the continuous type of outcome, we entered the number of participants in two groups and the mean and standard deviation (SD) of laboratory data. We used methods described in Luo et al. (2018) and Wan et al. (2014) to estimate mean and SD based on median (IQR). A meta-analysis was conducted for data on laboratory and clinical characteristics, comorbidities, and vital status when there were at least three between-group comparisons for each variable. Because studies used different measurement scales, we employed the standardized mean difference (SMD) as effect size (ES) measure. For categorical data, we used the dichotomous method to calculate the odds ratio (OR) with its 95% confidence interval as ES measure. As explained by Higgins and Green et al., the ES of 0.2, 0.5, and 0.8 represent small, moderate, and large effect estimates, respectively (Higgins and Wells 2011).
We investigated heterogeneity across studies by Cochran’s Q test, which is calculated by the weighted sum of squared differences between individual study ES estimates. A P-value of 0.10 or less indicates the presence of heterogeneity. The I2 index was used to get a more precise estimate of heterogeneity. If the I2 is less than 40%, the heterogeneity is considered to be not significant. The fixed-effects model is the preferred method of meta-analysis. However, if I2 estimates exceeded 40%, the random-effects approach was applied as the meta-analysis model. We based our analysis on the different outcome stratification including critical/ICU vs noncritical/non-ICU, severe/critical vs non-severe, and survivors vs non-survivors. Subgroup analysis was performed to explore the potential sources for heterogeneity. We also applied univariate meta-regression analyses to investigate the effect of potential moderators, e.g., sample size, the difference in the mean age of two groups (years), and percentage of female patients on the effect size and heterogeneity. Finally, to identify studies exerting excessive influence on the results or causing high heterogeneity among studies, we conducted a sensitivity analysis by systematically excluding each study at a time and then rerunning the analysis to assess the change in ES.
We assessed publication bias visually by the degree of funnel plot asymmetry and used Begg-Mazumdar Kendall’s tau (Begg and Mazumdar 1994) and Egger bias test to quantify it (Duval and Tweedie 2000). A P-value of 0.10 or less suggests the presence of publication bias across studies. The trim and fill method was used to adjust effect sizes in cases that there was evidence of publication bias (Duval and Tweedie 2000).
22.3 Results
22.3.1 Characteristics of Selected Studies
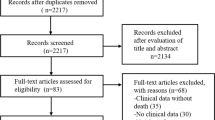
The database search resulted in 461 records. After the removal of duplicates (n = 60) and retrieval of additional 14 studies through hand search of additional references, the title/abstract of 415 search results was screened for potential eligibility. Three hundred forty papers were excluded through screening. Seventy-five full-text articles were reviewed in detail, and 32 met the inclusion criteria. Moreover, an updated search was conducted and resulted in the inclusion of 22 studies. In total, 54 studies were included in the meta-analysis. Figure 22.1 provides an overview of study selection for systematic review and meta-analysis according to PRISMA guidelines.
22.3.2 Study and Patient Characteristics
As summarized in Table 22.3, all included studies were from China, except two studies, which were from Singapore (Young et al. 2020; Fan et al. 2020). All studies were designed as retrospective cohort studies except two with a prospective cohort design (Liu et al. 2020b; Momtazmanesh et al. 2020) and one which was a cross-sectional study (Zhang et al. 2020b). Tables 22.4 and 22.5 show the results of the quality assessment for studies included in the data synthesis.
There were 16 studies that compared critical or severe vs non-severe patients (Zhao et al. 2020; Zhang et al. 2020a; Guan et al. 2020b; Li et al. 2020a; Zhou et al. 2020b; Cai et al.; Tian et al. 2020; Liu et al. 2020b, d; Wu et al. 2020; Feng et al. 2020c; Wan et al. , 2020; Xu et al. 2020; ; Han et al. 2020b; Wang et al. 2020d); 3 studies compared 3 groups of patients, namely, critical, severe, and non-severe (Feng et al. 2020b; Liu et al. 2020c; Han et al. 2020a); 20 studies compared severe vs non-severe patients (Qu et al. 2020; Zhang et al. 2020b, c; Wang et al. 2020e; Cao 2020; Gao et al. 2020; Chen et al. 2020a, e; Liu et al. 2020a; lei and Jian-ya 2020; Lu et al. 2020; Chu et al. 2020; Ji et al. 2020; Xie et al. 2020; Zheng et al. 2020a; c; Li et al. 2020c; Gong et al. 2020; Young et al. 2020; Qin et al. 2020; Nie et al. 2020); 6 studies compared critical/ICU vs noncritical/non-ICU patients (Wang et al. 2020a; Fan et al. 2020; Du et al. 2020; Yan et al. 2020; Huang et al. 2020; Chen et al. 2020b); and 9 studies compared survivor vs non-survivor patients (Tang et al. 2020; Yang et al. 2020; Zhou et al. 2020a; Wu et al. 2020; Momtazmanesh et al. 2020; Chen et al. 2020c, d; Li et al. 2020c; Wang et al. 2020c; Cao et al. 2020). Two studies were not entered into analysis because of the shared samples (Chen et al. 2020c; Huang et al. 2020). A total of 8603 patients were included in the meta-analysis. Tables 22.6, 22.7, 22.8, 22.9, 22.10, 22.11, and 22.12 provide a summary of the results of meta-analyses, subgroup analyses, and meta-regressions.
22.3.3 Laboratory Findings
22.3.3.1 Severity Analysis
Twenty-seven studies provided data on white blood cell (WBC) values for patients with severe (n = 1131) and non-severe (n = 3293) COVID-19. Meta-analysis showed that patients with non-severe outcome had lower WBC compared to patients with severe COVID-19 (SMD −0.40; 95% CI, −0.58, −0.21; P < 0.0001). There was no evidence of publication bias (Begg’s P = 0.77; Egger’s P = 0.10). Significant heterogeneity existed across studies (I2 = 84%), and therefore, the random-effects model was applied. Meta-regression analyses demonstrated no significant effect of percentages of female patients, the difference in the mean age, and sample size on the heterogeneity. Neither subgroup meta-analyses nor sensitivity analysis showed a significant effect of specific outcome or study on the effect size and related heterogeneity. Meta-analysis of data on four studies showed that critical COVID-19 patients (n = 131) had higher WBC than noncritical COVID-19 patients (n = 348) (SMD −0.58; 95% CI, −0.79, −0.37; P < 0.0001). There was no significant heterogeneity and evidence of publication bias as well.
