Abstract
Laparoscopic cholecystectomy is the “gold standard” in the treatment of symptomatic gallbladder’s lithiasis. Difficult gallbladder (DGB) means a procedure with an increased surgical risk and high conversion rate compared to standard cholecystectomy. Acute cholecystitis is the most frequent clinical condition and also scleroatrophic cholecystitis and cholecystectomy in cirrhosis represent a difficult gallbladder pattern. The conversion rate increases depending on the degree of gallbladder inflammation, patient comorbidities, and the skills of surgeon.
We propose a procedural algorithm built on the “Safe Step in Laparoscopic Cholecystectomy for Acute Cholecystitis” identified by Wakabayashi to identify possible critical issues and suggest solutions. Bailout techniques, including cholecystostomy, subtotal cholecystectomy, and conversion to open surgery, are alternative procedures recommended to avoid potential biliary/vascular injury. If bile appears in the operating field, an i.o. cholangiography is recommended to confirm and define the location and extent of the biliary injury. In case of limited biliary surgery experience, it is preferable to drain the abdomen, even in laparoscopy, and refer the patient to a dedicated hospital.
Whenever a DGB scenario is expected, the operating room should be prepared for possible conversion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Laparoscopic cholecystectomy
- Difficult gallbladder
- Cholecystitis
- Bailout technique
- Safety in cholecystectomy
10.1 Introduction
Laparoscopic cholecystectomy (LC) is the “gold standard” in the treatment of symptomatic gallbladder’s lithiasis and is the most performed laparoscopic abdominal surgery in the world.
It is associated with a morbidity rate of around 10% with an increased risk of bile duct injury (0.1–1.5%) when compared to open surgery [1, 2].
In elective procedures, the laparoscopic conversion rate in the open cholecystectomy is low with a range in the literature between 2% and 15% [3,4,5,6], while it increases up to 25% when the patient is operated on for acute cholecystitis [7, 8].
There are conditions in which it is not possible to complete the cholecystectomy safely and in such cases deviations from the standard surgical procedure are mandatory.
In the literature, this condition is generically identified as “difficult gallbladder” and represents a challenge for the laparoscopic surgeon.
“Difficult gallbladder” (DGB) corresponds to a procedure with an increased surgical risk compared to standard cholecystectomies and has been reported with an incidence up to 26% in large series [9, 10].
The concept of “difficult gallbladder” is mainly based on intraoperative findings and strongly depends on the surgeon’s skills. The pattern of “difficult gallbladder” is characterized by severe inflammation which makes dissection difficult, alters the anatomy, and increases the risk of bleeding [11, 12]. In 2016, among 2212 patients undergoing laparoscopic cholecystectomy in 10 years, Ashfaq A. et al. reported in 351 (15.8%) criteria of difficult gallbladder and among these the conversion rate was 19.9% [11].
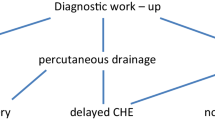
In case of acute cholecystitis, chronic or scleroatrophic cholecystitis and cirrhotic liver are more frequently faced with a difficult gallbladder scenario (Fig. 10.1).
Sample case: 89-year-old male. Admission to E.R. for abdominal pain for over 48 h. Abdominal U.S: acute acalculous cholecystitis with thickening of the walls and pericholecystic fluid. Medical therapy started. After 24 h worsening of clinical symptoms. CT scan: acute gangrenous cholecystitis, hydrops, peri-cholecystic abscess and perihepatic fluid , with irregularity of the gallbladder wall and the partial lack of contrast enhancement (perforation at the fundus level). Indication for emergency surgery. Laparoscopic approach but early conversion. Right subcostal laparotomy. Intraoperative evidence of: free corpuscular fluid in the abdomen; viscero-parietal and liver-diaphragmatic adhesion; abscess between cholecysts and «falciform ligament» and in the Morrison's pouch. The gallbladder is hydropic, with necrotic areas at the body and fundus level with at least two perforations. An anterograde cholecystectomy was performed leaving a small portion of gallbladder in the cholecystic bed
Acute cholecystitis is one of the main causes of acute abdominal hospitalization in the adult population and the most common indication for abdominal surgery in the elderly patient [13, 14]. Early laparoscopic cholecystectomy results in a shorter postoperative hospitalization period and lower healthcare costs [7, 15]. In a recent study by Kais H. et al., in 1658 laparoscopic cholecystectomies, the conversion rate increases more than tenfold depending on whether the surgery was performed in election or for acute cholecystitis from 2.1% to 24.8%, respectively [16]. In order to univocally define the acute cholecystitis patterns, several scores have been proposed, the most widespread of which are the Tokyo Guidelines in their last revision dated 2018 (TG18). The TG 18 also suggest a treatment flowchart [17,18,19,20].
A recent Swedish study reported a doubled risk of bile duct injury (BDI) in acute cholecystitis and showed a statistically significant association between the severity of cholecystitis according to TG18 and BDI risk [21].
Chronic or scleroatrophic cholecystitis can lead to a difficult gallbladder pattern. The Swiss Society of Laparoscopic and Thoracoscopic Surgery analyzed a database of 22,953 patients and found a conversion rate for acute cholecystitis of 15.9% versus 6.4% for chronic cholecystitis. Both are significantly higher than for standard cholecystectomy [3].
Prevalence of gallstones in patients with cirrhosis is estimated at 29–46% and is three times higher than that in patients without cirrhosis [22]. Laparoscopic cholecystectomy in cirrhosis is associated with a morbidity rate between 5% and 23% and a mortality rate between 7% and 20%.
In a review on cholecystectomy in cirrhotic patients, Laurence J.M et al. reported that mortality rates in LC was 0.74% while in open cholecystectomy it was 2%, the conversion rate was 5.8% while the overall complication rate was 17.6% in LC and 47.7% in open [23]. In another review, Machado N.O. documented a conversion rate of 4.58%, 17% morbidity, and 0.45% mortality [24].
Cholecystectomy is more hazardous in cirrhosis because of hemorrhage related to portal hypertension, coagulopathy, and thrombocytopenia [23].
Surgeons around the world, after an initial period in which the majority of them were convinced that the cholecystectomy had to be completed in laparoscopy, soon realized that the procedure had to be converted to avoid complications [25].
A large-scale multinational survey involving more than 500 participants from Japan, Korea, and Taiwan achieved that the commonly used indicators of surgical difficulty during LC, such as the duration of surgery, estimated blood loss, and open conversion rate, are inappropriate as they are surgeon- and workplace-dependent. Safety measures and recognition of landmarks and gallbladder anatomy during LC are performed at the surgeons’ discretion and are not yet standardized [26].
Laparoscopic surgeons have been found to have conversion rates to open surgery of one-fourth compared to others [27]. Laparoscopic surgeons tend to persist in laparoscopy even in case of significant lengthening of operating time caused by extensive viscerolysis, Calot fibrosis, or diffuse scarring of the gallbladder bed.
