Abstract
Vascular mechanobiology deals with the question of how different physical forces and changes in the mechanical properties of single cells and entire tissue structures contribute to cell differentiation, physiology, initiation, and progression of disease development. This review surveys new findings and progress in the research field of atherosclerosis in recent years. Moreover, it aims to integrate different aspects to demonstrate the interlacing and integration of certain mechanisms in the pathogenesis of atherosclerosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Blood vessels and their constituent endothelial cells are constantly exposed to mechanical stimuli. A striking feature of atherosclerosis is its highly heterogeneous distribution within the arterial tree [1]. Atherosclerotic lesions predominantly develop at branches and bifurcations. These sites are exposed to low or disturbed blood flow causing low or oscillatory shear stress on the vessel wall, respectively [2, 3]. In addition to this flow evoked wall shear stress, circumferential stress driven by the pulsatile blood pressure plays an important role in mechanical processes including angiogenesis, vascular remodeling, and atherogenesis [4]. Potential mechanosensors of endothelial cells, e.g., surface glycocalyx [5], integrins, mechanosensitive ion channels, or stretch sensitive focal adhesion proteins [6] sense different physiological or pathological intensities of mechanical stimuli on the cell surface. These different intensities are converted inside the cell into the activation of various pathways determining the fate of cells. Besides, changes in extracellular matrix proteins and mechanical properties of the vessel wall are related to arterial stiffening which may activate several mechanisms also involved in the process of atherosclerosis [7, 8]. This in turn enables the recruitment and diapedesis of circulating immune cells, predominantly monocytes, and T lymphocytes, starting the inflammatory process of atherosclerosis [9].
12.2 The Endothelial Glycocalyx is an Amplifying Mechanosensor
The glycocalyx not only represents a physical barrier to leukocyte–endothelium adhesion but rather plays an important role in sensing and transducing the magnitude and direction of mechanical forces into biochemical signals [5], thus governing the phenotype of endothelial cells at atherosclerosis-prone branch regions [10]. Glycocalyx thickness, which ranges from 200 to 2000 nm in thickness [11], and cell surface coverage were significantly decreased in the atheroprone region of the brachiocephalic artery in a high fat-fed ApoE−/− mouse model [12]. In ApoE/low-density lipoprotein receptor (LDLR) deficient mice glycocalyx degradation and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development was detectable [13]. The observed glycocalyx shedding increased lipid permeability and facilitated macrophage infiltration promoting lipid retention and the development of atherosclerotic plaques [12, 14, 15]. Present results indicate that biglycan as a small leucine-rich repeat proteoglycan plays a protective role during the progression of atherosclerosis by inhibiting thrombin activity, platelet activation, and finally macrophage-mediated plaque inflammation [16].
Histomorphometric analysis of vascular walls suggest a remodeling induced by low flow and high-pressure loading [17]. These findings are consistent with the classic hypertensive aortic phenotype characterized by a thicker and more rigid vascular wall as well as an increased aortic diameter [17]. It has been suggested that glycocalyx degradation occurs in atheroprone regions by TNF-α-dependent mechanisms involving activation of endothelial heparanase [18]. As a consequence, the normal nitric oxide/NF-κB negative feedback loop, i.e., nitric oxide production and NF-κB inhibition [19], is disrupted. Thus, decreased nitric oxide availability results in sustained activation of NF-κB in response to shear and increased intercellular adhesion molecule-1 (ICAM-1) expression. ICAM-1 interaction with the integrin LFA-1 (leukocyte function-associated antigen-1) found on leukocytes is crucial in mediating its transmigration [10].
The glycocalyx is a prerequisite for unidirectional shear stress-induced nitric oxide generation [20], thus modulating redox signaling in endothelial cells. Locally produced reactive oxygen species (ROS) generated by xanthine oxidoreductase has been shown to induce glycocalyx reduction [21]. Xanthine oxidase has been shown to bind to the glycocalyx through its heparin-binding domain [21]. Prolonged inhibition of NADPH oxidase with apocynin decreased xanthine oxidase protein levels and prevented endothelial superoxide generation in response to oscillatory shear stress [22]. These data suggest firstly that NADPH oxidases maintain endothelial cell xanthine oxidase levels and secondly that xanthine oxidase is responsible for increased ROS production in response to oscillatory shear stress. Interestingly, the NOX family of ROS-generating NADPH oxidases has been found to modulate the endothelial redox state in response to different forms of wall shear stress [23]. Unidirectional shear stress activates the NOX2–p47phox complex to activate endothelial nitric oxide synthase (NOS3) phosphorylation and nitric oxide formation [23]. In contrast, oscillatory shear stress activates the NOX1–NOXO1 (NADPH oxidase organizer 1) complex to uncouple NOS3 increasing the NOS3-dependent generation of ROS [23].
