Abstract
There exists a wide variety of pulmonary conditions which can be considered to mimic diffuse cystic lung diseases (DCLDs). Radiologically, a cyst is defined as a round parenchymal lucency with a well-defined interface with normal lung. Cysts are becoming increasingly noted due to improved imaging technologies and more routine use of high-resolution computed tomography (CT). Radiologically, the conditions that can mimic true DCLDs include, among others, emphysema, fibrotic lung disease, neoplastic processes, and a range of infections. Some of these disorders have a higher prevalence than true DCLDs, thus underscoring the need to pay careful attention to these conditions when evaluating a patient with DCLD. This chapter comprises a detailed discussion of a variety of common conditions, which mimic DCLDs. In the course of our discussion, we consider the radiographical and clinical nuances which can help to differentiate these conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Diffuse cystic lung diseases
- Pulmonary cyst
- Cyst mimics
- Emphysema
- Interstitial lung disease
- Bronchiectasis
- Neoplastic processes
- Alpha-one antitrypsin deficiency
- Cystic fibrosis
- Primary ciliary dyskinesia
Introduction
Pathologically, a cyst is defined as any round circumscribed space surrounded by a wall, either epithelial or fibrous, of varying thickness [1]. The Fleischner Society Guidelines define a pulmonary cyst radiographically as a round parenchymal lucency or low-attenuating area with a well-defined interface with normal lung [2]. Cysts can have variable wall thickness, though they are most often thin-walled with a mural diameter of less than 2 mm. Most commonly cysts contain air, but they also may be fluid-filled or contain solid material on occasion. Typically, cysts occur without associated emphysematous change.
There is considerable overlap between the radiological appearance of cysts and numerous other radiographical and pathological entities [3]. For example, a bulla is defined as a focal lucency measuring more than 1 cm in diameter, which is bordered by a thin wall no greater than 1 mm in thickness [4]. Bullae, in comparison to cysts, occur in the presence of emphysematous change. Moreover, a cavity is defined as a lucency or low-attenuation area, which tends to occur within an area of pulmonary consolidation, nodule, or mass [2]. Cavities may or may not contain air-fluid levels. Usually wall thickness of a cavity is greater than 4 mm though notably in the case of cavitating consolidation, the consolidation will often resolve leaving only a thin wall. Furthermore, a pneumatocele is a round thin-walled airspace in the lung, which occurs in response to various causative factors such as trauma, aspiration, or acute pneumonia by a process of parenchymal necrosis and check-valve airway obstruction [5].
Given the overlap with other radiological entities there is often diagnostic uncertainty in terms of the radiological diagnosis of cystic lung disease. In this chapter, we consider in more detail a range of common conditions whose radiographical features mimic those of the true diffuse cystic lung diseases (DCLDs).
Emphysema
Emphysema can be pathologically defined as a condition of the lung characterized by abnormal, permanent enlargement of airspaces distal to the terminal bronchiole, accompanied by the destruction of their walls, and without obvious fibrosis [6].
Emphysema is a term used interchangeably with chronic obstructive pulmonary disease (COPD) and is associated with a high burden of morbidity and mortality. COPD is currently the third leading cause of mortality worldwide [7, 8].
While emphysema tends to be strongly associated with smoking, there are other conditions that can cause this structural disorder in the lungs. Alpha-one antitrypsin deficiency (AATD) is a genetic disease characterized by low circulating levels of the alpha-1 antitrypsin protein (AAT) and is inherited in an autosomal co-dominant fashion. AAT is an important and potent protease inhibitor synthesized and secreted by the liver. It functions mainly in the lungs to protect elastic structures against proteases such as neutrophil elastase. Mutations in the SERPINA1 gene on chromosome 14q32, the gene that encodes for AAT , alter the configuration of the protein in multiple ways to inhibit its release from hepatocytes, thereby leading to reduced circulating levels systemically. Homozygosity for the PI*Z allele is associated with low AAT serum levels and high risk of clinical disease. The imbalance of protease and antiprotease caused by reduced AAT levels results in destruction of alveolar walls and interstitial tissues leading to early and rapidly progressive emphysema, particularly when compounded by cigarette smoking [9]. Radiologically it is associated with extensive basal-predominant panlobular emphysema [10, 11] and the distribution itself may trigger diagnosis of the disease as this is an unusual pattern in any other context [12]. Treatment options center around lifestyle modifications and treatment of ensuing COPD , cirrhosis, or panniculitis; however, intravenous AAT replacement therapy is employed in more severe cases in countries where it is available and is the only treatment known to target the underlying cause of the disease [13,14,15].
