Abstract
Recent progress has shown that Metal-organic Frameworks (MOFs) can be integrated with biomacromolecules. Depending on the synthesis protocol used, it is possible to control the spatial localization of the biomacromolecules in or on MOF biocomposites. When biomacromolecules are infiltrated or encapsulated within MOFs, the porous materials were found to form a protective coating around the bioactive entity, thus offering improved stability to non-native external environments that would normally lead to its degradation. When MOF particles are surface functionalized with biomacromolecules either via adsorption or grafting, enhanced targeting capabilities and improved biodistribution were observed. In this book chapter, we examine the principal synthetic methods for the preparation of MOF bio-composites. We discuss the progress toward combining the four main classes of biomacromolecules, such as proteins, carbohydrates, nucleic acids, and lipids, with MOFs, and disclose relevant examples for application to drug delivery, biopreservation, and biosensing. As MOF biocomposites composition has been extended from biomacromolecules to more complex biological systems, including viruses and cells, we illustrate how MOFs can be used for the preparation of protective exoskeletons for potential applications to vaccine transportation/delivery and regenerative medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- MOF
- Biocomposite
- Biomacromolecule
- Adsorption
- Grafting
- Encapsulation
- Infiltration
- Co-precipitation
- de novo
- Biomimetic mineralization
12.1 Introduction
Biomacromolecules play a key role in natural biological systems but are increasingly finding greater application as therapeutics, and as biomarkers for disease prognosis and monitoring ongoing treatment. Indeed, with the rapid development of new medical technologies and treatments, especially for age-related and emerging diseases, the need to cost effectively and reliably store or transport biomacromolecules (biopreservation) and improve detection (biosensing) continues to grow. A significant challenge in this area is that biomacromolecules are typically unstable when removed from their finely tuned biological environment and lose their functionality when exposed to elevated temperatures, nonaqueous media, or non-native pH.
Low-temperature storage and lyophilization (freeze-drying) are employed to protect biomacromolecules for storage and transport; however, these forms do not typically allow biomacromolecules to be directly used as therapeutics or sensing. Other strategies to improve biomolecule stability for applications in biomedicine include using more stable homologs from extremophiles, genetic engineering, and posttranslational (chemical) modification of biomacromolecules to provide access to more stable variants; however, these are not universal approaches. To this end, researchers have focused on developing more general approaches, for enhancing biomacromolecule stability and providing protection that also facilitate practical use of the biomolecule . Among these, porous materials have been actively researched for stabilizing biomacromolecules either by adsorbing or grafting them to the surface of the material, via pore infiltration or via sol-gel encapsulation [1,2,3]. Materials explored for this purpose range from soft hydrogels to hard inorganic materials like silica, metallophosphates, and metal oxides [1,2,3,4].
While a vast array of biocomposites have been prepared that confer stability to protein-based therapeutics and biomacromolecule-based sensing platforms, there is still a need to develop new strategies to stabilize and protect biomacromolecules. Although inorganic materials (e.g., silica) have been synthesize from biocompatible precursors (e.g., sodium silicates in water), some intrinsic problems, such as large degrees of shrinkage (up to 80%) or limited range of pore size tuneability, morphology and polarity, result in a limited range of biomacromolecules that can be immobilized [5]. The building block synthetic approach, pre- and post-synthetic chemical mutability, and intrinsic porosity of metal-organic frameworks (MOFs ) provide opportunities for application to biomacromolecule protection that solve or minimize some of the existing challenges of other materials. This includes tuneable biocompatibility, access to the biomacromolecule through a robust and regular pore network, and differing framework chemistry that can provide sustained and targeted biologically relevant release. MOF biocomposites are obtained by integrating biomacromolecules with MOFs , which has provided an emerging class of materials for biomedicine, biopreservation, biosensing, and biocatalysis [6,7,8].
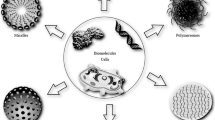
Depending on the synthesis protocol used and the spatial localization of the biomacromolecules in or on the MOF particle, different types of MOF biocomposites (Fig. 12.1) can be identified:
-
1.
Biomacromolecule-on-MOF: In this configuration, preformed MOF particles are surface-decorated with biomacromolecules. The preparation process is named surface immobilization and involves (a) adsorption via noncovalent interactions or (b) grafting (covalent bonding, also termed bioconjugation) of biomacromolecules on MOFs .
-
2.
Biomacromolecule@MOF: In this composite, biomacromolecules are embedded within the MOF particles. These composites can be obtained by (a) infiltration or (b) one-pot encapsulation methods.
In this book chapter, we first discuss the different preparation methods used to prepare MOF biocomposites, including the merits and challenges of each strategy. We then focus on the different MOF biocomposites by examining each single class of biomacromolecules (proteins, fatty acids, carbohydrates, and nucleic acids) and highlight the applications of these materials for biomedical applications.
12.2 Synthesis Methods
12.2.1 Surface Immobilization
Biomacromolecule-on-MOF composites are obtained by immobilizing biomacromolecules onto the surface of MOF particles by surface adsorption or grafting. These are processes heavily influenced by strategies for biomacromolecule immobilization on other materials and, while not directly dependent on the permanent porosity of MOFs , are enabled by the diverse chemical structures of MOFs and the mutability of their surface chemistry.
12.2.1.1 Adsorption of Biomacromolecules on MOFs
The easiest approach to prepare biomacromolecule-on-MOF composites is the surface adsorption of biomacromolecules on preformed MOF particles. This immobilization method depends on noncovalent interactions (e.g., electrostatic interactions, hydrophilic/hydrophobic interactions, or hydrogen bonds) between the biomacromolecules and the MOF surface.
Electrostatic interactions are often exploited to decorate surfaces with biomacromolecules [9, 10]. For example, in the case of proteins, surface-exposed amino acids determine the overall charge of the protein and the electrostatic properties of this system depend on the pH of the solution and on the pK values of the ionizable groups [11]. Thus, by selecting the appropriate biomolecule , adsorption conditions, and an MOF material with suitable functional groups, introduced pre- or postsynthetically, it is possible to control the attractive or repulsive forces. To favor attraction and adsorption, the pH and ionic strength of the solution should be tuned to induce opposite net charges on the MOF and on the biomacromolecule [12]. For example, Li et al. adsorbed pectinase on polymethacrylic acid (PMMA) decorated UiO-66-NH2 particles and showed that if the pH of the solution was significantly higher than the pectinase isoelectric point (pH 3.8), both the protein and the PMMA-decorated MOF surfaces were negatively charged and the immobilization was not effective [12]. Conversely, close to the isoelectric point, successful adsorption was achieved, allowing the biomacromolecule to form hydrogen bonds with the functional groups exposed on the PMMA decorated MOF surface [13]. These weak, but multipoint, interactions typically impede leaching (i.e., release of the biomacromolecules in solution) and stabilize the biomacromolecule but can be contingent on maintenance of the conditions [13]. Additionally, however, when the interaction with a surface is sufficiently strong, changes in the biomacromolecule structure (e.g., protein unfolding) and in its bioactivity can result [14].
Hydrophilic/hydrophobic surfaces can also be used to favor the adsorption of biomacromolecules. In general, proteins possess a high affinity for hydrophobic surfaces [15]; however, such interactions can perturb the proteins tertiary structure and result in loss of activity [16]. For example, Doonan and coworkers demonstrated that enzymes adsorbed on zeolitic imidazolate frameworks (ZIFs) of varied hydro-philicity/phobicity showed different enzymatic activities [17]. When catalase (CAT) was adsorbed on hydrophilic MAF-7 (synthesized from Zn2+ and 3-methyl-1,2,4-triazolate) or ZIF-90 (synthesized from Zn2+ and 2-imidazolatecarboxaldehyde), its enzymatic activity was largely maintained. Conversely, when CAT was adsorbed on hydrophobic ZIF-8 (synthesized from Zn2+ and 2-methyilmidazole (HmIM)), the enzymatic activity was inhibited. However, different biomacromolecules have different conformational sensitivities and the potential activity loss should be assessed case by case. For example, some antibodies and enzymes can be supported on different hydrophobic surfaces, without showing significant unfolding of the protein structure [18,19,20]., Furthermore, the interaction between an enzyme and the MOF surface can influence the orientation of the biomacromolecule on the surface, as determined by Pan et al. in the case of recombinant T4 phage lysozyme partially encapsulated into ZIF-8 particles [21]. Thus, for biomacromolecules-on-MOF biocomposites, understanding the interaction between the MOF surface and biomacromolecules is a fundamental research topic that needs to be further understood to allow for their development.
12.2.1.2 Grafting of Biomacromolecules on MOFs
A more robust method of biomacromolecule immobilization onto MOF surfaces is to form a covalent bond between specific functional groups on the surfaces of the biomolecule and MOF. This strategy, termed grafting, takes advantage of the vast library of covalent bond forming protocols available to combine proteins and MOFs . A widely used grafting procedure reacts carboxylic and amino groups via the N,N′-dicyclohexylcarbodiimide (DCC)-mediated coupling reaction or the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide/N-hydroxysuccinimide (EDC/NHS) method [22]. These protocols are widely used for the permanent immobilization of proteins [23], nucleic acid [23], carbohydrates [24], and cells [25] on different materials. As DCC is not soluble in water, the DCC coupling can be only be applied if the selected biomacromolecule is stable in the organic solvent used for the reaction. Conversely, the EDC/NHS method can be performed in water and buffer solutions, and this is the reason for the preferential use of this coupling reaction in the preparation of biomacromlecules-on-MOFs via grafting.
Carbodiimide coupling techniques were exploited to prepare biomacromolecules-on-MOF biocomposites with proteins [26, 27], carbohydrates [28,29,30] and nucleic acids [31]. As an example, Huang et al. [26] reported the DCC-mediated coupling of trypsin (a digestive enzyme) to the surface of MIL-88-NH2(Cr) (Fig. 12.2). In this case, due to the approach used, the authors suggested that the covalent bond was formed on the uncoordinated carboxylic acid group of the MOF linker. To use the amino-functionality in NH2-MIL-53(Al) for the immobilization of β-glucosidase, Falcaro and coworkers used a glutaraldehyde-mediated grafting procedure [27]. These examples demonstrate the versatility of the “crystals as molecules” reactivity of MOFs for grafting that could be further extended via postsynthetic modification (PSM) processes [32].
Schematic view of the trypsin immobilization onto DCC-activated MOFs . (Adapted with permission of Wiley from Ref. [26], © 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim)
12.2.1.3 General Considerations for Biomacromolecules-On-MOF Composites
This method is extremely versatile, and a variety of biomacromolecules-on-MOF composites can be prepared. The flexibility of this system derives from: (1) the large number of available MOFs ; (2) postsynthetic modification methods for fine-tuning of the chemical functional groups on the MOF surface; (3) number of conditions/protocols available for adsorption/bioconjugation; and, (4) the possibility to perform the bioconjugation under biocompatible conditions (e.g., in water or buffer solution). The final, solid, biocomposite can be easily recovered and recycled, thus facilitating the use of biomacromolecules mostly for biocatalytic, biosensing, and drug delivery applications [6, 27, 33]. It should be noted that not all the reports pioneering biomacromolecules-on-MOF composites exploit the porous properties of MOFs and, in many cases, only the external surface of the porous crystal is used, thus providing little difference to nonporous nanoparticles (aside from contributing slightly less mass to the biocomposite) [6]. However, it has been recently shown that surface functionalization methods can significantly improve the properties of MOF-based drug delivery systems where MOF pores are infiltrated with therapeutics. Indeed, several therapeutic@MOF systems have shown an improved colloidal stability , blood circulation time, and cellular uptake when their surface is bioconjugated with selected biomacromolecules [31, 34,35,36].
12.2.2 Embedding of Biomacromolecules in MOFs
Homogenous distribution of biomacromolecules within MOFs has been achieved either via infiltration or one-pot encapsulation. These biocomposites are named biomacromolecule @MOF.
12.2.2.1 Infiltration
Infiltration consists of the insertion of biomacromolecules into the pores of preformed MOF particles. For this approach, it is crucial that the pore-size distribution and connectivity can accommodate the selected biomacromolecules and, moreover, allow for the diffusion of the cofactors/substrates through the material once the biomacromolecule has been infiltrated. Because of the typical size of biomacromolecules, infiltration methods are usually limited to mesoporous MOFs . For example, successful infiltrations were performed with Aspergillus saitoi (2.85 nm) [37], organophosphorus acid anhydrolase (Fig. 12.3, 4.4 nm) [38], green fluorescent protein (4.5 nm) [39], microperoxidase-11 (3.3 nm) [40, 41] in MIL-101(Al)-NH2 (up to 3.6 nm) [37], NU-1003 (Fig. 12.3, up to 4.5 nm) [38], IRMOFs (up to 5 nm) [39], Tb-TATB (up to 4.7 nm) [40], respectively. Although MOFs with pores larger than biomacromolecules are desired, they are not strictly necessary. For example, it was observed that certain biomacromolecules with dimensions slightly bigger than the MOF pore aperture can undergo conformational changes that allow access to the MOF structure [42]. So far, the infiltration strategy has been applied to immobilize proteins [38, 39, 42, 43] and nucleic acids [44, 45] into MOFs .
(a) Schematic illustration of the infiltration of organophosphorus acid anhydrolase (OPAA) in the mesoporous channels of the MOF (NU-1003). (b) Time-resolved confocal laser scanning microscopy images of a single crystal of OPAA@NU-1003 collected during the infiltration process (scale bar is 10 μm). (Adapted with permission of ACS from Ref. [38], https://pubs.acs.org/doi/10.1021/acsnano.6b04996, further permissions related to the material excerpted should be directed to the ACS)
Experimentally, the infiltration protocol involves exposing the MOF crystals to a solution containing the biomacromolecule . To maximize the loading of the biomacromolecules via infiltration, the diffusion within the MOF pores should be facilitated; however, diffusion depends on: (1) adsorption–desorption equilibria process that is governed by the specific biomacromolecules–MOF affinity (e.g., electrostatic and hydrophobic/philic interactions) and by MOF particle size; and, (2) the size of the biomacromolecules with respect to the MOF pore size [44]. In addition, aspects such as the MOF structural stability and biomacromolecule structural preservation should be assessed for each protocol. For example, Hidalgo et al. infiltrated RNA molecules in MIL-100 and MIL-101-NH2 MOFs that possess two types of mesocages (25 & 29 or 29 & 34 Å, respectively) accessible through microporous windows (5 & 8.6 Å or 12 & 16 Å, respectively) [44]. In this case, to favor the infiltration, the anionic nature of the nucleic acids (negatively charged biomacromolecules) was paired with a cationic MOF nanoparticles (positively charged porous matrix). By changing the pH, the surface charge of the MOF particles was tuned, and it was found that for pH<5 the MOF nanoparticles were stable colloids, positively charged, and the infiltration was successful.
Compared to biomacromolecules-on-MOF, biomacromolecules@MOF can exploit advantages related to the porous structure of the MOF matrix. The porous framework can offer further protection against harsh environments by means of a physical stabilization of the biomacromolecules, due to the pore size constraint or by acting as a molecular sieve, typically preventing the diffusion of molecules larger than the MOF pore aperture (e.g., for conferring stability against hydrolytic enzymes). This molecular sieving functionality can be exploited for application to biocatalysis, biosensing, and drug delivery [6, 7]. In the case of biocatalysis and biosensing, the surface charge of the porous structure, combined with the defined pore size, can also act as charge and size-dependent sieve, influencing the local concentration of reactants in proximity of the immobilized enzyme [46]. Furthermore, the recyclability of the immobilized biomacromolecule is facilitated. Potential drawbacks could be related to the noncovalent nature of the infiltration process. For example, washing procedures or extensive recycling could lead to the leaching of the biomacromolecule , although the noncovalent interactions between the biomacromolecule and the MOF pore surface tend to minimize this. Moreover, the number of potential MOF candidates is limited because of the large pore size needed.
12.2.2.2 Encapsulation
The encapsulation of functional biomacromolecules in MOFs is obtained by self-assembly of an MOF shell around a biomacromolecule (Fig. 12.4). Since the MOF is formed in the presence of the biomacromolecule , it is possible to embed bioentities far greater in size than MOF micropores. Thus, proteins [47,48,49], nucleic acids [50], carbohydrates [51], viruses [52], and cells [53] can be successfully confined within an MOF matrix. The MOF growth conditions determine the final particle size and crystalline phase of the biocomposite [54]. Usually, the biocomposite particle size ranges from tens of nanometers to tens of micrometers, allowing the easy isolation and recyclability of the biocomposites. In the literature, biomacromolecule @MOF has been proposed for application to biocatalysis, biomedicine (e.g., drug delivery, biopreservation, and biosensing), and cell and virus manipulation [7, 8, 33, 55].
From an experimental point of view, the synthesis of biomacromolecule @MOF via encapsulation is a one-pot method that refers to the formation of MOF biocomposites by mixing the selected biomacromolecules with the MOF precursors. As for the previously discussed biocomposites, the encapsulation procedure should preserve the structure and function of the encapsulated biomacromolecules. Thus, the encapsulation protocols should be performed under biocompatible conditions and encapsulation could either be triggered by the biomacromolecule itself or by the presence of additives and/or solvents.
The first one-pot encapsulation of protein in MOFs was reported by Ge, Liu, and coworkers [47] (2014, Fig. 12.4): Cytochrome C (Cytc) modified with PolyVinylPyrrolidone (PVP) was added in a methanol solution of ZIF-8 precursors (Zn2+ and HmIM). Immediately after mixing, the authors observed the precipitation of Cytc@ZIF-8. SEM micrographs of the calcined biocomposite proved the presence of cavities within the bulk of ZIF-8 crystals that were hosting the enzyme. The activity of encapsulated enzyme showed a 10-fold enhancement compared to the same concentration of free enzyme in solution, demonstrating the potential of this method for biocatalysis. This synthetic protocol was named coprecipitation and was extended to different proteins (e.g., horseradish peroxidase (HRP) and lipase in HRP@ZIF-8 and lipase@ZIF-8) proving that the MOF shell can protect the encapsulated proteins. In 2015, Tshu and coworkers proposed the de novo approach: catalase was mixed to PVP and ZIF-90 precursors in water and CAT@ZIF-90 rapidly formed (Fig. 12.4) [48]. Compared to the first reported coprecipitation method, the replacement of methanol with water represented a salient advance through the use of more biocompatible conditions. In these cases, the encapsulation of the proteins was afforded by using PVP [47, 48], as a biocompatible agent that was used for the stabilization of the proteins [56], and for its role as a crystallization facilitator of ZIF materials [57, 58]. A further improvement was reported by Falcaro and coworker that noted the spontaneous crystallization of ZIF-8 around different biomacromolecules in water (Fig. 12.4) [49]. (patent WO2016/000032, earliest priority date 2014). We demonstrated that neither PVP nor alcohol was needed for the encapsulation of proteins and DNA in ZIF-8. This process was termed biomimetic mineralization, as the biomolecule acts as the seed for the heterogeneous nucleation of MOFs [17, 49, 59, 60]. This study reported that the enzymatic activity of the HRP@ZIF-8 and urease@ZIF-8 was maintained under inhospitable conditions (proteolytic agents, organic solvents and high temperature), demonstrating the protective properties of the ZIF matrix [49]. The protective capacity of ZIF-8 outperformed other porous carriers (i.e., CaCO3, mesoporous SiO2) when the enzymatic activity was measured under equivalent conditions [49]. Furthermore, the study reported the controlled release of the encapsulated biomacromolecules, triggered by the lowering of the pH to 6 and the dissolution of the ZIF-8 matrix, and moreover showed that the released biomolecules were active.