Pooled data from 22 studies, including 2215 non-severe and 1109 severe COVID-19 patients, demonstrated that polymorphonuclear (PMN) cell counts were increased in severe compared to non-severe patients (SMD −0.72; 95% CI, −0.88, −0.57; P < 0.0001). There was significant heterogeneity among studies (I2 = 64%). No evidence of publication bias was observed (Begg’s P = 0.498; Egger’s P = 0.496). Meta-analysis for data derived from four studies comparing critical and noncritical COVID-19 patients showed a similar result with the SMD of −0.74 (95% CI, −1.10 to −0.39; P < 0.0001). Critical patients had higher PMN compared to the noncritical group.
Patients with severe outcomes (n = 1890) had lower lymphocyte count compared to non-severe patients (n = 4035) (SMD 0.68; 95% CI, 0.53, 0.84; P < 0.0001). Heterogeneity was high (I2 = 80%). Also, critical patients had lower lymphocyte counts than noncritical patients.
Patients with severe disease (n = 2288) had significantly lower platelets than non-severe cases (n = 627) with an SMD of 0.21 (95% CI, 0.06, 0.35; P = 0.004). Moderate heterogeneity was present (I2 = 50.5%). No evidence of publication bias was observed (Begg’s P = 0.707; Egger’s P = 0.241).
Eight studies documented CD4 counts for 506 non-severe and 587 severe patients. Because of evidence of publication bias (Begg’s P = 0.009), the ES was adjusted using trim and fill method, resulting in an ES of 0.80 (95% CI, −0.06, 1.62), which showed no significant difference between severe and non-severe patients in regard to CD4 counts. Heterogeneity was high (I2 = 96%).
Total bilirubin (TB) was investigated in 15 studies, with a total of 1528 non-severe and 650 severe individuals. Severe patients had higher TB compared to the non-severe group (SMD −0.33; 95% CI, −0.46, −0.21, P < 0.0001), and no substantial heterogeneity was seen (I2 = 31%).
Alanine transaminase (ALT) was documented in 23 studies accounting for a total of 1875 non-severe and 775 severe patients. ALT levels were higher in severe patients than non-severe patients (SMD −0.52; 95% CI, −0.77, −0.27; P < 0.0001). There was evidence of publication bias (Begg’s P = 0.009). The ES was adjusted using the trim and fill method and estimated to be about −0.56 with 95% CI, −0.79, −0.32. There was high heterogeneity across studies (I2 = 85.5%). A sensitivity analysis showed that I2 dropped to 49% with the associated ES of −0.36 (95% CI, −0.50, −0.22) when the study (Zheng et al. 2020a) was excluded. Similarly, AST levels were higher in severe patients compared to non-severe patients (SMD −0.79; 95% CI, −1.00, −0.58; P < 0.0001). Heterogeneity was high (I2 = 74.5%). Critical patients had higher AST and ALT levels compared to noncritical patients (SMD −0.78; 95% CI, −1.27, −0.29; and SMD −0.45; 95% CI, −0.68, −0.22), respectively.
Creatinine was increased in severe patients (n = 751) compared to non-severe patients (n = 2320) (SMD −0.21; 95% CI, −0.34, −0.08; P = 0.001). No evidence of publication bias was found (Begg’s P = 0.785; Egger’s P = 0.282). Moderate heterogeneity was seen (I2 = 52%). Furthermore, BUN was higher in severe patients than non-severe patients (SMD −0.55; 95% CI, −0.93, −0.17; P < 0.0001).
Severe patients showed no difference in potassium levels compared with non-severe patients (SMD = 0.18, p = 0.269). However, the exclusion of a study (Feng et al. 2020b) could resolve heterogeneity (I2 = 0.0), and the ES became significant (SMD 0.25; 95% CI, 0.12, 0.39; P < 0.0001). Sodium levels were not different between severe and non-severe patients, and sensitivity analysis showed no significant effect of any individual study.
Troponin I (TnI) was higher in severe patients (n = 199) than non-severe patients (n = 585) (SMD −0.98; 95% CI, −1.18, −0.77). Thirteen studies reported data on albumin levels, including 868 severe patients and 1719 non-severe patients. Severe patients had lower albumin levels (SMD 1.02; 95% CI, 0.81, 1.23; P < 0.0001). Also, severe (n = 1170) patients had higher procalcitonin levels compared to non-severe patients (n = 707) (SMD −0.81; 95% CI, −1.07, −0.55; P < 0.0001).
A meta-analysis of 11 studies providing data on prothrombin time (PT) showed that patients with severe disease were not different from non-severe patients (SMD −0.30; 95% CI, −0.64, 0.03; P = 0.074). Also, PTT showed no significant difference between the two groups (SMD = 0.05; 95% CI, −0.29 to 0.39; P = 0.772). Critical patients had higher PT compared to noncritical patients (SMD −0.27; 95% CI, −0.50, −0.46, P = 0.019), though PTT was not different between two critical and noncritical groups (SMD 0.11; 95% CI, −0.12, 0.34; P = 0.347).
Seventeen studies reported data on LDH levels, and the meta-analysis revealed that severe patients had higher LDH levels than non-severe patients (SMD −1.33; 95% CI, −1.54, −1.17; P < 0.0001). There was moderate heterogeneity (I2 = 68.41). Also, severe patients had significantly higher D-dimer levels compared to non-severe patients (SMD −0.93; 95% CI, −1.12, −0.74; P < 0.0001). Heterogeneity among studies was relatively high (I2 = 72.77%), but meta-regression analysis could not find any significant potential moderators.
