Abstract
Astrocytes are specialized glial cells that are embedded in a framework of neurons and act as an interface between neurons and the vasculature in the brain. This privileged, interconnecting position has recently been shown to render these cells crucial in the central control of systemic metabolism by allowing them to sense and convey blood-borne information within the brain and, in turn, critically fine-tune properties of neuronal networks that calibrate energy intake and expenditure. For decades, however, these neuronal networks have largely occupied the limelight regarding the study of energy homeostasis. Accordingly, the aim of this chapter is to summarize the paradigm shift currently taking place in studies of the central control of energy balance occurring over the last years, from a rather “neurocentric” view towards a more holistic perspective in which the role of other cell types, such as astrocytes, is increasingly appreciated. Finally, we will discuss recent cutting-edge methodological approaches emerging in the field that allow for the study of astrocytes, presently or yet to be conceived, which will provide a further and more complete understanding of the central regulation of energy metabolism.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Astrocytes are a specialized type of glial cell—also known as “astroglia.” To note, the prefix “astro” refers to the star-like shape of these cells. Although neurons have long been the center of attention in neuroscience, it is important to highlight that an intimate and coordinated association between astrocytes and surrounding neurons is required to carry out all biochemical and physiological processes that occur in the brain. Beyond merely supporting neurons, astrocytes have recently started to draw more attention due to their involvement with neurons to ensure brain function in general, such as for the homeostatic regulation of body weight and systemic metabolism (Garcia-Caceres et al. 2019).
1.1 A Brief History of Astrocytes
The mammalian brain contains billions of cells, but the existence of cell types distinct from neurons only gained recognition around 1825. At that time, the common hypothesis among neuroscientists was that the primary function of the “mass” of cells, in which the main neural elements were nested, would be to serve as some kind of connective tissue. Accordingly, this mass of non-neuronal cells was conflated and jointly coined “neuroglia” by Rudolf Virchow in the 1850s and was thought to merely fulfill passive functions such as providing structural support. Eventually, between the end of the nineteenth century and the beginning of the twentieth century, Golgi described glia as round cells developing many fine processes in each and every direction. He also already determined using silver chromate staining that glial cells: (1) are diverse; (2) form networks; and (3) direct their glial endfeet to the blood vessels.
In 1895 the term “astrocyte” was introduced by von Lenhossék to describe a subtype of the parenchymal glia. Interestingly, the use of this term was mostly propagated after Ramón y Cajal developed a gold chloride-sublimate staining to label glial-fibrillary acidic protein (GFAP) , in both protoplasmic and fibrous astrocytes, which is still to this day the most utilized marker to visualize astrocytes.
In the mid-twentieth century, when A. L. Hodgkin, A. F. Huxley, and others elegantly described neuronal electrical properties, neurons became the predominant focus of investigation in nervous system function, heralding decades of research on the effects of ionic flow through neuronal membranes and action potentials. Consequentially, astrocytes were ignored for several decades, demoted to supporting actors for the neuronal leading roles. In recent decades, this imbalance has gradually diminished, and neurons and glial cells comprising the nervous system are now seen as vital and interacting partners in a sophisticated and well-coordinated communication network. Nowadays, recent advances in biology, optics, genetics, and pharmacology, combined with the use of genetically engineered animals, are establishing new strategies to further investigate the physiology and functional contribution of astrocytes to the regulation of neuronal function in health and disease.
2 Biology and Physiology of Astrocytes: Characteristics and Function
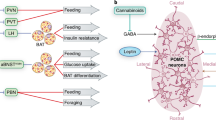
Astrocytes were thought to be less relevant than neurons, being considered merely as a scaffold for the latter. Yet, over time, the scientific community has uncovered and attributed an extensive number of functions to astrocytes. We now know that these glial cells are essential for: (a) maintaining homeostasis of the central nervous system (CNS) through modulation of neurotransmitter levels (glutamate–glutamine cycle), maintaining ionic equilibrium (K+ and H+ buffering), limiting reactive oxygen species (ROS) (in glutathione recycling), and participating in osmotic regulation; (b) regulating cerebral blood flow by interacting with endothelial cells of microvessels and pericytes that form the blood–brain barrier (BBB); (c) mounting the brain’s line of defense together with the meningeal lymphatic system and microglia (another type of glial cells); and (d) proper neuronal function, by performing critical activities such as providing neurons with energy substrates, and more generally by regulating synaptic sculpting (genesis, maturation, elimination), synaptic plasticity and synaptic transmission, which are crucial not solely during development, but also during adulthood (Fig. 6.1).
Fundamentals of astrocyte function in the CNS. Astrocytes take on a broad range of functions in the brain and are considered gatekeepers of central nervous system (CNS) homeostasis. (a) They encode information by means of intracellular Ca2+ signals, which eventually even spread to neighboring astrocytes via gap junctions, forming what resembles functional astrocyte networks. (b) Moreover, astrocytes ensheath synaptic connections with their fine processes to form the tripartite synapse, which is the trademark for regulating neuronal transmission. (c) In addition, astrocytes contain various types of intracellular vesicles, whose cargo can be released via Ca2+-regulated exocytosis; following fusion with the plasma membrane, these vesicles release their signaling cues, also called “gliotransmitters,” to act on surrounding cells. (d) Furthermore, astrocytes constitute an integral part of the neuro-glia-vascular unit. By directly sensing neuronal activity, astrocytes in turn guide cerebral blood flow towards activated brain regions to support locally increased energy demands. Astrocytes are also crucial for the development and maintenance of the blood–brain barrier together with endothelial cells and pericytes. (e) Lastly, astrocytes take center stage in sensing and integrating homeostatic feedback signals emanating from the periphery. A variety of receptors and transporters are distributed throughout astrocytic processes, which extensively cover the cerebral vasculature. Thus, astrocytes are ideally situated and equipped to detect blood-borne signals and modulate their entry into the brain
2.1 Astrocytes Are Critical for Energetics of the CNS
The brain is the most energy-demanding organ. While weighing only 2% of the total body mass, the brain requires 25% of the circulating glucose for maintaining its regular function under physiological conditions. Astrocytes, like neurons, uptake and metabolize glucose and other energy substrates to generate energy in the form of ATP, necessary for the normal functioning of the cell. Interestingly, glucose is the preferred energy substrate in the brain, and only astrocytes—during adulthood and under physiological conditions—are able to accumulate and store glucose in the form of glycogen , allowing them to secure the energy supply for neurons under conditions of decreasing circulating glucose levels.
In the adult CNS, under certain conditions associated with a glucose deficit, the brain activates one of its complex homeostatic mechanisms to maintain normal neuronal activity. This mechanism consists of astrocytes breaking down their glycogen stores into lactate, which is then provided to neurons to sustain oxidative metabolism. Glycogen is also used to support long-term potentiation in neurons, which experimentally correlates with learning and memory consolidation (Drulis-Fajdasz et al. 2015). This concept was introduced by Magistretti and Pellerin in 1994 when they proposed the existence of an astrocyte–neuron lactate shuttle for the supply of energy substrates to neurons in an activity-dependent, glutamate-mediated manner (Pellerin and Magistretti 1994). They demonstrated that cortical neurons use lactate or glucose indistinctly to support oxidative metabolism. Yet, other studies have questioned this astrocyte–neuron metabolic pathway and suggest that lactate could have an additional signaling role rather than being solely another energy source for neurons.