These pioneering studies on ZIF-based biocomposites paved the way for the development of several MOF-based biocomposites for biocatalysis, biomedicine, and biosensing [61]. Each synthetic parameter can play a significant role in the mechanism of formation and in the final properties of the biomacromolecule @MOF (e.g., bioactivity, protective properties and release profile). Given that the encapsulation methodology combines several steps of MOF biocomposite formation into one process (i.e., both MOF and composite synthesis), herein, we are examining this process in greater detail, as it is influenced by a number of interacting parameters. Hereafter, we examine how the different components can influence the synthesis, stability , and activity aspects of biomacromolecule @MOFs synthesized via one-pot encapsulation. In particular, we examine the surface chemistry and size of the biomacromolecules. Finally, we discuss the chemical and structural properties of the MOF and the role of different coprecipitation agents.
12.2.2.2.1 Influence of the Biomacromolecule Surface Chemistry on the Encapsulation Process
The surface chemistry of the biomacromolecule is crucial to the encapsulation process [62]. The electrostatics of proteins, carbohydrates, and viruses have been demonstrated to play a fundamental role in promoting or inhibiting its encapsulation within ZIF-8 [51, 52, 61, 63,64,65]. For instance, it was established that a negatively charged protein can enhance the concentration of Zn2+ cations at the protein surface, thus favoring the spontaneous formation of ZIF-8. Therefore, the nature of the protein will determine the success of the ZIF-8 biomimetic mineralization process (Fig. 12.5) [63]. However, it was also shown that it is possible to expand the process to positively charged molecules by chemical modification of the protein surface.
Plots of (a) the isoelectric points calculated for bovine serum albumin (BSA), pepsin, hemoglobin, and myoglobin, with and without the surface modifications; (b) the experimental zeta (ζ) potentials for the same biomacromolecules and their modified variants; and (c) the general changes in zeta potential for the chemical modifications used. (Adapted from Ref. [63] – Published by The Royal Society of Chemistry. https://creativecommons.org/licenses/by/3.0/)
This control over the surface charge of a biomacromolecule can promote (e.g., succinylation reactions) or inhibit (e.g., amination reactions) the rapid self-assembly of ZIFs formed via biomimetic mineralization [63]. The importance of the surface charge for the success of the biomineralization is not limited to proteins [51]. For example, Falcaro and coworkers found Dextran active as a nucleation seed for ZIF-8 growth only when functionalized with chemical groups with high affinity for Zn2+ (e.g., carboxyl functionalization) [51]. The understanding of this mechanism was crucial to develop biocomposites based on clinical Glycosaminoglycans and different ZIFs (e.g., ZIF-8, MAF-7 and ZIF-90) [64].
12.2.2.2.2 The Relative Size of Biomacromolecules and MOF Pores
A salient characteristic of the one-pot encapsulation strategies is that embedding occurs irrespective of biomacromolecule size. However, dimensions of the biomacromolecules, or assemblies thereof, could influence the spatial localization within the MOF biocomposite. For example, in the case of proteins, nucleic acids or carbohydrates, the typical biomacromolecule @MOF configuration is an MOF matrix with macromolecules localized in different pockets with distributions based on the chosen biomolecule [47, 49, 66]. However, micrometric biological entities, including viruses and cells, produce a core-shell configuration with an external polycrystalline coating of MOFs [52, 53, 55, 67]. Under optimal conditions, a continuous coating is formed and the perm-selective properties of the MOFs can be used to regulate the transport of molecules (e.g., glucose as nutrients) to the bioentity (e.g., yeast cell) [52, 67].
12.2.2.2.3 Influence of the Chemical Properties of the MOF on the Encapsulation Process
The MOF chemistry is relevant in the properties of biomacromolecule @MOF biocomposites. For example, it has been observed that the hydrophobicity/-philicity of the MOF matrix is a fundamental for the preservation of the biomolecular structure. In particular, Doonan and coworkers reported the encapsulation of enzymes in three different ZIF matrixes (ZIF-8, ZIF-90 and MAF-7) with different affinity to water [17]. The porous framework of ZIF-90 and MAF-7 is a hydrophilic environment that was shown to preserve the structure of the encapsulated enzymes and their catalytic activity. Conversely, the secondary structure of catalase encapsulated in ZIF-8 was degraded and its enzymatic activity severely reduced. This study highlighted the importance of MOF/biointerface chemistry for the preservation of the biological functions of encapsulated biomacromolecules.
12.2.2.2.4 Influence of Coprecipitation Agents on the Encapsulation Process
Coprecipitation agents are defined as additives that, when added to the building block solution of the biocomposite, facilitate the synthesis of biomacromolecules@MOFs . In the case of protein@ZIF-8 prepared via de novo [48] and coprecipitation [47] methods, PVP was used as an additive to stabilize the protein [56] and to facilitate the crystallization [57, 58] of the MOF. Therefore, the role of PVP can be envisaged as a coprecipitation agent. A different type of coprecipitation agent used in the water-based synthesis of MOFs are bases (e.g., NaOH, NH4OH). The addition of bases to the reaction mixture of MOFs is known to promote the ligand deprotonation and enhance the MOF self-assembly kinetics. Therefore, electron-pair donor compounds such as Lewis bases can be considered as coprecipitation agents and have been successfully applied to the synthesis of biomacromolecule @MOF biocomposites in water [17]. For example, Doonan and coworkers employed a small amount of ammonia (final [NH3]=0.1 M) to deprotonate 3-methyl-1,2,4-triazole and induce the formation of enzyme@MAF-7 biocomposites [17]. If a base is added to the reaction mixture, the stability of the biomacromolecule could be influenced by the high pH or by the nature of the used base [68]. These aspects should be investigated case by case. If the amount of base needed to deprotonate the MOF ligand does not affect the biomacromolecule structure, this strategy could be potentially extended to different MOFs that otherwise do not spontaneously crystallize around biomacromolecules in water.
12.2.2.2.5 Crystalline Phase of Biomacromolecules@ZIF-8
Most of the research on the direct encapsulation of biomacromolecules focuses on (sod) ZIF-8 as the MOF matrix [57]. However, by varying the relative concentration between metal cations, ligand and the biomacromolecule , or the overall precursor concentration, it was possible to produce ZIF-based biocomposites with different topologies including diamondoid (dia), ZIF-L or katsenite (kat) and with different crystalline phases, including ZIF-CO3-1 (aka ZIF-C, composed of Zn2+, HmIM, and CO32−), unidentified crystalline topologies (U12 and U13), and amorphous ZIF-based material [54, 69,70,71]. Notably, all these different phases spontaneously form in solution in the presence of protein (e.g., with no coprecipitation agents) if the proper concentrations of precursors are selected (Fig. 12.6a, b). Despite the same building blocks being used for MOF synthesis, these diverse phases afford different functional properties (e.g., porosity) and stability (e.g., dissolution kinetics in acid or in the presence of chelating agents, Fig. 12.6c) [54]. Investigations into the variety of different phases encountered for the encapsulation of biomacromolecules are at its infancy, but encouraging pioneering work suggests that engineering of ZIF crystalline phases will impel the progress of biomacromolecule @ZIF biocomposites. For example, Wu et al. demonstrated that the catalytic activity of the enzyme (e.g., glucose oxidase, GOx) encapsulated in amorphous ZIF was up to 20 times higher compared to the same enzyme encapsulated in ZIF-8 with sod topology [71]. The authors associated the higher performance of enzyme@amorphousZIF biocomposites with the presence of coordination defects and mesopores in the amorphous MOF particles that facilitated the reagents diffusion.
Ternary diagrams (by weight fraction) of bovine serum albumin (BSA), 2-methylimidazole (HmIM, labeled as ligand) and Zn(OAc)2·2H2O (labeled as metal). TD-H2O (a) represents the main phases (>50% wt) obtained by washing the sample with water. TD-EtOH (b) represents the main phases (>50% wt) obtained by washing the sample first with water and then with ethanol. (c) BSA release profiles from micrometric BSA@ZIF particles with different phases. (Adapted from Ref. [54] – Published by The Royal Society of Chemistry. https://creativecommons.org/licenses/by/3.0/)
12.2.2.2.6 Recent Developments of Encapsulation Synthetic Protocols
So far, all the discussed strategies for the synthesis of MOF biocomposites were solution-based syntheses performed in batch by mixing different reagents in a vial. Recently, in the field of direct encapsulation, two novelties were introduced: the syntheses using flow reactors and the mechanochemical syntheses (Fig. 12.7). Carraro et al. and Hu et al. simultaneously explored two different flow chemistry approaches for the synthesis of protein@ZIF-8 materials [72, 73]. Carraro et al. reported that the continuous flow synthesis of protein@ZIF-8 biocomposites could provide control over particle size (Fig. 12.7a) [72]. It was found that the synthesis of the protein@ZIF-8 biocomposite started with the formation of amorphous protein@ZIF-8 particles and that ethanol triggered the crystallization of the MOF. By using a simple flow setup (e.g., Y and T mixers, 1/16” PFA tubes), it was possible to control the residence time of the growing amorphous protein@ZIF-8 particles prior the introduction of an ethanol flow: this triggered the MOF crystallization and stopped the growth of the particle size. This strategy was employed to encapsulate a protein therapeutic (α1-antitrypsin) in ZIF-8. Hu et al. controlled the protein encapsulation in a microfluidic laminar flow system (PDMS chip) by tuning the residence time of the growing ZIF-8 particles prior to introducing a flow of the enzyme solution (Fig. 12.7b) [73]. This method yielded GOx@ZIF-8 that showed 98% of the activity of the native GOx, whereas bulk synthesized GOx@ZIF-8 showed less than 15% activity of the native GOx. The authors explained the enhanced activity by the presence of defects in the laminar flow synthesized MOF framework (e.g., mesopores due to Zn coordination defects) that were associated with an easier diffusion of reagents through the MOF matrix. We posit that the syntheses in flow could be applied to the high-throughput preparation of biomacromolecules@MOF composites with controlled properties (e.g., therapeutic dose and release profile).
(a) (i) Schematic representation of a microfluidic setup used for the preparation of protein@ZIF-8 composites; the residence time prior quenching can be varied by changing the length of the reactor or the flow rate. (ii) Average crystallite size of BSA@ZIF-8 obtained, versus the ethanol flow rate employed. The red line is the fitted exponential decay (crystallite size=a+b·e−x/t, with a=53±3, b=220±30, τ=0.6±0.1, x=flow rate ratio, R2=0.98). (iii) Average particle size obtained from AFM topography as a function of the residence time, including a power law fit of the experimental data (particle size=a+b·xc, with a=45±3, b=3±1, c=0.6±0.1, x=residence time, R2=0.97). (Adapted from Ref. [72], Published by Wiley-VCH Verlag GmbH & Co. KGaA. https://creativecommons.org/licenses/by/4.0/) (b) Top: schematic representation of the synthesis of enzyme@MOF biocomposites in a microfluidic laminar flow that lead to defective MOF particles, as reported in ref. [73]. Bottom: schematic representation of enzyme@MOF without defects obtained in bulk solution. (c) Schematic representation of the mechanochemical synthesis of enzyme@MOF biocomposites and of their biocatalytic activity and protective properties. (Adapted from Ref. [75], Copyright © 2019, Springer Nature https://creativecommons.org/licenses/by/4.0/)
In all these solution-based one-pot encapsulation strategies, the choice of the MOF matrix is limited to the compatibility of the MOF synthesis conditions and the stability of the biomacromolecules. This is particularly important in the case of proteins and nucleic acids that can irreversibly degrade if exposed to high temperatures, organic solvents, denaturing agents (e.g., urea), or extreme pHs [49]. Therefore, these wet approaches are generally only employed for MOFs that can assemble in mild conditions, like ZIFs [54]. To the best of our knowledge, there are no published examples of the one-pot encapsulation of biomacromolecules via solution-based synthesis in MOFs that are typically synthesized in harsh conditions (e.g., high temperature and organic solvents), such as UiOs [74]. As an alternative to solvent-based encapsulation strategies, mechanochemical synthesis was proposed for the direct encapsulation of biomacromolecules in MOFs (Fig. 12.7c) [75]. Mechanochemical processes (e.g., ball milling) are industrially scalable solvent-free methods and are commonly employed for the processing of different materials. Wei et al. reported the synthesis of enzymes@MOFs (e.g., β-glucosidase, invertase, catalase in ZIF-8, UiO-66-NH2, and Zn-MOF-74) via ball milling [75]. The powdered MOF precursors were added into a zirconia grinding jar containing lyophilized enzyme and the mixture was ground to obtain the enzyme@UiO-66-NH2 biocomposites. Once encapsulated, the enzymes maintained their enzymatic activity and showed increased resistance to proteases. Based on this result, ball milling processes are an attractive method to expand the choice of the MOF matrixes for the encapsulation of biomacromolecules. For each case, the compatibility of the ball-milling process with the stability of the biomacromolecule should be assessed.
12.2.2.2.7 General Considerations on Biomacromolecules@MOF Composites Obtained Via Encapsulation
When compared with surface immobilization of biomacromolecules on the MOF surface, encapsulation methods provide a high degree of protection against harsh environments (e.g., temperature, organic solvents, and proteolytic agents). For example, enzymes and antibodies encapsulated in MOFs are usually not affected by proteolytic agents, since the porous framework acts as a molecular sieve and blocks the access of these digestive agents [17, 76]. In comparison to infiltration methods, encapsulation has the advantage of being MOF pore size-independent, as the MOF grows around the biomacromolecules [49]. In fact, even micrometric bioentities, including virus and cells, can be encapsulated in MOF shells following one-pot encapsulation methods [52, 53, 55, 67]. In the case of smaller bioentities, their spatial distribution within MOF crystals is not defined a priori as in the case of infiltration strategies, but depends on the bioentity nature and synthesis conditions. The recyclability of the encapsulated biomacromolecule is usually improved when compared to biocomposites prepared via surface immobilization [33]. In fact, repeated washings of a biomacromolecule @MOF biocomposite typically do not show significant leaching [17, 48]. Conversely, in the case of protein adsorption, repeated washings, especially under conditions that weaken the non-covalent interactions, can lead to the removal of the adsorbed biomacromolecule [17, 48].
12.2.3 General Properties of MOFs Biocomposites
Prior to discussing the different class of MOF biocomposites and their applications, we introduce some fundamental concepts such as controlled release, biocompatibility, and particle size. These properties are often used to assess the performances of biocomposites for biomedicine and other biotechnological applications.
12.2.3.1 Controlled MOF Degradation and Cargo Release
Pharmacokinetics describes the fate of an administrated substance and includes the uptake by the body, its transformation, the biodistribution in the tissues, and finally, its removal from the organism [77]. To understand the therapeutic properties of a drug, pharmacokinetics studies are needed. The biodistribution is related to the transfer and accumulation of the drug/carrier within the body [78]. Studies of the localization of the drug delivery system provide information on the biodistribution that describes the capability of the system to target organs [79]. The use of nanocarriers for drug delivery can allow precise control over different aspects of pharmacokinetics and biodistribution, by modifying the physicochemical properties of the carrier.
An additional relevant aspect in drug delivery is the release profile that describes the amount of drug that is released from the carrier into the surrounding environment as a function of time [80]. The release kinetics can drastically influence the therapeutic effect of a drug and the efficacy of an administration method [80]. For example, a fast release is usually preferred for analgesics and anticoagulants [81]. Conversely, a slow release would be preferred for prolonged treatments that could replace frequent administration via parenteral route [82,83,84]. For example, treatments that require frequent injections and, consequently, pain and discomfort for the patient are protein-based treatments such as insulin, growth hormones, or oxytocin [84, 85]. Typically, the drug release profile from a carrier is characterized by a typical unwanted initial burst and followed by a slower sustained release [86]. In the case of MOF biocomposites, by tuning the carrier structure (e.g., different MOF topologies [8]), composition (e.g., different MOFs [8]), or the drug spatial localization (e.g., different drug immobilization methods as previously discussed) within the carrier, the burst effect could be minimized and a steady sustained drug release could be obtained [86].
Recently, research into drug delivery has moved from regular drug delivery systems (DDS) [87] that exploit nonspecific diffusion to active-targeting and stimuli-responsive materials that can control the carrier localization, release time, and dosage [87]. Internal stimuli (e.g., pH, chemical environment, and temperature) that are related to the local environment of the target cells/tissues could trigger the carrier decomposition and the drug release. Alternatively, the release could be regulated via external controls like light, magnetic field, or temperature [88]. In this context, MOFs possess properties that can be exploited for their use as carriers. By selecting the appropriate building blocks, it is possible to synthesize MOFs with different stabilities to chemical or physical stimuli and impart either regular DDS properties or triggered-release responses. For example, MOF-based systems have been shown to change their structure or to decompose under specific conditions including acidic pH, presence of certain anions, and irradiation with light [88].