One has to keep in mind that in isolated endothelial cells cultured for several passages, the glycocalyx appearance is altered or collapsed due to the treatment with enzymatic solutions containing collagenase or trypsin to separate cells from the vessel wall or tissue-culture plates. Interestingly, glycocalyx appears predominantly on the edge of endothelial cells in the early days in culture after seeding and within 1 week in the apical area of the cell membrane [24]. Most of the published research using isolated endothelial cells as a model is presumably performed with cells grown to confluence for 3–7 days. Thus, estimating the status and importance of the glycocalyx in cultured endothelial cells under these conditions seems to be meaningful only to a very limited extent. This limitation needs to be kept in mind extrapolating such in vitro experimental results to pathophysiological or clinical settings.
12.3 Mechano-redox Regulation of Antioxidant Enzymes
It is widely assumed that low and oscillatory wall shear stress plays a key role in the initiation and development of atherosclerosis but evidence for this is less robust than commonly assumed [2]. In addition to common atherosclerosis risk factors increasing ROS formation by endothelial and vascular smooth muscle cells, other physical forces seem to regulate vascular production of ROS [25]. Cyclic stretch modulates redox enzyme expression in endothelial cells through an increased ROS formation, mainly superoxide anions and hydrogen peroxide [26]. Superoxide anions and nitric oxide rapidly react to peroxynitrite, hence reducing the level of biologically active nitric oxide even further [27]. This reaction not only weakens the nitric oxide inhibition of pro-inflammatory gene expression but may exert additionally a plethora of potentially deleterious effects [28]. On exposure to cyclic stretch, there was a transient increase in intracellular ROS in cultured HUVECs returning to control levels after 24 h [29]. The observed concomitant rise in glutathione peroxidase-1 (GPx-1) and heme oxygenase-1 (HO-1) expression may comprise an adaptive mechanism through which the cells maintain their anti-atherosclerotic properties despite a decreased bioavailability of nitric oxide [30]. GPx-1 is a selenium-dependent enzyme that inactivates hydrogen peroxide as well as various lipid hydroperoxides [26]. The expression of the inducible stress protein HO-1 can be markedly augmented by a wide range of substances causing transient changes in the cellular redox state [31]. The sialic acid component of the glycocalyx and the HO-1 expression were differentially regulated by unidirectional and oscillatory shear stress compared to static cultures [32]. Removal of sialic acid with neuraminidase before unidirectional shear stress exposure abrogated HO-1 induction mediated by the transcription factor nuclear factor E2-related factor 2 (Nrf2). Subsequently, mitochondrial superoxide response to fluid shear stress was enhanced [32]. These findings demonstrate that fluid shear stress-sensitive Nrf2 signaling is dependent on mechanotransduction through the glycocalyx and restoration may normalize redox homeostasis in atheroprone vascular regions [32]. Moreover, in response to unidirectional shear stress, an adaptive reorganization of the endothelial glycocalyx associated with changes in membrane rafts and the actin cytoskeleton has been reported [27].
Although the glycocalyx is essential for maintaining vascular homeostasis, none of the common appropriate treatments for atherosclerosis including lipid-lowering and anti-platelet therapies target the endothelial glycocalyx [33]. Monitoring and protecting the endothelial glycocalyx in patients may lower the risk of cardiovascular comorbidities [34]. Current pharmacologic approaches aim at inhibiting multiple adverse factors and enzymatic attacks or reassembling glycocalyx components and have only been tested in animals (reviewed in [34]). The effectiveness of such strategies remain to be determined in clinical experiments while accounting for unpredictable compensatory responses [34].
12.4 Mechanical Force-Induced CD40 Signaling Results in Endothelial Dysfunction
CD40 receptor expression by endothelial cells and the pro-inflammatory response of these cells after receptor stimulation by its ligand CD154, suggest an important role for CD40-CD154 co-stimulation in atherosclerosis and other vasculopathies [35]. Using an appropriate ROS scavenger and a protein kinase inhibitor revealed that stretch-induced CD40 expression is ROS-dependent and is mediated via the JNK/AP-1 signaling pathway [28]. In areas of disturbed blood flow with oscillatory shear stress and increased circumferential wall stress, endothelial cell CD40 expression, which is normally suppressed by unidirectional shear stress, is disinhibited [28]. Peroxynitrite is a potent oxidant with deleterious tissue-oxidant effects, formed from the reaction between superoxide radicals and nitric oxide. Interestingly, in this case, this oxidative protein modification seems to be protective. CD40-tyrosine nitration by peroxynitrite functionally inactivates and disconnects the receptor from the intracellular signal transduction cascade to the nucleus, followed by internalization of the nitrated protein and subsequent accelerated degradation [28]. Internalized CD40 exhibits different patterns of tumor necrosis factor receptor-associated factors TRAF2/3/6 recruitment and Akt (also known as protein kinase B) phosphorylation from the membrane-anchored CD40 complex [36]. Through this posttranslational oxidative modification, endothelial cells may adapt to unfavorable hemodynamic conditions and maintain their anti-inflammatory phenotype.