The radiological evaluation of emphysema involves CT as the core imaging modality. While plain chest radiography can be utilized in the early stages of workup, it does not allow advanced assessment of the extent of emphysematous changes. Several distinct morphological subtypes of emphysema exist and can be characterized on CT (Fig. 14.1). There is a degree of correlation between certain clinical features of COPD and imaging abnormalities, which can help stratify clinical risk for patients [16, 17]. For example, centrilobular emphysema and panlobular emphysema are associated with greater dyspnea and reduced walk distance compared to paraseptal emphysema where there can be less of a symptom burden [16].
Emphysema . Panel A illustrates centrilobular emphysema with multiple areas of low attenuation representing central destruction of the secondary pulmonary lobule. Paraseptal emphysema is visible in panel B toward the posterior pleural surface. These areas of hypoattenuation represent selective destruction of the distal acinus. Panel C shows severe panlobular emphysema with diffuse destruction of lung parenchyma across the entirety of the secondary pulmonary lobule. Panel D demonstrates a large bulla in the left lung base
Centrilobular emphysema , the most common emphysematous subtype, predominantly affects the upper lobes and is defined by small areas of low attenuation. Pathologically, these low attenuation areas are representative of areas of destruction in the center of the secondary pulmonary lobule and are surrounded by normal lung. These areas tend to be poorly defined due to their thin walls and are traversed by centrilobular arteries or arterioles, which mark their center. The areas can range in size from 1 to 3 mm and correlate with pathologically defined centrilobular emphysema [18]. Centrilobular emphysema tends to occur in older patients and is exquisitely associated with smoking status [19]. As the disease progresses, there is increased destruction of the lobular unit and confluence of the observed areas of low attenuation on CT. At this stage, the surrounding walls become almost imperceptible or disappear altogether, and the centrilobular distribution becomes less apparent.
Paraseptal emphysema is the pattern observed when the distal acinus is selectively destroyed [20]. Lesions form near the pleural surface and in the interlobar fissures, and can often coalesce to form large hyperlucent areas bordered by interlobular septae, which are intact but thickened by associated fibrosis due to the high level of airway inflammation [21]. Paraseptal emphysema tends to occur in the middle and upper zone subpleural areas as well as along the mediastinal fissures. Minimal subpleural paraseptal emphysema can be encountered in nonsmokers [22]. Paraseptal emphysema can occur in rows where it can sometimes be confused with the honeycomb change characteristic of fibrotic interstitial lung diseases [23].
Panlobular emphysema refers to diffuse destruction of lung parenchyma across the entire secondary pulmonary lobule rather than the central destruction pathognomonic of centrilobular emphysema [24]. It tends to affect the lower lobes and is highly associated with AATD [25]. It can also occur in cigarette smokers, usually in combination with centrilobular emphysema, and also in those who abuse certain intravenous drugs [26].
Bullae are defined as avascular low-attenuation areas greater than 1 cm in diameter with a thin but perceptible wall [4] and are found in all subtypes of emphysema. They are most closely associated with paraseptal emphysema and are often found in the upper lobes of the lung [27]. Bullae may be associated with adjacent atelectasis whereby the bulla becomes so large it impairs the expansion of the adjacent normal lung [28].
The distinct morphological subtypes of the spectrum of emphysema on CT scanning can help to distinguish disease phenotypes as well as allow assessment of disease severity for patients [16, 17]. CT appearances of emphysema can mimic DCLDs as all subtypes are marked by hyper-lucency with some element of a surrounding wall. Careful observation of spirometric airflow limitation, documentation of patient exposure history, and assessment of symptomatic burden can lead to the correct diagnosis and identify potential treatment strategies for the patients affected by this disease.
Interstitial Lung Disease
The term “interstitial lung disease” (ILD) is a broad classification encompassing a range of lung diseases that cause diffuse alterations to the lung parenchyma over time. The term represents a diverse group of conditions of varying severity, etiology, and prognosis but which are unified by the pathophysiological end-points of acute lung injury leading to chronic interstitial inflammation, damage to parenchymal tissues, fibroblastic change, and eventual distortion of normal lung architecture [29]. Previously investigation of diffuse ILDs often required lung biopsy [30], but advanced CT techniques allow current diagnostic criteria to rely more on imaging interpretation , abrogating the need to histologically demonstrate the underlying pathology in a substantial proportion of patients [31, 32]. Fibrotic ILDs can present with numerous radiological patterns, and a number of these may involve cystic appearing structures such as honeycombing and clustered traction bronchiectasis.