An exemplary case of a responsive MOF for drug delivery is ZIF-8. The widespread interest in ZIF-8 is due to several reasons: (i) the encapsulation of drugs/biomacromolecules can be performed in aqueous media; (ii) the drug/biomacromolecule loading and release efficiency can reach 100%; (iii) ZIF-8 matrix can protect the cargo against harsh conditions; and (iv) the cargo release can be controlled either by exposing the ZIF biocomposite to pH below 6.5 or to chelating agents (e.g., ethylenediaminotetraacetic acid, EDTA) [89]. Since the cargo is released via the decomposition of ZIF-8, it is important to study the effect of different chemical environments on the MOF stability and the degradation mechanism. Only a profound understanding of these aspects will permit to design a ZIF-8 drug delivery system with precise controlled release properties. For example, Luzuriaga et al. showed that ZIF-8 particles are degraded in several buffer solutions that are commonly used to mimic the physical conditions [90]. Phosphate buffer solution 1X (PBS 1X) is commonly employed because it closely mimics the pH, osmolarity, and ion concentrations of the human body. In a detailed study, Velásquez-Hernández et al. investigated the mechanism of ZIF-8 particle degradation in PBS 1X (Fig. 12.8) [89]. It was found that the coordination equilibrium between Zn2+ and HmIM in solution is changed by the presence of a phosphate buffer. Due to having a high affinity for Lewis metal centers, the phosphates induce the formation of insoluble zinc phosphate by-products and favor the release of HmIM from the composite into solution. The pH of the buffer (pH = 7.4) may also favor this process, since under these conditions, the ligand can be protonated (pKa1 = 7.85; pKa2 = 15.1) and Zn2+ coordinating ability is compromised. These investigations into the stability of MOFs in buffer solutions and bodily fluids are fundamental to anticipate side effects for MOF-based DDS [7] and furthermore to assess biocatalytic and biosensing activity data for MOF biocomposites (e.g., enzyme@MOF) that are often tested or stored in different buffers and pHs conditions [91].
(a) EDX elemental maps of fresh ZIF-8 powder. (b) EDX elemental maps of the powder recovered after 24 h of incubation in PBS 1x pH 7.4. (c) 31P NMR of PBS prepared in D2O before (lowest trace) and after adding ZIF-8 particles (0.5 mg mL−1, 1 and 24 h, middle and upper trace, resp.). (d) Quantitative determination of 2-methylimidazole released after the incubation process in PBS (1 h, 3 h, 6 h and 24 h). (Reproduced with permission from Ref. [89] – Published by The Royal Society of Chemistry. https://creativecommons.org/licenses/by/3.0/)
12.2.3.2 MOF Biocompatibility
When an MOF biocomposite is used for drug delivery, the MOF is degraded and releases both the drug and the MOF building blocks (i.e., cations and ligands) in the body. Therefore, a fundamental step for the development of MOF biocomposites for biomedical applications is the assessment of the toxicity of MOF constituents. Horcajada, Serre, and coworkers suggested the use of MOFs made of nature-derived or biocompatible building blocks and named them bioMOFs (Fig. 12.9) [7]. Endogenous molecules (amino acids, peptides, proteins, nucleobases, carbohydrates, and porphyrins) or exogenous bioactive ingredients (nicotinic acid, curcumin, olsalazine, and some dicarboxylic acid including fumaric acid) were selected as ligand candidates [7]. For the metal nodes, cations that are part of the daily requirement of the human body would be the best choice [92]. Nevertheless, each metal has its own toxicity which is quantified by the median lethal dose (LD50). LD50 is defined as the amount of compound required to kill 50% of a tested population within a selected time [92]. Based on this, the metal cations with low LD50 values that can be used for the synthesis of biocompatible MOFs are Mg2+ (LD50 MgSO4 = 5000) > Ca2+ (LD50 CaCl2 = 1940) > Fe3+ (LD50 FeCl3 = 450) > Fe2+ (LD50 FeCl2 = 984) > Zn2+ (LD50 Zn(OAc)2 = 100–600) [7, 93]. Referring to ZIF-8, it has been determined that an excessive concentration of this MOF has a cytotoxic effect on different cell lines (i.e., HEK-293, MDA-MB-231, HaCaT, RAW 264.7, NIH/3T3, and MG-63) [94]. The reason proposed was that the released Zn2+ cations activate apoptotic pathways in cells; however, it was found that a concentration of up to 30 μg mL−1 only causes a small reduction of cell viability to approximately 80% (i.e., IC20) compared to the control, and thus any value below this threshold would be suitable for drug delivery applications [94].
Schematic view of the building blocks used for the synthesis of BioMOFs, the concept is explained in detail in Ref. [7]
These values are useful to perform a preliminary assessment of the amount of an MOF that could be administrated in one dose. However, MOFs that target clinical biomedical applications would need to be studied both in vitro and in vivo. In vitro studies provide fundamental information on some aspects of cytotoxicity, but the biocompatibility of a new material cannot be fully assessed without in vivo studies. In fact, inside a living system, there are several important aspects (e.g., interferences, permanence in the circulatory system, accumulation in organs, and immune response) that could influence the MOF toxicity or show side effects that are not predictable from in vitro studies [95].
12.2.3.2.1 Biocomposite Particle Size
Biocomposite-based DDS are appealing for different administration routes, including parenteral injection and inhalation [96]. The particle size and shape of biocomposites play a crucial role for blood circulation time, biodistribution, and cellular internalization [97, 98]. In the case of cellular internalization, different mechanisms, including phagocytosis, micropinocytosis, or caveolar-mediated endocytosis, are particle size-dependent [99, 100]. Small particles (<5 nm) can be rapidly removed from blood circulation through extravasation or renal clearance [97]. Conversely, larger particles (10 nm to 15 μm) accumulate mainly in the liver, spleen, and bone marrow and are then removed from circulation by cells of the reticuloendothelial system (RES) [97]. Finally, particles larger than ~15 μm can be removed from the blood circulation by mechanical filtration in capillaries [97]. However, microparticles can be used for other drug administration routes. For example, microparticles could be used for transdermal and subcutaneous administration [96, 101].
MOF nanoparticles in the tens to hundreds of nanometers size range, administered via intravenous/subcutaneous injections, have shown to be promising nanocarriers for imaging agents and drug molecules [95, 102, 103]. Nevertheless, very small nanoparticles would not be suitable for all biomedical applications. For example, it was reported that nanoparticles smaller than 10 nm will not penetrate through the stratum corneum into viable human skin if administrated transdermally, but rather have a tendency to accumulate in the hair follicle openings [104]. On the other hand, particles in the 300 nm–1.5 μm size range can be used for transdermal administration [105]. For example, biodegradable microparticles (0.3–2 μm) were investigated for vaccine delivery and some formulations are now in clinical trial [106, 107]. Furthermore, large particles could have an increased stability in physiological conditions and a reduced aggregation that could be exploited for different applications (e.g., prolonged release) [54, 89, 96, 108]. For these reasons, the control of the particle size is one of the key points for the further development of MOF biocomposites for biomedical applications. In general, we posit that the control of particle size is crucial in most of the MOF biocomposite applications, ranging from biocatalysis and biosensing (e.g., molecular diffusion of reagents or analyte within the bulk of the porous structure [38]) to biobanking (e.g., MOF degradation kinetics could depend on particle size [89]).
12.3 Applications of Biomacromolecules and MOF Biocomposites
12.3.1 Protein@MOF as Drug Delivery Systems
Proteins are biomacromolecules composed of amino acid building units and play an important role in regulating body homeostasis, as these biomacromolecules are involved in many cellular functions such as gene regulation, signaling, and immune response [109]. Protein disorders (e.g., deficit or dysfunction of specific proteins) can cause chronic diseases such as Parkinson, Alzheimer, or diabetes mellitus [110, 111]. So far, the best strategy to treat this class of degenerative diseases is through the administration of proteinaceous drugs. For instance, one of the most effective therapies for diabetes mellitus type I and type II is the administration of the protein insulin [83]. In general, protein-based therapeutics offer higher specificity and potency than small molecule equivalents [112, 113]. However, the properties of proteinaceous therapeutics such as specificity and bioactivity depend on their 3-dimensional structure, which can be easily altered with minor modification to their environment, thereby reducing the bioactivity and loss of therapeutic effectiveness [112, 113]. Furthermore, once administered in biological systems, the efficacy of such therapeutics can be reduced due to fast renal clearance, fast degradation by proteolytic agents, or difficulties in crossing cell membranes [84, 85]. A promising strategy to improve protein stability and enhance intracellular delivery is their embedding in MOF carriers either using infiltration or one-pot encapsulation strategies (Fig. 12.4) [6, 47, 49]. Here, we will predominantly discuss encapsulation methods. As noted, a considerable advantage of encapsulation is that proteins of any size and shape can be embedded into MOFs (i.e., the MOF pores size does not impose a restriction on the size of the encapsulated guest biomacromolecule ).
Among the various MOF-based drug delivery systems (DDS), protein@ZIFs for the intracellular delivery of proteinaceous therapeutics for cancer treatment have gained special attention. In fact, ZIFs are one of the most widely studied MOF class for DDS and it is particularly attractive as stimuli-responsive DDS [88]. The pioneering study by Qu et al. [114] demonstrated the feasibility of ZIF-8-based nanocarriers for intracellular delivery of ovalbumin (OVA), which is a protein antigen that can trigger a humoral and cellular immune response. The OVA@ZIF-8 biocomposite was prepared through the de novo approach (Fig. 12.4). Subsequently, the resultant OVA@ZIF-8 biocomposite was further functionalized by the adsorption of an immune adjuvant (cytosine-phosphate-guanine oligodeoxynucleotides, CpG ODNs) to afford a core-shell nanocomposite OVA@ZIF-8-CpG (particle size = 200 nm). The colocalization of the antigen and the immune adjuvant enhanced cellular uptake, whereas the acidic environment of lyso/endosomes triggered the cytosolic release of the cargo. This study demonstrated that the encapsulation of OVA within an MOF-shell enhanced the in vivo protection against blood proteases and the delivered OVA activated a systemic immune response. A relevant challenge in DDS for cancer therapy is the enhancement of the cellular uptake and improvement of targeting properties toward tumor cells [115]. One possible strategy for enhancing the target intracellular delivery of the cargo is coating the outer surface of NPs with extracellular vesicles or membranes [115, 116]. For instance, Chu and coworkers prepared BSA@ZIF-8 nanoparticles (ca. 90 nm) and subsequently coated the biocomposite with polyvinylpyrrolidone (PVP) [59]. The PVP coating was found to enhance the stability of protein@ZIF-8 in cell media and improved the cellular uptake. Once within the cell, the acidic environment triggered the ZIF-8 decomposition and the release of the protein cargo (Fig. 12.10a). Live-cell studies confirmed the rapid cellular uptake of PVP-coated BSA@ZIF-8 NPs and such nanocarriers were successfully transported from endo-lysosomes into the cytosol affording an efficient intracellular co-delivery of multiple active proteins.
(a) Schematic representation of the endo-lysosomal release of proteins using, as drug delivery system, protein@ZIF-8 nano-biocomposite functionalized with PVP. (Reprinted with permission from Ref. [108], Copyright 2018 American Chemical Society). (b) Preparation of cell-like biomimetic platform CAT-PS-ZIF@Mem for targeted photodynamic therapy. (c) In vivo fluorescence images and ex vivo tissue imaging of HeLa tumor-bearing mice after intravenous injection of A) CAT-PS-ZIF@Mem and B) CAT-PS-ZIF taken at different times: 0 h (bright field), 1 h, 4 h, 6 h, 8 h, 24 h, 48 h, 72 h, (from A1B1 to A8B8). Tissue imaging of the mice after 72 h post injection, in which the number of 1–7 representing muscle, heart, liver, spleen, lung, kidney, and tumor, respectively (A9, B9). (Adapted with permission from Ref. [118], Copyright 2016 John Wiley and Sons)
Similarly, Zheng et al. [117] reported the encapsulation of gelonin, a ribosome-inactivating polypeptide used as apoptotic agent, within ZIF-8 nanoparticles (ca. 80 nm). The resultant biocomposites were coated with an extracellular vesicle to assist the internalization within homotypic cells. Vesicle gelonin@ZIF-8 biocomposites improved the specificity of the treatment and allowed for a systemic drug administration without compromising the integrity of toxic gelonin. This strategy has been further explored for the localized treatment of malignant tumors using bioactive MOF composites capable of producing cytotoxic agents on demand. For a similar application, Cheng et al. [118] designed an MOF nano-biocomposite for the spatio-temporal controlled production of cytotoxic 1O2 species upon applying a near-infrared irradiation (NIR) stimulus (photodynamic therapy). The bioactive composite was obtained by the coencapsulation of catalase (CAT) and Al(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4), which acts as photosensitizer (PS), within a ZIF-8 shell. The resultant MOF biocomposite was coated with a cancer cell membrane (Mem) leading to CAT&PS@ZIF-8@Mem NPs (particle size = 110 nm) (Fig. 12.10b). The Mem coating on CAT&PS@ZIF-8 possessed specific adhesion properties toward tumor cells, which afforded targeting and cell uptake, leading to preferential accumulation within tumor cells (Fig. 12.10c). Then, once the nanocarrier was internalized, CAT catalyzed the decomposition of endogenous intracellular H2O2 into H2O and O2, which increased the level of O2 within the hypoxic tumor cells. The resultant O2 was transformed by the PS into 1O2 upon NIR irradiation, and this highly reactive species caused lethal cell damage.
MOF-biocomposites have been used for the treatment of other pathologies, including protein disorder-related diseases. For instance, Willner’s group [119], developed an MOF-based glucose-responsive carrier for the controlled release of insulin (In) for diabetic treatments. Glucose-responsive properties were obtained by coencapsulation of insulin and GOx in ZIF-8 to yield an In&GOx@ZIF-8 biocomposite. In this system, GOx was used to catalyze glucose and O2 into gluconic acid and H2O2. The porosity of the MOF matrix allowed the reagents to reach the encapsulated enzyme. This catalytic reaction resulted in an acidified microenvironment, which triggered the degradation of the ZIF-8 matrix, thus releasing the encapsulated insulin (Fig. 12.11a). As H2O2 could inhibit the GOx enzymatic function and act as a cytotoxic agent, catalase (CAT) was coembedded in ZIF-8 to decompose H2O2 into H2O and O2. In this study, the authors demonstrated that the enzymatic cascade GOx/CAT reaction could be activated or inhibited by varying the concentration of glucose, and thereby controlling the insulin release on-demand (Fig. 12.11b, c). More recently, Tang et al. [105] used In&GOx@ZIF-67 (ZIF-67 made of Co2+ and HmIM) for the fabrication of a stimuli-responsive transdermal insulin delivery system (microneedles patch). However, in this case, the ZIF-67 matrix, which exhibited a catalase-like activity due to the presence of Co2+ ions as inorganic nodes, acted as the H2O2 scavenger replacing the CAT in the multi-enzyme cascade process for the controlled release of insulin in presence of glucose (Fig. 12.11d). With respect to employing ZIF biocomposites for insulin delivery, Carraro et al. investigated crystalline phases beyond ZIF-8 and reported a systematic study of different crystalline phases of In@ZIF systems [54]. It was shown that different Zn-mIM-based polymorphs (dia=diamonoid, sod=sodalite, U13, and ZIF-C=ZIF-CO3-1 [120]) can be prepared by varying the relative amount of ligand, metal, and biomacromolecule . We examined the encapsulation efficiency (EE%) and drug release kinetic of selected In@ZIF composites upon applying an acidic stimulus (pH 5.5). A phase-dependent release profile was observed and the complete release of insulin under acidic conditions (pH 5.5) occurred between 40 and 300 min, depending on the crystalline phase of the MOF (e.g., In@U13 – 100% release time in 40 min; In@ZIF-8(dia) – 100% release time in 300 min) [54]. Thus, this work shows that the crystalline phase of the MOF matrix is a relevant parameter for the design of MOF carriers.
(a) Schematic synthesis of the In&GOx@ZIF-8 composites and the stimuli induced release of the cargo triggered by the enzymatic oxidation of glucose. (b) Fluorescence spectra of FITC-labeled insulin released upon subjecting the FITC- In&GOx@ZIF-8 composites to different concentrations of glucose for a fixed time interval of 1 h: (a) 0 mM, (b) 1 mM, (c) 5 mM, (d) 10 mM, (e) 50 mM. (c) Switchable time-dependent release of FITC-labeled insulin in the presence of high (15 mM, blue) and low (5 mM, yellow) concentrations of glucose. (Adapted with permission from Ref. [119], Copyright 2018 American Chemical Society). (d) Schematic representation of the transdermal delivery of insulin encapsulated within In&GOx@ZIF-67 biocomposite. (Reprinted with permission from Ref. [105], Copyright 2020 American Chemical Society)
Although triggered-release properties of ZIFs in DDS have mostly been tested with pH-changes, several groups showed that ZIF-8 can be slowly degraded in PBS media [89, 90, 121]. The degradation occurs because of the affinity of the phosphate anions for the Zn2+ cations, which leads to the formation of amorphous zinc phosphate. The biodegradability of ZIFs in the presence of phosphate groups has inspired the development of ATP-responsive drug carriers based on ZIF-90 biocomposites. ZIF-90 is a structural analog to ZIF-8 made of Zn2+ ions interconnected by imidazole-2-carboxaldehyde that was used by Mao et al. [50] for the encapsulation of RNase A and genome-editing Cas9 nuclease (protein) CRISPR/Cas9. Cas9 is an RNA-guided endonuclease protein used to edit the genome of mammalian cells. Due to the higher concentration of ATP in the cytosol (1–10 mM) than in the extracellular environment (<0.4 mM), the RNase A and CRISP/Cas9 genome editing complex was selectively delivered within the cells.
Recent studies have shown that the one-pot encapsulation of biomacromolecules within hybrid matrices can be extended to carboxylated ligand-based MOFs . For example, Liu and coworkers [122] developed a drug carrier comprised of meso-2,6-diaminopimelic acid (DAP) interconnected by Mn2+ ions for targeted drug delivery of OVA. It is worth noting that in this material, the DAP functioned as both ligand and adjuvant, as it acts as a Nod1 agonist. Therefore, the resultant nanoparticles OVA@Mn-DAP (ca. 150 nm) ensured the co-delivery of an antigen protein and the adjuvant, which improves the cancer immunotherapy by preventing the growth of melanoma tumors. This study demonstrated that the retention of the biotherapeutics (OVA and DAP) in the lymph nodes increases when using OVA@Mn-DAP as a DDS in comparison to the administration of the free species (OVA and DAP). A further interesting example by Sung, Chang, and coworkers encapsulated OVA within MIL-53(Al)-NH2 for oral administration [123]. To facilitate the permeation of the biocomposite through the mucosa barrier, the authors embedded OVA@Al-MOF particles within yeast-derived capsules. In vivo studies revealed that this coensembled arrangement functioned as “Trojan Horse”-like platform, allowing for the transepithelial transport of OVA@Al-MOF.
In summary, MOFs have emerged as a new platform for the design of protein-based drug delivery systems, with the encapsulation pathway commonly used to form such composites. Careful choice of the target biological system (e.g., cancer cells) and the MOF (e.g., ZIFs) allows the DDS to be engineered to dissolve under selected chemical conditions. In case of ZIF-based biocomposites, it was shown that systems where both enzymes and therapeutics are embedded can be precisely engineered to be responsive to specific chemical stimuli. Furthermore, the MOF surface can be functionalized, thus improving the target properties or the circulation time of the biocomposite. By controlling degradation conditions, particle size, and surface chemistry, important properties such as sustained and targeted delivery and biodistribution can be enhanced.