Otherwise, ligation of endothelial CD40 by platelet-bound CD154 or soluble CD154 triggers the release of ultra-large von Willebrand factor multimers stored in Weibel–Palade bodies of endothelial cells [30]. Platelets rapidly adhere to these multimers even under flow conditions and become activated. Presumably, through P-selectin–PSGL-1 interaction, they can trap circulating monocytes facilitating their diapedesis and differentiation into macrophages [30]. Interestingly, platelet hyaluronidase-2, stored in α-granules translocates to the surface upon activation [37] possibly to function in glycocalyx degradation facilitating monocyte transmigration, as discussed above.
12.5 Mechanosensitive Transcription Factors
Disturbed blood flow and unidirectional laminar flow have been shown to differently modulate a variety of mechanosensitive transcription factors on various levels, as reviewed recently [38]. In particular, the occurrence of spatial shear stress gradients may represent important local modulators of endothelial transcription factor expression and consecutively activation at sites predisposed to atherosclerotic development [39, 40]. Thus, it is hardly surprising that emerging studies show that transcription factors, e.g., KLF2 or NFR2, represent promising therapeutic targets for the prevention and treatment of atherosclerosis [38, 41].
Some transcription factors have not yet been a focus within the biomechanics research interest so far. Biomechanical stretch activates NFAT5 (nuclear factor of activated T cells-5), also known as TonEBP (tonicity-responsive enhancer-binding protein), a transcription factor regulating the expression of genes involved in osmotic stress [42]. In high extracellular tonicity environments, the COOH-terminal transactivation domain of NFAT5 becomes phosphorylated, resulting in nuclear translocation of the activated transcription factor [43]. NFAT5 has been shown to regulate the expression of tenascin-C [44] and ACTBL2 (beta-actin-like protein 2) in native and cultured VSMCs [45] contributing to an enhanced migration of these cells promoting maladaptive vascular remodeling processes. Interestingly, NFAT5 seems to be continuously expressed and degraded in resting VSMCs while expression and accumulation of the NFAT5 isoform C in the nucleus is facilitated during biomechanical stress [46]. Nuclear translocation required palmitoylation and specific phosphorylation at Y143 but was inhibited by phosphorylation at S1197. Finally, VSMC-specific knockout of NFAT5 in mice inhibited the proliferation of VSMCs and the thickening of the arterial wall during both flow-induced collateral remodeling and hypertension-mediated arterial hypertrophy [47].
Besides the activation of transcription factors by phosphorylation and translocation from the cytosol to the nucleus, mechanical signals can propagate through mechanically stiff structures like focal adhesions. The LIM domain protein Zyxin is localized primarily at focal adhesion plaques [48]. Zyxin binds Ena/VASP proteins (enabled/vasodilator-stimulated phosphoprotein) that, in turn, promote actin polymerization [49]. Growing evidence suggests that zyxin is a vital mechanotransductor and key regulator of stretch-induced gene expression as zyxin translocated into the nucleus of cultured cyclic stretched VSMCs [50]. Loss of zyxin drives VSMCs toward a synthetic phenotype switch, a process further consolidated by exaggerated stretch. VSMCs phenotypic modulation plays a key role in atherosclerosis and is classically defined as a switch from a contractile phenotype to a synthetic phenotype [51].
In cultured human endothelial cells and perfused femoral arteries isolated from wild-type and several knockout mouse strains, a multistep signaling pathway leading to zyxin activation has been characterized [52]. Cyclic stretch led to a transient receptor potential channel 3-mediated release of the endothelial vasoconstrictor peptide endothelin-1 (ET-1). Through autocrine activation of its B-type receptor, ET-1 elicited release of pro–atrial natriuretic peptide (ANP) causing autocrine activation of the ANP receptor guanylyl cyclase A (GC-A). GC-A activation provoked protein kinase G-mediated phosphorylation of zyxin at serine 142, thereby leading to translocation of zyxin into the nucleus and inducing stretch-dependent changes in gene expression. Almost all zyxin-dependent genes can be attributed to a set of well-defined pathways that, on the one hand, inhibit apoptosis and proliferation and strengthen cell–matrix interactions but, on the other hand, contribute to a broad range of pro-inflammatory responses, e.g., Interleukin-8, ICAM-1 and VCAM-1 [53].
Focal adhesion plaques are integrin-containing, multi-protein structures, and sites of transmembrane interaction between the extracellular matrix and the actin cytoskeleton. The discovery of a connection between endothelial cell integrins, extracellular matrix, and signaling events opened a new perspective in the understanding of the molecular mechanisms regulating vascular responses to the changes in blood flow [54]. Excessive accumulation of the endothelial glycocalyx component hyaluronan around the vascular smooth muscle cells results in increased aortic stiffness and strength and accelerated atherosclerosis in ApoE-knockout mice [55]. Changes in extracellular matrix proteins and the mechanical properties of the vessel wall related to arterial stiffening may activate several mechanisms involved also in the initiation of atherosclerotic lesions [7, 56]. Extracellular matrix proteins like fibronectin and laminin differentially regulate the Smad2 activation in vascular endothelial cells in response to disturbed flow [57]. Oscillatory shear stress-induced a sustained activation of Smad2 in endothelial cells cultured on fibronectin, but only a transient activation of Smad2 in endothelial cells on laminin resulting in a transient induction of NF-κB and pro-inflammatory gene expression [57].