Another aspect to be considered is the training of young surgeons who have less experience in open cholecystectomy. Approximately 20% of surgeons find that conversion to open surgery does not make the procedure safer but sometimes more difficult [26].
In case of a difficult cholecystectomy, it is necessary to make sure to be as much as possible in the condition in which the conversion is an “elective” choice of the operator before being forced to do it as a result of major intraoperative complications.
10.2 Risk Factors to Open Conversion
Three types of factor have been identified in the literature that predispose to the conversion of the procedure and are classified as: patient-related, disease-related, and surgeon-related.
The “patient-related” factors are: age over 65 [28], male gender, BMI over 30, history of upper abdominal surgery, ASA score, cirrhosis [24, 29], diabetes mellitus (which can cause a delayed perception of symptoms) [30].
The “disease-related” factors are: gallbladder wall thicknesses (suggesting that a gallbladder wall thicker than 4–5 mm on preoperative ultrasound is a risk factor for conversion.); acute cholecystitis [29, 31], recurrent biliary colic, acute biliary pancreatitis; Mirizzi syndrome (found in 0.3–3%); GB cancer (which should be kept in mind in over 65-year-old patients, female gender, and high level of alkaline phosphatase); lithiasis of the main bile duct [6]; and the following laboratory data: CRP [32], WBC, albumin, and liver function tests [31].
“Surgeon-related” factors are the experience and skills of the surgeon [3, 33,34,35]. Using these factors, scores and models have been built to predict the conversion risk [6, 31, 36,37,38].
The pattern observed from these risk scores and models is that the risk of conversion increased when more risk factors were presented.
Defining risk factors for conversion and complications is important when planning the procedure and deciding who should perform the cholecystectomy [39].
In 2018, using the Delphi consensus methodology, Iwashita Y. identified 25 aspects of intraoperative difficulty associated to conversion; among these, diffuse scaring in the Calot’s triangle area had the strongest impact on surgical difficulty. Surgeons agreed that the surgical difficulty increases as more fibrotic change and scarring develop [18].
A “universally recognized” codification of the steps of laparoscopic cholecystectomy is essential, in particular, in the difficult gallbladder to limit the risks of BDI and biliary-vascular injury (BVI) [17]. The training of young surgeons should aim at this result.
The primary target of laparoscopic cholecystectomy is “safety first, total cholecystectomy second,” and the surgeon should always keep in mind this culture of safety and remain vigilant to stay ahead of dangerous situations. Safe management of the difficult gallbladder is possible with technical adjustment and careful use of bailout procedures.
10.3 Safe Steps in Laparoscopic Cholecystectomy in Difficult Gallbladder Pattern and When to Evaluate the Use of Bailout Procedures
We will develop the discussion by proposing a procedural algorithm built on the “Safe Step in Laparoscopic Cholecystectomy for Acute Cholecystitis” identified by Wakabayashi in the TG 2018, inserting moments of “time-out” in which the most common difficulties that can be found in the different steps will be identified, and codified solutions will be proposed (Fig. 10.2). In the next section of the chapter, we will examine in detail the bailout techniques.
The first assessment of the procedure should be made before carrying the patient to the operating room. It is necessary to assess with the anesthesiologist, depending on the anamnesis (COPD, cardiovascular diseases, previous surgery, especially of the upper abdomen, etc.) and the patient’s condition at the time of surgery (grade of cholecystitis according to TG18), whether he is able to tolerate pneumoperitoneum for an adequate operative time, also evaluating in advance the possibility of a long and complex viscerolysis [12].
If the patient’s condition is very damaged, consider the possibility of using percutaneous cholecystostomy as a definitive management or “bridge” to surgery [40].
10.4 In Operating Room
10.4.1 Step 1
A specific patient position is not considered as being better (French Vs American) [19].
In patients who have already been operated on, the site of the first port must be carefully chosen [41], also in variance with what is considered, the standard setting used in the surgical unit, in order to reduce the risk of being in an adhesion tangle that could lead to visceral iatrogenic lesions from the time of peritoneal access [12]. The method of placement of the first trocar should be tailored to the patient’s characteristics. The open trocar insertion is a safe technique [42]. In cirrhotic patients, the first port was placed, with the open technique, in the midline sub-umbelically to avoid undetected enlarged collateral vessels [43].
Once the pneumoperitoneum has been established, it is necessary to evaluate the extent and characteristics of the adhesions to assess the complexity and risk of iatrogenic lesions from viscerolysis and the possibility of a significant increase in surgical time compared to an open procedure [44]. This is a good time to decide on an early conversion.
In a patient where the supramesocolic district is inaccessible due to adhesions, perhaps between the transverse colon and the parietal peritoneum, a right subcostal laparotomy could allow access to the cholecystic lodge avoiding prolonged and risky viscerolysis maneuvers. The same applies if there are adhesions that significantly hinder the setting of two or more trocar. Usually the lysis of adhesions caused by an acute cholecystitis is all the easier the earlier the intervention is performed compared to the onset, while the exacerbation on previous acute episodes usually leads to tenacious adhesions. During viscerolysis maneuvers, it is advisable to proceed as far as possible to dissect using cold scissors [41]. In case of the first episode of cholecystitis, especially if the intervention is performed early at the onset of symptoms, the adhesions are smoothed out. In case of relapse on chronic cholecystitis or in case of covered perforation, the adhesions can be tenacious. Viscerolysis should be performed particularly carefully to avoid injury to the duodenum, which may be attached to the gallbladder (Fig. 10.3).
10.4.2 Step 2
Once the gallbladder has been visualized, the possibility of its mobilization must be evaluated.
The mobilization of the gallbladder is a key point because it allows the correct exposure of the hepatocystic triangle. A correct mobilization involves a traction with fundus clamp towards the right shoulder of the patient associated with a traction with a second clamp positioned on the Hartmann pouch downwards and to the right [45]. Surgeons should keep in mind that decreasing the number of port and/or using smaller instruments may create technical challenges, due to more difficult retraction and triangulation [19]. In the case of an outstretched gallbladder (hydropic or emphysematous), prior needle aspiration results in decompression with minimal intraperitoneal contamination [46] (Fig. 10.4). Once aspirated, it is necessary to evaluate the thickness and consistency of the walls of the gallbladder. The use of an endograsper or the placement of one or more traction points on the bottom can mobilize the most demanding gallbladder. Usually the bleeding resulting from the trauma of the gallbladder wall is annoying but not significant.
Outstreatched gallbladder. (a) A suction needle is introduced from the port on the right side (always under direct vision). (b) The emptying of the gallbladder can be facilitated by cautious compression (milking) maneuvers performed with a clamp. (c) At the end of the procedure the prick area is grasped with the forceps in order to limit as much as possible the outflow of residual bile
The presence of large necrotic areas can preclude the possibility of performing a valid gallbladder traction, while the presence of perforations with constant outflow of pus, bile, and calculi can make the evaluation of the operating field very complex.