Among other energy substrates, astrocytes utilize fatty acids (FA) to generate ATP. Indeed, in the CNS, astrocytes are the major site of FA oxidation. As a matter of fact, it has been reported that astrocytes oxidize FA to meet their energy requirements during low-fat diet intake, whereas they switch their energy metabolism to generate ketone bodies from the excess FA during high-fat diet intake. Once produced, the ketone bodies leave the astrocytes via the monocarboxylate transporter (MCT)-1 and enter the neurons via MCT-2. In neurons, ketone bodies are metabolized by mitochondria as another metabolic fuel source, notably used in states of starvation (Le Foll and Levin 2016; Puchalska and Crawford 2017).
2.2 Astrocyte Networks and Diversity: Morphological and Molecular Hallmarks
Astrocyte Networks
Each astrocyte occupies a specific territory in non-overlapping domains defined by its finger-like processes that can interact with blood vessels, individual neighboring neurons, synapses, and other cells, thus forming a unique functional network structure. Apart from physically interacting with neurons and other cells, astrocytes are engaged in extensive astrocyte–astrocyte communication through gap-junction channels formed by connexins 43 and 30. These connexins-mediated gap junctions associate astrocytes to form specific astrocytic networks that act as functional metabolic units and are directly involved in activity-dependent trafficking of glucose and its metabolites from blood vessels to neurons imbedded into these astrocytic networks.
Astrocyte Diversity
An individual astrocyte is typically recognized as having a stellate-like morphology with fine, rather long, and numerous processes extending from the soma. In the early 1900s, astrocytes were grouped into two main sub-types: fibrous and protoplasmic astrocytes. Fibrous astrocytes are located in the white matter and display the “prototypic” star-like shape attributed to astrocytes, with rather regular contours and processes, whereas the protoplasmic astrocytes are located in the gray matter and characteristically display a more irregular shape that has been referred to as “bushy.” We now know that the majority of protoplasmic astrocytes show an extensive and elaborate arborization, which exhibits more of a sponge shape rather than that of a star.
Regardless of astrocytic diversity (Fig. 6.2), most studies are still primarily targeting astrocytes by using GFAP, retaining it as the prevailing astrocyte marker. By doing so, these studies might overlook the fact that GFAP only encompasses one of the several astrocyte populations, and there are other astrocyte-specific molecular markers that allow for the visualization of these glial cells (Table 6.1). Unfortunately, so far, no universal marker has been identified to visualize all astrocytes indistinctly.
Morphological diversity of astrocytes in the CNS. The central nervous system contains several, morphologically distinct subclasses of astrocytes. The images show two of the major astroglial “morphotypes” visualized in mice expressing enhanced green fluorescent protein (eGFP) under transcriptional control of the GFAP promoter. Protoplasmic astrocytes (found in gray matter) exhibit a round and bushy appearance with highly arborized processes. In contrast, fibrous astrocytes (found in white matter) appear elongated with long and less complex processes. Notably, the morphological complexity of astrocytes remained elusive for a long time since the most common visualization method relied on the marker GFAP (see Table 6.1), which only reveals the primary, star-shaped processes (inset; red). Scale bars: 10 μm
The variety of available markers supports the heterogeneity of astrocytes , which could also define some functional aspects of these subpopulations. In fact, an individual astrocyte usually does not express only one of these markers, but rather a combination of them (Verkhratsky et al. 2016), which can be influenced by the surrounding micro-environment and neighboring cells. This molecular diversity is indicative of the great heterogeneity characterizing astrocytes, with inter- and intra-regional features, both in their function and phenotype (Ben Haim and Rowitch 2017). Yet, recent evidence indicates that astrocytes can modify and adjust their molecular and functional properties depending on the surrounding neural circuits and stem from the energetic demands of the extracellular space in which they are located (Farmer et al. 2016; Hasel et al. 2017).
2.3 Astrocytic Ca2+ Signaling: The Trademark of Astrocyte Communication
The underlying principles of information processing by the brain remain one of the greatest enigmas in neuroscience. Despite being considered electrically silent, astrocytes actually do assist in neural encoding and utilize various mechanisms to propagate information to surrounding astrocytes through astroglial networks by employing a form of intracellular ion waves , mainly Ca2+ (Rusakov 2015) (Fig. 6.1 and Video 6.1). The Ca2+-dependent propagation of information from a single astrocyte to neighboring astrocytes allows for local and coordinated signaling synchronization with the surrounding cells, including neurons and endothelial cells of the microvasculature (Arcuino et al. 2002; Gordon et al. 2008; Schummers et al. 2008). Likewise, astrocytes can respond in a Ca2+-dependent manner to fluctuations in neuronal activity occurring in the surrounding synapses, and, as these glial cells are also in direct contact with the microvasculature, they consequently act as intermediates in translating neuronal function into changes in the local blood flow (Rossi 2006). Indeed, Ca2+ signaling in astrocytes also contributes to neurovascular coupling to regulate local cerebral blood flow by eliciting vasoconstriction or vasodilation of arterioles (Metea and Newman 2006). Interestingly, recent technical advances and improvements in real-time monitoring and manipulation of in vivo changes in Ca2+ signaling in astrocytes (Fig. 6.3 and Table 6.2—Ca2+ indicators) have revealed (a) that intracellular Ca2+ transients and oscillations in these glial cells differ substantially in timing and amplitude depending on whether changes in the Ca2+ waves occur in the astrocyte’s soma or its processes (Yu et al. 2020) and (b) the presence of subtle, asynchronous Ca2+ dynamics in microdomains of glial processes (Fig. 6.1). Importantly, alterations in astrocytic Ca2+ homeostasis have been reported to occur with brain injury, reflecting healing processes or pathophysiology (Hamby and Sofroniew 2010).