Recent genome-wide association studies identified that the JCAD (Junctional cadherin 5 associated, also known as KIAA1462, encoding a junctional protein associated with CAD) locus is associated with the risk of coronary artery disease and myocardial infarction [58]. JCAD deficiency attenuated high-fat diet-induced atherosclerosis in ApoE-deficient mice. Mechanistically, JCAD regulated the YAP/TAZ pathway, thus promoting endothelial dysfunction and the expression of downstream proatherogenic genes in human coronary artery endothelial cells [58]. YAP and TAZ are both effectors of the Hippo pathway. Dysregulation of the Hippo signaling pathway leads to different kinds of cardiovascular diseases, such as myocardial infarction, cardiac hypertrophy, neointima formation, and atherosclerosis [59]. Emerging evidence indicates that YAP and TAZ sense different blood flow patterns and regulate atherosclerotic lesions [60]. Disturbed flow leads to a significant decrease in YAP phosphorylation and marked increased adhesion molecule and TAZ expression [60]. Moreover, disturbed flow resulted in YAP/TAZ nuclear localization, whereas increased YAP/TAZ cytoplasmic retention was observed in HUVECs subjected to unidirectional shear stress [60]. In the latter study, methotrexate at therapeutically relevant concentrations inhibited disturbed flow-induced endothelial YAP/TAZ activation. Thus, it has been proposed that methotrexate can be regarded as an important therapeutic agent not only to treat rheumatic diseases but also to reduce cardiovascular risk and mortality [61].
12.6 Flow-Induced Epigenetic Mechanisms of Endothelial Gene Expression
Epigenetic mechanisms involve the interplay between signal-transduction pathways, transcription factors, and the genome chromatin packaging, determining the gene expression pattern of a cell. Recent research demonstrates that blood flow and pressure are hemodynamic cell environments that have been demonstrated to influence transcription via epigenetic mechanisms (c.f. recent review [62] and Chap. 9). Principal mechanisms are chemical modifications, e.g., methylation of cytosine DNA residues and amino acids of histone proteins associated with DNA in nucleosomes [62]. The promoter of several mechanosensitive genes, such as HoxA5, Klf3, and Klf4, were hypermethylated by disturbed blood flow but rescued by DNA methyltransferase inhibitors [63].
In addition, posttranslational modifications, including phosphorylation and SUMOylation, provide new perspectives on the disturbed flow-induced pathogenesis of atherosclerosis [64]. It has been shown that SUMOylation of DNA methyltransferase is induced by disturbed flow but not by steady laminar flow signaling [64]. Disturbed flow induces peroxynitrite production, which in turn activates protein kinase C ζ and its binding to the E3 SUMO (small ubiquitin-like modifier) ligase PIASy (protein inhibitor of activated STATy) [64, 65]. Thus, determining the interplay of each PTM and epigenetic event will provide a new paradigm to elucidate the difference between disturbed flow and steady laminar flow, which may lead to novel therapeutic intervention strategies to inhibit plaque formation [64].
In recent years, noncoding RNAs as a mode of epigenetic-related regulation at the transcriptional and post-transcriptional level have become an area of intensive investigation. Targeting mRNA by short microRNAs (miRNAs, 20–26 nucleotides) and long noncoding RNAs (lncRNAs, >200 nucleotides) facilitate transcript degradation. Since noncoding RNAs make up >97% of the transcriptome [66], there is huge potential for dynamic epigenomic regulation of gene expression including biomechanical stimulation of the epigenomic regulation. So far, several miRNAs such as miR-10a, miR-19a, miR-23b, miR-101, and miR-143/145 have been identified to be induced by high shear stress mediating an atheroprotective role [67]. Interestingly, changes in the expression profile of miR-21 and miR-92a by high shear stress are associated with an atheroprotective function, while low shear stress-induced expression of miR-21, miR-92a, and miR-663 results in a pathological endothelial cell phenotype [67]. This contradiction could be explained by the fact that these microRNAs are differentially regulated by diverging shear stress modes or additional factors beyond shear stress [67]. For example, overexpressing miR-21 in endothelial cells on one hand decreases apoptosis and increases NOS3 phosphorylation and subsequently nitric oxide formation. On the other hand, oscillatory shear stress and overexpression of miR-21 enhances VCAM-1, and monocyte chemotactic protein-1 (MCP-1) expression and thereby monocyte adhesion to the endothelial cells [68]. MiR-155 fulfills a wide range of functions in different regions of the vasculature. When exposed to different modes of shear stress it is elevated in pro-inflammatory macrophages and atherosclerotic lesions in vivo [69]. Flow disturbance induced by partial carotid ligation led to a lower expression of miR-23b and a higher endothelial cell proliferation in comparison with the pulsatile flow regions of unligated vessels [70].