12.3.2 Protein@MOFs for Biopreservation
The bioactivity of a protein depends on its 3-D conformation, and thus, structural changes caused by environmental stressors can lead to protein denaturation and bioactivity loss. The relatively “fragile” nature of biomacromolecules is the major issue that limits their application as therapeutics and biosensing components [112]. One strategy to preserve the bioactivity of proteins in solid state is through lyophilization or freeze drying [124]. However, a considerable amount of protein-based therapeutics are formulated as aqueous solutions. Those formulations are typically stored and transported at low temperatures (aka “cold-chain”) to improve their shelf life. Regarding another class of biological assemblies, that is, vaccines, the World Health Organization (WHO) suggests their storage at a temperature that ranges from 2 to 8° C [124, 125]. The infrastructure required for the cold-chain increases the shipping costs and hampers the distribution/storage of vaccines to geographically remote places. An emerging strategy to address these problems is the encapsulation of vaccine components within MOF materials, as this has been shown to enhance the stability of the biomolecule against harsh conditions including elevated temperatures, organic solvents, mechanical stress, and the presence of proteolytic agents. For instance, in 2015, Falcaro and coworkers studied the biopreservation capabilities of MOF-based biocomposites obtained via biomimetic mineralization approach (Fig. 12.4) [49]. In that work, the enzymatic activity of free HRP and HRP@ZIF-8 upon exposure to inhospitable environments was compared, including the presence of a proteolytic agent (trypsin) and hot solvents (water and DMF, Fig. 12.12a). According to this study, in the presence of trypsin, free enzyme exhibited only a 20% of its enzymatic activity for the conversion of pyrogallol to purpurogallin, whilst HRP encapsulated within a ZIF-8 exoskeleton retained 88% of its initial enzymatic activity. The protective properties of ZIF-8 were compared to other porous materials such as CaCO3 and mesoporous SiO2. For this purpose, free HRP, HRP@ZIF-8, HRP@CaCO3, and HRP@SiO2 were incubated in boiling water for one hour. The enzymatic assay performed after the incubation process revealed that the free enzyme lost its activity, while HRP@CaCO3 and HRP@SiO2 only retained 39% and 65% of the bioactivity, respectively.
(a) Schematic illustration of the biomimetic mineralization of HRP within ZIF-8. Biopreservation performance of different biocomposite materials upon exposed to drastic conditions. (Adapted with permission from Ref. [42], Copyright 2015 Nature communications). (b) Schematic representation of the cold-chain-based biospecimen preservation. (Reprinted with permission from Ref. [127], Copyright 2018 American Chemical Society). Biopreservation efficacy of insulin (c) and antibodies (d) encapsulated within different MOF shells upon being exposed under various environmental stressors. (Reprinted with permission from Refs. [130] and [76] respectively. Copyright 2018 John Wiley and Sons)
By contrast, HRP encapsulated within ZIF-8 preserved 88% of its initial activity (Fig. 12.12a). The superior stability afforded by the ZIF-8 exoskeleton compared with CaCO3 and SiO2 was experimentally correlated to the tight encapsulation of the enzyme within the MOF, where the biomolecules are enclosed in pockets on slightly larger than the size of the biomacromolecule . The presence of such mesopores was confirmed by a SAXS investigation on the ZIF-8-based biocomposites. The results revealed the formation of pockets within the MOF matrix that are 10–30% larger than the radius of gyration of the encapsulated biomacromolecule . Such confinement effects were postulated as the reason for the preservation of the biomacromolecules conformation and bioactivity [126].
The protective properties of ZIF-8 were further studied by Singamaneni et al. [127] for the preservation of biomarkers to improve their integrity during transport, storage, and handling. Two biospecimens were employed in this study: (i) neutrophil gelatinase-associated lipocalin (NGAL), a protein present in blood after acute kidney injury [128] and (ii) serum/plasma CA-125, a tumor marker from ovarian cancer cells [129]. The preparation of biomarker@ZIF-8 biocomposites was successfully conducted in different biological fluids including urine, serum, plasma, and blood. Once encapsulated, the samples were supported on paper substrates and stored in a dry state. The samples were transported around the United States (with a distance of over 2500 Km for 10 days using the regular US mailing service under unknown shipping and handling conditions, Fig. 12.12b). This study also demonstrated that the biomacromolecules are active even after being exposed to 40° C. The same research group demonstrated that this strategy could be further applied for the biopreservation of insulin (In) [130]. In that work, the authors compared the biological activity of free In and In@ZIF-8 after being exposed to various stressors, including high temperatures, (25, 40 and 60 °C for one week), mechanical agitation (200 rpm for 48 h), and incubation in organic solvents (ethyl acetate) (Fig. 12.4c). An immunoassay test and spectroscopic analysis demonstrated that the free In stored in a dry state at high temperatures (25, 40 and 60 °C) suffered a biological activity loss (≈70%, ≈60% and ≈50%; respectively). By contrast, the In released from In@ZIF-8 after being stored under the same temperatures preserved more than 80% of its initial activity (>95% at 25°C and 40°C, >80% at 60°C). A similar study was performed by Chen and coworkers [76] who tested the stability of polyclonal antibodies including human immunoglobulin G (IgG), polyclonal antibody (H-IgG), and goat anti BSA IgG (G-IgG) encapsulated within two different MOF matrices (ZIF-8 and ZIF-90). To evaluate the protection effect of the MOF matrix on G-IgG@ZIF-90 and G-IgG@ZIF-8 biocomposites, the samples were exposed to a series of environments that would typically lead to denaturation of proteins (i.e., high temperatures, organic solvents, and mechanical pressure). Subsequently, the bioactivity of the encapsulated and free G-IgG was assessed by enzyme-linked immunosorbent assay (ELISA) test. The results revealed that the free G-IgG antibody, stored at 75° C, lost its initial binding activity (< 10%) and presented severe aggregation (88%). By contrast, the G-IgG released from the MOF matrix retained its binding capability (>90%), and showed low aggregation (13–25%) after being exposed to 75° C for 20 min (Fig. 12.12d). These results highlight that MOF matrices can protect antibodies from thermal decomposition.
In summary, the preparation of biomacromolecules@MOFs was found to be an effective strategy for biospecimen preservation due to the unprecedented protection properties and on-demand degradability of the MOF matrices. Hormones, enzymes, biomarkers, vaccines, and antibodies have all been encapsulated within ZIF-8 and protected from temperature, solvents, and mechanical stress. After their release from the ZIF matrix, their activity was found always superior to the free biomacromolecules exposed to identical conditions. This represents an emerging attractive technology alternative to cold-chain transportation and storage of biotherapeutics, potentially reducing their shipping costs and enhancing their use.
12.3.3 Protein-On-MOFs and Proteins@MOFs Biocomposites in Assays
A biosensor is a self-contained integrated device capable of providing selective quantitative or semiquantitative analytical information [131]. The biosensor is constructed by placing a biological recognition element in direct spatial contact with a signal transducer, allowing it to convert a biological response mediated by enzymes, immunosystems, or cells into a quantified processable signal. The biological recognition unit acts as a chemical receptor that responds selectively to a target analyte, and this response is transformed by the transducer into an electrochemical, colorimetric, or optical signal [132,133,134,135,136]. Although different sensing, transduction, and integration methods are available, sensitivity and reproducibility remain the major challenges in current diagnostic technologies to facilitate early diagnoses and prompt treatments. In this sense, protein-based MOFs biocomposites are emerging materials for the design of new, highly sensitive, and cost-effective biosensors [137,138,139,140,141]. In such systems, the protein acts as a biorecognition element and it can be either embedded (protein@MOFs ) in or bioconjugated to MOFs (protein-on-MOFs) [138]. The use of MOF composites as detection probes permits the colocalization of the biorecognition element and a large number of signaling elements in one single particle, thereby improving considerably the detection threshold of the system. So far, MOF biocomposites have been extensively studied for sensing a wide variety of analytes ranging from small molecules (glucose, H2O2, phenol, etc.) – generally exploiting the catalytic activity of supported enzymes – to large biomolecules such as antigens, biomarker, infectious agents and exosomes – generally exploiting the targeting capabilities of supported antibodies [137,138,139,140,141].
12.3.3.1 Applications of Protein@MOF Biocomposites for Small Molecule Detection
In biochemistry, an analyte with molecular weight below 1000 Da is classified as small molecule. On this basis, most of the reports about the use of protein@MOF composites for small molecules sensing are focused on the detection of H2O2 and glucose, and are based on the catalytic activity of encapsulated enzymes. These proof of concepts are discussed below.
12.3.3.1.1 Protein@MOF as H2O2 Sensors
In biology, hydrogen peroxide (H2O2) is an important reactive oxygen species obtained as by-product of numerous metabolic reactions. Although H2O2 plays an important role in the transmission of cellular signals, H2O2 can decompose to hydroxyl radicals, which are strong oxidants capable of reacting with biological molecules and causing damage to cells and tissues. Therefore, it is important to develop new biosensing technologies for the detection of H2O2 in living organisms (e.g., determination of absolute rates of H2O2 production and steady-state concentrations in cells) [142]. A pioneering report by Ge and Liu et al. [47] in 2014 suggested the use of Cyt c encapsulated within ZIF-8 as fluorometric sensor to detect H2O2, methyl ethyl ketone peroxide (MEKP), and tert-butyl hydroperoxide (TBHP) in solution. The authors used N-acetyl-3,7-dihydroxyphenoxazine (Amplex Red, fluorogenic probe) as a signal molecule, since in the presence of the target peroxides Cyt c catalyzes the oxidation of Amplex Red to yield a fluorescent phenoxazine (i.e., resorufin) (Fig. 12.13) [143]. This work inspired the development of other protein@MOF biosensors for the detection of H2O2 [144, 145]. For instance, Yang et al. [145] designed a colorimetric biosensor encapsulating hemoglobin (BHb) in ZIF-8 particles; while H2O2 was detected by using 4-aminoantipyrine (AAP) as signal molecules, the peroxidase-like activity of BHb@ZIF-8 was used to perform the catalytic co-oxidation of phenol and AAP in the presence of H2O2 [146]. The catalytic activity of this system was 423% higher than that observed in the free BHb. Additionally, the BHb@ZIF-8 sensor showed a faster catalytic response (4 min) than the free enzyme (15 min), and a wide linear range (0–800 μM) for H2O2 with a limit of detection (LOD) of 1 μM.
(a) Schematic representation of the synthesis of Cyt c@ZIF-8 biocomposites and TEM image of the Cyt c@ZIF-8 composite. (b) Fluorometric detection of H2O2 using the enzymatic activity of Cyt c. The graph shows the relative peroxidase activity of Cyt c, Cyt c@ZIF-8 composite, PVP/Cyt c mixture, Cyt c/zinc ion mixture, Cyt c/2-methylimidazole mixture, and Cyt c/ZIF-8 mixture. (Adapted with permission from Ref. [47], Copyright 2014 American Chemical Society)
12.3.3.1.2 Protein@MOF as Glucose Sensors
The relevance of glucose detections relies on its relationship to diabetes. This disease results in abnormal levels of insulin in the body, due to either a malfunction of the pancreas (diabetes type 1) or the ineffective use of insulin by cells (diabetes type 2). Insulin is the hormone that regulates the level of glucose in the blood, and thus, its deficiency in diabetic patients can cause hypoglycemic or hyperglycemic conditions, leading to severe health issues including tissue damage, kidney failure, and blindness, among others [147]. As a consequence, regular glucose monitoring in diabetic patients can prevent further health complications. The use of MOF-based biocomposites for the enzymatic detection of glucose has been extensively explored mostly as colorimetric or electrochemical sensors [141]. Liu and coworkers reported the first example of a colorimetric glucose biosensor based on the coencapsulation of multiple enzymes (GOx and HRP) in ZIF-8 particles [148]. This multi-enzyme system (GOx&HRP@ZIF-8) operates via a biocatalytic cascade process: (1) GOx in the presence of O2 catalyzes the oxidation of glucose to yield gluconic acid and H2O2; (2) HRP consumes H2O2 for the oxidation of ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) into ABTS•+. The latter is a chromogenic agent that can be monitored by UV-vis spectroscopy at 420 nm. The reported limit of detection (LOD) was 0.5 μM, demonstrating a sensitivity higher than the most common colorimetric glucose sensors. Additionally, irrespective of interfering compounds (e.g., like fructose, maltose), GOx&HRP@ZIF-8 showed specificity toward glucose detection. It is worth mentioning that the two enzymes are randomly distributed through the MOF particle, but their close spatial location in the porous microenvironment facilitates molecular diffusion and enhances the efficiency of the enzymatic cascade reaction.
Recent studies further supported the importance of the spatial distribution of the enzymes within the MOF composites for enhanced multi-enzyme cascade catalysis [149]. For instance, Jiang et al. [150] demonstrated that the compartmentalization of GOx/HRP multicatalytic system within ZIF-8 is an effective strategy to improve the sensitivity and increase the linear range of colorimetric biosensors for glucose detection. The compartmentalization of the enzymes was achieved by mixing sodium deoxycholate (NaDC), HRP, and the Zn2+ precursor. This strategy permits the embedding of HRP in a hydrogel coating . Then, a second solution containing both HmIM and GOx was added to this mixture. The authors suggested that the hydrogel allowed for the spatial separation between enzymes and served as a soft template to form hollow ZIF-8 spheres. Thus, the HRP is located within the central cavity of the hollow MOF capsules, while the GOx is supported onto the outer region of the particle. The spatially controlled localization of enzymes promotes the efficient diffusion of products from GOx to HRP pulling the equilibrium toward the product formation.
The previous systems were all based on ZIF-8; however, recent reports have demonstrated that the enzymatic detection of glucose can be prepared by immobilization of a biorecognition element inside different MOFs (e.g., MAF-2 [151]) and on the outer surface of the MOF. The bioconjugation strategy allows for the use of a variety of preformed MOF materials with peroxidase-mimicking activity [152]. This strategy could exploit the enzymatic-like activity of the MOF to reduce issues related to the production of intermediates during multienzyme cascade reactions. This hypothesis was probed by Zhu et al. [153] who reported the fabrication of colorimetric glucose biosensor based on grafting (covalent immobilization) of GOx onto Fe-MIL-88B-NH2, an MOF that showed a peroxidase-like activity (Fig. 12.14a). In this catalytic process, first GOx catalyzes the glucose oxidation to yield gluconic acid and H2O2, then Fe-MIL-88B-NH2 consumes H2O2 to produce •OH, which oxidizes the chromogenic substrate 3,3′,5,5′- tetramethylbenzidine (TMB), into a green-blue colored ox-TMB intermediate (λmax 652 nm) (Fig. 12.14b). Accordingly, when GOx-on-Fe-MIL-88B-NH2 was used as a glucose biosensor, it presented high selectivity and displayed a linear response range of 1–500 μM, with an LOD of 0.478 μM (Fig. 12.14c). Furthermore, GOx-on-Fe-MIL-88B-NH2 showed higher tolerance to temperature and pH changes in comparison with the free enzyme system, and its reusability was tested up to five cycles. Based on these results, the authors tested this material for the detection of glucose in human serum. The GOx-on-Fe-MIL-88B-NH2 results were in good agreement with the results obtained with a commercial glucometer (<5% st. dev.).
(a) Schematic representation of the synthesis of a GOx-on-Fe-MIL-88B-NH2 biocomposite. (b) UV-vis spectra of the enzymatic-cascade reaction at different concentrations of glucose (1–500 uM). (c) Selectivity studies of the glucose biosensor in the presence of other carbohydrates. (Reprinted with permission from Ref. [153], Copyright 2019 American Chemical Society)
12.3.3.2 Protein-On-MOFs and Proteins@MOFs Biocomposites in Immunoassays
Early detection of specific biomarkers in biological fluids is crucial for a prompt medical diagnosis and successful therapeutic treatment. Immunodiagnostic tests are attractive systems for early detection. Enzyme-linked immunosorbent assay (ELISA) is a biochemical method designed to detect and quantify targeted biomacromolecules (examples of antigens are proteins, carbohydrates, nucleic acids, and viruses). ELISA tests use antibodies as biorecognition elements and an enzyme provides the signal response when the antigen-antibody complex is formed. The enzyme can be either directly or indirectly coupled to the biorecognition element (antibody) [134]. Because of the specificity of antibodies, this analytical technique has been extensively used in food and environmental analysis, and medical diagnosis. Nevertheless, the synthesis and purification of enzyme-antibodies conjugates require laborious protocols. This results in loss of the biorecognition properties (antibody) and catalytic activity of the tag (enzyme), which compromises the sensitivity of the method [154, 155]. These issues, together with the low concentration of biomarkers and infectious agents, limit the practical application of ELISA tests at the point of care (POC) [132, 134]. A strategy to enhance the sensitivity of immunodiagnostic tests is to increase either the antibody concentration or the number of signaling tags attached to the antibody. However, such modifications can alter the background and the specificity of the method.
An emerging strategy to improve sensitivity, stability , and selectivity is through the immobilization of antibodies on MOFs and the resulting antibody-on-MOF biocomposites can be used as probes. In fact, in such antibody-on-MOF systems, both signaling and bio-recognition components can be colocalized in the same particle. Li et al. [156] bioconjugated secondary antibodies (Ab2) on HKUST-1 (Cu-BTC; BTC = 1,3,5-benzenetricarboxylic acid), to develop a fluorogenic click immunoassay for the detection of hepatitis B virus antigen (HBsAg) in clinical serum samples (Fig. 12.15a). This sandwich assay is composed of a microplate with immobilized antibodies (Ab1) that can create a complex with biomarker antigen (HBsAg); upon antigen binding, the secondary antibodies, that is, the Ab2-on-HKUST-1 particles, will selectively bind to the immobilized HBsAg (Fig. 12.15b). Upon formation of this antigen-antibody assembly, Cu(II) in HKUST-1 can be easily reduced to Cu(I) by a sodium ascorbate solution. Thus, the reduced MOF can catalyze azide–alkyne cycloaddition reaction between fluorogenic azide and alkyne precursors to yield the corresponding triazole, which provides a fluorescence signal. The formation of multiple triazole molecules within the porous MOF network resulted in a system with a detection limit below 11.2 pg mL–1, which is comparable or superior to previously reported methods for HBsAg detection (Fig. 12.15c) [156].