Honeycombing is defined as subpleural, clustered, multi-layered, or multi-tiered cystic air spaces with well-defined walls [33]. It is often difficult to distinguish from paraseptal emphysema. While honeycombing involves stacked cystic structures, paraseptal emphysema presents as a linear arrangement of cysts without stacking. The cystic spaces in honeycomb lung are associated with irregularly thickened walls and range in diameter from 3 to 10 mm. Pathologically these cystic areas represent dilated and thickened terminal bronchioles and most commonly occur in patients with end-stage ILD [34]. Demonstration of honeycombing in association with peripheral subpleural basal-predominant reticular opacities and traction bronchiectasis leads to a radiological diagnosis of definite usual interstitial pneumonia (UIP) and negates the need for surgical lung biopsy as a confirmatory diagnostic test [35]. Probable UIP is marked by the abovementioned features but without demonstration of honeycombing. In these cases, the clinical context is combined with imaging results, and surgical biopsy may be employed if the risk: benefit ratio is favorable for the individual [35].
UIP is the typical radiological pattern observed in idiopathic pulmonary fibrosis (IPF) (Fig. 14.2a) but can also be observed in other disease processes, including rheumatoid arthritis-interstitial lung disease (RA-ILD) (Fig. 14.2b), drug-related ILD, sarcoidosis, and some occupational lung diseases [36]. Furthermore, honeycombing can occur in some cases of nonspecific interstitial pneumonia (Fig. 14.2c) though it is usually a very late sign in longstanding NSIP and tends to not be the predominant feature on imaging [37, 38]. Clusters of cystic lesions seen with ILDs such as NSIP and hypersensitivity pneumonitis can often represent traction bronchiectasis rather than true honeycombing. If bronchiectasis is the cause of these clusters, the cysts tend to be larger than the cysts of honeycombing and are more regular in size [38].
Interstitial lung disease. Panel A demonstrates the CT findings in a patient with usual interstitial pneumonia pattern associated with idiopathic pulmonary fibrosis. The constellation of findings includes honeycombing, traction bronchiectasis, and subpleural reticular opacities thus demonstrating definite UIP. Panel B illustrates honeycombing in a patient with rheumatoid arthritis associated interstitial lung disease. Also noted is an incidental left-sided spontaneous pneumothorax. Panel C shows significant architectural distortion associated with fibrotic nonspecific interstitial pneumonitis
Although cystic areas can be seen in a variety of fibrotic ILDs, careful demographic and clinical history-taking combined with the presence of other (noncystic) interstitial abnormalities on chest radiology often makes it rather straightforward to distinguish these ILDs from true DCLDs.
Bronchiectasis
Bronchiectasis is defined as irreversible abnormal dilatation of the bronchial tree, particularly the proximal and medium-sized bronchi [39]. Bronchiectasis most commonly occurs as a consequence of other conditions and involves destruction and weakening of the bronchial walls through multiple processes such as inflammation, edema, and fibrosis [40]. These factors combine to attract multiple pathogens to the areas of damaged lung, and over time bronchiectatic lungs can often become a nidus for colonization with resistant bacteria [41]. The end result is marked architectural distortion of lung tissue [42]. Causes of the disease are most likely infective, most notably primary lung infection, aspiration pneumonia, and immunodeficiency , but can also be caused by connective tissue disorders, genetic diseases such as cystic fibrosis, and toxin exposure [43]. Clinically bronchiectasis is suspected in patients who present with increased cough, sputum production, and hemoptysis.
Bronchiectasis is often characterized as cylindrical, cystic, or varicose, and differentiation of these three subtypes is made on CT imaging [44]. Airway dilatation is the most common finding on HRCT and can be seen either as parallel lines, so-called tram lines, or end-on where it is seen as ring-shaped shadows. If the airway diameter is more than one and a half times the accompanying vessel’s diameter, this indicates the presence of cylindrical bronchiectasis. This finding is also termed the “signet-ring” sign where the dilated airway is air filled and contiguous with the small opacity of the pulmonary artery [45] (Fig. 14.3a). In addition to dilatation of the airway, lack of tapering of the bronchus and bronchial wall thickening are seen and the combination of all three features is more specific for bronchiectasis than for other conditions that can also be associated with mild bronchial dilatation such as asthma.