In the cardiovascular system, lncRNA expression has been detected and characterized under normal physiological conditions and in disease states (c.f. recent review [71, 72]). The pro-angiogenic lncRNA MANTIS was tightly regulated by the mechanosensitive transcription factors KLF2 and KLF4 and limits the ICAM-1 mediated monocyte adhesion to endothelial cells and thus potentially atherosclerosis development in humans [73]. In contrast, the role of flow-dependent lncRNAs regulation in vascular dysfunction and atherosclerosis is widely unknown and an emerging research field [74]. First results about the flow-sensitive lncRNA STEEL along with other lncRNAs studied in the context of vascular pathophysiology and atherosclerosis have been reviewed very recently [74]. The role of lncRNAs as potential biomarkers in cardiovascular disease especially in atherosclerosis is still at its beginning [74].
12.7 Conclusions and Remaining Questions
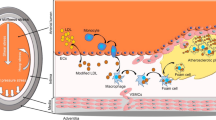
So obviously, on the cellular level, there appear to exist several mechanical force-dependent pro-atherosclerotic but also in parallel protective, closely integrated mechanisms to locally protect endothelial cells under unfavorable conditions present at atherosclerotic predilection sites (Fig. 12.1). So why do some people develop atherosclerotic plaques and others do not? At first, the individual lifestyle associated with a balanced diet may be important. Several epidemiological studies report promising protective effects of antioxidant foods. However, many unclear points are remaining regarding the contribution of the nutritional elements found in antioxidant foods to the prevention of atherosclerotic disease [31]. Thus, among other risk factors, genetic polymorphisms should be more prominently included in the individual risk profiling to develop atherosclerosis or coronary heart disease. For example, the C allele of a functional polymorphism in the Kozak consensus sequence of the CD40 gene has been associated with enhanced translational efficiency correlating to increased CD40 protein expression and an overall increased atherosclerosis risk [75]. The polymorphism genotype frequency of the oxidative stress detoxifying glutathione S-transferase mu 1 (gene name GSTM1) was significantly higher in individuals diagnosed with atherosclerosis and reported to smoke or being former smokers [76]. Single polymorphisms in other protective genes like superoxide dismutase (SOD2) [77] or NOS3 [78], or an association between NOS3 and GPx-1 gene polymorphisms [79] are independent risk factors for susceptibility to develop atherosclerosis or coronary artery disease in men. Interestingly, a fluid shear stress-induced rise in SOD2 expression [80] and enhanced release of the anti-inflammatory metabolite of the arachidonic acid pathway 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) [81] in NOS3 dysfunctional genotype endothelial cells effectively stabilizes their anti-atherosclerotic phenotype [80]. Thus, it must be concluded that there are individual and summed effects of high-risk genetic polymorphisms on the development of atherosclerosis.
Schematic summary demonstrating how individual risk factors along with local biomechanical forces provide a local predilection for the initiation of atherosclerosis at arterial bifurcations by stimulating (red) or inhibiting (green) atherogenic processes in endothelial cells (L-Arg, Arginine, CD40 cell surface receptor, EC endothelial cells, lncRNA long noncoding RNA, miRNA microRNA, NO nitric oxide, NOS3 nitric oxide synthase 3, (O2•−) superoxide, ONOO− peroxynitrite, TF transcription factor)
Abbreviations
- ApoE:
-
Apolipoprotein E
- ICAM-1:
-
Intercellular adhesion molecule-1
- LIM:
-
Named after their initial discovery in the proteins Lin11, Isl-1, Mec-3
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate hydrate
- NOS3:
-
Endothelial nitric oxide synthase (eNOS)
- NOX:
-
NADPH oxidase
- ROS:
-
Reactive oxygen species
- Smad:
-
Acronym refers to the Caenorhabditis elegans Sma (small worm phenotype) and the Drosophila Mad (mothers against decapentaplegic) gene family
- SUMO:
-
Small Ubiquitin-related Modifier
- TAZ:
-
Transcriptional co-activator with PDZ-binding motif
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular cell adhesion protein-1
- VSMCs:
-
Vascular smooth muscle cells
- YAP:
-
Yes-associated protein
References
Warboys CM, Ghim M, Weinberg PD (2019) Understanding mechanobiology in cultured endothelium: a review of the orbital shaker method. Atherosclerosis 285:170–177
Peiffer V, Sherwin SJ, Weinberg PD (2013) Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 99:242–250
Warboys CM, Amini N, de Luca A, Evans PC (2011) The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Med Rep 3:5