(a) Schematic representation of the synthesis of antibody Ab2 immobilized onto a Cu-MOF, HKUST-1, and the conceptual sandwich-type method for immunodetection of HBsAG. A fluorescence signal is detected at 395 nm due to the CuAAC click reaction catalyzed by the Cu-MOF. (b) Selectivity response of the immunoassay against different protein biomarkers. (c) Fluorescence emission spectra upon addition of different concentrations of HBsAg. (Adapted with permission from Ref. [156], Copyright American Chemical Society)
In summary, protein-based MOF biocomposites are emerging materials for the preparation of cost-effective biosensors with sensitivity and selectivity toward a variety of analytes ranging from small molecules such as H2O2 and glucose to large biomacromolecules such as biomarkers. Both protein@MOF and protein-on-MOF configurations have been shown to be relevant for the development of colorimetric [145], electrochemical [157], and fluorometric [156] biosensors. Notably, when enzymes are used for the detection of small molecules, the MOF matrix can stabilize and protect the protein structure (as discussed in the biopreservation section), while the porous framework allows the diffusion of analytes. Another important aspect of this class of composites is that the biorecognition element and the signaling components can be coimmobilized within the same MOF matrix. These aspects are expected to facilitate the implementation of the assays onto miniaturized biochips, reduce the time involved in the detection process, and enhance the LOD.
12.3.4 Carbohydrates@MOF and Carbohydrates-On-MOF Biocomposites as Drug Delivery Systems
Carbohydrates (CHs) are a class of biomolecules that play an important role in several biological functions, including cellular and intracellular interactions in the form of cell surface receptors, bacterial adhesives, and signaling molecules [158]. Recently, carbohydrate-based drugs were investigated as potential candidates for different therapies [159]. The most relevant commercial CH-based therapeutics are Glycosaminoglycans (GAGs) [160]. GAGs are unbranched high-molecular-weight polysaccharides comprised of uronic acid (D-glucuronic or L-iduronic acid) and amino sugars (D-glucosamine or D-galactosamine) [161]. Such biomacromolecules are negatively charged due to the presence of multiple carboxylate and sulfate moieties attached to the carbohydrate backbone. The applications of GAGs in biomedicine include their use as anti-inflammatories, anticoagulants, wound-healing agents, as well as drugs for the treatment of diabetes, osteoarthritis, and cancer [160,161,162]. However, the main drawbacks of GAG therapeutics are the low bioavailability and their poor stability [162]. For example, Heparin (HP), used for thromboembolic treatments, suffers from rapid degradation, fast serum clearance, and poor bioavailability [160, 163, 164]. Hyaluronic Acid (HA), a wound-healing therapeutic, is unstable in the presence of hyaluronidase and reactive oxygen species (ROS) [165, 166]. To overcome these limitations, MOFs have been investigated as carriers for CH-based therapeutics and it was reported that MOF particles improved the pharmacokinetics and bioavailability of the CH-based therapeutics [64, 167, 168]. In this section, we mainly describe the use of MOFs as DDS for CH-based drugs. In addition, because of the significant targeting ability and stability imparted by CHs coatings on MOFs , we include a brief discussion of selected CH-on-MOFs systems.
12.3.4.1 MOFs as Carriers for CH-Based Therapeutics
Recently, Falcaro and coworkers reported the encapsulation of CHs into MOFs , where CH@MOF biocomposites were prepared via the biomimetic mineralization approach (Fig. 12.16a) [51]. Several CHs including D-galactose, D-glucose, D-glucitol, D-mannose, meglumine, D-xylose, D-glucosamine, N-acetyl-D-glucosamine, sucrose, methyl-β-D-glucopyranoside, D-gluconic acid-δ-lactone, maltodextrin, dextran, carboxymethyl dextran (CM-dextran), diethylaminoethyl dextran (DEAE-dextran), and cellulose were assessed as potential biomimetic mineralization agents for ZIF-8. As for the case of negatively charged proteins [52, 63], it was shown that a negatively charged CH (i.e., CM-dextran, often used as model GAG therapeutic) [169] triggered the formation of the ZIF-8 biocomposite because of the increased concentration of Zn2+ around the COO− groups of the CH [51]. The CM-dextran@ZIF-8 biocomposite showed 100% encapsulation efficiency, 70 wt% loading capacity, and the CH release could be triggered by the exposure to EDTA. Based on this, studies were expanded to commercial GAG-based therapeutics. Heparin (HP), Hyaluronic Acid (HA), Chondroitin Sulfate (CS), Dermatan Sulfate (DS), GM-111 (anti-inflammatory drug), and HepSyl (anti-carcinogenic agents) were encapsulated within three different pH-responsive metal azolate frameworks: ZIF-8, ZIF-90, and MAF-7 (Fig. 12.16b) [64]. The resultant GAG@ZIFs demonstrated different crystalline phases (e.g., sod, dia, or amorphous structures) and particle size (50–7000 nm). The encapsulation efficiencies were measured in the 45–100% range and release kinetics (performed at pH = 6 up to 100% release) were found to vary from 20 min to 2.5 h depending on the specific systems. This proved that the release of GAG-based therapeutics could be modulated by selecting an appropriate MOF matrix. These discoveries should enable the progress for CH-based drug delivery using MOF carriers [64].
(a) Schematic illustration of the preparation of CH@ZIF-8. Negatively charged carbohydrates in a ZIF-8 precursor solution trigger the biomimetic mineralization that encapsulates carbohydrates in ZIF-8 [51]., Astria, Efwita, et al. “Carbohydrates@MOFs .” Materials Horizons 6.5 (2019): 969–977- Published by The Royal Society of Chemistry. (b) Schematic of one-pot synthesis of GAG@MOFs biocomposites by using three different metal azolate framework (ZIF-8, ZIF-90 and MAF-7). Reproduced with permission from Ref. [64]. (c) Schematic illustration of the external surface modification of MIL-100(Fe) NPs with heparin. Model drugs were loaded in Heparin coated MIL-100(Fe) NPs with two different approaches: Top (A) – Heparin was initially coated on the surface of NPs followed by caffeine loading and, Bottom (B) – NPs were preloaded with furazan and the coated by rhodamine-labeled heparin. (Reprinted with permission from Ref. [29] Copyright 2015 John Wiley and Sons) (d) Schematic illustration of the preparation of ICG-infiltrated HA-on-MIL-100(Fe) NPs, HA conjugation, ICG loading, and multimodal imaging-guided PTT of MIL-100(Fe) NPs. (Reprinted with permission from Ref. [171], Copyright (2017) American Chemical Society)
12.3.4.2 Carbohydrates-On-MOF Biocomposites for DDS
CHs have been employed as coatings of MOF-based DDS to improve both stability and targeting abilities [29, 170]. Horcajada and colleagues reported the surface modification of MIL-101(Fe) by using HP to enhance the colloidal stability in different biological relevant media (e.g., water, PBS, PBS containing albumin (5.4% w/v), and Roswell Park Memorial Institute (RPMI) medium) of the MOF particles (Fig. 12.16c) [29]. HP-on-MIL-100(Fe) demonstrated a drug loading capacity of up to 42 wt% for caffeine as a model drug. HP-on-MIL-100(Fe) also offered an improved control over the release of the cargo when compared to uncoated MIL-100(Fe). This was shown by monitoring the release kinetics of caffeine into PBS solution, where only 20 wt% was released within the first hour, when using HP-on-MIL-100(Fe) as the DDS, while for MIL-100(Fe), 56 wt% of caffeine was released over the same time period. This research revealed that the surface modification of MOF NPs with GAGs can enhance the properties of MOF-based DDS, therefore progressing MOF materials toward biomedical applications [29].
In another demonstration, HA-on-MIL-100(Fe) was used for multimodal imaging-guided cancer photothermal therapy (PTT) by Liu and coworkers (Fig. 12.16d) [171]. Indocyanine green (ICG), an FDA-approved near-infrared organic dye for clinical application, suffers from issues of low aqueous solubility, low cancer specificity, and unstable optical properties. ICG was infiltrated into MIL-100(Fe) NPs. Then, ICG@MIL-100(Fe) were coated with HA and the authors reported that the use of this MOF carrier improved the dye performance in PTT [171]. The HA-on-MIL-100(Fe) NPs showed 40 wt% loading capacity for ICG, low cytotoxicity, and photothermal stability under NIR light irradiation. Since HA has been reported as a good targeting agent for CD44, a transmembrane glycoprotein which is highly expressed in cancer cells [172, 173], the authors tested the targeting abilities of ICG-infiltrated HA-on-MIL-100(Fe) biocomposites toward CD44-positive MCF-7 tumor cells/xenograft [171]. ICG-infiltrated HA-on-MIL-100(Fe) showed selective targeting properties and thus the authors posit that this MOF system could be used as a theranostic nanoplatform for cancer-specific and imaging-guided delivery [171].
In summary, these examples have demonstrated that use of CHs for the formation of MOFs biocomposites is an attractive approach for the design of new DDSs. In such biocomposites, the CHs can be either encapsulated within an MOF shell (CH@MOFs ) or immobilized onto the outer surface of the material (CH-on-MOFs). In the first case, the CH is itself used as the therapeutic and the MOF is employed as protective carrier for preservation and triggered release of the cargo. Alternatively, in the CH-on-MOFs configuration, the CH is used as coating agent to facilitate the transport of the MOF carrier within the circulatory system. Despite only a few studies having explored the integration of CHs with MOF, these reports show that these biocomposites are of interest for biomedical applications. Thus, further exploratory and systematic studies are required to fully realize the potential of CH@MOFs and CH-on-MOFs.
12.3.5 Nucleic Acid and MOF Biocomposites
Nucleic acids (NAs) are naturally occurring polymers that can store and transfer genetic information. These macromolecules are constructed from units called nucleotides, monomers composed of three component parts: a 5-carbon sugar, a phosphate group, and a nitrogenous base [174]. NAs comprise both ribonucleic acid (RNA) and deoxyribonucleic acid (DNA), depending on the nature of the sugar unit, and take on various structures (oligomeric, single or double stranded, etc.) and topologies (e.g., linear, circular). These biomacromolecules have recently received considerable attention because of their potential for medical applications, including the development of innovative therapeutics and gene therapies, and biosensing [175,176,177]. For example, in gene therapy, small interfering RNA (siRNA) can treat diseases that are caused by the activity of specific genes like some viral infections [178, 179], cancers [180], or autoimmune diseases [181]. Another example is plasmid DNA (plDNA), which is the simplest vector for transport of DNA into the cell nucleus; plDNA can be used for gene transfer, passing new instructions to a cell (e.g., production of a lacking protein) [182, 183]. In recent years, there has been a growing research interest in viral and nonviral vectors for the active targeted delivery of nucleic acids [184]. In this field, pioneering works integrating NAs with MOFs have shown how new porous carriers can progress the delivery of genetic information.
The first example of a biocomposite based on nucleic acids and MOFs was reported by Lin et al [31]. The authors infiltrated a cisplatin prodrug (Cis) into the pores of a Zr-based UiO MOF nanoparticles (<100 nm, Fig. 12.17a). Then, the Cis@UiO NPs were functionalized with a multiple drug resistance (MDR) gene-silencing siRNA to form a siRNA-on-Cis@UiO biocomposite. The authors hypothesized that siRNA was bound to the MOF particle surface via multiple coordination bonds between vacant Zr sites and phosphate residues of the siRNA backbone. This siRNA-on-Cis@UiO biocomposite was shown to induce cell apoptosis in cisplatin-resistant ovarian cancer cells. This was attributed to the presence of the MDR gene-silencing siRNA on the surface of the drug-loaded particles that facilitated the cellular uptake, promoted siRNA escape from endosomes to silence MDR genes, and consequently permitted the in vitro chemotherapeutic efficacy of cisplatin [31]. After this foundational work, the surface of several other MOFs (e.g., MIL-101 [185], UiO-66 [186], UiO-66-NH2 [187], NU-1000 [188]) has been functionalized with different nucleic acids (e.g., siRNAs [185], oligonucleotides [186], DNAs [186,187,188]) to facilitate the cellular uptake of drug-loaded MOF carriers.
(a) Schematic representation of the infiltration of Cis into UiO NPs and of the surface functionalization with siRNA. (Adapted with permission from Ref. [193], copyright 2014 American Chemical Society, further permissions related to the material excerpted should be directed to the ACS). (b) Schematic representation of the ssDNA infiltration into the mesoporous MOF Ni-IRMOF-74-II. (Adapted with permission from Ref. [195]) (c) schematic representation of the plasmid@ZIF-8 synthesis via biomimetic mineralization and of the plasmid release via ZIF-8 decomposition in the cell nucleus, as described in Ref. [192]
NA-based MOF biocomposites include but are not limited to NA-on-MOF; indeed, NAs can be infiltrated and encapsulated within MOF particles [44, 189,190,191,192,193,194,195]. For example, Farha and coworkers selected a mesoporous zirconium-based MOFs (NU-1000) that could be infiltrated with siRNA molecules. The porous framework offered protection to the infiltrated siRNA against enzymatic degradation, enabling the release of functional siRNA to the cytoplasm of in vitro cultured cells [189]. Horcajada and coworkers infiltrated siRNA into nanoparticles of biocompatible iron(III) carboxylate MOFs (MIL-100 and MIL-101-NH2), to protect and release the siRNA for in vitro gene therapy tests [44]. Single-stranded DNA (ssDNA) was infiltrated by Peng et al. into a series of mesoporous Ni-IRMOFs-74 (hexagonal channels with size from 2.2 nm to 4.2 nm, Fig. 12.17b) [195]. By modulating the MOF pore size, the authors infiltrated ssDNA molecules with different lengths (from 11 to 53 nucleotides). The MOF offered protection against ssDNA degradation (e.g., exposure to 10% fetal bovine serum) and permitted the intracellular delivery of intact ssDNA molecules.
In comparison to the infiltration method, the encapsulation strategy allows the embedding of macromolecules into an MOF matrix where the biomolecule is larger than the pore aperture. Khashab and coworkers coencapsulated CRISPR-associated protein 9 (Cas9 [196], a bacterial RNA-guided endonuclease that uses base pairing to recognize and cleave target DNAs with complementarity to the guide RNA) and an engineered single guide RNA (sgRNA) within ZIF-8 particles (~100 nm) via biomimetic mineralization [190, 191]. The combination of Cas9 and sgRNA is a genome editing platform (aka CRISPR/Cas9) that can be used as a potential therapy for genetic diseases (e.g., targeted changes to the genome of living cells) [197]. The encapsulation within the porous framework protected both biomacromolecules from endosomal degradation and allowed for the controlled release of the cargo triggered by the exposure to acidic conditions (pH≤6) [190]. The gene editing properties of the Cas9-sgRNA@ZIF-8 biocomposite were assessed by the reduction of the gene expression of a model protein (e.g., green fluorescent protein) by 37% over a period of 4 days [190]. Using ZIF-8 and the biomimetic mineralization approach, Shukla and coworkers recently reported the encapsulation of a complete gene-set (6.5 kilo base-pairs, Fig. 12.17c) [192]. In this work, a green fluorescent protein (GFP) plasmid (plGFP) was used as a model genetic macromolecule, allowing the authors to demonstrate that the plGFP remained intact after release from the plGFP@ZIF-8 particles. Moreover, the successful transfection of the mammalian cancer cells was proven. Simultaneously, Tang and coworkers encapsulated in ZIF-8 an enhanced GFP plasmid (plEGFP, 4.7 kil base pairs), and the plEGFP@ZIF-8 was successfully tested for intracellular gene delivery and expression [193]. Another relevant example was reported by Wang et al. [194] involving encapsulation of a chlorin e6 (a photodynamic therapy agent) functionalized DNAzyme within particles of ZIF-8. The release of the cargo was triggered by the exposure of the biocomposite to acidic pH. The simultaneous release of the DNAzyme and Zn2+ ions was exploited as a messenger RNA-targeting agent and as a cofactor for the enzymatic activity of the DNAzyme to activate the gene therapy (DNAzyme-mediated cleavage reaction in MCF-7 cells). Furthermore, the photosensitizer chlorin e6 provided reactive oxygen species (ROS) and a fluorescence signal used for imaging-guided gene-photodynamic therapy [194].
In summary, biocomposites based on MOFs and nucleic acids (NAs) are promising materials for biomedical applications, as drug delivery systems, gene therapies, and biosensing [175,176,177, 198, 199]. Nucleic acids have been bioconjugated to the outer surface of drug-loaded MOF particles (NA-on-MOFs) to enhance their colloidal stability and their internalization efficiency. When encapsulated or infiltrated into MOF particles (NA@MOFs ), the porous framework offered protection to different environmental stressors that typically decompose NA molecules; thus, the structure and functionality of small interfering and plasmid DNA and RNA were preserved. Utilizing the additional stability offered, NA@MOFs were used for the intracellular delivery of NA molecules or the co-delivery of NAs and other biomolecules (e.g., endonuclease) for advanced gene therapies.
12.3.6 Lipid and MOF Biocomposites
Lipids are typically defined as a class of biomolecules that are soluble in nonpolar solvents [109]. Some commonly encountered examples of lipids are fatty acids, sterols, glycerolipids, and glycerophospholipids [200]. Lipids are involved in several relevant biological processes, including cell signaling and metabolism and are the major component of biological membranes (e.g., glycerophospholipids). Lipid-based drug delivery systems were investigated to improve the solubility and bioavailability of poorly water-soluble drugs, thus exploiting the amphiphilic properties of lipids. Some of these systems are the basis of current commercially viable strategies for the delivery of pharmaceuticals via topical, oral, pulmonary, or parenteral administration [200].
In the field of MOF biocomposites, lipids have been employed for the surface functionalization of MOF particles formed via bioconjugation. For example, Wang et al. functionalized a series of Zr-based MOF nanoparticles with 1,2-dioleoyl-sn-glycero-3-phosphate (DOPA) to allow preparation of stable colloidal dispersions while maintaining the structural properties of MOFs [201]. The preparation of a stable colloidal MOF dispersion is considered a crucial aspect for processing of MOF materials and the development of MOF-based devices [202].
Lipid coatings of MOF (i.e., lipid-on-MOFs) have been shown to enhance the performance of MOF-based drug delivery systems beyond colloidal stability . For example, it was shown that a lipid coating can improve the cellular uptake of MOF biocomposites. Wuttke at al. prepared MIL-100(Fe) and MIL-101(Cr) nanoparticles and loaded the MOF pores with a fluorescent dye [34], prior to coating the MOF nanoparticles with a lipid bilayer comprised of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC). For the preparation of the coating , the authors followed a controlled solvent-exchange protocol for deposition of the lipid onto the MOF surface: by increasing the water concentration, the lipids precipitate on the nanoparticle surface and form a lipid bilayer. In addition to the increased the colloidal stability of the MOF dispersion, the lipid bilayer prevented the uncontrolled release of the cargo (e.g., fluorescent dye) from the MOF particles. Furthermore, the authors demonstrated the superior cancer cell uptake of lipid-coated MOF particles when compared to bare MOF particles.