Even a bulky calculus impacted in the infundibulum can limit the possibility of mobilization of the gallbladder; often cautious maneuvers with the aspirator or with forceps allow the infundibular calculus to dislodge towards the gallbladder body [47].
Another element to take into account is the liver. The presence of segment 3 or 4 hypertrophy covering the hepatic pedicle may require the use of a retractor to visualize the cholecystic hilum or may force the use of incorrect traction to visualize the hepatocystic triangle (Fig. 10.5).
Liver texture is another important variable because tractions on a steatosic liver can lead to lacerations with subsequent bleeding while a cirrhotic liver can be very difficult to mobilize because it is hard and fibrotic [43]. The adhesions in the cirrhotic patient may be hypervascularized. Furthermore, the finding of signs of portal hypertension should lead to a careful reconsideration of the therapeutic strategy chosen for the high risk of bleeding [43].
10.4.3 Step 3
10.4.3.1 Critical View of Safety (CVS)
The CVS is not a dissection technique. It is the final view that is achieved after a thorough dissection of the hepatocystic triangle to delineate the cystic duct and the cystic artery before they are clipped and divided. The CVS should be seen clearly both from the front and the back to have a complete circumferential visualization of the cystic duct and the artery (doublet view). The anterior view is easily achievable by retracting the infundibulum inferolaterally towards the right (with segment 5 surface visible across window), while the posterior view requires the infundibulum to be retracted towards the umbilical fissure (with segment 4/quadrate lobe surface visible across window). Dissection has to lead to the creation of 2 windows, one between the cystic duct and the artery and one between the artery and the liver. The cystic plate must be clearly identified [48].
To obtain the CVS, the coded technique involves three steps [49]:
-
(a)
Clearance of the hepatocystic (HC) triangle: The HC triangle should be cleared of all the fibrofatty and soft areolar tissue. Once adequately cleared of all fibrofatty tissue, the under surface of the liver is easily seen across this triangle.
-
(b)
Exposure of the lower cystic plate: The gallbladder should be separated from its liver bed to expose at least the lower third of the cystic plate.
-
(c)
Two and only two tubular structures should be seen entering the gallbladder: The cystic duct and the cystic artery [19] (Fig. 10.6).
The CVS should be seen clearly both from front (a) and the back (b) to have complete circumferential visualization of cystic duct and artery (doublet view). The HC triangle has been cleared of fibroadipose tissue, the gallbladder has been removed from the bottom third of the cystic plate, and 2 and only 2 structures are seen entering the gallbladder
Because of inflammation of the tissues of the hepatocystic triangle, the maneuvers often cause bleeding. For persistent bleeding, the surgeon must achieve hemostasis primarily by compression and by avoiding the excessive use of electrocautery or clipping [18]. The same gallbladder should be used as a compression instrument for parenchyma; the use of gauze and/or local hemostatic (e.g., oxidized cellulose) should also be considered [50]. There is a low level of evidence in favor of recommending a source of energy compared to another regarding safety. Bipolar, monopolar, and ultrasonic devices are appropriate source of energy for safe cholecystectomy [19]. If a monopolar energy device (most of the one with hook cautery) is used, it is important to keep it at a low setting; divide a small amount of tissue at a time after a gentle pull to avoid injury to deeper structures by the heel of the hook cautery; use intermittent short bursts of current at 2–3 s intervals in order to avoid thermal spread to the bile duct; and avoid blind use of cautery in the case of brisk bleeding [48, 51].
Bipolar cautery is useful to control bleeding in the HC triangle and in the liver bed.
Sometimes, the volumetric increase in Mascagni’s lymph node (usually a landmark for cystic artery localization) makes it difficult to identify the cystic artery and gall bladder neck. In addition, in recurring inflammation, Rouviere’s sulcus is often erased and not displayed correctly. In these cases, it is desirable to start the dissection very high in contact with the wall of the gallbladder, to which one must always remain attached, and then proceed on both sides to the gallbladder bed [52] (Fig. 10.7).
In chronic cholecystitis, inflammatory adhesions can melt the wall of the gallbladder to the wall of the common liver duct. If a biliary-cholecystic fistula is suspected at this stage, strongly consider a bailout technique [53].
In presence of severe acute and/or chronic inflammation, secure ductal identification by the critical view of safety (CVS) may be very challenging.
During “Step 3,” it may also be useful to stop several times, widen the view of the surgical field, reevaluate our landmarks, and then resume the isolation maneuvers.
In accordance with the data in the literature, we have identified the warning signs, which we will call “red flags,” to which particular attention should be paid during the acquisition of the CVS [47].
10.4.3.2 Red Flags
-
(a)
More than two tubular structures entering GB [48].
-
(b)
An unusually large presumed cystic artery (this may be the hepatic artery). The cystic artery is usually single, originates from RHA, and most commonly traverses the HC triangle. If the presence of a short cystic artery (<1 cm) is not noted during surgery, the right hepatic artery may be clipped and divided [54]. Keeping the dissection of the artery close to the gallbladder on the right side of the cystic lymph node may prevent injury to the right hepatic artery [47]. It may be useful to isolate a longer-than-usual tract of the artery to check whether it has penetrated the hepatic parenchyma or ended up in the gallbladder.
-
(c)
Large artery pulsations behind the presumed cystic ducts (this duct may be common hepatic duct).
-
(d)
A medium-large clip fails to occlude the ductal lumen (this duct may be the common hepatic duct) [55].
-
(e)
Large ductal structure that can be traced behind the duodenum.
-
(f)
Excessive fibrofatty/lymphatic tissue noted around the presumed cystic duct.
-
(g)
Bile leak seen with intact GB: See dedicated paragraph.
-
(h)
Bleeding requiring blood transfusion.
In persisting doubt, ask for a second opinion if available [56].
10.4.3.3 Warning
Alternative techniques to the standard exist and are implemented to obtain access to the cystic duct but they do not respect the principles of CVS.
The most widespread is the infundibular approach [57].
In the infundibular technique, cystic duct identification is based on the appearance of the infundibulum–cystic duct junction as a funnel. When this junction is circumferentially exposed, the surgeon confirms the identification of the cystic duct and then proceeds with its division. Complete dissection in the HC triangle is not performed at this stage. In certain situations, this technique can be misleading. When the cystic duct is fused with CBD due to acute or chronic inflammation, when the cystic duct is very short or effaced by a large stone impacted in the infundibulum, or when there is difficultly in exposing the HC triangle due to inadequate retraction, the CBD may be misidentified as the cystic duct. Circumferential dissection then goes around the CBD rather than around the cystic duct across the HC triangle. This leads to classic BDI where the bile duct is divided twice before the gallbladder can be completely separated from the liver. Therefore, this technique of cystic duct identification does not protect against biliary injury in difficult situations [48, 58,59,60].