Kwak BR, Back M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC (2014) Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 35:3013–3020, 3020a–3020d
Zeng Y, Zhang XF, Fu BM, Tarbell JM (2018) The role of endothelial surface glycocalyx in mechanosensing and transduction. Adv Exp Med Biol 1097:1–27
Muhamed I, Chowdhury F, Maruthamuthu V (2017) Biophysical tools to study cellular mechanotransduction. Bioengineering (Basel) 4
Palombo C, Kozakova M (2016) Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol 77:1–7
Won BY, Park SG, Lee SH, Kim MJ, Chun H, Hong D, Kim YS (2020) Characteristics of metabolic factors related to arterial stiffness in young and old adults. Clin Exp Hypertens 42:225–232
Wolf D, Ley K (2019) Immunity and inflammation in atherosclerosis. Circ Res 124:315–327
McDonald KK, Cooper S, Danielzak L, Leask RL (2016) Glycocalyx degradation induces a proinflammatory phenotype and increased leukocyte adhesion in cultured endothelial cells under flow. PLoS One 11:e0167576
Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M (2010) Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res 87:300–310
Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM (2016) Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis 252:136–146
Bar A, Targosz-Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, Sternak M, Przybylo M, Kurpinska A, Walczak M, Kostogrys RB, Szymonski M, Chlopicki S (2019) Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/low-density lipoprotein receptor-deficient mice. J Am Heart Assoc 8:e011171
Constantinescu AA, Vink H, Spaan JA (2003) Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol 23:1541–1547
Fels B, Kusche-Vihrog K (2020) It takes more than two to tango: mechanosignaling of the endothelial surface. Pflugers Arch 472:419–433
Grandoch M, Kohlmorgen C, Melchior-Becker A, Feldmann K, Homann S, Muller J, Kiene LS, Zeng-Brouwers J, Schmitz F, Nagy N, Polzin A, Gowert NS, Elvers M, Skroblin P, Yin X, Mayr M, Schaefer L, Tannock LR, Fischer JW (2016) Loss of biglycan enhances thrombin generation in apolipoprotein E-deficient mice: implications for inflammation and atherosclerosis. Arterioscler Thromb Vasc Biol 36:e41–e50
Liu J, Kang H, Ma X, Sun A, Luan H, Deng X, Fan Y (2018) Vascular cell glycocalyx-mediated vascular remodeling induced by hemodynamic environmental alteration. Hypertension 71:1201–1209
Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L (2014) Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014:694312
Grumbach IM, Chen W, Mertens SA, Harrison DG (2005) A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J Mol Cell Cardiol 39:595–603
Bartosch AMW, Mathews R, Tarbell JM (2017) Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys J 113:101–108
Rubio-Gayosso I, Platts SH, Duling BR (2006) Reactive oxygen species mediate modification of glycocalyx during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 290:H2247–H2256
McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG (2003) Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol Heart Circ Physiol 285:H2290–H2297
Siu KL, Gao L, Cai H (2016) Differential roles of protein complexes NOX1-NOXO1 and NOX2-p47phox in mediating endothelial redox responses to oscillatory and unidirectional laminar shear stress. J Biol Chem 291:8653–8662
Bai K, Wang W (2012) Spatio-temporal development of the endothelial glycocalyx layer and its mechanical property in vitro. J R Soc Interface 9:2290–2298
Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H (2003) Role of oxidative stress in atherosclerosis. Am J Cardiol 91:7A–11A
Wagner AH, Kautz O, Fricke K, Zerr-Fouineau M, Demicheva E, Guldenzoph B, Bermejo JL, Korff T, Hecker M (2009) Upregulation of glutathione peroxidase offsets stretch-induced proatherogenic gene expression in human endothelial cells. Arterioscler Thromb Vasc Biol 29:1894–1901
Zeng Y, Tarbell JM (2014) The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS One 9:e86249
Wagner AH, Hildebrandt A, Baumgarten S, Jungmann A, Muller OJ, Sharov VS, Schoneich C, Hecker M (2011) Tyrosine nitration limits stretch-induced CD40 expression and disconnects CD40 signaling in human endothelial cells. Blood 118:3734–3742
Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H (2006) Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 290:H458–H452
Moller K, Adolph O, Grunow J, Elrod J, Popa M, Ghosh S, Schwarz M, Schwale C, Grassle S, Huck V, Bruehl C, Wieland T, Schneider SW, Nobiling R, Wagner AH, Hecker M (2015) Mechanism and functional impact of CD40 ligand-induced von Willebrand factor release from endothelial cells. Thromb Haemost 113:1095–1108
Motterlini R, Green CJ, Foresti R (2002) Regulation of heme oxygenase-1 by redox signals involving nitric oxide. Antioxid Redox Signal 4:615–624