Bronchiectasis . Panel A depicts bronchiectasis, dilated bronchioles can be seen throughout and there are examples of signet-ring formation where the dilated bronchiole is wider in diameter than the accompanying vessel. Panel B is from a patient with cystic fibrosis and demonstrates the severe apical cystic bronchiectasis associated with these cases
Varicose bronchiectasis is diagnosed wherever alternating areas of bronchial dilatation and constriction occur in nearby proximity [46]. Constricted areas can give rise to post-obstructive pneumonitis and thereby cause additional damage to lung parenchyma [47]. Cystic bronchiectasis occurs in more severe cases where unchecked inflammation can result in neovascularization and angiogenesis [48]. This causes cyst-like structures to form off the damaged bronchial wall, and these tend to cluster, often into areas which resemble clusters of grapes [47]. This bronchiectasis subtype mimics DCLDs most closely [2] (Fig. 14.3b).
The distribution on HRCT of bronchiectasis can be indicative of the underlying cause [49]. Cystic fibrosis is commonly associated with upper lobe predominant findings on CT, which tend to be particularly severe in terms of the level of parenchymal distortion [50] (see Fig. 14.3b). Primary ciliary dyskinesia, which causes bronchiectasis due to impaired mucus clearance, has a predilection for middle and lower zones, while idiopathic bronchiectasis tends to occur in the lower zones [51]. There is some evidence that the extent of bronchiectasis on HRCT correlates with disease severity with evidence of mucus plugging and bronchial wall thickening correlating with decline in FEV1 over time in a repeated measure study [52]; however, this assertion has not been borne out in subsequent studies [53]. The pattern of disease does not seem to correlate well with clinical severity measures except that cystic bronchiectasis can be associated with hemoptysis [44].
Neoplastic Processes
Cavitary and cystic change is an unusual but documented occurrence in lung neoplasms, and in certain cases cavitary lung tumors can be difficult to differentiate from DCLDs. Detailed description of the neoplastic causes of DCLDs is provided in Chap. 11 of this textbook. In those with primary lung malignancy, cavitary change has been detected in up to 22% of CT images [54, 55]. Squamous cell carcinoma accounts for the vast majority of the cases of cavitary primary lung malignancy, up to 80% in some reports [56] (Fig. 14.4). Adenocarcinoma represents the remainder of cases. In general, cavities associated with malignancy are solitary; however, very rarely bronchoalveolar cell carcinoma can present with multiple cavitary lesions [57]. The proposed mechanism for cavity and cyst genesis in lung cancer is that unchecked rapid growth of the tumor exceeds the tumor’s own blood supply and central necrosis occurs [55]. Recently it has been shown that a very high proportion of those primary tumors that cavitate exhibit overexpression of epidermal growth factor receptor (EGFR), which may contribute to their rapid growth and central necrosis and add some merit to this proposed mechanism [58]. Patients with a cavitating primary lung malignancy have a worse prognosis than those who do not have cavitary lesions, particularly for those patients with squamous cell carcinoma [59].
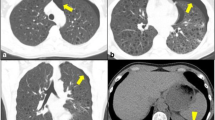
Neoplastic processes . Panel A illustrates a cavitary mass in a patient with primary squamous cell carcinoma of the lung. Panel B illustrates a similar cyst-like structure in a patient with metastatic endometrial sarcoma. Panel C demonstrates a metastatic urothelial cancer with a moderate-sized metastasis in the posterior right lower lobe. This metastasis has become cystic after treatment of the urothelial cancer, panel D
In addition to cavitating primary lung malignancies, cystic change can be frequently associated with metastatic malignancies, especially sarcomas arising from a variety of different sites [59]. Cystic metastases associated with sarcoma carry a particularly poor prognosis due to the lesions’ tendency toward pneumothorax formation [60]. Direct extension of the metastasis to the pleural space is the most common mechanism by which this occurs. The cystic metastasis associated with rare endometrial stromal sarcomas (see Fig. 14.4) can very closely resemble DCLDs on chest imaging, and should be a key differential diagnosis when evaluating DCLD patients [61].