The fundus first approach has been described as an alternative technique to complete LC in the presence of severe inflammation in the HC triangle. The gallbladder is dissected off its liver bed and then the cystic duct and the artery are identified and divided. However, the surgeons must be wary of this technique, they should have clear understanding of the cystic plate anatomy and pathological alteration affecting it, they should remain very close to the gallbladder throughout the dissection, and when such dissection does not seem possible, they should resort to bailout techniques like subtotal cholecystectomy (see dedicated paragraph).
Surgeons using this technique should be aware of this possible mishap [47].
Only after obtaining the CVS, surgeons should proceed to dissecting the artery and cystic duct [61].
10.4.4 Step 4
GB separation from its bed leaving behind the cystic plate attached to the liver.
Also, in this phase surgeons should pay attention especially in cirrhotic liver, scleroatrophic cholecystitis, and in partially intrahepatic and necrotic cholecystitis. It is better to leave a small portion of gallbladder in the cholecystic bed rather than enter the hepatic parenchyma in that site [43, 62].
Firstly, there might be troublesome bleeding from liver parenchyma, especially if the terminal tributaries of the middle hepatic vein (which lie in this location) are injured. This type of bleeding may sometimes require a conversion to open surgery for its control [62].
Secondly, sub-vesical bile ducts may be injured, causing a postoperative bile leak [63].
Such a breach is more likely to occur in chronic cholecystitis where the gallbladder may be densely adherent to the underlying liver without distinct dissection planes [64].
10.4.5 Special Issue: Bile Leak Seen with Intact GB
If bile appears in the operating field, you must identify the source of the leak. Do not proceed with blind dissections in search of the possible source [65], but try to obtain CVS. Once the cystic duct has been identified, perform an i.o. cholangiography to confirm and define the location and extent of the biliary injury [66, 67]. There is no evidence that IOC could prevent BDI [68,69,70], but IOC is recommended in order to define unclear anatomy [19]. In extreme cases, a cholangiography by direct puncture of the gallbladder can also be attempted. After the staging of the injury, you should make an evaluation of your experience in biliary surgery and decide how to proceed. In case of limited biliary surgery experience, it is preferable to drain the abdomen, even in laparoscopy, and refer the patient to a dedicated hospital. In case of partial lesion of the common bile duct, an attempt to suture on a tutor may be indicated, but always consider the technical experience of the surgeon and the stage of inflammation. In case of complete sections of CBD or complex lesions, it is advisable to stop the procedure, drain the abdomen, and send the patient to a reference center [67, 71].
If you convert the laparoscopic procedure into open surgery to define the extent of the lesion, you should not proceed with an extensive dissection of the hepatocystic triangle if you are not sure that the problem can be properly solved.
10.5 Bailout Procedures
In situation of a difficult gallbladder, when the target (cystic duct, cystic artery, and CBD) identification with the CVS cannot be properly achieved, it is not important to push ahead with the goal of a complete cholecystectomy while risking the patient’s safety due to potential biliary/vascular injury. It is important to perform an alternative procedure that allows the surgeon to complete the procedure in a safe manner [47].
Bail-out techniques include: cholecystostomy, partial cholecystectomy and conversion to open surgery. In addition to these one should consider the fundus first approach, which is a hybrid way to try to complete the cholecystectomy laparoscopically, adopting the open surgery approach from the bottom of the gallbladder, with the possibility to conclude in a partial cholecystectomy.
The use of these methods should not be considered as a failure, but on the contrary as an integral and responsible part of the patient’s path of care and as a way to safeguard his/her health.
The operating surgeon should not hesitate to seek a second opinion whenever needed, and this should be considered as a sign of good clinical practice rather than a sign of surgical incompetence.
10.5.1 Cholecystostomy
Tube cholecystostomy could be a simple bridge procedure to provide symptomatic relief until a definitive procedure can be performed. It can be done percutaneously, laparoscopically, or after conversion to open surgery [40, 72,73,74].
10.5.1.1 Percutaneous Cholecystostomy
A percutaneous cholecystostomy tube serves an important function for patients with cholecystitis who are unable to undergo immediate cholecystectomy safely. An increasing TG18 Grade and comorbidity status are the primary predictors of need for cholecystostomy [75]. If the patient is a poor candidate for general anesthesia, has had symptoms for more than 72 h, or has an advanced metastatic disease, PTC is considered [73, 74]. A trans hepatic approach is typically chosen to enter the gallbladder, with moderate sedation used for nearly all cases. Tube placement procedures were performed by the interventional radiologist with US or CT guidance.
10.5.1.2 Laparoscopically Cholecystostomy
During a laparoscopic approach, the decision to proceed with cholecystostomy is related to the finding of a gangrenous gallbladder and severe inflammation of the HC triangle in a patient in severe general condition. Laparoscopic cholecystostomy is performed when the intraoperative finding shows a condition in which a cholecystectomy cannot be performed safely [76]. The procedure can be facilitated by the use of the laparoscopic ultrasound guidance. An 18-gauge needle was inserted in subcostal position at the midclavicular line into the gallbladder. Once the bile has been aspirated, a guidewire is placed within the lumen of the gallbladder. Over the guidewire, a 14 F catheter is placed into the lumen of the gallbladder and secured to the skin. If there is a rupture on the gallbladder wall, it is easier to empty the gallbladder with the aspirator and then place a catheter with a balloon inside the gallbladder so that it can be safely fixed (without spillage) to the abdominal wall [77, 78].
10.5.1.3 Open Cholecystostomy
The same procedures performed in laparoscopy are also available in open surgery [47].
In most cases when a surgical cholecystostomy is performed, the preoperative assessment is probably not adequate.
10.5.2 Fundus First
In case of diffuse inflammation of Calot’s triangle, continued dissection to obtain the CVS might result in BDI [79] and an anterograde approach may represent an alternative to immediate conversion to open cholecystectomy [80,81,82].
Requirements for a safe dome-down technique are: (a) clear understanding of the anatomy of the cystic and hilar plates [83]; (b) the dissection should be maintained along the subserosal-inner layer to avoid vascular and/or biliary injury [19].
The procedure involves the following steps:
-
1.
The gallbladder is dissected away from the gallbladder bed from the fundus down towards the cystic duct [83].
-
2.
Dissection then continues along the gallbladder [84]. The cystic artery is identified, isolated, ligated, and transected.
-
3.
The cystic duct is positively identified and isolated, creating a 360-degree view of the gallbladder–cystic duct junction.
-
4.
The cystic duct is ligated and divided.
This technique poses a technical challenge in handling the gallbladder, as it tends to twist once separated completely from the liver, and also in liver retracting.
In chronic cholecystitis with a small and contracted gallbladder, the longitudinal length of the cystic plate from the fundus to its attachment with right portal pedicle sheath becomes short. Without appreciating this pathologic shortening, the surgeon may enter into the right portal pedicle sheath soon after dissecting the fundus/body of the gallbladder. This may cause injury to the right portal pedicle structures causing serious VBI [64].