Visualizing astrocyte Ca2+ responses to diverse stimuli using genetically encoded Ca2+ indicators (GECIs) in Ca2+ brain slices. Pseudocolor images representing fluorescence intensities indicative of Ca2+ responses in dorsolateral striatum resident astrocytes expressing GCaMP6f (part of the GECIs, coupled with the green fluorescent protein; see Table 6.2) before (left) and after (right) (a) application of clozapine-N oxide, an agonist of the hM3Dq DREADD (designer receptors exclusively activated by designer drugs; see Table 6.3) or (b) electrical stimulation of corticostriatal axons to evoke astrocyte calcium-dependent signal
2.4 Astrocytes: Secretory Cells Within the CNS
At the beginning of the 1900s, a pioneering study of neuroglia by Held revealed the presence of granular inclusions in astrocytes, which hinted towards a putative secretory pathway in these cells. This suggested that astrocytes constitute actively communicating cells that respond and signal to the surrounding cellular partners via the release of diverse chemical substances (Held 1909). A century later, this early hypothesis of a secretory compartment present in astrocytes was ultimately confirmed: astrocytes were shown to release signaling cues, also referred to as gliotransmitters (ATP, glutamate, D-serine), to neighboring neurons and other glial cells to regulate synaptic function, a process currently known as gliotransmission (Araque et al. 2014). Therefore, astrocytes, like neurons, are secretory cells with the ability to send molecules and ions back and forth between themselves and neurons, other glial cells, and blood vessels, and to control all physiological processes in the brain, including the activity and plasticity of local neuronal networks. In fact, neuron-derived transmitters can activate G protein-coupled receptors (GPCRs) in astrocytes, resulting in elevation of intracellular Ca2+ concentrations, which induces a fine-tuned and rapid exocytosis of gliotransmitters in a Ca2+-dependent manner. Upon activation, astrocytes can also release glutamate, which regulates the dynamics of neuronal responses by controlling synaptic strength between neurons (Araque et al. 2014).
Gliotransmitter Release from Astrocytes
Astroglial secretion is mainly regulated by cytoplasmic Ca2+ and Na+ signals, but several alternative pathways by which astrocytes signal to neighboring cells also appear to exist. In fact, a given molecule might be released through several of these pathways, which comprise: (1) vesicle-mediated exocytosis (Box 6.1); (2) diffusion through pores/channels; and (3) extrusion by transporters.
Box 6.1: Astrocytes Release Gliotransmitters via Ca2+-Regulated Exocytosis
Vesicle-mediated exocytosis is primarily induced in response to increases in cytosolic free Ca2+. The coupling of transient changes in intracellular Ca2+ and vesicle fusion relies on proteins of the so-called SNARE (soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor) family (Fig. 6.1). Upon surpassing a given Ca2+ threshold, the SNARE complex initiates a dramatic change in its molecular conformation, which ultimately triggers the fusion of vesicles with the astrocytic plasma membrane to release its cargo. The exocytotic machinery of astrocytes generally displays a lower sensitivity compared to neurons, which results in a rather lethargic stimulus-secretion coupling. Not only do exocytotic events in astrocytes occur with quite some delay, their overall number is also substantially lower compared to neurons (maximal secretion: 0.1–2/s in astrocytes and 3–6000/s in neurons) (Verkhratsky et al. 2016).
Types of Vesicles. At the ultrastructural level, two main distinct secretory organelles have been described: (1) synaptic-like microvesicles (SLMV) and (2) large dense-core vesicles (LDCV). SLMVs typically have a diameter of 30–100 nm and contain the aminergic “gliotransmitters” glutamate and/or D-serine (Fig. 6.1). Importantly, they are neither as densely packed nor as numerous as their neuronal counterparts and exist in small groups (ca. 15 vesicles) directly adjacent to neuronal structures. While SLMV appear small and clear (or electron-lucent) under electron microscopy inspection, a separate family of vesicles can be easily distinguished given their larger dimensions and higher electron-density. Referred to as LDCVs, these pools of vesicles typically harbor neuropeptides, hormones, and ATP and are generated at the trans-Golgi network. Astroglial LDCVs have a diameter of 100–600 nm and carry numerous and diverse substances, including neuropeptide Y (NPY), atrial natriuretic peptide (ANP), octadecaneuropeptide (ODN), brain-derived neurotrophic factor (BDNF), and ATP. Intriguingly, the SLMV and LDCV pools engage separate SNARE isoforms and non-overlapping mechanisms for regulated exocytosis.
2.5 Astrocytes Regulate Synaptic Plasticity and Transmission
The concept of “tripartite synapse ” to define a bidirectional and rapid dialog between neurons and surrounding astrocytes was first used by Araque and colleagues in 1999 (Araque et al. 1999) (Fig. 6.1). Astrocytes project terminal processes to neighboring neurons, and both cell types exchange encoded information in a rapid, plastic, bidirectional manner through an extensive number of receptors, ion channels, and transporters expressed along their membranes. Neuronal activity triggers the release of neurotransmitters in the synaptic cleft that can induce the Ca2+-dependent activation of proximal astrocytes, which in turn secrete gliotransmitters to ultimately modulate neuronal communication (Fig. 6.1). Several studies have also pointed out that the degree of astroglial ensheathment of the neuronal membrane influences the number and type of synapses—in the pre-, post-, and extra-synaptic elements—and shapes the local neuronal networks (Fig. 6.1). The perisynaptic astroglial processes rapidly remodel to strengthen or weaken synapses, which means that astrocytes influence synaptic plasticity. Additionally, and further supporting that astrocytes can influence synaptic events, astrocytes locally control neurotransmitter homeostasis by buffering the concentrations of presynaptically released glutamate and GABA at the synaptic cleft.
Until very recently, technical limitations had impeded further advancement in understanding how astrocytes regulate synaptic activity through the release of gliotransmitters. However, advances in targeting non-neuronal cells based on remotely controlling in vivo astrocyte activity and gliotransmitters release, in combination with novel bioengineered technologies applied in neuroscience (Table 6.3), now allow us to focus on fully understanding the relevance of astrocyte–neuron interactions in the control of brain function.
2.6 Astrocytes as Integral Components of the Neuro-Glio-Vascular Unit
Local regulation of cerebral blood flow is a crucial element for brain activity, especially in conditions of increased neuronal firing when vasodilation of the surrounding microvessels is required to respond to neuronal energy demands, also termed functional hyperemia (Fig. 6.1). Astrocytes are thought to be key active regulators of cerebral blood flow, as they are the first barrier line between the blood and neurons. Indeed, in the later part of the 1800s, the neuroanatomists Camillo Golgi and Santiago Ramón y Cajal had already speculated that astrocytes, due to their unique anatomical positioning within the brain, would be perfect candidates to elicit various functions. Localized between the vasculature and neurons, astrocytes are ideally placed to govern the brain–body interface and integrate homeostatic feedback from the periphery. In fact, astrocytes line the entire vasculature of the brain and provide complete blood vessel coverage by morphological specializations called perivascular endfeet (Fig. 6.1). Through both this physical contact and by releasing an array of soluble factors with vasoregulatory properties, astrocytes contribute to the formation and maintenance of the BBB (Fig. 6.1). Moreover, astrocytes tune the properties of the endothelium in order to regulate the entry of nutrients and hormones. As previously mentioned, by forming the first line of cells behind the BBB, astrocytes are ideally positioned to rapidly sense and adjust to changing levels of nutrients and other factors. Astrocytes are fully equipped to act as putative “metabolic sensors ,” given that they express a wide array of receptors and transporters distributed throughout their extensive cell surface area. While the BBB-associated astrocytes effectively shield the brain from changes in the blood milieu that could be devastating, they equally hamper the intended delivery of drugs to the brain—including potential candidates for the treatment of brain diseases.