Psefteli P-M, Fowler M, Mann GE, Siow RC (2018) Redox regulation in human endothelial cells: a critical role for the glycocalyx in mechanotransduction of fluid shear stress. Free Radical Biol Med 120:S26
Mitra R, O’Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE (2017) Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep 19:63
Cao RN, Tang L, Xia ZY, Xia R (2019) Endothelial glycocalyx as a potential theriapeutic target in organ injuries. Chin Med J (Engl) 132:963–975
Schonbeck U, Libby P (2001) The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci 58:4–43
Chen Y, Chen J, Xiong Y, Da Q, Xu Y, Jiang X, Tang H (2006) Internalization of CD40 regulates its signal transduction in vascular endothelial cells. Biochem Biophys Res Commun 345:106–117
Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA (2015) Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood 125:1460–1469
Niu N, Xu S, Xu Y, Little PJ, Jin ZG (2019) Targeting mechanosensitive transcription factors in atherosclerosis. Trends Pharmacol Sci 40:253–266
Nagel T, Resnick N, Dewey CF Jr, Gimbrone MA Jr (1999) Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler Thromb Vasc Biol 19:1825–1834
Min E, Schwartz MA (2019) Translocating transcription factors in fluid shear stress-mediated vascular remodeling and disease. Exp Cell Res 376:92–97
Hecker M, Wagner AH (2017) Transcription factor decoy technology: a therapeutic update. Biochem Pharmacol 144:29–34
Kultz D, Csonka L (1999) What sets the TonE during osmotic stress? Proc Natl Acad Sci U S A 96:1814–1816
Halterman JA, Kwon HM, Wamhoff BR (2012) Tonicity-independent regulation of the osmosensitive transcription factor TonEBP (NFAT5). Am J Physiol Cell Physiol 302:C1–C8
Scherer C, Pfisterer L, Wagner AH, Hodebeck M, Cattaruzza M, Hecker M, Korff T (2014) Arterial wall stress controls NFAT5 activity in vascular smooth muscle cells. J Am Heart Assoc 3:e000626
Hodebeck M, Scherer C, Wagner AH, Hecker M, Korff T (2014) TonEBP/NFAT5 regulates ACTBL2 expression in biomechanically activated vascular smooth muscle cells. Front Physiol 5:467
Zappe M, Feldner A, Arnold C, Sticht C, Hecker M, Korff T (2018) NFAT5 isoform C controls biomechanical stress responses of vascular smooth muscle cells. Front Physiol 9:1190
Arnold C, Feldner A, Zappe M, Komljenovic D, De La Torre C, Ruzicka P, Hecker M, Neuhofer W, Korff T (2019) Genetic ablation of NFAT5/TonEBP in smooth muscle cells impairs flow- and pressure-induced arterial remodeling in mice. FASEB J 33:3364–3377
Wang YX, Wang DY, Guo YC, Guo J (2019) Zyxin: a mechanotransductor to regulate gene expression. Eur Rev Med Pharmacol Sci 23:413–425
Schwartz MA (2010) Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol 2:a005066
Ghosh S, Kollar B, Nahar T, Suresh Babu S, Wojtowicz A, Sticht C, Gretz N, Wagner AH, Korff T, Hecker M (2015) Loss of the mechanotransducer zyxin promotes a synthetic phenotype of vascular smooth muscle cells. J Am Heart Assoc 4:e001712
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702
Suresh Babu S, Wojtowicz A, Freichel M, Birnbaumer L, Hecker M, Cattaruzza M (2012) Mechanism of stretch-induced activation of the mechanotransducer zyxin in vascular cells. Sci Signal 5:ra91
Wojtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M (2010) Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circ Res 107:898–902
Zaragoza C, Marquez S, Saura M (2012) Endothelial mechanosensors of shear stress as regulators of atherogenesis. Curr Opin Lipidol 23:446–452
Lorentzen KA, Chai S, Chen H, Danielsen CC, Simonsen U, Wogensen L (2016) Mechanisms involved in extracellular matrix remodeling and arterial stiffness induced by hyaluronan accumulation. Atherosclerosis 244:195–203
Ohayon J, Gharib AM, Garcia A, Heroux J, Yazdani SK, Malve M, Tracqui P, Martinez MA, Doblare M, Finet G, Pettigrew RI (2011) Is arterial wall-strain stiffening an additional process responsible for atherosclerosis in coronary bifurcations?: an in vivo study based on dynamic CT and MRI. Am J Physiol Heart Circ Physiol 301:H1097–H1106
Yang TL, Lee PL, Lee DY, Wang WL, Wei SY, Lee CI, Chiu JJ (2018) Differential regulations of fibronectin and laminin in Smad2 activation in vascular endothelial cells in response to disturbed flow. J Biomed Sci 25:1
Xu S, Xu Y, Liu P, Zhang S, Liu H, Slavin S, Kumar S, Koroleva M, Luo J, Wu X, Rahman A, Pelisek J, Jo H, Si S, Miller CL, Jin ZG (2019) The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis. Eur Heart J 40:2398–2408
Wang YY, Yu W, Zhou B (2017) Hippo signaling pathway in cardiovascular development and diseases. Yi Chuan 39:576–587