10.5.3 SubTotal Cholecystectomy
In 1954, McElmoyle first [85] described and illustrated the principles and technique of this operation when performed specifically for the prevention of bile duct or vascular injury during a difficult cholecystectomy. No attempt is made to dissect the cystic duct or artery when inflammation obscures the neck of the gallbladder. The gallbladder is opened and the redundant portions excised. The cystic duct and the portions of the body, neck, and infundibulum lying above and to the left side are left in situ as a shield to the vulnerable structures, then renamed “Shield of McElmoyle.” The cystic duct is not closed; its mucosa is ablated and a drain is placed.
Thirty years later, Bornman and Terblanche [86] described their experience in managing difficult gallbladders in cases of severe cholecystitis. Bickel and Shtamler in 1993 describe their successful experience in the treatment of six patients with the use of laparoscopic subtotal cholecystectomy [87].
In literature, “partial,” “subtotal,” “insufficient,” and “uncompleted” are different terms used to define the same concept. Strasberg in 2016 suggested that the term “subtotal” should be preferred since it expresses the nearly complete removal of the gallbladder.
It is important to remove all stones from the gallbladder, to ablate the mucosa of the gallbladder stump (with diathermy or argon plasma coagulator) and leave this stump as small as possible.
10.5.3.1 Technique [88]
The modern technique to perform these operations is clear and can be performed both laparoscopically and in open surgery. The gallbladder is opened along its long axis and emptied of stones, including those in the lumen of the gallbladder neck and cystic duct if possible. Surgeons are recommended to remove all stones from the peritoneal cavity, if necessary, by placing them in an endo-bag. The portion of the gallbladder adherent to the liver is usually left in situ and ablated. The latter may be done with electrocautery, a bipolar forceps, an argon beam, or saline-linked radiofrequency ablation. Alternatively, some or all of the gallbladder attached to the liver may be removed. When this is done, the gallbladder wall and cystic plate may be removed down to the bare liver or the cystic plate may be left in situ. In some cases, the gallbladder will be gangrenous. If so, the gangrenous portion should be excised without widening the extent of the subtotal resection. In a cirrhotic patient, the risk of bleeding from the liver bed may be theoretically avoided in subtotal cholecystectomy by not removing the posterior GB wall.
The area should be carefully drained. The gallbladder lip is usually somewhat larger and the lumen is closed by sutures [88, 89].
The patient must be informed that a gallbladder remnant may result in the formation of new stones with the risk of a new cholecystitis with a possibly challenging preoperative cholecystectomy [90].
10.5.3.2 Cross-Check the Different Techniques
Based on the meta-analysis of Elshaer and coworkers, on a sample of 1231 subtotal cholecystectomies, in 72.9% of cases the procedure was performed laparoscopically. They found low rate of postoperative BDI (0.08%) but higher rates of bile leak (18%), particularly in open procedures. These fistulas seem to resolve spontaneously in most cases within 2 weeks [89].
More patients whose surgery ended with a drainage left under the cholecystic bed because the stump of the neck of the gallbladder was not closed underwent postoperative ERCP compared to those in whom closure was successfully performed, but there was no change in the rate of complications [91].
Based on the meta-analysis of Elshaer and coworkers, it seems that a subtotal fenestrating cholecystectomy is more likely to be done when an open approach is used [89].
Paradoxically, in their data, bile leaks were more common after a laparoscopic procedure. Possibly, this is due to the improved ability to suture the cystic duct orifice when the procedure is done in open surgery [89].
Based on presently available information, it would seem that the fenestrating type of subtotal cholecystectomy would be preferable, but knowledge in this area is very incomplete.
There is no enough information regarding the incidence of symptomatic gallbladder remnants after subtotal cholecystectomies [91].
Laparoscopic subtotal cholecystectomies generally produced better outcomes compared with open subtotal cholecystectomies, but nonsignificant differences were found between the technique of closure or nonclosure of the cystic duct or gallbladder stump and removal compared to the nonremoval of the gallbladder posterior wall [89].
10.5.4 Open Conversion
In the past, difficult cholecystectomy was strongly associated with conversion to open surgery. More recently due to decreasing experience in open surgery, alternative tricks are considered before resorting to conversion [19, 92].
The need for conversion is often related to a problem of gallbladder mobilization or the need to control a massive bleeding.
It is important to realize that simply converting to an open procedure does not safeguard against bile duct/vascular injury [93]. A difficult procedure may remain difficult even after conversion to open surgery with no effect on postoperative complications [94].
When setting up the operating room for acute cholecystitis or when we suspect a difficult gallbladder, it is a good rule to prepare the operating bed for the placement of poles to anchor the retractor for the right hypochondrium.
When converting the procedure into open surgery, you need to have a few landmarks in mind.
-
(a)
The abdomen should be deflated before incision, as the pneumoperitoneum distorts the abdominal wall anatomy. Subcostal laparotomy is made 2 to 3 fingerbreadths below the ribs (Fig. 10.8). The incision should allow ideal access to the hepatic pedicle; if necessary, it is preferable to extend it to the left [62].
-
(b)
When the cause of conversion is the difficulty in identifying or releasing the gallbladder, the first point to find is the anterior margin of the liver. It is necessary to begin the release possibly starting from the right side of the gallbladder and then moving to the left. When the bottom of the gallbladder is discovered, if possible, it is grasped with a ring clamp and the release continues on the lower face of the gallbladder, descending towards the Winslow which the surgeon should explore using his index finger. The round ligament then is divided (except in cirrhotic patients), and a retractor is placed. In the most difficult cases, where adhesions due to inflammation or previous surgery have made the structures unrecognizable, more effort is required; the hepatic flexure of the colon and duodenum may be mobilized to the left.
Placement of sponges behind the liver to lift it forward is often helpful with exposure.
Use the Bismuth maneuver of straightening the hepatic peduncle for the correct identification of CBD: downward traction of the duodenum with a sponge and upward traction of the S4 base [52].
-
(c)
When the rearrangement of the hepatic pedicle makes the management of the cystic pedicle dangerous, it is advisable to continue by anterograde approach [95].
Depending on the adherent inflammation pattern, the detachment of the gallbladder from its bed can be started at the intermediate portion.
In chronic cholecystitis with a small and contracted gallbladder, the longitudinal length of the cystic plate from the fundus to its attachment with the right portal pedicle sheath becomes short. This may cause injury to the right portal pedicle structures causing serious BDI/VBI when the surgeon enters into the right portal pedicle sheath.
Despite the dome-down approach, if it is not possible to safely isolate and manage the cystic duct and cystic artery, consider performing a subtotal cholecystectomy.
-
(d)
Recurrent episodes of cholecystitis can lead to the formation of biliodigestive fistulas (with the duodenum or colon). Cholecystic duodenal fistulas are the most common [96]. The finding is in most cases occasional, and the presence of a fistula must be suspected when you are locked on a very tight adhesion. We proceed by isolating the fistula on both sides. The detachment between the two organs will occur on the cholecystic side. Usually, the fistula is small, and once the healthy margins of the duodenum are prepared, a suture can be performed.