22.3.3.2 Survival Analysis
A total of five studies reported the white blood cell count concerning survival outcomes. Non-survivor patients (n = 707) had significantly higher WBC than survivors (n = 365) with an ES of −1.01 (95% CI, −1.33, −0.69). No evidence of publication bias was observed (Begg’s P = 0.327; Egger’s P = 0.356). Heterogeneity was high (I2 = 79%). Sensitivity analysis was performed, leaving out a study that only included patients with ARDS (acute respiratory syndrome) (Wu et al. 2020), and as a result, heterogeneity was abolished, and ES became −1.18 (95% CI, −1.33, −1.03).
Analysis of four studies with a total number of 570 survivors and 311 non-survivors revealed significantly higher PMN counts in non-survivor patients compared to survivors (SMD −1.05; 95% CI, −1.53, −0.58; P < 0.0001). Heterogeneity was high (I2 = 87.76%). When removing the abovementioned study (Wu et al. 2020), heterogeneity dropped to 55%, and the ES remained significant −1.32 (SMD −1.32; 95% CI, −1.59, −1.06).
Patients who survived (n = 727) had significantly higher lymphocyte than non-survivors (n = 397) (SMD 0.97; 95% CI, 0.52, 1.42). Significant publication bias was observed (Begg’s P = 0.024 Egger’s P = 0.043). The trim and fill method was used to adjust the ES (SMD 1.20; 95% CI, 1.06, 1.34). No individual study showed a significant influence on the ES.
Compared to non-survivors (n = 376), survivors had significantly higher platelet counts (SMD 0.45; 95% CI, 0.19, 0.70; P = 0.001). Heterogeneity was relatively high (I2 = 67.56%). When the study (Yang et al. 2020) was removed, there was no heterogeneity across studies further (I2 = 0%), while the pooled effect remained significant.
Non-survivor patients showed higher ALT (SMD −0.36, P < 0.0001) and total bilirubin (SMD = −0.79, p < 0.0001) than survivors. Albumin levels were measured in five studies. Survivor patients showed higher albumin levels compared to non-survivors (SMD = 0.88, p < 0.0001). However, heterogeneity was high (I2 = 87.95). Sensitivity analysis showed a robust ES. Additionally, with the exclusion of two studies (Wu et al. 2020; Momtazmanesh et al. 2020), I2 reduced to 58.41% (Wu et al. 2020; Momtazmanesh et al. 2020).
IL-6 (SMD −1.22; P < 0.0001) and procalcitonin (SMD −1.05; P < 0.0001) levels were significantly higher in non-survivors than survivors. For both analyses, there was no evidence of publication bias and significant heterogeneity.
A meta-analysis of data from five studies showed no difference between non-survivors (n = 343) and survivors (n = 590) regarding creatinine levels (SMD −0.34; 95% CI, −0.77, 0.09; P = 0.123). However, there was high heterogeneity (I2 = 87.55). Sensitivity analysis excluding two studies reduced heterogeneity to I2 = 0%, though the ES remained not significant (SMD 0.016; 95% CI, −0.18, 0.21; P = 0.876).
PT was significantly higher in non-survivors than survivors (SMD −0.78; 95% CI, −1.89, −0.37; P < 0.0001). However, there was no difference regarding PTT between survivors and non-survivors (SMD 0.26; 95% CI, −0.62, 0.41; P = 0.695).
22.3.4 Symptoms and Vital Status
22.3.4.1 Severity Analysis
Compared to severe patients, non-severe patients were less likely to have chills (OR 0.62; 95% CI, 0.42, 0.93), sputum production (OR 0.66; 95% CI, 0.54 to 0.79), dyspnea (OR 0.22; 95% CI, 0.14, 0.33), hemoptysis (OR 0.25; 95% CI, 0.11, 0.55), fatigue (OR 0.71; 95% CI, 0.52, 0.97), and abdominal pain (OR 0.49; 95% CI, 0.24, 0.98). Patients with severe outcomes had significantly higher fever than non-severe patients (SMD −0.38; 95% CI, −0.67, −0.09; P = 0.01).
22.3.4.2 Survival Analysis
The only symptoms that showed a difference between survivors and non-survivors were dyspnea and fatigue, which were less likely to occur in survivors (OR 0.34; 95% CI 0.25, 0.46; and OR 0.75; 95% CI 0.57, 0.90).
22.3.5 Comorbidities and Demographic Characteristics
22.3.5.1 Severity Analysis
Severe patients (n = 4006) were significantly older than non-severe patients (n = 1493) (SMD −0.59; 95% CI, −0.76, −0.41). A meta-analysis of data derived from nine studies showed that non-severe patients were less likely to be smokers than severe patients (OR 0.53; 95% CI, 0.38, 0.73). Male patients had a higher risk of developing the severe disease compared to females (OR 1.32; 95% CI, 1.17, 1.49). Non-severe patients were less likely to have diabetes (OR 0.36; 95% CI, 0.26 to 0.52), chronic heart disease (OR 0.34; 95% CI, 0.25, 0.45), COPD (OR 0.20; 95% CI, 0.12, 0.33), cerebrovascular disease (OR 0.30; 95% CI, 0.18, 0.50), hypertension (OR 0.40; 95% CI, 0.30, 0.52), CKD (OR 0.35; 95% CI, 0.18, 0.68), and malignancy (OR 0.42; 95% CI, 0.24, 0.72) than severe patients. For all analyses, there was low to moderate heterogeneity, with I2 ranging from 0 to 47%.
22.3.5.2 Survival Analysis
A meta-analysis of data from eight studies demonstrated that non-survivors (n = 435) were older than survivors (n = 974) with the SMD of −1.07 (95% CI, −1.35, −0.78; P < 0.0001). Male patients were not different from females concerning survival (p = 0.066; I2 = 51.46).
Patients who survived were less likely to have diabetes (OR 0.49, 0.36 to 0.67), chronic heart disease (OR 0.31; 95% CI, 0.13–0.72), COPD (OR 0.13; 95% CI, 0.052–0.34), cerebrovascular disease (OR 0.125; 95% CI, 0.034–0.46), and CKD (OR 0.095; 95% CI, 0.023–0.39). For all analyses, there was low to moderate heterogeneity, with I2 ranging from 0 to 61%.