Wuttke’s group further investigated the combination of drug-loaded MOF particles and exosomes [35]. Exosomes are extracellular vesicles that are produced by eukaryotic cells and they have a very similar membrane structures to cell membranes [203]. These liposomes are commonly present in body fluids and they are supposed to be non-immunogenic [35]. Therefore, it has been proposed that they could be used as a protective coating for nanoparticles to improve the biocompatibility (e.g., shielding the particles from the immune system) and the circulation time of particles. Wuttke et al. coated the surface of drug-loaded (e.g., calcein) MIL-88A nanoparticles with exosomes and investigated their performance as a drug delivery system. This MOF biocomposite showed a high drug encapsulation efficiency and negligible premature drug leakage. Furthermore, the successful intracellular delivery into HeLa cells was attributed to endogenous exosomal release mechanisms.
In summary, the research field of lipids and MOFs bicomposites is in its infancy; however, lipid-on-MOFs have already shown their potential for drug-delivery application. The rich chemistry of lipids could be exploited to impart different functionality to MOF particles: for example, lipid-based capping systems could be tailored to address targeted release or improved biocompatibility and circulation time. Furthermore, the lipid-coating is independent from both the MOF and its cargo which suggests that a variety of combinations are possible for different biotechnological applications such as bioimaging, and biosensing.
12.3.7 Large Bioentities (Cells, Viruses) for Biopreservation and Cell and Virus Manipulation
The encapsulation of cells and viruses within a synthetic shell offers additional protection from environmental factors such as cytotoxic compounds, mechanical stress, and UV radiation. Applications that require ex vivo handling and manipulation of cells, such as cell therapy and tissue engineering, can expose cells to physical forces and strong mechanical stress [204,205,206,207,208]. This is critical for mammalian cells, since they lack the structural support of an exoskeletal shell or strong cell wall [204]. Therefore, the encapsulation of cells has been of increasing interest in the research community as a solution for the protection of these large and fragile bioentities. Typically, cell encapsulation methods are based on materials such as hydrogels, soft polymers, mineral shells, and supramolecular assembles of coordination complexes [209, 210]. Different types of microbial and mammalian cells have been successfully encapsulated with these materials [8] and studied for application such as probiotic delivery, cell therapy, and biocatalysis [7]. However, typical encapsulation methods lack precise control over perm-selective properties, and degradation of the shell on demand under biocompatible conditions can be an issue [210, 211]. In this context, MOFs are highly attractive materials since their pore size and connectivity can be tuned and their degradation can be triggered in biological media. In addition, MOFs can be assembled under biological conditions with biocompatible building blocks [6, 55, 211]. Finally, as selected examples discussed below will illustrate, depending on the synthetic strategy used, a flexible or rigid MOF shell can be constructed around cells.
12.3.7.1 Encapsulation of Cells in MOFs
In 2016, Liang et al. reported for the first time the encapsulation of live cells in an MOF shell performed via self-assembly of ZIF-8 on the surface of Saccharomyces cerevisiae (baker’s yeast) and Micrococcus luteus (bacterium) (Fig. 12.18a, b) [53]. The synthesis, carried out in water, led to the spontaneous MOF formation on the surface of the cells. Under the conditions used, cell viability studies and standard assays indicated that the ZIF-8 shell is non-toxic to yeast cells [53, 212]. The porous MOF exoskeleton was found to possess interesting perm-selective properties: it was permeable to nutrients (glucose) but impermeable to both Lyticase (a cytotoxic enzyme) and Filipin (a polyene macrolide antibiotic) [213]. Another important property was the triggered degradation of the cell by adding a biocompatible chelating agent (EDTA), after which the normal reproductive rate of free yeast cells was recovered (Fig. 12.18c). In follow-up work, the β-galactosidase enzyme was immobilized on the surface of yeast cells and then a ZIF-8 coating was formed. Remarkably, the bioactive functionality of both cell and enzymes was preserved by the ZIF-8 coating [67]. In an environment with lactose, a carbohydrate that yeast cell cannot metabolize, the β-galactosidase in the cell surface coating converted the disaccharide into monosaccharides, thus providing a nutrient to the yeast cells. Under these conditions, protected yeast cells survived for more than 7 days in this nutrient-deficient environment (Fig. 12.18d). An interesting observation noted during the synthesis of the ZIF-8 coating was the preferential growth of ZIF-8 on yeast cells. These cell membranes, which are rich in glycoproteins and peptidoglycans, could presumably enhance the local concentration of Zn2+ and seed the ZIF-8 formation. This effect was also observed for a number of negatively charged carbohydrates [51, 64].
(a) Schematic of biomimetic mineralization of an MOF coating on living cells. (b) CLSM cross-section of the ZIF-8-coated yeast cells, showing live yeast cells (FDA-labeled, green) and ZIF-8 coating (Alexa Fluor 647-labeled, red). (c) Comparison of yeast growth before and after removal of MOF coatings by EDTA for naked yeast (blue) and yeast@ZIF-8 (red). (Reprinted with permission from Ref. [53]. Copyright 2016 John Wiley and Sons). (d) Relative cell viability (%) of the yeast@ β-gal/ZIF-8 biocomposites in oligotrophic and inhospitable environments (in presence of lactose and cytotoxic enzymes). (Adapted with permission from Ref. [67]. Copyright 2017 John Wiley and Sons). (e) Formation of artificial exoskeletons on living cells by SupraCell synthesis of various materials (MOFs and inorganic coatings). (f) Mechanical response test: Comparison of elastic modulus and stiffness of HeLa cells and SupraCells-MIL-100(Fe) or ZIF-8 (M or Z refers to MIL-100(Fe) or ZIF-8, respectively); with different coating cycles (numbers 1–3). The inset shows a schematic of the experiment imposing a load P onto cells and SupraCells with a Berkovich Indenter. (Reprinted with permission from Ref [214]. Copyright 2018 John Wiley and Sons)
More recent studies have focused on different cell types and MOFs . A versatile method was developed by Brinker and coworkers in 2019, termed “SupraCells” [214], which relied on the colloidal deposition of pre-synthesized MOF nanoparticles (e.g., ZIF-8, MIL-100 (Fe), UiO-66-NH2 and MET-3-Fe) via a tannic acid-mediated crosslinking step. This afforded robust artificial exoskeletons on live mammalian cells that permitted the permeability of nutrients, metabolites, and signaling molecules to the cell undisturbed, thus preserving the cell viability and metabolic functions (Fig. 12.18e). Furthermore, this strategy was able to prevent typical endocytotic nanoparticle (NP) internalization pathways. The SupraCell synthesis was successfully applied to obtain continuous MOF exoskeletons on different types of mammalian cells: HeLa cells, adenocarcinomic human alveolar basal epithelial cell (A549 cells), human promyelocytic leukemia cells (HL-60 cells), and Raw 264.7 cell line. The authors showed that these artificial coatings increased the resistance of cells to environmental stressors such as pH, reactive oxygen species (ROS), UV irradiation, osmotic pressure, and toxic NPs. The authors also demonstrated that the functionality of the MOF NP-based coatings could be tuned to confer abiotic properties to the cells, for example, increased mechanical stability (Fig. 12.18f). Therefore, the authors propose this MOF coating for tissue engineering, regenerative medicine, immunotherapy, and cell-based sensing.
A similar concept based on pre-synthesized MOF NPs was proposed by Yang, Yaghi and coworkers in 2018, and applied to Morella thermoacetica, an anaerobic bacteria [215]. The authors formed artificial exoskeletons of the MOF [Zr6O4(OH)4(BTB)2(OH)6(H2O)6; BTB = 1,3,5-benzenetribenzoate] on the bacteria by adding pre-synthesized MOF NPs into the culture media. This allowed for the self-assembly of a flexible monolayer (1–2 nm) that adapted to cell wall alterations, such as growth and reproduction. Remarkably, under excess MOF in the culture media, newly grown cells were spontaneously coated with the MOF NP coating . Cell viability studies showed that the MOF-coated anaerobic bacteria could survive up to 3 days in the presence of O2 and H2O2, obtaining comparable results to the uncoated cells in their natural anaerobic environment. The authors suggested that this protection from oxidative stress observed for the MOF-coated cells is due to the catalytic activity (decomposition of ROS) of zirconium clusters in the MOF.
12.3.7.2 Encapsulation of Viruses in MOFs
The encapsulation of a virus in an MOF shell was first reported by Gassensmith and coworkers in 2016 [216]. A model virus (tobacco mosaic virus, TMV) was encapsulated in ZIF-8 via biomimetic mineralization (Fig. 12.19). The TMV@ZIF-8 biocomposites were shown to remain stable in the presence of different environmental stressors such as polar solvents (methanol, DMF, and DCM) and high temperatures (boiling water). Additionally, after dissolving the MOF shell with an EDTA aqueous solution, it was possible to recover the undamaged virus. The authors showed control over the thickness of the MOF coating in the 70–100 nm range by changing the metal to ligand ratio (Zn2+:2-methylimidazole). In a follow-up work, the authors explored different ligand-to-metal (L/M) ratios and precursor concentrations and found two different encapsulating morphologies: small core-shell bionanoparticles (CSBN), with the same underlying morphology as the virus (~300 nm × 18 nm rods), and several TMV particles enclosed in micrometric ZIF-8 single crystals (rhombic dodecahedra) (Fig. 12.19b). The authors also investigated the influence of surface charge on the formation mechanism by inducing bioconjugation reactions on the virus surface. The results showed that the CSBN formed in good yields (~90%) under most circumstances except for highly positively charged surfaces for which there was a slight drop in the yield (~70%). This effect was attributed to the increased concentration of zinc cations in the proximity of the TMV, and it is supported by other studies that elucidate the relevance of electrostatics in the biomimetic mineralization process [63].
(a) Schematic of the tobacco mosaic virus (TMV) with its typical sizes (300 nm × 18 nm diameter × 4 nm) and of the TMV-templated ZIF-8 mineralization process, illustrating the two possible morphologies of the TMV@ZIF-8 obtained. (b) SEM images showing the effect of different ligand/metal ratios on the resultant morphology of the TMV@MOF particles (at 20 mM metal concentration, with and without TMV). (Reprinted with permission from Ref. [52], Copyright 2018 American Chemical Society. Further permissions for this material must be directed to the ACS). (c) Tobacco plants (N. benthamiana) 10 days after inoculation with (1) 0.1 M pH 7.4 potassium phosphate buffer (negative control), (2) TMV@ZIF, (3) exfoliated TMV@ZIF, and (4) native TMV (positive control). (d) ELISA tests on naked and encapsulated TMV under no stress (i), heating (ii), methanol (iii), 6 M guanidinium chloride (iv), and ethyl acetate (v). (e) In vivo experiments after subcutaneous injection of a fluorescent-tagged ZIF-8-encapsulated TMV virus (Cy5-TMV) on mice. Time point images after injection of Cy5-TMV or Cy5-TMV@ZIF. (Adapted with permission from Ref. [217]. Copyright 2019 American Chemical Society.)
In 2019, the same team studied the viability of using MOF encapsulation to protect and administer vaccines (using the previously reported TMV@ZIF-8 biocomposites) [217]. In this latter study, the authors analyzed by ELISA the integrity of the virus surface after exposure to various stressors (Fig. 12.19c, d), as well as conducting in vivo studies on live animal models to assess the immune response, biocompatibility, release profile and bioaccumulation in the organs (Fig. 12.19e). The viral recovery studies found that the RNA remained active after inoculation of N. benthamiana (tobacco) plants with the TMV@ZIF-8, suggesting that the porous coating did not affect significantly the RNA or the protein structure. The in vivo studies demonstrated that the administration of TMV@ZIF-8 to animals produced no changes in tissue morphology at the injection site or distal organs, and no illness, deaths, or behavioral changes were observed. The authors propose that this method could offer a safe and reliable platform for the delivery of protein-based drugs and vaccines.
12.3.7.3 General Considerations for Large Bioentities and MOF Biocomposites
In this section, we have discussed the advantages, challenges, and progress made on the encapsulation and protection of large bioentities such as cells and viruses within MOF coatings. MOFs offer unique advantages, such as a precise control over the permeability of nutrients and stimulants while blocking cytotoxic agents [53, 55]. Regarding the encapsulation of viruses and virus-like particles (VLP), it has been proposed that MOFs could be a key step for the protection of virus-based therapies and vaccines, which need constant refrigeration (a cold chain) to prevent protein denaturation [55, 216, 217]. However, to realize the full potential of these MOF-based coatings, the research should continue to explore other MOF systems and different cells and viruses types (e.g., enveloped and nonenveloped) [55]. Systematic studies should be conducted to master precise control over the MOF shell thickness and composition (i.e., MOF biocomposite shells). In addition, most of the research has been performed on cells with robust walls such as fungi and bacteria; however, the application of MOFs to fragile mammalian cells [218] will progress biomedical technologies such as tissue engineering and cell therapy [55, 210].
12.4 Summary
In this chapter, we introduced the concept of integrating biomacromolecules with MOFs and how this could lead to improved drug delivery systems; biopreservation to obviate use of cold chains; and for practical biosensing concepts. Given the emerging nature of this field, we first considered the types of biocomposites and the methods used to prepare them; two possible configurations are possible with the biomacromolecules either interacting with the MOF surface or embedded into the MOF particle. In the former case, namely biomacromolecules-on-MOF systems, we outlined the two approaches used to prepare these which we distinguished as adsorption (non-covalent interactions) and grafting (covalent bonding, also termed bioconjugation). For the synthesis of biomacromolecule @MOF composites, we discussed embedding protocols that we categorized as infiltration and one-pot encapsulation. Each of these methods has particular advantages and limitations that confer different properties to the resulting biocomposite. Due to the importance of one-pot encapsulation methods in this emerging field of biomacromolecules/MOF composites, we have concentrated on providing a detailed understanding of the factors that govern successful encapsulation and the properties of these systems that lend them to biomedical applications.
In the second part of the chapter, we examined selected examples of MOF biocomposites in which proteins, carbohydrates, nucleic acids, lipids, or larger bioentities (i.e., cells and viruses) have been combined with MOFs . For such systems, we focused on their properties for drug delivery and protection, and specifically illustrated their potential for their utilization in biomedical applications. This section demonstrated that both configurations – biomacromolecules-on-MOF and biomacromolecules@MOF systems – are applicable in biomedicine. As this field is in its infancy, additional exploratory and systematic studies are required before these composites may be used in clinical settings.