Less often the situation can be more complex: the duodenum is fused between a scleroatrophic gallbladder and the CBD. It is advisable to empty the gallbladder and try a cholangiographic study before deciding what strategy to take.
Cholecystocolic fistulae usually affect the bottom of the gallbladder. Their isolation is easier. They can be treated with either a mechanical suture or a direct suture on the colic side [97].
Fistulas between gallbladder and bile duct deserve separate treatment and will not be the subject of this work.
-
(e)
In case of bleeding from the HC triangle not controlled by compression, consider using the Pringle maneuver as an alternative to achieve temporary hemostasis and identify the source of bleeding. Sometimes it is enough to compress the pedicle between the fingers. Avoid placing stitches blindly or extensive blind coagulation.
In case of bleeding from the gallbladder bed, try compression with gauze and contact hemostatic. As far as possible try to identify whether the source of bleeding is from the roots of the suprahepatic vein [50].
10.6 Conclusions
Laparoscopic cholecystectomy is the gold standard for the treatment of gallbladder symptomatic lithiasis. To decrease the incidence of BDI during LC, an effective surgical education system is imperative [98]. While it is easy to underestimate LC as a basic general surgical procedure, the operation may actually be one of the most difficult challenges unexpectedly facing the general surgeon, who should adopt a mindset of “preparing for the worst.” In nearly 20% of cases, there is a difficult gallbladder situation that makes the procedure a challenge for the surgeon.
We should always keep in mind that the procedure is performed for a benign pathology. Routine adoption of Culture of Safety in Cholecystectomy (COSIC) may help reduce the incidence of post cholecystectomy biliary/vascular injury [47].
While strict adherence to the CVS is important to decrease BDI, it is only one part of the COSIC, which mandates safety to be at the forefront. Besides achieving the CVS in cases of total cholecystectomy, COSIC also requires an appropriate selection and workup of the patient, the adjustment of the surgical technique in the setting of nonroutine cases, the use of bailout procedures, and the avoidance of complex cases when appropriate experience is not available [99].
A key concept when performing a difficult cholecystectomy is to promptly recognize that change in surgical strategy may result in minor risk of bile duct injury. The conversion of a laparoscopic cholecystectomy to an open procedure should not be experienced as a failure.
Despite overall low incidence of adverse events during LC, the high rate of LC leads to a significant absolute number of patients who suffer from long-term adverse events, one of the most significant being BDI. Otto Von Bismarck once said “Fools say they learn from experience; I prefer to learn from the experience of others.”
References
Harboe KM, Bardram L. The quality of cholecystectomy in Denmark: outcome and risk factors for 20,307 patients from the national database. Surg Endosc. 2011;25:1630–41.
Navez B, Ungureanu F, Michiels M, et al. Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc. 2012;26:2436–45.
Giger UF, Michel JM, Opitz I, et al. Risk factors for perioperative complications in patients undergoing laparoscopic cholecystectomy: analysis of 22,953 consecutive cases from the Swiss Association of Laparoscopic and Thoracoscopic Surgery database. J Am Coll Surg. 2006;203:723–8.
Rosen M, Brody F, Ponsky J, et al. Predictive factors for conversion of laparoscopic cholecystectomy. Am J Surg. 2002;184:254–8.
Zhang W, Li J, Wu G, et al. Risk factors affecting conversion in patients undergoing laparoscopic cholecystectomy. ANZ J Surg. 2008;78(11):973–6.
Sutcliffe RP, Hollyman M, Hodson J, et al. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: a validated risk score derived from a prospective UK database of 8820 patients. J Hepatobiliary Pancreat Sci. 2016;18(11):922–8.
Gurusamy K, Samraj K, Gluud C, et al. Meta-analysis of randomized controlled trials on the safety and effectiveness of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:141–50.
Shingu Y, Komatsu S, Norimizu S, et al. Laparoscopic subtotal cholecystectomy for severe cholecystitis. Surg Endosc. 2016;30(2):526–31.
Simorov A, Ranade A, Parcells J, et al. Emergent cholecystostomy is superior to open cholecystectomy in extremely ill patients with acalculous cholecystitis: a large multicenter outcome study. Am J Surg. 2013;206(6):935–41.
Salky BA, Edye MB. The difficult cholecystectomy: problems related to concomitant diseases. Semin Laparosc Surg. 1998;5(2):107–14.
Ashfaq A, Ahmadieh K, Shah AA, et al. The difficult gall bladder: outcomes following laparoscopic cholecystectomy and the need for open conversion. Am J Surg. 2016;212:1261–4.
Santos BF, Brunt LM, Pucci MJ. The difficult gallbladder: a safe approach to a dangerous problem. J Laparoendosc Adv Surg Tech A. 2017;27:571–8.
Miettinen P, Pasanen P, Lahtinen J, et al. Acute abdominal pain in adults. Ann Chir Gynaecol. 1996;85:5–9.
Ukkonen M, Kivivuori A, Rantanen T, et al. Emergency abdominal operations in the elderly: a multivariate regression analysis of 430Consecutive patients with acute abdomen. World J Surg. 2015;39:2854–61.
Wilson E, Gurusamy K, Gluud C, et al. Cost-utility and value-of information analysis of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Br J Surg. 2010;97:210–9.
Kais H, Hershkovitz Y, Abu-Snina Y, et al. Different setups of laparoscopic cholecystectomy: conversion and complication rates: a retrospective cohort study. Int J Surg. 2014;12(12):1258–61.
Wakabayashi G, Iwashita Y, Hibi T, et al. Tokyo guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:73–86.
Iwashita Y, Hibi T, Ohyama T, et al. Delphi consensus on bile duct injuries during laparoscopic cholecystectomy: an evolutionary cul-de-sac or the birth pangs of a new technical framework? J Hepatobiliary Pancreat Sci. 2017;24:591–602.
Conrad C, Wakabayashi G, Asbun HJ, et al. IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 2017;24:603–15.
Ansaloni L, Pisano M, Coccolini F, et al. 2016 WSES guidelines on acute calculous cholecystitis. World J Emerg Surg. 2016;11:25.
Tornqvist B, Waage A, Zheng Z, et al. Severity of acute cholecystitis and risk of iatrogenic bile duct injury during cholecystectomy, a population-based case-control study. World J Surg. 2016;40:1060–7.
Conte D, Fraquelli M, Fornari F, et al. Close relation between cirrhosis and gallstones: cross-sectional and longitudinal survey. Arch Intern Med. 1999;159:49–52.
Laurence JM, Tran PD, Richardson AJ, et al. Laparoscopic or open cholecystectomy in cirrhosis: a systematic review of outcomes and meta-analysis of randomized trials. HPB. 2012;14:153–61.