22.4 Discussion
Hundreds of thousands of deaths due to COVID-19 during only a few months have placed a heavy burden on the shoulder of each medical researcher by explicitly proving that our knowledge, at the present condition of the pandemic, can seldom offer a useful product to humanity unless all data are collected from other disciplines and then it would be entirely fair to arrive at conclusions from the accumulation of this data (Momtazmanesh et al. 2020; Mohamed et al. 2020a; Rzymski et al. 2020; Moradian et al. 2020; Kafieh et al. 2020). For example, medical doctors initially described COVID-19 as a disease of the respiratory system. It did not take long when a wave of studies reporting anosmia in patients with COVID-19 emerged, putting forward the neurotropism of COVID-19 (Yazdanpanah et al. 2020b; Jahanshahlu and Rezaei 2020a; Saleki et al. 2020). The next wave was actually of two sources of studies in parallel. One was studies that reported stroke events in young patients with COVID-19, and the other was studies that provided evidence of a high incidence of thromboembolic events in patients with COVID-19. Therefore, the second wave strongly confirmed that the involvement of the central nervous system might be why patients with COVID-19 die from acute respiratory failure in a few days as well as suggested coagulopathies as another significant contributor to death from COVID-19. In the meantime, many disciplinary perspectives on the immunology of COVID-19 come into the competition, and the perspective which is already dominant deals inflammatory cells and molecules widely distributed throughout the critical organs to mediate COVID-19 pathogenesis (Pourahmad et al. 2020; Bahrami et al. 2020; Yazdanpanah et al. 2020a; Mansourabadi et al. 2020; Saghazadeh and Rezaei 2020a; Pashaei and Rezaei 2020; Nasab et al. 2020; Saghazadeh and Rezaei 2020b; Rokni et al. 2020). As a result, taking a look at the current evidence links us to a variety of biomarkers and clinical features that might predict the prognosis of COVID-19. The present chapter aimed to offer the most reliable predictors, and for this, we performed a systematic synthesis of the data.
We have stratified patients according to their diverse outcomes: survivor vs non-survivor, critical vs noncritical, and severe vs non-severe. The analysis by severity outcome demonstrated that higher levels of WBC with PMN dominancy might be related to disease severity and subsequent mortality. In the analysis of the WBC in severe patients, a relatively high amount of heterogeneity was observed. Meta-regression of the sample size could only explain 28% of that heterogeneity, and the two other moderators, including the difference in age between two groups and percentages of female patients, could not explain heterogeneity significantly.
We could not find a significant difference regarding CD4+ and CD8+ cell counts between severe and non-severe patients. However, previous investigations have shown that the depletion of T cells occurs during the COVID-19 course (Diao et al. 2020; Fathi and Rezaei 2020). The study (Zheng et al. 2020b) has shown extensive lymphocyte subset reduction in the blood of patients infected with COVID-19 compared to patients with other types of pneumonia. CD45+ lymphocytes, CD3+ lymphocytes, CD4+ T cells, CD8+ T cells, and CD19+ B cells were significantly lower in patients with COVID-19 than those in non-COVID-19-infected pneumonia patients with the same radiological stage.
In line with previous studies, thrombocytopenia was found to be associated with the severity, mortality, and critical outcomes of COVID-19 (Lippi et al. 2020). However, the most remarkable effect size was observed for comparison between survivors and non-survivor patients, which showed moderate ES. Previous literature recognized thrombocytopenia as an indicator that occurs in critically ill patients and is associated with mortality (Williamson et al. 2013). Therefore, COVID-19 can be more challenging in patients whom themselves suffer from immune thrombocytopenia (Sahu et al. 2020).
Acute-phase reactants, including ESR, CRP, ferritin, and procalcitonin, were significantly increased in patients with severe disease compared to patients with the non-severe disease and in patients who died compared to patients who survived. In contrast, negative phase reactants such as albumin, on the other hand, were decreased in patients with severe disease and in patients who died.
Liver enzymes were increased in severe, critical, and deceased patients compared with their counterparts without adverse outcomes. Given the relatively low prevalence of chronic liver disease before infection with COVID-19, studies suggest that increased liver enzymes, as seen in severe patients, might result from a dysregulated immune response against the virus rather than underlying liver comorbidity (Mantovani et al. 2020). Studies postulating the same hypothesis emphasize an inflammatory storm having played a role in immune dysregulation. Other studies believe that respiratory distress syndrome-induced hypoxia and subsequent hepatic ischemia and hypoxia-reperfusion dysfunction might contribute to liver dysfunction in COVID-19 (Feng et al. 2020a).
Our findings pointed to a higher level of TnI in both severe patients and non-survivors. A cohort of 416 hospitalized patients with COVID-19 established that 19.7% of these patients had a cardiac injury and showed an independent correlation between cardiac injury and a higher risk of mortality in patients with COVID-19 (Shi et al. 2020). Several mechanisms could be responsible for cardiac injury, including increased myocardial demand relative to the blood supply and hypoxia, systemic inflammation derived from cytokines, and direct cardiac damage due to COVID-19 (Guo et al. 2020).
On the other hand, there was not a significant difference regarding creatinine neither between severe and non-severe patients nor between survivors and non-survivors. BUN, on the other hand, was significantly increased in severe patients compared to the non-severe group. The authors in Wang et al. (2020b) evaluated the kidney function of patients with COVID-19 over 4 weeks and found that patients with COVID-19 experienced no acute renal injury and only showed a mildly elevated BUN and creatinine. However, there is controversy around acute kidney injury in severe patients.
Electrolytes, including sodium and potassium, showed no significant difference between severe and non-severe patients. However, there was high heterogeneity in studies reporting potassium, and during sensitivity analysis, a significant ES was observed indicating hypokalemia in severe cases. Studies report hypokalemia to be present in a majority of critically ill patients. It can be attributed to increased urine potassium excretion due to the renin-angiotensin system (RAS) disturbances and enhanced delivery of sodium and water to distal tubules (Li et al. 2020b).