Infectious and Inflammatory Causes
Multiple infectious and inflammatory processes can contribute to cyst formation (see Chap. 10 for a more in-depth discussion of the infectious etiologies of DCLDs). Many of these processes involve cavitation, which can further evolve into cystic change. The radiologic appearances of these various processes can overlap significantly with DCLDs to confer considerable diagnostic uncertainty. Cavitation can occur with a variety of organisms and form via a process of suppurative necrosis, especially in the case of bacterial pneumonia. Radiographically these cavities are surrounded by areas of consolidation and can often transform further to form lung abscesses, clearly circumscribed collections of pus occurring due to liquefactive necrosis of lung tissues [62]. Classically pulmonary abscesses are easily visualized on chest radiograph due to the presence of a thick-walled cavity containing an air-fluid level (Fig. 14.5). With treatment, the air-fluid level will resolve, consolidation will reduce and eventually disappear, and the wall of the cavity will become thinner. However, the cavity can persist for months after treatment of the active infection and can on occasion be mistaken for cystic lung disease [62].
Infectious processes. Panel A shows cavitary transformation in a case of necrotizing pneumonia due to the Rhizopus fungus. Panel B demonstrates a thick-walled cavity in the right upper lobe in a patient with pulmonary Mycobacterium tuberculosis infection. Panel C shows multiple septic emboli that have cavitated
Mycobacterium tuberculosis (TB) , the leading cause of infectious disease mortality worldwide, is a common cause of cavitary lung disease, though TB can often take any radiographic form on imaging investigations [63]. Multiple components of the M. tuberculosis cell wall combine to make the bacterium highly antigenic and so a cascade of cytokines is initiated that results in formation of a protective granuloma [64]. Continual development of these TB granulomas leads to central caseating necrosis, which are the pathological hallmark of TB infection in all tissues but most notably in the lungs [65]. Cavities associated with pulmonary tuberculosis tend to be large and located in the apical regions. They are most often associated with a thick wall and surrounding consolidation and may contain an air-fluid level, which represents active infection [63] (see Fig. 14.5). Occasionally cavities can be small and numerous and over time, with treatment, may resolve their thick wall and surrounding consolidation leaving them to more closely mimic DCLDs [66]. With treatment, much of the radiographical evidence of infection will resolve, but it is common for patients to continue to display changes of pulmonary tuberculosis on imaging many years after their infection has been treated.
Septic pulmonary emboli occur when blood clots containing infectious materials embolize from their source to the lungs via the pulmonary arterial vasculature. They can occur as sequalae of a spectrum of infectious conditions; risk factors include intravenous drug use, indwelling vascular catheters, thrombophlebitis, and suppurative diseases of the head and neck [67]. The embolus itself leads to focal infarction in the pulmonary vasculature similar to nonseptic emboli [68]. However, because they contain material from an infected primary source, release of infective materials leads to the formation of a focal abscess. These tend to cavitate centrally and can be numerous giving the appearance of multiple pulmonary cysts [68] (see Fig. 14.5).
A pneumatocele is a space in the lung, which is surrounded by a thin wall and filled with gas and as such there is often very high overlap between the radiologic appearance of pneumatoceles and pulmonary cysts [2]. Most commonly, pneumatoceles occur as sequelae of infection such as Pneumocystis jiroveci and Staphylococcal aureus, but they can also occur in the setting of blunt trauma to the thorax, ingestion of hydrocarbon, and following prolonged positive pressure ventilation most commonly in neonates [69, 70]. Pneumatoceles tend to be transient and radiographically appear as smooth, rounded, thin-walled air spaces [2] (Fig. 14.6). Generally, patients with pneumatoceles are asymptomatic; however, very rarely these can rupture, resulting in pneumothorax [71]. Pneumatoceles usually resolve within 6 weeks, but rarely they can become secondarily infected and persist [72]. On a very rare occasion, pneumatoceles can cause significant tension, in which case drainage may be needed to improve hemodynamic instability [73].
Conclusion
Cysts are increasingly visualized on CT imaging of the lungs and can pose a diagnostic challenge. Diagnosis involves careful attention to the existence of such cyst mimics and the knowledge that these mimics are more common than true diffuse cystic lung diseases. If these conditions have been ruled out following careful clinical and radiological correlation, patients can be investigated for processes, which cause true cystic lung disease.
Key Learning Points
-
Pulmonary cysts and cyst mimics are increasingly identified due to the routine use of chest CT scanning in clinical practice.
-
The most common situations where radiologic findings can mimic DCLDs include emphysema, bronchiectasis, and neoplastic and infectious etiologies.
-
Certain genetic diseases such as alpha-one antitrypsin deficiency, cystic fibrosis, and primary ciliary dyskinesia can cause radiological patterns that mimic DCLDs. While alpha-one antitrypsin deficiency causes panlobular emphysema, both cystic fibrosis and primary ciliary dyskinesia can cause bronchiectasis.