2.7 Astrocytes in the Brain Control of Systemic Metabolism
Astrocytes Regulate Glucose Entry into the Brain via Insulin Signaling
As previously mentioned, in order to maintain proper brain function, it is of utmost importance to guarantee a constant and uninterrupted supply of glucose from the periphery to the brain to be used as its major source of energy (Box 6.2). In response to a meal, the body regulates glucose homeostasis to a large extent by secreting insulin from pancreatic β-cells, which is used by the cells of peripheral tissues (e.g., liver, adipose tissue, skeletal muscle) to take up glucose to generate energy. Unlike the rest of the body, glucose utilization by the brain was believed to be regulated independently of insulin, attributing the existence of abundantly expressed insulin receptors (IRs) within the brain to other roles of this hormone not related to glucose homeostasis. Yet, if neurons themselves do not rely on insulin signaling to utilize glucose, it remains possible that the entry of glucose into the brain via other cellular components, especially those forming the intricate body–brain interface (endothelial cells, astrocytes, pericytes), might depend on this specific signaling. Indeed, it was recently uncovered that insulin acts in astrocytes as a signal to regulate glucose entry from the periphery into the brain. In fact, the ablation of IRs from astrocytes induces a decrease in the astrocytic uptake of glucose, resulting from a reduced expression of the glucose transporter 1 (GLUT-1), and is associated with a lower glycolytic rate together with a decreased L-lactate efflux (Garcia-Caceres et al. 2016; Hernandez-Garzon et al. 2016). Such changes in cellular energy metabolism in astrocytes promote fatty acid β-oxidation. Aside from the impact of insulin on astrocytic bioenergetics, insulin signaling in astrocytes was reported to be determinant of how these glial cells functionally engage with, and are integrated into, hypothalamic neuronal circuits that are key in the control of metabolism. Specifically, astrocytes lacking IRs failed to properly ensheath pro-opiomelanocortin (POMC)-expressing neurons, which, in turn, rendered this otherwise glucose-responsive population insensitive to elevated blood glucose levels. Therefore, these findings support the notion that, contrary to what was previously assumed, glucose metabolism in the brain involves local insulin-dependent pathways (Fig. 6.1).
Box 6.2: Central Regulation of Glucose Homeostasis
The notion that the brain might be crucially involved in the regulation of blood glucose concentrations actually dates back to 1854, when the French physiologist Claude Bernard induced diabetes simply by puncturing the floor of the fourth ventricle (“piqûre diabétique”). Nowadays, it is well established that intricate glucoregulatory systems exist in the brain, which readily respond to both hypoglycemia and hyperglycemia. Distinct populations of neurons have been described to reside in the hypothalamus and brainstem that are either excited or inhibited by glucose. Such glucoregulatory system is crucially important for surveying circulating levels of glucose and subsequently eliciting immediate counterregulatory mechanisms. Intriguingly, these glucose-sensitive neurons share the molecular machinery that has been described to allow pancreatic β-cells to monitor blood glucose levels. As soon as glucose enters the cells, it gets phosphorylated by a distinct isoform of the hexokinase enzyme (hexokinase IV or glucokinase), which exhibits a relatively low affinity to glucose and is thus well within the range to act as a glucose-sensing enzyme. Lastly, these cells have incorporated the ATP-dependent potassium channel (KATP), which is a universal sensor linking cellular energy status—for example, impacted by glucose fluxes—with membrane depolarization.
Astrocytes Control Feeding via Leptin Signaling
Leptin is a well-known adipocyte-derived hormone that plays a pivotal role in energy balance and the control of body weight. As its concentration in blood correlates with adiposity, leptin is a reflective measure of energy reserves and provides an anorexigenic feedback signal that is sensed by the brain, in particular at the level of the hypothalamus. Most emphasis has been placed on how leptin affects neuronal activity and how neurons process and convey this information in order to calibrate food intake, energy expenditure, and ultimately body weight. However, it was revealed that astrocytes constitute additional, functionally relevant targets of blood-borne leptin (Kim et al. 2014; Wang et al. 2015). Specifically, Kim and colleagues reported that the inducible loss of the functional long form of the leptin receptor (LepR) impairs astrocyte–neuron spatial interactions in the hypothalamus. Similar to what has been observed in mice deficient in astrocytic insulin signaling, the inducible loss of LepR led to pronounced effects on the synaptic organization of feeding circuits located in the arcuate nucleus of the hypothalamus, namely the anorexigenic POMC neurons and the orexigenic neurons that co-express both Agouti-related peptide and neuropeptide Y (known as AgRP/NPY neurons), which are considered paramount for energy homeostasis (Box 6.3). Interestingly, leptin treatment failed to suppress food intake as efficiently in mice devoid of astrocytic LepR than in control ones. Mice lacking astrocytic LepR showed further feeding alterations such as a potentiated food intake in response to fasting or the fasting-mimicking hormone ghrelin (Kim et al. 2014). Notably, the subsequent study by Wang and colleagues similarly reported that the loss of LepR in astrocytes impairs leptin signaling in the brain, as evidenced by reduced phosphorylation of signal-transducer and activator of transcription 3 (pSTAT3) (Wang et al. 2015). Interestingly, this was observed even when leptin was administered centrally by directly infusing it into the cerebral ventricle, suggesting a central role of astrocytic LepR that is independent of hormonal transport across the BBB. In summary, these studies further support the observation that astrocytes are crucially important for integrating hormonal feedback signals to shape and tune the homeostatic neurocircuits in the hypothalamus (Fig. 6.1).
Box 6.3: Astrocyte–Neuron Interactions in the Arcuate Nucleus of the Hypothalamus
Over the past several decades, substantial research effort has been placed on the mapping of neurocircuitries controlling energy balance and body weight. In the course of this endeavor, two distinct populations of neurons emerged as crucial players, with both of them coexisting within the same brain region, the arcuate nucleus of the hypothalamus (ARC) (Fig. 6.4). By being situated directly adjacent to the median eminence, a circumventricular organ, ARC neurons and surrounding astrocytes have a privileged direct access to circulating feedback signals entering through local fenestrated blood vessels. Thus, ARC neurons can constantly monitor the metabolic state of the body, signaled, for instance, by means of circulating hormones such as ghrelin, leptin, or insulin. Importantly, two populations of ARC neurons can be characterized by their distinct molecular signature, with one subset expressing Agouti-related peptide/neuropeptide Y (AgRP/NPY) and the other population identified by expression of the pro-opiomelanocortin (POMC) precursor neuropeptide. Each set of ARC neurons exerts opposing effects on feeding behavior and energy expenditure and is arranged in an antagonizing relationship to the other. On the one hand, AgRP/NPY neurons get activated in the context of food deprivation and are necessary and sufficient to trigger feeding by increasing the consummatory drive. On the other hand, POMC neurons are activated by signals of energy surplus and reduce food intake while increasing energy expenditure. Intriguingly, the projection patterns of AgRP/NPY and POMC neurons are overlapping and exert opposing effects. Clearly, the circuit of AgRP/NPY and POMC neurons plays an integral role in controlling energy homeostasis. More recently, however, other cell types present in the ARC have stepped into the limelight. Among them, local astrocytes were attributed particular significance given that they show various region-specific properties not found elsewhere in the brain. In response to environmental cues such as nutrients and hormones, for instance, those astrocytes residing in the ARC were shown to undergo rapid changes in morphology and function. This in turn results in profound changes in synaptic function in the local AgRP/NPY and POMC neurocircuit. In summary, the traditional AgRP/NPY and POMC neurocircuit is nowadays known to be structurally and functionally influenced by the local ARC-residing astrocytes.