Liu D, Lv H, Liu Q, Sun Y, Hou S, Zhang L, Yang M, Han B, Wang G, Wang X, Du W, Nie H, Zhang R, Huang X, Hou J, Yu B (2019) Atheroprotective effects of methotrexate via the inhibition of YAP/TAZ under disturbed flow. J Transl Med 17:378
Balanescu AR, Bojinca VC, Bojinca M, Donisan T, Balanescu SM (2019) Cardiovascular effects of methotrexate in immune-mediated inflammatory diseases. Exp Ther Med 17:1024–1029
Davies PF, Manduchi E, Jimenez JM, Jiang YZ (2017) Biofluids, cell mechanics and epigenetics: flow-induced epigenetic mechanisms of endothelial gene expression. J Biomech 50:3–10
Dunn J, Thabet S, Jo H (2015) Flow-dependent epigenetic DNA methylation in endothelial gene expression and atherosclerosis. Arterioscler Thromb Vasc Biol 35:1562–1569
Heo KS, Berk BC, Abe J (2016) Disturbed flow-induced endothelial proatherogenic signaling via regulating post-translational modifications and epigenetic events. Antioxid Redox Signal 25:435–450
Takabe W, Alberts-Grill N, Jo H (2011) Disturbed flow: p53 SUMOylation in the turnover of endothelial cells. J Cell Biol 193:805–807
Liu X, Ma Y, Yin K, Li W, Chen W, Zhang Y, Zhu C, Li T, Han B, Wang S, Zhou Z (2019) Long non-coding and coding RNA profiling using strand-specific RNA-seq in human hypertrophic cardiomyopathy. Sci Data 6:90
Neth P, Nazari-Jahantigh M, Schober A, Weber C (2013) MicroRNAs in flow-dependent vascular remodelling. Cardiovasc Res 99:294–303
Kumarswamy R, Volkmann I, Thum T (2011) Regulation and function of miRNA-21 in health and disease. RNA Biol 8:706–713
Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A (2013) MicroRNA-126, -145, and -155: a therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc Biol 33:449–454
Wang KC, Nguyen P, Weiss A, Yeh YT, Chien HS, Lee A, Teng D, Subramaniam S, Li YS, Chien S (2014) MicroRNA-23b regulates cyclin-dependent kinase-activating kinase complex through cyclin H repression to modulate endothelial transcription and growth under flow. Arterioscler Thromb Vasc Biol 34:1437–1445
Uchida S, Dimmeler S (2015) Long noncoding RNAs in cardiovascular diseases. Circ Res 116:737–750
Jae N, Heumuller AW, Fouani Y, Dimmeler S (2019) Long non-coding RNAs in vascular biology and disease. Vascul Pharmacol 114:13–22
Leisegang MS, Bibli SI, Gunther S, Pfluger-Muller B, Oo JA, Hoper C, Seredinski S, Yekelchyk M, Schmitz-Rixen T, Schurmann C, Hu J, Looso M, Sigala F, Boon RA, Fleming I, Brandes RP (2019) Pleiotropic effects of laminar flow and statins depend on the Kruppel-like factor-induced lncRNA MANTIS. Eur Heart J
Kumar S, Williams D, Sur S, Wang JY, Jo H (2019) Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vascul Pharmacol 114:76–92
Yun Y, Ma C, Ma X (2014) The SNP rs1883832 in CD40 gene and risk of atherosclerosis in Chinese population: a meta-analysis. PLoS One 9:e97289
Rodrigues DA, Martins JV, KSE S, Costa IR, Lagares MH, Campedelli FL, Barbosa AM, de Morais MP, Moura KK (2017) GSTM1 polymorphism in patients with clinical manifestations of atherosclerosis. Genet Mol Res 16
Yeh HL, Kuo LT, Sung FC, Yeh CC (2018) Association between polymorphisms of antioxidant gene (MnSOD, CAT, and GPx1) and risk of coronary artery disease. Biomed Res Int 2018:5086869
Cattaruzza M, Guzik TJ, Slodowski W, Pelvan A, Becker J, Halle M, Buchwald AB, Channon KM, Hecker M (2004) Shear stress insensitivity of endothelial nitric oxide synthase expression as a genetic risk factor for coronary heart disease. Circ Res 95:841–847
Shuvalova YA, Kaminnyi AI, Meshkov AN, Shirokov RO, Samko AN (2012) Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Mol Cell Biochem 370:241–249
Asif AR, Hecker M, Cattaruzza M (2009) Disinhibition of SOD-2 expression to compensate for a genetically determined NO deficit in endothelial cells–brief report. Arterioscler Thromb Vasc Biol 29:1890–1893
Urban I, Turinsky M, Gehrmann S, Morgenstern J, Brune M, Milewski MR, Wagner AH, Rumig C, Fleming TH, Leuschner F, Gleissner CA, Hecker M (2019) 15-Deoxy-delta12,14-prostaglandin J2 reinforces the anti-inflammatory capacity of endothelial cells with a genetically determined nitric oxide deficit. Circ Res
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest The author declares that he has no conflict of interest.
Ethical Approval This chapter does not contain any studies with human participants or animals.
Sources of Funding None.
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wagner, A.H. (2021). Mechanobiology of Atherosclerosis. In: Hecker, M., Duncker, D.J. (eds) Vascular Mechanobiology in Physiology and Disease. Cardiac and Vascular Biology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-63164-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-63164-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-63163-5
Online ISBN: 978-3-030-63164-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)