-
Honeycomb change and traction bronchiectasis associated with fibrotic ILDs can also mimic the cysts of DCLDs. However, the other associated radiological interstitial findings typically make it easy to distinguish between the cystic change of ILDs from the typical DCLDs.
-
Pneumatoceles can be seen following certain infections, trauma, or aspiration events and tend to follow a benign course with spontaneous resolution in a few weeks.
References
Genereux GP. The end-stage lung: pathogenesis, pathology, and radiology. Radiology. 1975;116(02):279–89.
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722.
Jawad H, Walker CM, Wu CC, Chung JH. Cystic interstitial lung diseases: recognizing the common and uncommon entities. Curr Probl Diagn Radiol. 2014;43(3):115–27.
Stern EJ, Frank MS. CT of the lung in patients with pulmonary emphysema: diagnosis, quantification, and correlation with pathologic and physiologic findings. AJR Am J Roentgenol. 1994;162(4):791–8.
Flaherty RA, Keegan JM, Sturtevant HN. Post-pneumonic pulmonary pneumatoceles. Radiology. 1960;74:50–3.
The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, division of lung diseases workshop. Am Rev Respir Dis. 1985;132(1):182–5.
Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet. 1997;349(9064):1498–504.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–128.
Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246–59.
Brantly ML, Paul LD, Miller BH, Falk RT, Wu M, Crystal RG. Clinical features and history of the destructive lung disease associated with alpha-1-antitrypsin deficiency of adults with pulmonary symptoms. Am Rev Respir Dis. 1988;138(2):327–36.
Gishen P, Saunders AJ, Tobin MJ, Hutchison DC. Alpha 1-antitrypsin deficiency: the radiological features of pulmonary emphysema in subjects of pi type Z and pi type SZ: a survey by the British Thoracic association. Clin Radiol. 1982;33(4):371–7.
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900.
Chapman KR, Burdon JG, Piitulainen E, Sandhaus RA, Seersholm N, Stocks JM, et al. Intravenous augmentation treatment and lung density in severe alpha1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(9991):360–8.
McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. Long-term efficacy and safety of alpha1 proteinase inhibitor treatment for emphysema caused by severe alpha1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–60.
Chapman KR, Chorostowska-Wynimko J, Koczulla AR, Ferrarotti I, McElvaney NG. Alpha 1 antitrypsin to treat lung disease in alpha 1 antitrypsin deficiency: recent developments and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:419–32.
Smith BM, Austin JH, Newell JD Jr, D’Souza BM, Rozenshtein A, Hoffman EA, et al. Pulmonary emphysema subtypes on computed tomography: the MESA COPD study. Am J Med. 2014;127(1):94.e7–23.
Gurney JW, Jones KK, Robbins RA, Gossman GL, Nelson KJ, Daughton D, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology. 1992;183(2):457–63.
Foster WL Jr, Pratt PC, Roggli VL, Godwin JD, Halvorsen RA Jr, Putman CE. Centrilobular emphysema: CT-pathologic correlation. Radiology. 1986;159(1):27–32.
Cosio MG, Hale KA, Niewoehner DE. Morphologic and morphometric effects of prolonged cigarette smoking on the small airways. Am Rev Respir Dis. 1980;122(2):265–21.
Takahashi M, Fukuoka J, Nitta N, Takazakura R, Nagatani Y, Murakami Y, et al. Imaging of pulmonary emphysema: a pictorial review. Int J Chron Obstruct Pulmon Dis. 2008;3(2):193–204.
Swensen SJ, Aughenbaugh GL, Douglas WW, Myers JL. High-resolution CT of the lungs: findings in various pulmonary diseases. AJR Am J Roentgenol. 1992;158(5):971–9.
Mets OM, van Hulst RA, Jacobs C, van Ginneken B, de Jong PA. Normal range of emphysema and air trapping on CT in young men. AJR Am J Roentgenol. 2012;199(2):336–40.
Juhl KS, Bendstrup E, Rasmussen F, Hilberg O. Emphysema mimicking interstitial lung disease: two case reports. Respir Med Case Rep. 2015;15:24–6.
Dirksen A, Wille MM. Computed tomography-based subclassification of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(Suppl 2):S114–7.
Foster WL Jr, Gimenez EI, Roubidoux MA, Sherrier RH, Shannon RH, Roggli VL, et al. The emphysemas: radiologic-pathologic correlations. Radiographics. 1993;13(2):311–28.