References
Fried DI, Brieler FJ, Fröba M (2013) Designing inorganic porous materials for enzyme adsorption and applications in biocatalysis. ChemCatChem 5:862–884
Yang X-Y, Chen L-H, Li Y, Rooke JC, Sanchez C, Su B-L (2017) Hierarchically porous materials: synthesis strategies and structure design. Chem Soc Rev 46:481–558
Pierre AC (2004) The sol-gel encapsulation of enzymes. Biocatal Biotransform 22:145–170
Hudson S, Cooney J, Magner E (2008) Proteins in mesoporous silicates. Angew Chem Int Ed 47:8582–8594
Jin W, Brennan JD (2002) Properties and applications of proteins encapsulated within sol–gel derived materials. Anal Chim Acta 461:1–36
Doonan C, Riccò R, Liang K, Bradshaw D, Falcaro P (2017) Metal–organic frameworks at the biointerface: synthetic strategies and applications. Acc Chem Res 50:1423–1432
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, Férey G, Morris RE, Serre C (2012) Metal–organic frameworks in biomedicine. Chem Rev 112:1232–1268
Horcajada P, Serre C, Vallet-Regí M, Sebban M, Taulelle F, Férey G (2006) Metal–organic frameworks as efficient materials for drug delivery. Angew Chem Int Ed 45:5974–5978
Hartvig RA, van de Weert M, Østergaard J, Jorgensen L, Jensen H (2011) Protein adsorption at charged surfaces: the role of electrostatic interactions and interfacial charge regulation. Langmuir 27:2634–2643
Brynda E, Houska M (1996) Multiple alternating molecular layers of albumin and heparin on solid surfaces. J Colloid Interface Sci 183:18–25
Shaw KL, Grimsley GR, Yakovlev GI, Makarov AA, Pace CN (2001) The effect of net charge on the solubility, activity, and stability of ribonuclease Sa. Protein Sci 10:1206–1215
Li Y, Liu J, Zhang K, Lei L, Lei Z (2018) UiO-66-NH2@PMAA: a hybrid polymer–MOFs architecture for pectinase immobilization. Ind Eng Chem Res 57:559. https://doi.org/10.1021/acs.iecr.7b03398
Ma W, Jiang Q, Yu P, Yang L, Mao L (2013) Zeolitic imidazolate framework-based electrochemical biosensor for in vivo electrochemical measurements. Anal Chem 85:7550–7557
Pan H, Qin M, Meng W, Cao Y, Wang W (2012) How do proteins unfold upon adsorption on nanoparticle surfaces? Langmuir 28:12779–12787
Rabe M, Verdes D, Seeger S (2011) Understanding protein adsorption phenomena at solid surfaces. Adv Colloid Interf Sci 162:87–106
Hoarau M, Badieyan S, Marsh ENG (2017) Immobilized enzymes: understanding enzyme – surface interactions at the molecular level. Org Biomol Chem 15:9539–9551
Liang W, Xu H, Carraro F et al (2019) Enhanced activity of enzymes encapsulated in hydrophilic metal–organic frameworks. J Am Chem Soc 141:2348–2355
Couston RG, Skoda MW, Uddin S, van der Walle CF (2013) Adsorption behavior of a human monoclonal antibody at hydrophilic and hydrophobic surfaces. MAbs 5:126–139
Wiseman ME, Frank CW (2012) Antibody adsorption and orientation on hydrophobic surfaces. Langmuir 28:1765–1774
Hu Y, Dai L, Liu D, Du W (2018) Rationally designing hydrophobic UiO-66 support for the enhanced enzymatic performance of immobilized lipase. Green Chem 20:4500–4506
Pan Y, Li H, Farmakes J, Xiao F, Chen B, Ma S, Yang Z (2018) How do enzymes orient when trapped on metal–organic framework (MOF) surfaces? J Am Chem Soc 140:16032–16036
Hermanson GT (2013) Chapter 4 – Zero-length crosslinkers. In: Hermanson GT (ed) Bioconjugate techniques, 3rd edn. Academic, Boston, pp 259–273
Wong SS, Jameson DM (2011) Chemistry of protein and nucleic acid cross-linking and conjugation. CRC Press, Boca Raton
Stine KJ (2015) Carbohydrate nanotechnology. Wiley, Hoboken
Khang G (2012) Handbook of intelligent scaffold for tissue engineering and regenerative medicine. CRC Press, Boca Raton
Shih Y-H, Lo S-H, Yang N-S, Singco B, Cheng Y-J, Wu C-Y, Chang I-H, Huang H-Y, Lin C-H (2012) Trypsin-immobilized metal–organic framework as a biocatalyst in proteomics analysis. ChemPlusChem 77:982–986
Doherty CM, Grenci G, Riccò R, Mardel JI, Reboul J, Furukawa S, Kitagawa S, Hill AJ, Falcaro P (2013) Combining UV lithography and an imprinting technique for patterning metal-organic frameworks. Adv Mater 25:4701–4705
Qiu J, Li X, Gref R, Vargas-Berenguel A (2020) Chapter 20 – Carbohydrates in metal organic frameworks: supramolecular assembly and surface modification for biomedical applications. In: Mozafari M (ed) Metal-organic frameworks for biomedical applications. Woodhead Publishing, Dordrecht, pp 445–465
Bellido E, Hidalgo T, Lozano MV, Guillevic M, Simón-Vázquez R, Santander-Ortega MJ, González-Fernández Á, Serre C, Alonso MJ, Horcajada P (2015) Heparin-engineered mesoporous iron metal-organic framework nanoparticles: toward stealth drug nanocarriers. Adv Healthc Mater 4:1246–1257
Zhang Y, Sun P, Zhang L, Wang Z, Wang F, Dong K, Liu Z, Ren J, Qu X (2019) Silver-infused porphyrinic metal–organic framework: surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection. Adv Funct Mater 29:1808594
He C, Lu K, Liu D, Lin W (2014) Nanoscale metal–organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J Am Chem Soc 136:5181–5184
Jung S, Park S (2017) Dual-surface functionalization of metal-organic frameworks for enhancing the catalytic activity of candida antarctica lipase B in polar organic media. ACS Catal 7:438–442
Lian X, Fang Y, Joseph E, Wang Q, Li J, Banerjee S, Lollar C, Wang X, Zhou H-C (2017) Enzyme–MOF (metal–organic framework) composites. Chem Soc Rev 46:3386–3401
Wuttke S, Braig S, Preiß T, Zimpel A, Sicklinger J, Bellomo C, Rädler JO, Vollmar AM, Bein T (2015) MOF nanoparticles coated by lipid bilayers and their uptake by cancer cells. Chem Commun 51:15752–15755
Illes B, Hirschle P, Barnert S, Cauda V, Wuttke S, Engelke H (2017) Exosome-coated metal–organic framework nanoparticles: an efficient drug delivery platform. Chem Mater 29:8042–8046
Qi X, Chang Z, Zhang D, Binder KJ, Shen S, Huang YYS, Bai Y, Wheatley AEH, Liu H (2017) Harnessing surface-functionalized metal–organic frameworks for selective tumor cell capture. Chem Mater 29:8052–8056
Navarro-Sánchez J, Almora-Barrios N, Lerma-Berlanga B, Ruiz-Pernía JJ, Lorenz-Fonfria VA, Tuñón I, Martí-Gastaldo C (2019) Translocation of enzymes into a mesoporous MOF for enhanced catalytic activity under extreme conditions. Chem Sci 10:4082–4088
Li P, Moon S-Y, Guelta MA, Lin L, Gómez-Gualdrón DA, Snurr RQ, Harvey SP, Hupp JT, Farha OK (2016) Nanosizing a metal–organic framework enzyme carrier for accelerating nerve agent hydrolysis. ACS Nano 10:9174–9182
Deng H, Grunder S, Cordova KE et al (2012) Large-pore apertures in a series of metal-organic frameworks. Science 336:1018–1023
Lykourinou V, Chen Y, Wang X-S, Meng L, Hoang T, Ming L-J, Musselman RL, Ma S (2011) Immobilization of MP-11 into a mesoporous metal–organic framework, MP-11@mesoMOF: a new platform for enzymatic catalysis. J Am Chem Soc 133:10382–10385
Feng D, Liu T-F, Su J et al (2015) Stable metal-organic frameworks containing single-molecule traps for enzyme encapsulation. Nat Commun 6:5979
Chen Y, Lykourinou V, Vetromile C, Hoang T, Ming L-J, Larsen RW, Ma S (2012) How can proteins enter the interior of a MOF? Investigation of cytochrome c translocation into a MOF consisting of mesoporous cages with microporous windows. J Am Chem Soc 134:13188–13191
Li P, Chen Q, Wang TC et al (2018) Hierarchically engineered mesoporous metal-organic frameworks toward cell-free immobilized enzyme systems. Chem 4:1022–1034
Hidalgo T, Alonso-Nocelo M, Bouzo BL, Reimondez-Troitiño S, Abuin-Redondo C, de la Fuente M, Horcajada P (2020) Biocompatible iron(III) carboxylate metal–organic frameworks as promising RNA nanocarriers. Nanoscale 12:4839–4845
Peng S, Bie B, Sun Y, Liu M, Cong H, Zhou W, Xia Y, Tang H, Deng H, Zhou X (2018) Metal-organic frameworks for precise inclusion of single-stranded DNA and transfection in immune cells. Nat Commun 9:1–10
Gkaniatsou E, Sicard C, Ricoux R, Benahmed L, Bourdreux F, Zhang Q, Serre C, Mahy J-P, Steunou N (2018) Enzyme encapsulation in mesoporous metal–organic frameworks for selective biodegradation of harmful dye molecules. Angew Chem Int Ed 57:16141–16146
Lyu F, Zhang Y, Zare RN, Ge J, Liu Z (2014) One-pot synthesis of protein-embedded metal–organic frameworks with enhanced biological activities. Nano Lett 14:5761–5765
Shieh F-K, Wang S-C, Yen C-I et al (2015) Imparting functionality to biocatalysts via embedding enzymes into nanoporous materials by a de Novo approach: size-selective sheltering of catalase in metal–organic framework microcrystals. J Am Chem Soc 137:4276–4279
Liang K, Ricco R, Doherty CM et al (2015) Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat Commun 6:7240
Yang X, Tang Q, Jiang Y, Zhang M, Wang M, Mao L (2019) Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J Am Chem Soc 141:3782–3786
Astria E, Thonhofer M, Ricco R et al (2019) Carbohydrates@MOFs. Mater Horiz 6:969–977
Li S, Dharmarwardana M, Welch RP, Benjamin CE, Shamir AM, Nielsen SO, Gassensmith JJ (2018) Investigation of controlled growth of metal–organic frameworks on anisotropic virus particles. ACS Appl Mater Interfaces 10:18161–18169
Liang K, Richardson JJ, Cui J, Caruso F, Doonan CJ, Falcaro P (2016) Metal–organic framework coatings as cytoprotective exoskeletons for living cells. Adv Mater 28:7910–7914
Carraro F, Velásquez-Hernández M d J, Astria E et al (2020) Phase dependent encapsulation and release profile of ZIF-based biocomposites. Chem Sci 11:3397–3404
Riccò R, Liang W, Li S, Gassensmith JJ, Caruso F, Doonan C, Falcaro P (2018) Metal–organic frameworks for cell and virus biology: a perspective. ACS Nano 12:13–23
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Lee Y-R, Jang M-S, Cho H-Y, Kwon H-J, Kim S, Ahn W-S (2015) ZIF-8: a comparison of synthesis methods. Chem Eng J 271:276–280
Lu G, Li S, Guo Z et al (2012) Imparting functionality to a metal–organic framework material by controlled nanoparticle encapsulation. Nat Chem 4:310–316
Chen T-T, Yi J-T, Zhao Y-Y, Chu X (2018) Biomineralized metal–organic framework nanoparticles enable intracellular delivery and endo-lysosomal release of native active proteins. J Am Chem Soc 140:9912–9920
Pei X, Wu Y, Wang J, Chen Z, Liu W, Su W, Liu F (2020) Biomimetic mineralization of nitrile hydratase into a mesoporous cobalt-based metal–organic framework for efficient biocatalysis. Nanoscale 12:967–972
Chen G, Kou X, Huang S, Tong L, Shen Y, Zhu W, Zhu F, Ouyang G (2020) Modulating the biofunctionality of metal–organic-framework-encapsulated enzymes through controllable embedding patterns. Angew Chem 132:2889–2896
Zoungrana T, Findenegg GH, Norde W (1997) Structure, stability, and activity of adsorbed enzymes. J Colloid Interface Sci 190:437–448
Maddigan NK, Tarzia A, Huang DM, Sumby CJ, Bell SG, Falcaro P, Doonan CJ (2018) Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8. Chem Sci 9:4217–4223
Velasquez M, Astria E, Winkler S et al (2020) Modulation of metal-azolate frameworks for the tunable release of encapsulated glycosaminoglycans. Chem Sci 11:10835–10843
Wang H, Han L, Zheng D et al (2020) Protein-structure-directed metal–organic zeolite-like networks as biomacromolecule carriers. Angew Chem Int Ed 59:6263–6267
Liang K, Coghlan CJ, Bell SG, Doonan C, Falcaro P (2016) Enzyme encapsulation in zeolitic imidazolate frameworks: a comparison between controlled co-precipitation and biomimetic mineralisation. Chem Commun 52:473–476
Liang K, Richardson JJ, Doonan CJ, Mulet X, Ju Y, Cui J, Caruso F, Falcaro P (2017) An enzyme-coated metal–organic framework shell for synthetically adaptive cell survival. Angew Chem Int Ed 56:8510–8515
Yang A-S, Honig B (1993) On the pH dependence of protein stability. J Mol Biol 231:459–474
Liang W, Ricco R, Maddigan NK, Dickinson RP, Xu H, Li Q, Sumby CJ, Bell SG, Falcaro P, Doonan CJ (2018) Control of structure topology and spatial distribution of biomacromolecules in protein@ZIF-8 biocomposites. Chem Mater 30:1069–1077
Zhang S, Du M, Shao P, Wang L, Ye J, Chen J, Chen J (2018) Carbonic anhydrase enzyme-MOFs composite with a superior catalytic performance to promote CO2 absorption into tertiary amine solution. Environ Sci Technol 52:12708–12716
Wu X, Yue H, Zhang Y et al (2019) Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat Commun 10:1–8
Carraro F, Williams J, Linares-Moreau M, Parise C, Liang W, Amenitsch H, Doonan C, Kappe CO, Falcaro P (2020) Continuous flow synthesis of ZIF-8 biocomposites with tuneable particle size. Angew Chem Int Ed 59:8123. https://doi.org/10.1002/anie.202000678
Hu C, Bai Y, Hou M et al (2020) Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis. Sci Adv 6:eaax5785
Katz MJ, Brown ZJ, Colón YJ, Siu PW, Scheidt KA, Snurr RQ, Hupp JT, Farha OK (2013) A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem Commun 49:9449–9451
Wei T-H, Wu S-H, Huang Y-D et al (2019) Rapid mechanochemical encapsulation of biocatalysts into robust metal–organic frameworks. Nat Commun 10:1–8
Feng Y, Wang H, Zhang S et al (2019) Antibodies@MOFs: an in vitro protective coating for preparation and storage of biopharmaceuticals. Adv Mater 31:1805148
Nordberg M, Duffus J, Templeton DM (2004) Glossary of terms used in toxicokinetics (IUPAC Recommendations 2003). Pure Appl Chem 76:1033–1082
Singh AK (2016) Engineered nanoparticles: structure, properties and mechanisms of toxicity. Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam/Boston
Silva Lima B, Videira MA (2018) Toxicology and biodistribution: the clinical value of animal biodistribution studies. Mol Ther – Methods Clin Dev 8:183–197
Lakshmi BA, Kim S (2019) Current and emerging applications of nanostructured metal–organic frameworks in cancer-targeted theranostics. Mater Sci Eng C 105:110091
Ghosh R, Bhuiyan MA, Dewan I, Ghosh DR, Islam A (2012) Immediate release drug delivery system (Tablets): an overview. Int Res J Pharm Appl Sci 2:88–94
Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J (2003) Reticular synthesis and the design of new materials. Nature 423:705–714
Leader B, Baca QJ, Golan DE (2008) Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 7:21–39
Vermonden T, Censi R, Hennink WE (2012) Hydrogels for protein delivery. Chem Rev 112:2853–2888
Szlachcic A, Zakrzewska M, Otlewski J (2011) Longer action means better drug: tuning up protein therapeutics. Biotechnol Adv 29:436–441
Pathak Y, Thassu D (eds) (2016) Drug delivery nanoparticles formulation and characterization, 0th edn. https://doi.org/10.3109/9781420078053
Mura S, Nicolas J, Couvreur P (2013) Stimuli-responsive nanocarriers for drug delivery. Nat Mater 12:991–1003
Cai W, Wang J, Chu C, Chen W, Wu C, Liu G (2019) Metal-organic framework-based stimuli-responsive systems for drug delivery. Adv Sci 6:1801526
Velásquez-Hernández M d J, Ricco R, Carraro F et al (2019) Degradation of ZIF-8 in phosphate buffered saline media. CrystEngComm 21:4538–4544
Luzuriaga MA, Benjamin CE, Gaertner MW, Lee H, Herbert FC, Mallick S, Gassensmith JJ (2019) ZIF-8 degrades in cell media, serum, and some—but not all—common laboratory buffers. Supramol Chem 0:1–6
Gkaniatsou E, Sicard C, Ricoux R, Mahy J-P, Steunou N, Serre C (2017) Metal–organic frameworks: a novel host platform for enzymatic catalysis and detection. Mater Horiz 4:55–63
Horcajada P, Serre C, McKinlay AC, Morris RE (2011) Biomedical applications of metal-organic frameworks. In: Farrusseng D (ed) Metal-organic frameworks. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 213–250
Rojas S, Arenas-Vivo A, Horcajada P (2019) Metal-organic frameworks: a novel platform for combined advanced therapies. Coord Chem Rev 388:202–226
Hoop M, Walde CF, Riccò R et al (2018) Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl Mater Today 11:13–21
Simon-Yarza T, Mielcarek A, Couvreur P, Serre C (2018) Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv Mater 30:1707365
Kohane DS (2007) Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng 96:203–209
Petros RA, DeSimone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9:615–627
Yu W, Liu R, Zhou Y, Gao H (2020) Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci 6:100–116
Rejman J, Oberle V, Zuhorn IS, Hoekstra D (2004) Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J 377:159–169
Hillaireau H, Couvreur P (2009) Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci 66:2873–2896
Anselmo AC, Mitragotri S (2014) An overview of clinical and commercial impact of drug delivery systems. J Control Release 190:15–28
Horcajada P, Chalati T, Serre C et al (2010) Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater 9:172–178
Della Rocca J, Liu D, Lin W (2011) Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc Chem Res 44:957–968
Prow TW, Grice JE, Lin LL et al (2011) Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev 63:470–491
Yang X-X, Feng P, Cao J, Liu W, Tang Y (2020) Composition-engineered metal–organic framework-based microneedles for glucose-mediated transdermal insulin delivery. ACS Appl Mater Interfaces 12:13613–13621
Tiwari G, Tiwari R, Bannerjee S, Bhati L, Pandey S, Pandey P, Sriwastawa B (2012) Drug delivery systems: an updated review. Int J Pharma Investig 2:2
Singh M, Chakrapani A, O’Hagan D (2007) Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines 6:797–808
Li X, Lachmanski L, Safi S, Sene S, Serre C, Grenèche JM, Zhang J, Gref R (2017) New insights into the degradation mechanism of metal-organic frameworks drug carriers. Sci Rep 7:13142
Moss GP, Smith PAS, Tavernier D (1995) Glossary of class names of organic compounds and reactivity intermediates based on structure (IUPAC Recommendations 1995). Pure Appl Chem 67:1307–1375
Ng C-H, Guan MSH, Koh C, Ouyang X, Yu F, Tan E-K, O’Neill SP, Zhang X, Chung J, Lim K-L (2012) AMP Kinase activation mitigates dopaminergic dysfunction and mitochondrial abnormalities in drosophila models of Parkinson’s disease. J Neurosci 7:14311
Du F, Yu Q, Yan S, Hu G, Lue L-F, Walker DG, Wu L, Yan SF, Tieu K, Yan SS (2017) PINK1 signalling rescues amyloid pathology and mitochondrial dysfunction in Alzheimer’s disease. Brain 140:3233–3251
Mitragotri S, Burke PA, Langer R (2014) Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13:655–672
Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769
Zhang Y, Wang F, Ju E, Liu Z, Chen Z, Ren J, Qu X (2016) Metal-organic-framework-based vaccine platforms for enhanced systemic immune and memory response. Adv Funct Mater 26:6454–6461