Machado NO. Laparoscopic cholecystectomy in cirrhotics. J Soc Laparoendosc Surg. 2012;16:392–400.
Pucher PH, Brunt LM, Davies N, et al. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc. 2018;32:2175–83.
Hibi T, Iwashita Y, Ohyama T, et al. The ‘right’ way is not always popular: comparison of surgeons’ perceptions during laparoscopic cholecystectomy for acute cholecystitis among experts from Japan, Korea and Taiwan. J Hepatobiliary Pancreat Sci. 2017;24:24–32.
Kortram K, Reinders JS, van Ramshorst B, et al. Laparoscopic cholecystectomy for acute cholecystitis should be performed by a laparoscopic surgeon. Surg Endosc. 2010;24:2206–9.
Yang TF, Guo L, Wang Q. Evaluation of preoperative risk factor for converting laparoscopic to open cholecystectomy: a meta-analysis. Hepato-Gastroenterology. 2014;61(132):958–65.
Philip Rothman J, Burcharth J, Pommergaard HC, et al. Preoperative risk factors for conversion of laparoscopic cholecystectomy to open surgery—a systematic review and meta-analysis of observational studies. Dig Surg. 2016;33(5):414–23.
Paajanen H, Suuronen S, Nordstrom P, et al. Laparoscopic versus open cholecystectomy in diabetic patients and postoperative outcome. Surg Endosc. 2011;25(3):764–70.
Goonawardena J, Gunnarsson R, de Costa A. Predicting conversion from laparoscopicto open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am J Surg. 2015;210(3):492–500.
Terho PM, Leppäniemi AK, Mentula PJ. Laparoscopic cholecystectomy for acute calculous cholecystitis: a retrospective study assessing risk factors for conversion and complications. World J Emerg Surg. 2016;11:54.
Andrews S. Does concentration of surgical expertise improve outcomes for laparoscopic cholecystectomy? 9-year audit cycle. Surgeon. 2013;11(6):309–12.
Madani A, Watanabe Y, Feldman LS, et al. Expert intraoperative judgment and decision-making: defining the cognitive competencies for safe laparoscopic cholecystectomy. J Am Coll Surg. 2015;221(5):931–40 e8.
Beliaev AM, Booth M. An association between conversion of laparoscopic cholecystectomy to open surgery and intra-abdominal organ injury. ANZ J Surg. 2016;86(7–8):625.
Kologlu M, Tutuncu T, Yuksek YN, et al. Using a risk score for conversion from laparoscopic to open cholecystectomy in resident training. Surgery. 2004;135:282–7.
Lipman JM, Claridge JA, Haridas M, et al. Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery. 2007;142:556–63.
Sugrue M, Sahebally SM, Ansaloni L, et al. Grading operative findings at laparoscopic cholecystectomy- a new scoring system. World J Emerg Surg. 2015;10:14.
Hobbs MS, Mai Q, Knuiman MW, et al. Surgeon experience and trends in intraoperative complications in laparoscopic cholecystectomy. Br J Surg. 2006;93(7):844–53.
Stanek A, Dohan A, Barkun J, et al. Percutaneous cholecystostomy: a simple bridge to surgery or an alternative option for the management of acute cholecystitis? Am J Surg. 2018;216(3):595–603.
Balasuriya HD, Dunn DJ, Joseph MG. How to gain safe entry for laparoscopic cholecystectomy in the multi-scarred abdomen. ANZ J Surg. 2018;88(4):376–7.
Ahmad G, Gent D, Henderson D, et al. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2015;8:CD006583.
Palanivelu C, Rajan PS, Jani K, et al. Laparoscopic cholecystectomy in cirrhotic patients: the role of subtotal cholecystectomy and its variants. J Am Coll Surg. 2006;203(2):145–51.
Ercan M, Bostanci EB, Ulas M, et al. Effects of previous abdominal surgery incision type on complications and conversion rate in laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2009;19:373–8.
Eikermann M, Siegel R, Broeders I, et al. Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 2012;26(11):3003–39.
Giger U, Michel JM, Vonlanthen R, et al. Laparoscopic cholecystectomy in acute cholecystitis: indication, technique, risk and outcome. Langenbeck’s Arch Surg. 2005;390:373–80.
Gupta V, Jain G. Safe laparoscopic cholecystectomy: adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg. 2019;11(2):62–84.
Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211:132–8.
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 1995;180:101–25.
Masci E, Faillace G, Longoni M. Use of oxidized regenerated cellulose to achieve hemostasis during laparoscopic cholecystectomy: a retrospective cohort analysis. BMC Res Note. 2018;11(1):239.
Singh R, Brunt L. Critical view of safety-its feasibility and efficacy in preventing bile duct injuries. Ann Laparosc Endosc Surg. 2018;3:2.
Nuzzo G, Giuliante F, Giovannini I, et al. Le lesioni iatrogene della via biliare principale. Collana Monografica della Società Italiana di Chirurgia - N. 18, anno 2002.
Strasberg SM, Eagon CJ, Drebin JA. The “hidden cystic duct” syndrome and the infundibular technique of laparoscopic cholecystectomy--the danger of the false infundibulum. J Am Coll Surg. 2000;191:661–7.
Andall RG, Matusz P, du Plessis M, Ward R, Tubbs RS, Loukas M. The clinical anatomy of cystic artery variations: a review of over 9800 cases. Surg Radiol Anat. 2016;38:529–39.
Dixon E, Vollmer CM Jr, May GR. Management of benign biliary stenosis and injury. Switzerland: Springer; 2015. p. 165–86.
GuptaV. ABCD of safe laparoscopic cholecystectomy: imbibing universal culture of safety in cholecystectomy. Indian J Surg. 2018;81:203–4.
Vettoretto N, Saronni C, Harbi A, et al. Critical view of safety during laparoscopic cholecystectomy. JSLS. 2011;15:322–5.
Strasberg SM. Biliary injury in laparoscopic surgery: part 2. Changing the culture of cholecystectomy. J Am Coll Surg. 2005;201:604–11.
Strasberg SM. Error traps and vasculo-biliary injury in laparoscopic and open cholecystectomy. J Hepatobiliary Pancreat Surg. 2008;15:284–92.
Strasberg SM. Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepato-Biliary-Pancreat Surg. 2002;9(5):543–7.
Nijssen MA, Schreinemakers JM, Meyer Z, et al. Complications after laparoscopic cholecystectomy: a video evaluation study of whether the critical view of safety was reached. World J Surg. 2015;39(7):1798–803.
Sanford DE. An update on technical aspects of cholecystectomy. Surg Clin North Am. 2019;99(2):245–58.
Schnelldorfer T, Sarr MG, Adams DB. What is the duct of Luschka?--a systematic review. J Gastrointest Surg. 2012;16(3):656–62.
Strasberg SM, Gouma DJ. ‘Extreme’ vasculobiliary injuries: association with fundus-down cholecystectomy in severely inflamed gallbladders. HPB (Oxford). 2012;14(1):1–8.