Astrocyte–neuron interactions in the arcuate nucleus of the hypothalamus. (a, b) The arcuate nucleus of the hypothalamus is ideally located to serve as a metabolic sensing hub (hormones, nutrients) and also hosts two neuronal populations of utmost importance for the control of energy balance and body weight: the neurons expressing the orexigenic neuropeptides Agouti-related peptide and neuropeptide Y (aka. AgRP/NPY neurons, in red) and the neurons expressing pro-opiomelanocortin (POMC neurons, in green), a precursor of anorexigenic neuropeptides (for more details, see Box 6.3). Surrounding these neurons are astrocytes (in blue—confocal microscopy image—scale bar: 20 μm), which are emerging as another cell type that plays a key role in the regulation of the activity of these hypothalamic neuronal circuits. (c) Astroglial processes ensheath the surrounding neurons to regulate their activity, as shown in this electron microscopy image where a glial process (in blue) is covering the soma of a POMC neuron (in green). Scale bar: 1 μm
Other Emerging Roles of Astrocytes in Metabolic Control
Additional studies suggest other functions or have further confirmed the role of astrocytes in the central regulation of whole-body energy metabolism. Interestingly, Gao and colleagues reported the metabolic relevance of the capacity of astrocytes to uptake FA by generating a mouse model deficient in lipoprotein lipase specifically in GFAP-positive astrocytes (Gao et al. 2017b). The authors found that such alteration promotes ceramide accumulation in hypothalamic neurons, which in excessive amounts has been reported to induce detrimental effects in the brain, such as lipotoxicity and neuronal dysfunction (Chaurasia and Summers 2015). A recent study, led by Bouyakdan and colleagues, reported another interesting aspect, namely the role of astrocytic endozepines in the central control of energy balance (Bouyakdan et al. 2019). Endozepines are generally defined as endogenous ligands for the benzodiazepine receptor and it was previously shown that central exogenous delivery of a specific endozepine, ODN, both reduced food intake and improved glucose tolerance. In that study, the authors revealed that the deletion, specifically in GFAP-positive astrocytes, of the endogenous acyl-CoA-binding protein (ACBP), from which endozepines can be derived, is sufficient to promote food intake in both males and females (Bouyakdan et al. 2019). Interestingly, by placing ACBP-positive astrocytes in contact with the anorexigenic POMC neurons, the authors were able to show that ODN can activate these neurons. Furthermore, overexpressing ACBP in the ARC decreased food intake and weight gain. These results highlight ACBP as a gliopeptide that plays a central role in the control of energy balance by exercising an anorectic effect through interaction with the melanocortin system (Bouyakdan et al. 2019).
Other studies recently highlighted the active role of astrocytes in metabolic control by using recently developed techniques to manipulate astrocytic activity in the hypothalamus (e.g., chemo- (DREADDs, designer receptors exclusively activated by designer drugs) and opto-genetics technologies). Specifically, studies employing these techniques have shown that Ca2+-dependent activation of astrocytes located in the mediobasal hypothalamus (MBH) is determinant for the reduction in food intake, both in basal conditions and in a ghrelin-induced food intake paradigm (Yang et al. 2015), which is independent of the emotional state of the animal (Sweeney et al. 2016). Likewise, these studies have allowed for the identification of astrocyte-derived adenosine as the molecule mediating the inactivation of AgRP neurons via adenosine A1 receptors (Yang et al. 2015). Intriguingly, another group using a similar approach recently reported that activation of astrocytes in the ARC of the hypothalamus (located in the MBH) was associated with an increase in food intake (Chen et al. 2016), which is in contradiction to the study published by Yang and colleagues. The authors attributed these food intake-promoting effects of activating astrocytes to a sequential activation of AgRP neurons, while no direct changes in POMC neuronal activity were observed (Chen et al. 2016). Overall these opposing findings suggest that the functionality of astrocytes in the control of metabolism could be determined by the local network in which they are embedded and also highlight the necessity of being extremely cautious with the experimental setup, especially when it comes to using relatively new tools, but also with the conclusions we draw from the results that are obtained.
3 Astrocytes in Pathological Conditions
Over the last decades, substantial progress has been made in elucidating the roles of astrocytes in CNS disorders and pathologies. Astrocytes are plastic cells that respond dynamically to environmental stimuli, thereby allowing versatile alterations in their morphological, molecular, and functional properties. However, such alterations vary depending on the nature of the stimulus and can even be accompanied by a conspicuous structural change, which is frequently observed in activated or reactive astrocytes in response to CNS injury and/or disease. Astrocytosis or astrogliosis is known as the characteristic hypertrophic (reactive), and at times proliferative, phenotype that these glial cells adopt, undergoing an increase in GFAP and vimentin expression, associated with alterations in astrocytic Ca2+ homeostasis, all of which can reflect either healing processes or pathophysiology. Therefore activated/reactive astrocytes undergo morphological, functional, and molecular changes that occur to a greater or lesser extent depending on the severity and/or nature of stimuli. In severe cases, these modifications observed in astrocytes could even lead to a pronounced overlapping of astrocytic domains and the generation of a dense, narrow, and compact glial scar. The generation of a glial scar is characterized by the accumulation of hypertrophic resident astrocytes and the production, by all CNS cell types, of cytokines, mediators of innate immunity (e.g., toll-like receptor ligands), chemokines, neurotransmitters (e.g., glutamate, noradrenaline), growth factors, hypoxia, and neurotrophic factors, among others. Although many groups have extensively studied the formation of a glial scar in response to a stab wound, it is still unclear if it serves as a defense mechanism or participates in the propagation of further CNS insult and dysfunction. Initially, a glial scar was thought to be due to a negative and maladaptive response of the CNS, which inhibits axonal regeneration and in turn impedes functional neuronal recovery, contributing to the initiation and progression of neurological complications. However, accumulating evidence rather indicates that the formation of this physical barrier could avoid the propagation of inflammatory factors, for example, from the lesion core to the healthier surrounding tissue. The types of CNS insult able to induce astrogliosis are very heterogeneous. This insult can be mechanical, resulting from a wound injury or stroke, or rather related to neurodegenerative diseases. Signs of astrogliosis have been reported in Alzheimer’s, Parkinson’s, and Huntington’s diseases, amyotrophic lateral sclerosis, multiple sclerosis, dementia, and so on. In these chronic pathologies, the formation of a glial scar is less systematic, although it has also been reported.