Stern EJ, Frank MS, Schmutz JF, Glenny RW, Schmidt RA, Godwin JD. Panlobular pulmonary emphysema caused by i.v. injection of methylphenidate (Ritalin): findings on chest radiographs and CT scans. AJR Am J Roentgenol. 1994;162(3):555–60.
Goldberg C, Carey KE. Bullous lung disease. West J Emerg Med. 2013;14(5):450–1.
Siddiqui NA, Nookala V. Bullous emphysema. Treasure Island: StatPearls; 2019.
Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24(135):102–14.
Hutchinson JP, McKeever TM, Fogarty AW, Navaratnam V, Hubbard RB. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997–2008. Eur Respir J. 2016;48(5):1453–61.
Brownell R, Moua T, Henry TS, Elicker BM, White D, Vittinghoff E, et al. The use of pretest probability increases the value of high-resolution CT in diagnosing usual interstitial pneumonia. Thorax. 2017;72(5):424–9.
Hunninghake GW, Zimmerman MB, Schwartz DA, King TE Jr, Lynch J, Hegele R, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–6.
Johkoh T, Sakai F, Noma S, Akira M, Fujimoto K, Watadani T, et al. Honeycombing on CT; its definition, pathologic correlation, and future direction of its diagnosis. Eur J Radiol. 2014;83(1):27–31.
Wuyts WA, Cavazza A, Rossi G, Bonella F, Sverzellati N, Spagnolo P. Differential diagnosis of usual interstitial pneumonia: when is it truly idiopathic? Eur Respir Rev. 2014;23(133):308–19.
American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304.
Chung JH, Lynch DA. The value of a multidisciplinary approach to the diagnosis of usual interstitial pneumonitis and idiopathic pulmonary fibrosis: radiology, pathology, and clinical correlation. AJR Am J Roentgenol. 2016;206(3):463–71.
Lee HY, Lee KS, Jeong YJ, Hwang JH, Kim HJ, Chung MP, et al. High-resolution CT findings in fibrotic idiopathic interstitial pneumonias with little honeycombing: serial changes and prognostic implications. AJR Am J Roentgenol. 2012;199(5):982–9.
Akira M, Inoue Y, Arai T, Okuma T, Kawata Y. Long-term follow-up high-resolution CT findings in non-specific interstitial pneumonia. Thorax. 2011;66(1):61–5.
Magis-Escurra C, Reijers MH. Bronchiectasis. BMJ Clin Evid. 2015:1507.
Boyton RJ, Altmann DM. Bronchiectasis: current concepts in pathogenesis, immunology, and microbiology. Annu Rev Pathol. 2016;11:523–54.
Menendez R, Mendez R, Polverino E, Rosales-Mayor E, Amara-Elori I, Reyes S, et al. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect Dis. 2017;17(1):659.
Ooi GC, Khong PL, Chan-Yeung M, Ho JC, Chan PK, Lee JC, et al. High-resolution CT quantification of bronchiectasis: clinical and functional correlation. Radiology. 2002;225(3):663–72.
Araujo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon T, et al. Standardised classification of the aetiology of bronchiectasis using an objective algorithm. Eur Respir J. 2017;50(6):1701289.
Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Ann Thorac Med. 2011;6(3):131–6.
Milliron B, Henry TS, Veeraraghavan S, Little BP. Bronchiectasis: mechanisms and imaging clues of associated common and uncommon diseases. Radiographics. 2015;35(4):1011–30.
Cantin L, Bankier AA, Eisenberg RL. Bronchiectasis. AJR Am J Roentgenol. 2009;193(3):W158–71.
Moulton BC, Barker AF. Pathogenesis of bronchiectasis. Clin Chest Med. 2012;33(2):211–7.
Ward C, Rydell-Tormanen K, Westergren-Thorsson G, Eriksson LT, Walters H. Infection and remodelling: a 21st century model of bronchiectasis? Eur Respir J. 2011;38(4):758–60.
Bueno J, Flors L. The role of imaging in the diagnosis of bronchiectasis: the key is in the distribution. Radiologia. 2018;60(1):39–48.
Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519–31.
Robinson P, Morgan L. Bronchiectasis in PCD looks different to CF on CT scan. Multidiscip Respir Med. 2018;13(Suppl 1):24.