Parrilla P, Robles R, Varo E, et al. Liver transplantation for bile duct injury after open and laparoscopic cholecystectomy. Br J Surg. 2014;101(2):63–8.
Tornqvist B, Stromberg C, Persson G, et al. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population-based cohort study. BMJ. 2012;345:e6457.
Nuzzo G, Giuliante F, Giovannini I, et al. Bile duct injury during laparoscopic cholecystectomy. Results of an Italian national survey on 56591 cholecystectomies. Arch Surg. 2005;140:986–92.
Nuzzo G, Giuliante F, Giovannini I, et al. Re: role of intraoperative cholangiography in avoiding bile duct injury. J Am Coll Surg. 2007;205(4):e5–6; author reply e6.
Ford JA, Soop M, Du J, et al. Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 2012;99:160–7.
Sajid MS, Leaver C, Haider Z, et al. Routine on-table cholangiography during cholecystectomy: a systematic review. Ann R Coll Surg Engl. 2012;94:375–80.
Nuzzo G, Giuliante F, Giovannini I, et al. Advantages of multidisciplinary management of bile duct injuries occurring during cholecystectomy. Am J Surg. 2008;195(6):763–9.
Akhan O, Akinci D, Ozmen MN. Percutaneous cholecystostomy. Eur J Radiol. 2002;43(3):229e236.
Colonna AL, Griffiths TM, Robison DC, et al. Cholecystostomy: are we using it correctly? Am J Surg. 2019;217(6):1010–5.
Gurusamy KS, Rossi M, Davidson BR. Percutaneous cholecystostomy for high-risk surgical patients with acute calculous cholecystitis. Cochrane Database Syst Rev 2013;(8):CD007088.
Okamoto K, Suzuki K, Takada T, et al. Tokyo guidelines 2018: flowchart for the management of acute cholecystitis. J Hepatobiliary Pancreat Sci. 2018;25:55–72.
Haicken BN. Laparoscopic tube cholecystostomy. Surg Endosc. 1992;6(6):285–8.
Berber E, Engle KL, String A. Selective use of tube cholecystostomy with interval laparoscopic cholecystectomy in acute cholecystitis. Arch Surg. 2000;135(3):341–6.
Cherng N, Witkowski ET, Sneider EB, et al. Use of cholecystostomy tubes in the management of patients with primary diagnosis of acute cholecystitis. J Am Coll Surg. 2012;214(2):196–201.
Iwashita Y, Ohyama T, Honda G, et al. What are the appropriate indicators of surgical difficulty during laparoscopic cholecystectomy? Results from a Japan– Korea–Taiwan multinational survey. J Hepatobiliary Pancreat Sci. 2016;23:533–47.
Kelly MD. Laparoscopic retrograde (fundus first) cholecystectomy. BMC Surg. 2009;9:19.
Fullum TM, Kim S, Dan D, et al. Laparoscopic “dome-down” cholecystectomy with the LCS-5 harmonic scalpel. JSLS. 2005;9(1):51–7.
Huang SM, Hsiao KM, Pan H, et al. Overcoming the difficulties in laparoscopic management of contracted gallbladders with gallstones: possible role of fundus-down approach. Surg Endosc. 2011;25(1):284–91.
Honda G, Iwanaga T, Kurata M. Dissection of the gallbladder from the liver bed during laparoscopic cholecystectomy for acute or subacute cholecystitis. J Hepato-Biliary-Pancreat Surg. 2008;15(3):293–6.
Honda G, Iwanaga T, Kurata M, et al. The critical view of safety in laparoscopic cholecystectomy is optimized by exposing the inner layer of the subserosal layer. J Hepato-Biliary-Pancreat Surg. 2009;16(4):445–9.
McElmoyle WA. Cholecystectomy: a method for the difficult gall-bladder. Lancet. 1954;266(6826):1320–3.
Bornman PC, Terblanche J. Subtotal cholecystectomy: for the difficult gallbladder in portal hypertension and cholecystitis. Surgery. 1985;98:1):1–6.
Bickel A, Shtamler B. Laparoscopic subtotal cholecystectomy. J Laparoendosc Surg. 1993;3(4):365–7.
Strasberg SM, Pucci MJ, Brunt LM, et al. Subtotal cholecystectomy-“Fenestrating” vs “Reconstituting” subtypes and the prevention of bile duct injury: definition of the optimal procedure in difficult operative conditions. J Am Coll Surg. 2016;222(1):89–96.
Elshaer M, Gravante G, Thomas K, et al. Subtotal cholecystectomy for “difficult gallbladders”: systematic review and meta-analysis. JAMA Surg. 2015;150(2):159–68.
Pernice LM, Andreoli F. Laparoscopic treatment of stone recurrence in a gallbladder remnant: report of an additional case and literature review. J Gastrointest Surg. 2009;13(11):2084–91.
Henneman D, da Costa DW, Vrouenraets BC, et al. Laparoscopic partial cholecystectomy for the difficult gallbladder: a systematic review. Surg Endosc. 2013;27(2):351–8.
Lengyel BI, Azagury D, Varban O, et al. Laparoscopic cholecystectomy after a quarter century: why do we still convert? Surg Endosc. 2012;26(2):508–13.
Mangieri CW, Hendren BP, Strode MA, et al. Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 2019;33(3):724–30.
Borzellino G, Sauerland S, Minicozzi AM, et al. Laparoscopic cholecystectomy for severe acute cholecystitis. A meta-analysis of results. Surg Endosc. 2008;22(1):8–15.
Jenkins PJ, Paterson HM, Parks RW, et al. Open cholecystectomy in the laparoscopic era. Br J Surg. 2007;94(11):1382–5.
Tantia O, Bandyopadhyay SK, Sen B, et al. Pericholecystic fistula: a study of 64 cases. Int Surg. 2002;87(2):90–3.
Mourot J. Colecistectomia per via laparotomica per litiasi della colecisti. In: Tecniche Chirurgiche-Addominale. Paris: EMC (Elsevier Masson SAS); 2006. p. 40–920.
McKinley SK, Brunt LM, Schwaitzberg SD. Prevention of bile duct injury: the case for incorporating educational theories of expertise. Surg Endosc. 2014;28:3385–91.
Chen CB, Palazzo F, Doane SM, et al. Increasing resident utilization and recognition of the critical view of safety during laparoscopic cholecystectomy: a pilot study from an academic medical center. Surg Endosc. 2017;31:1627–35.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
D’Acapito, F., La Barba, G., Togni, C., Ercolani, G. (2021). Difficult Laparoscopic Cholecystectomy: When to Convert to Open Technique. In: Di Carlo, I. (eds) Difficult Acute Cholecystitis. Springer, Cham. https://doi.org/10.1007/978-3-030-62102-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-62102-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62101-8
Online ISBN: 978-3-030-62102-5
eBook Packages: MedicineMedicine (R0)