Being associated with CNS insult, the presence of astrogliosis is linked to inflammatory states that are more or less pronounced. Interestingly, the laboratory of Ben Barres coined as “A1-reactive astrocytes ” a subtype of astrocytes that are rendered reactive via neuroinflammation (Liddelow et al. 2017). These A1-reactive astrocytes have been found in patients with neurodegenerative diseases and are believed to be involved in promoting neuronal and oligodendrocyte cell death through the secretion of a yet-to-be identified toxin and via the loss of many of the normal astrocytic functions (Liddelow et al. 2017). Conversely, aging is also considered a driver of astrogliosis leading to improper astrocyte functionality, which results in defects in the astrocyte ability to properly maintain a healthy CNS environment, affecting their interaction with neighboring cells and ultimately contributing to the development of an inflammatory state associated with aging.
3.1 Reactive Astrocytes in Obesity
Astrogliosis is a hallmark of the tissue inflammation and/or injury that underlies neurological diseases. Interestingly, studies have pointed out that obesity might be a brain disease, also showing signs of inflammation and astrogliosis that were until recently solely reported in neurogenerative diseases (Fig. 6.5). In 2005, De Souza and colleagues were the first to demonstrate that obesity, at least in rodents, is associated with an increase in inflammatory signaling in the hypothalamus (De Souza et al. 2005). This led to studies aiming to further understand which aspects of inflammation were involved in the progression of metabolic disease. In 2010, experiments led by Horvath and colleagues demonstrated that diet-induced obese mice exhibited an upregulation of GFAP in astrocytes, particularly in the hypothalamus, which was associated with changes in the physical interactions of astrocytes with endothelial cells and neurons, contributing to alterations of the cytoarchitecture and synaptology of hypothalamic circuits (Horvath et al. 2010). Interestingly, such inflammatory hallmarks, including increased cytokines in the hypothalamus, were detected prior to any changes in peripheral inflammation and body weight gain (Thaler et al. 2012), suggesting their potential role in hypothalamic dysfunction associated with astrogliosis to promote obesity pathogenesis (Fig. 6.5). Further studies have provided supplementary evidence that confirmed the presence of reactive glia in the hypothalamus in monogenic models of obesity (Buckman et al. 2013; Hsuchou et al. 2009; Pan et al. 2008), but also in response to maternal or neonatal overnutrition (Fuente-Martin et al. 2012; Garcia-Caceres et al. 2011). Importantly, hypothalamic astrogliosis, as detected by magnetic resonance imaging, has also been reported to occur in humans with high body mass index (BMI) (Thaler et al. 2012).
Diet-induced astrogliosis in the arcuate nucleus of the hypothalamus. Consumption of a high-calorie diet can trigger profound changes in the hypothalamic cytoarchitecture, including the rapid upregulation of GFAP (glial-fibrillary acidic protein) in local astrocytes. By acquiring a more “bushy,” hypertrophic morphology as a consequence, these now so-called reactive astrocytes are believed to: (a) disrupt the synaptology and function of local neurocircuits in the hypothalamus controlling energy balance and (b) to hamper the entry of homeostatic feedback signals emanating from the periphery. However, more functional studies are yet warranted to support such a claim, which currently remains based mainly on descriptive reports
The astrogliosis associated with the consumption of hypercaloric diet was reported to be a reversible event, since the resumption of a normal chow diet restrains hypothalamic astrogliosis in association with a reduction in body weight (Berkseth et al. 2014). Yet, not all calorie-dense diets induce the same changes in hypothalamic glial activity, which indicates a certain heterogeneity in the response of glial cells depending on the composition of the diet.
According to Gao and colleagues, the combination of dietary fat and sugars, but not fat or obesity per se, is a determinant for the induction of microglial activity. Yet, changes in the expression of GFAP did not seem to depend on the combination of high-carbohydrates and high-fat in the diet, but rather solely on increased levels of fat in the diet (Gao et al. 2017a). Overall, these findings suggest the existence of hypothalamic specific responses from the different types of glia to distinct diet components in the context of hypercaloric diets.
In 2014, Morselli and colleagues highlighted a sex discrepancy in the development of hypercaloric diet feeding-associated astrogliosis with the observation that male mice—but not females—exhibited hypothalamic astrogliosis and upregulation of cytokines, despite both sexes exhibiting excessive weight gain (Morselli et al. 2014). Thus, these findings suggest the existence of sexual differences in diet-induced responses of hypothalamic astrocytes. Furthermore, astrogliosis associated with the consumption of a hypercaloric diet was also reported to affect extra-hypothalamic areas such as the hippocampus and the thalamus (Buckman et al. 2013), although the time and the composition of the diet that are needed to induce astrogliosis can differ depending on the brain area. Interestingly, microglia are thought to be involved in some of the astrocytic responses elicited by a hypercaloric diet and are considered the first responders, producing inflammatory factors that would, in turn, simultaneously activate astrocytes and trigger neuronal stress (Thaler et al. 2012; Valdearcos et al. 2014) (see Chap. 7).
Astrocytic Pathways Mediate Hypothalamic Astrocytosis Associated with Diet-Induced Obesity
Recent work by Pfuhlmann and colleagues has demonstrated that hypercaloric diets trigger hypothalamic astrocytosis by activation of the Ca2+/calmodulin-activated serine/threonine phosphatase calcineurin (Pfuhlmann et al. 2018). Conversely, other studies have proposed inhibition of pro-inflammatory pathways in astrocytes as a means to prevent low-grade hypothalamic inflammation, including astrocytosis, associated with the consumption of energy-dense diets and obesity. In this regard, Douglass and colleagues have reported that blocking the IκB kinase (IKK) β/NF-κB pathway, involved in most inflammatory signaling, specifically in astrocytes is sufficient to attenuate diet-induced astrogliosis, as well as the upregulation of inflammatory factors and the impairment of leptin and insulin sensitivity occurring within the hypothalamus. Importantly, these findings were associated with a decrease in food intake and an increase in energy expenditure in mice fed with a hypercaloric diet (Douglass et al. 2017). Other studies are aligned with these observations underlining the relevance of inhibiting the inflammatory IKKβ/NF-κB pathway in astrocytes to improve whole-body energy homeostasis under obesogenic conditions (Zhang et al. 2017). Interestingly, these studies reported dynamic changes in astrocytic morphology depending on the feeding status of mice. Chronic overnutrition, together with the upregulation of the IKKβ/NF-κB pathway, induced long-lasting shortening of astrocytic processes that was accompanied by glucose intolerance and an increase in blood glucose levels, fat accumulation, and total body weight (Zhang et al. 2017). Furthermore, these authors reported that the IKKβ/NF-κB pathway in astrocytes mediates the astrocytic regulation of extracellular levels of GABA and BDNF (brain-derived neurotrophic factor), which was partially responsible for the metabolic syndrome observed in these mice on a hypercaloric diet (Zhang et al. 2017).