Sheehan RE, Wells AU, Copley SJ, Desai SR, Howling SJ, Cole PJ, et al. A comparison of serial computed tomography and functional change in bronchiectasis. Eur Respir J. 2002;20(3):581–7.
Eshed I, Minski I, Katz R, Jones PW, Priel IE. Bronchiectasis: correlation of high-resolution CT findings with health-related quality of life. Clin Radiol. 2007;62(2):152–9.
Mouroux J, Padovani B, Elkaim D, Richelme H. Should cavitated bronchopulmonary cancers be considered a separate entity? Ann Thorac Surg. 1996;61(2):530–2.
Onn A, Choe DH, Herbst RS, Correa AM, Munden RF, Truong MT, et al. Tumor cavitation in stage I non-small cell lung cancer: epidermal growth factor receptor expression and prediction of poor outcome. Radiology. 2005;237(1):342–7.
Chaudhuri MR. Primary pulmonary cavitating carcinomas. Thorax. 1973;28(3):354–66.
Edwards CW. Alveolar carcinoma: a review. Thorax. 1984;39(3):166–74.
Kolodziejski LS, Dyczek S, Duda K, Goralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66–73.
Dodd GD, Boyle JJ. Excavating pulmonary metastases. Am J Roentgenol Radium Therapy, Nucl Med. 1961;85:277–93.
Hoshi M, Oebisu N, Iwai T, Ieguchi M, Ban Y, Nakamura H. An unusual presentation of pneumothorax associated with cystic lung metastasis from epithelioid sarcoma: a case report and review of the literature. Oncol Lett. 2018;15(4):4531–4.
Aubry MC, Myers JL, Colby TV, Leslie KO, Tazelaar HD. Endometrial stromal sarcoma metastatic to the lung: a detailed analysis of 16 patients. Am J Surg Pathol. 2002;26(4):440–9.
Gross I, Gordon O, Cohen-Cymberknoh M, Reiter J, Tsabari R, Gileles-Hillel A, et al. Giant lung cysts following necrotizing pneumonia: resolution with conservative treatment. Pediatr Pulmonol. 2019;54(6):901–6.
McAdams HP, Erasmus J, Winter JA. Radiologic manifestations of pulmonary tuberculosis. Radiol Clin N Am. 1995;33(4):655–78.
Ong CW, Elkington PT, Friedland JS. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am J Respir Crit Care Med. 2014;190(1):9–18.
Dannenberg AM Jr, Sugimoto M. Liquefaction of caseous foci in tuberculosis. Am Rev Respir Dis. 1976;113(3):257–9.
Ray A, Suri JC, Sen MK, Khanna A. Cystic lung disease in tuberculosis: an unusual presentation. Lung India. 2013;30(4):351–3.
Stawicki SP, Firstenberg MS, Lyaker MR, Russell SB, Evans DC, Bergese SD, et al. Septic embolism in the intensive care unit. Int J Crit Illn Inj Sci. 2013;3(1):58–63.
Jaffe RB, Koschmann EB. Septic pulmonary emboli. Radiology. 1970;96(3):527–32.
Arora P, Kalra VK, Natarajan G. Pneumatoceles in infants in the neonatal intensive care unit: clinical characteristics and outcomes. Am J Perinatol. 2013;30(8):689–94.
Hussain N, Noce T, Sharma P, Jagjivan B, Hegde P, Pappagallo M, et al. Pneumatoceles in preterm infants-incidence and outcome in the post-surfactant era. J Perinatol. 2010;30(5):330–6.
Thukral A, Tiwari DN, Tripathi K. Pneumatocele in an adult. J Assoc Physicians India. 2011;59:186–7.
Hirata S. A case of traumatic pneumatocele revealed by CT 36 years after blunt chest trauma. Nihon Kokyuki Gakkai Zasshi. 2008;46(12):1070–4.
Park TH, Kim JK. Nonsurgical management of an enlarging pneumatocele by fibrin sealant injection via pigtail catheter. Pediatr Pulmonol. 2016;51(2):E5–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
O’Carroll, O., Murphy, D.J., McCarthy, C. (2021). Mimics, Impersonators, and Semblances of Pulmonary Cysts. In: Gupta, N., Wikenheiser-Brokamp, K.A., McCormack, F.X. (eds) Diffuse Cystic Lung Diseases. Respiratory Medicine. Humana, Cham. https://doi.org/10.1007/978-3-030-63365-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-63365-3_14
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-63364-6
Online ISBN: 978-3-030-63365-3
eBook Packages: MedicineMedicine (R0)