Also, PT and PTT in severe patients were not significantly different from non-severe patients. However, PT was higher in critical patients and non-survivors compared to noncritical and survivors, respectively. It might be due to the measurement time of coagulation parameters, which mostly was at the time of admission, while it would undergo significant change over the disease course. D-dimer, on the other hand, was significantly increased in patients with poor outcome.
Patients with poor outcomes, e.g., severe disease and mortality, were more likely to have COPD, CHD, DM, HTN, CKD, cerebrovascular disease, and underlying malignancy compared to their counterparts. A nationwide analysis of 1590 patients in China reported that after adjusting for age and smoking status, COPD, diabetes, hypertension, and malignancy predicted increased admission to ICU, need for invasive ventilation, and death rate (Guan et al. 2020a).
Finally, patients with dyspnea at baseline were about four times more likely to progress to severe or critical condition. Also, hemoptysis increased the likelihood of developing a severe disease by about four times.
22.5 Conclusion
The current pandemic of COVID-19 has affected the healthcare system profoundly (Moazzami et al. 2020), and this effect can take a long time to compensate for it, and as the risk of reinfection is possible (Jabbari and Rezaei 2020), we must work at practice management. Early and efficient detection of high-risk patients is a priority for maximizing the utilization of limited resources. The present chapter aimed to offer the most reliable predictors, and for this, we performed a systematic synthesis of the data. Meta-analyses identified several laboratory parameters (WBC, PMN, IL-6, total bilirubin, ALT, creatinine, troponin, procalcitonin, LDH, and D-dimer), comorbidities (diabetes, chronic heart disease, COPD, cerebrovascular disease, hypertension, CKD, and malignancy), and symptoms (dyspnea and hemoptysis) that might help the prediction of severe, critical, and lethal phenotypes of COVID-19. These parameters correlate with the immune system function, inflammation, coagulation, and liver and kidney function, supporting the view that COVID-19 is a multisystem disorder.
References
Ahanchian H, Moazzen N, Faroughi MSD, Khalighi N, Khoshkhui M, Aelami MH, Haghi NSM, Rezaei N (2020) COVID-19 in a child with primary specific antibody deficiency
Ahmadi M, Saffarzadeh N, Habibi MA, Hajiesmaeili F, Rezaei N (2020) Colon cancer and SARS-CoV-2: impact of ACE2 expression in susceptibility to COVID-19. bioRxiv
Babaha F, Rezaei N (2020) Primary immunodeficiency diseases in COVID-19 pandemic: a predisposing or protective factor? Am J Med Sci. https://doi.org/10.1016/j.amjms.2020.07.027
Bahrami A, Vafapour M, Moazzami B, Rezaei N (2020) Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. https://doi.org/10.1111/jpc.15048
Basiri A, Heidari A, Nadi MF, Fallahy MTP, Nezamabadi SS, Sedighi M, Saghazadeh A, Rezaei N (2020a) Microfluidic devices for detection of RNA viruses. Rev Med Virol n/a (n/a):e2154. https://doi.org/10.1002/rmv.2154
Basiri A, Pazhouhnia Z, Beheshtizadeh N, Hoseinpour M, Saghazadeh A, Rezaei N (2020b) Regenerative medicine in COVID-19 treatment: real opportunities and range of promises. Stem Cell Rev Rep:1–13. https://doi.org/10.1007/s12015-020-09994-5
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication Bias. Biometrics 50(4):1088–1101. https://doi.org/10.2307/2533446
Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy n/a (n/a) https://doi.org/10.1111/all.14309
Cao W (2020) Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv:2020.2002.2023.20026963. https://doi.org/10.1101/2020.02.23.20026963
Cao J, Tu W-J, Cheng W, Yu L, Liu Y-K, Hu X, Liu Q (2020) Clinical features and short-term outcomes of 102 patients with Corona virus disease 2019 in Wuhan, China. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa243
Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R (2020) Features, evaluation and treatment coronavirus (COVID-19). In: Statpearls [internet]. StatPearls Publishing
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q (2020a) Clinical and immunologic features in severe and moderate forms of Coronavirus disease 2019. medRxiv:2020.2002.2016.20023903. https://doi.org/10.1101/2020.02.16.20023903
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H (2020b) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130(5)
Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H (2020c) Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. https://doi.org/10.1016/j.jinf.2020.03.004
Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, Chen X, Luo M, Liang K, Gao S, Zhang Y, Deng L, Xiong Y (2020d) Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol Ser A. https://doi.org/10.1093/gerona/glaa089
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H (2020e) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ:368
Chu J, Yang N, Wei Y, Yue H, Zhang F, Zhao J, He L, Sheng G, Chen P, Li G, Wu S, Zhang B, Zhang S, Wang C, Miao X, Li J, Liu W, Zhang H (2020) Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. https://doi.org/10.1002/jmv.25793
Darbeheshti F, Rezaei N (2020) Genetic predisposition models to COVID-19 infection. Med Hypotheses 142:109818. https://doi.org/10.1016/j.mehy.2020.109818
de Wit E, van Doremalen N, Falzarano D, Munster VJ (2016) SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14(8):523
Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Wu Y, Chen Y (2020) Reduction and functional exhaustion of T Cells in patients with Coronavirus disease 2019 (COVID-19). medRxiv:2020.2002.2018.20024364. https://doi.org/10.1101/2020.02.18.20024364