Other signaling pathways in astrocytes have been identified to be involved in the generation of astrogliosis, such as Stat-3 and ErB, but none of these has yet been studied in the context of diet-induced obesity, leaving ample opportunity for further mechanistic understanding in this regard.
4 Perspectives
At the end of the nineteenth century, Ramon y Cajal’s pioneering studies were the first to reveal that an intimate and coordinated association between glia and neurons is required for normal brain function. Despite the undeniable essential role of astrocytes in the brain, a simplistic view, solely concerned with exploring neuronal activity, prevailed during the previous decades, ignoring the presence and active role of other cells in the brain. This has likely hindered the progression of knowledge towards forming a complete understanding of how the brain controls the many processes that are under its jurisdiction, including the control of systemic metabolism. We now know that the regulation of brain function cannot be operated or explained by neurons alone, and the notion that astrocytes play an important role in metabolic control is currently gaining momentum. Moreover, the implication of astroglia in this process has brought these cells into the spotlight and has resulted in advances in our understanding of their role in the physiological control of metabolism, but also in the pathophysiology of metabolic diseases. However, there is still much to be learned regarding astrogliosis in both diet-induced obesity and dietary challenges. Indeed, scientists need to continue putting effort into identifying new markers and generating new tools that are less invasive and allow higher resolution, which will allow us to abate the difficulties and eventually grant new exciting discoveries. Hence, one continuing challenge is to determine the relationship between the different inflammatory and glial responses in the hypothalamus and their implication in the perpetuation of weight gain, as well as the associated secondary complications. Understanding these processes may lead to new therapeutic targets to treat CNS diseases, including obesity.
5 Key Literature
-
Araque et al. (1999) [Review discussing the integral role of astrocytes within synapses].
-
Garcia-Caceres et al. (2016) [Original article reporting the importance of astrocytic insulin signaling for the control of both central glucose sensing and systemic glucose metabolism by modulating the entry of glucose across the blood–brain barrier, depending on the overall metabolic status].
-
Garcia-Caceres et al. (2019) [Review discussing the importance of non-neuronal partners in the central control of systemic metabolism].
-
Horvath et al. (2010) [Original article reporting that diet-induced obese mice exhibit hypothalamic astrocytic reactivity which is associated with changes in the physical interactions of astrocytes with endothelial cells and neurons, contributing to alterations of the cytoarchitecture and synaptology of hypothalamic circuits].
-
Verkhratsky et al. (2016) [Review summarizing the features of astrocytic secretion of signaling molecules].
Change history
07 December 2023
A correction has been published.
References
Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22(5):208–215
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81(4):728–739. https://doi.org/10.1016/j.neuron.2014.02.007
Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M (2002) Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A 99(15):9840–9845. https://doi.org/10.1073/pnas.152588599
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18(1):31–41. https://doi.org/10.1038/nrn.2016.159
Berkseth KE, Guyenet SJ, Melhorn SJ, Lee D, Thaler JP, Schur EA, Schwartz MW (2014) Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology 155(8):2858–2867. https://doi.org/10.1210/en.2014-1121
Bouyakdan K, Martin H, Lienard F, Budry L, Taib B, Rodaros D, Chretien C, Biron E, Husson Z, Cota D, Penicaud L, Fulton S, Fioramonti X, Alquier T (2019) The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J Clin Invest 130:2417–2430. https://doi.org/10.1172/JCI123454
Buckman LB, Thompson MM, Moreno HN, Ellacott KL (2013) Regional astrogliosis in the mouse hypothalamus in response to obesity. J Comp Neurol 521(6):1322–1333. https://doi.org/10.1002/cne.23233
Chaurasia B, Summers SA (2015) Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 26(10):538–550. https://doi.org/10.1016/j.tem.2015.07.006
Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, Han W (2016) Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife 5. https://doi.org/10.7554/eLife.18716
De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA (2005) Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146(10):4192–4199. https://doi.org/10.1210/en.2004-1520
Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP (2017) Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6(4):366–373. https://doi.org/10.1016/j.molmet.2017.01.010
Drulis-Fajdasz D, Wojtowicz T, Wawrzyniak M, Wlodarczyk J, Mozrzymas JW, Rakus D (2015) Involvement of cellular metabolism in age-related LTP modifications in rat hippocampal slices. Oncotarget 6(16):14065–14081. https://doi.org/10.18632/oncotarget.4188
Farmer WT, Abrahamsson T, Chierzi S, Lui C, Zaelzer C, Jones EV, Bally BP, Chen GG, Theroux JF, Peng J, Bourque CW, Charron F, Ernst C, Sjostrom PJ, Murai KK (2016) Neurons diversify astrocytes in the adult brain through sonic hedgehog signaling. Science 351(6275):849–854. https://doi.org/10.1126/science.aab3103
Fuente-Martin E, Garcia-Caceres C, Granado M, de Ceballos ML, Sanchez-Garrido MA, Sarman B, Liu ZW, Dietrich MO, Tena-Sempere M, Argente-Arizon P, Diaz F, Argente J, Horvath TL, Chowen JA (2012) Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122(11):3900–3913. https://doi.org/10.1172/JCI64102
Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, Guzman-Ruiz M, Layritz C, Legutko B, Zinser E, Garcia-Caceres C, Buijs RM, Woods SC, Kalsbeek A, Seeley RJ, Nawroth PP, Bidlingmaier M, Tschop MH, Yi CX (2017a) Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 6(8):897–908. https://doi.org/10.1016/j.molmet.2017.06.008
Gao Y, Layritz C, Legutko B, Eichmann TO, Laperrousaz E, Moulle VS, Cruciani-Guglielmacci C, Magnan C, Luquet S, Woods SC, Eckel RH, Yi CX, Garcia-Caceres C, Tschop MH (2017b) Disruption of lipid uptake in astroglia exacerbates diet-induced obesity. Diabetes 66(10):2555–2563. https://doi.org/10.2337/db16-1278
Garcia-Caceres C, Fuente-Martin E, Burgos-Ramos E, Granado M, Frago LM, Barrios V, Horvath T, Argente J, Chowen JA (2011) Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 152(5):1809–1818. https://doi.org/10.1210/en.2010-1252
Garcia-Caceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P, Ninkovic J, Yi CX, Le Thuc O, Szigeti-Buck K, Cai W, Meyer CW, Pfluger PT, Fernandez AM, Luquet S, Woods SC, Torres-Aleman I, Kahn CR, Gotz M, Horvath TL, Tschop MH (2016) Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell 166(4):867–880. https://doi.org/10.1016/j.cell.2016.07.028
Garcia-Caceres C, Balland E, Prevot V, Luquet S, Woods SC, Koch M, Horvath TL, Yi CX, Chowen JA, Verkhratsky A, Araque A, Bechmann I, Tschop MH (2019) Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci 22(1):7–14. https://doi.org/10.1038/s41593-018-0286-y
Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA (2008) Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456(7223):745–749. https://doi.org/10.1038/nature07525
Hamby ME, Sofroniew MV (2010) Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 7(4):494–506. https://doi.org/10.1016/j.nurt.2010.07.003
Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, Markus NM, McQueen J, Hampton DW, Torvell M, Tiwari SS, McKay S, Eraso-Pichot A, Zorzano A, Masgrau R, Galea E, Chandran S, Wyllie DJA, Simpson TI, Hardingham GE (2017) Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 8:15132. https://doi.org/10.1038/ncomms15132
Held H (1909) Über die Neuroglia marginalis der menschlichen Grosshirnrinde. Monatschr f Psychol u Neurol 26 Rdg:360–416
Hernandez-Garzon E, Fernandez AM, Perez-Alvarez A, Genis L, Bascunana P, Fernandez de la Rosa R, Delgado M, Angel Pozo M, Moreno E, McCormick PJ, Santi A, Trueba-Saiz A, Garcia-Caceres C, Tschop MH, Araque A, Martin ED, Torres Aleman I (2016) The insulin-like growth factor I receptor regulates glucose transport by astrocytes. Glia 64(11):1962–1971. https://doi.org/10.1002/glia.23035
Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107(33):14875–14880. https://doi.org/10.1073/pnas.1004282107
Hsuchou H, He Y, Kastin AJ, Tu H, Markadakis EN, Rogers RC, Fossier PB, Pan W (2009) Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain 132(Pt 4):889–902. https://doi.org/10.1093/brain/awp029
Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR, Jeong JK, Szigeti-Buck K, Gao Y, Garcia-Caceres C, Yi CX, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschop MH, Horvath TL (2014) Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17(7):908–910. https://doi.org/10.1038/nn.3725
Le Foll C, Levin BE (2016) Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Comp Physiol 310(11):R1186–R1192. https://doi.org/10.1152/ajpregu.00113.2016
Li D, Agulhon C, Schmidt E, Oheim M, Ropert N (2013) New tools for investigating astrocyte-to-neuron communication. Front Cell Neurosci 7:193. https://doi.org/10.3389/fncel.2013.00193
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, Kaelin V, Zuend M, San Martin A, Romero-Gomez I, Baeza-Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF, Weber B (2016) In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab 23 (1):94-102. doi:https://doi.org/10.1016/j.cmet.2015.10.010
Metea MR, Newman EA (2006) Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26(11):2862–2870. https://doi.org/10.1523/JNEUROSCI.4048-05.2006
Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, Navas CR, Gordillo R, Neinast M, Kalainayakan SP, Li DL, Gao Y, Yi CX, Hahner L, Palmer BF, Tschop MH, Clegg DJ (2014) Hypothalamic PGC-1alpha protects against high-fat diet exposure by regulating ERalpha. Cell Rep 9(2):633–645. https://doi.org/10.1016/j.celrep.2014.09.025
Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ (2008) Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology 149(6):2798–2806. https://doi.org/10.1210/en.2007-1673
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91(22):10625–10629. https://doi.org/10.1073/pnas.91.22.10625
Pfuhlmann K, Schriever SC, Legutko B, Baumann P, Harrison L, Kabra DG, Baumgart EV, Tschop MH, Garcia-Caceres C, Pfluger PT (2018) Calcineurin A beta deficiency ameliorates HFD-induced hypothalamic astrocytosis in mice. J Neuroinflammation 15(1):35. https://doi.org/10.1186/s12974-018-1076-x
Puchalska P, Crawford PA (2017) Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 25(2):262–284. https://doi.org/10.1016/j.cmet.2016.12.022
Rossi DJ (2006) Another BOLD role for astrocytes: coupling blood flow to neural activity. Nat Neurosci 9(2):159–161. https://doi.org/10.1038/nn0206-159
Rusakov DA (2015) Disentangling calcium-driven astrocyte physiology. Nat Rev Neurosci 16(4):226–233. https://doi.org/10.1038/nrn3878
Sahlender DA, Savtchouk I, Volterra A (2014) What do we know about gliotransmitter release from astrocytes? Philos Trans R Soc Lond B Biol Sci 369(1654):20130592. https://doi.org/10.1098/rstb.2013.0592
Schummers J, Yu H, Sur M (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320(5883):1638–1643. https://doi.org/10.1126/science.1156120
Sweeney P, Qi Y, Xu Z, Yang Y (2016) Activation of hypothalamic astrocytes suppresses feeding without altering emotional states. Glia 64(12):2263–2273. https://doi.org/10.1002/glia.23073
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122(1):153–162. https://doi.org/10.1172/JCI59660
Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK (2014) Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9(6):2124–2138. https://doi.org/10.1016/j.celrep.2014.11.018
Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R (2016) Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J 35(3):239–257. https://doi.org/10.15252/embj.201592705
Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W (2015) Role of astrocytes in leptin signaling. J Mol Neurosci 56(4):829–839. https://doi.org/10.1007/s12031-015-0518-5
Yang L, Qi Y, Yang Y (2015) Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep 11(5):798–807. https://doi.org/10.1016/j.celrep.2015.04.002
Yu X, Nagai J, Khakh BS (2020) Improved tools to study astrocytes. Nat Rev Neurosci 21(3):121–138. https://doi.org/10.1038/s41583-020-0264-8
Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP, Cai D (2017) Astrocytic process plasticity and IKKbeta/NF-kappaB in central control of blood glucose, blood pressure, and body weight. Cell Metab 25(5):1091–1102. e1094. https://doi.org/10.1016/j.cmet.2017.04.002
Acknowledgements
The authors thank Alfonso Araque and Tamas Horvath for kindly providing illustrative imaging material included in Fig. 6.3 and Video 6.1, and Fig. 6.4, respectively. This work was supported by the European Research Council ERC (AdG grant Hypoflam # 695054 and STG grant AstroNeuroCrosstalk # 757393) and the German Research Foundation DFG under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy—ID 390857198) and Helmholtz Excellence Network.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Video 6.1
Ca2+ imaging in hippocampal astrocytes. Astrocytes located in the hippocampus were infected with viral vectors in order to express GCaMP6, a genetically encoded Ca2+ indicator (see Table 6.2), as well as the DREADD hM3Dq (Table 6.3). Receptor activation of hM3Dq by application of its selective ligand clozapine-N oxide (CNO) robustly evokes intracellular Ca2+ transients in astrocytes that translates into fluorescence changes of GCaMP6, as displayed in this video of a hippocampal brain preparation (AVI 22204 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this chapter
Cite this chapter
Le Thuc, O., Gruber, T., Tschöp, M.H., García-Cáceres, C. (2021). Control of Systemic Metabolism by Astrocytes in the Brain. In: Tasker, J.G., Bains, J.S., Chowen, J.A. (eds) Glial-Neuronal Signaling in Neuroendocrine Systems. Masterclass in Neuroendocrinology, vol 11. Springer, Cham. https://doi.org/10.1007/978-3-030-62383-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-62383-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62382-1
Online ISBN: 978-3-030-62383-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)