Qin X, Yu C, Wei J, Li L, Zhang C, Wu Q, Liu J, Yao SQ, Huang W (2019) Rational design of nanocarriers for intracellular protein delivery. Adv Mater 31:1902791
Wang Z, Zhou X, Li J et al (2013) Suppression of hepatoma tumor growth by systemic administration of the phytotoxin gelonin driven by the survivin promoter. Neoplasma 60:469–479
Cheng G, Li W, Ha L et al (2018) Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J Am Chem Soc 140:7282–7291
Cheng H, Zhu J-Y, Li S-Y, Zeng J-Y, Lei Q, Chen K-W, Zhang C, Zhang X-Z (2016) An O 2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv Funct Mater 26:7847–7860
Chen W-H, Luo G-F, Vázquez-González M, Cazelles R, Sohn YS, Nechushtai R, Mandel Y, Willner I (2018) Glucose-responsive metal–organic-framework nanoparticles act as “smart” sense-and-treat carriers. ACS Nano 12:7538–7545
Huang Z, Ge M, Carraro F, Doonan CJ, Falcaro P, Zou X (2020) Can 3D electron diffraction provide accurate atomic structures of metal-organic frameworks? Faraday Discuss. https://doi.org/10.1039/D0FD00015A
Allegretto JA, Dostalek J, Rafti M, Menges B, Azzaroni O, Knoll W (2019) Shedding light on the dark corners of metal–organic framework thin films: growth and structural stability of ZIF-8 layers probed by optical waveguide spectroscopy. J Phys Chem A 123:1100–1109
Zhao H, Xu J, Li Y, Guan X, Han X, Xu Y, Zhou H, Peng R, Wang J, Liu Z (2019) Nanoscale coordination polymer based nanovaccine for tumor immunotherapy. ACS Nano 13:13127–13135
Miao Y, Pan W, Chen K, Wei H, Mi F, Lu M, Chang Y, Sung H (2019) Engineering a nanoscale Al-MOF-armored antigen carried by a “Trojan Horse”-like platform for oral vaccination to induce potent and long-lasting immunity. Adv Funct Mater 29:1904828
Welch RP, Lee H, Luzuriaga MA, Brohlin OR, Gassensmith JJ (2018) Protein–polymer delivery: chemistry from the cold chain to the clinic. Bioconjug Chem 29:2867–2883
Kristensen D, Chen D, Cummings R (2011) Vaccine stabilization: research, commercialization, and potential impact. Vaccine 29:7122–7124
Wilkins DK, Grimshaw SB, Receveur V, Dobson CM, Jones JA, Smith LJ (1999) Hydrodynamic radii of native and denatured proteins measured by pulse field gradient NMR techniques†. Biochemistry 38:16424–16431
Wang C, Sun H, Luan J, Jiang Q, Tadepalli S, Morrissey JJ, Kharasch ED, Singamaneni S (2018) Metal–organic framework encapsulation for biospecimen preservation. Chem Mater 30:1291–1300
Devarajan P (2008) Neutrophil gelatinase-associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest 68:89–94
Scholler N, Urban N (2007) CA125 in ovarian cancer. Biomark Med 1:513–523
Wang C, Sudlow G, Wang Z, Cao S, Jiang Q, Neiner A, Morrissey JJ, Kharasch ED, Achilefu S, Singamaneni S (2018) Metal-organic framework encapsulation preserves the bioactivity of protein therapeutics. Adv Healthc Mater 7:1800950
Thévenot DR, Toth K, Durst RA, Wilson GS (2001) Electrochemical biosensors: recommended definitions and classification 1 International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytical Chemistry).1. Biosens Bioelectron 16:121–131
Gutiérrez-Capitán M, Baldi A, Fernández-Sánchez C (2020) Electrochemical paper-based biosensor devices for rapid detection of biomarkers. Sensors 20:967
Peltomaa R, Glahn-Martínez B, Benito-Peña E, Moreno-Bondi M (2018) Optical biosensors for label-free detection of small molecules. Sensors 18:4126
Byrne B, Stack E, Gilmartin N, O’Kennedy R (2009) Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors 9:4407–4445
Mehrotra P (2016) Biosensors and their applications – A review. J Oral Biol Craniofacial Res 6:153–159
Turner APF (2013) Biosensors: sense and sensibility. Chem Soc Rev 42:3184
Miller SE, Teplensky MH, Moghadam PZ, Fairen-Jimenez D (2016) Metal-organic frameworks as biosensors for luminescence-based detection and imaging. Interface Focus 6:20160027
Bilal M, Adeel M, Rasheed T, Iqbal HMN (2019) Multifunctional metal–organic frameworks-based biocatalytic platforms: recent developments and future prospects. J Mater Res Technol 8:2359–2371
Wang H-S (2017) Metal–organic frameworks for biosensing and bioimaging applications. Coord Chem Rev 349:139–155
Carrasco S (2018) Metal-organic frameworks for the development of biosensors: a current overview. Biosensors 8:92
Liu C-S, Li J, Pang H (2020) Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coord Chem Rev 410:213222
Winterbourn CC (2018) Biological production, detection, and fate of hydrogen peroxide. Antioxid Redox Signal 29:541–551
Dębski D, Smulik R, Zielonka J, Michałowski B, Jakubowska M, Dębowska K, Adamus J, Marcinek A, Kalyanaraman B, Sikora A (2016) Mechanism of oxidative conversion of Amplex® Red to resorufin: pulse radiolysis and enzymatic studies. Free Radic Biol Med 95:323–332
Gong C, Shen Y, Chen J, Song Y, Chen S, Song Y, Wang L (2017) Microperoxidase-11@PCN-333 (Al)/three-dimensional macroporous carbon electrode for sensing hydrogen peroxide. Sensors Actuators B Chem 239:890–897
Yin Y, Gao C, Xiao Q, Lin G, Lin Z, Cai Z, Yang H (2016) Protein-metal organic framework hybrid composites with intrinsic peroxidase-like activity as a colorimetric biosensing platform. ACS Appl Mater Interfaces 8:29052–29061
Metelitza DI, Litvinchuk AV, Savenkova MI (1991) Peroxidase-catalyzed co-oxidation of halogen-substituted phenols and 4-aminoantipyrine. J Mol Catal 67:401–411
Khawandanah J (2019) Double or hybrid diabetes: a systematic review on disease prevalence, characteristics and risk factors. Nutr Diabetes 9:33
Wu X, Ge J, Yang C, Hou M, Liu Z (2015) Facile synthesis of multiple enzyme-containing metal–organic frameworks in a biomolecule-friendly environment. Chem Commun 51:13408–13411
Chen W-H, Vázquez-González M, Zoabi A, Abu-Reziq R, Willner I (2018) Biocatalytic cascades driven by enzymes encapsulated in metal–organic framework nanoparticles. Nat Catal 1:689–695
Liu H, Du Y, Gao J, Zhou L, He Y, Ma L, Liu G, Huang Z, Jiang Y (2020) Compartmentalization of biocatalysts by immobilizing bienzyme in hollow ZIF-8 for colorimetric detection of glucose and phenol. Ind Eng Chem Res 59:42–51
Xu Y, Liu S-Y, Liu J, Zhang L, Chen D, Chen J, Ma Y, Zhang J-P, Dai Z, Zou X (2019) In situ enzyme immobilization with oxygen-sensitive luminescent metal–organic frameworks to realize “All-in-One” multifunctions. Chem Eur J 25:5463–5471
Patra S, Crespo TH, Permyakova A, Sicard C, Serre C, Chaussé A, Steunou N, Legrand L (2015) Design of metal organic framework–enzyme based bioelectrodes as a novel and highly sensitive biosensing platform. J Mater Chem B 3:8983–8992
Xu W, Jiao L, Yan H, Wu Y, Chen L, Gu W, Du D, Lin Y, Zhu C (2019) Glucose oxidase-integrated metal–organic framework hybrids as biomimetic cascade nanozymes for ultrasensitive glucose biosensing. ACS Appl Mater Interfaces 11:22096–22101
Jeanson A, Cloes J-M, Bouchet M, Rentier B (1988) Comparison of conjugation procedures for the preparation of monoclonal antibody-enzyme conjugates. J Immunol Methods 111:261–270
Presentini R (2017) A new covalent peroxidase conjugation method using bis(sulfosuccinimidyl) suberate as cross-linking reagent in a two-step procedure. J Immunoass Immunochem 38:100–113
Xue L, Yang Y, Wu S, Huang Y, Li J, Xiang Y, Li G (2020) In situ reduction of porous copper metal–organic frameworks for three-dimensional catalytic click immunoassay. Anal Chem 92:2972–2978
Liu T-Z, Hu R, Zhang X, Zhang K-L, Liu Y, Zhang X-B, Bai R-Y, Li D, Yang Y-H (2016) Metal–organic framework nanomaterials as novel signal probes for electron transfer mediated ultrasensitive electrochemical immunoassay. Anal Chem 88:12516–12523
Mishra S, Upadhaya K, Mishra KB, Shukla AK, Tripathi RP, Tiwari VK (2016) Carbohydrate-based therapeutics. In: Studies in natural products chemistry. Elsevier, Cambridge, pp 307–361
Osborn HMI, Evans PG, Gemmell N, Osborne SD (2004) Carbohydrate-based therapeutics. J Pharm Pharmacol 56:691–702
Pomin V, Mulloy B (2018) Glycosaminoglycans and proteoglycans. Pharmaceuticals 11:27
Gandhi NS, Mancera RL (2008) The structure of glycosaminoglycans and their interactions with proteins. Chem Biol Drug Des 72:455–482
Wong C (ed) (2003) Carbohydrate-based drug discovery, 1st edn. Wiley-VCH, Weinheim. https://doi.org/10.1002/3527602437
Aich U, Meledeo MA, Sampathkumar S-G et al (2010) Development of delivery methods for carbohydrate-based drugs: controlled release of biologically-active short chain fatty acid-hexosamine analogs. Glycoconj J 27:445–459
Hirsh J, Anand SS, Halperin JL, Fuster V (2001) Guide to anticoagulant therapy: heparin: a statement for healthcare professionals from the American Heart Association. Circulation 103:2994–3018
Lee H-Y, Hwang C-H, Kim H-E, Jeong S-H (2018) Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr Polym 186:290–298
Stern R, Kogan G, Jedrzejas MJ, Šoltés L (2007) The many ways to cleave hyaluronan. Biotechnol Adv 25:537–557
Vinogradov VV, Drozdov AS, Mingabudinova LR et al (2018) Composites based on heparin and MIL-101(Fe): the drug releasing depot for anticoagulant therapy and advanced medical nanofabrication. J Mater Chem B 6:2450–2459
Abednejad A, Ghaee A, Nourmohammadi J, Mehrizi AA (2019) Hyaluronic acid/ carboxylated Zeolitic Imidazolate Framework film with improved mechanical and antibacterial properties. Carbohydr Polym 222:115033
Makyła-Juzak K, Chachaj-Brekiesz A, Dynarowicz-Latka P, Dąbczyński P, Zemla J (2018) The effect of dextran sulfate—As model glycosaminoglycan analogue—on membrane lipids: DPPC, cholesterol, and DPPC–cholesterol mixture. The Monolayer Study. J Membr Biol 251:641–651
Misra S, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Ghatak S (2015) Utilization of glycosaminoglycans/proteoglycans as carriers for targeted therapy delivery. Int J Cell Biol 2015:1–25
Cai W, Gao H, Chu C, Wang X, Wang J, Zhang P, Lin G, Li W, Liu G, Chen X (2017) Engineering phototheranostic nanoscale metal–organic frameworks for multimodal imaging-guided cancer therapy. ACS Appl Mater Interfaces 9:2040–2051
Wickens JM, Alsaab HO, Kesharwani P, Bhise K, Amin MCIM, Tekade RK, Gupta U, Iyer AK (2017) Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov Today 22:665–680
Chen C, Zhao S, Karnad A, Freeman JW (2018) The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol 11:64
Blackstock JC (1989) Chapter 7 – Nucleic Acids. In: Blackstock JC (ed) Guide to biochemistry. Butterworth-Heinemann, Oxford, pp 78–90
Anderson W (1992) Human gene therapy. Science 256:808–813
Mulligan R (1993) The basic science of gene therapy. Science 260:926–932
Schena M (2002) Microarray analysis. Wiley, Hoboken
DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A (2010) A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci 107:8800–8805
Subramanya S, Kim S-S, Manjunath N, Shankar P (2010) RNA interference-based therapeutics for human immunodeficiency virus HIV-1 treatment: synthetic siRNA or vector-based shRNA? Expert Opin Biol Ther 10:201–213
Pai SI, Lin Y-Y, Macaes B, Meneshian A, Hung C-F, Wu T-C (2006) Prospects of RNA interference therapy for cancer. Gene Ther 13:464–477
Courties G, Presumey J, Duroux-Richard I, Jorgensen C, Apparailly F (2009) RNA interference-based gene therapy for successful treatment of rheumatoid arthritis. Expert Opin Biol Ther 9:535–538
Williams PD, Kingston PA (2011) Plasmid-mediated gene therapy for cardiovascular disease. Cardiovasc Res 91:565–576
Council NR (1984) Genetic engineering of plants: agricultural research opportunities and policy concerns. The National Academies Press, Washington, DC
Ramamoorth M, Narvekar A (2015) Non viral vectors in gene therapy – An overview. J Clin Diagn Res 9:GE01–GE06
Chen Q, Xu M, Zheng W, Xu T, Deng H, Liu J (2017) Se/Ru-decorated porous metal–organic framework nanoparticles for the delivery of pooled siRNAs to reversing multidrug resistance in taxol-resistant breast cancer cells. ACS Appl Mater Interfaces 9:6712–6724
Wang S, McGuirk CM, Ross MB, Wang S, Chen P, Xing H, Liu Y, Mirkin CA (2017) General and direct method for preparing oligonucleotide-functionalized metal–organic framework nanoparticles. J Am Chem Soc 139:9827–9830
Wang Z, Fu Y, Kang Z et al (2017) Organelle-specific triggered release of immunostimulatory oligonucleotides from intrinsically coordinated DNA–metal–organic frameworks with soluble exoskeleton. J Am Chem Soc 139:15784–15791
Wang S, Chen Y, Wang S, Li P, Mirkin CA, Farha OK (2019) DNA-functionalized metal–organic framework nanoparticles for intracellular delivery of proteins. J Am Chem Soc 141:2215–2219
Teplensky MH, Fantham M, Poudel C et al (2019) A highly porous metal-organic framework system to deliver payloads for gene knockdown. Chem 5:2926–2941
Alsaiari SK, Patil S, Alyami M, Alamoudi KO, Aleisa FA, Merzaban JS, Li M, Khashab NM (2018) Endosomal escape and delivery of CRISPR/Cas9 genome editing machinery enabled by nanoscale zeolitic imidazolate framework. J Am Chem Soc 140:143–146
Alyami MZ, Alsaiari SK, Li Y, Qutub SS, Aleisa FA, Sougrat R, Merzaban JS, Khashab NM (2020) Cell-type-specific CRISPR/Cas9 delivery by biomimetic metal organic frameworks. J Am Chem Soc 142:1715–1720
Poddar A, Conesa JJ, Liang K et al (2019) Encapsulation, visualization and expression of genes with biomimetically mineralized zeolitic imidazolate framework-8 (ZIF-8). Small 15:1902268
Li Y, Zhang K, Liu P, Chen M, Zhong Y, Ye Q, Wei MQ, Zhao H, Tang Z (2019) Encapsulation of plasmid DNA by nanoscale metal-organic frameworks for efficient gene transportation and expression. Adv Mater Weinheim 31:e1901570
Wang H, Chen Y, Wang H, Liu X, Zhou X, Wang F (2019) DNAzyme-loaded metal–organic frameworks (MOFs) for self-sufficient gene therapy. Angew Chem Int Ed 58:7380–7384
Peng S, Bie B, Sun Y, Liu M, Cong H, Zhou W, Xia Y, Tang H, Deng H, Zhou X (2018) Metal-organic frameworks for precise inclusion of single-stranded DNA and transfection in immune cells. Nat Commun 9:1293
Anders C, Jinek M (2014) Chapter One – In vitro enzymology of Cas9. In: Doudna JA, Sontheimer EJ (eds) Methods in enzymology. Academic, Orlando, pp 1–20
Cong L, Ran FA, Cox D et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823
Wu Y, Han J, Xue P, Xu R, Kang Y (2015) Nano metal–organic framework (NMOF)-based strategies for multiplexed microRNA detection in solution and living cancer cells. Nanoscale 7:1753–1759
Zhang H-T, Zhang J-W, Huang G, Du Z-Y, Jiang H-L (2014) An amine-functionalized metal–organic framework as a sensing platform for DNA detection. Chem Commun 50:12069–12072
Shrestha H, Bala R, Arora S (2014) Lipid-based drug delivery systems. J Pharma 2014:e801820
Wang S, Morris W, Liu Y, McGuirk CM, Zhou Y, Hupp JT, Farha OK, Mirkin CA (2015) Surface-specific functionalization of nanoscale metal–organic frameworks. Angew Chem Int Ed 54:14738–14742
Horcajada P, Serre C, Grosso D, Boissière C, Perruchas S, Sanchez C, Férey G (2009) Colloidal route for preparing optical thin films of nanoporous metal–organic frameworks. Adv Mater 21:1931–1935
van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 19:213–228
Hasturk O, Kaplan DL (2019) Cell armor for protection against environmental stress: advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater 95:3–31
Blaeser A, Duarte Campos DF, Puster U, Richtering W, Stevens MM, Fischer H (2016) Controlling shear stress in 3D bioprinting is a key factor to balance printing resolution and stem cell integrity. Adv Healthc Mater 5:326–333
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32:773–785
Matsuzawa A, Matsusaki M, Akashi M (2013) Effectiveness of nanometer-sized extracellular matrix layer-by-layer assembled films for a cell membrane coating protecting cells from physical stress. Langmuir 29:7362–7368
Levesque MJ, Sprague EA, Schwartz CJ, Nerem RM (1989) The influence of shear stress on cultured vascular endothelial cells: the stress response of an anchorage-dependent mammalian cell. Biotechnol Prog 5:1–8
Park JH, Hong D, Lee J, Choi IS (2016) Cell-in-shell hybrids: chemical nanoencapsulation of individual cells. Acc Chem Res 49:792–800
Kim BJ, Cho H, Park JH, Mano JF, Choi IS (2018) Strategic advances in formation of cell-in-shell structures: from syntheses to applications. Adv Mater 30:1706063
Dutta S, Kim J, Hsieh P, Hsu Y, Kaneti YV, Shieh F, Yamauchi Y, Wu KC-W (2019) Nanoarchitectonics of biofunctionalized metal–organic frameworks with biological macromolecules and living cells. Small Methods 3:1900213
Chen W, Kong S, Lu M, Chen F, Cai W, Du L, Wang J, Wu C (2020) Comparison of different zinc precursors for the construction of zeolitic imidazolate framework-8 artificial shells on living cells. Soft Matter 16:270–275
Hamilton-Miller JM (1973) Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev 37:166–196
Zhu W, Guo J, Amini S et al (2019) SupraCells: living mammalian cells protected within functional modular nanoparticle-based exoskeletons. Adv Mater 31:1900545
Ji Z, Zhang H, Liu H, Yaghi OM, Yang P (2018) Cytoprotective metal-organic frameworks for anaerobic bacteria. Proc Natl Acad Sci U S A 115:10582–10587
Li S, Dharmarwardana M, Welch RP, Ren Y, Thompson CM, Smaldone RA, Gassensmith JJ (2016) Template-directed synthesis of porous and protective core-shell bionanoparticles. Angew Chem Int Ed 55:10691–10696
Luzuriaga MA, Welch RP, Dharmarwardana M, Benjamin CE, Li S, Shahrivarkevishahi A, Popal S, Tuong LH, Creswell CT, Gassensmith JJ (2019) Enhanced stability and controlled delivery of MOF-encapsulated vaccines and their immunogenic response in vivo. ACS Appl Mater Interfaces 11:9740–9746
Lee J, Cho H, Choi J, Kim D, Hong D, Park JH, Yang SH, Choi IS (2015) Chemical sporulation and germination: cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid–Fe III complex. Nanoscale 7:18918–18922
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Carraro, F. et al. (2021). MOFs and Biomacromolecules for Biomedical Applications. In: Horcajada Cortés, P., Rojas Macías, S. (eds) Metal-Organic Frameworks in Biomedical and Environmental Field. Springer, Cham. https://doi.org/10.1007/978-3-030-63380-6_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-63380-6_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63379-0
Online ISBN: 978-3-030-63380-6
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)