Du R-H, Liu L-M, Yin W, Wang W, Guan L-L, Yuan M-L, Li Y-L, Hu Y, Li X-Y, Sun B (2020) Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication Bias in meta-analysis. Biometrics 56(2):455–463. https://doi.org/10.1111/j.0006-341X.2000.00455.x
Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, Kuperan P, Ong KH (2020) Hematologic parameters in patients with COVID-19 infection. Am J Hematol n/a (n/a) https://doi.org/10.1002/ajh.25774
Fathi N, Rezaei N (2020) Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol Int 44(9):1792–1797. https://doi.org/10.1002/cbin.11403
Feng G, Zheng KI, Yan Q-Q, Rios RS, Targher G, Byrne CD, Van Poucke S, Liu W-Y, Zheng M-H (2020a) COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol 8(1):18
Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F (2020b) COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med
Feng Z, Yu Q, Yao S, Luo L, Duan J, Yan Z, Yang M, Tan H, Ma M, Li T, Yi D, Mi Z, Zhao H, Jiang Y, He Z, Li H, Nie W, Liu Y, Zhao J, Wang W (2020c) Early prediction of disease progression in 2019 Novel Coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. https://doi.org/10.1101/2020.02.19.20025296
Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L (2020) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. https://doi.org/10.1002/jmv.25770
Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, Cao J, Tan M, Xu W, Zheng F (2020) Multicenter development and validation of a novel risk nomogram for early prediction of severe 2019-novel coronavirus pneumonia. Available at SSRN 3551365
Guan W-j, Liang W-h, Zhao Y, Liang H-r, Chen Z-s, Li Y-m, Liu X-q, Chen R-c, Tang C-l, Wang T, Ou C-q, Li L, Chen P-y, Sang L, Wang W, Li J-f, Li C-c, Ou L-m, Cheng B, Xiong S, Ni Z-y, Xiang J, Hu Y, Liu L, Shan H, Lei C-l, Peng Y-x, Wei L, Liu Y, Hu Y-h, Peng P, Wang J-m, Liu J-y, Chen Z, Li G, Zheng Z-j, Qiu S-q, Luo J, Ye C-j, Zhu S-y, Cheng L-l, Ye F, Li S-y, Zheng J-p, Zhang N-f, Zhong N-s, He J-x (2020a) Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur Respir J:2000547. https://doi.org/10.1183/13993003.00547-2020
Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, Liu L, Shan H, Lei C-L, Hui DSC, Du B, Li L-J, Zeng G, Yuen K-Y, Chen R-C, Tang C-L, Wang T, Chen P-Y, Xiang J, Li S-Y, Wang J-L, Liang Z-J, Peng Y-X, Wei L, Liu Y, Hu Y-H, Peng P, Wang J-M, Liu J-Y, Chen Z, Li G, Zheng Z-J, Qiu S-Q, Luo J, Ye C-J, Zhu S-Y, Zhong N-S (2020b) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol
Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C (2020a) Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol
Han Y, Zhang H, Mu S, Wei W, Jin C, Xue Y, Tong C, Zha Y, Song Z, Gu G (2020b) Lactate dehydrogenase, a risk factor of severe COVID-19 patients. medRxiv
Hanaei S, Rezaei N (2020) COVID-19: developing from an outbreak to a pandemic. Arch Med Res. https://doi.org/10.1016/j.arcmed.2020.04.021
Higgins J, Wells G (2011) Cochrane handbook for systematic reviews of interventions
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Jabbari P, Rezaei N (2020) With risk of reinfection, is COVID-19 here to stay? Disaster Med Public Health Prep:1. https://doi.org/10.1017/dmp.2020.274
Jabbari P, Jabbari F, Ebrahimi S, Rezaei N (2020) COVID-19: a chimera of two pandemics. Disaster Med Public Health Prep:1–2. https://doi.org/10.1017/dmp.2020.223
Jahanshahlu L, Rezaei N (2020a) Central nervous system involvement in COVID-19. Arch Med Res. https://doi.org/10.1016/j.arcmed.2020.05.016
Jahanshahlu L, Rezaei N (2020b) Monoclonal antibody as a potential anti-COVID-19. Biomed Pharmacother Biomed Pharmacotherapie 129:110337. https://doi.org/10.1016/j.biopha.2020.110337
Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, Chen G, Cheng G, Wang Y, Bi J, Tan L, Lau G, Qin E (2020) Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa414
Kafieh R, Arian R, Saeedizadeh N, Minaee S, Yadav SK, Vaezi A, Rezaei N, Javanmard SH (2020) COVID-19 in Iran: a deeper look into the future. medRxiv
Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W (2003) A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 348(20):1953–1966
Lei I, Jian-Ya G (2020) Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv:2020.2002.2020.20025536. https://doi.org/10.1101/2020.02.20.20025536
Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C (2020a) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig Radiol
Li X, Hu C, Su F, Dai J (2020b) Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). MedRxiv
Li YK, Peng S, Li LQ, Wang Q, Ping W, Zhang N, Fu XN (2020c) Clinical and transmission characteristics of Covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. https://doi.org/10.1007/s11596-020-2176-2
Lippi G, Plebani M, Henry BM (2020) Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta
Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X (2020a) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv:2020.2002.2016.20023671. https://doi.org/10.1101/2020.02.16.20023671
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C, Zhang M, Tan J, Xu Y, Song R, Song M, Wang L, Zhang W, Han B, Yang L, Wang X, Zhou G, Zhang T, Li B, Wang Y, Chen Z, Wang X (2020b) Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv:2020.2002.2010.20021584. https://doi.org/10.1101/2020.02.10.20021584
Liu KC, Xu P, Lv WF, Qiu XH, Yao JL, Gu JF, Wei W (2020c) CT manifestations of coronavirus disease-2019: a retrospective analysis of 73 cases by disease severity. Eur J Radiol 126. https://doi.org/10.1016/j.ejrad.2020.108941
Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, Yu L (2020d) Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv
Lotfi M, Rezaei N (2020) SARS-CoV-2: a comprehensive review from pathogenicity of the virus to clinical consequences. J Med Virol. https://doi.org/10.1002/jmv.26123
Lotfi M, Hamblin MR, Rezaei N (2020) COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta 508:254–266. https://doi.org/10.1016/j.cca.2020.05.044
Lu J, Hu S, Fan R, Liu Z, Yin X, Wang Q, Lv Q, Cai Z, Li H, Hu Y, Han Y, Hu H, Gao W, Feng S, Liu Q, Li H, Sun J, Peng J, Yi X, Zhou Z, Guo Y, Hou J (2020) ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv:2020.2002.2020.20025510. https://doi.org/10.1101/2020.02.20.20025510
Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27(6):1785–1805
Mansourabadi AH, Sadeghalvad M, Mohammadi-Motlagh HR, Rezaei N (2020) The immune system as a target for therapy of SARS-CoV-2: a systematic review of the current immunotherapies for COVID-19. Life Sci 258:118185. https://doi.org/10.1016/j.lfs.2020.118185
Mantovani A, Beatrice G, Dalbeni A (2020) Coronavirus disease 2019 (COVID-19) and prevalence of chronic liver disease: a meta-analysis. Liver Int
Mirbeyk M, Rezaei N (2020) The impact of COVID-19 on pregnancy and neonatal health: a systematic review
Moazzami B, Razavi-Khorasani N, Dooghaie Moghadam A, Farokhi E, Rezaei N (2020) COVID-19 and telemedicine: immediate action required for maintaining healthcare providers well-being. J Clin Virol 126:104345. https://doi.org/10.1016/j.jcv.2020.104345
Mohamed K, Rodríguez-Román E, Rahmani F, Zhang H, Ivanovska M, Makka SA, Joya M, Makuku R, Islam MS, Radwan N, Rahmah L, Goda R, Abarikwu SO, Shaw M, Zoghi S, Irtsyan S, Ling I, Cseprekal O, Faten AB, Hazar Sayar E, Soloukey C, Grancini G, Rezaei N (2020a) Borderless collaboration is needed for COVID-19-A disease that knows no borders. Infect Control Hosp Epidemiol:1–2. https://doi.org/10.1017/ice.2020.162
Mohamed K, Yazdanpanah N, Saghazadeh A, Rezaei N (2020b) Computational drug discovery and repurposing for the treatment of Covid-19: a systematic review. Available at SSRN 3583748
Momtazmanesh S, Ochs HD, Uddin LQ, Perc M, Routes JM, Vieira DN, Al-Herz W, Baris S, Prando C, Rosivall L, Abdul Latiff AH, Ulrichs T, Roudenok V, Aldave Becerra JC, Salunke DB, Goudouris E, Condino-Neto A, Stashchak A, Kryvenko O, Stashchak M, Bondarenko A, Rezaei N (2020) All together to fight COVID-19. Am J Trop Med Hyg 102(6):1181–1183. https://doi.org/10.4269/ajtmh.20-0281
Moradian N, Ochs HD, Sedikies C, Hamblin MR, Camargo CA Jr, Martinez JA, Biamonte JD, Abdollahi M, Torres PJ, Nieto JJ, Ogino S, Seymour JF, Abraham A, Cauda V, Gupta S, Ramakrishna S, Sellke FW, Sorooshian A, Wallace Hayes A, Martinez-Urbistondo M, Gupta M, Azadbakht L, Esmaillzadeh A, Kelishadi R, Esteghamati A, Emam-Djomeh Z, Majdzadeh R, Palit P, Badali H, Rao I, Saboury AA, Jagan Mohan Rao L, Ahmadieh H, Montazeri A, Fadini GP, Pauly D, Thomas S, Moosavi-Movahed AA, Aghamohammadi A, Behmanesh M, Rahimi-Movaghar V, Ghavami S, Mehran R, Uddin LQ, Von Herrath M, Mobasher B, Rezaei N (2020) The urgent need for integrated science to fight COVID-19 pandemic and beyond. J Transl Med 18(1):205. https://doi.org/10.1186/s12967-020-02364-2
Nasab MG, Saghazadeh A, Rezaei N (2020) SARS-CoV-2-a tough opponent for the immune system. Arch Med Res. https://doi.org/10.1016/j.arcmed.2020.05.020
Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z (2020) Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv
Pashaei M, Rezaei N (2020) Immunotherapy for SARS-CoV-2: potential opportunities. Expert Opin Biol Ther:1–5. https://doi.org/10.1080/14712598.2020.1807933
Pourahmad R, Moazzami B, Rezaei N (2020) Efficacy of Plasmapheresis and immunoglobulin replacement therapy (IVIG) on patients with COVID-19. SN Compr Clin Med:1–5
Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis
Qu R, Ling Y, Zhang Y, Ly W, Chen X, Li X, Xy L, Hm L, Guo Z, Ren H (2020) Platelet-to-lymphocyte ratio is associated with prognosis in patients with Corona virus Disease-19. J Med Virol
Rabiee N, Rabiee M, Bagherzadeh M, Rezaei N (2020) COVID-19 and picotechnology: potential opportunities. Med Hypotheses 144:109917. https://doi.org/10.1016/j.mehy.2020.109917
Rezaei N (2020a) COVID-19 affects healthy pediatricians more than pediatric patients. Infect Control Hosp Epidemiol 1. https://doi.org/10.1017/ice.2020.139
Rezaei N (2020b) COVID-19 and medical biotechnology. Avicenna J Med biotechnol 12(3):139
Rokni M, Hamblin MR, Rezaei N (2020) Cytokines and COVID-19: friends or foes? Hum Vaccin Immunother:1–3
Rzymski P, Nowicki M, Mullin GE, Abraham A, Rodríguez-Román E, Petzold MB, Bendau A, Sahu KK, Ather A, Naviaux A-F (2020) Quantity does not equal quality: scientific principles cannot be sacrificed. Int Immunopharmacol 86:106711
Saghazadeh A, Rezaei N (2020a) Immune-epidemiological parameters of the novel coronavirus - a perspective. Expert Rev Clin Immunol 16(5):465–470. https://doi.org/10.1080/1744666x.2020.1750954
Saghazadeh A, Rezaei N (2020b) Towards treatment planning of COVID-19: rationale and hypothesis for the use of multiple immunosuppressive agents: anti-antibodies, immunoglobulins, and corticosteroids. Int Immunopharmacol 84:106560. https://doi.org/10.1016/j.intimp.2020.106560
Sahu KK, Siddiqui AD, Rezaei N, Cerny J (2020) Challenges for management of immune thrombocytopenia during COVID-19 pandemic. J Med Virol. https://doi.org/10.1002/jmv.26251
Saleki K, Banazadeh M, Saghazadeh A, Rezaei N (2020) The involvement of the central nervous system in patients with COVID-19. Rev Neurosci 31(4):453–456. https://doi.org/10.1515/revneuro-2020-0026
Shamshirian D, Rezaei N (2020) Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis
Sharifkashani S, Bafrani MA, Khaboushan AS, Pirzadeh M, Kheirandish A, Yavarpour Bali H, Hessami A, Saghazadeh A, Rezaei N (2020) Angiotensin-converting enzyme 2 (ACE2) receptor and SARS-CoV-2: potential therapeutic targeting. Eur J Pharmacol 884:173455. https://doi.org/10.1016/j.ejphar.2020.173455
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q (2020) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost
The Novel Coronavirus Pneumonia Emergency Response Epidemiology T (2020) The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China, 2020. China CDC Wkly 2(8):113–122. https://doi.org/10.46234/ccdcw2020.032
Tian S, Hu N, Lou J, Chen K, Kang X, Xiang Z, Chen H, Wang D, Liu N, Liu D (2020) Characteristics of COVID-19 infection in Beijing. J Infect
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14(1):135. https://doi.org/10.1186/1471-2288-14-135
Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, Xiang J, Zhang B, Chen Y (2020) Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). https://doi.org/10.1101/2020.02.10.20021832
Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol n/a (n/a) https://doi.org/10.1002/jmv.25783
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y (2020a) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA
Wang L, Li X, Chen H, Yan S, Li Y, Li D, Gong Z (2020b) SARS-CoV-2 infection does not significantly cause acute renal injury: an analysis of 116 hospitalized patients with COVID-19 in a single hospital, Wuhan, China medRxiv:20202002.2019.20025288. https://doi.org/10.1101/2020.02.19.20025288
Wang Y, Lu X, Chen H, Chen T, Su N, Huang F, Zhou J, Zhang B, Li Y, Yan F, Wang J (2020c) Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202003-0736LE
Wang Y, Yao L, Zhang J-P, Tang P-J, Ye Z-J, Shen X-H, Xu J-C, Wu M-Y, Yu X (2020d) Clinical Characteristics and Laboratory Indicator Analysis of 69 COVID-19 Pneumonia Patients in Suzhou, China. China (3/3/2020)
Wang Z, Yang B, Li Q, Wen L, Zhang R (2020e) Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis
Williamson DR, Lesur O, Tétrault J-P, Nault V, Pilon D (2013) Thrombocytopenia in the critically ill: prevalence, incidence, risk factors, and clinical outcomes. Can J Anesth 60(7):641–651. https://doi.org/10.1007/s12630-013-9933-7
Wu C, Chen X, Cai Y, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med
Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H (2020) Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. https://doi.org/10.1111/liv.14449
Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, Dong L, Ma X, Zhang HJ, Gao BL (2020) Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. https://doi.org/10.1016/j.jinf.2020.02.017
Yan S, Song X, Lin F, Zhu H, Wang X, Li M, Ruan J, Lin C, Liu X, Wu Q, Luo Z, Fu W, Chen S, Yuan Y, Liu S, Yao J, Lv C (2020) Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv:2020.2003.2019.20038539. doi:https://doi.org/10.1101/2020.03.19.20038539
Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med
Yazdanpanah F, Hamblin MR, Rezaei N (2020a) The immune system and COVID-19: friend or foe? Life Sci 256:117900. https://doi.org/10.1016/j.lfs.2020.117900
Yazdanpanah N, Saghazadeh A, Rezaei N (2020b) Anosmia: a missing link in the neuroimmunology of coronavirus disease 2019 (COVID-19). Rev Neurosci. https://doi.org/10.1515/revneuro-2020-0039
Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng O-T, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI-C, Chan M, Vasoo S, Wang L-F, Tan BH, Lin RTP, Lee VJM, Leo Y-S, Lye DC, Team ftSNCOR (2020) Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. https://doi.org/10.1001/jama.2020.3204
Yousefzadegan S, Rezaei N (2020) Case report: death due to COVID-19 in three brothers. Am J Trop Med Hyg 102(6):1203–1204. https://doi.org/10.4269/ajtmh.20-0240
Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H (2020a) Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv
Zhang H, Wang X, Fu Z, Luo M, Zhang Z, Zhang K, He Y, Wan D, Zhang L, Wang J (2020b) Potential factors for prediction of disease severity of COVID-19 patients
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD (2020c) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. https://doi.org/10.1111/all.14238
Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am J Roentgenol:1–6
Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG (2020a) Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci 24(6):3404–3410. https://doi.org/10.26355/eurrev_202003_20711
Zheng Y, Huang Z, Ying G, Zhang X, Ye W, Hu Z, Hu C, Wei H, Zeng Y, Chi Y (2020b) Comparative study of the lymphocyte change between COVID-19 and non-COVID-19 pneumonia cases suggesting uncontrolled inflammation might not be the main reason of tissue injury. medRxiv
Zheng Y, Xu H, Yang M, Zeng Y, Chen H, Liu R, Li Q, Zhang N, Wang D (2020c) Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J Clin Virol 104366
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X (2020a) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet
Zhou Y, Yang Z, Guo Y, Geng S, Gao S, Ye S, Hu Y, Wang Y (2020b) A new predictor of disease severity in patients with COVID-19 in Wuhan, China. medRxiv
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R (2020) A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gholami, B. et al. (2021). Clinical and Laboratory Predictors of Severity, Criticality, and Mortality in COVID-19: A Multisystem Disease. In: Rezaei, N. (eds) Coronavirus Disease - COVID-19. Advances in Experimental Medicine and Biology, vol 1318. Springer, Cham. https://doi.org/10.1007/978-3-030-63761-3_22
Download citation
DOI: https://doi.org/10.1007/978-3-030-63761-3_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63760-6
Online ISBN: 978-3-030-63761-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)